Abstract
Understanding the neurochemical composition of the enteric nervous system (ENS) is critical for elucidating neurological function in the gastrointestinal (GI) tract in health and disease. Despite their status as the closest models of human neurological systems, relatively little is known about enteric neurochemistry in nonhuman primates. We describe neurochemical coding of the enteric nervous system, specifically the myenteric plexus, of the rhesus monkey (Macaca mulatta) by immunohistochemistry and directly compare it to human tissues. There are considerable differences in the myenteric plexus along different segments of the monkey GI tract. While acetylcholine neurons make up the majority of myenteric neurons in the stomach (70%), they are a minority in the rectum (47%). Conversely, only 22% of gastric myenteric neurons express nitric oxide synthase (NOS) compared to 52% in the rectum. Vasoactive intestinal peptide (VIP) is more prominent in the stomach (37%) versus the rest of the GI tract (10%), and catecholamine neurons are rare (1%). There is significant coexpression of NOS and VIP in myenteric neurons that is more prominent in the proximal GI tract. Taken as a whole, these data provide insight into the neurochemical anatomy underlying GI motility. While overall similarity to other mammalian species is clear, there are some notable differences between the ENS of rhesus monkeys, humans, and other species that will be important to take into account when evaluating models of human diseases in animals.
Keywords: enteric, gastrointestinal, nitric oxide, vasoactive intestinal peptide, catecholamine, acetylcholine
Gastrointestinal (GI) function is controlled in large part by a semiautonomous complex network of neurons embedded in the wall of the GI tract called the enteric nervous system (ENS). The ENS is comprised mainly of two parts: an inner plexus (Meissner's or submucosal) in the submucosal layer, mainly involved in regulating secretion, and an outer plexus (Auerbach's or myenteric) between the intestinal muscular layers, mainly involved in controlling smooth muscle motility (Wedel et al., 1999; Furness, 2006). There is extensive interaction between the plex-uses and between different levels of the GI tract.
The ENS has been estimated to contain nearly 200-500 million neurons in humans, making it the largest collection of neurons outside the brain (Furness, 2006). ENS neurons have been classified in numerous ways including shape, anatomical projections, electrophysiological properties, function, and neurotransmitter content (Bornstein et al., 2004; Furness, 2006; Schemann, 2005; Costa and Brookes, 2008). The neurochemical phenotype of enteric neurons is critical to their function and has been extensively studied in guinea pigs, mice, rats, and humans (Gabella, 1987; Ekblad et al., 1988, 1994a; Costa et al., 1992; Wattchow et al., 1997, 2008; Porter et al., 1997; 2002; Lomax and Furness, 2000; Furness, 2000, 2006; Anlauf et al., 2003; Neunlist et al., 2003; Pimont et al., 2003; Bornstein et al., 2004; Ganns et al., 2006; Murphy et al., 2007; Qu et al., 2008; Ippolito et al., 2009; Mongardi Fantaguzzi et al., 2009). While essentially every classical neurotransmitter and neuropeptide is expressed in the ENS, particularly prominent roles in mammalian enteric neurotransmission have been ascribed to nitric oxide (NO), vasoactive intestinal peptide (VIP), catecholamines, and acetylcholine (Ach). Reports have demonstrated that the neurochemical constituents of the ENS are qualitatively, although not necessarily quantitatively conserved across species (Anlauf et al., 2003; Schemann, 2005; Furness, 2006; Qu et al., 2008).
Primate models of human disease have provided valuable information for decades. In fact, abnormalities of GI function are frequently modeled in nonhuman primates. These experiments have included models of simian immunodeficiency virus-induced enteropathy (Orandle et al., 2007; Raffatellu et al., 2008) and inflammatory bowel disease and colitis (McKenna et al., 2008; Raffatellu et al., 2009), as well as study of pharmacological agents affecting GI motility (Yogo et al., 2008). Additionally, extensive investigation into neurodegenerative diseases, such as Alzheimer's disease, Parkinson's disease, and Huntington's disease has been performed in nonhuman primates. Now that effects of these disorders are beginning to be recognized outside the central nervous system, exploration into peripheral systems, such as the ENS, are critical (Bar et al., 2008; Bjorkqvist et al., 2008; Khan and Alkon, 2008; Lebouvier et al., 2008, 2009; Lees, 2009).
Despite the reliance on primates as the closest model of human neurological systems, relatively little is known about the composition of the ENS in nonhuman primates. Even basic information concerning the neurochemical coding of the ENS is lacking. Only one report has recently been published investigating the neurochemical composition of enteric neurons in nonhuman primates, and that study was limited to proximal colon neurons in a monkey model of Parkinson's disease (Chaumette et al., 2009).
We report quantitative neurochemical phenotyping of the ENS of the rhesus monkey along the entire GI tract using markers of NO, VIP, catecholamine, and Ach neurons. We focused our investigation on the myenteric plexus and made a direct comparison with human samples. These data indicate considerable neurochemical differences in myenteric plexus composition in different segments of the monkey GI tract. While overall similarity to other mammalian species is clear, there are some notable differences between the ENS of rhesus monkeys, humans, and other species that will be important to take into account when evaluating models of human diseases in animals.
MATERIALS AND METHODS
Tissue samples
Six-micron formalin-fixed paraffin-embedded tissue sections from five 5-year-old rhesus monkeys (Macaca mulatta) were obtained from the Yerkes National Primate Research Center. Regions examined included stomach, duodenum, jejunum, ileum, transverse colon, and rectum. Hematoxylin and eosin-stained sections were examined from each sample to confirm GI tract segment and tissue integrity. At Yerkes, animals were housed in paired housing with free access to standard primate chow and water. All experimental procedures were performed in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Experimental Animals and approved by the Emory University Institutional Animal Care and Use Committee.
Six-micron formalin-fixed paraffin-embedded tissue sections from eight control individuals (Table 1) were obtained from the Arizona Parkinson's Disease Consortium and the Banner Sun Health Brain and Body Donation Program in Sun City, Arizona. Regions examined included stomach, duodenum, and transverse colon. Hematoxylin and eosin-stained sections were examined from each sample to confirm GI tract segment and tissue integrity. All protocols related to the Brain and Body Donation Program were approved by the Banner Sun Health Research Institute Institutional Review Board.
TABLE 1.
Patient Characteristics for Postmortem Samples
| Age | Cause of death | Postmortem interval (hours) |
|---|---|---|
| 87 | Congestive heart failure, aortic stenosis | 2.5 |
| 73 | Acute myeloid leukemia due to myelodysplastic syndrome | 2.5 |
| 82 | Acute myeloid leukemia | 2.5 |
| 88 | Complications of stroke | 2.5 |
| 89 | Metastatic pancreatic cancer | 3.3 |
| 87 | Cardiopulmonary arrest, ischemic heart disease, pulmonary embolism | 10.3 |
| 92 | Cardiorespiratory failure due to severe progressive anemia | 3.0 |
| 75 | Pulmonary embolus, malignancy - unknown primary | 2.8 |
Antibody characterization
Please see Table 2 for a list of all primary antibodies used. The rabbit anti-NOS antibody recognized a band of 161 kD on western blots of human brain (manufacturer's data sheet). The mouse anti-NOS antibody recognized a band of 155 kD on western blots of rat brain (manufacturer's data sheet). Staining was eliminated by preincubation of the diluted antibody (1:500) with 25 μg/ml of recombinant rat protein. Staining for both anti-NOS antibodies colocalized fully when incubated together on intestine slides. Both antibodies stained a similar pattern of cellular morphology and distribution in the monkey and human ENS that was comparable to previous reports concerning NO neurons (Chaumette et al., 2009; Bernard et al., 2009; Greene et al., 2009).
TABLE 2.
