Abstract
This study explored the antioxidant and immunomodulatory potential of ethnomedicinally valuable species, namely, Arisaema jacquemontii of north-western Himalayan region. The tubers, leaves, and fruits of this plant were subjected to extraction using different solvents. In vitro antioxidant studies were performed in terms of chelation power on ferrous ions and FRAP assay. The crude methanol extract of leaves was found to harbour better chelating capacity (58% at 100 μg/mL) and reducing power (FRAP value 1085.4 ± 0.11 μMFe3+/g dry wt.) than all the other extracts. The crude methanol extract was thus further partitioned with solvents to yield five fractions. Antioxidant study of fractions suggested that the methanol fraction possessed significant chelation capacity (49.7% at 100 μg/mL) and reducing power with FRAP value of 1435.4 μM/g dry wt. The fractions were also studied for immune modulating potential where it was observed that hexane fraction had significant suppressive effect on mitogen induced T-cell and B-cell proliferation and remarkable stimulating effect on humoral response by 141% and on DTH response by 168% in immune suppressed mice as compared to the controls. Therefore, it can be concluded that A. jacquemontii leaves hold considerable antioxidant and immunomodulating potential and they can be explored further for the identification of their chemical composition for a better understanding of their biological activities.
1. Introduction
Natural products from plants have gathered significant attention due to the recent evidences suggesting their capacity to ameliorate oxidative stress and other related diseases. Plant secondary metabolites such as phenolics, flavonoids, and terpenoids play an important role as natural antioxidants and immunomodulators [1]. In biological systems, it is often considered that reactive oxygen species (ROS) originate from the interaction of iron with enzymatically and/or nonenzymatically generated superoxide (O2 ∙−, Haber-Weiss reaction) and/or hydrogen peroxide (H2O2, Fenton reaction) [2]. The human body harbours enzymatic defence mechanisms to counter oxidative stress induced by free radicals which involve superoxide dismutase, catalase, and glutathione peroxidase. Apart from innate defence system, synthetic drugs are also available which improve the capacity of the body to counter oxidative stress and other diseases. But owing to the harmful side effects of synthetic drugs, research on natural products has taken a leap in recent years.
Phytochemicals have also proved to enhance immunity by modulating the innate and adaptive immune responses. Immunomodulation using medicinal plants can provide an alternative to conventional chemotherapy for a variety of diseases, especially when the host defence mechanism has to be activated under conditions of impaired immune response or when a selective immunosuppression is desired in situations such as autoimmune disorders. Several types of immunomodulators have been identified, including substances isolated and purified from natural sources such as plants including microorganisms. Polyphenolic compounds such as phenolic acids, flavonoids, anthocyanidins, and tannins, produced as secondary metabolites by plants, possess remarkable antioxidant and immunomodulatory activities [3].
The genus Arisaema (commonly known as “Cobra Lilies”) is made up of more than 250 herbaceous species, which are distributed throughout temperate to tropical areas. Arisaema sp. are traditionally documented and are known to be used by the indigenous people of India and China as antinematodal, anti-inflammatory, analgesic, and antidote and also for treating rheumatoid arthritis [4–7]. A. heterophyllum, A. peninsulae, A. robustum, A. consanguineum,and A. japonicum were frequently used in Chinese herbal medicine as an anticonvulsant [8]. Rhizome of A. jacquemontii grounded with edible oil forms a paste which is used for massage to regain muscular strength and in skin problems such as blisters and pimples [9].
Arisaema jacquemontii is commonly found in the Himalayan forests at an altitude of 2,300–4,300 m. It also occurs in the Nilgiri Hills in southern India and the Khasi Hills region of north-east India. This plant has not been scientifically evaluated for its antioxidant and immunomodulatory potential. Owing to the lack of availability of substantial reports, the present study was planned to explore the biological potential of this traditionally documented plant.
2. Materials and Methods
2.1. Preparation of Extracts and Fractions
A. jacquemontii was collected from high altitude (2500–3500 m) from north-western Himalayan region and was identified by the taxonomist of University of Jammu. The leaves, fruits, and tubers were dried separately and grounded in a blender to make fine powder. A total of 100 g powder of each of the leaves, fruits, and tubers were extracted with 500 mL of three different solvents, that is, methanol (AJL1), water (AJL2), and chloroform (AJL3). Methanol and chloroform extracts were obtained by continuous stirring at room temperature for 6 h whereas water extract was prepared at 60°C overnight. This treatment was repeated thrice and the extracts were pooled, filtered, and evaporated using rotary vacuum evaporator. Active crude extract was further sequentially partitioned with different solvents like hexane (AJL1-H), chloroform (AJL1-C), ethyl acetate (AJL1-E), acetone (AJL1-A), and methanol (AJL1-M) to obtain fractions (Figure 3). All the fractions were freeze-dried using rotary vacuum evaporator and lyophilized.
