Abstract
Domoic acid (DA), an excitatory amino acid produced by diatoms belonging to the genus Pseudo-nitzschia, is a glutamate analog responsible for the neurologic condition referred to as amnesic shellfish poisoning. To date, the renal effects of DA have been underappreciated, although renal filtration is the primary route of systemic elimination and the kidney expresses ionotropic glutamate receptors. To characterize the renal effects of DA, we administered either a neurotoxic dose of DA or doses below the recognized limit of toxicity to adult Sv128/Black Swiss mice. DA preferentially accumulated in the kidney and elicited marked renal vascular and tubular damage consistent with acute tubular necrosis, apoptosis, and renal tubular cell desquamation, with toxic vacuolization and mitochondrial swelling as hallmarks of the cellular damage. Doses≥0.1 mg/kg DA elevated the renal injury biomarkers kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin, and doses≥0.005 mg/kg induced the early response genes c-fos and junb. Coadministration of DA with the broad spectrum excitatory amino acid antagonist kynurenic acid inhibited induction of c-fos, junb, and neutrophil gelatinase-associated lipocalin. These findings suggest that the kidney may be susceptible to excitotoxic agonists, and renal effects should be considered when examining glutamate receptor activation. Additionally, these results indicate that DA is a potent nephrotoxicant, and potential renal toxicity may require consideration when determining safe levels for human exposure.
Domoic acid (DA), a water-soluble, heat-stable tricarboxylic acid produced by diatoms belonging to the genus Pseudo-nitzschia, is responsible for a condition known as amnesic shellfish poisoning in humans.1–4 Shellfish, such as clams and mussels, and fish that accumulate DA serve as vectors of exposure to various species of birds and aquatic mammals in addition to humans.5,6 Initially recognized as a human toxicant when more than 100 people became ill after eating contaminated mussels in eastern Canada in 1987, DA poisoning was defined by the occurrence of gastrointestinal or neurologic symptoms ranging from abdominal cramps and headache to more severe cases of memory loss, seizures, coma, and even death.2,4 Increased awareness and governmental regulation, which set a limit of 20 μg DA/g in shellfish tissue, has reduced the incidence of DA toxicity in humans since the 1987 outbreak. However, there is concern that exposure will increase because of the growing presence of toxic diatom-producing algal blooms, which are often attributed to human factors, such as pollution, shipping, and global warming, leading to greater nutrient availability, greater distribution of algal species, and longer growth periods.7–14 Although the overt gastrointestinal and neurologic manifestations have defined the disease, emerging evidence from animal and human studies support previously unrecognized threats and novel toxicologic syndromes caused by subclinical toxicity from acute and chronic DA exposures, which may ultimately challenge the adequacy of the current acceptable limit.15–18
DA is a potent activator of kainate receptors (KRs) as well as a subpopulation of α-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid receptors (AMPARs).19 The toxic response produced by DA is a coordinated effort, which involves initial activation of KR and AMPAR by DA and secondary activation of N-methyl-D-aspartate receptors (NMDARs) by glutamate, and it is associated with an influx of Ca2+ across the plasma membrane, inflammation, neuronal cell injury and death, and neurobehavioral alterations.19–22 Although they are extensively characterized in the central nervous system, glutamate receptors are also expressed at peripheral sites and have been shown to exhibit toxicity in multiple tissues, including the kidney, where NMDARs contribute to organ damage in models of ischemia-reperfusion injury and gentamicin nephrotoxicity.23–26 There is limited information about the effects of DA on the kidney; however, oral dosing in coho salmon has shown that the kidney is a primary site of DA accumulation in this species, and studies in rodents have shown that renal excretion is the exclusive route of systemic DA elimination.27,28 Examination of sea lions after DA poisoning has also revealed some evidence of interstitial nephritis, renal edema, and elevated BUN, although the exact cause of these findings cannot be definitively attributed to DA toxicity.29,30 Furthermore, sea lions with acute DA toxicosis seem to have an elevated hematocrit,31 suggesting that water reabsorption or red blood cell production could be affected, both of which are functions of the kidney. Despite this circumstantial evidence, a detailed examination of the renal response to DA administration has not been fully explored. The purpose of the current study was to characterize the acute renal effects of DA at doses that produce neurotoxicity and neurobehavioral changes (1.0–2.5 mg/kg) as well as several lower doses (0.0005–0.5 mg/kg), which are considered below the limit of toxicity.
Results
DA Preferentially Accumulates in the Murine Kidney
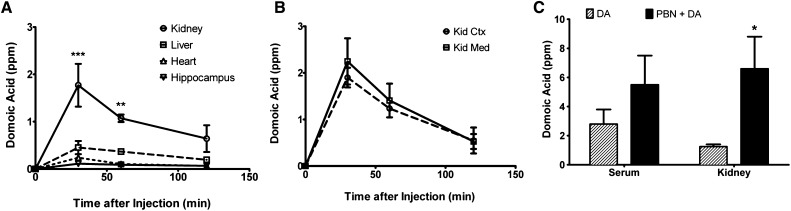
Thirty minutes after injection, DA concentration ([DA]) in the kidney was fourfold higher compared with the liver, heart, and hippocampus. Although it decreased between 30 and 120 minutes, [DA] was still highest in the kidney at each time point, and it was undetectable after 24 hours (not shown). There was no difference in [DA] in the renal cortex compared with the medulla (Figure 1B). To determine the effect of renal secretion on DA elimination and tissue deposition, the organic anion transport inhibitor probenecid (PBN; 600 mg/kg) was administered 10 minutes before DA injection, and mice were euthanized 30 minutes after the injection of DA. At this time point, there were significantly higher levels of [DA] in the kidneys of mice exposed to PBN+DA compared with DA alone (Figure 1C). These results suggest that the route of elimination of DA by the kidney not only involves filtration but also tubular secretion.
Figure 1.
DA preferentially accumulated in the murine kidney. (A) DA levels were measured in the kidney, liver, heart, and hippocampus at 30, 60, and 120 minutes after administration of DA. (B) DA levels were measured in the kidney cortex (Kid Ctx) and medulla (Kid Med) at 30, 60, and 120 minutes after administration of DA. DA was undetectable at 24 hours. (C) DA concentrations were measured in the serum and kidney 30 minutes after exposure to DA alone or PBN+DA. *Data were significantly different from (A) the 0-minute time point or (C) DA groups (n=4, *P<0.05; **P<0.01; ***P<0.001, ANOVA followed by Newman–Keuls for individual group comparisons).
