Abstract
Human pluripotent stem cells (hPSCs) can generate a diversity of cell types, but few methods have been developed to derive cells of the kidney lineage. Here, we report a highly efficient system for differentiating human embryonic stem cells and induced pluripotent stem cells (referred to collectively as hPSCs) into cells expressing markers of the intermediate mesoderm (IM) that subsequently form tubule-like structures. Treatment of hPSCs with the glycogen synthase kinase-3β inhibitor CHIR99021 induced BRACHYURY+MIXL1+ mesendoderm differentiation with nearly 100% efficiency. In the absence of additional exogenous factors, CHIR99021-induced mesendodermal cells preferentially differentiated into cells expressing markers of lateral plate mesoderm with minimal IM differentiation. However, the sequential treatment of hPSCs with CHIR99021 followed by fibroblast growth factor-2 and retinoic acid generated PAX2+LHX1+ cells with 70%–80% efficiency after 3 days of differentiation. Upon growth factor withdrawal, these PAX2+LHX1+ cells gave rise to apically ciliated tubular structures that coexpressed the proximal tubule markers Lotus tetragonolobus lectin, N-cadherin, and kidney-specific protein and partially integrated into embryonic kidney explant cultures. With the addition of FGF9 and activin, PAX2+LHX1+ cells specifically differentiated into cells expressing SIX2, SALL1, and WT1, markers of cap mesenchyme nephron progenitor cells. Our findings demonstrate the effective role of fibroblast growth factor signaling in inducing IM differentiation in hPSCs and establish the most rapid and efficient system whereby hPSCs can be differentiated into cells with features characteristic of kidney lineage cells.
CKD is a significant global public health problem1 and is the leading risk factor for cardiovascular disease. Despite advances in the quality of dialysis therapy, patients with CKD experience significant morbidity and mortality and reduced quality of life. For selected patients, kidney transplantation is an alternative renal replacement therapy to dialysis; however, this option is limited by the shortage of compatible organs and requires the use of lifelong immunosuppressive medication to prevent graft rejection. For these reasons, research in regenerative medicine, with the ultimate aim of generating functional replacement kidney tissue or even a whole kidney from a patient’s own tissue, offers the potential for new therapeutic strategies to treat CKD and ESRD. Human pluripotent stem cells (hPSCs) have the potential to revolutionize our ability to generate functional cells and tissues for purposes of regenerative medicine and disease modeling. Both human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs), collectively referred to as hPSCs in this manuscript, possess the ability to self-renew and to differentiate into cells of all three germ layers of the embryo,2,3 making them ideal starting substrates for generating cells of the kidney lineage.
While other organs, such as the heart, liver, pancreas, and central nervous system, have benefited from more established differentiation protocols for deriving their functional cell types from hPSCs, considerably fewer methods have been developed to effect kidney differentiation. This may be partly explained by the complex architecture of the kidney and its functional units, nephrons, which are composed of highly specialized epithelial cell types, such as glomerular podocytes, proximal tubular epithelial cells, cells of the thick and thin limbs of the loop of Henle, distal convoluted tubule, and collecting duct cells. No single protocol is likely to generate the multitude of these cell types, but a system to differentiate hPSCs into the nephron progenitor cell populations, namely the intermediate mesoderm (IM) and the metanephric mesenchyme, may offer a common point from which more specific kidney lineages can be derived.
Although several studies have attempted to differentiate mouse ESCs into kidney cells,4–15 only a few studies have reported protocols in hESCs and hiPSCs.16–19 These previous reports have produced cells that share characteristics expected of human kidney progenitor or epithelial cells, although the identities of these differentiated cells have yet to be conclusively verified. In addition, the efficiencies of these protocols for generating cells of the renal lineage are low, necessitating the use of cell sorting to enrich populations of cells using markers that are not entirely specific to the kidney. For example, OSR1, used as a marker by Mae and colleagues to label cells of the intermediate mesoderm,17 is also expressed in lateral plate mesoderm,20 which gives rise during embryonic development to the adult heart, hematopoietic system, and vasculature. Narayanan and colleagues isolated populations of AQP1+ proximal tubular-like cells,18 but this marker is expressed not only in the kidney but also broadly in the gastrointestinal system, lungs, and blood cells.21 In both instances, the sorted cells were heterogeneous and included a small percentage of cells that exhibited properties and behaviors of cells of the kidney lineage. While these and earlier studies have suggested a role for Wnt, activin, bone morphogenetic protein (BMP), and retinoic acid signaling in the induction of cells of the kidney lineage, inductive effects of other signaling pathways, such as fibroblast growth factor (FGF) signaling, on kidney differentiation from hPSCs have not been reported.
Here we report a simple, efficient, and highly reproducible system to induce IM differentiation in hESCs and hiPSCs under chemically defined, monolayer culture conditions. Chemical induction with the potent small molecule inhibitor of GSK-3β, CHIR99021 (CHIR), robustly and rapidly differentiates hPSCs into multipotent cells expressing mesendodermal markers in a manner that recapitulates mesendoderm formation in the developing embryo. We demonstrate that hPSCs treated with CHIR preferentially differentiate into cells expressing lateral plate mesoderm markers. With the precisely timed addition of specific growth factors, this default fate can be diverted into definitive endoderm or other types of mesoderm. Importantly, we show that in cells treated with CHIR, the combination of FGF2 and retinoic acid (RA) efficiently generates cells coexpressing PAX2 and LHX1 (markers of IM) within 3 days of differentiation. This is the most efficient method to generate PAX2+LHX1+ IM cells and the first demonstration of the role of FGF signaling as a potent inducer of IM-specific PAX2 expression in differentiating hPSCs. These PAX2+LHX1+ cells spontaneously form tubular-like structures that express apical cilia and markers of proximal tubular epithelial cells and integrate into mouse embryonic kidney explant cultures. PAX2+LHX1+ cells can also be specifically differentiated into cells expressing SIX2, SALL1, and WT1, markers of the multipotent nephron progenitor cells of the cap mesenchyme (CM), further demonstrating their capacity to give rise to IM derivatives. When placed under the kidney capsule, cells express aquaporin-1.
Results
CHIR Efficiently Induces hPSCs to Differentiate into Cells Expressing Markers Characteristic of Mesendoderm
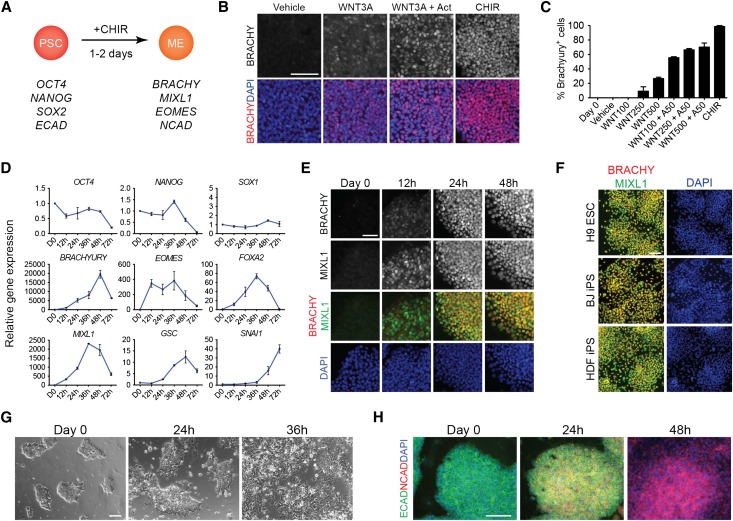
To develop a platform to target generation of IM, it was vital to first identify which conditions most efficiently differentiate hPSCs into mesendoderm (Figure 1A). During gastrulation, cells of the epiblast ingress through the primitive streak to form the endodermal and mesodermal germ layers of the developing embryo,22 and this process depends on the secreted molecules of the Wnt and nodal/activin signaling pathways.23,24 To determine the minimal signals required to induce mesendoderm gene expression in hPSCs, we differentiated cells under serum-free, feeder-free conditions in the absence of other chemical additives except for the precise signals tested. We first tested the efficacy of WNT3A and activin A to induce expression of the primitive streak marker BRACHYURY (BRACHY). WNT3A alone, at a concentration of 500 ng/ml, induced BRACHY expression in 26.7%±1.9% of cells after 24 hours of treatment (Figure 1, B and C). The addition of activin A to WNT3A increased the percentage of BRACHY+ cells to 70.3%±5.5% of the total population. However, when cells were treated with 5 μM CHIR, a potent GSK-3β inhibitor/Wnt pathway agonist, 98.7%±1.3% of cells expressed BRACHY at 24 hours (Figure 1, B and C). Gene expression profiling of CHIR-induced cells revealed that expression of primitive streak genes (BRACHY, MIXL1, EOMES, FOXA2, and GSC) was rapidly upregulated within 24 hours of treatment, peaked between 36–48 hours, and decreased by 72 hours, a pattern consistent with the transient expression of these genes during gastrulation. In parallel, the induction of mesendoderm genes was accompanied by the loss of pluripotency, as reflected by a reduction of OCT4 and NANOG mRNA (Figure 1D). Protein levels paralleled this gene expression pattern, as immunostaining for BRACHY and MIXL1 over the first 48 hours of differentiation revealed coexpression of these markers in CHIR-induced cells as early as 12 hours with coexpression peaking at 48 hours (Figure 1, E and F). As cells migrate through the primitive streak during development, they express SNAI1, downregulate E-cadherin expression, upregulate N-cadherin expression, and undergo an epithelial–mesenchymal transition.25,26 Consistent with these observations, CHIR induction resulted in the upregulation of SNAI1 as early as 24 hours after treatment (Figure 1D). This was associated with epithelial–mesenchymal transition with waves of cells migrating away from the periphery of differentiating colonies between 36 and 48 hours of differentiation (Figure 1G) and with a switch in cadherin expression from E- to N-cadherin (Figure 1H). Together, these findings demonstrated the potency of CHIR to induce differentiation of hPSCs into mesendoderm-like cells via a program that mimics normal development in vivo.