Primary Antibodies Used in Monkey and Human Tissue
| Antigen | Immunogen | Source | Dilution |
|---|---|---|---|
| Choline acetyl transferase (ChAT) | Human placental enzyme | Millipore | 1:100 |
| Goat Polyclonal | |||
| Cat: AB144P | |||
| Lot: 0608037072 | |||
| Human neuronal protein HuC/HuD (HuC/D) | 12-Residue synthetic peptide representing the carboxy-terminal domain of human HuD from amino acids 240–251 | Invitrogen | 1:200 |
| Mouse Monoclonal | |||
| Cat: A21271 | |||
| Lot: 53877A | |||
| Neuronal nitric oxide synthase (nNOS) | Human nNOS, aa1095–1289, C-terminal | BD Biosciences Pharmingen | 1:500 |
| Mouse Monoclonal | |||
| Cat: 610308 | |||
| Lot:0000077630 | |||
| Neuronal nitric oxide synthase (nNOS) | Recombinant human nNOS | Millipore | 1:500 |
| Rabbit Polyclonal | |||
| Cat: AB5380 | |||
| Lot: LV1547827 | |||
| Tyrosine hydroxylase (TH) | TH purified from PC12 cells | Millipore | 1:100 |
| Mouse Monoclonal | |||
| Cat: MAB318 | |||
| Lot: 0505000106 | |||
| Tyrosine hydroxylase (TH) | Denatured TH from rat pheochromocytoma | Millipore | 1:100 |
| Rabbit Polyclonal | |||
| Cat: AB152 | |||
| Lot: LV1458671 | |||
| Vasoactive intestinal peptide (VIP) | Human VIP, aa1–95, N-terminal | Santa Cruz | 1:100 |
| Biotechnology | |||
| Rabbit Polyclonal | |||
| Cat: sc-20727 | |||
| Lot: A0203 | |||
| Vasoactive intestinal peptide (VIP) | 16-Residue synthetic peptide representing the carboxy-terminal domain of human VIP from amino acids 125–140 | Santa Cruz Biotechnology | 1:100 |
| Goat Polyclonal | |||
| Cat: sc-21041 | |||
| Lot: E2506 |
The rabbit anti-VIP antibody recognized a single band of 20 kD on western blots of mouse brain (manufacturer's data sheets). The goat anti-VIP antibody recognized a single band of 20 kD on western blots of mouse brain (manufacturer's data sheet); staining was eliminated by preincubation of the diluted antibody (1:100) with 20 μg/ml of the recombinant protein. Staining for both anti-VIP antibodies colocalized fully when incubated together on intestine slides. Both antibodies stained a similar pattern of cellular morphology and distribution in the monkey and human ENS that was comparable to previous reports concerning VIP neurons (Anlauf et al., 2003; Greene et al., 2009; Chaumette et al., 2009).
The rabbit and mouse anti-tyrosine hydroxylase (TH) antibodies each recognized a band of 60 kD on western blots of rat brain (manufacturer's data sheets). Staining for both anti-TH antibodies colocalized fully when incubated together on monkey intestine slides. Both antibodies stained a similar pattern of cellular morphology and distribution in the monkey and human ENS that was comparable to previous reports concerning catecholaminergic neurons (Anlauf et al., 2003; Greene et al., 2009).
The goat anti-choline acetyltransferase (ChAT) antibody recognized a band of 70 kD on western blots of rat brain (manufacturer's data sheet). Staining was eliminated by preincubation of the diluted antibody (1:100) with 50 μg/ml of the recombinant protein. The antibody stained a similar pattern of cellular morphology and distribution in the monkey and human ENS that was comparable to previous reports concerning cholinergic neurons (Anlauf et al., 2003; Chaumette et al., 2009; Bernard et al., 2009).
The mouse anti-HuC/D antibody recognized bands of 36, 40, and 42 kD on western blots of rat brain (Pascale et al., 2004). The antibody stained a similar pattern of cellular morphology and distribution in the monkey and human ENS that was comparable to previous reports (Phillips et al., 2004; Murphy et al., 2007; Hoff et al., 2008; Bernard et al., 2009; Greene et al., 2009; Chaumette et al., 2009).
Immunostaining
After dewaxing and washing the sections in distilled water, antigen retrieval was performed using boiling 10 mM sodium citrate solution for 10 minutes. Sections were cooled to room temperature, washed with distilled water and 1% Tris-buffered saline pH 7.3 (TBS), blocked in 5% normal donkey serum (NDS), and incubated with primary antibody solution overnight at 4° C.
The following day, sections were washed with TBS and incubated with secondary antibody for 1-2 hours. Rabbit primary antibodies against nNOS, VIP, and TH were visualized with AlexaFluor488-conjugated goat antirabbit secondary antibody (1:200, Invitrogen, La Jolla, CA) or Cy3-conjugated donkey antirabbit secondary (1:200, Jackson Immunolabs, West Grove, PA). Goat anti-VIP was visualized with AlexaFluor488-conjugated donkey anti-goat secondary antibody (1:200, Invitrogen). Mouse primary antibodies against nNOS and TH were visualized with Cy3-conjugated donkey antimouse secondary (1:200, Jackson Immunolabs), and mouse anti-HuC/D was visualized with Cy3-conjugated donkey or goat anti-mouse secondary (1:200, Jackson). Immunofluorescent samples were washed with TBS and coverslipped using Aquamount.
The secondary antibody for goat anti-ChAT was biotinconjugated donkey antigoat (1:200, Jackson) and detected using enzyme-linked immunohistochemistry. After incubation with secondary antibody, sections were washed with TBS and exposed to ABC solution (Vector Labs, Burlingame, CA) for 45 minutes at room temperature. They were then washed with TBS and incubated with 3, 3′-diaminobenzidine (DAB) tetrachloride for 30 minutes. For histochemically stained sections, colabeling was performed serially, so after washing in TBS sections were incubated with mouse anti-HuC/D overnight at 4° C. On the third day the sections were washed with TBS, incubated with secondary antibody (Cy3 Donkey anti-mouse, 1:200) for an hour, washed, and coverslipped.
No labeling was observed when protocols were completed without exposure to primary antibody. Control experiments were performed to ensure there was no crossreactivity of secondary antibodies in colabeling experiments.
Neuron counting
Counting was performed on an upright Olympus BX-60 microscope by blinded observers. Neuronal cell body and nuclear diameters were measured using Slidebook imaging software (Intelligent Imaging Innovations). For chemical coding using double-label immunofluorescence, all positive neurons in each segment were counted and divided by total number of HuC/D-positive cells in each segment. For ChAT staining the total number of neurons was calculated as the sum of ChAT-positive and HuC/D-positive cell numbers because immunohisto-chemical deposition of DAB quenches HuC/D fluorescence. For evaluation of NOS/VIP colabeling, neurons were characterized as NOS-only, VIP-only, or NOS/VIP. In each monkey and human case, two to three sections from each GI segment were examined using each staining protocol. Percentages for each section were calculated individually from counts corrected for differences in object size using Abercrombie's formula (T/T + h, where T was section thickness [6 μm] and h was mean nuclear diameter for the neuron type of interest) (Abercrombie, 1946; Guillery, 2002). Values from individual sections were averaged to obtain a single value for each case. Values for each case were averaged together and data are presented as mean ± standard error (n number of cases). Linear regression analysis was used to compare trends across GI segments in monkeys. Statistical comparisons between individual GI tract segments were made using repeated-measures analysis of variance (ANOVA) with post-hoc Bonferroni tests. P < 0.05 was considered significant.
Photomicrographs
Representative pictures were taken on an upright Olympus BX-60 microscope using an Olympus QColor 3 color camera. Adobe Photoshop (San Jose, CA) was used to adjust image size, brightness, and contrast.
RESULTS
Qualitative examination confirmed integrity of intestinal layers from mucosa to serosa, low levels of background staining, and discernable immunostaining for all sections and labeling techniques.
NOS, VIP, TH, and ChAT antibodies all labeled neuronal cell bodies in the monkey myenteric plexus (Figs. 1, 5; Supplemental Figures 1-3). Perikaryal TH and VIP staining tended to have a granular pattern, whereas NOS and ChAT immunoreactivity were more diffuse in the cell body. NOS immunoreactivity was uniformly robust in neuronal cell bodies. As has been previously reported, cytoplasmic ChAT staining was variable in intensity. NOS, VIP, and TH staining was qualitatively similar between monkey and human samples.
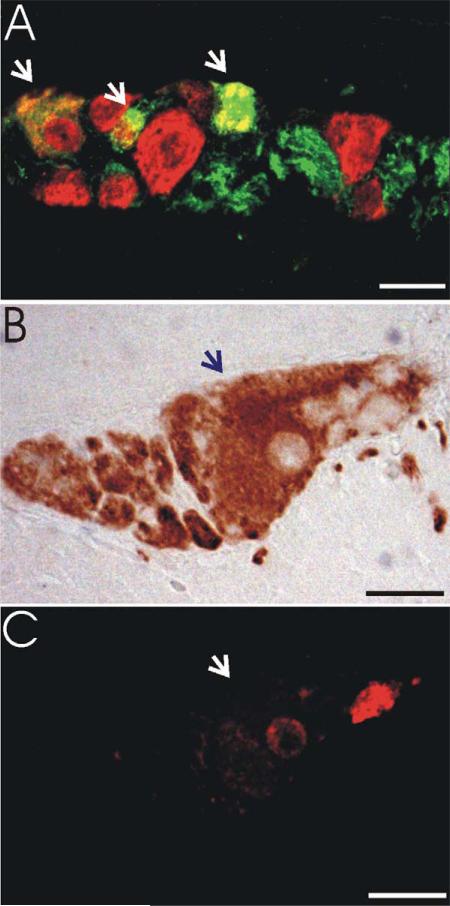
Figure 1.