KR Expression in the Kidney
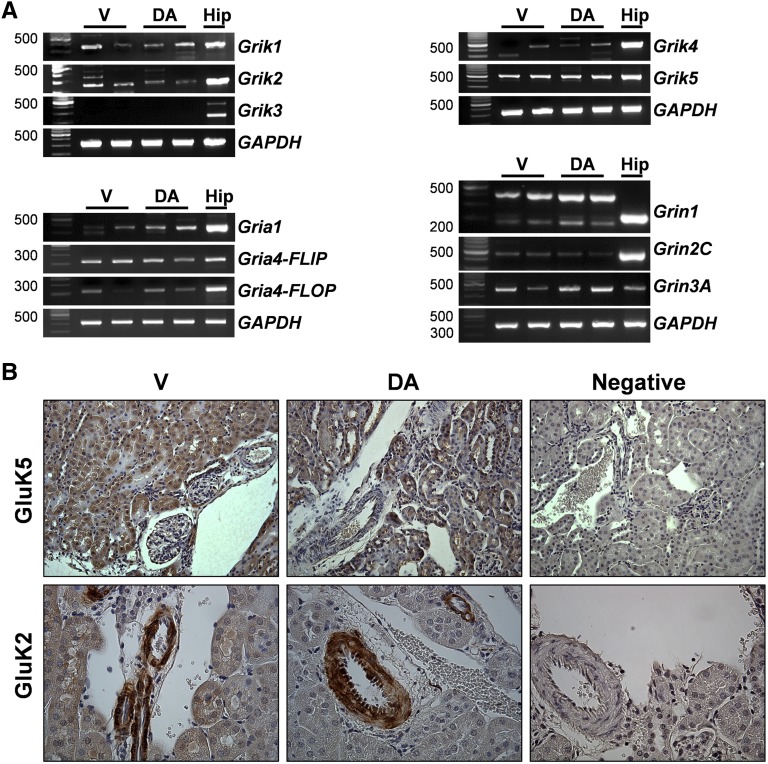
Ionotropic glutamate receptors are expressed in the kidney24,32; however, only a select number have been examined in detail, and a more complete analysis of the KR was necessary. Grik1 and Grik2 mRNA, which encodes the catalytic KR subtypes GluK1 and GluK2, respectively, were detected at varying levels in vehicle- and DA-treated kidneys, and Grik3 was not detected (Figure 2A). Grik4 and Grik5, which encode the high-affinity KR GluK4 and GluK5, respectively, were also detected in vehicle- and DA-treated kidneys (Figure 2A). AMPARs and NMDARs were also examined and detected by RT-PCR analysis, and there were no obvious differences between vehicle- and DA-treated kidneys (Figure 2A). By immunoblot analysis, both GluK2 and GluK5 proteins were also detected in the kidney (Supplemental Figure 1). The distribution of GluK5 and GluK2 proteins was analyzed by immunohistochemistry (IHC) in kidney sections from vehicle- and DA-treated animals after 72 hours of exposure (Figure 2B). GluK5 was expressed throughout the cortex as well as the medullary region (primarily in tubules) in both vehicle- and DA-treated animals (Figure 2B, Supplemental Figure 1). GluK2 protein was expressed predominantly in the renal vasculature (Figure 2B).
Figure 2.
Glutamate receptor mRNA and protein were detected in the murine kidney. (A) RT-PCR mRNA analysis of KR (Grik1, Grik2, Grik3, Grik4, and Grik5), AMPAR (Gria1, Gria4-FLIP, and Gria4-FLOP), and NMDAR subtypes (Grin1, Grin2C, and Grin3A) in the kidneys of vehicle- and DA-treated mice. Hippocampal lysates were used as a positive control for each gene product. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; Hip, hippocampal lysate; V, vehicle. (B) GluK5 and GluK2 expressions were examined by IHC in the renal cortex of kidneys from V- and DA-treated mice. Note the intense tubular staining of GluK5 in both V- and DA-treated kidneys and the predominantly vascular staining of GluK2 in renal tissue. Diaminobenzidine chromogen with hematoxylin counterstain was used. Diluent without antibody was applied to the negative control sections.
Renal Tissue Damage and Cell Death
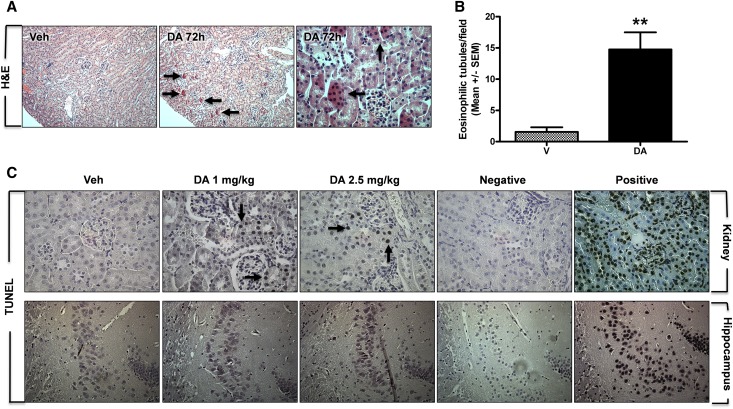
Administration of DA (2.5 mg/kg) for 3 consecutive days produced histopathological alterations (Figure 3A), including cytoplasmic hypereosinophilia in proximal tubules (Figure 3, A, arrows, and B), which presented sporadically throughout the renal cortex, consistent with acute tubular cell death. Higher-magnification images showed eosinophilic cells within the luminal space that are indicative proximal tubule cell desquamation after DA-induced cell death (Figure 3A, arrows). Similar lesions were detected in animals administered 1 mg/kg DA, which could be visualized in hematoxylin and eosin– and Masson trichrome–stained sections (Supplemental Figure 2). Cell death was also examined by TUNEL assay, a marker of DNA damage and apoptosis (Figure 3C). TUNEL-positive cells were not detected in vehicle-treated kidneys (Figure 3C). In DA-treated kidneys, there were regions of TUNEL-positive nuclei, primarily within proximal tubules, at both 1.0- and 2.5-mg/kg doses (Figure 3C, arrows). At the time point examined, there was no evidence of TUNEL-positive staining in the CA3 region of the hippocampus from vehicle- or DA-treated mice (Figure 3C).
Figure 3.
Acute DA administration induced cell death and renal histopathology. (A and B) Renal histopathology was examined by hematoxylin and eosin (H&E). (A) Note the intense cytoplasmic eosinophilia of proximal tubule cells, which is indicative of cell death at 72 hours after 2.5 mg/kg DA (arrows in center panel); it was not present in vehicle kidneys (Veh; left panel). Higher-magnification view of the renal cortex depicts eosinophilic cells within the luminal space, which is indicative of tubule cell desquamation after injury (arrows in right panel). (B) Quantification of eosinophilic tubules in vehicle- and DA-treated kidneys (n=3, 8 fields per n, **P<0.01, t test). V, vehicle. (C) Apoptosis was assessed by TUNEL staining in kidney and brain sections from vehicle- and DA-treated animals. Note TUNEL-positive renal tubule cells after exposure to 1.0 (arrow) and 2.5 mg/kg DA (arrows). No TUNEL-positive cells were detected in the CA3 region of the hippocampus after DA. Negative controls were processed without terminal deoxynucleotidyl transferase enzyme, and positive controls were treated with DNase I at the beginning of the protocol. Veh, vehicle.