Figure 1.
CHIR99021 efficiently induces human pluripotent stem cells to differentiate into mesendoderm-like cells. (A) Diagram of differentiation of human PSCs into mesendoderm using CHIR. (B) hPSCs treated with DMSO (vehicle), Wnt3a 500 ng/ml, Wnt3a 500 ng/ml+activin 50 ng/ml, or CHIR 5 μM were immunostained for BRACHYURY after 24 hours of differentiation. (C) Quantification of cells with positive immunofluorescence staining for BRACHYURY after treatment with vehicle, Wnt3a at increasing doses, Wnt3a at increasing doses with activin, or CHIR for 24 hours. Data represent mean±SEM (n=3). (D) Time course of gene expression in hPSCs treated with CHIR. Quantitative RT-PCR of genes expressed in mesendoderm and in pluripotent stem cells. Data represent mean±SEM (n>3). (E) Immunofluorescence staining for BRACHYURY and MIXL1 in CHIR-treated hPSCs over the first 48 hours of differentiation. (F) Immunofluorescence staining for BRACHYURY and MIXL1 after 24 hours of differentiation in hESC and hiPSC lines treated with CHIR. (G) Phase contrast images of CHIR-treated human PSCs over 36 hours showing epithelial to mesenchymal transition. (H) Immunofluorescence staining for E-cadherin and N-cadherin in CHIR-treated human PSCs. Scale bars, 100 μm. BRACHY, BRACHYURY; CHIR, CHIR99021; ME, mesendoderm; PSC, pluripotent stem cell. BJ, iPS cells derived from human foreskin fibroblasts; DAPI, 4′,6-diamidino-2-phenylindole; HDF, iPS cells derived from adult human dermal fibroblasts.
Timed Addition of Exogenous Factors Modulates Cell Fate of CHIR-Induced hPSCs
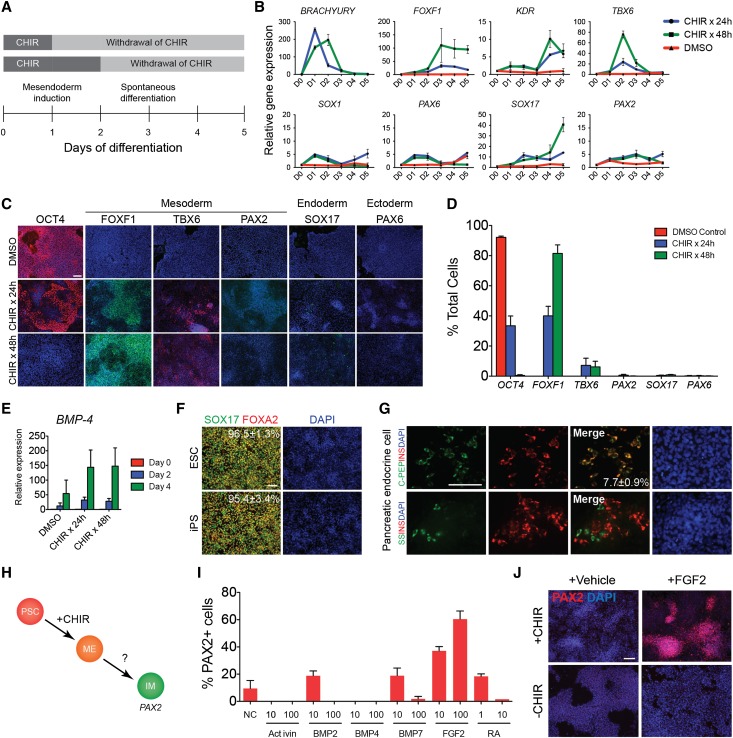
To determine whether treatment with CHIR alone was sufficient to induce differentiation toward IM, we next tested the intrinsic multipotency of CHIR-induced cells by withdrawing CHIR from the culture media after 24 or 48 hours of induction and allowing the cells to differentiate for another 3–4 days (Figure 2A). CHIR-induced cells spontaneously differentiated into heterogeneous populations of cells expressing transcriptional markers of definitive endoderm (SOX17) and mesodermal subtypes (FOXF1, KDR, TBX6) without significant upregulation of the IM marker PAX2 or neuroectodermal markers (SOX1, PAX6) (Figure 2B). Immunocytochemistry of these cell cultures revealed a dependence of cell fate on the duration of CHIR treatment. The hPSCs pulsed with CHIR for 24 hours generated approximately 40% FOXF1+ cells, a marker that is characteristic of lateral plate mesoderm (Figure 2, C and D). A significant proportion of cells (30%–40%), however, expressed the pluripotency marker OCT4 (Figure 2, C and D), suggesting that the withdrawal of CHIR after 24 hours may have resulted in the reversion of incompletely differentiated cells back to the pluripotent state. Prolonging the duration of CHIR treatment to 48 hours eliminated the appearance of OCT4+ cells, with >80% of cells staining positive for FOXF1. The protein expression of the paraxial mesoderm marker TBX6 and the IM marker PAX2 was seen in <10% and 1% of cells, respectively, signifying that CHIR treatment was insufficient to efficiently induce these mesodermal subtypes (Figure 2C). Similarly, <1% of cells expressed SOX17 or PAX6, suggesting that endodermal or ectodermal differentiation was not significantly induced by CHIR treatment. Because proper differentiation of lateral plate mesoderm during embryonic development depends on higher BMP-4 signaling gradients, we evaluated the expression of BMP-4 transcripts in CHIR-induced cells by quantitative RT-PCR. The hPSCs treated with CHIR for 24 or 48 hours significantly upregulated BMP-4 on day 4 compared with DMSO-treated controls, demonstrating that induction with CHIR stimulated endogenous expression of BMP-4 (Figure 2E).
Figure 2.
Timed addition of exogenous factors modulates cell fate of CHIR-induced hPSCs. (A) Diagram depicting time course of differentiation. (B) Time course of gene expression in human PSCs treated with CHIR for 24 hours, CHIR for 48 hours, or DMSO (vehicle). Data shown represent mean±SEM (n=4). Representative immunostaining (C) and quantification (D) of markers of pluripotency, mesoderm, definitive endoderm, and ectoderm in human PSCs treated with DMSO (vehicle), CHIR for 24 hours, or CHIR for 48 hours, day 4 of differentiation. Data shown in graph represent mean±SEM (n>5). (E) Expression of BMP-4 by quantitative RT-PCR in hPSCs treated with CHIR for 24 hours, CHIR for 48 hours, or DMSO. Data shown represent mean±SEM (n=3). (F) Representative immunostaining for SOX17 and FOXA2 in hESCs and hiPSCs treated with CHIR for 24 hours followed by activin A 100 ng/ml for 3 days. Numbers represent the mean percentage±SEM of SOX17+ cells from at least two independent experiments. (G) Cells at the definitive endoderm stage were differentiated using a three-step protocol into hormone-expressing pancreatic endocrine cells producing insulin, proinsulin C-peptide, and somatostatin. Number represents the mean percentage±SEM of insulin+C-peptide+ cells from at least two independent experiments. (H) Diagram of directed differentiation of hPSCs into intermediate mesoderm. (I) Quantification of cells with positive immunostaining for PAX2. Data shown in the graph represent mean±SEM (n=2). (J) Immunostaining for PAX2 in hPSCs treated with or without CHIR for 24 hours followed by FGF2 100 ng/ml for 3 days, day 4. Scale bars, 100 μm. DAPI, 4′,6-diamidino-2-phenylindole.
To determine whether we could divert CHIR-induced cells from the default lateral plate mesoderm fate, we applied high doses of activin A to hPSCs after 24 hours of CHIR treatment and successfully generated cells expressing SOX17+FOXA2+, markers of definitive endoderm, with >95% efficiency after 2–3 days of subsequent differentiation (Figure 2F, Supplemental Figure 1, A–C). These cells could be differentiated further into a variety of endodermal cells expressing the pancreatic markers insulin and somatostatin27,28 (Figure 2G), the mature hepatocyte marker albumin,29 and markers of anterior foregut endoderm (precursors to lung and thyroid tissue)30 or hindgut endoderm (precursors to intestine)31 (Supplemental Figure 1, D–F). However, when activin A was added after 48 hours of CHIR treatment, the number of SOX17+ cells markedly decreased after 4 days of differentiation (Supplemental Figure 1B), indicating a distinct window of time during which CHIR-induced cells could be differentiated into different mesendodermal lineages. Collectively, these results highlighted the critical importance of timing and duration of signaling factors with regards to cell fate determination in our differentiation system.
FGF2 Induces PAX2 Expression in CHIR-Induced Cells
Having demonstrated that we could manipulate the fate of CHIR-treated cells with the time-sensitive addition of specific inducing factors, we then screened candidate growth factors for the ability to induce the expression of the IM marker PAX2. PAX2 is an early marker of IM, and, unlike the markers OSR1 or LHX1, which are also expressed in the adjacent lateral plate mesoderm, PAX2 expression is restricted in mesoderm to the IM.20 hPSCs were induced with CHIR for 24 hours, at which time CHIR was withdrawn and cells were treated with increasing doses of activin A, BMP-2, BMP-4, BMP7, FGF2, or RA. On day 4 of differentiation, modest increases in PAX2 expression, compared with a vehicle control, were seen in cells treated with low doses of BMP-2, BMP7, and RA, and no PAX2 expression was seen in cells treated with activin or BMP-4 (Figure 2I). We observed, however, that approximately 50%–60% of cells treated with FGF2 at a dose of 100 ng/ml were positive for PAX2 by immunostaining, suggesting that FGF2 could be a potential inducer of IM. The ability of FGF2 to induce PAX2 expression depended on cells being pretreated with CHIR. The addition of FGF2 to hPSCs not initially induced with CHIR resulted in the absence of PAX2 expression on day 4 of differentiation (Figure 2J).