Figure 3.
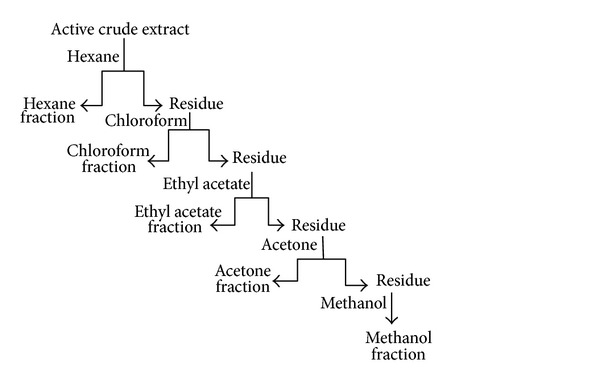
Flowchart of sequential partitioning of active crude methanol extract of leaves of A. jacquemontii to obtain fractions.
2.2. Antioxidant Studies
2.2.1. Chelation Power on Ferrous (Fe2+) Ions
The chelating effect on ferrous ions of the prepared extracts was estimated by the method of Dinis with slight modifications [10]. Briefly, 100 μL of each test sample (1 mg/mL) was taken and raised to 3 mL with methanol. 740 μL of methanol was added to 20 μL of 2 mM FeCl2. The reaction was initiated by the addition of 40 μL of 5 mM ferrozine into the mixture, which was then left at room temperature for 10 min and then the absorbance of the mixture was determined at 562 nm.
(i) Ferric Ion Reducing Antioxidant Power (FRAP Assay). FRAP activity was measured according to the method of Benzie and Strain [11]. Briefly, acetate buffer (300 mM, pH 3.6), TPTZ (2,4,6-tripyridyl-s-triazine) 10 mM in 40 mM HCl and FeCl3·6H2O (20 mM) were mixed in the ratio of 10 : 1 : 1 to obtain the working FRAP reagent. Test sample (0.5 mL) was mixed with 3 mL of working FRAP reagent and absorbance was measured at 593 nm after vortexing. Methanol solutions of FeSO4·7H2O ranging from 100 to 2000 μM were prepared and used for the preparation of the calibration curve of known Fe2+ concentration. The parameter equivalent concentration was defined as the concentration of antioxidant having a Ferric-TPTZ reducing ability equivalent to that of 1 mM FeSO4·7H2O.
2.3. Acute Toxicity Study
Acute toxicity study of fractions was conducted as per OECD guidelines 420 (fixed dose procedure) using Swiss mice. Each animal was administered test samples at a dose of 2000 mg/kg by oral route. The animals were observed for any changes continuously for the first 4 h and up to 24 h for any mortality. The animals were then kept for 14 days to observe daily cage side observations and mortality [12].
2.4. Immunomodulatory Studies
2.4.1. Mitogen Activity Test by MTT Assay
Mitogen activity test was based on the method described by Mosmann [13]. Animals (balb/c mice, 18–22 g) were sacrificed; their spleens were removed in sterile conditions. A single cell suspension was prepared in 5 mL of incomplete RPMI. The cell suspension was centrifuged at 1200 rpm for 10 min and supernatant was discarded. RBCs were lysed by Trisammonium chloride treatment. The cells were centrifuged at 1200 rpm for 10 min, after centrifuging, supernatant was discarded and cell pellet was resuspended in complete RPMI. The viability of cells was checked with trypan blue. 1 × 106 cells/mL suspension was prepared and 100 mL of it was poured in each well of 96-well microtitre plate. An aliquot of 50 mL of standard mitogens, that is, Concanavalin A (Con A) (1/4) 10 mg/mL and Lipopolysaccharide (LPS) (1/4) 10 mg/mL, and test materials were added according to the experimental setup. The extracts in different concentrations (10−4, 10−5, and 10−6 M) were dissolved in DMSO and added to each well of flat bottom microtitre 96-well plate. Plates were placed on a shaker for 5 min. The plates were incubated for 48 h in CO2 incubator (37°C, 5% CO2, and 90% relative humidity). After 48 h of incubation, plates were taken out from the CO2 incubator and reading was taken on ELISA plate reader at 540 nm. Thereafter, 10 μL of MTT solution (5 mg/mL in PBS) was added to each well. The contents were placed on a shaker for 5 min and plates were incubated for 4–6 h in CO2 incubator (37°C, 5% CO2, and 90% relative humidity) to allow the MTT to be metabolized. After incubation, the plates were inverted on a paper towel to remove the medium. The formazan crystals (MTT byproduct) were resuspended in 100 mL DMSO and reading was measured at a wavelength of 570 nm.
