Abstract
Mitochondrial biogenesis may be an adaptive response necessary for meeting the increased metabolic and energy demands during organ recovery after acute injury, and renal mitochondrial dysfunction has been implicated in the pathogenesis of AKI. We proposed that stimulation of mitochondrial biogenesis 24 hours after ischemia/reperfusion (I/R)–induced AKI, when renal dysfunction is maximal, would accelerate recovery of mitochondrial and renal function in mice. We recently showed that formoterol, a potent, highly specific, and long-acting β2-adrenergic agonist, induces renal mitochondrial biogenesis in naive mice. Animals were subjected to sham or I/R-induced AKI, followed by once-daily intraperitoneal injection with vehicle or formoterol beginning 24 hours after surgery and continuing through 144 hours after surgery. Treatment with formoterol restored renal function, rescued renal tubules from injury, and diminished necrosis after I/R-induced AKI. Concomitantly, formoterol stimulated mitochondrial biogenesis and restored the expression and function of mitochondrial proteins. Taken together, these results provide proof of principle that a novel drug therapy to treat AKI, and potentially other acute organ failures, works by restoring mitochondrial function and accelerating the recovery of renal function after injury has occurred.
Keywords: acute renal failure, ischemia-reperfusion, proximal tubule, renal function, renal tubular epithelial cells, mitochondria
AKI is a clinical disorder characterized by a rapid decrease in kidney excretory function and subsequent retention of nitrogenous waste products and metabolic acids and increased potassium and phosphate concentrations.1 AKI incidence is increasing, and the prevalence is approximately 60% in patients during intensive care admission; in the past 50 years, mortality rates have remained unchanged, ranging from 50% to 70%.1–3 In addition, AKI is costly to treat and poses a significant financial burden on the health care system.4 Current treatments are limited to mechanical support by dialysis. Historically, most drug research efforts for AKI have focused on pretreatment. However, clinical translation of the outcomes obtained from this approach is limited because AKI primarily presents with an unpredictable acute onset. Taken together, the high mortality rates, financial burden, and limitations in treatment demonstrate a significant clinical need for discovery of novel approaches to therapeutics that promote recovery of renal function following AKI.
A common cause of AKI is ischemia/reperfusion (I/R) injury, and it is now recognized that tubular mitochondrial dysfunction contributes to oxidative stress, persistent energy depletion, impairment of energy-dependent repair mechanisms, and cell death in AKI.1,5–10 Investigation into renal mitochondrial dysfunction in glycerol, sepsis, and I/R models of AKI in rodents revealed a persistent elevation in serum creatinine concomitant with continual suppression of mitochondrial- and nuclear-encoded genes and proteins of the electron transport chain (ETC) and mitochondrial function.6,11,12
Mitochondrial biogenesis (MB) is a complex physiologic process by which cells form new mitochondria to increase energy production in response to environmental stimuli or physiologic stress.13 Peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) is referred to as the master regulator of MB and is abundantly expressed in tissues with high metabolic demand (e.g., heart, skeletal muscle, and kidneys).14–17 It is highly inducible by physiologic and pathologic stimuli, including exercise, caloric restriction, sepsis, and hypoxia.18–21 The ability of PGC-1α to respond to numerous stimuli and alter the metabolic profile of the cell makes it a target for pharmacologic intervention in a variety of disease states.
Our laboratory previously demonstrated in oxidant-induced renal proximal tubular cell (RPTC) injury that PGC-1α is upregulated after injury and that overexpression of PGC-1α after injury promotes the recovery of mitochondrial and cellular functions.22,23 Tran et al. reported that renal-specific PGC-1α null mice subjected to sepsis-induced AKI were unable to recover from injury, in contrast to their wild-type littermates.11 Other evidence from in vivo studies supports the hypothesis that induction of PGC-1α and subsequent MB is a crucial adaptive response aimed at sustaining metabolic and energy demands required for recovery from acute organ injury.24,25
A recent high-throughput screen performed by our laboratory revealed that the specific and long-acting β2-adrenergic receptor agonist formoterol was a potent inducer of MB in RPTC and in the kidneys of mice.26 Subsequently, with use of RPTC, the ability a structurally diverse panel of β2-adrenoceptor agonists to stimulate MB was assayed and cheminformatic profiling elucidated four essential chemical moieties to stimulate MB, which was shared by formoterol.27 Here, we carried out experiments evaluating the efficacy of formoterol to restore mitochondrial and kidney function after an ischemic insult in a mouse model of I/R-induced AKI.
