Abstract
Background
Chloride intracellular channel 4 (CLIC4) is highly expressed in the endothelium of remodelled pulmonary vessels and plexiform lesions of patients with pulmonary arterial hypertension (PAH). CLIC4 regulates vasculogenesis through endothelial tube formation. Aberrant CLIC4 expression may contribute to the vascular pathology of PAH.
Methods and Results
CLIC4 protein expression was increased in plasma and blood-derived endothelial cells from patients with idiopathic PAH (IPAH) and in the pulmonary vascular endothelium of 3 rat models of pulmonary hypertension. CLIC4 gene deletion markedly attenuated the development of chronic hypoxia-induced pulmonary hypertension in mice. Adenoviral overexpression of CLIC4 in cultured human pulmonary artery endothelial cells compromised pulmonary endothelial barrier function and enhanced their survival and angiogenic capacity, while CLIC4 shRNA had an inhibitory effect. Similarly, inhibition of CLIC4 expression in blood-derived endothelial cells from patients with IPAH attenuated the abnormal angiogenic behaviour that characterises these cells. The mechanism of CLIC4 effects involves p65-mediated activation of nuclear factor-κB, followed by stabilisation of hypoxia-inducible factor-1α and increased downstream production of vascular endothelial growth factor and endothelin-1.
Conclusions
Increased CLIC4 expression is an early manifestation and mediator of endothelial dysfunction in pulmonary hypertension.
Keywords: Angiogenesis, endothelium, endothelin, hypoxia & hypertension, pulmonary
INTRODUCTION
Chloride intracellular channel 4 (CLIC4) is a member of a family of six highly conserved redox-sensitive proteins (CLIC1–6) homologous to glutathione transferases1. In its soluble form, CLIC4 is essential for normal vasculogenesis through endothelial tubulogenesis2,3. Knockout of the murine CLIC4 gene results in reduced collateral vessel formation in vivo, associated with a reduction in ischemia-induced expression of hypoxia inducible factor-1α (HIF-1α) and downstream targets, such as (VEGF) and angiopoietin-24.
CLIC4 is highly expressed in the lungs of patients with pulmonary arterial hypertension (PAH), compared with healthy lungs5. Increased CLIC4 expression is evident in the vascular endothelium and, in particular, in the occlusive and plexiform lesions that result from endothelial cell proliferation, impaired apoptosis and disorganised angiogenesis that characterise the disease6. The expression of CLIC4 is influenced by reactive oxygen species (ROS), DNA damage and cytokines, such as tumour necrosis factor-α (TNF-α) and transforming growth factor-β (TGF-β)7,8. CLIC4 is an integral component of TGF-β signalling through prevention of the dephosphorylation of phospho-Smad2 and 3 in the nucleus, as well as several other pathways that have been implicated in the pathogenesis of PAH, including TNF-α, bone morphogenetic protein (BMP) and Rho signalling9,10.
We hypothesized that aberrant CLIC4 expression may contribute to pulmonary endothelial dysfunction and vascular remodelling in PAH. To address this, we investigated (a) the expression of CLIC4 in the plasma of idiopathic PAH (IPAH) patients and 3 experimental models of PAH, (b) the effect of CLIC4 knockout on pulmonary vascular homeostasis in the hypoxic mouse, (c) the role of CLIC4 in the regulation of endothelial junctional integrity and function in human pulmonary artery endothelial cells and endothelial cells derived from circulating progenitors in patients with IPAH and (d) the molecular mechanisms mediating CLIC4-induced effects.
MATERIALS AND METHODS
An expanded Materials and Methods section is available in the Online Data Supplement.
Human pulmonary arterial endothelial cell (HPAEC) and smooth muscle cell (HPASMC) culture
HPAECs were cultured in endothelial growth medium-2 (Promocell) while HPASMCs were cultured in smooth muscle cell growth medium-2 (Promocell). The cells were incubated under normoxic (20% O2, 5% CO2) or hypoxic (2% O2, 5% CO2, 93% N2) conditions for 1–24 hr at 37°C. Hypoxia was used as means of manipulating HPAEC phenotype, protein expression and angiogenic potential in vitro. In some experiments, 5 μmol/L diphenylene iodonium (DPI, Sigma) or 5 μmol/L rotenone (Sigma), were added to the cells 1 hr prior to hypoxic exposure.
Plasma and blood-derived human endothelial cell from IPAH patients
Venous blood samples were obtained with local ethics committee approval and informed written consent from healthy volunteers and IPAH patients (Tables S1–S2 in the Online Data Supplement). Plasma samples were obtained from 123 consecutive patients, between 2011 and 2013, and endothelial colony forming cells (ECFCs) were derived as previously described11,12.
Animals and Experimental Design
All experiments were conducted in accordance to the UK Home Office Animals (Scientific Procedures) Act 1986 (London, UK) and the Colorado State University Animal Care Use Committee. To study the effect of CLIC4 gene knockout on the development of pulmonary hypertension, 6 weeks old wild-type or CLIC4 knockout adult male mice (CD-1 background)13, were either housed in (1) normoxic conditions at sea level, (2) at Denver altitude (5280 ft; Pb= 630 mmHg) for 13 weeks or (3) at Denver altitude for 10 weeks followed by 3 weeks hypoxia (normobaric; Fio2=10%) (n=4–6/group). At 19 weeks, animals were weighed and anesthetized (Hypnorm 0.25 mL/kg; Midazolam 25 mg/kg IP), then the right ventricular systolic pressure (RVSP) was measured and the right ventricle to the left ventricle plus septum ratios (RV/LV+S) were determined. The left lungs were fixed, embedded in paraffin, and stained by the elastic van Gieson’s method. Vascular muscularisation was defined as the proportion of vessels (<50 μm diameter) with double elastic lamina over the total number of vessels stained with elastin. In addition, CLIC4 expression was studied in the lungs of rats with chronic hypoxia-, monocrotaline- and Sugen 5416/hypoxia models of pulmonary hypertension. Sugen 5416/hypoxia14 lung sections were a kind gift of Professor Nobert Voelkel (Virginia Commonwealth University, Richmond, VA, USA).
CLIC4 protein expression and localization
Plasma CLIC4 levels were measured using a sandwich enzyme immunoassay (Antibodies Online Inc., Aachen, Germany), according to the manufacturer’s protocol. Tissue CLIC4 protein expression and localization was investigated by western blot analysis, immunostaining of tissue sections and cultured endothelial cells followed by confocal microscopy. Relative quantification of CLIC4 expression in the intimal and medial layers of pulmonary vessels from healthy and pulmonary hypertension animal models was performed using Image J.
Manipulation of CLIC4 expression and activity in cultured cells
Overexpression CLIC4 was induced by adenoviral gene transfer15. The inhibition of CLIC4 expression was achieved using adenoviral gene transfer of CLIC4 shRNA.
Endothelial cell permeability and morphology
Endothelial permeability and morphology were studied as previously described16. In some experiments, mouse monoclonal VEGF function-blocking antibodies (10 μg/mL; R&D Systems) were added to the upper chamber of Transwell dishes 24 hr before permeability measurement.
In vitro angiogenesis assays
Matrigel tube formation assay was used. Total tube length was determined using Image J software.
Cell metabolic activity
CellTiter 96 proliferation assay (Promega) was used to assess metabolic activity associated with cell proliferation and migration17.
Cell survival assay
HPAECs were grown in full medium or medium deprived of serum and growth factors. Menadione (50 μM, Sigma) was added to cells for 6 hr as a positive control. Cells were incubated with MitoProbe™ DiOC2(3) (Life Technologies, Invitrogen, USA) for 45 min and images captured using an Olympus IX70 inverted fluorescent microscope, with 10× objective F-view Soft Imaging System camera. Fluorescence intensity, corresponding to the number of live cells was measured with Image J.
Short interference (si)RNA knockdown
Cells were transfected with HIF-1α siRNA or BMPR2 siRNA or scrambled siRNA, and the levels of either CLIC4 or HIF-1α were measured by western blotting and/or by quantitative reverse transcription polymerase chain rwaction 48 hr post-transfection.