2.4.2. Effect on Humoral and Cellular Response in Immune Suppressed Mice
Swiss albino mice (Mus musculus) 10–12 weeks old, with 20–25 g body weight, and male Charles Foster rats (Rattus norvegicus) 10–12 weeks, with 100–150 g body weight, in groups of six were employed for study. In every experiment, one group of animals was used as a vehicle control while another received a standard drug Azathioprine (Aza). The test sample was freshly prepared as a homogenised suspension in 1% w/v acacia gum administered orally daily once a day for the duration of the experiment.
(1) Antigen (SRBC). Fresh sheep red blood cells (SRBC) collected aseptically from jugular vein of sheep were stored in cold sterile Alsever's solution, washed three times with pyrogen free sterile normal saline (0.9% NaCl w/v), and adjusted to a concentration of 5 × 109 cells/mL for immunization and challenge at the required time schedule.
(2) Humoral Antibody Response (Hab). Groups of six mice each were immunized by injecting 0.2 mL of 5 × 109 SRBC/mL intraperitoneally (i.p.) on day 0 and challenged 7 days later by injecting an equal volume of SRBC i.p. Blood samples were collected on day +7 (before challenge) for primary antibody titre. Hemagglutination antibody titres were determined following the microtitration technique described by Nelson and Mildenhall [14]. The value of the highest serum dilution causing haemagglutination was taken as a titre. BSA saline alone served as a control.
(3) Delayed Type Hypersensitivity Response (DTH). The method of Doherty was followed to assess SRBC induced DTH response in mice [15]. Mice were immunized by injecting 20 μL of 5 × 109 SRBC/mL subcutaneously into the right hind foot pad. Seven days later, the thickness of the left hind foot was measured with a spheromicrometer (0.01 mm pitch) and was considered as a control. These mice were then challenged by injecting the same amount of SRBC intradermally into the left hind foot pad. The foot thickness was measured again at 0, 4, and 24 hr after challenge.
2.5. Phytochemical Analysis
The fractions obtained from the crude methanol extract of leaves were also investigated for the presence of secondary metabolites like terpenoids, coumarins, glycosides, quinones, saponins, tannins, anthraquinones, alkaloids, phenols, and flavonoids.
2.6. Statistical Analysis
All experiments were carried out in triplicate. Data values are expressed as mean ± standard deviation.
3. Results and Discussion
Diverse species of Arisaema hold ethnomedicinal importance in different areas of Asian subcontinent for treating various medical ailments. The rhizomes or tubers of A. calcareum, A. serratum, A. asperatum, A. heterophyllum, and A. amurense are used as analgesic, antitumor, and pesticide agents in traditional Chinese medicine [16]. Chinese herbal traditional medicine system uses A. cumbile for treating dementia and neurological symptoms [17]. Later on, studies explained that A. cumbile inhibits the production of proinflammatory cytokines including interleukin (IL-)1β, IL-6, and tumor necrosis factor (TNF-)α [18]. Antihepatotoxic cerebrosides have also been isolated from A. amurense [8]. A. erubescens is a widely distributed species in China and it is used as a medicinal herb against damp phlegm, convulsions, and swelling [19]. Pharmacological study has also proved that this species harbors anticonvulsant and anticancer effects [20]. Paeonol, a phenolic compound which possesses antimutagenic, anticonvulsant, and anti-inflammatory activities, has been isolated from A. erubescens [21].
However, sufficient scientific data regarding the biological potential of A. jacquemontii is still lacking. In view of this fact, our study focussed on unearthing the antioxidant and immunomodulating potential of A. jacquemontii, which is found in abundance in the high altitude forests of Himalayas. Extracts of each of tubers, leaves, and fruits of A. jacquemontii were prepared in chloroform, methanol, and water and tested for antioxidant potential by undertaking in vitro chemical assays, mainly chelation power on ferrous ions and ferric ion reducing antioxidant power (FRAP).
