Abstract
AKI is characterized by increased catecholamine levels and hypertension. Renalase, a secretory flavoprotein that oxidizes catecholamines, attenuates ischemic injury and the associated increase in catecholamine levels in mice. However, whether the amine oxidase activity of renalase is involved in preventing ischemic injury is debated. In this study, recombinant renalase protected human proximal tubular (HK-2) cells against cisplatin- and hydrogen peroxide–induced necrosis. Similarly, genetic depletion of renalase in mice (renalase knockout) exacerbated kidney injury in animals subjected to cisplatin-induced AKI. Interestingly, compared with the intact renalase protein, a 20–amino acid peptide (RP-220), which is conserved in all known renalase isoforms, but lacks detectable oxidase activity, was equally effective at protecting HK-2 cells against toxic injury and preventing ischemic injury in wild-type mice. Furthermore, in vitro treatment with RP-220 or recombinant renalase rapidly activated Akt, extracellular signal-regulated kinase, and p38 mitogen-activated protein kinases and downregulated c-Jun N-terminal kinase. In summary, renalase promotes cell survival and protects against renal injury in mice through the activation of intracellular signaling cascades, independent of its ability to metabolize catecholamines, and we have identified the region of renalase required for these effects. Renalase and related peptides show potential as therapeutic agents for the prevention and treatment of AKI.
AKI is a common clinical condition affecting up to 20% of hospitalized patients and is frequently associated with sepsis, surgery, and certain drugs. Epidemiologic data indicate a positive association between the severity of AKI and in-hospital and long-term mortality.1,2 Unfortunately, the development of effective therapy for AKI has been hampered by (1) an inherent delay in diagnosis, a consequence of relying on serum creatinine, which only increases 48–72 hours after the original insult, and (2) the paucity of validated targets of therapy. There is an urgent need to identify novel therapeutic modalities.
Renalase is a novel secretory flavoprotein with amine oxidase activity.3–5 In vitro, renalase metabolizes epinephrine, norepinephrine, and dopamine and also possesses significant intrinsic nicotinamide adenine dinucleotide (NADH) oxidase activity.6,7 Other investigators have questioned the amine oxidase activity of renalase.8,9 We had proposed that, in contrast to the classic amine oxidases, renalase reacts with oxygen to generate superoxide anions and hydrogen peroxide, with subsequent oxidation of catecholamines to their respective aminochromes. Recent results indicate that renalase functions as an oxidase/anomerase, using molecular oxygen to convert α-NAD(P)H to β-NAD+, with hydrogen peroxide as reaction byproduct.10 Because it was also shown to bind epinephrine, the authors pointed out that the hydrogen peroxide (H2O2) generated from the anomerase reaction will drive the oxidation of epinephrine to adrenochrome, albeit at a slower rate. Single-nucleotide polymorphisms present in the gene are associated with hypertension, cardiac disease, and diabetes.6,11–14 The administration of renalase in wild-type (WT) mice lowers plasma catecholamines and systemic BP. In contrast, the deficiency of renalase in renalase knockout (KO) mice raises catecholamine levels and BP.15 Renalase also modulates the severity of renal ischemia and reperfusion injury.16 In WT mice, the administration of recombinant human renalase before induction of renal ischemia significantly blunts the severity of renal injury, with less renal tubular necrosis, inflammation, and apoptosis. In contrast, the lack of renalase in renalase KO mice exacerbates the renal damage after similar ischemic injury.
AKI is characterized by an elevation in plasma catecholamine levels. It has been postulated that, in addition to causing hypertension, excess catecholamines in AKI may produce an inflammatory response, aggravating tissue damage and contributing to multiorgan dysfunction.17 Interestingly, renalase levels in the blood and kidneys of WT mice are reduced following acute renal ischemia. Because the renalase in blood is secreted from the kidneys and is thought to metabolize circulating catecholamines, the excess catecholamines in AKI may be a direct consequence of the concurrent renalase deficiency. Notably, the administration of renalase to WT mice before induction of ischemia, which greatly attenuates ischemic renal injury, dampens the rise in blood catecholamine levels. On the basis of these findings, it is inviting to speculate that the renal protective effect in ischemic injury of renalase and its hemodynamic effect stem from its amine oxidase activity.
The role of amine oxidase activity of renalase in mediating its hemodynamic effect has been questioned. First, measurement of the amine oxidase activity of renalase relies on the production of H2O2 as an indirect measure of oxidase activity. Because the measured rate of H2O2 synthesis is low, the putative oxidase activity of renalase has been deemed unlikely to have physiologic significance.9 Second, the recombinant renalase synthesized in Escherichia coli with a histidine-tag possesses no detectable oxidase activity, and yet it markedly lowers BP when injected into rats.8,18
In this study, we sought to clarify the role of amine oxidase activity in the renal protective effects of renalase and to explore an additional mechanism of action of renalase that is independent of its oxidase activity.