Assessment of myenteric neuron proportions. Relative proportions of myenteric neuron phenotypes were assessed using double-label immunofluorescence or combined immunohistochemistry/immunofluorescence.A: Myenteric ganglion from jejunum demonstrating double-label immunofluorescence for NOS and HuC/D. Double-labeled NOS-positive neurons (arrows) are yellow/orange. NOS-negative neurons (HuC/D only) are red. NOS-positive processes are green. B: Myenteric ganglion from jejunum demonstrating ChAT immunohistochemistry. C: Fluorescent image of the same ganglion shown in B demonstrating quenched HuC/ D fluorescence in the ChAT-positive neuron (arrow) and a ChAT-negative (HuC/D only) neuron in red. Magenta-green version depicted in Supplemental Figure 1. Scale bars =20 μm.
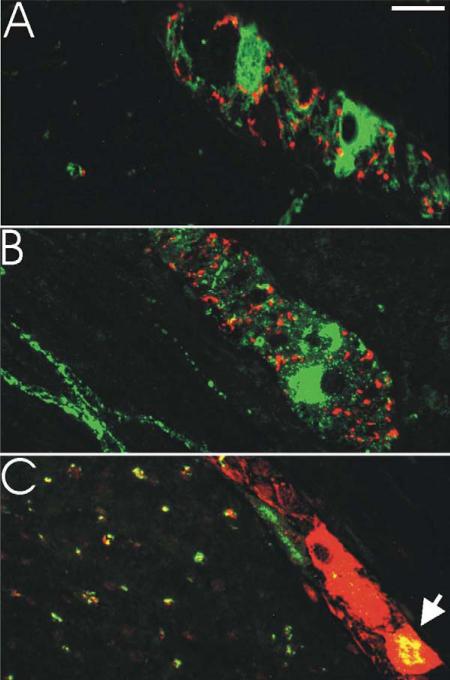
Figure 5.
Coexpression of neurotransmitter markers in myenteric plexus in rhesus monkey. A: Colabeling for TH (red) and NOS (green) revealed minimal overlap in all GI segments examined. Section depicted is from duodenum. B: Colabeling for TH (red) and VIP (green) revealed minimal overlap in all GI segments examined. Section depicted is from colon. C: Colabeling for NOS (red) and VIP (green) revealed significant overlap (orange) that varied by segment. Section depicted is from duodenum. Overlap in circular muscle processes is also prominent. Magenta-green version depicted in Supplemental Figure 2. Scale bar = μm.
The panneuronal antibody HuC/D was used to label all neurons in the myenteric plexus as the basis for relative quantification of neurochemical phenotype. For NOS, VIP, and TH, double-label immunofluorescence was used to quantify the proportion of each subtype (Fig. 1A). All neurons positive for NOS, VIP, or TH were also HuC/D-positive. For cholinergic neurons, combined immunohisto-chemistry (ChAT) and immunofluorescence (HuC/D), whereby ChAT-immunoreactivity quenched HuC/D fluorescence, was used to quantify proportions (Fig. 1B,C). The total number of neuron profiles counted per section for individual samples was comparable for all staining protocols. Table 3 demonstrates neuronal cell body and nuclear diameters were very similar for all neuronal sub-types in monkey and human myenteric plexus.
TABLE 3.
Average ± SEM Diameter in Microns (N)
| Monkey |
Human |
|||
|---|---|---|---|---|
| Neuron type | Soma | Nucleus | Soma | Nucleus |
| ChAT | 22 ± 0.6 (51) | 11 ± 0.3 (51) | ND | ND |
| NOS | 23 ± 1.3 (21) | 11 ± 0.5 (21) | 23 ± 1.6 (24) | 10 ± 0.4 (24) |
| VIP | 22 ± 1.0 (27) | 11 ± 0.6 (27) | 23 ± 0.8 (24) | 11 ± 0.3 (24) |
| TH | 22 ± 2.2 (7) | 10 ± 1.4 (7) | 23 ± 1.4 (11) | 11 ± 0.6 (11) |
| HuC/D | 23 ± 2.2 (71) | 11 ± 0.3 (71) | 23 ± 0.8 (28) | 11 ± 0.3 (28) |
ChAT, choline acetyltransferase; NOS, nitric oxide synthase; VIP, vasoactive intestinal peptide; TH, tyrosine hydroxylase; ND, not determined.
Table 4 shows the number of monkey neuron profiles counted for each segment and neuron subtype. On average per case (± SEM) there were 154 ± 36 counted in the stomach, 250 ± 27 in the duodenum, 151 ± 13 in the jejunum, 209 ± 51 in the ileum, 270 ± 50 in the colon, and 429 ± 95 in rectum sections for each staining protocol. Table 5 shows the number of human neuron profiles counted for each segment and neuron subtype. On average per case (±SEM) there were 72 ± 27 counted in the stomach, 150 ± 37 in the duodenum, and 315 ± 68 in the colon for each staining protocol. While each of the specimens examined were of approximately the same length, these data should not be interpreted with regard to absolute numbers of neurons per segment. However, they do demonstrate the extent of the counting performed to provide context for the proportional data.
TABLE 4.
Average ± SEM Number of Monkey Neuron Profiles Counted per Case (Range)
| Stomach | Duodenum | Jejunum | Ileum | Colon | Rectum | |
|---|---|---|---|---|---|---|
| ChAT | 102 ± 30 (52–202) | 157 ± 19 (122–218) | 61 ± 14 (37–85) | 98 ± 34 (34–204) | 140 ± 30 (76–217) | 171 ± 54 (39–289) |
| NOS | 31 ± 7 (12–49) | 52 ± 9 (38–80) | 40 ± 7 (26–57) | 74 ± 23 (31–150) | 105 ± 21 (61–161) | 234 ± 47 (102–330) |
| VIP | 49 ± 8 (26–67) | 26 ± 5 (15–40) | 10 ± 3 (5–19) | 18 ± 5 (7–34) | 18 ± 7 (5–35) | 54 ± 8 (32–72) |
| TH | 2 ± 1.2 (0–6) | 0.6 ± 0.7 (0–3) | 1.2 ± 0.9 (0–4) | 6.0 ± 3.2 (0–16) | 0.6 ± 0.4 (0–2) | 0.8 ± 0.4 (0–2) |
| Total (HuC/D) | 154 ± 36 (84–260) | 250 ± 27 (182–326) | 151 ± 13 (119–179) | 209 ± 51 (128–373) | 270 ± 50 (148–385) | 429 ± 95 (176–601) |
ChAT, choline acetyltransferase; NOS, nitric oxide synthase; VIP, vasoactive intestinal peptide; TH, tyrosine hydroxylase.
TABLE 5.
Average ± SEM Number of Human Neuron Profiles Counted per Case (Range)
| Stomach | Duodenum | Colon | |
|---|---|---|---|
| NOS | 22 ± 6 (5–48) | 54 ± 11 (16–92) | 157 ± 33 (5–299) |
| VIP | 35 ± 11 (9–82) | 28 ± 5 (8–49) | 28 ± 13 (0–104) |
| TH | 9 ± 3 (0–22) | 9 ± 2 (0–17) | 18 ± 7 (0–52) |
| Total (HuC/D) | 76 ± 26 (24–215) | 161 ± 32 (43–291) | 315 ± 68 (12–592) |
NOS, nitric oxide synthase; VIP, vasoactive intestinal peptide; TH, tyrosine hydroxylase.