HIF-1α stabilization, endothelin-1 (ET-1) and VEGF production
Human osteosarcoma cells stably expressing a luciferase reporter construct under the control of a hypoxia response element (U2OS-HRE-luc)18 were used to assess the effects of CLIC4 on HIF stabilization. Expression of HIF proteins was determined by western blotting and inhibition of HIF-1α expression was achieved by transfecting HPAECs with siRNA (details in the Online Data Supplement). Production of VEGF and other angiogenic factors was studied using Proteome Profiler Human Angiogenesis Array Kit® (R&D Systems) in untreated and CLIC4 overexpressing cells cultured under both normoxic and hypoxic conditions for 24 hr. Potential modulation of VEGF receptor type 2 (VEGFR2) expression was measured using a PathScan® Total VEGFR-2 Sandwich ELISA kit (Cell Signaling). Levels of ET-1 were determined using Human Endothelin-1 QuantiGLO® ELISa kit (R&D Systems).
p65 expression, phosphorylation and NFκB activity
p65 expression and phosphorylation were analysed by western blotting. The antibodies were from Cell Signaling Technology, Denver USA. Adenoviral NFκB luciferase reporter construct (AdNFκB-Luc) and Luciferase Assay System (Promega)19 were used to measure NFκB activity. In some experiments, the NFκB inhibitor, BAY 117085 (10 μM/L; Sigma) was added to the U2OS-HRE-luc cells 24 hr before luciferase assay.
Statistical analysis
All experiments were performed at least in triplicate. Data are presented as the mean±standard error of mean. Comparisons between 2 groups were made using Student’s t-test (except for analysis of plasma CLIC4 levels which was by the Mann-Whitney U-test) while 3 or more groups were compared with ANOVA and Tukey’s post-hoc test using GraphPad Prism version 5.0. All key findings were confirmed by non-parametric statistical analyses (Mann-Whitney U-test or Kruskal-Wallis test as appropriate). Statistical significance was accepted for P<0.05.
RESULTS
Increased CLIC4 levels in plasma and cells from IPAH patients
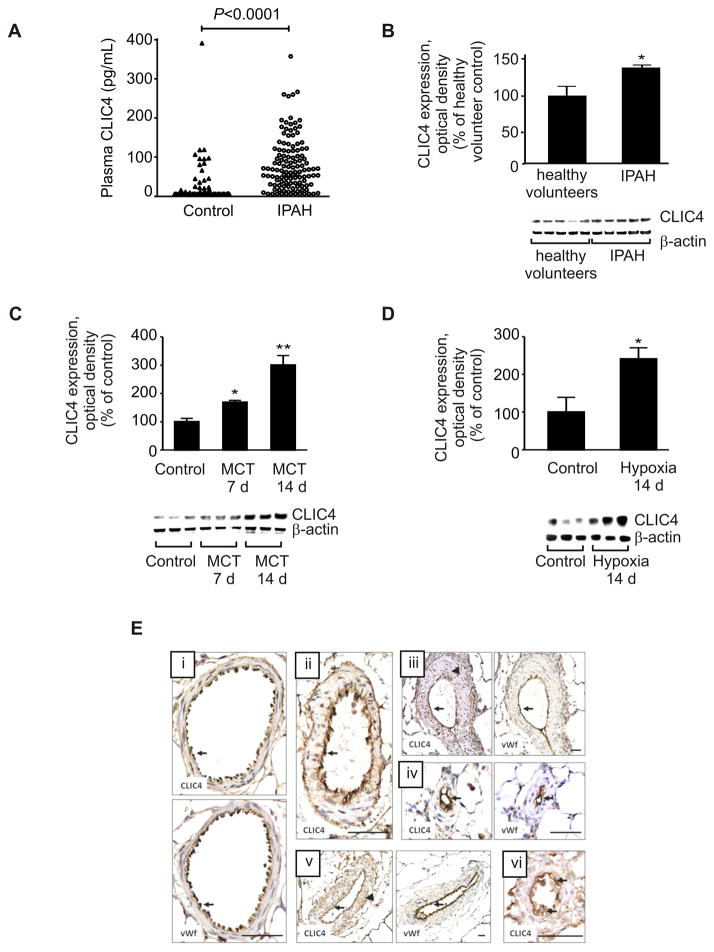
Following on from our previous finding of increased CLIC4 protein expression in lung tissues from PAH patients5, we measured circulating CLIC4 and found that plasma CLIC4 levels were significantly higher in IPAH patients (n=123) compared with healthy volunteers (n=64) (Figure 1A). Levels were elevated across all four WHO functional classes, independent of clinical measures of disease severity.
Figure 1.
CLIC4 protein expression in patient plasma, human ECFCs and rat lung. (A) CLIC4 protein levels in plasma samples from IPAH patients (n=123) and healthy volunteers (n=64). As these data show a skewed distribution they were analysed using the Mann-Whitney U-test. (B) Levels determined in blood-derived endothelial cells from IPAH patients (n=4–5). (C) CLIC4 protein expression in the lungs of rats treated with monocrotaline (MCT) or (D) exposed to hypoxia (n= 3–8). Data are presented as mean±SEM. *P<0.05; **P<0.01, compared with controls. Lung sections from (E i) control, (E ii) hypoxia-, (E iii–iv) MCT and (E v–vi) Sugen 5416/hypoxia-induced pulmonary hypertensive rats, demonstrating co-localisation of CLIC4 and von Willebrand factor (vWf) in the pulmonary endothelium (arrows) of normal and remodelled pulmonary arteries. Arrowheads (E iii, v) indicate CLIC4 immunoreactivity in smooth muscle cells. Bar=50 μm.
There is interest in the role of circulating endothelial progenitor cells in the pathogenesis of pulmonary vascular disease6,20,21. Cells derived from the peripheral blood of IPAH patients and healthy volunteers exhibited similar immunostaining for the endothelial markers, CD31, VE-cadherin and von Willebrand factor and were indistinguishable from mature endothelial cells (Figure S1 in the Online Data Supplement). Flow cytometry also demonstrated that these cells were positive for the endothelial markers CD31 (99.4±0.1%, n=16) and VEGFR-2 (87.1±1.8%, n=16), but negative (<0.1%) for the haematopoietic/monocytic marker CD45 and CD133. Consistent with the findings in lung tissues and plasma samples, CLIC4 protein levels were also significantly elevated in blood-derived endothelial cells from IPAH patients (Figure 1B).
Increased CLIC4 expression in the lungs of pulmonary hypertensive rats
CLIC4 protein was examined in the lungs of rats exposed to monocrotaline, chronic hypoxia and hypoxia-combined with Sugen. To understand better the relationship of CLIC4 to pulmonary hypertension, lung CLIC4 levels were measured during the development of pulmonary hypertension in monocrotaline- and hypoxia-exposed rats. An early increase was detected in both models, before pulmonary hypertension is established (typically 3–4 weeks in the monocrotaline rat22,23 and 2 weeks in the hypoxic rat24 (Figure 1C–D). Further studies in the hypoxic rat showed that this response appears to be lung-specific, as renal and cardiac CLIC4 levels were reduced in hypoxic rats (Figure S2 in the Online Data Supplement). CLIC4 was localized predominantly to the endothelium in the rat pulmonary vasculature (Figure 1E). Sections of lung from all 3 rat models of pulmonary hypertension exhibited characteristic medial wall thickening, distal muscularization and occlusion of pulmonary arteries, with prominent CLIC4 immunostaining of the endothelium, showing a similar distribution to the endothelial marker, von Willebrand factor (Figure 1E ii–vi). Cells in the hypertrophied medial layer of pulmonary arteries displayed weaker and more variable CLIC4 immunostaining (Figure 1E iii, v). Semi-quantitative analysis of immunostained lung sections indicated significantly increased CLIC4 expression in the intimal layer of intrapulmonary arteries in all three animal models of pulmonary hypertension, while the lower levels of CLIC4 immunostaining in the medial layer only increased significantly in the monocrotaline model (Figure S3 in the Online Data Supplement).