Results
Recombinant Renalase Protects HK-2 Cells against Oxidant and Cisplatin Injury
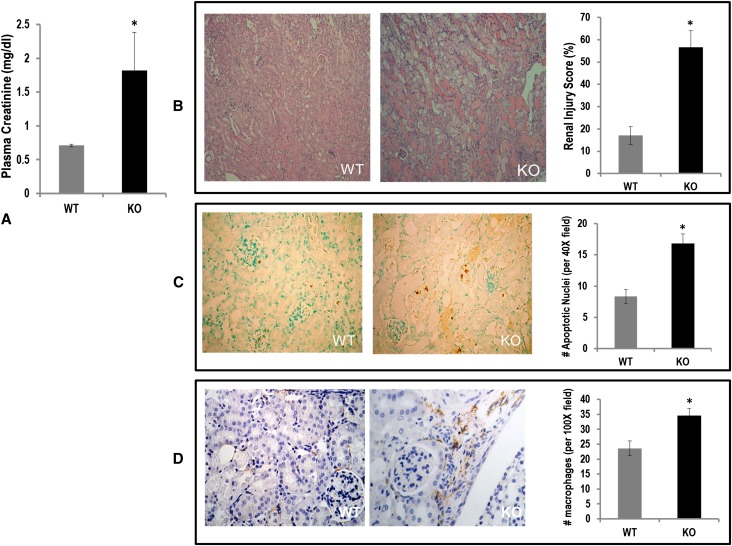
We had previously shown that renalase deficiency aggravates ischemic AKI.16 In this study, we asked whether renalase deficiency would similarly exacerbate toxic AKI from cisplatin.19 Three days after treatment with cisplatin (20 mg/kg, by intraperitoneal injection), plasma creatinine was significantly higher in renalase KO mice than in WT mice (1.82±0.56 versus 0.71±0.02 mg/dl, n=6; P=0.02) (Figure 1A). Renalase-deficient mice developed worse renal histologic injury compared with renalase WT mice (Figure 1B). Renalase KO mice showed more severe acute tubular necrosis compared with WT mice (injury scores: KO, 56.62%±7.38%, n=6; WT, 17.07%±4.03%; n=5; P=0.002). The number of apoptotic renal cells (reddish stain) was increased two-fold in cisplatin-treated KO mice compared with WT mice (Figure 1C). Likewise, renal macrophage infiltration was significantly increased (brown stain) in KO mice compared with WT mice (Figure 1D).
Figure 1.
Renalase deficiency aggravates cisplatin AKI. (A) Plasma creatinine levels in WT and renalase KO mice 3 days after administration of cisplatin (n=6, *P<0.05). (B) Left panel: representative photomicrographs for hematoxylin and eosin (H&E) staining of kidney sections of WT mice treated with cisplatin. Middle panel: representative photomicrograph of H&E staining of kidney from renalase KO mice treated with cisplatin. Right panel: renal injury score (n=6, *P<0.05). (C) Left panel: representative photomicrographs of TUNEL staining of kidney sections of WT mice treated with cisplatin (n=6). Middle panel: representative photomicrograph of TUNEL staining of kidney from renalase KO mice treated with cisplatin (n=6). Right panel: number of apoptotic nuclei per ×40 field (n=6, *P<0.05). (D) Left panel: representative photomicrographs of macrophage (F4/80) staining of kidney sections of WT mice treated with cisplatin (n=5). Middle panel: representative photomicrograph of macrophage (F4/80) staining of kidney from renalase KO mice treated with cisplatin (n=5;). Right panel: number of macrophages per ×100 field (n=5, *P<0.05).
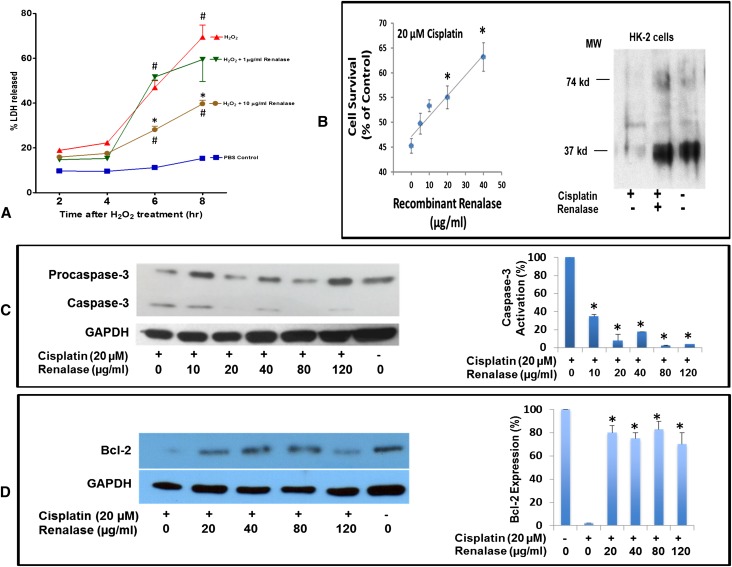
To elucidate the mechanism of renal protection by renalase, we asked whether recombinant renalase could protect cells in culture. In HK-2 cells, recombinant renalase significantly reduced necrosis induced with 2 mM H2O2 compared with vehicle-treated HK-2 cells (Figure 2A). HK-2 cells exposed to cisplatin for 24 hours showed decreased cell viability (Figure 2B, left panel) and renalase expression (Figure 2B, right panel), both of which were reversed by the addition of exogenous renalase. Renalase treatment inhibited caspase-3 activation (Figure 2C) and increased Bcl-2 expression (Figure 2D). These in vitro cell data suggest that renalase’s in vivo protective effect may not be mediated solely by a reduction in circulating catecholamines.
Figure 2.
Recombinant renalase protects HK-2 cells against oxidant and cisplatin mediated injury. (A) HK-2 cell treated with 2 mM H2O2 with and without renalase for indicated time (n=8, *P<0.05 for control versus renalase; #P<0.05 for control versus H2O2). (B) HK-2 cells treated with 20 µM cisplatin with and without renalase for 24 hours. Left panel: cell survival measured by the WST-1 method (n=6, *P<0.05). Right panel: renalase expression measured by Western blot; representative blot shown (n=3). (C) HK-2 cells treated as in B. Left panel: caspase activation measured by Western blot. Right panel: quantification by densitometry (n=3, *P<0.05). (D) HK-2 cells treated as in B. Left panel: Bcl-2 expression measured by Western blot. Right panel: quantification by densitometry (n=3, *P<0.05). GAPDH, glyceraldehyde 3-phosphate dehydrogenase; WST-1, 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium.