Proportion of neuron phenotypes in the myenteric plexus
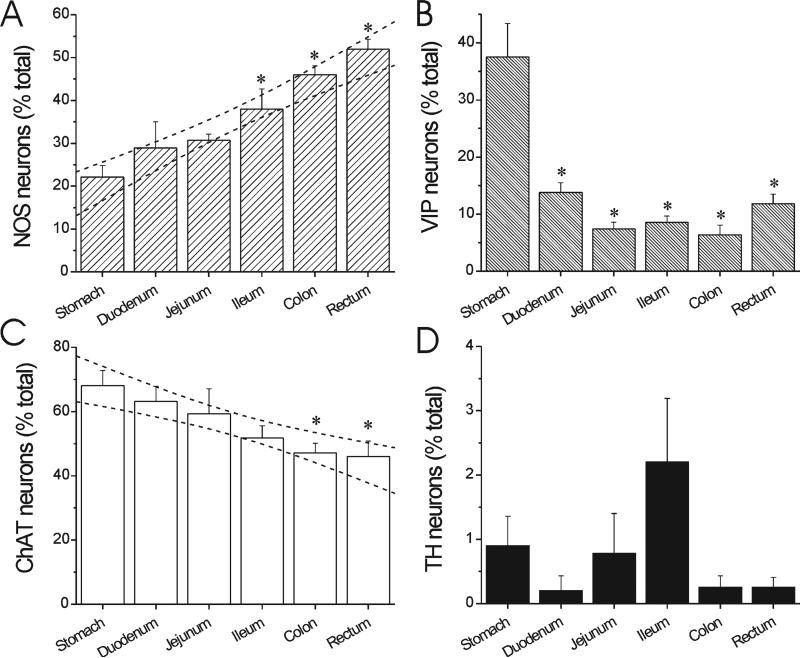
There are striking overall patterns in the neurochemical coding of the monkey myenteric plexus as depicted by the quantitative distribution of each major neuron subtype (Fig. 2). In particular, there is a graded increase in the proportion of nitric oxide myenteric neurons from the stomach, where they are in low abundance at 22 ± 3%, to the rectum, where they comprise about half of the myenteric neuron population (52 ± 2%) (Fig. 2A). Conversely, VIP neurons are relatively abundant in the stomach (37 6 6%), but a minor population (6-14%) in the rest of the GI tract (Fig. 2B). There is a decrease in the proportion of cholinergic myenteric neurons from the stomach, where they are the majority at 70 ± 5%, to the rectum, where they comprise a minority (47 ± 5%) (Fig. 2C). Catecholaminergic neurons are rare in the monkey myenteric plexus, on the order of 0.2-2.2%, depending on segment (Fig. 2D).
Figure 2.
Distribution of major neuronal subtypes in rhesus monkey myenteric plexus. A: NOS myenteric neuron distribution. B: VIP myenteric neuron distribution. C: Cholinergic (ChAT) myenteric neuron distribution. D: TH myenteric neuron distribution. Bars are mean ± SEM from five animals. The proportion of neuron subtypes varies based on GI tract segment. There is a progressive increase in abundance of NO neurons (R2 = 0.72, F = 70.3, P < 0.0001 for slope > 0) and a decrease in ChAT neurons (R2 0.44, F = 22.3, P < 0.0001 for slope < 0). Dashed lines represent 95% confidence bands for linear regression. *P < 0.05 vs. stomach, repeated measures ANOVA with post-hoc Bonferroni test.
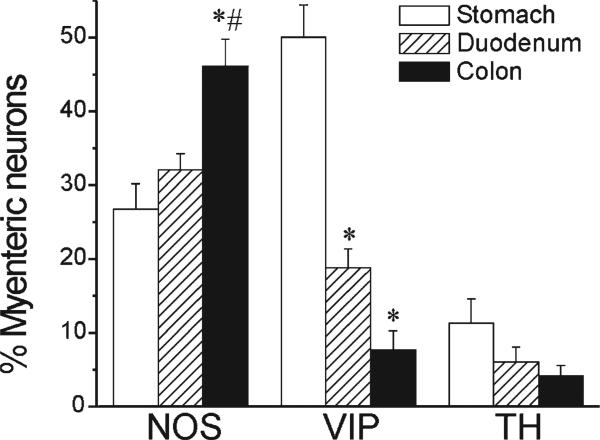
Comparing the abundance of certain myenteric neurons in rhesus monkey to that from human samples highlights several interesting similarities and differences. In general, as in the monkey, the proportion of NO neurons in the human myenteric plexus is higher in transverse colon (46 ± 4%) than proximal segments (27 ± 3% in stomach and 32 ± 2 in duodenum; Fig. 3). Conversely, but again as in the monkey, VIP neurons are more prominent in the proximal GI tract, particularly the stomach, where they account for half (50 ± 4%) of myenteric neurons. For both NO and VIP the relative abundances in human are comparable to that in the monkey. In contrast, the proportion of catecholaminergic neurons is significantly higher in the human myenteric plexus (Fig. 3). In humans, a significant proportion of myenteric neurons were TH-positive (11 ± 3% in stomach; 6 ± 2% in duodenum; and 4 ± 1% in colon), whereas almost no TH-positive neurons were detected in the monkey (0.9 ± 0.5% in stomach; 0.2 ± 0.2% in duodenum; and 0.3 ± 0.2% in colon).
Figure 3.
Comparative distribution of neuron subtypes in human myenteric plexus. Bars are mean ± SEM from eight cases. All segments were examined in every case. Note the similar relative distribution of NOS- and VIP-positive neurons in human and rhesus monkey. TH-positive neurons are more abundant in human samples. *P < 0.05 vs. stomach, ANOVA with post-hoc Bonferroni; #P < 0.05 vs. duodenum, ANOVA with post-hoc Bonferroni.
Qualitative assessment of different neural phenotypes innervating the gut wall
NOS, VIP, TH, and ChAT antibodies labeled varicose processes innervating every segment of the monkey GI tract. Without more quantitative methods, detailed conclusions about relative distribution of innervation were precluded; however, several critical points were clear.
First, myenteric ganglia at almost every level of the monkey GI tract were surrounded by processes of all four neuronal phenotypes (Fig. 4). Second, processes expressing NOS, VIP, and ChAT were very prominent in the circular muscle layer and less obvious in the longitudinal muscle layer in all GI tract segments, while processes expressing TH were nearly absent from the muscle layers (Fig. 4). Third, TH-positive processes were commonly observed surrounding submucosal blood vessels (Fig. 4K, inset). Fourth, VIP-positive processes were frequently found in the mucosal layer and villi (Fig. 4E, inset).
Figure 4.
Differential distribution of NOS-, VIP-, ChAT-, and TH-positive processes in the GI tract. Proximate sections from stomach (A,D,G,J), jejunum (B,E,H,K), and colon (C,F,I,L) from one monkey stained for NOS (A-C), VIP (D-F), ChAT (G-I), and TH (J-L). Circular muscle contains a substantial number of processes expressing NOS, VIP, and ChAT, but is sparsely innervated by TH. Arrows in A and J indicate a single neuron stained for both NOS and TH on adjacent sections. In each image, longitudinal muscle (LM) is to the left and circular muscle (CM) is to the right with myenteric ganglion cell layer (G) in between for cross-sections perpendicular to the long-axis of the GI tract. Inset in E, VIP-positive processes (arrowheads) in jejunal mucosa. Inset in K, TH-positive processes (arrowheads) surrounding jejunal submucosal blood vessels. Scale bars = 100 μm in C, E, inset; 25 μm in K, inset.
Colocalized phenotypes in the myenteric plexus
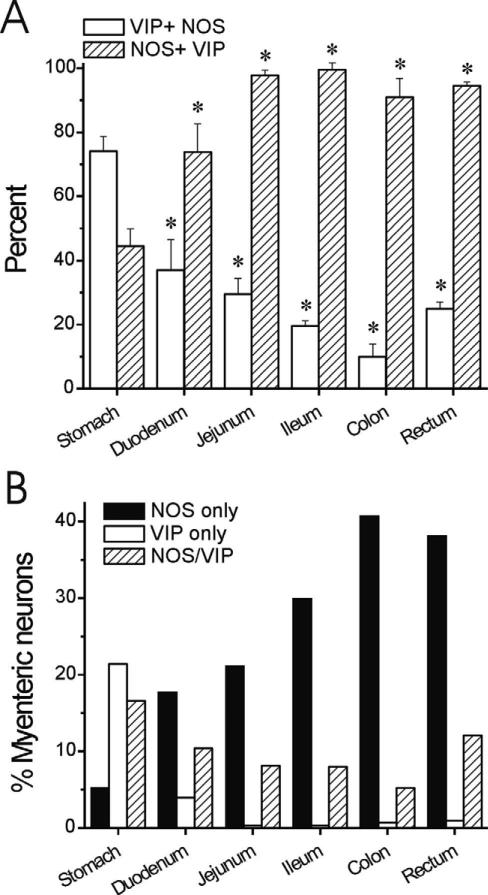
We performed colocalization studies for NOS, VIP, and TH. Our staining showed no consistent coexpression of either NOS or VIP with TH in either cell bodies or processes in the GI tract of rhesus monkey (Fig. 5A,B). The only possible exception was one myenteric neuron in the stomach that appeared to be detected on adjacent sections as positive for NOS and TH (Fig. 4A,J). NOS and VIP demonstrated a considerable degree of overlap in myenteric neurons and circular muscle processes (Fig. 5C); 10-74% of NO neurons were positive for VIP. This coexpression was higher in proximal GI tract (74 ± 5% in the stomach) and lower distally (10 ± 4% in the colon and 25 ± 2% in the rectum) (Fig. 6A). On the other hand, the proportion of VIP neurons that were NOS-positive trended in the opposite direction, with 45 ± 5% of VIP neurons expressing NOS in the stomach and 91-99% of VIP neurons expressing NOS distal to the duodenum (Fig. 6A). These data indicate that the greater relative abundance of myenteric NO neurons in the distal GI tract is primarily a reflection of higher numbers of NOS-only neurons (Fig. 6B).