CLIC4 knockout mice are protected against development of chronic hypoxia-induced pulmonary hypertension
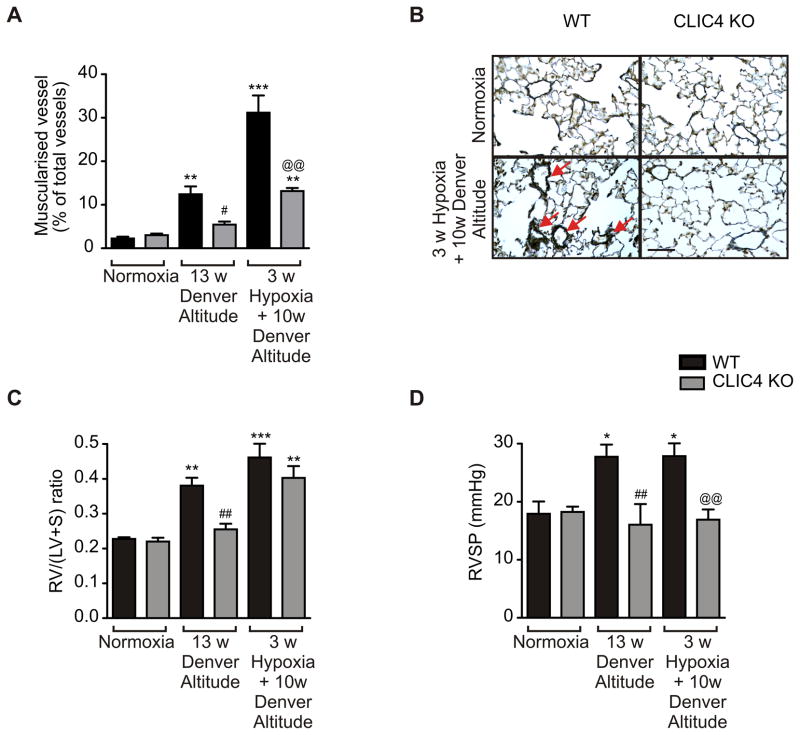
To determine the importance of CLIC4 signalling in vivo, we studied the development of chronic hypoxia-induced pulmonary hypertension in CLIC4 knockout (CLIC4 KO) mice, compared with wildtype (WT) controls. The absence of CLIC4 in CLIC4 KO mice was confirmed by western blotting and immunohistochemical analyses (Figure S4A–B in the Online Data Supplement). Knockout of CLIC4 protein did not alter the expression of the closely related CLIC1 or CLIC5 proteins (Figure S4A in the Online Data Supplement). WT mice kept for 13 weeks at Denver altitude (5280 feet above sea level) developed pulmonary hypertension and vascular remodelling, evident by a significant increase in RVSP, RV/LV+S ratio and vessel muscularization (Figure 2). In contrast, these measurements showed little change in CLIC4 knockout mice maintained under identical conditions. Exposure of these mice to an additional 3 weeks of hypoxia (10 % O2) further increased vessel muscularisation and RV hypertrophy while RVSP remained unchanged. The effects of hypoxia on vessel muscularization, right heart hypertrophy and RSVP were significantly attenuated in CLIC4 KO mice (Figure 2). These results suggest that CLIC4 knockdown is protective in early stages of pulmonary hypertension.
Figure 2.
CLIC4 gene knockout attenuates development of chronic hypoxia-induced pulmonary hypertension. (A) Percentage of muscularized vessels diameter ≤50 μm in lung sections of normoxic and hypoxic wildtype (WT; black bars) and CLIC4 knockout (CLIC4 KO; grey bars) mice. (B) Representative sections showing α-smooth muscle actin staining in normoxic or hypoxic WT or CLIC4 KO mice. Red arrows point to the fully muscularized peripheral artery with double elastic lamina staining. Bar=50 μm. (C) Right ventricular hypertrophy (RV/(LV+S)) and (D) right ventricular systolic pressure (RVSP) were measured in the WT control and CLIC4 KO mice following exposure to normoxia, 13 weeks at Denver altitude or 10 weeks (w) of Denver altitude followed by 3 weeks of hypoxia. *P<0.05, **P<0.01, ***P<0.01, compared to normoxic WT; #P<0.05, ##P<0.01 compared to WT at Denver altitude and @@P<0.01, WT versus CLIC4 KO group at 3w Hypoxia+10w Denver Altitude; (C). In (A–C) n=4–8.
CLIC4 affects HPAEC morphology in vitro
The intracellular localization of CLIC4 is an important determinant of its function15. Soluble, cytoplasmic CLIC4 can localize to the cell membrane where it interacts with cytoskeletal proteins involved in lamellipodia and filopodia formation, while nuclear-targeted CLIC4 promotes apoptosis15. CLIC4 was localised predominantly in the cytoplasm of cultured HPAECs (Figure S5 in the Online Data Supplement), consistent with its distribution in cells in plexiform lesions5. Hypoxia increased CLIC4 protein expression 2-fold, with some translocation of protein to the cell periphery (Figure S5A, B in the Online Data Supplement). Hypoxia-induced increase in CLIC4 expression in endothelial cells was prevented by the inhibitors of intracellular ROS production, DPI and rotenone (Figure S5B in the Online Data Supplement). In contrast, hypoxia did not significantly affect CLIC4 expression in HPASMCs (Figure S6 in the Online Data Supplement). We have also compared the relative levels of CLIC4 protein expression in HPAECs and HPASMCs cultured in normoxic or in hypoxic conditions. The results show that normoxic and hypoxic levels of CLIC4 expression in HPAECs were significantly higher than CLIC4 levels in HPASMCs (Figure S6 in the Online Data Supplement).
To examine the role of CLIC4 in modulating pulmonary endothelial cell phenotype, CLIC4 was overexpressed in HPAECs via adenoviral gene transfer. The CLIC4-overexpressing cells showed enhanced cortical F-actin polymerization and the formation of membrane ruffles, absent in the normal, untreated endothelium (Figure S7A–D in the Online Data Supplement). The morphological effects of CLIC4 overexpression were inhibited in cells co-transfected with CLIC4 shRNA (Figure S7E–F in the Online Data Supplement).
CLIC4 affects hypoxia-induced endothelial permeability
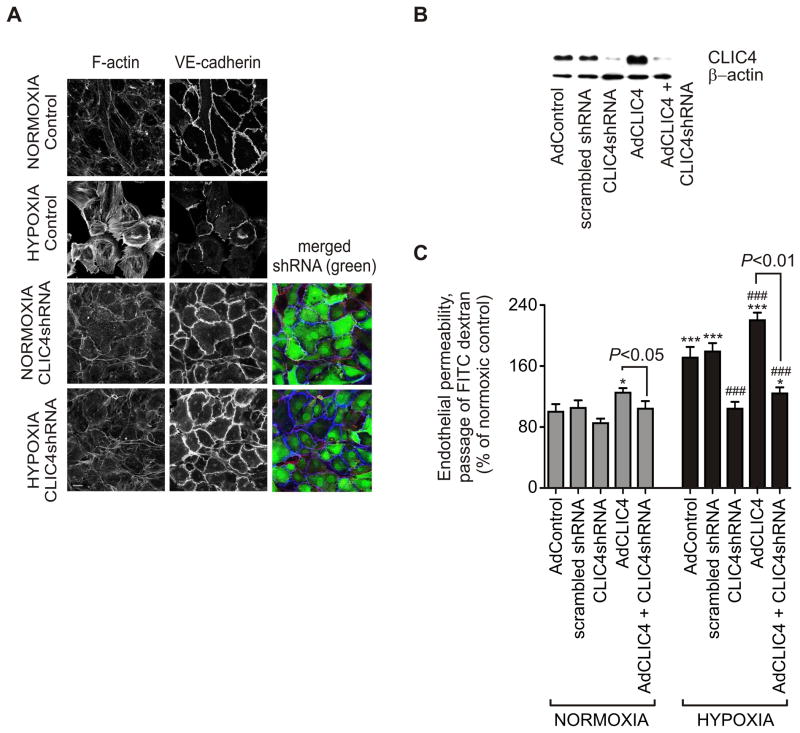
We hypothesised that CLIC4, through association with cytoskeletal proteins and regulation of cell morphology could influence vascular integrity. Increased endothelial permeability is thought to be an early event in the pathogenesis of pulmonary hypertension, permitting growth factors access to cells in the vascular wall. Hypoxia affected both HPAEC cell morphology and permeability (Figure 3A, B). In confluent cells under normal oxygen tension, F-actin was localized mainly at the cell periphery together with vascular endothelial (VE)-cadherin (Figure 3A), a major component of adherence junctions16. Changes in CLIC4 protein levels following adenoviral infection were confirmed by western blotting (Figure 3B). Hypoxia (24 hr) induced the formation of actomyosin filaments and disruption of intercellular junctions and these changes were accompanied by a 1.7-fold increase in endothelial permeability (Figures 3A, C). Overexpression of CLIC4 compromised endothelial barrier function under normoxic conditions and further increased hypoxia-induced permeability (Figure 3C). Conversely, the effects of hypoxia were inhibited by CLIC4 shRNA (Figures 3A, C).
Figure 3.