Cellular Damage Assessed by Transmission Electron Microscopy
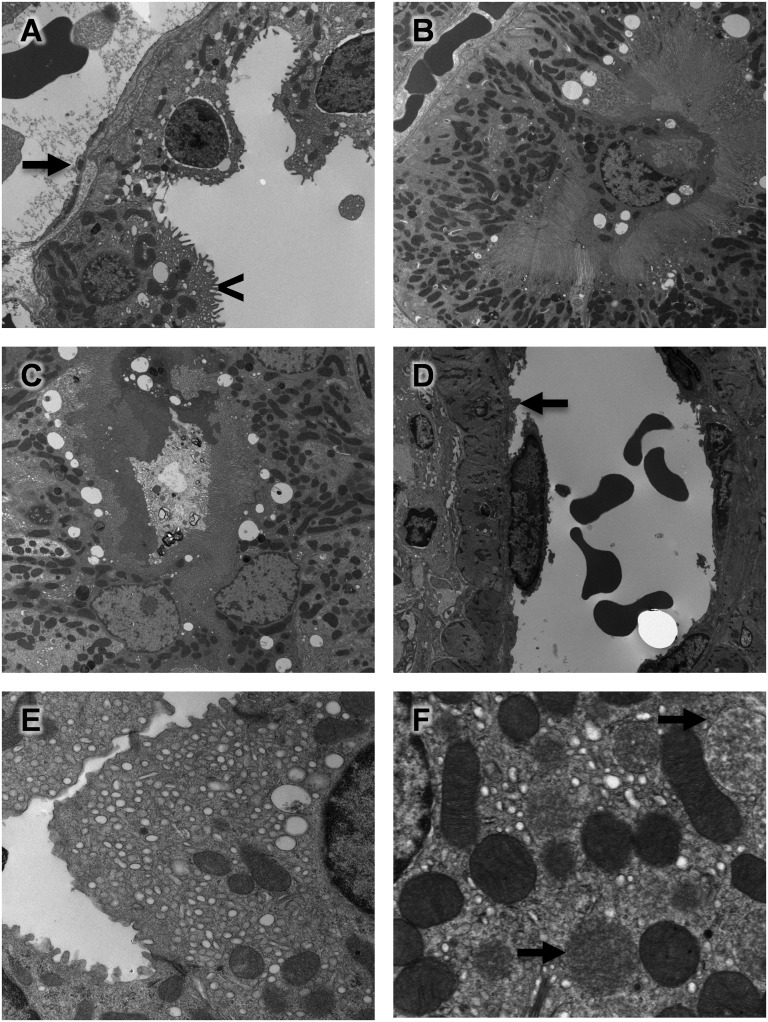
With transmission electron microscopy (TEM), cellular damage was observed at 72 hours (Figure 4), including vascular toxicity, with evidence of endothelial cell swelling (Figure 4A, arrow) and epithelial cell vacuolization (Figure 4A, arrowhead). Additionally, we observed evidence of proximal tubule cell detachment from the basement membrane (Figure 4B) and the presence of luminal proteinaceous material and cellular debris after DA administration (Figure 4C). Higher-magnification views provided clear evidence of endothelial cell damage (Figure 4D, arrow), toxic vacuolization (Figure 4E), and mitochondrial swelling (Figure 4F, arrows), which likely contributed to the renal injury.
Figure 4.
DA administration produced subcellular renal damage. TEM of renal cell injury revealed vascular damage, including (A, arrow) endothelial cell swelling, (A, arrowhead) epithelial cell vacuolization, (B) proximal tubule cell detachment, and (C) luminal protenaceous material and cellular debris. Higher-magnification views more clearly showed (D, arrow) endothelial cell damage, (E) toxic vacuolization, and (F, arrows) mitochondrial swelling after DA administration.
AKI Biomarkers
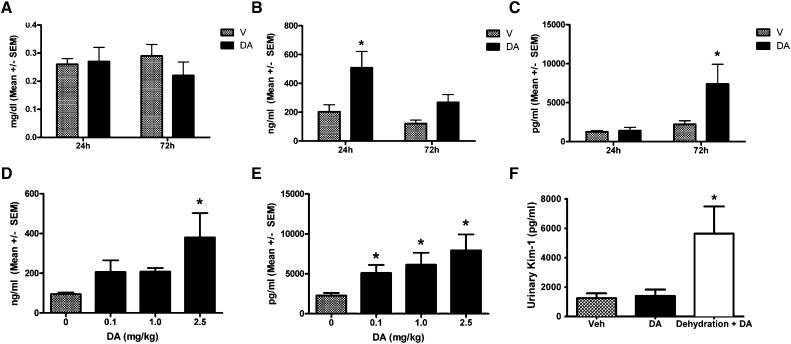
Serum creatinine (sCr) was not altered at 24 or 72 hours after DA administration (Figure 5A). To assess renal tubule damage from DA, urinary markers of tubule injury, neutrophil gelatinase-associated lipocalin (uNGAL), and kidney injury molecule-1 (uKIM-1) were examined. Both uNGAL and uKIM-1 were elevated at 24 and 72 hours after initial dose (Figure 5, B and C). A dose–response curve was generated by measuring uNGAL at 24 hours and uKIM-1 at 72 hours after 0.1 and 1.0 mg/kg DA exposure in addition to the 2.5-mg/kg dose (Figure 5, D and E). uNGAL elevated with 0.1 and 1.0 mg/kg but was only significantly increased with 2.5 mg/kg DA (Figure 5D). uKIM-1 increased with 0.1, 1.0, and 2.5 mg/kg DA administration (Figure 5E). There is reason to believe that water restriction may exacerbate the toxic effects of DA on the kidney, and to test this finding, mice were dehydrated for 12 hours before DA exposure (2.5 mg/kg). Twenty-four hours after injection, uKIM-1 levels were elevated in the water-restricted animals exposed to DA compared with vehicle-treated mice or animals allowed full access to water before DA (Figure 5F). KIM-1 and NGAL were also examined by RT-PCR and immunoblot analysis in kidney tissue lysates, and both were found to be elevated in kidney tissue (Supplemental Figure 3).
Figure 5.