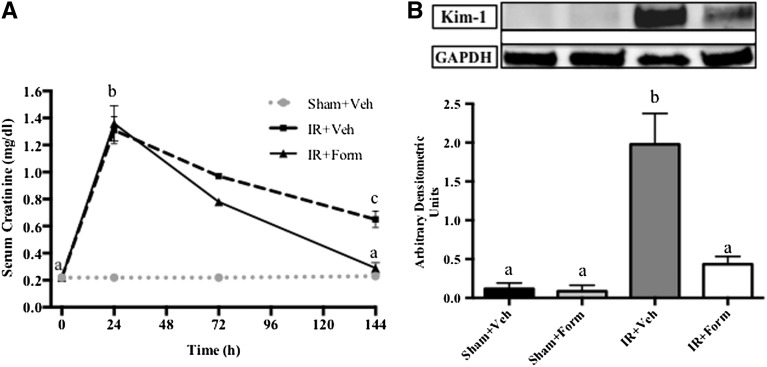
C57BL/6 mice were divided into four groups that were subjected to sham or I/R surgery, followed by once-daily intraperitoneal administration with formoterol (0.3 mg/kg) or vehicle (0.3% DMSO in normal saline) between 24 and 144 hours after reperfusion. Before injury, serum creatinine (SCr) was approximately 0.2 mg/dl in all animals and increased to approximately 1.3 mg/dl 24 hours after I/R (Figure 1A). Treatment was randomly initiated 24 hours after reperfusion, when SCr was maximally elevated; therefore, intervention was not initiated until after AKI was established. After I/R, SCr partially recovered in mice receiving vehicle treatment (IR+Veh); however, SCr was persistently elevated compared with preinjury levels at approximately 0.7 mg/dl at 144 hours after reperfusion, indicating persistent injury. In contrast, after five daily doses of formoterol following I/R (IR+Form), SCr completely recovered by 144 hours (Figure 1A). SCr did not change following sham operation in either vehicle (Sham+Veh)-treated or formoterol (Sham+Form)-treated animals.
Figure 1.
Treatment with formoterol restored kidney function and mitigated proximal tubule injury. Mice were subjected to sham or I/R surgery and subsequent treatment with vehicle (Veh) or formoterol (Form). Kidney function was assessed via serum creatinine (A) and tubular injury via KIM-1 immunoblot analysis (B). KIM-1 protein was measured in kidneys from mice 144 hours after injury and quantified by densitometry. Samples were analyzed via one-way ANOVA followed by a Student–Newman–Keuls post hoc test to evaluate differences between groups. Data points are mean±SEM; with bars with different superscripts are significantly different from one another (n=5, P<0.01). GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Kidney injury molecule-1 (KIM-1) is a highly sensitive and specific biomarker of renal tubular injury that is minimally detected in healthy kidneys.28 In cortical lysates from IR+Veh kidneys, KIM-1 protein was elevated compared with levels in sham animals at 144 hours, and formoterol treatment attenuated KIM-1 protein expression to levels of the control animals (Figure 1B).