Hypoxia-induced changes in human pulmonary endothelial phenotype and permeability. (A) Images showing the organization of F-actin and VE-cadherin in confluent HPAECs under normoxic or hypoxic (24 hr) conditions. The cells were left untreated or overexpressed CLIC4 shRNA, as indicated. Merged images show F-actin (red), VE-cadherin (blue) and GFP co-expressed with CLIC4 shRNA (green). Bar=10 μm. (B) The adenoviral manipulation of CLIC4 expression was confirmed by western blot analysis of cell extracts. (C) Pulmonary endothelial cell permeability in normoxic and hypoxic cells expressing scrambled shRNA, CLIC4 shRNA or CLIC4, as indicated; In (C) data are expressed as % of control and each bar represents mean±SEM (n=4). *P<0.05; ***P<0.001, compared with normoxic AdControl; ###P<0.001, compared with hypoxic AdControl. Comparisons between AdCLIC4 and CLIC4 shRNA + AdCLIC4 are also indicated.
CLIC4 expression influences HPAEC metabolic activity, cell survival and angiogenesis in vitro
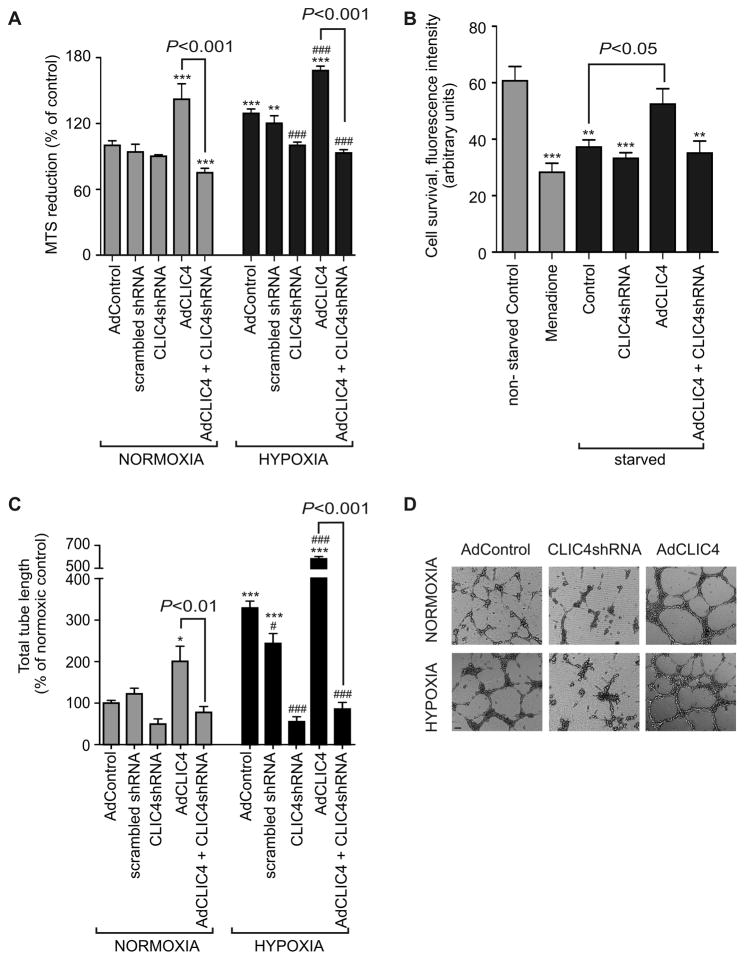
The reduction of MTS tertazolium compound into coloured formazan measures the metabolic activity of viable cells, which increases with cell proliferation and migration, irrespective of the presence of oxidative respiration25. Overexpression of CLIC4 increased cell metabolic activity in both normoxic and hypoxic conditions (Figure 4A). Endogenous CLIC4 expression was essential for hypoxia-induced cell activation and was inhibited by treatment with CLIC4 shRNA (Figure 4A). CLIC4 overexpression significantly improved survival of HPAECs cultured in media deprived of serum and growth factors (Figure 4B), indicating its possible role in the emergence of apoptosis-resistant endothelial cells in the distal pulmonary vasculature.
Figure 4.
Effects of CLIC4 expression on HPAEC metabolic activity, cell survival and angiogenesis. (A) Cell metabolic activity under normoxic and hypoxic conditions and following overexpression of CLIC4, scrambled shRNA (shRNA control) or CLIC4 shRNA, as indicated; MTS reduction assay. (B) HPAEC viability in cells cultured in full media, treated with apoptosis-inducer, menadione (6 hr, 10 μM) or serum-starved for 48 hr. The cells were overexpressing CLIC4 or CLIC4 and CLIC4 shRNA, as indicated. (C) Changes in total endothelial tube length (expressed as % of normoxic control) in cells treated, as indicated. (D) Representative images showing the effects AdCLIC4 and AdCLIC4 shRNA on tube formation in normoxic and hypoxic HPAECs. Bar=500 μm. In (A–C) each bar represents mean±SEM; (n=4–5). *P<0.05, **P<0.01, ***P<0.001, compared with normoxic control (A–C) or non-starved control (B); ###P<0.001, compared with hypoxic AdControl (A, C). Comparisons between AdCLIC4 and AdCLIC4 + CLIC4 shRNA groups are also indicated.
Hypoxia, a known stimulus of angiogenesis, induced a 3-fold increase in tube formation by HPAECs in Matrigel, compared with normoxic controls. Overexpression of CLIC4 increased tube formation in both normoxic (2-fold) and hypoxic (5.8-fold) conditions compared to controls. The effects of hypoxia and CLIC4 overexpression were prevented by CLIC4 shRNA (Figure 4C, D).
CLIC4 expression is not affected by BMPR2 knockdown
Reduction in BMPR2 signaling is associated with the pathogenesis of PAH2. We used BMPR2 siRNA to inhibit expression of the receptor in both HPAECs and blood-derived endothelial cells but found no change in CLIC4 protein levels in either cell type, despite a marked reduction in BMPR2 mRNA and protein levels (Figure S8 in the Online Data Supplement).
CLIC4 stabilises HIF-1α and increases production of ET-1 and VEGF in HPAECs
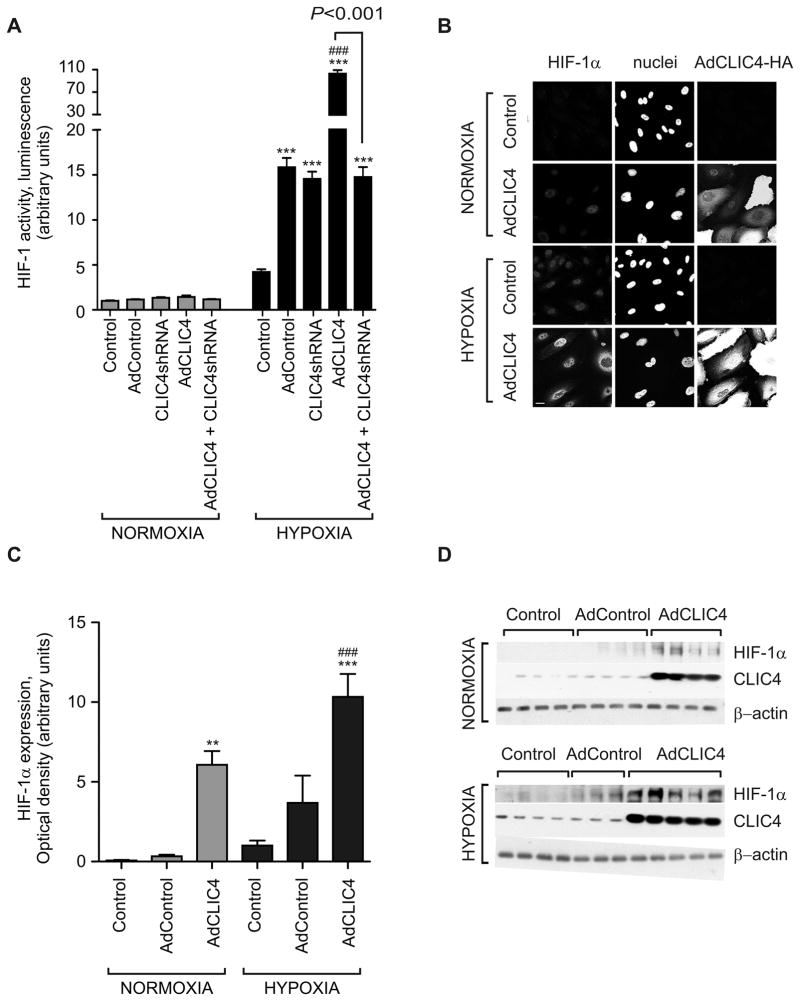
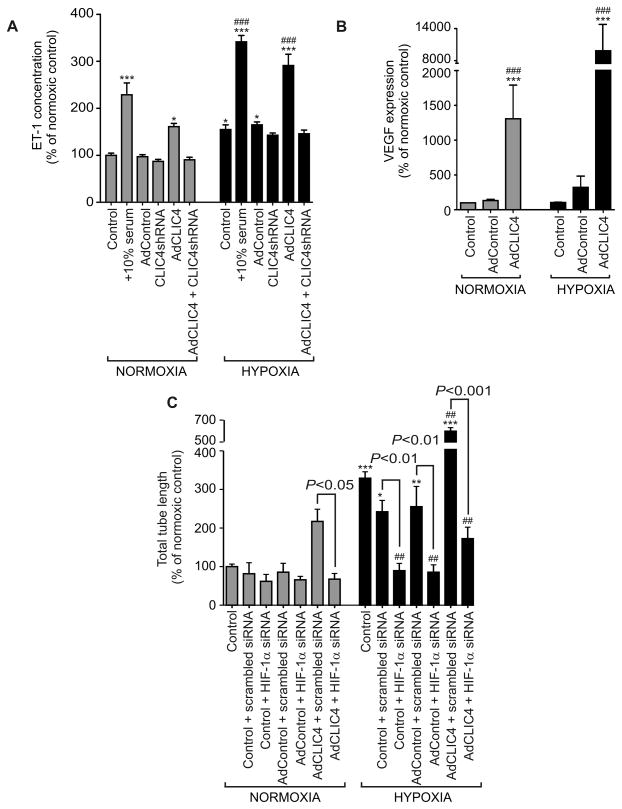