Urinary AKI biomarkers were elevated following acute DA administration. (A) No change in sCr was seen at 24 or 72 hours (n=6). V, vehicle. (B) uNGAL and (C) uKIM-1 were elevated at 24 and 72 hours after DA exposure, respectively (n=6). V, vehicle. (D) Dose–response curve was generated by measuring uNGAL at 24 hours after exposure to 0.1, 1.0, and 2.5 mg/kg (n=6–10). V, vehicle. (E) Dose–response curve was generated by measuring uKIM-1 at 72 hours after exposure to 0.1, 1.0, and 2.5 mg/kg (n=6–10). V, vehicle. (F) After 12 hours of dehydration, mice were exposed to 2.5 mg/kg DA, and uKIM-1 was measured in urine collected overnight after the single dose. *Data were significantly different from vehicle (P<0.05, ANOVA followed by Newman–Keuls for individual group comparisons). Veh, vehicle.
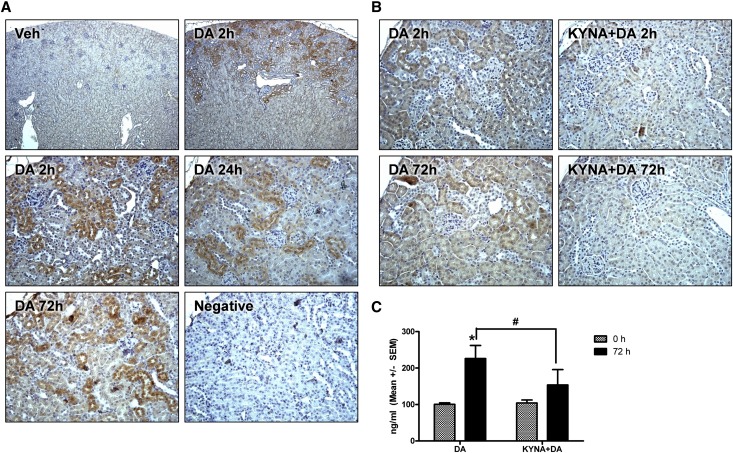
Renal NGAL expression was examined by IHC to more specifically characterize the site(s) of injury and the source of NGAL production (Figure 6). Low-powered images revealed NGAL immunoreactivity primarily localized to the renal cortex within 2 hours of DA administration (2.5 mg/kg) (Figure 6A). From higher-magnification images, NGAL expression was noted in cortical tubules at 2, 24, and 72 hours after DA administration (Figure 6A). To determine if NGAL induction was driven by renal glutamate receptor activation, mice were pretreated with the broad spectrum excitatory amino acid antagonist kynurenic acid (KYNA; 12.5 mg/kg intraperitoneally) or vehicle 5 minutes before DA administration (1.0 mg/kg). KYNA pretreatment suppressed renal NGAL expression at 2 and 72 hours (Figure 6B). Consistent with this observation, uNGAL was also reduced in KYNA+DA animals compared with vehicle+DA (Figure 6C).
Figure 6.
Acute DA administration induced renal cortical NGAL expression. (A) NGAL IHC at 2, 24, or 72 hours after DA or vehicle treatment in the renal cortex as viewed in low- (top panels; 5× objective) or higher-powered images (bottom panels; 20× objective). Diaminobenzidine chromogen with hematoxylin counterstain was used. Veh, vehicle. (B) NGAL IHC at 2 or 72 hours after DA or KYNA+DA treatment (20× objective). (C) uNGAL excretion 72 hours after DA or KYNA+DA treatment. *Data were significantly different from the 0-hour time point. #KYNA+DA treatment was significantly different from DA (n=3, P<0.05, ANOVA followed by Newman–Keuls for individual group comparisons).
Renal c-fos and junb Induction
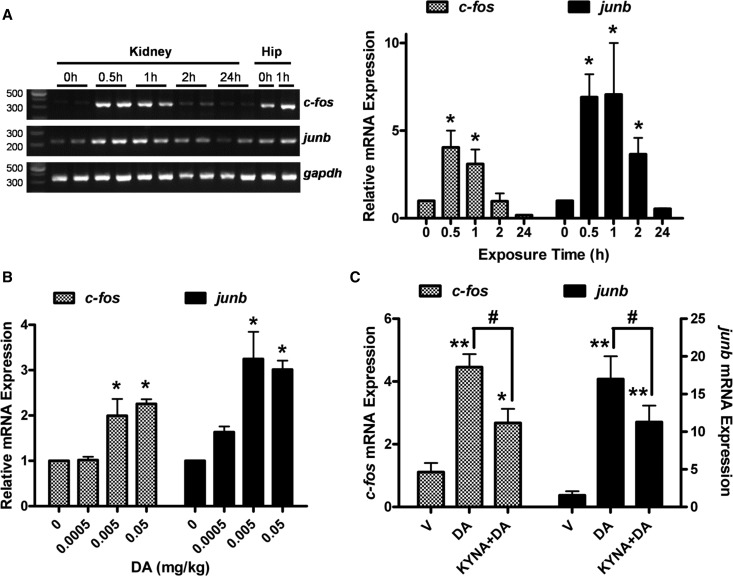
To further characterize the cellular response to DA in the kidney, the acute-phase genes c-fos and junb, which are induced in the brain after DA exposure,33,34 were examined by quantitative RT-PCR. c-fos mRNA was minimally expressed in the kidney before DA treatment but robustly elevated at 30 and 60 minutes after DA injection (2.5 mg/kg), and it was back to normal by 2 hours (Figure 7A). Similarly, junb was elevated at 30, 60, and 120 minutes after DA administration but back to normal by 24 hours (Figure 7A). Because we had observed evidence of nephrotoxicity as low as 0.1 mg/kg, we examined c-fos and junb expressions at this dose as well as doses several fold lower (Figure 7B). At 30 minutes, both c-fos and junb mRNA, were induced after as little as 0.005 and 0.05 mg/kg DA administration. Expectedly, induction at the lower doses was not as robust as it was at the 2.5-mg/kg dose, and induction was more transient as well, because mRNA was rapidly lost after 30 minutes. To examine the effect of glutamate receptor inhibition, c-fos and junb mRNA were examined in kidneys from mice pretreated with KYNA before DA administration. Thirty minutes after DA injection, c-fos and junb mRNA were elevated in both the KYNA+DA and the DA groups; however, KYNA pretreatment attenuated the response, indicating that the response was, at least partially, dependent on ionotropic glutamate receptor activity (Figure 7C).
Figure 7.