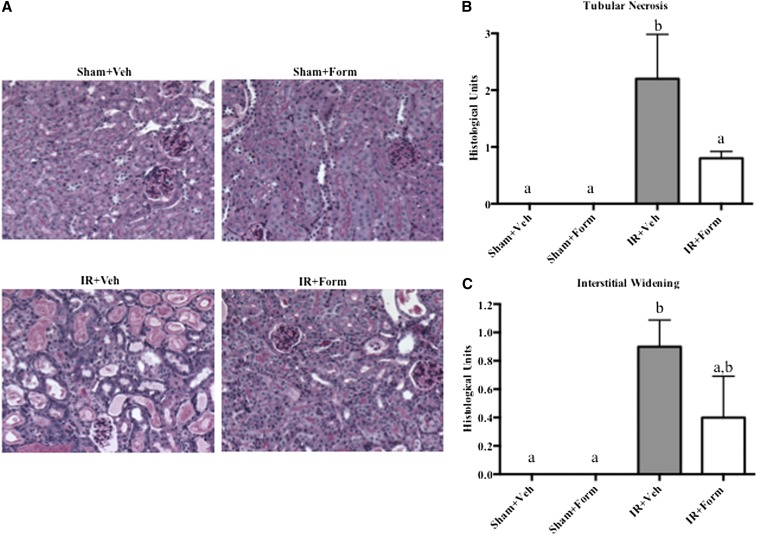
Renal histopathology was assessed using periodic acid–Schiff (PAS) and hematoxylin and eosin staining. Kidneys from IR+Veh and IR+Form mice displayed proximal tubule dilation, brush-border damage, and the presence of proteinaceous casts (Figure 2A). Kidneys from IR+Veh mice displayed evidence of persistent tubular necrosis at 144 hours, which was attenuated with formoterol treatment (Figure 2B). Additionally, there was evidence of interstitial widening, an early sign of renal fibrosis, in IR+Veh kidneys that was not as prevalent in IR+Form mice (Figure 2C).
Figure 2.
Treatment with formoterol (Form) improved tubule histology. Mice were subjected to sham or I/R surgery, treated with vehicle (Veh) or formoterol 24 hours later, and were euthanized 144 hours after surgery. (A) PAS stain of representative slides of renal cortical tissue (magnification, ×10). (B) Scoring of tubular necrosis. (C) Scoring of interstitial widening. Samples were analyzed via one-way ANOVA followed by a Student–Newman–Keuls post hoc test to evaluate differences between groups. Data points are mean±SEM; bars with different superscripts are significantly different from one another (n=5, P<0.05).
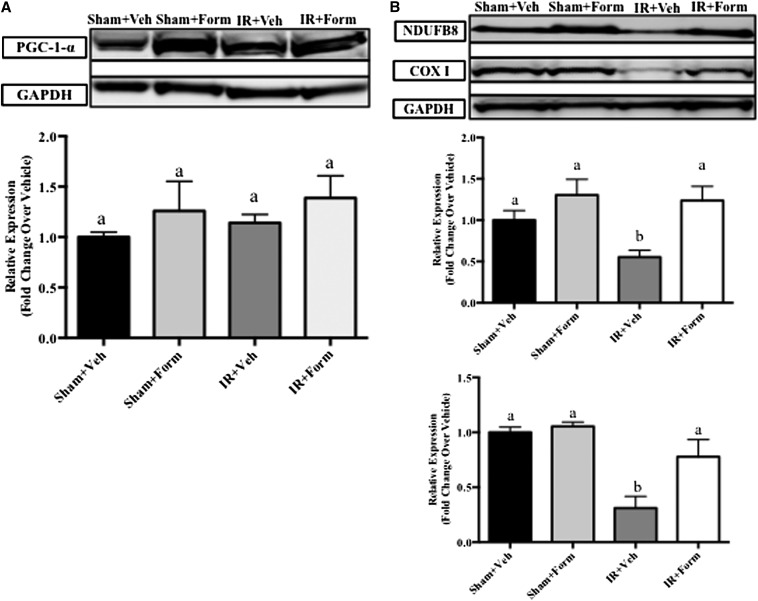
Using our I/R model, we have previously shown that essential components of the ETC, reduced form of nicotinamide adenine dinucleotide dehydrogenase (ubiquinone)-1β subcomplex 8 (NDUFB8) and mitochondrial cytochrome c oxidase subunit I (COX I), decreased within 24 hours of I/R and remain decreased through 144 hours.6 If the improved renal function and decreased tissue injury stimulated by formoterol is the result of renal MB, then renal mitochondrial proteins should be restored and mitochondrial function improved compared with I/R mice. At 144 hours, PGC-1α protein expression did not change with treatment or after injury (Figure 3A); however, nuclear-encoded NDUFB8 and mitochondrial-encoded COX I ETC proteins were decreased 144 hours after reperfusion in IR+Veh kidneys (Figure 3B). Treatment with formoterol after I/R restored NDUFB8 and COX I protein abundance to levels of the control animals (Figure 3B).