Most reactive oxygen species (ROS) are generated as by-products during mitochondrial electron transport and other metabolic reactions. In addition, ROS are formed as necessary intermediates of metal catalyzed oxidation reactions. The transition metal ion Fe2+ possesses the ability to perpetuate the formation of free radicals by gain or loss of electrons. Therefore, the reduction of the formation of reactive oxygen species can be achieved by the chelation of metal ions with chelating agents. Chelation power assay was carried out to assess the chelation capacity of the crude extracts which illustrated that the crude methanol extract of leaves of A. jacquemontii possessed remarkable chelation power at 100 μg/mL (58%) as compared to tuber (12%) and fruit extracts (34%) (Table 1). However, the aqueous and chloroform extracts of tubers, leaves, and fruits showed negligible activity. Therefore, active crude methanol extract of leaves was subjected to sequential fractionation yielding five fractions, that is, hexane (AJL1-H), chloroform (AJL1-C), ethyl acetate (AJL1-E), acetone (AJL1-A), and methanol (AJL1-M). Chelation power of fractions was also analysed and it was observed that the methanol fraction exhibited significant capacity to chelate ferrous ions in comparison to other fractions with the value of 49.7% at 100 μg/mL (Figure 1). Excess of metal ions can lead to various anomalies in the body. The iron (II) chelating activity of plant extracts is of great significance, because it has been proposed that the transition metal ions contribute to the oxidative damage in neurodegenerative disorders like Alzheimer's and Parkinson's diseases [22]. Also, chelation therapy is a common practice of neutralising iron overload in the body especially in cases of treatment of Thalassemia and other anemias [23]. The current scenario suggests that the chelation therapy makes use of synthetic compounds which have certain side effects as well. Therefore, chelation of metal ions by natural phytochemicals from Arisaema sp. can prove to be of therapeutic importance.
Table 1.
Chelation power on ferrous ions and ferric reducing antioxidant power (FRAP) of A. jacquemontii crude extracts.
| Extracts | Chelation power on ferrous ions (% at 100 µg/mL) | FRAP (μM/g dry wt.) | ||||
|---|---|---|---|---|---|---|
| A. jacquemontii | Tuber | Leaves | Fruit | Tuber | Leaves | Fruit |
| Methanol (AJL1) | 12 | 58 | 34 | 37 ± 0.021 | 1085.4 ± 0.11 | 635.4 ± 0.032 |
| Aqueous (AJL2) | 9 | 28 | 20 | 77.4 ± 0.41 | 805 ± 0.26 | 159.5 ± 0.015 |
| Chloroform (AJL3) | 13 | 32 | 22 | na | na | na |
*Values are expressed as mean ± standard deviation. na: not active.
Figure 1.
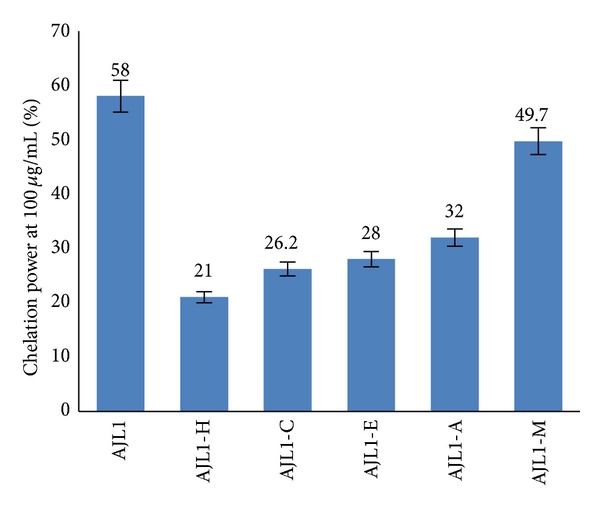
Chelation power on ferrous ions of A. jacquemontii crude methanol leaf extract and its fractions. AJL1: crude methanol leaf extract, AJL1-H: hexane fraction, AJL1-C: chloroform fraction, AJL1-E: ethyl acetate fraction, AJL1-A: acetone fraction, and AJL1-M: methanol fraction.
Another assay, that is, ferric reducing antioxidant power (FRAP), was conducted on all the extracts and fractions of A. jacquemontii to confirm its antioxidant potential. In this assay, reduction of ferric tripyridyl triazine (Fe3+-TPTZ) complex to ferrous form which has an intense blue colour can be monitored by measuring the change in absorption at 593 nm. The results of this experiment were similar to that of chelation power assay; that is, crude methanol extract of A. jacquemontii leaves showed noteworthy FRAP activity with reducing the value of 1085.4 ± 0.11 μM/g dry wt. However, tuber and fruit methanol extracts possessed very low FRAP activity with values of 37 ± 0.021 μM/g dry wt. and 635.4 ± 0.032 μM/g dry wt., respectively (Table 1). Also, further FRAP analysis of fractions showed that methanol fraction (AJL1-M) possessed better reducing power with FRAP value of 1435.4 μM/g dry wt. in comparison to other fractions (Figure 2).