Figure 6.
Distribution and colocalization of NOS and VIP in myenteric neurons along the GI tract from rhesus monkey. A: Percentage of VIP neurons also expressing NOS (hatched bars) is higher distal to the duodenum. The percentage of NOS neurons also expressing VIP (white bars) is lower distal to the stomach. *P < 0.05 vs. stomach, ANOVA with post-hoc Bonferroni. B: Absolute percentage of neurons was calculated based on percentages determined with HuC/D colabeling (Fig. 2). There is a significant increase in the abundance of NOS-only myenteric neurons in the distal GI tract.
DISCUSSION
The present study demonstrates the neurochemical coding of myenteric plexus neurons in different segments of the GI tract in rhesus monkey for the first time.
The main findings of this study are: 1) the relative distribution and abundance of NO, VIP, and Ach neurons in the monkey myenteric plexus are similar to other mammalian species, including humans; and 2) NO and VIP are sometimes cotransmitters in the monkey ENS. In general, the neurochemical anatomy of myenteric neurons in relationship to different segments of the GI tract is remarkably conserved across mammalian species, which has led us to focus our discussion on the potential implications of neurochemical differences in the myenteric plexus on overall function of the GI tract. We will first discuss the relative distribution of NO and VIP neurons in different segments of the GI tract, their anatomical overlap in the myenteric plexus, and how their relationship as cotransmitters changes in a proximal to distal gradient. Second, we will address the relatively low abundance of catecholaminergic myenteric neurons and its potential implications. Finally, we discuss the relative abundance of excitatory Ach neurons and its relationship to the other neuronal phenotypes examined throughout the length of the GI tract.
NOS distribution in the myenteric plexus
These data show that NO is a prominent neurotransmitter in the monkey ENS. Not only do a significant proportion of myenteric plexus neurons express NOS in neuronal cell bodies, but fibers innervating circular muscle and myenteric ganglia are dense. This anatomy implies a role in the monkey for NO as a transmitter in efferent motor neurons and enteric interneurons, similar to that observed in other species. Interestingly, there is a progressive increase in the proportion of myenteric neurons expressing NOS from proximal to distal GI segments. NO fibers demonstrate a similar pattern. The quantitative difference is considerable in that about one out of five myenteric neurons in the stomach body express NOS, while half use NO as a neurotransmitter in the rectum. Results from human myenteric plexus were qualitatively similar, with the proportion of myenteric neurons expressing NOS higher in the colon (46%) than the duodenum (32%) and stomach body (27%).
Our results are in agreement with a single recent report that 51% of colonic myenteric neurons are NOS-positive in rhesus monkey (Chaumette et al., 2009). Until now, direct comparison of the proportion of NOS neurons among GI tract segments within primate (human or non-human) subjects has not been made. Data from separate earlier studies support our results, including percentages of NOS myenteric neurons in humans ranging from 43-51% in colon (Porter et al., 1997, 2002; Murphy et al., 2007) to 40% in gastric fundus (Pimont et al., 2003). Estimates in small intestine have ranged from 20-38% in limited samples (Belai and Burnstock, 1999; Brehmer et al., 2006). In a study of rat ENS, 25% of stomach neurons, 21% from small intestine, and 36% from large intestine were positive for NOS (Ekblad et al., 1994a,b); these relative proportions can vary with strain (Jarvinen et al., 1999; Greene et al., 2009). Similarly, Sang and Young (1996) reported that 26% and 35% of neurons in small and large intestine of mice, respectively, were NOS-positive. In general, results suggest that an increase in the proportion of myenteric neurons expressing NOS in a proximal to distal GI gradient is a fairly consistent finding across mammalian species.
The prominence of NOS in the colon and rectum suggests NO is important for maintenance of normal colon motility, colonic reflexes, and defecation. Tracing studies have implicated NO as a transmitter in primary descending motor neurons in the myenteric plexus, and pharmacological or genetic blockade of NOS activity causes uncoordinated spasm of intestinal smooth muscle accompanied behaviorally by slowed GI transit (Costa et al., 1992; Mizuta et al., 1999; Mashimo et al., 2000; Brehmer et al., 2006; Sivarao et al., 2008). Both results suggest that direct inhibition of smooth muscle contraction is an important function of NO in the ENS in many species; NOS-positive processes innervating circular muscle demonstrate an anatomical substrate likely underlying a similar function in monkeys and humans.
VIP distribution in the myenteric plexus
Our data show a distribution of VIP in the monkey ENS that mirrors that of NOS. VIP is expressed in nearly 40% of myenteric neurons in the stomach body of the monkey but a significantly lower percentage in subsequent segments. VIP distribution in human myenteric neurons paralleled that in the monkey and confirmed previous results; 50% of human myenteric neurons in the stomach expressed VIP, and less than 10% of colonic myenteric neurons were VIP-positive. Similar to NOS, VIP fibers were dense in the circular muscle and myenteric ganglia; however, VIP fibers were also frequent in mucosal and submucosal layers. Anlauf et al. (2003) demonstrated that myenteric VIP neurons were higher in number in the proximal GI tract and tended to decrease toward distal segments (59% in the stomach vs. 13% in colon). The relatively dense mucosal and submucosal VIP innervation observed in both that and the present study is likely derived from VIP neurons in the submucosal plexus where VIP-positive neurons have been described to be the majority in humans (Anlauf et al., 2003). Other reports support the conclusion that VIP-positive neurons are significantly more abundant in the stomach than in other levels of the GI tract in humans and rodents (Neunlist et al., 2003; Pimont et al., 2003; Greene et al., 2009).
Studies have implicated VIP as a transmitter in primary muscle motor neurons, secretomotor neurons, interneurons, and possibly intestinofugal neurons in the myenteric plexus, which are all consistent with the present results as well as previously reported anatomical data in humans (Furness, 2000; Anlauf et al., 2003; Brehmer et al., 2006). With respect to neuromuscular transmission, VIP is predominantly inhibitory and causes relaxation (Costa et al., 1996). Conversely, VIP can be excitatory to myenteric neurons and secretory epithelium. Pharmacological or genetic blockade of VIP has been shown to slow GI transit, potentially by disrupting VIP neurotransmission at any of those three sites (Shi and Sarna, 2008). The anatomical pattern observed in monkey and human myenteric plexus suggests that VIP may be especially important for the modulation of proximal gut motility, particularly gastric motility and emptying.
NO and VIP are cotransmitters in the monkey myenteric plexus
Our colabeling studies show that there is significant overlap between NOS and VIP in the monkey myenteric plexus. For demonstration purposes, we divided neurons with regard to VIP or NOS expression into three categories: NOS-only, VIP-only, NOS/VIP. Of course, neurons expressing NOS, but not VIP, may express other neuro-transmitters or neuromodulators. For that matter, so might neurons expressing VIP, but not NOS, or those expressing both.
Colocalization of NOS and VIP has been reported previously in enteric neurons from rats, guinea pigs, and humans (Costa et al., 1992; Aimi et al., 1993; Pimont et al., 2003; Brehmer et al., 2006). In general, these neurons are thought to be descending inhibitory motor neurons, and the colocalization we observed in circular muscle processes supports a similar role in monkeys. In guinea pigs, some NOS/VIP neurons may be excitatory descending interneurons, also raising that possibility in primates (Furness, 2000; Brehmer et al., 2006). Limited human data indicate that the percentage of VIP neurons that express NOS is relatively stable across GI segments while the percentage of NOS neurons expressing VIP is lower and more variable (Porter et al., 1997; Pimont et al., 2003; Brehmer et al., 2006). The present results from monkey add interesting anatomical context to the relationship between NOS and VIP in the myenteric plexus. In particular, myenteric neurons expressing NOS without VIP increase dramatically in abundance in a proximal to distal gradient, essentially accounting for the entire disparity in NOS neurons between the stomach and rectum. An increase in NOS+/VIP– processes in circular muscle parallels the change in myenteric neuronal cell body coding. Neurons expressing both NOS and VIP or VIP alone follow the VIP pattern of greater abundance in the stomach and less in more distal GI segments.