Immediate response genes c-fos and junb were elevated following DA administration. (A) c-fos and junb mRNA were examined by quantitative RT-PCR in kidneys from mice at 0.5, 1, 2, and 24 hours after DA exposure (2.5 mg/kg), with representative gel images shown in left panel and quantitative PCR analysis shown in right panel. Hip, hippocampus. (B) c-fos and junb mRNA were examined by quantitative RT-PCR in kidneys 30 minutes after exposure to 0.0005, 0.005, 0.05, or 2.5 mg/kg DA. (C) c-fos and junb mRNA were examined in kidneys by quantitative PCR analysis 30 minutes after mice were pretreated with KYNA (12.5 mg/kg) followed by DA administration (2.5 mg/kg). V, vehicle. *Data were significantly different from control (0 hour time point or V treatment); *P<0.05; **P<0.01. #KYNA+DA was significantly different from DA (P<0.05). ANOVA followed by Newman–Keuls for individual group comparisons (n=3).
Discussion
After the 1987 outbreak, Health Canada established a limit of 20 ppm DA in shellfish for human consumption, which was subsequently adopted by the US Food and Drug Administration among other governing agencies. The limit is primarily based on a calculated safety factor determined by no observed adverse effect levels from neurologic and gastrointestinal case reports.35–37 Additionally, DA is poorly absorbed, because the majority ingested is excreted in the feces, it does not readily cross the blood–brain barrier, and it is rapidly cleared from systemic circulation; therefore, there are several physiologic factors in addition to the regulatory factors that limit DA toxicity.38,39 However, there is evidence of peripheral toxicity, which may precede neurotoxicity, as well as an emerging concern over the effects of subclinical toxicity in the absence of overt organ toxicity or acute neurobehavioral changes.40–42 In the current study, DA preferentially accumulated in the kidneys of mice, with limited amounts detected in the liver, heart, and hippocampus, and this observation alone provided a compelling reason to examine the effects of DA on the kidney. This finding was consistent with the findings in a study by Lefebvre et al.,27 which found preferential accumulation in the kidneys of coho salmon after oral DA exposure, although the duration of tissue deposition in the fish was quite different compared with the time frame reported here.27
Despite previous reports containing circumstantial evidence of renal effects after DA exposure, including renal edema and elevated hematocrit levels,29,31 there has not been a systematic examination of the kidney after DA exposure. In the current study, renal toxicity was evident from histologic, electron microscopy, and biomarker analyses after intraperitoneal administration of 1.0 or 2.5 mg/kg. It was also discovered that doses as low as 0.1 mg/kg DA produced a toxic response based on uKIM-1 excretion, and 0.005 mg/kg DA induced c-fos and junb mRNA in the kidney. Presently, the cellular response that led to the induction of c-fos and junb in the kidney after DA administration is not known, although it is likely a stress response to elevated intracellular calcium, cellular depolarization, and/or a proliferative response, all of which have been previously described in models of renal ischemia and toxicosis.43–45 In the brain, induction of c-fos has been described as a marker of seizure activity and an early sign of neuronal excitotoxicity after exposure to glutamate, kainic acid, and DA.33,46,47 Studies from Peng and Ramsdell47 and Xi et al.48 showed that c-fos mRNA was induced in the brain at concentrations as low as 1.0 mg/kg DA (with nuclear immunolocalization at 0.5 mg/kg) in adult mice and as low as 0.1 mg/kg in neonates. Determining a lower limit of DA toxicity is essential to understanding the potential risks of exposure, although extrapolating results obtained in rodent studies to equivalent DA exposure levels in humans or other mammals is difficult. Most experimental rodent models use parenteral administration instead of oral dosing, and rodents are more resistant to the effects of DA compared with humans, nonhuman primates, and even sea mammals.41 It is possible that the renal toxicity observed in the current study was subsequent to altered central nervous system activity. However, because the doses used were well below levels shown to produce neurologic effects, it is assumed that the effects were directly related to DA on the kidney rather than a secondary effect of seizure activity, which has been suggested in DA-induced cardiotoxicity.49
Renal NGAL expression was isolated to tubules of the outer cortex after DA administration. This observation, combined with excretion of uKIM-1 and uNGAL and the localization of histopathology and TUNEL reactivity, suggests that cortical proximal tubules were the primary target of acute DA nephrotoxicity. It should be noted, however, that KIM-1 and NGAL are primarily associated with proximal tubule injury, and therefore, there may be additional injury to lower segments of the tubule that was not detected without markers specific to these regions. DA was equally distributed between the renal cortex and the medulla, and KRs were expressed in tubules from both regions; therefore, it is unclear why the cortex would be the primary region affected. Because GluK5, a high-affinity KR, must form a heteromer with the catalytic subtypes GluK1 or GluK2, it is possible that functional channels are limited to the cortex. Additionally, studies in the brain have determined that DA-induced neurotoxicity involves a concerted effort from both KRs/AMPARs as well as NMDARs,19–22 and thus, the relative distribution of these receptors would need to be considered as well. Using IHC techniques, Gill et al.24 found that GluN1 was preferentially distributed to the renal cortex; however, other groups have shown equal or higher levels in the outer medullary region.25 Currently, it is not known which receptors are necessary for DA-induced nephrotoxicity.
To our knowledge, this study is the first study to show renal toxicity associated with exposure to a KR agonist. By PCR and IHC analysis, it was determined that various subtypes of KR were expressed in the kidney. Furthermore, coadministration with the broad spectrum excitatory amino acid inhibitor KYNA before DA suppressed acute-phase gene induction and biomarker expression/excretion. These data would suggest that injury was mediated, at least partially, through glutamate receptor activation, and it is likely that renal KRs were involved; however, it is possible that DA activated an alternative effector site and that KYNA had effects outside of excitatory amino acid inhibition. In addition to its inhibitory effects at ionotropic glutamate receptors, KYNA may also act as an inhibitor at α7-nicotinic cholinergic receptors or as an agonist at G protein–coupled receptor GPR35, which is thought to inhibit cytokine release from immune cells and suppress inflammation,50 although this mechanism of inhibition would seem unlikely considering the time course of the effects reported here.
In addition to acute DA toxicity, there is concern over the long-term consequences from repeated exposure, such as the chronic neurologic effects that have been identified in sea lions exposed to sublethal levels of DA, characterized by hippocampal atrophy and development of epilepsy.40 With respect to the kidney, there is convincing evidence that AKI incidence is a risk factor for CKD and ESRD.51,52 Because there was no change in renal function (i.e., sCr) and there was only modest histopathology, the clinical relevance associated with DA nephrotoxicity is unclear at this time. Even the AKI biomarkers KIM-1 and NGAL were only moderately elevated compared with insults that produce overt renal damage, such as ischemia-reperfusion injury or cisplatin nephrotoxicity. However, recent evidence suggests that even subclinical toxicity might lead to tubule damage and an overall decline in renal function.53–56 Indeed, elevated uNGAL or plasma NGAL, even in the absence of detectable changes in sCr, has been associated with the need for RRT and higher mortality rates.53 It is our position that subclinical renal toxicity associated with repeated DA exposure, particularly as an undetected injury from an unrecognized source, could contribute to the progression of renal disease. Furthermore, it is conceivable that the effects of DA may be compounded, or even masked, in populations with underlying renal disease or compromised renal function, such as aged or diabetic populations. Validation of these findings in other systems and species is needed, and confirmation may necessitate new discussions regarding acceptable exposure to humans. Finally, these results draw attention to the kidney as a potentially unrecognized target of excitatory drugs, toxins, and amino acids, and renal effects should be considered in studies examining these effector sites.