Figure 3.
Formoterol (Form) restored mitochondrial protein expression after I/R-induced AKI. Mice were subjected to sham or I/R surgery and subsequent treatment with vehicle (Veh) or formoterol. Markers for MB were evaluated via immunoblot 144 hours after surgery. Shown are renal cortical lysate PGC-1α (A) and mitochondrial ETC proteins (B) NDUFB8 (middle graph) and COX I (bottom graph). Densitometric semiquantification is shown below the representative blots. Samples were analyzed via one-way ANOVA followed by a Student–Newman–Keuls post hoc test to evaluate differences between groups. Bars with different superscripts are significantly different from one another. Data points are mean±SEM and are relative values compared with control (n=6, P<0.05).
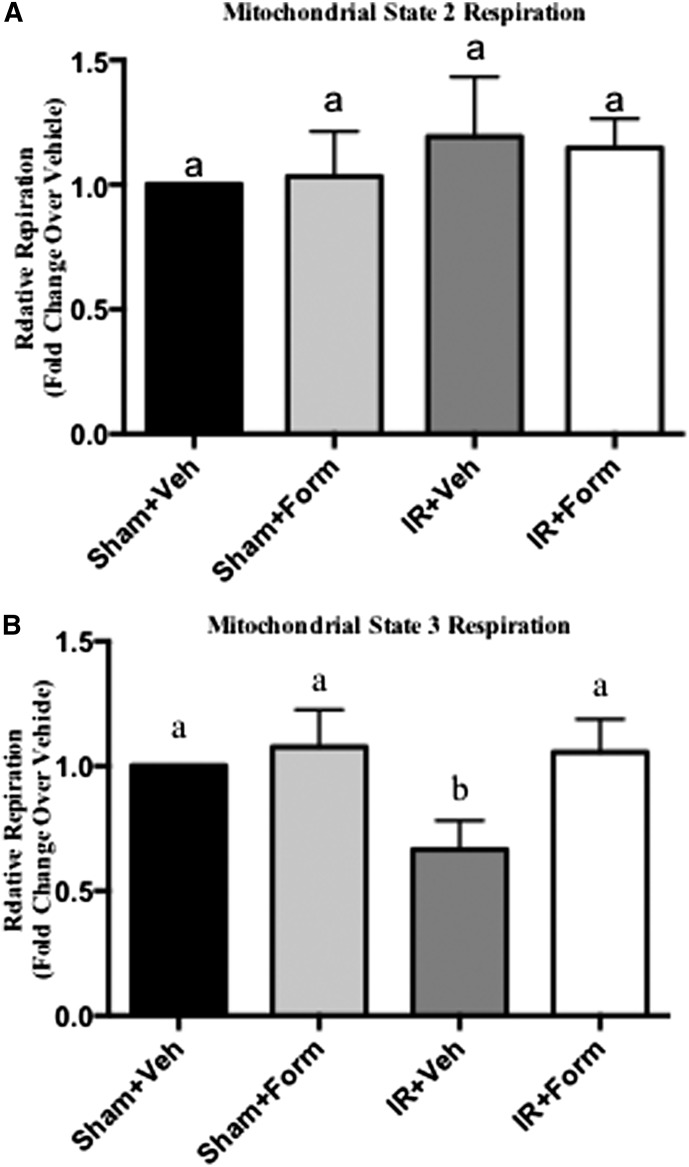
Previous in vivo and in vitro research in hypoxic and I/R-induced models of AKI identified dysfunctional mitochondria, in the presence of suppressed ETC protein expression, in reduced kidney function.6 Renal mitochondria were isolated from mice at 144 hours and mitochondrial function was determined. State 2 (basal respiratory rate) was not altered under any conditions (Figure 4A). State 3 (ADP-stimulated respiratory rate) respiration was reduced in mitochondria from IR+Veh kidneys, which indicated sustained mitochondrial dysfunction (Figure 4B), and was restored in IR+Form kidneys (Figure 4B). These results demonstrate that formoterol induced MB and restored mitochondrial function following I/R in concert with the return of renal function.