FGF2 and RA Induce PAX2+LHX1+ Intermediate Mesoderm Cells
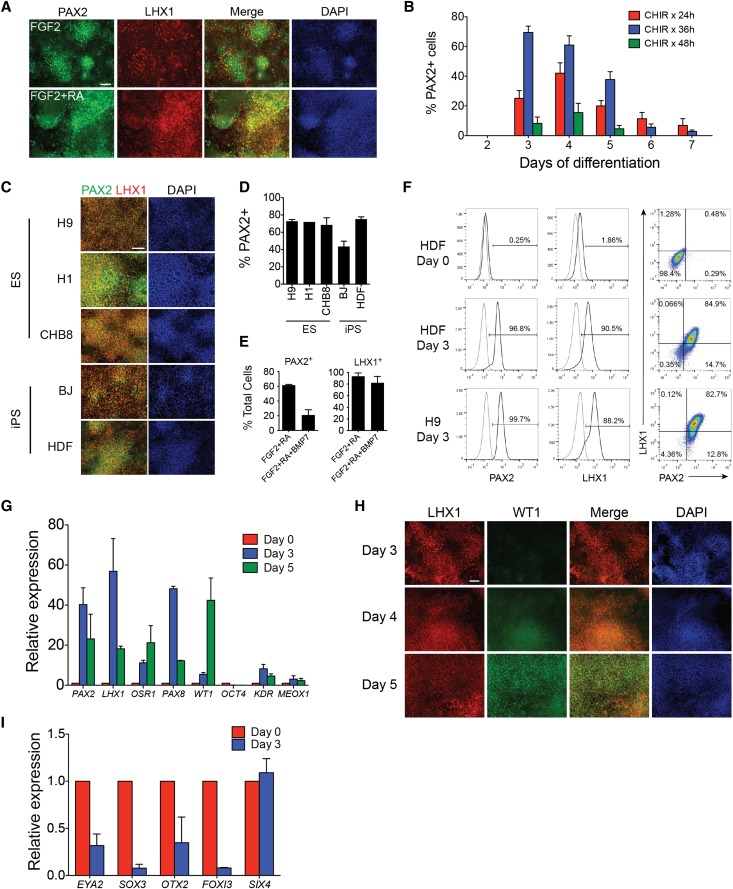
Retinoic acid signaling has previously been demonstrated to play an important role in the early stages of kidney development.32,33 Because we observed a small inductive effect on PAX2 expression with RA, we then tested low-dose RA in combination with FGF2 to determine whether it could have a synergistic effect on PAX2 expression. Although we did not see a significant increase in PAX2 expression, the addition of 1 μM RA to FGF2 resulted in a marked increase in LHX1 expression, with most cells coexpressing both PAX2 and LHX1 (Figure 3A). Because PAX2 and LHX1 are both expressed in the developing IM, we considered these cells as a putative IM cell population.
Figure 3.
FGF2 and RA induce PAX2+LHX1+ intermediate mesoderm cells. (A) Immunostaining for PAX2 and LHX1 in hPSCs cells treated with CHIR for 24 hours followed by FGF2 or FGF2+RA for 3 days, day 4 of differentiation. (B) Quantification of PAX2+ cells on days 2–7 of differentiation in hPSCs treated with CHIR for 24, 36, or 48 hours followed by FGF2+RA. Data shown represent mean±SEM (n>4). (C) Immunostaining for PAX2 and LHX1 and (D) quantification of PAX2+ cells in three hESC lines and two hiPSC lines treated with CHIR for 36 hours followed by FGF2+RA (ChFR), day 3. Data shown represent mean±SEM (n=3). (E) Quantification of PAX2+ or LHX1+ cells in hPSCs treated with CHIR for 36 hours followed by FGF2+RA or FGF2+RA+BMP7, day 3. Data shown represent mean±SEM (n=3). (F) Quantification of PAX2+ and LHX1+ cells by flow cytometry. (G) Quantitative RT-PCR of intermediate mesoderm genes in hPSCs treated with ChFR. Data shown represent mean±SEM (n=3). (H) Immunostaining for LHX1 and WT1 in hPSCs treated with ChFR. (I) Quantitative RT-PCR of non-IM genes in hPSCs treated with ChFR. Data shown represent mean±SEM (n=2). Scale bar, 100 μm. DAPI, 4′,6-diamidino-2-phenylindole.
We then sought to optimize the efficiency of generating IM cells. We first tested the effects of different durations of CHIR pretreatment and assayed PAX2 expression from days 2 through 7 of differentiation. Induction of hPSCs with CHIR for 36 hours followed by FGF2 and RA resulted in PAX2 expression in >70% of cells as early as day 3 of differentiation (Figure 3B). Longer pretreatment with CHIR for 48 hours resulted in less PAX2 expression at all time points compared with CHIR treatment for 24 or 36 hours. Regardless of the duration of CHIR pretreatment, the proportion of cells expressing PAX2 significantly decreased after day 4 of differentiation, with <10% of cells at day 7 retaining PAX2 expression. Because previous studies have identified a role for BMP7 in inducing IM cells,5,14,16,17 we next determined whether the addition of BMP7 to FGF2 and RA could enhance IM cell differentiation. In contrast to these other reports, we found that the addition of BMP7 significantly decreased the percentage of cells expressing PAX2 but had a minimal effect on the expression of LHX1 (Figure 3E).
To determine the reproducibility of our protocol in different hPSC lines, we tested the combination of CHIR induction for 36 hours followed by the addition of FGF2 and RA in three hESC lines and two hiPSC lines. Similar patterns of costaining for PAX2 and LHX1 were observed in all cell lines with nearly identical efficiencies of differentiation (70%–80%) in four of the five cell lines and a slightly reduced differentiation efficiency in one hiPSC line (Figure 3, C and D). To confirm these findings, we used flow cytometry to quantify PAX2 and LHX1 expression in hESCs and hiPSCs treated with this protocol. Interestingly, even higher proportions of differentiated cells were positive for PAX2 or LHX1 by flow cytometry, and 80%–85% of cells were double positive for PAX2 and LHX1 (Figure 3F).
We then performed gene expression profiling of these putative IM cells using quantitative RT-PCR. Consistent with our protein expression data, we observed a marked upregulation of IM genes, including PAX2, LHX1, OSR1, and PAX8, on day 3 of differentiation, followed by a reduction in gene expression at day 5 (Figure 3G), suggesting that IM differentiation in hPSCs may be a transient state which can be rapidly induced but lasts only 2–3 days. The expression of lateral plate and paraxial mesoderm markers KDR and MEOX1, respectively, was not significantly upregulated by our IM induction protocol, and, as expected with differentiation, we noted downregulation of the pluripotency marker OCT4. Expression of WT1, a marker expressed first in the IM and then in the urogenital ridge, which is derived from IM,34 was expressed at a low level on day 3 and was strongly upregulated on day 5 of differentiation (Figure 3, G and H). Because PAX2 and LHX1 are also expressed in the developing ear, eye, and central nervous system during embryogenesis,35,36 we evaluated our IM cells for the expression of markers (EYA2, SOX3, OTX2, FOXI3, SIX4) that are coexpressed with PAX2 and/or LHX1 at relevant stages of neuroectodermal development. We found that their expression was downregulated or unchanged compared with undifferentiated hPSCs (Figure 3I). We therefore concluded that our PAX2+LHX1+ cell population was most likely to be representative of IM.