Concise Methods
Animals
All studies involving animals were done in accordance with the institutional guidelines of the Medical University of South Carolina. The study was performed using adult 128Sv/Black Swiss mice weighing approximately 25–30 g.
DA Administration
For tissue distribution studies, mice were exposed to DA (2.5 mg/kg intraperitoneally) and euthanized 30, 60, or 120 minutes after exposure; kidneys, liver, heart, hippocampus, and serum were harvested. For 72-hour toxicity studies, mice were exposed to a single daily dose of DA for 3 consecutive days (0.1–2.5 mg/kg intraperitoneally) or an equal volume of diluents (isotonic saline), and they were euthanized 24 hours after the third dose. For immediate response gene studies, mice were exposed to a single dose of DA (0.0005–2.5 mg/kg intraperitoneally) and euthanized 30 minutes after injection; kidneys and hippocampus were harvested and flash frozen. For PBN pretreatment studies, mice were given PBN (600 mg/kg intraperitoneally) or vehicle (saline) 5 minutes before DA (2.5 mg/kg intraperitoneally) and euthanized 30 minutes after DA injection; organs were collected for DA measurements. For KYNA pretreatment studies, mice were injected with KYNA (12.5 mg/kg intraperitoneally) or vehicle (isotonic saline) 5 minutes before DA (2.5 mg/kg intraperitoneally) and euthanized 30 minutes after DA injection; kidneys were harvested and flash frozen for RNA processing.
DA Measurements
DA concentration was measured by ELISA (Mercury Science, Durham, NC) using the method as previously described.57
RNA Isolation and RT-PCR
RNA was obtained using the RNeasy RNA Isolation Kit (Qiagen), and reverse transcription was performed with the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). RT-PCR experiments assessing ionotropic glutamate receptor expression were performed using GoTaq Green PCR Master Mix (Promega, Madison, WI) and primer sequences that have been previously reported.58 Quantitative RT-PCR experiments assessing c-fos and junb expression were performed using SsoAdvanced SYBR Green PCR Mix (Bio-Rad) and primer sequences that have been previously reported.34
Kidney Function and Injury Measurements
Terminal blood was collected by venepuncture of the inferior vena cava at time of euthanasia. Serum was extracted from collected blood and used to measure creatinine (QuantiChrom Creatinine Assay Kit; BioAssay Systems, Hayward, CA). Urine collected overnight after the first and third injections, was used to measure KIM-1 (Quantikine KIM-1 ELISA; R&D Systems, Indianapolis, IN) and NGAL (Bioporto, Denmark).
Histology and IHC
Mice were exposed to DA for 3 days. At the time of euthanasia, organs were immersion-fixed in 10% formalin and paraffin-embedded. Histologic evaluations were determined from hematoxylin and eosin- and Masson trichrome-stained sections. For IHC experiments, tissue sections were dewaxed in xylenes followed by rehydration in a graded ethanol series, heat-mediated antigen retrieval in sodium citrate buffer, and blocking for 1 hour in 5% normal goat serum/1% BSA. Primary antibodies were incubated for 2 hours at room temperature using antibodies against GluK5 (Abcam, Cambridge, MA), GluK2 (Aviva Systems Biology, San Diego, CA), and NGAL (Lipocalin2; Abcam). After antibody incubation, the sections were incubated in biotinylated secondary antibody and horseradish peroxidase-conjugated avidin–biotin complex and developed with diaminobenzidine exposure using a Vectastain ABC Kit according to the manufacturer’s instructions (Vector Labs, Burlingame, CA). Sections were counterstained with hematoxylin (Vector Labs).
TUNEL Assay
Paraffin-embedded kidney sections from mice treated for 72 hours with 1.0 or 2.5 mg/kg DA were used for detection of apoptosis by TUNEL assay. The assay was performed using an ApopTag Peroxidase In Situ Apoptosis Detection Kit (Millipore, Billerica, MA) according to the manufacturer’s instructions and developed with diaminobenzidine. Negative control sections were incubated in buffer without terminal deoxynucleotidyl transferase enzyme. Positive control sections were incubated with DNase I at the beginning of the protocol.
TEM
After 72 hours of DA exposure, kidneys were harvested and immediately placed in a dish containing modified Karnovsky’s buffer (2.5% glutaraldehyde/2.5% formalin in PBS) followed by processing into resin blocks (Embed 812; Electron Microscopy Sciences, Hatfield, PA). The resin blocks are thick-sectioned at 1 μm with a histodiamond knife using an Ultracut UCT 7 (Leica, Bannockburn, IL); sections are then collected on slides and stained with Toluidine Blue. The appropriate blocks are then thin-sectioned using a diamond knife (Diatome; Electron Microscopy Sciences) at 70–90 nm (silver to pale gold using color interference), and sections are placed on copper grids. After drying, the sections are stained with the heavy metals uranyl acetate and lead citrate for contrast. After drying, the grids are then viewed on a Tecnai Spirit 120kv TEM (FEI, Hillsboro, OR). Digital images are taken with an AMT CCD camera.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Fran Van Dolah and Jennifer Maucher-Fuquay for generating preliminary data for domoic acid levels in mouse tissues during the early inception of this project. We also thank Melissa Foley Chimento and Edward Phillips at the High Resolution Imaging Facility at the University of Alabama at Birmingham for their assistance in generating transmission electron microscopy images.
J.A.F. was supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Training Grant T32DK007752 in Renal Function and Disease. M.G.J. was supported by Office of Naval Research Award N000140810341. This study was supported by Veterans Affairs Merit Grant P30 DK074038 and funds from Dialysis Clinic (to P.D.B.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013080836/-/DCSupplemental.