Figure 4.
Formoterol (Form) restored mitochondrial function in the kidney after I/R-induced AKI. Kidneys were excised followed by isolation of mitochondria. Shown are relative state 2 respiration (non-ADP–stimulated respiration) (A) and relative state 3 respiration (ADP-stimulated respiration) (B). These results of respiration are expressed as the mean±SEM and are relative values compared with control. Bars with different superscripts are significantly different from one another (n=7, P< 0.05). Veh, vehicle.
Currently, no pharmacologic therapies are approved for AKI, and most drug discovery research for AKI has historically focused on prevention. Therefore, an animal model in which treatment begins after establishment of AKI is more clinically relevant.1,2 In the current study, we sought to discover a pharmacotherapeutic approach focused on accelerating recovery of kidney function in a mouse model of AKI.
Several studies have demonstrated that mitochondrial dysfunction is a key component of AKI,7–10 and more recent studies have shown persistent mitochondrial dysfunction after injury.6,11 Previous proof-of-principle studies were conducted using oxidant injury in RPTC and demonstrated that overexpression or pharmacologic activation of PGC-1α after oxidant injury accelerated recovery of mitochondrial and cellular function.5,23 Furthermore, formoterol, a US Food and Drug Administration–approved, long-acting, specific β2-adrenergic receptor agonist, was shown to induce MB in RPTC and mice.26
Treatment with formoterol after I/R-induced AKI completely restored kidney function, attenuated tubule injury, and reduced renal cell necrosis. Concurrently, formoterol induced MB and restored mitochondrial proteins and function after injury. These data define formoterol as a first-in-class agent that successfully promotes full recovery of renal function after maximal injury. Furthermore, because mitochondrial dysfunction is common in many acute organ injuries/failures, our approach may be extrapolated to other tissues.
In this model and others, renal function, as measured by serum creatinine, improves over several days following I/R6 but does not fully recover by 6 days. Of note, formoterol treatment resulted in the complete return of renal function and recovery of renal proximal tubular injury as measured by KIM-1, which was associated with the recovery of mitochondrial function. Thus, we speculate that recovery of mitochondrial function is critical for complete recovery of the proximal tubule and kidney function. In addition, because the extent of AKI and subsequent prolonged injury to the proximal tubules have been linked to the development of CKD,29,30 assuagement of proximal tubule injury by formoterol after AKI may not be limited to short-term benefits by restoring kidney function, but may also have long-term benefits by modulating the progression to CKD due to a reduction in both extent and duration of proximal tubule damage.
Concise Methods
Eight-week-old male C57BL/6 mice weighing 25–30 g were subjected to bilateral renal pedicle ligation for 20 minutes, as described previously.6 Dosing was initiated 24 hours after reperfusion, and mice were given a daily injection of 0.3 mg/kg formoterol fumarate dihydrate (Sigma-Aldrich F9552) or vehicle (0.3% DMSO in normal saline) via intraperitoneal injection. All procedures involving animals were performed with approval from the Institutional Animal Care and Use Committee in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.
Blood was collected by retro-orbital eye bleed. Serum was isolated from each blood sample, and serum creatinine levels were measured using a Quantichrom Creatinine Assay Kit (BioAssay Systems, Hayward, CA) according to the manufacturer’s protocol.
Kidney sections approximately 5–6 microns from animals at 144 hours after I/R or sham surgery were stained with hematoxylin and eosin and PAS, and the degree of morphologic changes was determined by light microscopy in a blinded fashion. The following measures were chosen as an indication of morphologic damage to the kidney after treatment with vehicle or formoterol: proximal tubule dilation, brush-border damage, proteinaceous casts, interstitial widening, and necrosis. These measures were evaluated on a scale from 0 to 4, which ranged from not present (0), mild (1), moderate (2), severe (3), and very severe (4).