Increased HIF-1α stabilization and activity has been reported in the pulmonary vasculature and endothelial cells isolated from patients with IPAH26. Knockout of the murine CLIC4 is associated with a reduction of ischemia-induced HIF-1α signalling4. We postulated that the effects of CLIC4 in HPAECs may reflect, at least in part, modulation of HIF-1α activity and expression. To measure changes in HIF activation, we used the human U2OS-HRE-luc cell line, which had been optimised and validated for the use in high-throughput screens of HIF inhibitors and studies on hypoxia-induced HIF activation18. Overexpression of CLIC4 led to a marked (30-fold) increase in U2OS luciferase activity under hypoxic conditions, which was reduced to adenovirus control levels by pre-treatment with CLIC4 shRNA (Figure 5A). In keeping with a role for CLIC4 in the regulation of HIF stabilization, overexpression of CLIC4 was accompanied by the nuclear translocation of HIF-1α in HPAECs (Figures 5B) and a marked increase of HIF-1α protein levels (Figure 5C). Consistent with the changes in HIF-1 activity, CLIC4 increased production of ET-1 in cells, while CLIC4 shRNA had an inhibitory effect (Figure 6A). The overexpression of CLIC4 was also associated with increased VEGF production in HPAECs (Figure 6B and Figure S9A in the Online Data Supplement), with no apparent change in VEGFR-2 levels (Figure S9B in the Online Data Supplement). VEGF function-blocking antibodies prevented the CLIC4-induced increase in endothelial permeability, implicating secreted VEGF as a critical mediator of CLIC4-induced changes in endothelial junctional integrity (Figure S9C). siRNA-mediated knockdown of HIF-1α (Figure S10A in the Online Data Supplement) completely prevented the pro-angiogenic effects of CLIC4 (Figures 6C), reinforcing the concept of HIF-1α acting as a downstream mediator of CLIC4 effects. Consistent with the postulated role for CLIC4 in the regulation of HIF stability, CLIC4 knockout mice showed reduced levels of HIF-1α (Figure S10B in the Online Data Supplement). Interestingly, the levels of HIF-2α in these mice were also reduced, suggesting common regulatory mechanisms (Figure S10B in the Online Data Supplement).
Figure 5.
CLIC4 regulates endothelial HIF activity and stabilization. Overexpression of CLIC4 (24 hr) or exposure to hypoxia (A) stabilizes HIF in U2OS-HRE-luc cells, luciferase reporter assay (n=12); (B) increases nuclear translocation of HIF-1α and (C) increases HIF-1α levels in HPAECs (n=4–5). Each bar represents mean±SEM ***P<0.001, **P<0.01, compared with normoxic AdControl; ###P<0.001, compared with hypoxic AdControl. Comparisons between AdCLIC4 and CLIC4 shRNA + AdCLIC4, are also indicated
Figure 6.
The effect of CLIC4 on HIF-dependent protein expression and tube formation in HPAECs. Production of (A) ET-1 and (B) VEGF in normoxic and hypoxic HPAECs overexpressing CLIC4 or CLIC4 shRNA (n=3–5). (C) HPAEC tube formation following siRNA-mediated HIF-1α knockdown in cells treated, as indicated (n=5). Each bar represents mean±SEM. *P<0.05; **P<0.01, ***P<0.001 compared with normoxic AdControl; ##P<0.01, ###P<0.001 compared with hypoxic AdControl. Comparisons between AdCLIC4 and CLIC4 shRNA or between scrambled siRNA and HIF-1α siRNA groups are also indicated.
HIF-1α and tube formation are dysregulated in blood-derived endothelial cells from IPAH patients
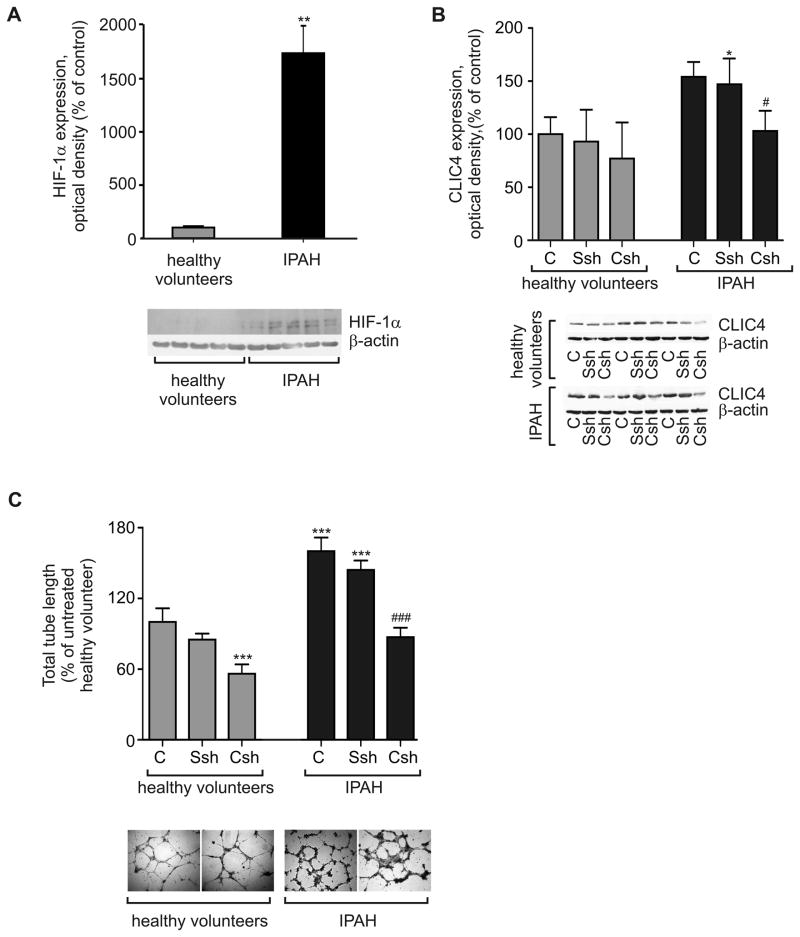
Cells derived from circulating progenitor cells in IPAH patients showed significantly higher levels of CLIC4 and HIF-1α proteins, compared with cells isolated from healthy volunteers (Figure 7A–B). In keeping with the proposed pro-angiogenic role of CLIC4, endothelial cells from IPAH patients also showed an enhanced capacity to form endothelial tubes in Matrigel. Transfection with CLIC4 shRNA reduced CLIC4 expression and tube formation to a level comparable to that seen with cells derived from healthy volunteers (Figures 7C).
Figure 7.