Renalase’s Protection against Cellular and Organ Injury Is Independent of Its Enzymatic Function
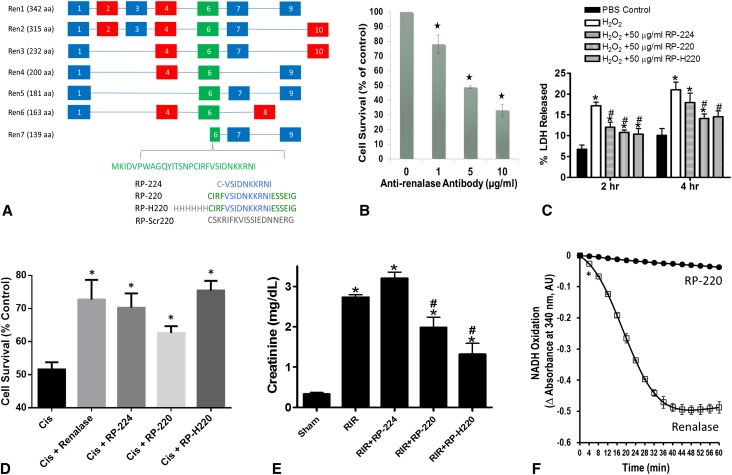
There is a single renalase GENE (10 exons), which undergoes alternative splicing to give rise to at least 6 isoforms (Renalase 1–7) as documented by PCR of human tissue20 and gene sequencing data (GENBANK) (Figure 3A). The carboxy terminal half of exon 6 is present in all spliced isoforms detected to date. Examination of renalase’s crystal structure indicates that renalase peptide 220 (RP-220; Figure 3A) is partly solvent exposed and located on the external surface of the protein.8. CCL-119 cells (CCF-MEC, acute lymphoblastic leukemic cell line; American Type Culture Collection) divide rapidly and express the highest level of renalase (approximately 3.8-fold over mean; microarray data from BioGPS.org) among the cells making up the NCI-60 panel, which contains the most frequently studied human tumor cell lines in cancer research. Although the monoclonal antibody raised against RP-220 did not alter the amine oxidase activity of renalase, the antibody was cytotoxic to CCL-119 cells (Figure 3B). These findings indicated that the epitope is accessible to the antibody and is likely located on the external surface of the protein. The cytotoxic effect of the antibody in the absence of altered amine oxidase activity also suggested that the antibody might decrease cell survival by interfering with the interaction of renalase with a putative binding partner, perhaps a membrane receptor.
Figure 3.
Renalase’s protective effect is independent of its enzymatic activity. (A) Renalase isoforms Ren1–7; exons numbered from 1 to 10; RP-224, renalase peptide amino acid 224–233 of Ren1 or Ren2; RP-220, amino acids 220–239; RP-H220, histidine tagged RP-220; RP-Scr220, scrambled RP-220. (B) CCL-119 cells in culture treated with antirenalase monoclonal antibody for 24 hours; cell survival measured by the WST-1 method (n=3, *P<0.05). (C) HK-2 cell treated with 2 mM H2O2 with and without renalase peptides for indicated time (n=6, *P<0.05 for control versus renalase peptide). (D) HK-2 cells treated with 20 µM cisplatin with and without recombinant renalase and renalase peptides for 24 hours (*P<0.05 for control versus renalase peptide). (E) Plasma creatinine levels from mice subjected to sham surgery or to renal ischemia reperfusion; renalase WT mice were subjected to sham surgery or to 30-minute renal ischemia and reperfusion. For mice subjected to renal ischemia and reperfusion, RP-H220 or vehicle (saline) was injected 10 minutes before renal ischemia. RP-H220 produced significant renal protection in renalase WT mice (n=4–6 per group, *P<0.05 versus vehicle-treated mice subjected to sham surgery; #P<0.05 versus vehicle-treated WT mice subjected to renal ischemia and reperfusion. (F) Renalase peptides do not oxidize NADH; recombinant renalase or peptides in 25 mM Tris (pH 7.5), 5 mM NaCl, and 150 µM NADH at 37°C (*P<0.05). WST-1, 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium.
We hypothesized that RP-220 could represent the point of contact between renalase and its cognate receptor, and tested whether RP-220, RP-H220 and RP-224 would mimic the cytoprotective action of renalase in vitro. In HK-2 cells incubated with 2 mM H2O2, RP-220 and RP-H220 significantly reduced necrosis compared with vehicle-treated cells (Figure 3C) at 2 and 4 hours. RP-224 was also protective at 2 hours but not at 4 hours. The scrambled version of RP-220 (RP-Scr220) had no effect lactate dehydrogenase (LDH) release from cells exposed to H2O2 for hours (H2O2: 23.2%±1.8% versus H2O2+RP-Scr220: 25.3%±2.1%; n=3, P=NS). Similar results were obtained with HK-2 cells exposed to cisplatin for 24 hours. As shown in Figure 3D, renalase, RP-224, RP-220, and RP-H220 improved HK-2 cell survival. In control studies, RP-Scr220 had no effect on the survival of cells treated with cisplatin (cisplatin: 49.5%±5.6%; cisplatin+RP-Scr220: 51%±6.2%; n=6, P=NS).