hPSC-Derived PAX2+LHX1+ Cells Form Tubule Structures That Express Proximal Tubular Markers
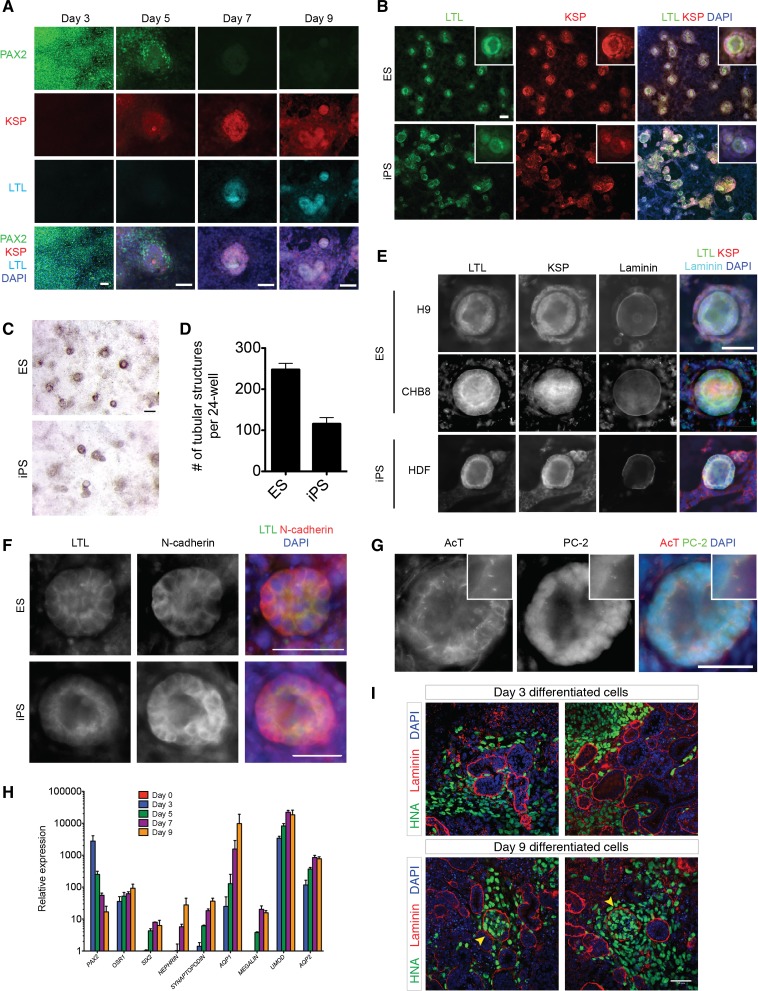
To determine whether hPSC-derived PAX2+LHX1+ IM cells have the capacity to give rise to more differentiated cell and tissue derivatives of IM, we withdrew FGF2 and RA from the culture media on day 3 of differentiation and cultured them in serum-free media, containing no additional growth factors or chemicals, for another week. Cell growth and proliferation continued under these conditions, and as early as day 7, tubular epithelial structures formed in parallel with the downregulation of PAX2 expression (Figure 4, A–D). Immunostaining for markers of more differentiated kidney cell types revealed that the cells comprising these tubular structures expressed the following: Lotus tetragonolobus lectin (LTL), which localizes to the apical surface of kidney proximal tubules; N-cadherin, which is the predominant cadherin expressed on proximal tubular cells37,38; and kidney-specific protein (KSP), a cadherin that is known to be expressed on all kidney tubular epithelial cells and marks mouse embryonic stem cell–derived kidney tubular cells15,39,40 (Figure 4, E and F). The formation of laminin-bounded tubular structures coexpressing LTL, KSP, and N-cadherin was reproducible in structures derived from both hESC and hiPSC lines (Figure 4, E and F). Furthermore, we observed the presence of primary cilia expressing the ciliary protein polycystin-2 on the apical surface of many of the tubular structures (Figure 4G), another feature of polarized kidney tubules that confirms the polarity of the epithelial cells.41,42 We then evaluated the expression of more differentiated kidney markers in cells treated with our IM-inducing protocol from days 0 to 9 and observed a significant, time-dependent upregulation in the expression of SIX2, a marker for multipotent nephron progenitor cells of the metanephric mesenchyme, and markers of mature kidney epithelial cells, such as NEPHRIN (podocyte), SYNAPTOPODIN (podocyte), AQP1 (proximal tubule), MEGALIN (proximal tubule), UMOD (loop of Henle), and AQP2 (collecting duct) (Figure 4H).
Figure 4.
PAX2+LHX1+ intermediate mesoderm cells form polar tubular structures expressing polycystin-2+ cilia and kidney proximal tubular markers. (A) Immunostaining time course from days 3 to 9 for PAX2, KSP, and LTL in hPSCs treated with FGF2+RA (ChFR) for 3 days, then cultured in media without additional growth factors for an additional 6 days. Scale bar, 50 μm. (B) Immunostaining for KSP and LTL in tubular structures formed by PAX2+LHX1+ IM cells, day 9. Inset shows tubular structure at higher magnification. Scale bar, 50 μm. (C) Brightfield stereomicroscope imaging of tubular structures, day 9. Scale bar, 200 μm. (D) Quantification of tubular structures formed from PAX2+LHX1+ IM cells, day 9. Data shown represent mean±SEM (n=4 for one hESC and n=4 for one hiPSC line). (E) Immunostaining for KSP, LTL, and laminin in tubular structures derived from two hESC lines and one iPSC line, day 9. Scale bar, 50 μm. (F) Immunostaining for LTL and N-cadherin in tubular structures derived from one hESC and one iPSC line, day 9. Scale bar, 50 μm. (G) Immunostaining for acetylated α-tubulin and polycystin-2 in tubular structures, day 9. Inset shows higher magnification of cilia localized to the apical surface. Scale bar, 50 μm. (H) Quantitative RT-PCR of genes associated with kidney development and mature kidney epithelial cells. Data shown represent mean±SEM (n=2). (I) Whole-mount immunohistochemistry for anti–human nuclear antigen (HNA) and laminin in chimeric kidney explant cultures. Dissociated hPSC-derived IM cells on day 3 (n=10) and day 9 (n=3) of differentiation were mixed with dissociated E12.5 mouse embryonic kidneys in single cell suspension, reaggregated by centrifugation, and cultured for 3 days in kidney explant culture. Arrowhead, laminin-bounded structures containing human and mouse cells. Scale bar, 50 μm. AQP1, aquaporin-1; AQP2, aquaporin-2; DAPI, 4′,6-diamidino-2-phenylindole; HNA, human nuclear antigen; UMOD, uromodulin.
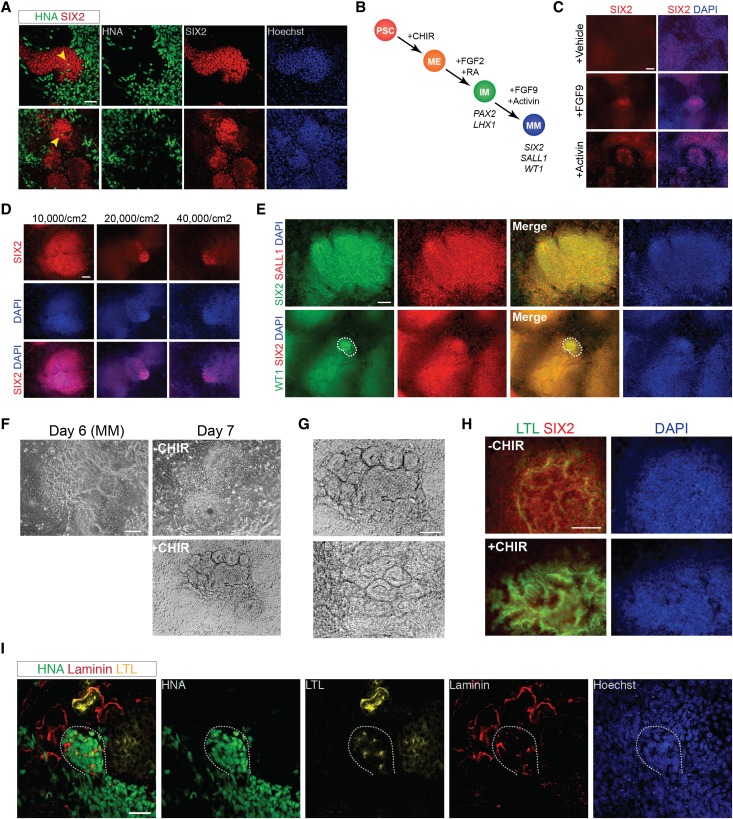
To further confirm the identity of these cells as embryonic kidney cells, we subjected them to kidney explant reaggregation assays.43 Cells expressing IM markers were dissociated on day 3 (PAX2+LHX1+) or 9 (LTL+KSP+) of differentiation and were recombined with dissociated cells from wild-type E12.5 mouse embryonic kidneys. Human cells from day 3 were incorporated into mouse metanephric tissues, distributing in the interstitium; however, no tubular integration was observed. Human cells from day 9 were found not only in the mouse metanephric interstitium but also within organized laminin-bounded structures that contained mouse cells (Figure 4I). These structures were similar in morphology to other laminin-bounded structures containing only mouse metanephric cells in the coculture reaggregate. However, tubular-like structures lined by human cells could not be visualized, suggesting that their integration into the mouse metanephric tissue was incomplete. When LTL+KSP+ tubular cells were implanted beneath the kidney capsule of immunodeficient mice, they generated human growths expressing AQP1 (Supplemental Figure 2, A–C).
FGF9 and Activin A Induce Expression of CM Markers in PAX2+LHX1+ Cells
During embryonic kidney development, the CM comprises a population of multipotent nephron progenitor stem cells that express the transcription factor SIX2 and give rise to nearly all the epithelial cells of the nephron, with the exception of the collecting duct cells.44 Although SIX2 mRNA levels increased during stochastic differentiation into tubular structures (Figure 4H), SIX2 protein was not clearly detectable by immunofluorescence, suggesting that its stable expression may require additional factors.44 In addition to forming chimeric laminin-bounded structures in recombination explant culture (Figure 4I), stochastically differentiated human PAX2+LHX1+ cells incorporated into clusters of Six2+ mouse metanephric cells but did not express SIX2 protein by immunofluorescence (Figure 5A). To identify conditions that promote and sustain a SIX2+ cell population in vitro, we screened growth factors added on day 3 of differentiation for the ability to induce SIX2 expression detectable by immunofluorescence (Figure 5B). From our initial screen, we observed small populations of SIX2+ cells when PAX2+LHX1+ cells were treated with FGF9 100 ng/ml or activin A 10 ng/ml for 5 additional days, whereas no SIX2+ cells were seen with treatment with a vehicle control (Figure 5C). The combination of FGF9 and activin A, together with decreasing the initial cell plating density, markedly improved the efficiency of SIX2 induction and demonstrated that SIX2 expression could be seen as early as day 6 of differentiation (Figure 5D). To confirm that this SIX2 expression was consistent with differentiation toward CM, we evaluated the expression of SALL1 and WT1, two other important markers of CM.34,45 Nearly all SIX2+ cells coexpressed SALL1 as seen by immunocytochemistry, and a subset of SIX2+ cells also coexpressed WT1 (Figure 5E).