Figure 2.
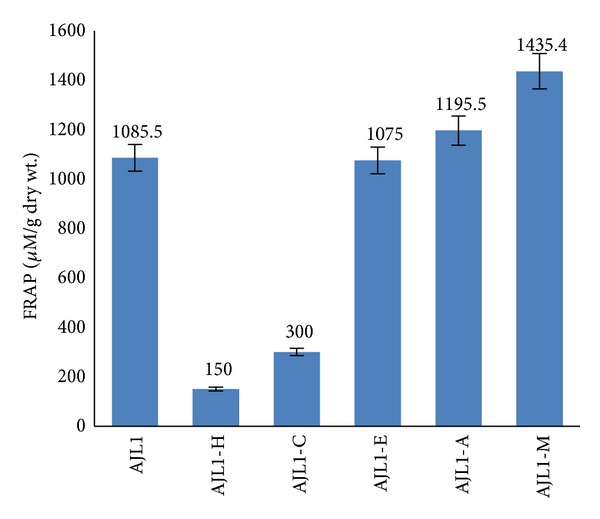
Ferric reducing antioxidant power (FRAP) of A. jacquemontii crude methanol leaf extract and its fractions. AJL1: crude methanol leaf extract, AJL1-H: hexane fraction, AJL1-C: chloroform fraction, AJL1-E: ethyl acetate fraction, AJL1-A: acetone fraction, and AJL1-M: methanol fraction.
Antioxidant studies suggested that the crude methanol extract of leaves (AJL1) and its subsequent methanol fraction (AJL1-M) possessed promising chelating and reducing antioxidant power as compared to all the other extracts of tubers and fruits. All the fractions were also studied for the effects of acute toxicity and behavioural changes using Swiss mice as per OECD guidelines 420 (Fixed dose procedure). It was observed that there was no mortality and noticeable behavioural changes in treated animals as compared to control animals. All the fractions were found to be safe up to 2000 mg/kg body weight p.o.
Many medicinal plants are a rich source of substances which induce para-immunity, non-specific activation of granulocytes, macrophages, natural killer cells and the complement system. Confirming the safety levels of all the fractions by acute toxicity study in mice, they were further analysed for immune modulating potential by studying the effect on Con A and LPS induced murine lymphocyte proliferation (MTT assay). Concanavalin A and Lipopolysaccharide (LPS) are the mitogens which activate T-cell and B-cell proliferation, respectively, in the splenocytes. MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay is based on the ability of a mitochondrial dehydrogenase enzyme from viable cells to cleave the tetrazolium rings of the pale yellow MTT and form a dark blue formazan crystals which is largely impermeable to cell membranes, thus resulting in its accumulation within healthy cells. The number of surviving cells is directly proportional to the level of the formazan product created which can then be quantified using a simple colorimetric assay [13]. Hexane (AJL1-H) and ethyl acetate fraction (AJL1-E) showed significant suppressive effect on LPS induced B-cell proliferation and moderate effect on Con-A induced T-cell proliferation. However, methanol fraction (AJL1-M) also depicted considerable immune suppression on both B-cell and T-cell proliferation (Table 2). Immune suppression finds application in the treatment of autoimmune disorders such as rheumatoid arthritis and multiple sclerosis [24].
Table 2.
Effect of different concentrations of fractions of crude methanol extract of A. jacquemontii leaves on Con A and LPS induced murine lymphocyte proliferation.