We had previously shown that recombinant renalase protects mice against ischemic AKI by reducing apoptosis, necrosis, and inflammation.16 The efficacy of the renalase peptides was tested in WT mice subjected to 30 minutes of renal ischemia followed by 24-hour reperfusion. RP-220 and RP-H220, administered 30 min before ischemia, reduced renal injury (Figure 3E). RP-H220 was more effective than RP-220, while RP-224 was ineffective. Plasma norepinephrine was not significantly different between control mice receiving saline or RP-H220–treated mice (control: 0.521±0.169; RP-H220: 0.423±0.221; n=4, P=0.73). In addition, none of these peptides had any detectable amine or NADH oxidase activity (Figure 3F), suggesting that the in vitro cytoprotective action of renalase is independent of its ability to metabolize catecholamines.
AKT and MAPK Activation Critical for the Protective Effect of Renalase Peptides
The finding that renalase peptides with no detectable oxidase activity were equally effective as recombinant renalase in protecting mice against AKI led us to search for mechanisms unrelated to catecholamine metabolism. Mitogen-activated protein kinase (MAPK) signal transduction pathways coordinate cellular responses to various signals, including hormones, cytokines, and growth factors,21 and modulate the development and severity of experimental AKI.22 Therefore, we hypothesized that renalase and related peptides signal via protein kinase B (AKT) and MAPK, and that such signaling is critical for their protective action against AKI.
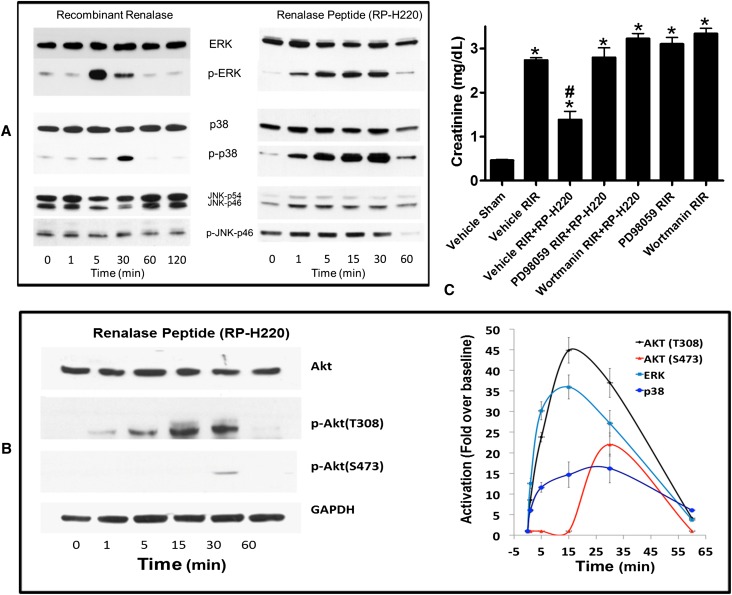
The addition of recombinant renalase to HK-2 cells in culture caused a rapid and transient increase in phosphorylated extracellular signal-regulated kinase 1 and 2 (ERK) and p38 MAPK (Figure 4A, left panel). Likewise, RP220 induced rapid and transient phosphorylation of ERK, p38 (Figure 4A, right panel), and AKT (Figure 4B, left panel). Increased phosphorylation was detectable within 1 minute of adding RP-H220, with a return to baseline within 60 minutes (Figure 4B, right panel). Phosphorylation of c-Jun N-terminal kinases decreased at 60 minutes. Similar results were obtained with RP-220. In control studies, RP-Scr220, a scrambled version of RP-220, did not activate the AKT and MAPK signaling pathways (percentage change in phosphorylation over baseline: AKT, 2%±1%; ERK, −1%±0.4%; n=3, P=NS).
Figure 4.
MAPK activation is critical for the protective effect renalase peptides. (A) MAPK signaling by renalase and renalase peptides. Western blot analysis; representative blot (n=3). JNK, c-Jun N-terminal kinases; p, phosphorylated, activated proteins. (B) Renalase activates AKT. Left panel: Western blot analysis; representative blot (n=3). Right panel: signals normalized to glyceraldehyde 3-phosphate dehydrogenase loading control (n=3). Change over baseline statistically significant at P<0.05 from 1 to 60 minutes for ERK, p38, and AKT (T308) and at 30 minutes only for AKT (S473). (C) ERK or AKT inhibition abrogates protective effect of RP-H220. Plasma creatinine levels from mice subjected to sham surgery or to renal ischemia and reperfusion (RIR); renalase WT mice were subjected to sham surgery or to 30 minutes of renal ischemia and reperfusion. For mice subjected to renal ischemia and reperfusion, RP-H220 or vehicle (saline) was injected 10 minutes before renal ischemia. Some animals were pretreated with the ERK inhibitor PD98059 or the PI3K/AKT inhibitor wortmannin (n=6–8 per group, *P<0.05 versus vehicle-treated mice subjected to sham surgery; #P<0.05 versus vehicle-treated WT mice subjected to renal ischemia and reperfusion).
Chemical inhibition of ERK and AKT signaling was used to test whether these molecules were important mediators of RP-H220’s protective effect in AKI. As shown above, RP-H220 ameliorated the renal ischemic injury in WT mice. The mitogen-activated protein kinase kinase 1 inhibitor 2′-amino 3′-methoxyflavone (PD98059) completely abrogated the peptide’s protective action (Figure 4C). A similar result was obtained with phosphatidylinositol 3-kinase (PI3K)/AKT inhibition by wortmannin (Figure 4C). In control studies, pretreatment with PD98059 or wortmannin alone without the peptides had no effect on the severity of AKI in ischemic WT mice (Figure 4C). These results support the hypothesis that the protective effect of renalase in ischemic AKI is mediated, at least in part, through ERK1/2 and PI3K/AKT signaling.