Figure 5.
FGF-9 and activin differentiate PAX2+LHX1+ cells into cells expressing markers of CM. (A) Whole-mount immunohistochemistry for anti–human nuclear antigen (HNA) and SIX2 in chimeric kidney explant cultures. Dissociated hESC-derived IM cells on day 9 (n=3) of differentiation were mixed with dissociated E12.5 mouse embryonic kidneys in single cell suspension, reaggregated by centrifugation, and cultured for 5 days in kidney explant culture. Arrowhead, HNA+ cells present within clusters of mouse Six2+ cells. Scale bar, 50 μm. (B) Diagram showing the stepwise differentiation of hESCs into metanephric CM. (C) Immunostaining for SIX2 in hESCs treated with FGF2+RA (ChFR) for 3 days then either 100 ng/ml FGF-9, 10 ng/ml activin, or vehicle for 3 days, day 6. Scale bar, 100 μm. (D) Immunostaining for SIX2 in hESCs plated at different densities and treated with ChFR for 3 days then 100 ng/ml FGF-9+10 ng/ml activin for 3 days, day 6. Scale bar, 100 μm. (E) Immunostaining for SIX2, SALL1, and WT1 in hESCs treated with ChFR for 3 days then 100 ng/ml FGF-9+10 ng/ml activin for 3 days, day 6. Dashed line encompasses the population of cells that stain positive for WT1. Scale bar, 100 μm. (F) Brightfield microscopy of hESCs treated with ChFR for 3 days, 100 ng/ml FGF-9+10 ng/ml activin for 3 days, then either 5 μM CHIR (+CHIR) or FGF-9+activin (−CHIR) for 24 hours. Scale bar, 100 μm. (G) Higher magnification of tubular epithelial-like structures seen in SIX2+ cells treated on day 6 with 5 μM CHIR for 24 hours, day 7. Scale bar, 100 μm. (H) Immunostaining (day 8) for SIX2 and LTL in hESCs treated with ChFR for 3 days, 100 ng/ml FGF-9+10 ng/ml activin for 3 days, then either 5 μM CHIR (+CHIR) or FGF9+activin (−CHIR) for 24 hours. Scale bar, 100 μm. (I) Whole-mount immunohistochemistry for anti–human nuclear antigen (HNA), laminin, and LTL in chimeric kidney explant cultures. Dissociated hESC-derived SIX2+ cells on day 6 (n=10) of differentiation were mixed with dissociated E12.5 mouse embryonic kidneys in single cell suspension, reaggregated by centrifugation, and cultured for 3 days in kidney explant culture. Dashed line encompasses an organizing cluster of HNA+ cells, which express laminin and LTL. Scale bar, 50 μm. DAPI, 4′,6-diamidino-2-phenylindole; ME, mesendoderm; MM, metanephric cap mesenchyme; PSC, pluripotent stem cell.
During embryonic kidney development, Wnt signals from the ureteric bud induce the CM to condense into a pretubular aggregate, which subsequently develops into a renal vesicle, comma-shaped body, S-shaped body, and ultimately a nephron.20 In vitro, FACS isolated mouse Six2+ cells transiently induced by Wnt signaling (using the GSK-3β inhibitor BIO) undergo epithelialization and begin expressing markers of nephron development.46 To test the competence of hPSC-derived SIX2+ cells to respond to canonical Wnt signaling, we treated cells on day 6 of differentiation with 5 μM CHIR for 24 hours, followed by withdrawal of CHIR. Within 24 hours of CHIR treatment, we observed distinct changes in cell morphology and the formation of tubular-like structures (Figure 5, F and G). Immunocytochemistry of these structures on day 8 of differentiation revealed a downregulation of SIX2 expression and increased expression of the proximal tubule marker LTL in CHIR-induced cells compared with cells not treated with CHIR (Figure 5H), suggesting that treatment with CHIR had induced changes similar to that seen with induction of CM and the initiation of tubulogenesis in vivo. Furthermore, hPSC-derived SIX2+ cells explanted into dissociated-reaggregated mouse embryonic kidneys formed organizing clusters of cells that expressed LTL (Figure 5I). We therefore concluded that the ability of hPSC-derived PAX2+LHX1+ cells to be differentiated into cells expressing multiple markers of kidney CM and that could form tubule-like structures in response to Wnt signaling was consistent with the behavior and function of nephrogenic IM cells.
Discussion
We report a rapid, efficient, and highly reproducible system to induce intermediate mesoderm cells from hESCs and hiPSCs under precise, chemically defined, monolayer culture conditions. Robust generation of a BRACHYURY+MIXL1+ cell population with the use of CHIR confirmed the potency of GSK-3β inhibitors to generate mesendoderm-like cells47–49 and established the proper platform for us to screen compounds which could effectively promote IM differentiation. By investigating the differentiation kinetics of CHIR-treated hPSCs, we established that increasing exposure to CHIR resulted in differentiation toward a lateral plate mesoderm fate, but this default pathway could be altered by the precisely-timed addition of fate-altering growth factors. This affirms the findings of prior reports demonstrating that mesodermal and endodermal cell fates are determined by a delicate time- and dose-dependent balance of Wnt, activin, BMP, and FGF signaling.24,25,47,50–62 Our findings, however, contrast with those of a recent study by Tan and colleagues, who showed that prolonged exposure to GSK-3β inhibition, specifically with CHIR, promoted an endodermal rather than mesodermal fate.49 With our differentiation conditions, definitive endoderm differentiation could be achieved only with the synergistic effect of CHIR and high-dose activin.
Although the differentiation of hPSCs into cells of the cardiac, hepatic, pancreatic, and neuronal lineages has been widely reported, relatively few previous studies have attempted to derive cells of the kidney lineage from hPSCs.16–19 An alternative to directed differentiation was recently demonstrated by means of direct reprogramming of immortalized human kidney cells into nephron progenitor–like cells; however, the efficiency of integration into kidney explant cultures was reportedly low.63 Mae and colleagues demonstrated induction of an intermediate mesoderm population using a stepwise combination of CHIR, activin, and BMP7 signaling in engineered OSR1-GFP hiPSC cell lines, achieving efficiencies of >90% of OSR1-GFP+ cells after 11–18 days of differentiation.17 Because OSR1 is expressed in both the lateral plate and the intermediate mesoderm during early mesoderm specification,20 this expression pattern does not distinguish intermediate from lateral plate mesoderm, and the proportion of OSR1+ cells that coexpressed other important IM markers, such as PAX2 or WT1, was comparatively low. We selected PAX2 and LHX1 as more specific markers of IM for the purpose of defining IM inducing culture conditions. It is important to note that the expression of either PAX2 or LHX1 is not limited to the developing kidney during embryogenesis and can be seen at other stages of development in the eye, ear, and central nervous system35,36; however, coexpression of PAX2 and LHX1 within the same domain has been described only in the developing kidney and dorsal spinal cord.20,64 Importantly, we identified for the first time that FGF2 is a potent factor in inducing PAX2 expression in CHIR-induced mesendodermal cells, and when combined with RA, we were able to robustly generate a PAX2+LHX1+ IM cell population as confirmed by both immunocytochemistry and flow cytometry.
Our protocol was capable of achieving efficient IM differentiation within 3 days, which is considerably quicker than existing protocols while maintaining a high level of efficiency, and was highly reproducible in multiple hESC and hiPSC lines without the need for flow sorting. Interestingly, with our culture conditions we observed that the addition of BMP7, which has been used as a component of other kidney-lineage differentiation protocols,5,16,17 did not have a synergistic effect in inducing IM differentiation. Although the precise conditions for specifically generating other IM derivatives, including the adrenal cortices and gonads, have yet to be defined, we demonstrated that inducing PAX2+LHX1+ cells is sufficient for these cells to autonomously express WT1, a later marker of IM differentiation, and to form polarized, ciliated tubular structures that express markers of kidney proximal tubular cells and partially integrate into mouse metanephric cultures. As demonstrated by Hendry et al., this property is a strong indication that the cells may be nephron progenitors because other types of cells do not typically integrate into this compartment.63 These polarized tubular structures could reproducibly form in monolayer culture, in contrast to previous reports in which tubular structures derived from differentiated hPSCs cells could form only with three-dimensional culture in vitro or after incorporation into mouse metanephric kidneys ex vivo.17,18 Furthermore, using the combination of FGF9 and activin, we could specifically direct the differentiation of PAX2+LHX1+ cells into cells coexpressing multiple markers of nephron progenitor cells in the CM, particularly SIX2, SALL1, and WT1.
To our knowledge, this is the first report of the generation of SIX2+ cells from hPSCs, and our method of using FGF9 to induce SIX2 expression is consistent with the important role of FGF9 in maintaining the nephron progenitor population during embryonic kidney development.65 When these SIX2+ cells were transplanted ex vivo into mouse metanephric cultures, they organized into structures that expressed LTL and laminin. In parallel, activation of canonical Wnt signaling in the SIX2+ cell population using CHIR resulted in the rapid formation of tubule-like structures in vitro in which cells downregulated SIX2 and expressed LTL. Although this result suggests that hPSC-derived SIX2+ cells can be induced to condense and epithelialize in a manner similar to that seen with CM in vivo, further studies are needed to determine the precise conditions for activating a program of kidney tubulogenesis.