It is likely the differing ratio between NO and VIP at different levels has significant impact on GI tract motility and coordination. As discussed above, the relative distributions of the two transmitters would suggest that VIP is critical in the proximal GI tract while NOS plays a more prominent role distally. Of course, the relationship is assuredly more complex because not only do the relative proportions of myenteric neurons change, but the interrelationship between NOS and VIP changes as well. Distal to the duodenum, the majority of NO neurons express NOS, but not VIP. The relative lack of coexpressing neurons in the large intestine suggests that the role of NOS/ VIP cotransmission may be less central to motility in the distal GI tract than in the stomach, although even a small subpopulation of NOS/VIP neurons could be critical to maintaining normal colon function.
TH distribution in the myenteric plexus
Myenteric neurons in mice and humans expressing TH have been previously reported to lack dopamine-β-bhydroxylase, indicating they are dopaminergic (Anlauf et al., 2003; Li et al., 2004). Conversely, many TH-positive fibers innervating the myenteric plexus and other layers of the GI tract (e.g., submucosal blood vessels) are noradrenergic and represent extrinsic sympathetic innervation (Anlauf et al., 2003). The current experiments examined only TH immunoreactivity, so we used the broader term “catecholaminergic” in discussing TH-positive innervation.
While they were a significant minority (<4-11%) in human myentric plexus, we observed intrinsic catecholaminergic neurons to be extremely rare (0-2%) in the monkey myenteric plexus. Catecholaminergic innervation of the myenteric ganglia was dense, possibly representing primarily extrinsic sympathetic input. In rodents, certain populations of enteric neurons have been thought to be transiently catecholaminergic during development, but presumed to convert to other neuronal subtypes or be lost in adults (Teitelman, 1981; Gershon, 1984; Baetge, 1990). Recently, as much as 10% of enteric neurons in adult mice have been described to be dopaminergic (Li et al., 2004). We demonstrated a small contingent of persistently catecholaminergic neurons in the myenteric plexus of adult mice and rats. In mice, we reported that about 0.5% of myenteric neurons in the ileum and jejunum express TH, which is in agreement with others (Anderson et al., 2007; Qu et al., 2008). The proportions we detected in rats tend to be lower in the ileum and colon (<2%) than in the stomach (about 15%) (Greene et al., 2009). Anlauf et al. (2003) reported a similar distribution in humans, where as many as 20% of myenteric neurons in the stomach, but fewer than 5% in the colon, are catecholaminergic; our present results confirm that qualitative distribution in human samples (Fig. 3). A recent report concerning the colon ENS in MPTP-treated primates suggested that, in contrast to our results, over 10% of colonic myenteric neurons expressed TH (Chaumette et al., 2009). We are confident that our labeling protocol is sufficiently sensitive, given the robust and clear positivity of a small population of myenteric neurons for TH in monkeys, as well as the close agreement of our human investigations with previously published results. One potential cause of this discrepancy, as well as the differing reports in rodents, is the recently described plasticity of enteric neurons with regard to TH expression (Chevalier et al., 2008). Additionally, we observed marked plasticity of TH in an enteric neuronal cell line and primary enteric neuron culture in response to toxic insults and growth factors (Anitha et al., 2009). The results in general suggest that there might exist in the ENS a population of neurons that have the potential to significantly increase TH expression (from either zero or very low levels) depending on the situation. These results need to be taken into careful consideration when discussing the role of enteric catecholaminergic neurons in GI motility and the effects of human disease states, such as diabetes and Parkinson's disease, on enteric neurons.
ChAT distribution in the myenteric plexus
In this study, cholinergic neurons were most numerous in proximal GI tract in monkeys and decreased in abundance distally; cholinergic fibers were dense in circular muscle and myenteric ganglia throughout the GI tract. The proportion of ChAT-positive neurons in the colon myenteric plexus detected in this study is slightly higher than that reported in the single published report in the rhesus monkey (Chaumette et al., 2009). In humans, results are somewhat variable, but gastric fundus has been reported to have a higher density of myenteric ChAT neurons than colon, 58% versus 33-48%, respectively (Neunlist et al., 2003; Pimont et al., 2003; Murphy et al., 2007). In guinea pig, two-thirds of myenteric neurons in the stomach express ChAT (Schemann et al., 1995). Vesicular acetylcholine transporter (VAchT) as a marker detected a higher proportion of cholinergic neurons in human myenteric plexus on the order of 65-72% (Anlauf et al., 2003). That result may indicate a higher sensitivity of VAchT for detection of enteric cholinergic neurons; however, that study employed PGP 9.5 as a general neuronal marker, which may not be as sensitive as HuC/D (Phillips et al., 2004; Murphy et al., 2007). Dense cholinergic processes detected in this and other studies innervating both muscle layers and the myenteric plexus most likely represent a combination of fibers from intrinsic neurons and extrinsic parasym-pathetic fibers from the dorsal motor nucleus of the vagus nerve and sacral spinal nuclei (Porter et al., 1996, 1997; Anlauf et al., 2003; Furness, 2006; Murphy et al., 2007).
This distribution and innervation pattern is consistent with the idea that cholinergic myenteric neurons are the primary excitatory motor neuron in the monkey myenteric plexus, as they are in humans and rodents (Anlauf et al., 2003; Furness, 2006). The prominence and density of ChAT staining in the monkey is also consistent with a broad role of Ach in the ENS beyond efferent motor neurons to enteric interneurons, secretomotor neurons, intrinsic primary afferent neurons, and intestinofugal neurons that has been described in rodents (Furness, 2006; Qu et al., 2008).
In the stomach, cholinergic myenteric neurons significantly outnumber NOS neurons by over a 3:1 margin, but the ratio is more balanced in the colon and rectum, where NOS neurons are more abundant. The exact implications of this proximal to distal gradient are unknown, but a review of data available in the literature suggests that it is preserved across species and perhaps made more complex by the finding that a small population of myenteric neurons has been reported to coexpress both markers (Murphy et al., 2007). A similar increase in the relative proportion of non-adrenergic, non-cholinergic (NANC) neurons has been described in the human myenteric plexus (Anlauf et al., 2003; Beck et al., 2009). As discussed above, comparable results have been reported for guinea pigs, mice, and rats (Matini et al., 1995; Sang and Young, 1996; Porter et al., 1997; Jarvinen et al., 1999; Pimont et al., 2003; Brehmer et al., 2006; Furness, 2006; Murphy et al., 2007). Regional differences in the neuro-chemistry of regulatory neurons in each region have implications with regard to the specific function of that region. For example, in Hirschsprung's disease, where there is aganglionosis of the distal GI tract, obstructive contractions prevent transit of contents, indicating greater reliance on neural-mediated muscle relaxation in the colon and rectum. In addition, the relaxation that precedes a food bolus in the colon has been reported to be greater than in the small intestine (Frigo and Lecchini, 1970). Both findings highlight the importance of muscular inhibition in the distal GI tract and correlate with the neuro-chemical anatomy (Furness, 2006).
SUMMARY
We directly compared the relative abundance of several myenteric neurochemical phenotypes between GI segments in rhesus monkeys and humans. In comparison with other species, most notably humans, the proximal to distal gradients of cholinergic, NO, and VIP neurons in the myenteric plexus of the rhesus monkey are similar. NO and VIP are cotransmitters in the monkey myenteric plexus, but their relationship changes substantially along the GI tract also in a proximal to distal gradient. Intrinsic TH-positive neurons are rarer in rhesus monkeys than in humans, but it is possible that their abundance varies based on GI activity or other factors. In the future, exploring the distribution and colocalization patterns of other prominent enteric neurotransmitters, such as substance P, ATP, and others, would help to generate a more complete characterization of neurochemical phenotypes in the monkey myenteric plexus.
Differences in digestive and storage functions of different segments of the GI tract are reflected in significant differences in neurochemical coding of myenteric neurons. Based on these findings, when exploring the effect of disease states on the neurochemical coding of the ENS, regional differences must be taken into account. Given the similarity of enteric neurochemical coding relative to humans, rhesus monkeys are likely to be a valid model for the study of enteric neuronopathies and other gastrointestinal diseases affecting the ENS in humans.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Shanthi Srinivasan, MD, for critical appraisal of the article and Shawn Arshad for technical assistance. We thank the Banner Sun Health Research Institute Brain and Body Donation Program of Sun City, Arizona for the provision of the human specimens. The Brain and Body Donation Program is supported by the National Institute on Aging (P30 AG19610 Arizona Alzheimer's Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer's Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, and 05-901 to the Arizona Parkinson's Disease Consortium) and the Prescott Family Initiative of the Michael J. Fox Foundation for Parkinson's Research.