Disrupted RP-220–Mediated ERK Signaling in Renalase KO Kidney
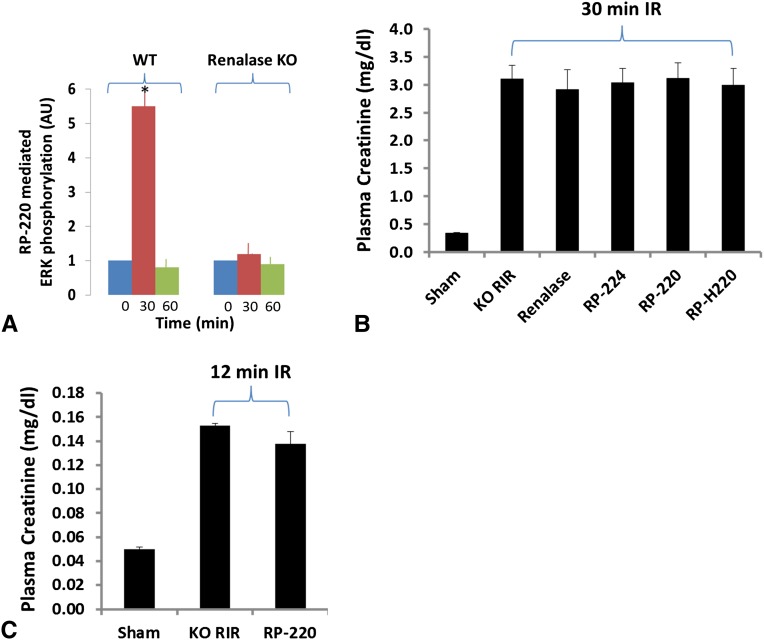
We examined whether renalase-mediated MAPK signaling in kidney differed between WT and KO. RP-220 (1 mg/kg) or saline control was administered by subcutaneous injection to WT or KO mice. The animals were euthanized 30 minutes later, and ERK phosphorylation was determined in whole kidney lysate. Compared with saline, RP-220 administration was associated with a 5-fold increase in ERK phosphorylation in WT kidney at 30 minutes after injection, whereas it had no detectable effect on ERK phosphorylation in renalase KO (Figure 5A). Because ERK inhibition abrogates the protective effect of RP-220, we examined whether recombinant renalase or renalase peptides modified the extent of ischemic renal injury in the renalase KO. Neither renalase (1 mg/kg) nor the renalase peptides (1 mg/kg) had a protective effect in renalase KO mice subjected to severe (30 minutes; n=6 for each group) renal ischemia reperfusion injury (Figure 5B). The effect of RP-220 was also tested in renalase KO exposed to less severe injury (renal ischemia and reperfusion, 12 minutes; n=5 for each group), and again, RP-220 administration was not protective (Figure 5C).
Figure 5.
Disrupted RP-220 mediated ERK signaling in kidney of renalase KO mice, and RP-220 lacked efficacy against ischemic AKI in KO mice. (A) RP-220 (1 mg/kg) given subcutaneously to WT or KO mice; kidneys were harvested 30 minutes later, and ERK phosphorylation was determined in whole kidney lysate by Western blotting; signals were normalized to glyceraldehyde 3-phosphate dehydrogenase loading control and quantified by densitometry (n=3, *P<0.05). (B) Plasma creatinine levels (enzymatic creatinine reagent kit) from mice subjected to sham surgery or to renal ischemia and reperfusion (RIR); renalase KO mice were subjected to sham surgery or to 30 minutes of renal ischemia and reperfusion. For mice subjected to renal ischemia and reperfusion, buffer, renalase, or renalase peptides were injected 10 minutes before renal ischemia (n=6 per group). No significant differences detected between treated and untreated animals. (C) As in B, except plasma creatinine was measured by HPLC, and renal ischemia and reperfusion lasted 12 minutes. No significant differences were detected between treated and untreated animals.
Discussion
The novel findings are that renalase and short renalase peptides devoid of amine oxidase activity signal via the AKT and MAPK pathways and that the peptides fully mimic the cytoprotective actions of recombinant renalase.
In the original description of renalase, we reported the recombinant protein, which was synthesized as a GST fusion protein in E. coli. It possessed amine oxidase activity, metabolizing epinephrine, norepinephrine, and dopamine in vitro. It caused a marked decrease in BP when injected into rodents.4 We subsequently showed recombinant renalase required NAD(P)H for full activity, and the rate of epinephrine (renalase’s preferred substrate) metabolism increased 18-fold in the presence of NADH. An extensive search for putative renalase substrates identified only catecholamines and catecholamine-like compounds.7 We concluded that the effects of recombinant renalase were mediated by decreased circulating catecholamines.
The validity of our conclusion, however, has been questioned. The method used to assess renalase activity relied on the production of H2O2 as an indirect measure of oxidase activity. Because the measured rate of H2O2 synthesis was low, the putative oxidase activity of renalase was deemed unlikely to have physiologic significance.9 In addition, recombinant renalase synthesized in E. coli as a Histidine-tagged protein had no detectable oxidase activity and yet caused a marked decrease in BP when injected into rats.8,18 The authors concluded that the hemodynamic effects of renalase were independent of catecholamine metabolism and were mediated instead by the metabolism of a yet-to-be-identified renalase substrate.