In conclusion, we demonstrate that sequential treatment with CHIR99021 and FGF2 and RA induces efficient differentiation of hPSCs into PAX2+LHX1+ cells characteristic of intermediate mesoderm and that these cells are capable of spontaneously forming polar ciliated tubular structures that express markers of kidney proximal tubular epithelial cells. Stochastically differentiated PAX2+LHX1+ cells also express markers of multiple differentiated, mature kidney cell types. The addition of FGF-9 and activin more specifically differentiates PAX2+LHX1+ cells into cells expressing SIX2, SALL1, and WT1, markers of the nephron progenitor stem cell pool in the CM, further demonstrating that PAX2+LHX1+ cells have the potential to give rise to IM derivatives. The establishment of this system will facilitate and improve the directed differentiation of hPSCs into cells of the kidney lineage for the purposes of bioengineering kidney tissue and iPS cell disease modeling.41
Concise Methods
hES and hiPS Cell Culture
Human foreskin (American Type Culture Collection) and human dermal fibroblast-α (Invitrogen) fibroblasts were reprogrammed into iPS cells by two rounds of overnight transduction with murine stem cell virus–internal ribosome entry site–green fluorescent protein retroviruses for OCT4, SOX2, KLF4, and c-MYC (Addgene) produced in 293FT cells (Invitrogen). H1, H9, and CHB8-H2B-GFP hESCs (passages 30–50) and human foreskin and human dermal fibroblast iPSCs (passages 12–40) were routinely cultured on irradiated mouse embryonic fibroblasts (GlobalStem) in DMEM/F12 (Invitrogen) supplemented with 20% KnockOut Serum Replacement (Invitrogen), 1 mM nonessential amino acids (Invitrogen), 2 mM GlutaMAX (Invitrogen), 0.55 mM 2-mercaptoethanol (Invitrogen), penicillin/streptomycin (Invitrogen), and 10 ng/ml recombinant human bFGF/FGF2 (Invitrogen). Cultures were passaged using collagenase type IV (STEMCELL Technologies) at a 1:3 split ratio every 5–7 days. For feeder-free culture, hESCs grown on mouse embryonic feeder fibroblasts were initially passaged using collagenase type IV onto plates coated with Geltrex hESC-qualified reduced growth factor basement membrane matrix (Invitrogen) according to manufacturer’s instructions and cultured in mTeSR1 medium (STEMCELL Technologies) supplemented with penicillin/streptomycin or ReproFF2 medium (ReproCELL) supplemented with FGF2.
Differentiation
For all differentiation experiments, hESCs or hiPSCs grown on Geltrex were washed once with PBS and dissociated into single cells with Accutase (STEMCELL Technologies). Cells were then plated at a density of 4×104 cells/cm2 onto Geltrex-coated plates in mTeSR1 medium supplemented with the ROCK inhibitor Y27632 10 μM (Stemgent). Cells were then fed daily with mTeSR1 without Y27632 for 2–3 days until they reached 50% confluency. To induce mesendoderm differentiation, the media were changed to Advanced RPMI (A-RPMI; Invitrogen) supplemented with 1× l-GlutaMAX (l-glu) and 1× penicillin/streptomycin (P/S) and CHIR (Stemgent), human Wnt3a (R&D systems), and/or human activin A (R&D Systems) at the doses described. To induce definitive endoderm differentiation, cells were treated with A-RPMI+1× l-glu+1× P/S+5 μM CHIR for 24 hours, then A-RPMI+1× l-glu+1×P/S+100 ng/ml activin A for 2–3 days. For hepatic differentiation, cells at the definitive endoderm stage were treated with A-RPMI+1× l-glu+1× P/S+1× B27 supplement (Invitrogen)+20 ng/ml BMP-4 (R&D systems)+10 ng/ml FGF2 (Invitrogen) for 5 days, then A-RPMI+1× l-glu+1× P/S+1× B27+10 ng/ml hepatocyte growth factor for 5 days, then hepatocyte culture medium (Lonza) supplemented with 20 ng/ml Oncostatin M and 10 ng/ml hepatocyte growth factor (Peprotech) for 5 days. For pancreatic differentiation, cells at the definitive endoderm stage were treated with DMEM/F12+2% FBS (Hyclone)+50 ng/ml FGF-7 (R&D systems) for 2 days, then high-glucose DMEM (Mediatech)+1% B27+2 μM retinoic acid (Sigma-Aldrich)+0.25 μM 3-keto-N-(aminoethyl-aminocaproyl-dihydrocinnamoyl)-cyclopamine (EMD Millipore)+100 ng/ml recombinant human Noggin (R&D Systems) for 4 days, then high-glucose DMEM+1% B27+100 ng/ml Noggin+300 nM indolactam V (Stemgent)+1 μM ALK5 inhibitor II (Axxora) for 4 days. For anterior foregut endoderm differentiation, cells at the definitive endoderm stage were treated with DMEM/F12+1× l-glu+1× B27+200 ng/ml Noggin+10 μM SB431542 (Stemgent) for 3 days. For hindgut endoderm differentiation, cells at the definitive endoderm stage were treated with A-RPMI+1× l-glu+1× P/S+2% FBS+500 ng/ml FGF4 (R&D Systems)+5μM CHIR for 4 days. For intermediate mesoderm differentiation, cells were treated with A-RPMI+1× l-glu+1× P/S+5 μM CHIR for 24, 36, or 48 hours, then A-RPMI+1× l-glu+1× P/S+100 ng/ml FGF2+1 μM retinoic acid for 2–3 days. For CM differentiation, cells were treated with A-RPMI+1× l-glu+1× P/S+5 μM CHIR for 36 hours, then A-RPMI+1× l-glu+1× P/S+100 ng/ml FGF2+1 μM retinoic acid for 36–42 hours, then A-RPMI+1× l-glu+1× P/S+100 ng/ml FGF-9 (R&D Systems)+10 ng/ml activin A for 3 days.
Immunofluorescence
For immunofluorescence studies, cultures were washed once with PBS (Invitrogen) and fixed in 4% paraformaldehyde for 15 minutes at room temperature (RT). Fixed cells were then washed three times in PBS and incubated in blocking buffer (0.3% Triton X-100 [Fisher Scientific] and 5% normal donkey serum [EMD Millipore] in PBS) for 1 hour at RT. The cells were then incubated with primary antibody overnight at 4°C or for 2 hours at RT in antibody dilution buffer (0.3% Triton X-100 and 1% BSA [Roche] in PBS). Cells were then washed three times in PBS and incubated with Alexa Fluor 488–, 555–, or 647–conjugated secondary antibodies (1:500) (Molecular Probes) in antibody dilution buffer for 1 hour at RT. For immunostaining with biotinylated LTL (Vector Labs), a streptavidin/biotin blocking kit (Vector Labs) and Alexa Fluor 488- or 647-conjugated streptavidin (Molecular Probes) were used according to manufacturer’s instructions. Nuclei were counterstained with DAPI (Sigma-Aldrich). A list of primary antibodies can be found in Supplemental Table 1. Immunofluorescence was visualized using an inverted fluorescence microscope (Nikon Eclipse Ti, Tokyo, Japan). Quantification was performed by counting a minimum of five random fields at ×10 magnification.
Quantitative RT-PCR
Total RNA was harvested and isolated from cells using the RNeasy Mini Kit (Qiagen). One microgram of RNA was used for reverse transcription with the M-MLV Reverse Transcription System (Promega), or 500 ng of RNA was used for High Capacity cDNA Reverse Transcription Kits (Applied Biosystems). RT-PCR reactions were run in duplicate using cDNA (diluted 1:10), 300 or 400 nM forward and reverse primers, and iQ SYBR Green Supermix (Bio-Rad) or iTAQ SYBR Green Supermix (Bio-Rad). Quantitative RT-PCR was performed using the iQ5 Multicolor Real-Time PCR Detection System (Bio-Rad). All samples were run with two technical replicates. β-Actin was used as the housekeeping gene. Primer sequences are listed in Supplemental Table 2.
Flow Cytometry
Cells were dissociated using Accutase for 15 minutes, and cell clumps were removed with a 40-μm cell strainer (BD Biosciences). Cells were fixed with 2% paraformaldehyde for 15 minutes on ice and then permeabilized with 0.1% Triton for 15 minutes on ice. Cells were then blocked with PBS+5% donkey serum for 15 minutes on ice and incubated with primary antibodies (PAX2 1:1000, LHX1 1:100) for 30 minutes. After washing three times with 1% BSA in PBS, cells were incubated with secondary antibodies (Alexa Fluor 488–conjugated donkey anti-rabbit 1:5000, Cy5-conjugated donkey anti-mouse 1:2500 [Jackson ImmunoResearch]) for 20 minutes on ice. Cells were then washed three times with 1% BSA in PBS. Flow cytometry was performed using MACSQuant (Miltenyi Biotec), and data analysis was performed using FlowJo software. Optimal dilution ratios of antibodies were determined using a negative control human proximal tubular cell line (HKC-8) that does not express PAX2 or LHX1. HKC-8 was kindly provided by Dr. Lorraine Racusen (Johns Hopkins Hospital).