References
- 1.Perl TM, Bédard L, Kosatsky T, Hockin JC, Todd EC, McNutt LA, Remis RS: Amnesic shellfish poisoning: A new clinical syndrome due to domoic acid. Can Dis Wkly Rep 16[Suppl 1E]: 7–8, 1990 [PubMed] [Google Scholar]
- 2.Perl TM, Bédard L, Kosatsky T, Hockin JC, Todd EC, Remis RS: An outbreak of toxic encephalopathy caused by eating mussels contaminated with domoic acid. N Engl J Med 322: 1775–1780, 1990 [DOI] [PubMed] [Google Scholar]
- 3.Perl TM, Teitelbaum J, Hockin J, Todd EC: Domoic acid toxicity. Panel discussion: Definition of the syndrome. Can Dis Wkly Rep 16[Suppl 1E]: 41–45, 1990 [PubMed] [Google Scholar]
- 4.Teitelbaum JS, Zatorre RJ, Carpenter S, Gendron D, Evans AC, Gjedde A, Cashman NR: Neurologic sequelae of domoic acid intoxication due to the ingestion of contaminated mussels. N Engl J Med 322: 1781–1787, 1990 [DOI] [PubMed] [Google Scholar]
- 5.Costa PR, Rosa R, Duarte-Silva A, Brotas V, Sampayo MA: Accumulation, transformation and tissue distribution of domoic acid, the amnesic shellfish poisoning toxin, in the common cuttlefish, Sepia officinalis. Aquat Toxicol 74: 82–91, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Scallet AC, Schmued LC, Johannessen JN: Neurohistochemical biomarkers of the marine neurotoxicant, domoic acid. Neurotoxicol Teratol 27: 745–752, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Silver MW, Bargu S, Coale SL, Benitez-Nelson CR, Garcia AC, Roberts KJ, Sekula-Wood E, Bruland KW, Coale KH: Toxic diatoms and domoic acid in natural and iron enriched waters of the oceanic Pacific. Proc Natl Acad Sci USA 107: 20762–20767, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trick CG, Bill BD, Cochlan WP, Wells ML, Trainer VL, Pickell LD: Iron enrichment stimulates toxic diatom production in high-nitrate, low-chlorophyll areas. Proc Natl Acad Sci U S A 107: 5887–5892, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Twiner MJ, Fire S, Schwacke L, Davidson L, Wang Z, Morton S, Roth S, Balmer B, Rowles TK, Wells RS: Concurrent exposure of bottlenose dolphins (Tursiops truncatus) to multiple algal toxins in Sarasota Bay, Florida, USA. PLoS One 6: e17394, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Twiner MJ, Flewelling LJ, Fire SE, Bowen-Stevens SR, Gaydos JK, Johnson CK, Landsberg JH, Leighfield TA, Mase-Guthrie B, Schwacke L, Van Dolah FM, Wang Z, Rowles TK: Comparative analysis of three brevetoxin-associated bottlenose dolphin (Tursiops truncatus) mortality events in the Florida Panhandle region (USA). PLoS One 7: e42974, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson DM: Red tides. Sci Am 271: 62–68, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Hallegraeff GM: A review of harmful algal blooms and their apparent global increase. Phycologia 32: 79–99, 1993 [Google Scholar]
- 13.Mos L: Domoic acid: A fascinating marine toxin. Environ Toxicol Pharmacol 9: 79–85, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Parsons ML, Dorthch Q, Turner RE: Sedimentological evidence of an increase in Pseudo-nitzschia (Bacillariophyceae) abundance in response to coastal eutrophication. Limnol Oceanogr 47: 551–558, 2002 [Google Scholar]
- 15.Lefebvre KA, Frame ER, Gulland F, Hansen JD, Kendrick PS, Beyer RP, Bammler TK, Farin FM, Hiolski EM, Smith DR, Marcinek DJ: A novel antibody-based biomarker for chronic algal toxin exposure and sub-acute neurotoxicity. PLoS One 7: e36213, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lefebvre KA, Tilton SC, Bammler TK, Beyer RP, Srinouanprachan S, Stapleton PL, Farin FM, Gallagher EP: Gene expression profiles in zebrafish brain after acute exposure to domoic acid at symptomatic and asymptomatic doses. Toxicol Sci 107: 65–77, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grattan LM, Leosing M, King A, Silbergeld E, Morris JG: Human health effects of exposure to domoic acid in the Pacific northwest: A preliminary study. Presented at the Behavioral Toxicology Society Conference, 2003 [Google Scholar]
- 18.Grattan LM, Roberts S, Trainer V, Boushey C, Burbacher TM, Grant KS, Tracy K, Morris JG: Domoic acid neurotoxicity in Native Americans in the Pacific Northwest: Human health project methods and update. Presented at the Fourth Symposium on Harmful Algae in the United States, 2007 [Google Scholar]
- 19.Hampson DR, Manalo JL: The activation of glutamate receptors by kainic acid and domoic acid. Nat Toxins 6: 153–158, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Tasker RA, Strain SM: Synergism between NMDA and domoic acid in a murine model of behavioural neurotoxicity. Neurotoxicology 19: 593–597, 1998 [PubMed] [Google Scholar]
- 21.Tasker RA, Strain SM, Drejer J: Selective reduction in domoic acid toxicity in vivo by a novel non-N-methyl-D-aspartate receptor antagonist. Can J Physiol Pharmacol 74: 1047–1054, 1996 [PubMed] [Google Scholar]
- 22.Berman FW, Murray TF: Domoic acid neurotoxicity in cultured cerebellar granule neurons is mediated predominantly by NMDA receptors that are activated as a consequence of excitatory amino acid release. J Neurochem 69: 693–703, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Deng A, Valdivielso JM, Munger KA, Blantz RC, Thomson SC: Vasodilatory N-methyl-D-aspartate receptors are constitutively expressed in rat kidney. J Am Soc Nephrol 13: 1381–1384, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Gill SS, Mueller RW, McGuire PF, Pulido OM: Potential target sites in peripheral tissues for excitatory neurotransmission and excitotoxicity. Toxicol Pathol 28: 277–284, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Leung JC, Marphis T, Craver RD, Silverstein DM: Altered NMDA receptor expression in renal toxicity: Protection with a receptor antagonist. Kidney Int 66: 167–176, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Yang CC, Chien CT, Wu MH, Ma MC, Chen CF: NMDA receptor blocker ameliorates ischemia-reperfusion-induced renal dysfunction in rat kidneys. Am J Physiol Renal Physiol 294: F1433–F1440, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Lefebvre KA, Noren DP, Schultz IR, Bogard SM, Wilson J, Eberhart BT: Uptake, tissue distribution and excretion of domoic acid after oral exposure in coho salmon (Oncorhynchus kisutch). Aquat Toxicol 81: 266–274, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Suzuki CA, Hierlihy SL: Renal clearance of domoic acid in the rat. Food Chem Toxicol 31: 701–706, 1993 [DOI] [PubMed] [Google Scholar]