We took advantage of additional clues pointing to a nonenzymatic mechanism of action of renalase. First, CCRF-CEM (CCL-119) is a T-lymphoblastoid cell line derived from the peripheral blood buffy coat of a patient with acute lymphoblastic leukemia.23 CCL-119 expresses renalase and is sensitive to cytotoxicity by a monoclonal antibody raised against RP220. Notably, the antibody did not change the oxidase activity of renalase, and, therefore, altered catecholamine metabolism could not account for its cytotoxicity. Unable to attribute the antibody’s cytotoxicity to an alteration in the enzymatic activity of renalase, we then postulated that the cytotoxicity might have resulted from a disruption by the antibody in the interaction of renalase with its binding partner, the interaction being critical for cell survival. Second, mutation of conserved cysteines to alanines reduced the enzymatic activity of recombinant renalase. Interestingly, certain mutants, such as C54A, even with markedly diminished enzymatic activity (<5% compared with WT renalase), still produced a significant reduction in BP of approximately 11 mmHg (versus approximately 28 mmHg for WT renalase).7 Third, recombinant renalase protected against ischemic AKI by reducing necrosis, apoptosis, and inflammation.16 This improvement was associated with a decrease in circulating catecholamines, which could have been accounted for by degradation of circulating catecholamines by recombinant renalase. Alternatively, vasoactive peptides, such as atrial natriuretic peptide, have been shown to downregulate the renin angiotensin and sympathetic nervous systems and to decrease the production and release of circulating catecholamines. The renalase’s effect on plasma catecholamines could have been mediated by a similar mechanism.
Renalase isoforms 3–7 lack large portions of the putative amine oxidase domain, are significantly shorter than isoforms 1 and 2, and are unlikely to possess oxidase activity. RP-220 is conserved in all renalase isoforms, including the shortest isoform 7, which lacks the first 4 exons. Isoform 1 and RP-220 signal rapidly through the PI3K and MAPK pathways to activate AKT, ERK, and p38, suggesting they both act by binding to a specific cell surface receptor. AKT and ERK activation is generally protective in ischemic AKI.24 Pharmacologic inhibition of both AKT and ERK abrogated the RP-220’s protective effect against ischemic AKI, confirming their protective role in ischemic injury.
As is the case in ischemic cardiac injury and ischemic AKI, renalase deficiency aggravates cisplatin AKI. Renalase and RP-H220 protect against cisplatin AKI. In this sense, renalase and the peptides derived from it would act as general survival factors interdicting pathways that lead to cell death under stress. Principal among those pathways are the MAPKs and AKT.20,25 By shifting the balance between ERK and c-Jun N-terminal kinase activation toward greater activation of ERK and AKT, cells can resist oxidant or toxic exposure.25–27 Cisplatin-induced cell death is EGFR/Src/ERK signaling–dependent in mouse proximal tubule cells. It would appear that renalase and its derived active peptides engage these pathways in a manner similar to that of other peptides more classically associated with growth and survival under stress. These findings strongly suggest that an as-yet-undiscovered surface receptor may be responsible for its prosurvival activity.
RP-H220 was more effective than RP-220 against ischemic AKI. We speculate that the N terminal histidine tag present in RP-H220 alters the conformation of the active moiety (RP-220), in a way that facilitates receptor binding. This could be tested once the receptor is identified, which we plan to do by screening for interaction with known receptors or by purification using standard biochemical methods. Although we could not detect any difference in the signaling patterns of the two peptides in HK-2 cells, it is possible that subtle differences exist in the overall pattern of AKT and MAPK signaling, accounting for the greater efficacy of RP-H220 in ischemic AKI.
In marked contrast to what we have observed in WT mice, neither renalase nor RP-220 protected renalase KO mice against mild or severe ischemic AKI. Interestingly, and not unexpectedly, renalase peptide administration also failed to increase ERK signaling when administered to KO mice. These results suggest that the interaction of RP-220 with its cognate receptor is disrupted in the renalase KO. This could be the result of a signaling defect or a decrease in receptor gene or protein expression, brought about by deletion of the renalase gene. These hypotheses will be best examined by determining the correlation of protection by renalase peptides with different levels of receptor protein expression in vivo and in vitro, once the renalase receptor is identified.
The findings described here represent a major advance in our understanding of the physiology of renalase. We have identified the critical region of renalase molecule that mediates its cytoprotective effects. Finally, renalase protects against toxic and ischemic injury not by its amine oxidase property but rather by its interaction with an as-yet-unidentified receptor that activates intracellular signaling in a manner that promotes cell survival. Key unresolved questions are (1) the identity of renalase receptor and (2) whether one or more receptors mediate the cytoprotective effects of renalase. We hope this work will serve as a foundation for the eventual identification of the renalase receptor, and will catalyze the development of novel therapeutic agents for the treatment of AKI.
Concise Methods
Synthesis and Analysis of Recombinant Human Renalase and Renalase Peptides
Human recombinant renalase was synthesized as described elsewhere.7 Renalase peptides were acetylated at the amino terminus and purified to 98% homogeneity (United Peptides, Herndon, VA). Renalase enzymatic activity was measured as previously described.7 Renalase expression was detected using an antirenalase monoclonal antibody generated against the renalase peptide RP-220 (amino acid 220–239 of hRenalase1).7
Murine Model of Cisplatin AKI
Cisplatin (15–20 mg/kg) was administered by intraperitoneal injection to WT or renalase KO mice under brief isoflurane anesthesia. The animals were euthanized 3 days later. Blood was collected for BUN and creatinine measurements, and kidneys were harvested for histologic examination, immunofluorescence, and Western blotting. One renal pathologist, masked to the identity of the study animal, reviewed each kidney specimen. Pathologic features were scored using an ordinal rating scale of 0–4 (0, none; 1, ≤25%; 2, 26%–50%; 3, 51%–75%; 4, 76%–100%) for the presence of tubular necrosis. Morphometry of renal cortex and medulla was performed using the point counting technique. Points falling on injured tubules were counted, and the percentage of lesion area was calculated as the percentage of total points counted.