Chimeric Kidney Explant Cultures
Chimeric kidney explants were created using published reaggregation techniques.43,66 Briefly, embryonic kidneys at stage E12.5 (day of plug=E0.5) were isolated from timed pregnant females (Charles River). Complete urogenital systems were placed in DMEM (Corning Cellgro) without serum and further dissected to isolate single E12.5 kidneys. Four to six kidneys were incubated in TrypLE Express (Invitrogen) at 37°C for 4 minutes. The enzyme was then quenched by adding kidney culture media (DMEM + 1× P/S + 10% FBS or renal epithelial cell basal media [Lonza] with 0.5% FBS) and incubating at 37°C for 10 minutes for recovery. Digested kidney rudiments were then transferred to a microcentrifuge tube with additional kidney culture medium and dissociated by repeated trituration. The cell suspension was then passed through a 100-μm-pore-size cell strainer before visualizing to confirm single cell suspension and counting. Differentiated human pluripotent stem cells from day 3, day 6, or day 9 were dissociated with TrypLE, visualized, and counted. Reaggregation was done by mixing 130,000 dissociated mouse kidney cells with 13,000 differentiated human cells in a microcentrifuge tube and centrifuging the chimeric mixture into a pellet at 700g. The resulting chimeric pellet was then placed onto a Nucleopore Track-Etch Membrane filter disk (pore size=1 μm) (Whatman),67 and the filter was floated on 1 ml kidney culture medium (or renal epithelial cell basal media)+10 μM Y2763268 in a 24-well tissue culture plate (two explants per 13-mm circular filter) and incubated for 24 hours. After the initial incubation, the media with Y27632 were replaced with kidney culture medium (or renal epithelial cell basal media) only and cultured for an additional 2 days. No qualitative difference in morphology or growth rate was observed between the two media recipes.
Whole-Mount Immunohistochemistry of Cultured Kidneys
Our immunochemistry protocol is based on previous methods for kidney explant cultures.69 Filters with cultured explants were rinsed once with PBS, then fixed with 4% paraformaldehyde in PBS for 30 minutes in a 24-well plate. Care was taken to submerge the explants to ensure even fixation. Following fixation, explants were washed three times in PBT (PBS with 0.1% Triton X-100) for 5 minutes each at room temperature with gentle rocking. Explants were then incubated in blocking solution (PBT with 5% donkey serum) at room temperature with rocking. Blocking solution was then removed and replaced with a primary antibody solution of diluted antibodies in PBT with 1% donkey serum and samples incubated overnight at 4°C. Antibody dilutions used were mouse anti–human nuclear antigen, 1:250 (Millipore), and rabbit anti-laminin, 1:500 (Sigma-Aldrich). Explants were then washed with PBT three times for 1 hour each with rocking at room temperature. Secondary antibody solution (PBT+1% donkey serum) with 1:250 dilutions of Alexa Fluor 488–conjugated donkey anti-mouse and Alexa Fluor 568–conjugated donkey anti-rabbit antibodies (Invitrogen) were added and incubated for 1–2 hours at room temperature. Samples were then washed with PBT three times for 30 minutes each at room temperature, followed by a 10-minute incubation with DAPI, and three additional 5-minute washes with PBS. Explant samples were then mounted with Vectashield (Vector Labs) and examined using a Nikon C1 confocal microscope.
Supcapsular Implantation
Two wells of a confluent 24-well plate containing LTL+KSP+ tubular structures were scored by needle, scraped off in clumps in 100 µl of DMEM/F12, and injected through an insulin syringe beneath the kidney capsule of adult NOD-SCID mice. Growths were harvested 3 weeks later, photodocumented, fixed in 4% paraformaldehyde, and paraffin-embedded or cryopreserved for serial sectioning.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank Dr. Andrew Elefanty for the rat anti-MIXL1 antibody and Drs. Hiroshi Itoh and Toshiaki Monkawa for the mouse anti-KSP antibody.
This study was supported by National Institutes of Health grants RC1 DK0864406 (J.V.B., A.Q.L.), R01 DK39773 (J.V.B.), F32 DK084692 (A.Q.L.), F32 DK092036 and NIH LRP (B.S.F.), and R00HD061981 (P.L.); American Heart Association grant 11FTF7320023 (A.Q.L.); the Harvard Stem Cell Institute (A.Q.L., M.T.V., J.V.B.) and the Uehara Memorial Foundation (R.M.); and a Grant-in-Aid for JSPS Postdoctoral Fellowship for Research Abroad (R.M.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013080831/-/DCSupplemental.
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S: Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131: 861–872, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM: Embryonic stem cell lines derived from human blastocysts. Science 282: 1145–1147, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Ren X, Zhang J, Gong X, Niu X, Zhang X, Chen P, Zhang X: Differentiation of murine embryonic stem cells toward renal lineages by conditioned medium from ureteric bud cells in vitro. Acta Biochim Biophys Sin (Shanghai) 42: 464–471, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Kim D, Dressler GR: Nephrogenic factors promote differentiation of mouse embryonic stem cells into renal epithelia. J Am Soc Nephrol 16: 3527–3534, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Ross EA, Williams MJ, Hamazaki T, Terada N, Clapp WL, Adin C, Ellison GW, Jorgensen M, Batich CD: Embryonic stem cells proliferate and differentiate when seeded into kidney scaffolds. J Am Soc Nephrol 20: 2338–2347, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rak-Raszewska A, Wilm B, Edgar D, Kenny S, Woolf AS, Murray P: Development of embryonic stem cells in recombinant kidneys. Organogenesis 8: 125–136, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vigneau C, Polgar K, Striker G, Elliott J, Hyink D, Weber O, Fehling HJ, Keller G, Burrow C, Wilson P: Mouse embryonic stem cell-derived embryoid bodies generate progenitors that integrate long term into renal proximal tubules in vivo. J Am Soc Nephrol 18: 1709–1720, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Nakane A, Kojima Y, Hayashi Y, Kohri K, Masui S, Nishinakamura R: Pax2 overexpression in embryoid bodies induces upregulation of integrin alpha8 and aquaporin-1. In Vitro Cell Dev Biol Anim 45: 62–68, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Bruce SJ, Rea RW, Steptoe AL, Busslinger M, Bertram JF, Perkins AC: In vitro differentiation of murine embryonic stem cells toward a renal lineage. Differentiation 75: 337–349, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Usui J, Kobayashi T, Yamaguchi T, Knisely AS, Nishinakamura R, Nakauchi H: Generation of kidney from pluripotent stem cells via blastocyst complementation. Am J Pathol 180: 2417–2426, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Nishikawa M, Yanagawa N, Kojima N, Yuri S, Hauser PV, Jo OD, Yanagawa N: Stepwise renal lineage differentiation of mouse embryonic stem cells tracing in vivo development. Biochem Biophys Res Commun 417: 897–902, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Morizane R, Monkawa T, Itoh H: Differentiation of murine embryonic stem and induced pluripotent stem cells to renal lineage in vitro. Biochem Biophys Res Commun 390: 1334–1339, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Mae S, Shirasawa S, Yoshie S, Sato F, Kanoh Y, Ichikawa H, Yokoyama T, Yue F, Tomotsune D, Sasaki K: Combination of small molecules enhances differentiation of mouse embryonic stem cells into intermediate mesoderm through BMP7-positive cells. Biochem Biophys Res Commun 393: 877–882, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Morizane R, Monkawa T, Fujii S, Yamaguchi S, Homma K, Matsuzaki Y, Okano H, Itoh H: Kidney specific protein-positive cells derived from embryonic stem cells reproduce tubular structures in vitro and differentiate into renal tubular cells. PLoS ONE 8: e64843, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Batchelder CA, Lee CC, Matsell DG, Yoder MC, Tarantal AF: Renal ontogeny in the rhesus monkey (Macaca mulatta) and directed differentiation of human embryonic stem cells towards kidney precursors. Differentiation 78: 45–56, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mae S, Shono A, Shiota F, Yasuno T, Kajiwara M, Gotoda-Nishimura N, Arai S, Sato-Otubo A, Toyoda T, Takahashi K, Nakayama N, Cowan CA, Aoi T, Ogawa S, McMahon AP, Yamanaka S, Osafune K: Monitoring and robust induction of nephrogenic intermediate mesoderm from human pluripotent stem cells. Nature Comm 4: 1367, 2013 [DOI] [PMC free article] [PubMed]
- 18.Narayanan K, Schumacher KM, Tasnim F, Kandasamy K, Schumacher A, Ni M, Gao S, Gopalan B, Zink D, Ying JY: Human embryonic stem cells differentiate into functional renal proximal tubular-like cells. Kidney Int 83: 593–603, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Lin SA, Kolle G, Grimmond SM, Zhou Q, Doust E, Little MH, Aronow B, Ricardo SD, Pera MF, Bertram JF, Laslett AL: Subfractionation of differentiating human embryonic stem cell populations allows the isolation of a mesodermal population enriched for intermediate mesoderm and putative renal progenitors. Stem Cells Dev 19: 1637–1648, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dressler GR: Advances in early kidney specification, development and patterning. Development 136: 3863–3874, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knepper MA: The aquaporin family of molecular water channels. Proc Natl Acad Sci U S A 91: 6255–6258, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tam PP, Loebel DA: Gene function in mouse embryogenesis: Get set for gastrulation. Nat Rev Genet 8: 368–381, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Conlon FL, Lyons KM, Takaesu N, Barth KS, Kispert A, Herrmann B, Robertson EJ: A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse. Development 120: 1919–1928, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A: Requirement for Wnt3 in vertebrate axis formation. Nat Genet 22: 361–365, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Gadue P, Huber TL, Paddison PJ, Keller GM: Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc Natl Acad Sci U S A 103: 16806–16811, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ip YT, Gridley T: Cell movements during gastrulation: Snail dependent and independent pathways. Curr Opin Genet Dev 12: 423–429, 2002 [DOI] [PubMed] [Google Scholar]