| Serial number | Samples | Conc. (M) |
Con A mean ± S.E. | Con A induced T-cell proliferation rate (%) | LPS mean ± S.E. | LPS induced B-cell proliferation rate (%) |
|---|---|---|---|---|---|---|
| (1) | Control | — | 0.75 ± 0.50 | — | 1.92 ± 0.05 | — |
|
| ||||||
| (2) | AJL1-H | 10−4 | 0.34 ± 0.04 | −54.66 | 0.39 ± 0.01 | −79.68 |
| 10−5 | 0.76 ± 0.03 | +1.33 | 0.44 ± 0.01 | −77.08 | ||
| 10−6 | 0.58 ± 0.02 | −22.66 | 0.60 ± 0.01 | −68.75 | ||
|
| ||||||
| (3) | AJL1-C | 10−4 | 0.69 ± 0.12 | −22.47 | 0.82 ± 0.17 | −16.32 |
| 10−5 | 0.81 ± 0.08 | −8.98 | 0.85 ± 0.06 | −13.26 | ||
| 10−6 | 0.82 ± 0.14 | −7.86 | 0.83 ± 0.09 | −15.30 | ||
|
| ||||||
| (4) | AJL1-E | 10−4 | 0.60 ± 0.11 | −20.00 | 0.36 ± 0.01 | −81.25 |
| 10−5 | 0.54 ± 0.03 | −28.00 | 0.48 ± 0.01 | −75.00 | ||
| 10−6 | 0.71 ± 0.00 | −5.33 | 0.59 ± 0.01 | −69.27 | ||
|
| ||||||
| (5) | AJL1-A | 10−4 | 0.43 ± 0.02 | −42.66 | 0.54 ± 0.30 | −72.21 |
| 10−5 | 0.71 ± 0.05 | −5.33 | 0.62 ± 0.30 | −67.75 | ||
| 10−6 | 0.64 ± 0.03 | −14.66 | 0.60 ± 0.17 | −68.12 | ||
|
| ||||||
| (6) | AJL1-M | 10−4 | 0.59 ± 0.14 | −33.70 | 0.48 ± 0.05 | −75.00 |
| 10−5 | 0.70 ± 0.01 | −21.34 | 0.52 ± 0.04 | −72.91 | ||
| 10−6 | 0.59 ± 0.14 | −33.70 | 0.55 ± 0.09 | −71.35 | ||
+ indicates immune stimulant agents, while − indicates immunosuppressive agents. Results are mean standard error (SE) of three separate experiments. The bold values are shown for those compounds which have proved to be active and those in normal font represent the least significant.
Furthermore, the effect of fractions on humoral and cell mediated immune response in immune suppressed mice was also studied (Table 3). The results after 7 days of oral administration of fractions to the immune suppressed mice showed some interesting results. The hexane fraction (AJL1-H) was observed to enhance the humoral and cell-mediated immune response in immune suppressed mice (Table 3). The humoral antibody titre at the concentration of 100 mg/mL was observed to be 141% which was more than that of standard drug, Levamisole (133%). Also, AJL1-H showed noteworthy positive DTH response at all three concentrations of 100 mg/mL (168%), 50 mg/mL (100%), and 25 mg/mL (128%). Also, acetone fraction (AJL1-A) showed moderate humoral and DTH stimulating responses at concentration of 100 mg/mL and 50 mg/mL and methanol fraction (AJL1-M) demonstrated immune stimulating activity only at concentration of 25 mg/mL (Table 3). The other fractions were not found to be much effective as immune stimulants. Therefore, it can be deduced from the study that hexane fraction of A. jacquemontii leaves (AJL1-H) possesses significant immune stimulating potential as it showed potent abrogative effect on humoral antibody response and delayed type hypersensitivity response in immune suppressed balb/c mice and these observations are suggestive of possible therapeutic usefulness in immune compromised patients.
Table 3.
Effect of active methanol fraction of A. jacquemontii leaves on humoral and cell mediated immune response.
| Sample | Conc. mg/kg p.o. |
Antibody titre Mean ± S.E. |
% Activity |
DTH Mean ± S.E. |
% Activity |
|---|---|---|---|---|---|
| Control | — | 6.5 ± 0.21 | — | 0.80 ± 0.16 | — |
|
| |||||
| Cyclophosphamide | 200 | 4.5 ± 0.21 | −30.7 | — | — |
|
| |||||
| Cyclosporine | 5 | — | — | 0.35 ± 0.10 | −56.25 |
|
| |||||
| Levamisole | 2.5 | 7.16 ± 0.22 | +133 | 1.11 ± 0.22 | +168 |
|
| |||||
| AJL1-H (mg/mL) | 100 | 7.33 ± 0.21 | +141 | 1.11 ± 0.22 | +168 |
| 50 | 5.83 ± 0.16 | +67 | 0.80 ± 0.16 | +100 | |
| 25 | 3.83 ± 0.16 | +33 | 0.91 ± 0.16 | +124 | |
|
| |||||
| AJL1-C (mg/mL) | 100 | 5.83 ± 0.16 | +67 | 0.48 ± 0.21 | −29 |
| 50 | 4.66 ± 0.21 | +8 | 0.45 ± 0.22 | +23 | |
| 25 | 5.16 ± 0.16 | +33 | 0.60 ± 0.20 | +56 | |
|
| |||||
| AJL1-E (mg/mL) | 100 | 5.66 ± 0.21 | +58 | 0.63 ± 0.21 | +63 |
| 50 | 5.16 ± 0.16 | +33 | 0.41 ± 0.16 | +14 | |
| 25 | 5.33 ± 0.21 | +42 | 0.50 ± 0.22 | +34 | |
|
| |||||
| AJL1-A (mg/mL) | 100 | 6.33 ± 0.21 | +91 | 0.80 ± 0.16 | +100 |
| 50 | 6.16 ± 0.40 | +83 | 0.83 ± 0.16 | +106 | |
| 25 | 5.33 ± 0.21 | +42 | 0.53 ± 0.21 | +40 | |
|
| |||||
| AJL1-M (mg/mL) | 100 | 5.16 ± 0.16 | +33 | 0.58 ± 0.16 | +52 |
| 50 | 4.16 ± 0.16 | −17 | 0.38 ± 0.22 | −7 | |
| 25 | 6.5 ± 0.22 | +100 | 0.93 ± 0.21 | +128 | |
Values are expressed as mean ± standard deviation.