Murine Model of Renal Ischemia Reperfusion Injury
After Columbia University Institutional Animal Care and use Committee approval, we subjected adult male C57BL/6 (Harlan Labs, Indianapolis, IN) to sham operation or to 30 minutes of renal ischemia followed by 24 hours of reperfusion, as previously described.28 To test the renal protective effects of renalase peptides (RP-220, RP-H220, and RP-224), we pretreated mice with saline (vehicle) or with renalase peptides (100 µg subcutaneously) 10–30 minutes before renal ischemia. Some mice were treated 30 minutes before renal ischemia and reperfusion with PD98059 (1 mg/kg intraperitoneally), an inhibitor of MAPK mitogen-activated protein kinase kinase 1, to inhibit ERK phosphorylation, or with wortmannin (1 mg/kg intraperitoneally), an inhibitor of PI3K to inhibit AKT phosphorylation. The doses and mode of administration were chosen on the basis of previous in vivo studies.29
In Vitro Model of Cisplatin and Hydrogen Peroxide Toxicity
HK-2 cells (human proximal tubular line) obtained from American Type Culture Collection (ATCC, Manassas, VA) were cultured in DMEM/F12 supplemented with glutamine, 10% FBS, and antibiotics and were maintained at 37°C in 5% CO2. Cells were exposed to cisplatin (20 µM) in the presence or absence of renalase for 24 hours, and cell viability was assessed by the WST-1 [2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium] method (Roche Applied Science, Germany). Cells were then harvested in radioimmunoprecipitation assay buffer (20 mM Tris-HCl [pH, 7.5], 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% NP-40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin) supplemented with a protease and phosphatase inhibitor cocktail (Roche Applied Science, Germany). Proteins were separated by SDS-PAGE, and Western blotting was carried out using the following antibodies: anti-renalase monoclonal,7,16 anti–caspase-3 (Cell Signaling Technology, Danvers, MA), and anti-Bcl2 (Thermo Scientific).
Necrotic injury in HK-2 cells (ATCC) was induced with exposure to 2 mM H2O2 for 2–8 hours and LDH released into cell culture media was measured as described using a commercial LDH assay kit (Promega, Madison, WI).30
Measurement of Renal Function
Blood was collected from mice anesthetized with isoflurane via cardiac puncture into a heparinized syringe and was subsequently centrifuged to separate plasma. Samples were submitted to the Yale O’Brien Kidney Center for measurement of BUN and creatinine. Plasma creatinine was measured by an enzymatic creatinine reagent kit according to the manufacturer’s instructions (Thermo Fisher Scientific, Waltham, MA) or by HPLC. These methods of creatinine measurement largely eliminate the interferences from mouse plasma chromagens, well known to the Jaffe method.
Norepinephrine Measurement
Plasma norepinephrine levels in mice subjected to renal ischemia reperfusion after vehicle (saline) treatment or to renal ischemia reperfusion after 100 μg RP-H220 treatment were measured with a commercial ELISA kit according to the manufacturer’s instructions (Rocky Mountain Diagnostics, Colorado Springs, CO). To determine the enzymatic activity of RP H220 in vitro, 4 or 8 ng/ml norepinephrine was treated with saline or with 500 pg/ml RP H220 for 30 minutes at room temperature before being subjected to norepinephrine ELISA.
Histologic Detection of Apoptosis and Macrophage Infiltration
Kidney apoptosis was detected in WT and KO mice with TUNEL staining as described using a commercially available in situ apoptosis kit (EMD Millipore, Billerica, MA) according to the instructions provided by the manufacturer. Kidney macrophage infiltrations were assessed with immunohistochemistry 72 hours after treatment with cisplatin or renalase treatment using anti-F4/80 (Abcam, Cambridge, MA) staining. The primary antibody was 1:80 diluted. It was subsequently detected with horseradish peroxidase–conjugated Rat IgG, localized with 3,3′-diaminobenzidine, counterstained with 3,3′-diaminobenzidine, and mounted with resinous mounting media. The kidney TUNEL staining and macrophage infiltration were quantified in five randomly chosen microscope image fields in the cortico–medullary junction.
Statistical Analyses
When appropriate, the Kruskal–Wallis one-way ANOVA by ranks was used to evaluate statistical significance. When the Kruskal–Wallis test revealed statistical significance, the Mann–Whitney test was used for pairwise comparisons. All data are the mean±SEM, and P<0.05 was accepted as indicating a statistically significant difference. Statistical analysis was carried out using GraphPad Prism (GraphPad Software, Inc.).
Disclosures
None.
Acknowledgments
This work was supported in part by Veterans Affairs Connecticut Healthcare System (H.V., R.S., and G.V.D.); National Institutes of Health grants RC1DK086465, RC1DK086402, and R01DK081037 (G.V.D.); National Basic Research Program of China 973 Program 2012CB517600, 2012CB517602; Shanghai Health Bureau ZXSNXD-CC-ZDYJ002 (W.L.); and Department of Anesthesiology, Columbia University (H.T.L.)