HIF-1α levels and tube formation in blood-derived endothelial cells. (A) Blood-derived endothelial cells from IPAH patients showed greater protein expression of HIF-1α compared to healthy controls. (B) CLIC4 shRNA (CSh; 48h) reduces CLIC4 levels in IPAH cells to control levels, while scrambled shRNA (Ssh) has no effect. (C) CLIC4 shRNA inhibits tube formation in IPAH endothelial cells. Each bar represents mean±SEM (n=3–4). Representative images of tube formation in healthy and IPAH cells are shown below the graph; Bar=100 μm. In (A–D) *P<0.05, **P<0.01, ***P<0.001, compared with untreated cells derived from healthy volunteers. #P<0.05, ###P<0.01 comparison with untreated IPAH cells.
CLIC4-induced activation of NFκB is required for HIF activation
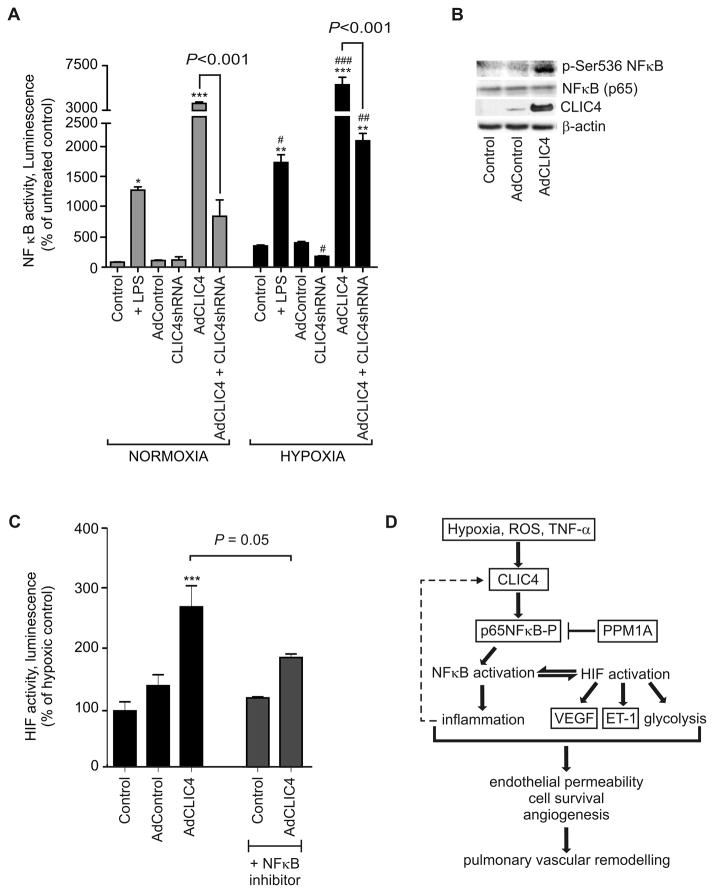
The pro-inflammatory transcription factor NFκB is a known inducer of HIF activity27. Using affinity purification followed by liquid chromatography and tandem mass spectrometry (LC-MS/MS) analyses (data not shown), the approach employed to identify CLIC4 in human lung5, we identified a p65 subunit of NFκB (RelA) as one of the proteins interacting with CLIC4. Phosphorylation of p65 facilitates nuclear translocation and transcriptional activity of NFκB28 and we hypothesised that CLIC4 overexpression may affect NFκB activity.
CLIC4 overexpression in HPAECs significantly increased NFκB activity in normoxic and in hypoxic conditions (Figure 8A). NFκB activation in CLIC4 overexpressing cells was accompanied by increased phosphorylation of p65 on Ser536 (Figure 8B), which is known to facilitate nuclear translocation and transcriptional activation of NFκB25. Consistent with the postulated role of CLIC4/NFκB in the regulation of HIF activity, treatment with an NFκB inhibitor, BAY-117085, attenuated CLIC4-induced HIF activation in hypoxic HPAECs (Figure 8C) – suggesting NFκB as an upstream regulator of HIF in the CLIC4 signalling pathway.
Figure 8.
CLIC4 regulates NFκB activity in HPAECs. (A) NFκB activity in HPAECs treated with lipopolysacharide (LPS), control adenovirus (AdControl), AdCLIC4 or AdCLIC4 shRNA in normoxic (grey bars) or hypoxic (black bars) conditions, as indicated; luciferase reporter assay. (B) CLIC4 increases phosphorylation of p65 on Serine 536, western blotting. (C) NFκB inhibitor, BAY 117085 prevents HIF activation in CLIC4- overexpressing HPAECs (30 hr overexpression) in hypoxia. n=4; *P<0.05; **P<0.01, ***P<0.001, compared with untreated controls; #P<0.05, ##P <0.01, ###P <0.001, comparison with hypoxic controls. (D) The proposed CLIC4 signalling pathway.
DISCUSSION
This study demonstrates a critical role for CLIC4 in the regulation of pulmonary endothelial function and vascular homeostasis. Increased CLIC4 levels were observed in plasma and blood-derived endothelial cells from IPAH patients and in the vascular endothelium of three rodent models of pulmonary hypertension. CLIC4 gene knockout in mice attenuated the development of chronic hypoxia-induced pulmonary hypertension. Overexpression of CLIC4 in HPAECs increased endothelial permeability and influenced their survival and angiogenic capacity, these effects being dependent in part on the stabilisation of HIF-1α. Selective inhibition of CLIC4 expression in endothelial cells derived from circulating progenitors from IPAH patients inhibited the aberrant tube formation characteristic of these cells. The mechanism of CLIC4-induced effects involves p65-dependent NFκB activation followed by stabilisation of HIF. Coupled with our previous observation of increased CLIC4 expression in the remodelled pulmonary vasculature of PAH patients5, our data provide compelling evidence implicating CLIC4 in the pathobiology of the disease.
As in IPAH lungs, increased CLIC4 expression was observed most consistently in the endothelium of the pulmonary vasculature of all 3 rat models of pulmonary hypertension - chronic exposure to hypoxia, monocrotaline and the combination of Sugen 5416 and hypoxia, a model that exhibits similarities to the pathological features of advanced human IPAH14. Nonetheless, while the endothelium appears to be a predominant site of CLIC4 protein expression in the hypertensive human and animal lung, we cannot exclude the possibility that CLIC4 may also affect other cell types. We observed lower levels of CLIC4 immunostaining in the pulmonary vascular medial layer, but this was increased in the vessels of monocrotaline-treated rats and CLIC4 has been implicated in the migratory responses of cultured HPASMCs downstream of BMPRII/BMP-2 signaling10.
The endothelial layer of the pulmonary vasculature acts as a barrier to the underlying tissue, limiting the influx of inflammatory cells, growth factors and cytokines. Changes in vascular endothelial permeability are thought to occur early in the pathogenesis of pulmonary hypertension, both in animal models and human PAH29,30. Consistent with a role for CLIC4 in compromising endothelial integrity early in the disease, we found that CLIC4 expression is increased (by 7 days) in both the monocrotaline and hypoxia-induced rat models of pulmonary hypertension, before pulmonary hypertension and vascular remodelling is fully established in these models (3–4 weeks for the monocrotaline rat and around 2 weeks for the hypoxic rat).
In contrast to the lung, CLIC4 levels were reduced in renal and myocardial tissues of chronically hypoxic animals. This suggests an organ-specific response to hypoxia and the differential regulation of CLIC4 expression in pulmonary and systemic vascular beds. The potential drivers of increased CLIC4 expression in the lung in pulmonary hypertension include oxidative stress, growth factors and pro-inflammatory cytokines (e.g. TNF-α and TGF-β)4,8,26,31–33. Hypoxia-response element consensus sequences have also been identified in the CLIC4 gene and may be a regulatory mechanism contributing to increased CLIC4 expression in ischemia or hypoxic conditions4. Our preliminary observations suggest that ROS generation may play a role in the regulation of CLIC4 expression in pulmonary endothelium, consistent with observations in ovarian cancer8. The inhibitor of NADPH-oxidase-induced ROS formation, DPI, and the inhibitor of mitochondrial ROS, rotenone, both attenuated the hypoxia-induced increase in CLIC4 expression in HPAECs.
As heterozygous germline BMPR2 mutations contribute to heritable forms of PAH34 and down regulation of BMP signalling has been implicated in the development of experimental pulmonary hypertension35, we examined whether BMPR2 expression influenced CLIC4 protein levels. Despite a marked suppression of BMPR2 mRNA and protein expression, we found no significant change in CLIC4 protein levels in either HPAECs or blood-derived endothelial cells. This is consistent with the results of an earlier study on the regulation of CLIC4 mRNA expression in isolated smooth muscle cells10 and suggests that the enhanced expression of CLIC4 found in endothelial cells from IPAH patients5 and lungs from rats with pulmonary hypertension is not a direct manifestation of altered BMPR2 expression.