The phytochemical analysis revealed that all the fractions contain high amount of terpenoids. Coumarins, quinones, and glycosides were present in moderate amount (Table 4). Alkaloids, anthraquinones, and flavonoids were found in low quantity and phenols were detected in moderate amount only in acetone and methanol fractions. This was a preliminary analysis of the phytoconstituents present in the fractions. However, the phytochemicals responsible for the antioxidant and immune modulating potential of A. jacquemontii leaves still need to be identified.
Table 4.
Preliminary phytochemical analysis of fractions of crude methanol extract of A. jacquemontii leaves.
| Phytochemicals | Fractions | ||||
|---|---|---|---|---|---|
| Hexane (AJL1-H) | Chloroform (AJL1-C) | Ethyl acetate (AJL1-E) | Acetone (AJL1-A) | Methanol (AJL1-M) | |
| Terpenoids | ++++ | ++ | +++ | +++ | +++ |
| Coumarins | + | ++ | + | + | + |
| Quinones | ++ | ++ | + | + | + |
| Saponins | − | − | − | − | − |
| Glycosides | ++ | + | +++ | ++ | − |
| Tannins | − | − | − | − | − |
| Alkaloids | + | + | − | + | + |
| Anthraquinones | + | + | − | − | − |
| Phenols | − | − | + | ++ | ++ |
| Flavonoids | − | − | − | − | + |
++++ indicates high amount; ++ indicates moderate amount; + indicates low amount; − indicates absence.
4. Conclusion
This work is an attempt to identify the biological potential of A. jacquemontii growing in the Himalayan region for the first time. The methanol fraction obtained from crude methanol extract of leaves was observed to harbour considerable antioxidant potential and, in case of immune modulating studies, the hexane fraction was observed to show remarkable immune suppressive as well as immune stimulating potential, which could prove to be of immense value in autoimmune diseases as well as in immune compromised patients. However, the phytoconstituents responsible for their significant activity are still unknown. The results obtained in this work provide the basis for designing future experimentation on this species for better understanding of its antioxidative and immunomodulatory system and discovering the phytochemicals responsible for these properties.
Acknowledgments
This work was supported by a research grant from the University Grants Commission, New Delhi, India. The authors extend their regards to Dr. Harish Dutt (Assistant Professor, Department of Botany, University of Jammu) for his guidance. They would also like to acknowledge the School of Biotechnology and Department of Bioinformatics, University of Jammu, for their support.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Estrada MJ, Contreras CV, Escobar AG, et al. In vitro antioxidant and antiproliferative activities of plants of the ethnopharmacopeia from northwest of Mexico. BMC Complementary and Alternative Medicine. 2013;13, article 12 doi: 10.1186/1472-6882-13-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel RM. Ferrous ion chelating activity (FICA)- a comparative antioxidant activity evaluation of extracts of eleven naturally growing plants of Gujarat, India. International Journal of Scientific Research. 2013;2(8) [Google Scholar]
- 3.Kumar UA, Manjunath C, Thaminzhmani T, Kiran YR, Brahmaiah Y. A review on immunomodulatory activity plants. Indian Journal of Novel Drug Delivery. 2012;4(2):93–103. [Google Scholar]
- 4.Agoreyo O, Okoro NC, Choudhary MI. Preliminary phytochemical analyses of two varieties of Adenia lobata (jacq) and the antioxidant activity of their various solvent fractions. Bayero Journal of Pure and Applied Sciences. 2012;5(1):182–186. [Google Scholar]
- 5.Balekar N, Bodhankar SL, Jain DK. Evaluation of Immunomodulatory Activity of Medicinal Plants: Ellagic Acid and Mangiferin. Lap Lambert Academic Publishing; 2010. [Google Scholar]
- 6.Choudhary K, Singh M, Pillai U. Ethnobotanical survey of Rajasthan—an update. The American-Eurasian Journal of Botany. 2008;1(2):38–45. [Google Scholar]