Grant sponsor: National Institutes of Health (NIH); Grant number: K08 NS048858 (to J.G.G.); Grant sponsor: Michael J. Fox Foundation for Parkinson's Research (to J.G.G.).
Footnotes
Additional Supporting Information may be found in the online version of this article.
LITERATURE CITED
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Aimi Y, Kimura H, Kinoshita T, Minami Y, Fujimura M, Vincent SR. Histochemical localization of nitric oxide synthase in rat enteric nervous system. Neuroscience. 1993;53:553–560. doi: 10.1016/0306-4522(93)90220-a. [DOI] [PubMed] [Google Scholar]
- Anderson G, Noorian AR, Taylor G, Anitha M, Bernhard D, Srinivasan S, Greene JG. Loss of enteric dopaminergic neurons and associated changes in colon motility in an MPTP mouse model of Parkinson's disease. Exp Neurol. 2007;207:4–12. doi: 10.1016/j.expneurol.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anitha M, Shahnavaz N, Qayed E, Joseph I, Gossrau G, Mwangi S, Sitaraman SV, Greene JG, Srinivasan S. BMP2 promotes differentiation of nitrergic and catecholaminergic enteric neurons through a Smad1 dependent pathway. Am J Physiol Gastrointest Liver Physiol. 2009 doi: 10.1152/ajpgi.00343.2009. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anlauf M, Schafer MK, Eiden L, Weihe E. Chemical coding of the human gastrointestinal nervous system: cholinergic, VIPergic, and catecholaminergic phenotypes. J Comp Neurol. 2003;459:90–111. doi: 10.1002/cne.10599. [DOI] [PubMed] [Google Scholar]
- Bar KJ, Boettger MK, Andrich J, Epplen JT, Fischer F, Cordes J, Koschke M, Agelink MW. Cardiovagal modulation upon postural change is altered in Huntington's disease. Eur J Neurol. 2008;15:869–871. doi: 10.1111/j.1468-1331.2008.02173.x. [DOI] [PubMed] [Google Scholar]
- Beck M, Schlabrakowski A, Schrodl F, Neuhuber W, Brehmer A. ChAT and NOS in human myenteric neurons: coexistence and co-absence. Cell Tissue Res. 2009;338:37–51. doi: 10.1007/s00441-009-0852-4. [DOI] [PubMed] [Google Scholar]
- Belai A, Burnstock G. Distribution and colocalization of nitric oxide synthase and calretinin in myenteric neurons of developing, aging, and Crohn's disease human small intestine. Dig Dis Sci. 1999;44:1579–1587. doi: 10.1023/a:1026658826010. [DOI] [PubMed] [Google Scholar]
- Bernard CE, Gibbons SJ, Gomez-Pinilla PJ, Lurken MS, Schmalz PF, Roeder JL, Linden D, Cima RR, Dozois EJ, Larson DW, Camilleri M, Zinsmeister AR, Pozo MJ, Hicks GA, Farrugia G. Effect of age on the enteric nervous system of the human colon. Neurogastroenterol Motil. 2009;21:746–e746. doi: 10.1111/j.1365-2982.2008.01245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkqvist M, Wild EJ, Thiele J, Silvestroni A, Andre R, Lahiri N, Raibon E, Lee RV, Benn CL, Soulet D, Magnusson A, Woodman B, Landles C, Pouladi MA, Hayden MR, Khalili-Shirazi A, Lowdell MW, Brundin P, Bates GP, Leavitt BR, Moller T, Tabrizi SJ. A novel pathogenic pathway of immune activation detectable before clinical onset in Huntington's disease. J Exp Med. 2008;205:1869–1877. doi: 10.1084/jem.20080178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein JC, Costa M, Grider JR. Enteric motor and interneuronal circuits controlling motility. Neurogastroenterol Motil. 2004;16(Suppl 1):34–38. doi: 10.1111/j.1743-3150.2004.00472.x. [DOI] [PubMed] [Google Scholar]
- Brehmer A, Schrodl F, Neuhuber W. Morphology of VIP/nNOS-immunoreactive myenteric neurons in the human gut. Histochem Cell Biol. 2006;125:557–565. doi: 10.1007/s00418-005-0107-8. [DOI] [PubMed] [Google Scholar]
- Chaumette T, Lebouvier T, Aubert P, Lardeux B, Qin C, Li Q, Accary D, Bezard E, Bruley des Varannes S, Derkinderen P, Neunlist M. Neurochemical plasticity in the enteric nervous system of a primate animal model of experimental Parkinsonism. Neurogastroenterol Motil. 2009;21:215–222. doi: 10.1111/j.1365-2982.2008.01226.x. [DOI] [PubMed] [Google Scholar]
- Chevalier J, Derkinderen P, Gomes P, Thinard R, Naveilhan P, Vanden Berghe P, Neunlist M. Activity-dependent regulation of tyrosine hydroxylase expression in the enteric nervous system. J Physiol. 2008;586:1963–1975. doi: 10.1113/jphysiol.2007.149815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M, Brookes SH. Architecture of enteric neural circuits involved in intestinal motility. Eur Rev Medical Pharmacol Sci. 2008;12(Suppl 1):3–19. [PubMed] [Google Scholar]
- Costa M, Furness JB, Pompolo S, Brookes SJ, Bornstein JC, Bredt DS, Snyder SH. Projections and chemical coding of neurons with immunoreactivity for nitric oxide synthase in the guinea-pig small intestine. Neurosci Lett. 1992;148:121–125. doi: 10.1016/0304-3940(92)90819-s. [DOI] [PubMed] [Google Scholar]
- Costa M, Brookes SJ, Steele PA, Gibbins I, Burcher E, Kandiah CJ. Neurochemical classification of myenteric neurons in the guinea-pig ileum. Neuroscience. 1996;75:949–967. doi: 10.1016/0306-4522(96)00275-8. [DOI] [PubMed] [Google Scholar]
- Ekblad E, Ekman R, Hakanson R, Sundler F. Projections of peptide-containing neurons in rat colon. Neuroscience. 1988;27:655–674. doi: 10.1016/0306-4522(88)90296-5. [DOI] [PubMed] [Google Scholar]
- Ekblad E, Alm P, Sundler F. Distribution, origin and projections of nitric oxide synthase-containing neurons in gut and pancreas. Neuroscience. 1994a;63:233–248. doi: 10.1016/0306-4522(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Ekblad E, Mulder H, Uddman R, Sundler F. NOS-containing neurons in the rat gut and coeliac ganglia. Neuro-pharmacology. 1994b;33:1323–1331. doi: 10.1016/0028-3908(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Frigo GM, Lecchini S. An improved method for studying the peristaltic reflex in the isolated colon. Br J Pharmacol. 1970;39:346–356. doi: 10.1111/j.1476-5381.1970.tb12898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness JB. Types of neurons in the enteric nervous system. J Autonom Nerv Sys. 2000;81:87–96. doi: 10.1016/s0165-1838(00)00127-2. [DOI] [PubMed] [Google Scholar]
- Furness JB. The enteric nervous system. Blackwell; Malden, UK: 2006. [Google Scholar]
- Gabella G. The number of neurons in the small intestine of mice, guinea-pigs and sheep. Neuroscience. 1987;22:737–752. doi: 10.1016/0306-4522(87)90369-1. [DOI] [PubMed] [Google Scholar]
- Ganns D, Schrodl F, Neuhuber W, Brehmer A. Investigation of general and cytoskeletal markers to estimate numbers and proportions of neurons in the human intestine. Histol Histopathol. 2006;21:41–51. doi: 10.14670/HH-21.41. [DOI] [PubMed] [Google Scholar]
- Greene JG, Noorian AR, Srinivasan S. Delayed gastric emptying and enteric nervous system dysfunction in the rotenone model of Parkinson's disease. Exp Neurol. 2009;218:154–161. doi: 10.1016/j.expneurol.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillery RW. On counting and counting errors. J Comp Neurol. 2002;447:1–7. doi: 10.1002/cne.10221. [DOI] [PubMed] [Google Scholar]
- Hoff S, Zeller F, von Weyhern CW, Wegner M, Schemann M, Michel K, Ruhl A. Quantitative assessment of glial cells in the human and guinea pig enteric nervous system with an anti-Sox8/9/10 antibody. J Comp Neurol. 2008;509:356–371. doi: 10.1002/cne.21769. [DOI] [PubMed] [Google Scholar]
- Ippolito C, Segnani C, De Giorgio R, Blandizzi C, Mattii L, Castagna M, Moscato S, Dolfi A, Bernardini N. Quantitative evaluation of myenteric ganglion cells in normal human left colon: implications for histopathological analysis. Cell Tissue Res. 2009;336:191–201. doi: 10.1007/s00441-009-0770-5. [DOI] [PubMed] [Google Scholar]