In addition to increasing permeability, CLIC4-induced endothelial junctional remodelling is an important step in angiogenesis, as breaking off inter-endothelial junctions in activated endothelium is a prerequisite for endothelial cell migration and new vessel formation. Key mediators of the effects of increased CLIC4 expression are HIF and angiogenic factors, such as VEGF. Hypoxia-inducible transcription factors have been implicated in both the development of experimental pulmonary hypertension and the altered endothelial cell phenotype found in IPAH patients26. HIF-1α regulates release of pro-angiogenic factors such as VEGF30 and vasoactive mediators such as ET-136, both of which are elevated in PAH30,37. HIF-1α has also been reported to mediate the effects of hypoxia on endothelial permeability38. We found that CLIC4 overexpression in HPAECs led to HIF activation in normoxia and to a marked increase in HIF activity under hypoxic conditions. This was accompanied by nuclear translocation of HIF-1α and increased VEGF and ET-1 production, while VEGF inhibition reduced the barrier-compromising effects of CLIC4. Consistent with these observations, CLIC4 knockout mice show impaired ischemia-induced expression of HIF-1α, VEGF and angiopoetin-24. We cannot exclude the possibility that HIF-2 may also play a role. HIF-2 is induced by hypoxia and regulates endothelial cell proliferation and angiogenesis39,40 and we observed a reduction in HIF-2 expression in CLIC4 knockout mice, contemporaneous with the reduction in HIF-1 expression.
Finally, we identified the p65 subunit of the pro-inflammatory transcription factor NFκB as one of the CLIC4-interacting proteins in endothelial cells. Phosphorylation of p65 on serine residues S536 and Ser276 is critical for full activation of NFκB28. CLIC4 competes with phosphatase PPM1A, known to de-phosphorylate p65 and other signalling proteins, including Smad 2/341. We observed significant activation of NFκB following overexpression of CLIC4, which correlated with increased phosphorylation of p65 on Ser536. More importantly, the NFκB inhibitor BAY117085, known to inhibit nuclear translocation of p65 in endothelial cells42, significantly attenuated CLIC4-induced activation of HIF. It is well documented that NFκB regulates HIF activity in a variety of pathological states27. These findings suggest NFκB acts as an upstream activator of HIF in the CLIC4 signalling pathway. A proposed CLIC4 signalling pathway is shown in Figure 8D.
CONCLUSION
Pulmonary endothelial CLIC4 is up-regulated in human PAH and experimental models of pulmonary hypertension. CLIC4 regulates the activity of two key transcription factors, NFκB and HIF-1, that drive endothelial responses to inflammatory and angiogenic stimuli. CLIC4 inhibition serves to maintain pulmonary endothelial function under hypoxic stress and attenuates the development of pulmonary hypertension in the chronically hypoxic mouse. Approaches that reverse increased pulmonary endothelial CLIC4 expression may be beneficial in patients with PAH.
Supplementary Material
Acknowledgments
We thank the staff of the NIHR/Wellcome Trust-Imperial Clinical Research Facility, Hammersmith Hospital (London UK) for their help and support and Dr Margaret Ashcroft (University College London, UK) and the Institute for Cancer Research, London, UK for the use of U2OS cells.
Funding Sources: The clinical research was supported by the National Institute for Health Research (NIHR) Imperial Clinical Research Facility. This research was supported by a PhD studentship (KHL), a project grant (PG 11/13/28765) from the British Heart Foundation, a Young Investigator Award from Pfizer UK (VBA-S) and an NIH Grant 5RO1HL92131 (JCE).
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Littler DR, Harrop SJ, Goodchild SC, Phang JM, Mynott AV, Jiang L, Valenzuela SM, Mazzanti M, Brown LJ, Breit SN, Curmi PM. The enigma of the CLIC proteins: Ion channels, redox proteins, enzymes, scaffolding proteins? FEBS Lett. 2010;584:2093–2101. doi: 10.1016/j.febslet.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 2.Bohman S, Matsumoto T, Suh K, Dimberg A, Jakobsson L, Yuspa S, Claesson-Welsh L. Proteomic analysis of vascular endothelial growth factor-induced endothelial cell differentiation reveals a role for chloride intracellular channel 4 (CLIC4) in tubular morphogenesis. J Biol Chem. 2005;280:42397–42404. doi: 10.1074/jbc.M506724200. [DOI] [PubMed] [Google Scholar]
- 3.Tung JJ, Hobert O, Berryman M, Kitajewski J. Chloride intracellular channel 4 is involved in endothelial proliferation and morphogenesis in vitro. Angiogenesis. 2009;12:209–220. doi: 10.1007/s10456-009-9139-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chalothorn D, Zhang H, Smith JE, Edwards JC, Faber JE. Chloride intracellular channel-4 is a determinant of native collateral formation in skeletal muscle and brain. Circ Res. 2009;105:89–98. doi: 10.1161/CIRCRESAHA.109.197145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdul-Salam VB, Wharton J, Cupitt J, Berryman M, Edwards RJ, Wilkins MR. Proteomic analysis of lung tissues from patients with pulmonary arterial hypertension. Circulation. 2010;122:2058–2067. doi: 10.1161/CIRCULATIONAHA.110.972745. [DOI] [PubMed] [Google Scholar]
- 6.Tuder RM, Voelkel NF. Angiogenesis and pulmonary hypertension: A unique process in a unique disease. Antioxid Redox Signal. 2002;4:833–843. doi: 10.1089/152308602760598990. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez-Salas E, Sagar M, Cheng C, Yuspa SH, Weinberg WC. P53 and tumor necrosis factor alpha regulate the expression of a mitochondrial chloride channel protein. J Biol Chem. 1999;274:36488–36497. doi: 10.1074/jbc.274.51.36488. [DOI] [PubMed] [Google Scholar]
- 8.Yao Q, Qu X, Yang Q, Wei M, Kong B. Clic4 mediates tgf-beta1-induced fibroblast-to-myofibroblast transdifferentiation in ovarian cancer. Oncol Rep. 2009;22:541–548. doi: 10.3892/or_00000469. [DOI] [PubMed] [Google Scholar]
- 9.Suh KS, Mutoh M, Gerdes M, Yuspa SH. Clic4, an intracellular chloride channel protein, is a novel molecular target for cancer therapy. J Investig Dermatol Symp Proc. 2005;10:105–109. doi: 10.1111/j.1087-0024.2005.200402.x. [DOI] [PubMed] [Google Scholar]
- 10.Spiekerkoetter E, Guignabert C, de Jesus Perez V, Alastalo TP, Powers JM, Wang L, Lawrie A, Ambartsumian N, Schmidt AM, Berryman M, Ashley RH, Rabinovitch M. S100a4 and bone morphogenetic protein-2 codependently induce vascular smooth muscle cell migration via phospho-extracellular signal-regulated kinase and chloride intracellular channel 4. Circ Res. 2009;105:639–647. doi: 10.1161/CIRCRESAHA.109.205120. 613 p following 647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 13.Ulmasov B, Bruno J, Gordon N, Hartnett ME, Edwards JC. Chloride intracellular channel protein-4 functions in angiogenesis by supporting acidification of vacuoles along the intracellular tubulogenic pathway. Am J Pathol. 2009;174:1084–1096. doi: 10.2353/ajpath.2009.080625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abe K, Toba M, Alzoubi A, Ito M, Fagan KA, Cool CD, Voelkel NF, McMurtry IF, Oka M. Formation of plexiform lesions in experimental severe pulmonary arterial hypertension. Circulation. 2010;121:2747–2754. doi: 10.1161/CIRCULATIONAHA.109.927681. [DOI] [PubMed] [Google Scholar]
- 15.Suh KS, Mutoh M, Nagashima K, Fernandez-Salas E, Edwards LE, Hayes DD, Crutchley JM, Marin KG, Dumont RA, Levy JM, Cheng C, Garfield S, Yuspa SH. The organellular chloride channel protein CLIC4/mtclic translocates to the nucleus in response to cellular stress and accelerates apoptosis. J Biol Chem. 2004;279:4632–4641. doi: 10.1074/jbc.M311632200. [DOI] [PubMed] [Google Scholar]