- 27.D’Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE: Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol 24: 1392–1401, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Blum B, Hrvatin SS, Schuetz C, Bonal C, Rezania A, Melton DA: Functional beta-cell maturation is marked by an increased glucose threshold and by expression of urocortin 3. Nat Biotechnol 30: 261–264, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Si-Tayeb K, Noto FK, Nagaoka M, Li J, Battle MA, Duris C, North PE, Dalton S, Duncan SA: Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology 51: 297–305, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Green MD, Chen A, Nostro MC, d’Souza SL, Schaniel C, Lemischka IR, Gouon-Evans V, Keller G, Snoeck HW: Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells. Nat Biotechnol 29: 267–272, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, Shroyer NF, Wells JM: Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470: 105–109, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taira M, Otani H, Jamrich M, Dawid IB: Expression of the LIM class homeobox gene Xlim-1 in pronephros and CNS cell lineages of Xenopus embryos is affected by retinoic acid and exogastrulation. Development 120: 1525–1536, 1994 [DOI] [PubMed] [Google Scholar]
- 33.Cartry J, Nichane M, Ribes V, Colas A, Riou JF, Pieler T, Dollé P, Bellefroid EJ, Umbhauer M: Retinoic acid signalling is required for specification of pronephric cell fate. Dev Biol 299: 35–51, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Armstrong JF, Pritchard-Jones K, Bickmore WA, Hastie ND, Bard JB: The expression of the Wilms’ tumour gene, WT1, in the developing mammalian embryo. Mech Dev 40: 85–97, 1993 [DOI] [PubMed] [Google Scholar]
- 35.Fujii T, Pichel JG, Taira M, Toyama R, Dawid IB, Westphal H: Expression patterns of the murine LIM class homeobox gene lim1 in the developing brain and excretory system. Development Dynam 199: 73–83, 1994 [DOI] [PubMed]
- 36.Püschel AW, Westerfield M, Dressler GR: Comparative analysis of Pax-2 protein distributions during neurulation in mice and zebrafish. Mech Dev 38: 197–208, 1992 [DOI] [PubMed] [Google Scholar]
- 37.Prozialeck WC, Lamar PC, Appelt DM: Differential expression of E-cadherin, N-cadherin and beta-catenin in proximal and distal segments of the rat nephron. BMC Physiol 4: 10, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nouwen EJ, Dauwe S, van der Biest I, De Broe ME: Stage- and segment-specific expression of cell-adhesion molecules N-CAM, A-CAM, and L-CAM in the kidney. Kidney Int 44: 147–158, 1993 [DOI] [PubMed] [Google Scholar]
- 39.Wertz K, Herrmann BG: Kidney-specific cadherin (cdh16) is expressed in embryonic kidney, lung, and sex ducts. Mech Dev 84: 185–188, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Thomson RB, Igarashi P, Biemesderfer D, Kim R, Abu-Alfa A, Soleimani M, Aronson PS: Isolation and cDNA cloning of Ksp-cadherin, a novel kidney-specific member of the cadherin multigene family. J Biol Chem 270: 17594–17601, 1995 [DOI] [PubMed] [Google Scholar]
- 41.Freedman BS, Lam AQ, Sundsbak JL, Iatrino R, Su X, Koon SJ, Wu M, Daheron L, Harris PC, Zhou J, Bonventre JV: Reduced ciliary polycystin-2 in induced pluripotent stem cells from polycystic kidney disease patients with PKD1 mutations. J Am Soc Nephrol 24: 1571–1586, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoder BK, Hou X, Guay-Woodford LM: The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol 13: 2508–2516, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Davies JA, Unbekandt M, Ineson J, Lusis M, Little MH: Dissociation of embryonic kidney followed by re-aggregation as a method for chimeric analysis. Methods Mol Biol 886: 135–146, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, McMahon AP: Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 3: 169–181, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nishinakamura R, Uchiyama Y, Sakaguchi M, Fujimura S: Nephron progenitors in the metanephric mesenchyme. Pediatr Nephrol 26: 1463–1467, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Park JS, Ma W, O’Brien LL, Chung E, Guo JJ, Cheng JG, Valerius MT, McMahon JA, Wong WH, McMahon AP: Six2 and Wnt regulate self-renewal and commitment of nephron progenitors through shared gene regulatory networks. Dev Cell 23: 637–651, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sumi T, Tsuneyoshi N, Nakatsuji N, Suemori H: Defining early lineage specification of human embryonic stem cells by the orchestrated balance of canonical Wnt/beta-catenin, Activin/Nodal and BMP signaling. Development 135: 2969–2979, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Bone HK, Nelson AS, Goldring CE, Tosh D, Welham MJ: A novel chemically directed route for the generation of definitive endoderm from human embryonic stem cells based on inhibition of GSK-3. J Cell Sci 124: 1992–2000, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tan JY, Sriram G, Rufaihah AJ, Neoh KG, Cao T: Efficient derivation of lateral plate and paraxial mesoderm subtypes from human embryonic stem cells through GSKi-mediated differentiation. Stem Cells Dev 22: 1893–1906, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jackson SA, Schiesser J, Stanley EG, Elefanty AG: Differentiating embryonic stem cells pass through ‘temporal windows’ that mark responsiveness to exogenous and paracrine mesendoderm inducing signals. PLoS ONE 5: e10706, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Evseenko D, Zhu Y, Schenke-Layland K, Kuo J, Latour B, Ge S, Scholes J, Dravid G, Li X, MacLellan WR, Crooks GM: Mapping the first stages of mesoderm commitment during differentiation of human embryonic stem cells. Proc Natl Acad Sci U S A 107: 13742–13747, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vallier L, Touboul T, Brown S, Cho C, Bilican B, Alexander M, Cedervall J, Chandran S, Ahrlund-Richter L, Weber A, Pedersen RA: Signaling pathways controlling pluripotency and early cell fate decisions of human induced pluripotent stem cells. Stem Cells 27: 2655–2666, 2009 [DOI] [PubMed] [Google Scholar]
- 53.Fehling HJ, Lacaud G, Kubo A, Kennedy M, Robertson S, Keller G, Kouskoff V: Tracking mesoderm induction and its specification to the hemangioblast during embryonic stem cell differentiation. Development 130: 4217–4227, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Kubo A, Shinozaki K, Shannon JM, Kouskoff V, Kennedy M, Woo S, Fehling HJ, Keller G: Development of definitive endoderm from embryonic stem cells in culture. Development 131: 1651–1662, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Chng Z, Teo A, Pedersen RA, Vallier L: SIP1 mediates cell-fate decisions between neuroectoderm and mesendoderm in human pluripotent stem cells. Cell Stem Cell 6: 59–70, 2010 [DOI] [PubMed] [Google Scholar]
- 56.Nakanishi M, Kurisaki A, Hayashi Y, Warashina M, Ishiura S, Kusuda-Furue M, Asashima M: Directed induction of anterior and posterior primitive streak by Wnt from embryonic stem cells cultured in a chemically defined serum-free medium. FASEB J 23: 114–122, 2009 [DOI] [PubMed] [Google Scholar]
- 57.ten Berge D, Koole W, Fuerer C, Fish M, Eroglu E, Nusse R: Wnt signaling mediates self-organization and axis formation in embryoid bodies. Cell Stem Cell 3: 508–518, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thomson M, Liu SJ, Zou LN, Smith Z, Meissner A, Ramanathan S: Pluripotency factors in embryonic stem cells regulate differentiation into germ layers. Cell 145: 875–889, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.D’Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE: Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol 23: 1534–1541, 2005 [DOI] [PubMed] [Google Scholar]
- 60.Zhang P, Li J, Tan Z, Wang C, Liu T, Chen L, Yong J, Jiang W, Sun X, Du L, Ding M, Deng H: Short-term BMP-4 treatment initiates mesoderm induction in human embryonic stem cells. Blood 111: 1933–1941, 2008 [DOI] [PubMed] [Google Scholar]
- 61.Nostro MC, Cheng X, Keller GM, Gadue P: Wnt, activin, and BMP signaling regulate distinct stages in the developmental pathway from embryonic stem cells to blood. Cell Stem Cell 2: 60–71, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pearson S, Sroczynska P, Lacaud G, Kouskoff V: The stepwise specification of embryonic stem cells to hematopoietic fate is driven by sequential exposure to Bmp4, activin A, bFGF and VEGF. Development 135: 1525–1535, 2008 [DOI] [PubMed] [Google Scholar]
- 63.Hendry CE, Vanslambrouck JM, Ineson J, Suhaimi N, Takasato M, Rae F, Little MH: Direct transcriptional reprogramming of adult cells to embryonic nephron progenitors. J Am Soc Nephrol 24: 1424–1434, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pillai A, Mansouri A, Behringer R, Westphal H, Goulding M: Lhx1 and Lhx5 maintain the inhibitory-neurotransmitter status of interneurons in the dorsal spinal cord. Development 134: 357–366, 2007 [DOI] [PubMed] [Google Scholar]
- 65.Barak H, Huh SH, Chen S, Jeanpierre C, Martinovic J, Parisot M, Bole-Feysot C, Nitschké P, Salomon R, Antignac C, Ornitz DM, Kopan R: FGF9 and FGF20 maintain the stemness of nephron progenitors in mice and man. Dev Cell 22: 1191–1207, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lusis M, Li J, Ineson J, Christensen ME, Rice A, Little MH: Isolation of clonogenic, long-term self renewing embryonic renal stem cells. Stem Cell Res (Amst) 5: 23–39, 2010 [DOI] [PubMed] [Google Scholar]
- 67.Park JS, Valerius MT, McMahon AP: Wnt/beta-catenin signaling regulates nephron induction during mouse kidney development. Development 134: 2533–2539, 2007 [DOI] [PubMed] [Google Scholar]
- 68.Unbekandt M, Davies JA: Dissociation of embryonic kidneys followed by reaggregation allows the formation of renal tissues. Kidney Int 77: 407–416, 2010 [DOI] [PubMed] [Google Scholar]
- 69.Barak H, Boyle SC: Organ culture and immunostaining of mouse embryonic kidneys. Cold Spring Harbor Protocols, 2011: pdb prot5558, 2011 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.