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Ricci Z, Cruz D, Ronco C: The RIFLE criteria and mortality in acute kidney injury: A systematic review. Kidney Int 73: 538–546, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Coca SG, Yalavarthy R, Concato J, Parikh CR: Biomarkers for the diagnosis and risk stratification of acute kidney injury: A systematic review. Kidney Int 73: 1008–1016, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Desir GV, Wang L, Peixoto AJ: Human renalase: A review of its biology, function, and implications for hypertension. J Am Soc Hypertens 6: 417–426, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Xu J, Li G, Wang P, Velazquez H, Yao X, Li Y, Wu Y, Peixoto A, Crowley S, Desir GV: Renalase is a novel, soluble monoamine oxidase that regulates cardiac function and blood pressure. J Clin Invest 115: 1275–1280, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li G, Xu J, Wang P, Velazquez H, Li Y, Wu Y, Desir GV: Catecholamines regulate the activity, secretion, and synthesis of renalase. Circulation 117: 1277–1282, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Farzaneh-Far R, Desir GV, Na B, Schiller NB, Whooley MA: A functional polymorphism in renalase (Glu37Asp) is associated with cardiac hypertrophy, dysfunction, and ischemia: Data from the heart and soul study. PLoS ONE 5: e13496, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desir GV, Tang L, Wang P, Li G, Sampaio-Maia B, Quelhas-Santos J, Pestana M, Velazquez H: Renalase lowers ambulatory blood pressure by metabolizing circulating adrenaline. J Am Heart Assoc 1: e002634, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milani M, Ciriello F, Baroni S, Pandini V, Canevari G, Bolognesi M, Aliverti A: FAD-binding site and NADP reactivity in human renalase: A new enzyme involved in blood pressure regulation. J Mol Biol 411: 463–473, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Boomsma F, Tipton KF: Renalase, a catecholamine-metabolising enzyme? J Neural Transm 114: 775–776, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beaupre BA, Carmichael BR, Hoag MR, Shah DD, Moran GR: Renalase is an α-NAD(P)H oxidase/anomerase. J Am Chem Soc 135: 13980–13987, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Barrett JC, Clayton DG, Concannon P, Akolkar B, Cooper JD, Erlich HA, Julier C, Morahan G, Nerup J, Nierras C, Plagnol V, Pociot F, Schuilenburg H, Smyth DJ, Stevens H, Todd JA, Walker NM, Rich SS, Type 1 Diabetes Genetics Consortium : Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet 41: 703–707, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buraczynska M, Zukowski P, Buraczynska K, Mozul S, Ksiazek A: Renalase gene polymorphisms in patients with type 2 diabetes, hypertension and stroke. Neuromolecular Med 13: 321–327, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malyszko J, Koc-Zorawska E, Malyszko JS, Kozminski P, Zbroch E, Mysliwiec M: Renalase, stroke, and hypertension in hemodialyzed patients. Ren Fail 34: 727–731, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Zhao Q, Fan Z, He J, Chen S, Li H, Zhang P, Wang L, Hu D, Huang J, Qiang B, Gu D: Renalase gene is a novel susceptibility gene for essential hypertension: A two-stage association study in northern Han Chinese population. J Mol Med (Berl) 85: 877–885, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Wu Y, Xu J, Velazquez H, Wang P, Li G, Liu D, Sampaio-Maia B, Quelhas-Santos J, Russell K, Russell R, Flavell RA, Pestana M, Giordano F, Desir GV: Renalase deficiency aggravates ischemic myocardial damage. Kidney Int 79: 853–860, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Lee HT, Kim JY, Kim M, Wang P, Tang L, Baroni S, D’Agati VD, Desir GV: Renalase protects against ischemic AKI. J Am Soc Nephrol 24: 445–455, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yap SC, Lee HT: Acute kidney injury and extrarenal organ dysfunction: New concepts and experimental evidence. Anesthesiology, 116: 1139–1148, 2012 [DOI] [PubMed]
- 18.Pandini V, Ciriello F, Tedeschi G, Rossoni G, Zanetti G, Aliverti A: Synthesis of human renalase1 in Escherichia coli and its purification as a FAD-containing holoprotein. Protein Expr Purif 72: 244–253, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Safirstein RL: Acute renal failure: from renal physiology to the renal transcriptome. Kidney Int Suppl S62–S66, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Hennebry SC, Eikelis N, Socratous F, Desir G, Lambert G, Schlaich M: Renalase, a novel soluble FAD-dependent protein, is synthesized in the brain and peripheral nerves. Mol Psychiatry 15: 234–236, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Kyriakis JM, Avruch J: Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol Rev 92: 689–737, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Bonventre JV, Yang L: Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foley GE, Lazarus H, Farber S, Uzman BG, Boone BA, McCarthy RE: Continuous culture of human lymphoblasts from peripheral blood of a child with acute leukemia. Cancer 18: 522–529, 1965 [DOI] [PubMed] [Google Scholar]
- 24.Arany I, Safirstein RL: Cisplatin nephrotoxicity. Semin Nephrol 23: 460–464, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Arany I, Megyesi JK, Kaneto H, Price PM, Safirstein RL: Cisplatin-induced cell death is EGFR/src/ERK signaling dependent in mouse proximal tubule cells. Am J Physiol Renal Physiol 287: F543–F549, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Arany I, Megyesi JK, Kaneto H, Tanaka S, Safirstein RL: Activation of ERK or inhibition of JNK ameliorates H(2)O(2) cytotoxicity in mouse renal proximal tubule cells. Kidney Int 65: 1231–1239, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Pabla N, Dong Z: Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int 73: 994–1007, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Lee HT, Xu H, Nasr SH, Schnermann J, Emala CW: A1 adenosine receptor knockout mice exhibit increased renal injury following ischemia and reperfusion. Am J Physiol Renal Physiol 286: F298–F306, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Joo JD, Kim M, D’Agati VD, Lee HT: Ischemic preconditioning provides both acute and delayed protection against renal ischemia and reperfusion injury in mice. J Am Soc Nephrol 17: 3115–3123, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Lee HT, Emala CW: Adenosine attenuates oxidant injury in human proximal tubular cells via A(1) and A(2a) adenosine receptors. Am J Physiol Renal Physiol 282: F844–F852, 2002 [DOI] [PubMed] [Google Scholar]