Renal cortical tissue from flash-frozen kidneys was lysed in RIPA buffer–containing cocktail protease and phosphatase inhibitors. Forty micrograms of total protein were loaded into SDS-PAGE gels and immunoblots were performed as previously described.22 Antibodies used for immunoblot studies were obtained from the following vendors: glyceraldehyde 3-phosphate dehydrogenase (Fitzgerald Antibodies), COX I and NDUFB8 (Invitrogen), PGC-1α (Calbiochem), and KIM-1 (R&D Systems).
Kidney mitochondria were isolated from male C57BL/6 mice. The whole kidney was minced and homogenized in ice-cold isolation buffer (250 mM sucrose, 1 mM EGTA, 10 mM HEPES, 1 mg/ml fatty acid-free BSA [pH 7.4], 300 mOsm/kg H2O). Nuclei and cellular debris were pelleted by centrifugation at 1000g for 10 minutes. The supernatant was centrifuged at 10,000g for 5 minutes, resulting in a crude mitochondrial pellet. The pellet was washed once in ice-cold isolation buffer and resuspended in assay buffer (220 mM mannitol, 70 mM sucrose, 5 mM MgCl2, 5 mM KH2PO4, 10 mM HEPES, 1 mg/ml fatty acid-free BSA [pH 7.4], 330 mOsm/kg H2O). Crude mitochondria were then diluted 1/10–1/100 in buffer B (137 mM KCl, 2 mM KH2PO4, 2.5 mM MgCl2, 20 mM HEPES, 0.5 mM EGTA, 0.2% FA-free BSA [pH 7.4], 330 mOsm/kg H2O) and 180 μl diluted mitochondria were added to triplicate wells of a Seahorse XF96 assay plate on ice. The plate was spun down at 3000g for 7 minutes at 4°C and immediately loaded into the XF96 Bioanalyzer. Oxygen consumption rate was normalized to mitochondrial protein per well.
Results were expressed as mean±SEM (n=3–7). Data were analyzed using a one-way ANOVA, and post hoc tests (Student–Newman–Keuls or Fisher least-significant difference where noted in the figure legend) were used to compare I/R untreated and sham and I/R compound-treated groups to vehicle. The level of significance was set at P<0.05.
Disclosures
None.
Acknowledgments
This study was supported by the National Institutes of Health (R01 GM084147 to R.G.S., T32 HL007260-35 to S.R.J and L.J.S., F32DK098053 to L.J.S., C06 RR015455 and SC COBRE in Oxidants, Redox Balance and Stress Signaling P20 GM103542-02), and by the Biomedical Laboratory Research and Development Program of the Department of Veterans Affairs (BX-000851). This publication was supported, in part, by the South Carolina Clinical and Translational Research Institute, with an academic home at the Medical University of South Carolina CTSA, and funded by the National Institutes of Health (UL1 RR029882).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Bellomo R, Kellum JA, Ronco C: Acute kidney injury. Lancet 380: 756–766, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Kellum JA, Bellomo R, Ronco C: Kidney attack. JAMA 307: 2265–2266, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Shusterman N, Strom BL, Murray TG, Morrison G, West SL, Maislin G: Risk factors and outcome of hospital-acquired acute renal failure. Clinical epidemiologic study. Am J Med 83: 65–71, 1987 [DOI] [PubMed] [Google Scholar]
- 4.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW: Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Funk JA, Odejinmi S, Schnellmann RG: SRT1720 induces mitochondrial biogenesis and rescues mitochondrial function after oxidant injury in renal proximal tubule cells. J Pharmacol Exp Ther 333: 593–601, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Funk JA, Schnellmann RG: Persistent disruption of mitochondrial homeostasis after acute kidney injury. Am J Physiol Renal Physiol 302: F853–F864, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall AM, Unwin RJ, Parker N, Duchen MR: Multiphoton imaging reveals differences in mitochondrial function between nephron segments. J Am Soc Nephrol 20: 1293–1302, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nowak G, Aleo MD, Morgan JA, Schnellmann RG: Recovery of cellular functions following oxidant injury. Am J Physiol 274: F509–F515, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Sharfuddin AA, Molitoris BA: Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol 7: 189–200, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Weinberg JM, Roeser NF, Davis JA, Venkatachalam MA: Glycine-protected, hypoxic, proximal tubules develop severely compromised