- 7.Chunxia C, Peng Z, Huifang P, Hanli R, Zehua H, Jizhou W. Extracts of Arisaema rhizomatum C.E.C. Fischer attenuate inflammatory response on collagen-induced arthritis in BALB/c mice. Journal of Ethnopharmacology. 2011;133(2):573–582. doi: 10.1016/j.jep.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 8.Jung JH, Lee CO, Kim YC, Kang SS. New bioactive cerebrosides from Arisaema amurense . Journal of Natural Products. 1996;59(3):319–322. doi: 10.1021/np960201+. [DOI] [PubMed] [Google Scholar]
- 9.Bibi Y, Nisa S, Waheed A, et al. Evaluation of Viburnum foetens for anticancer and antibacterial potential and phytochemical analysis. African Journal of Biotechnology. 2010;9(34):5611–5615. [Google Scholar]
- 10.Dinis TCP, Madeira VMC, Almeida LM. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Archives of Biochemistry and Biophysics. 1994;315(1):161–169. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- 11.Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Analytical Biochemistry. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 12.Guideline for Testing of Chemicals 420 Acute Oral Toxicity. The Organization of Economic Co-operation & Development (OECD), Paris, France, pp. 1–14, 2001.
- 13.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods. 1983;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 14.Nelson DS, Mildenhall P. Studies on cytophilic antibodies. 1. The production by mice of macrophage cytophilic antibodies to sheep erythrocytes: relationship to the production of other antibodies and the development of delayed-type hypersensitivity. The Australian journal of experimental biology and medical science. 1967;45(2):113–130. [PubMed] [Google Scholar]
- 15.Doherty NS. Selective effects of immunosuppressive agents against the delayed hypersensitivity response and humoral response to sheep red blood cells in mice. Agents and Actions. 1981;11(3):237–242. doi: 10.1007/BF01967620. [DOI] [PubMed] [Google Scholar]
- 16. Chinese Pharmacopoeia, Chinese Pharmacopoeia Committee, Chemical Industry Press, Beijing, China, 2005.
- 17.Hu R, Yin CL, Wu N, et al. Traditional Chinese herb Dihuang Yinzi (DY) plays neuroprotective and anti-dementia role in rats of ischemic brain injury. Journal of Ethnopharmacology. 2009;121(3):444–450. doi: 10.1016/j.jep.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 18.Ahn CB, Je JY. Anti-inflammatory Activity of the Oriental Herb Medicine, Arisaema cum Bile, in LPS-Induced PMA-Differentiated THP-1 Cells. Immunopharmacology and Immunotoxicology. 2011;34(4):661–666. doi: 10.3109/08923973.2011.608683. [DOI] [PubMed] [Google Scholar]
- 19. Encyclopedia of Chinese Medicinal Substances, Jiangsu New Medical College, In: Shanghai People's Publisher: Shanghai, China, pp. 329–333, 1986.
- 20.Yang ZH, Yi JY, Wei ZR, Yan WQ. Effect of extract of Rhizoma Arisaematis on languish and mechanism of SMMC-7721 cell of human liver cancer lines. Chinese Medicine of Old People. 2007;27:142–144. [Google Scholar]
- 21.Ducki S, Hadfield JA, Lawrence NJ, Zhang X, McGown AT. Isolation of paeonol from Arisaema erubescens . Planta Medica. 1995;61(6):586–587. doi: 10.1055/s-2006-959390. [DOI] [PubMed] [Google Scholar]
- 22.Aparadh VT, Naik VV, Karadge BA. Antioxidative properties (TPC, DPPH, FRAP, metal chelating ability, reducing power and TAC) within some Cleome species. Annali di Botanica. 2012;2:49–56. [Google Scholar]
- 23.Ebrahimzadeh MA, Pourmorad F, Bekhradnia AR. Iron chelating activity, phenol and flavonoid content of some medicinal plants from Iran. African Journal of Biotechnology. 2008;7(18):3188–3192. [Google Scholar]
- 24.Katiyar CK, Brindavanam NB, Tiwari P, Narayana DBA. Immunomodulator products from Ayurveda: current status and future perspectives. In: Upadhyaya SN, editor. Immunomodulation. New Delhi, India: Narosa Publishing House; 1997. pp. 163–187. [Google Scholar]