- 29.Gulland FM, Haulena M, Fauquier D, Langlois G, Lander ME, Zabka T, Duerr R: Domoic acid toxicity in Californian sea lions (Zalophus californianus): Clinical signs, treatment and survival. Vet Rec 150: 475–480, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Gulland F: Domoic Acid Toxicity in California Sea Lions (Zalophus californicans) Stranded along the Central California Coast, May–October 1998, Report to the National Marine Fisheries Service Working Group on Unusual Marine Mammal Mortality Events, Washington, DC, NOAA, 2000 [Google Scholar]
- 31.Neely BA, Soper JL, Greig DJ, Carlin KP, Favre EG, Gulland FM, Almeida JS, Janech MG: Serum profiling by MALDI-TOF mass spectrometry as a diagnostic tool for domoic acid toxicosis in California sea lions. Proteome Sci 10: 18, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deng A, Thomson SC: Renal NMDA receptors independently stimulate proximal reabsorption and glomerular filtration. Am J Physiol Renal Physiol 296: F976–F982, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robertson H, Renton K, Kohn J, White T: Patterns of Fos expression suggest similar mechanisms of action for the excitotoxins domoic and kainic acid. Ann N Y Acad Sci 648: 330–334, 1992 [DOI] [PubMed] [Google Scholar]
- 34.Ryan JC, Morey JS, Ramsdell JS, Van Dolah FM: Acute phase gene expression in mice exposed to the marine neurotoxin domoic acid. Neuroscience 136: 1121–1132, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Kumar KP, Kumar SP, Nair GA: Risk assessment of the amnesic shellfish poison, domoic acid, on animals and humans. J Environ Biol 30: 319–325, 2009 [PubMed] [Google Scholar]
- 36.Mariën K: Establishing tolerable dungeness crab (Cancer magister) and razor clam (Siliqua patula) domoic acid contaminant levels. Environ Health Perspect 104: 1230–1236, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wekell JC, Hurst J, Lefebvre KA: The origin of the regulatory limits for PSP and ASP toxins in shellfish. J Shellfish Res 23: 927–930, 2004 [Google Scholar]
- 38.Preston E, Hynie I: Transfer constants for blood-brain barrier permeation of the neuroexcitatory shellfish toxin, domoic acid. Can J Neurol Sci 18: 39–44, 1991 [DOI] [PubMed] [Google Scholar]
- 39.Iverson F, Truelove J, Nera E, Tryphonas L, Campbell J, Lok E: Domoic acid poisoning and mussel-associated intoxication: Preliminary investigations into the response of mice and rats to toxic mussel extract. Food Chem Toxicol 27: 377–384, 1989 [DOI] [PubMed] [Google Scholar]
- 40.Goldstein T, Mazet JA, Zabka TS, Langlois G, Colegrove KM, Silver M, Bargu S, Van Dolah F, Leighfield T, Conrad PA, Barakos J, Williams DC, Dennison S, Haulena M, Gulland FM: Novel symptomatology and changing epidemiology of domoic acid toxicosis in California sea lions (Zalophus californianus): An increasing risk to marine mammal health. Proc Biol Sci 275: 267–276, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lefebvre KA, Robertson A: Domoic acid and human exposure risks: A review. Toxicon 56: 218–230, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Zabka TS, Goldstein T, Cross C, Mueller RW, Kreuder-Johnson C, Gill S, Gulland FM: Characterization of a degenerative cardiomyopathy associated with domoic acid toxicity in California sea lions (Zalophus californianus). Vet Pathol 46: 105–119, 2009 [DOI] [PubMed] [Google Scholar]
- 43.DiMari J, Megyesi J, Udvarhelyi N, Price P, Davis R, Safirstein R: N-acetyl cysteine ameliorates ischemic renal failure. Am J Physiol 272: F292–F298, 1997 [DOI] [PubMed] [Google Scholar]
- 44.Wainford RD, Weaver RJ, Hawksworth GM: The immediate early genes, c-fos, c-jun and AP-1, are early markers of platinum analogue toxicity in human proximal tubular cell primary cultures. Toxicol In Vitro 23: 780–788, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Witzgall R, Brown D, Schwarz C, Bonventre JV: Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Invest 93: 2175–2188, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morgan JI, Cohen DR, Hempstead JL, Curran T: Mapping patterns of c-fos expression in the central nervous system after seizure. Science 237: 192–197, 1987 [DOI] [PubMed] [Google Scholar]
- 47.Peng YG, Ramsdell JS: Brain Fos induction is a sensitive biomarker for the lowest observed neuroexcitatory effects of domoic acid. Fundam Appl Toxicol 31: 162–168, 1996 [DOI] [PubMed] [Google Scholar]
- 48.Xi D, Peng YG, Ramsdell JS: Domoic acid is a potent neurotoxin to neonatal rats. Nat Toxins 5: 74–79, 1997 [DOI] [PubMed] [Google Scholar]
- 49.Vranyac-Tramoundanas A, Harrison JC, Sawant PM, Kerr DS, Sammut IA: Ischemic cardiomyopathy following seizure induction by domoic Acid. Am J Pathol 179: 141–154, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moroni F, Cozzi A, Sili M, Mannaioni G: Kynurenic acid: A metabolite with multiple actions and multiple targets in brain and periphery. J Neural Transm 119: 133–139, 2012 [DOI] [PubMed] [Google Scholar]
- 51.Coca SG, Singanamala S, Parikh CR: Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int 81: 442–448, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Venkatachalam MA, Griffin KA, Lan R, Geng H, Saikumar P, Bidani AK: Acute kidney injury: A springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol 298: F1078–F1094, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haase M, Devarajan P, Haase-Fielitz A, Bellomo R, Cruz DN, Wagener G, Krawczeski CD, Koyner JL, Murray P, Zappitelli M, Goldstein SL, Makris K, Ronco C, Martensson J, Martling CR, Venge P, Siew E, Ware LB, Ikizler TA, Mertens PR: The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: A multicenter pooled analysis of prospective studies. J Am Coll Cardiol 57: 1752–1761, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haase M, Kellum JA, Ronco C: Subclinical AKI—an emerging syndrome with important consequences. Nat Rev Nephrol 8: 735–739, 2012 [DOI] [PubMed] [Google Scholar]
- 55.Ronco C, Kellum JA, Haase M: Subclinical AKI is still AKI. Crit Care 16: 313, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ronco C, Rosner MH: Acute kidney injury and residual renal function. Crit Care 16: 144, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Litaker RW, Stewart TN, Eberhart BT, Wekell JC, Trainer V, Kudela RM, Miller PE, Roberts A, Hertz C, Johnson TA, Frankfurter G, Smith GJ, Schnetzer A, Schumacker J, Bastian JL, Odell A, Gentien P, Legal D, Hardison DR, Tester PA: Rapid enzyme-linked immunosorbant assay for detection of the algal toxin domoic acid. J Shellfish Res 27: 1301–1310, 2008 [Google Scholar]
- 58.Paarmann I, Frermann D, Keller BU, Hollmann M: Expression of 15 glutamate receptor subunits and various splice variants in tissue slices and single neurons of brainstem nuclei and potential functional implications. J Neurochem 74: 1335–1345, 2000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.