energetic function. Kidney Int 52: 140–151, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Tran M, Tam D, Bardia A, Bhasin M, Rowe GC, Kher A, Zsengeller ZK, Akhavan-Sharif MR, Khankin EV, Saintgeniez M, David S, Burstein D, Karumanchi SA, Stillman IE, Arany Z, Parikh SM: PGC-1α promotes recovery after acute kidney injury during systemic inflammation in mice. J Clin Invest 121: 4003–4014, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szeto HH, Liu S, Soong Y, Wu D, Darrah SF, Cheng FY, Zhao Z, Ganger M, Tow CY, Seshan SV: Mitochondria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury. J Am Soc Nephrol 22: 1041–1052, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puigserver P, Spiegelman BM: Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): Transcriptional coactivator and metabolic regulator. Endocr Rev 24: 78–90, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Liu C, Lin JD: PGC-1 coactivators in the control of energy metabolism. Acta Biochim Biophys Sin (Shanghai) 43: 248–257, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM: A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92: 829–839, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Scarpulla RC: Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev 88: 611–638, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM: Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98: 115–124, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, Baek KH, Rosenzweig A, Spiegelman BM: HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature 451: 1008–1012, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, Wang Y, Raederstorff D, Morrow JD, Leeuwenburgh C, Allison DB, Saupe KW, Cartee GD, Weindruch R, Prolla TA: A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS ONE 3: e2264, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suliman HB, Carraway MS, Welty-Wolf KE, Whorton AR, Piantadosi CA: Lipopolysaccharide stimulates mitochondrial biogenesis via activation of nuclear respiratory factor-1. J Biol Chem 278: 41510–41518, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Sutherland LN, Bomhof MR, Capozzi LC, Basaraba SA, Wright DC: Exercise and adrenaline increase PGC-1alpha mRNA expression in rat adipose tissue. J Physiol 587: 1607–1617, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rasbach KA, Schnellmann RG: Signaling of mitochondrial biogenesis following oxidant injury. J Biol Chem 282: 2355–2362, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Rasbach KA, Schnellmann RG: PGC-1alpha over-expression promotes recovery from mitochondrial dysfunction and cell injury. Biochem Biophys Res Commun 355: 734–739, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Wang H, Peiris TH, Mowery A, Le Lay J, Gao Y, Greenbaum LE: CCAAT/enhancer binding protein-beta is a transcriptional regulator of peroxisome-proliferator-activated receptor-gamma coactivator-1alpha in the regenerating liver. Mol Endocrinol 22: 1596–1605, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin W, Signore AP, Iwai M, Cao G, Gao Y, Chen J: Rapidly increased neuronal mitochondrial biogenesis after hypoxic-ischemic brain injury. Stroke 39: 3057–3063, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wills LP, Trager RE, Beeson GC, Lindsey CC, Peterson YK, Beeson CC, Schnellmann RG: The β2-adrenoceptor agonist formoterol stimulates mitochondrial biogenesis. J Pharmacol Exp Ther 342: 106–118, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peterson YK, Cameron RB, Wills LP, Trager RE, Lindsey CC, Beeson CC, Schnellmann RG: β2-Adrenoceptor agonists in the regulation of mitochondrial biogenesis. Bioorg Med Chem Lett 23: 5376–5381, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Timmeren MM, van den Heuvel MC, Bailly V, Bakker SJ, van Goor H, Stegeman CA: Tubular kidney injury molecule-1 (KIM-1) in human renal disease. J Pathol 212: 209–217, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Chawla LS, Amdur RL, Amodeo S, Kimmel PL, Palant CE: The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int 79: 1361–1369, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakhoul N, Batuman V: Role of proximal tubules in the pathogenesis of kidney disease. Contrib Nephrol 169: 37–50, 2011 [DOI] [PubMed] [Google Scholar]