- 16.Wojciak-Stothard B, Tsang LY, Haworth SG. Rac and rho play opposing roles in the regulation of hypoxia/reoxygenation-induced permeability changes in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;288:L749–760. doi: 10.1152/ajplung.00361.2004. [DOI] [PubMed] [Google Scholar]
- 17.Fiedler LR, Bachetti T, Leiper J, Zachary I, Chen L, Renne T, Wojciak-Stothard B. The ADMA/DDAH pathway regulates vegf-mediated angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:2117–2124. doi: 10.1161/ATVBAHA.109.194035. [DOI] [PubMed] [Google Scholar]
- 18.Chau NM, Rogers P, Aherne W, Carroll V, Collins I, McDonald E, Workman P, Ashcroft M. Identification of novel small molecule inhibitors of hypoxia-inducible factor-1 that differentially block hypoxia-inducible factor-1 activity and hypoxia-inducible factor-1alpha induction in response to hypoxic stress and growth factors. Cancer Res. 2005;65:4918–4928. doi: 10.1158/0008-5472.CAN-04-4453. [DOI] [PubMed] [Google Scholar]
- 19.Wojciak-Stothard B, Haworth SG. Perinatal changes in pulmonary vascular endothelial function. Pharmacol Ther. 2006;109:78–91. doi: 10.1016/j.pharmthera.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 20.Toshner M, Voswinckel R, Southwood M, Al-Lamki R, Howard LS, Marchesan D, Yang J, Suntharalingam J, Soon E, Exley A, Stewart S, Hecker M, Zhu Z, Gehling U, Seeger W, Pepke-Zaba J, Morrell NW. Evidence of dysfunction of endothelial progenitors in pulmonary arterial hypertension. Am J Resp Crit Care Med. 2009;180:780–787. doi: 10.1164/rccm.200810-1662OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diller GP, van Eijl S, Okonko DO, Howard LS, Ali O, Thum T, Wort SJ, Bedard E, Gibbs JS, Bauersachs J, Hobbs AJ, Wilkins MR, Gatzoulis MA, Wharton J. Circulating endothelial progenitor cells in patients with eisenmenger syndrome and idiopathic pulmonary arterial hypertension. Circulation. 2008;117:3020–3030. doi: 10.1161/CIRCULATIONAHA.108.769646. [DOI] [PubMed] [Google Scholar]
- 22.Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, Sydykov A, Lai YJ, Weissmann N, Seeger W, Grimminger F. Reversal of experimental pulmonary hypertension by pdgf inhibition. J Clin Invest. 2005;115:2811–2821. doi: 10.1172/JCI24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hess P, Clozel M, Clozel JP. Telemetry monitoring of pulmonary arterial pressure in freely moving rats. J Appl Physiol. 1996;81:1027–1032. doi: 10.1152/jappl.1996.81.2.1027. [DOI] [PubMed] [Google Scholar]
- 24.Sebkhi A, Strange JW, Phillips SC, Wharton J, Wilkins MR. Phosphodiesterase type 5 as a target for the treatment of hypoxia-induced pulmonary hypertension. Circulation. 2003;107:3230–3235. doi: 10.1161/01.CIR.0000074226.20466.B1. [DOI] [PubMed] [Google Scholar]
- 25.Berridge MV, Herst PM, Tan AS. Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction. Biotechnol Annu Rev. 2005;11:127–152. doi: 10.1016/S1387-2656(05)11004-7. [DOI] [PubMed] [Google Scholar]
- 26.Fijalkowska I, Xu W, Comhair SA, Janocha AJ, Mavrakis LA, Krishnamachary B, Zhen L, Mao T, Richter A, Erzurum SC, Tuder RM. HIF1α regulates the metabolic shift of pulmonary hypertensive endothelial cells. Am J Pathol. 2010;176:1130–1138. doi: 10.2353/ajpath.2010.090832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorlach A, Bonello S. The cross-talk between NFKβ and HIF-1: Further evidence for a significant liaison. Biochem J. 2008;412:e17–19. doi: 10.1042/BJ20080920. [DOI] [PubMed] [Google Scholar]
- 28.Lu X, An H, Jin R, Zou M, Guo Y, Su PF, Liu D, Shyr Y, Yarbrough WG. PPM1A is a Rel1A phosphatase with tumor suppressor-like activity. Oncogene. 2013 Jul 1; doi: 10.1038/onc.2013.246. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burton VJ, Ciuclan LI, Holmes AM, Rodman DM, Walker C, Budd DC. Bone morphogenetic protein receptor II regulates pulmonary artery endothelial cell barrier function. Blood. 2011;117:333–341. doi: 10.1182/blood-2010-05-285973. [DOI] [PubMed] [Google Scholar]
- 30.Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation. 2004;109:159–165. doi: 10.1161/01.CIR.0000102381.57477.50. [DOI] [PubMed] [Google Scholar]
- 31.Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF. Animal models of pulmonary arterial hypertension: The hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1013–1032. doi: 10.1152/ajplung.00217.2009. [DOI] [PubMed] [Google Scholar]
- 32.Bowers R, Cool C, Murphy RC, Tuder RM, Hopken MW, Flores SC, Voelkel NF. Oxidative stress in severe pulmonary hypertension. Am J Resp Crit Care Med. 2004;169:764–769. doi: 10.1164/rccm.200301-147OC. [DOI] [PubMed] [Google Scholar]
- 33.Burke DL, Frid MG, Kunrath CL, Karoor V, Anwar A, Wagner BD, Strassheim D, Stenmark KR. Sustained hypoxia promotes the development of a pulmonary artery-specific chronic inflammatory microenvironment. Am J Physiol Lung Cell Mol Physiol. 2009;297:L238–250. doi: 10.1152/ajplung.90591.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Machado RD, Eickelberg O, Elliott CG, Geraci MW, Hanaoka M, Loyd JE, Newman JH, Phillips JA, 3rd, Soubrier F, Trembath RC, Chung WK. Genetics and genomics of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S32–42. doi: 10.1016/j.jacc.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morty RE, Nejman B, Kwapiszewska G, Hecker M, Zakrzewicz A, Kouri FM, Peters DM, Dumitrascu R, Seeger W, Knaus P, Schermuly RT, Eickelberg O. Dysregulated bone morphogenetic protein signaling in monocrotaline-induced pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol. 2007;27:1072–1078. doi: 10.1161/ATVBAHA.107.141200. [DOI] [PubMed] [Google Scholar]
- 36.Kourembanas S, Marsden PA, McQuillan LP, Faller DV. Hypoxia induces endothelin gene expression and secretion in cultured human endothelium. J Clin Invest. 1991;88:1054–1057. doi: 10.1172/JCI115367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tuder RM, Chacon M, Alger L, Wang J, Taraseviciene-Stewart L, Kasahara Y, Cool CD, Bishop AE, Geraci M, Semenza GL, Yacoub M, Polak JM, Voelkel NF. Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: Evidence for a process of disordered angiogenesis. J Pathol. 2001;195:367–374. doi: 10.1002/path.953. [DOI] [PubMed] [Google Scholar]
- 38.Yeh WL, Lu DY, Lin CJ, Liou HC, Fu WM. Inhibition of hypoxia-induced increase of blood-brain barrier permeability by yc-1 through the antagonism of HIF1α accumulation and vegf expression. Mol Pharmacol. 2007;72:440–449. doi: 10.1124/mol.107.036418. [DOI] [PubMed] [Google Scholar]
- 39.Skuli N, Simon MC. Hif-1alpha versus hif-2alpha in endothelial cells and vascular functions: Is there a master in angiogenesis regulation? Cell cycle. 2009;8:3252–3253. doi: 10.4161/cc.8.20.9618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skuli N, Liu L, Runge A, Wang T, Yuan L, Patel S, Iruela-Arispe L, Simon MC, Keith B. Endothelial deletion of hypoxia-inducible factor-2alpha (HIF2α) alters vascular function and tumor angiogenesis. Blood. 2009;114:469–477. doi: 10.1182/blood-2008-12-193581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shukla A, Yuspa SH. CLIC4 and Schnurri-2: A dynamic duo in TGFβ signaling with broader implications in cellular homeostasis and disease. Nucleus. 2010;1:144–149. doi: 10.4161/nucl.1.2.10920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu X, Janssen WE, Moscinski LC, Bryington M, Dangsupa A, Rezai-Zadeh N, Babbin BA, Zuckerman KS. An IKβ inhibitor causes leukemia cell death through a p38 MAP-Kinase-dependent, NFKβ-independent mechanism. Cancer Res. 2001;61:6290–6296. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.