- Jarvinen MK, Wollmann WJ, Powrozek TA, Schultz JA, Powley TL. Nitric oxide synthase-containing neurons in the myenteric plexus of the rat gastrointestinal tract: distribution and regional density. Anat Embryol. 1999;199:99–112. doi: 10.1007/s004290050213. [DOI] [PubMed] [Google Scholar]
- Khan TK, Alkon DL. Early diagnostic accuracy and pathophysiologic relevance of an autopsy-confirmed Alzheimer's disease peripheral biomarker. Neurobiol Aging. 2008;31:889–900. doi: 10.1016/j.neurobiolaging.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Lebouvier T, Chaumette T, Damier P, Coron E, Touchefeu Y, Vrignaud S, Naveilhan P, Galmiche JP, Bruley des Varannes S, Derkinderen P, Neunlist M. Pathological lesions in colonic biopsies during Parkinson's disease. Gut. 2008;57:1741–1743. doi: 10.1136/gut.2008.162503. [DOI] [PubMed] [Google Scholar]
- Lebouvier T, Chaumette T, Paillusson S, Duyckaerts C, Bruley des Varannes S, Neunlist M, Derkinderen P. The second brain and Parkinson's disease. Eur J Neurosci. 2009;30:735–741. doi: 10.1111/j.1460-9568.2009.06873.x. [DOI] [PubMed] [Google Scholar]
- Lees AJ. The Parkinson chimera. Neurology. 2009;72:S2–11. doi: 10.1212/WNL.0b013e318198daec. [DOI] [PubMed] [Google Scholar]
- Li ZS, Pham TD, Tamir H, Chen JJ, Gershon MD. Enteric dopaminergic neurons: definition, developmental lineage, and effects of extrinsic denervation. J Neurosci. 2004;24:1330–1339. doi: 10.1523/JNEUROSCI.3982-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax AE, Furness JB. Neurochemical classification of enteric neurons in the guinea-pig distal colon. Cell Tissue Res. 2000;302:59–72. doi: 10.1007/s004410000260. [DOI] [PubMed] [Google Scholar]
- Mashimo H, Kjellin A, Goyal RK. Gastric stasis in neuronal nitric oxide synthase-deficient knockout mice. Gastroenterology. 2000;119:766–773. doi: 10.1053/gast.2000.16509. [DOI] [PubMed] [Google Scholar]
- Matini P, Faussone-Pellegrini MS, Cortesini C, Mayer B. Vasoactive intestinal polypeptide and nitric oxide synthase distribution in the enteric plexuses of the human colon: an histochemical study and quantitative analysis. Histochem Cell Biol. 1995;103:415–423. doi: 10.1007/BF01457541. [DOI] [PubMed] [Google Scholar]
- McKenna P, Hoffmann C, Minkah N, Aye PP, Lackner A, Liu Z, Lozupone CA, Hamady M, Knight R, Bushman FD. The macaque gut microbiome in health, lentiviral infection, and chronic enterocolitis. PLoS Pathogens. 2008;4:e20. doi: 10.1371/journal.ppat.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuta Y, Takahashi T, Owyang C. Nitrergic regulation of colonic transit in rats. Am J Physiol. 1999;277(2 Pt 1):G275–279. doi: 10.1152/ajpgi.1999.277.2.G275. [DOI] [PubMed] [Google Scholar]
- Mongardi Fantaguzzi C, Thacker M, Chiocchetti R, Furness JB. Identification of neuron types in the submucosal ganglia of the mouse ileum. Cell Tissue Res. 2009;336:179–189. doi: 10.1007/s00441-009-0773-2. [DOI] [PubMed] [Google Scholar]
- Murphy EM, Defontgalland D, Costa M, Brookes SJ, Wattchow DA. Quantification of subclasses of human colonic myenteric neurons by immunoreactivity to Hu, choline acetyltransferase and nitric oxide synthase. Neurogastroenterol Motil. 2007;19:126–134. doi: 10.1111/j.1365-2982.2006.00843.x. [DOI] [PubMed] [Google Scholar]
- Neunlist M, Aubert P, Toquet C, Oreshkova T, Barouk J, Lehur PA, Schemann M, Galmiche JP. Changes in chemical coding of myenteric neurones in ulcerative colitis. Gut. 2003;52:84–90. doi: 10.1136/gut.52.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orandle MS, Veazey RS, Lackner AA. Enteric ganglionitis in rhesus macaques infected with simian immunodeficiency virus. J Virol. 2007;81:6265–6275. doi: 10.1128/JVI.02671-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascale A, Gusev PA, Amadio M, Dottorini T, Govoni S, Alkon DL, Quattrone A. Increase of the RNA-binding protein HuD and posttranscriptional up-regulation of the GAP-43 gene during spatial memory. Proc Natl Acad Sci U S A. 2004;101:1217–1222. doi: 10.1073/pnas.0307674100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RJ, Hargrave SL, Rhodes BS, Zopf DA, Powley TL. Quantification of neurons in the myenteric plexus: an evaluation of putative pan-neuronal markers. J Neurosci Methods. 2004;133:99–107. doi: 10.1016/j.jneumeth.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Pimont S, Bruley Des Varannes S, Le Neel JC, Aubert P, Galmiche JP, Neunlist M. Neurochemical coding of myenteric neurones in the human gastric fundus. Neurogastroenterol Motil. 2003;15:655–662. doi: 10.1046/j.1350-1925.2003.00442.x. [DOI] [PubMed] [Google Scholar]
- Porter AJ, Wattchow DA, Brookes SJ, Schemann M, Costa M. Choline acetyltransferase immunoreactivity in the human small and large intestine. Gastroenterology. 1996;111:401–408. doi: 10.1053/gast.1996.v111.pm8690205. [DOI] [PubMed] [Google Scholar]
- Porter AJ, Wattchow DA, Brookes SJ, Costa M. The neurochemical coding and projections of circular muscle motor neurons in the human colon. Gastroenterology. 1997;113:1916–1923. doi: 10.1016/s0016-5085(97)70011-8. [DOI] [PubMed] [Google Scholar]
- Porter AJ, Wattchow DA, Brookes SJ, Costa M. Cholinergic and nitrergic interneurones in the myenteric plexus of the human colon. Gut. 2002;51:70–75. doi: 10.1136/gut.51.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu ZD, Thacker M, Castelucci P, Bagyanszki M, Epstein ML, Furness JB. Immunohistochemical analysis of neuron types in the mouse small intestine. Cell Tissue Res. 2008;334:147–161. doi: 10.1007/s00441-008-0684-7. [DOI] [PubMed] [Google Scholar]
- Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, Winter SE, Godinez I, Sankaran S, Paixao TA, Gordon MA, Kolls JK, Dandekar S, Baumler AJ. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008;14:421–428. doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffatellu M, George MD, Akiyama Y, Hornsby MJ, Nuccio SP, Paixao TA, Butler BP, Chu H, Santos RL, Berger T, Mak TW, Tsolis RM, Bevins CL, Solnick JV, Dandekar S, Baumler AJ. Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine. Cell Host Microbe. 2009;5:476–486. doi: 10.1016/j.chom.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang Q, Young HM. Chemical coding of neurons in the myenteric plexus and external muscle of the small and large intestine of the mouse. Cell Tissue Res. 1996;284:39–53. doi: 10.1007/s004410050565. [DOI] [PubMed] [Google Scholar]
- Schemann M. Control of gastrointestinal motility by the “gut brain”—the enteric nervous system. J Pediatr Gastroenterol Nutr. 2005;41(Suppl 1):S4–6. doi: 10.1097/01.scs.0000180285.51365.55. [DOI] [PubMed] [Google Scholar]
- Schemann M, Schaaf C, Mader M. Neurochemical coding of enteric neurons in the guinea pig stomach. J Comp Neurol. 1995;353:161–178. doi: 10.1002/cne.903530202. [DOI] [PubMed] [Google Scholar]
- Shi XZ, Sarna SK. Gene therapy of Cav1.2 channel with VIP and VIP receptor agonists and antagonists: a novel approach to designing promotility and antimotility agents. Am J Physiol Gastrointest Liver Physiol. 2008;295:G187–G196. doi: 10.1152/ajpgi.00047.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivarao DV, Mashimo H, Goyal RK. Pyloric sphincter dysfunction in nNOS−/− and W/Wv mutant mice: animal models of gastroparesis and duodenogastric reflux. Gastroenterology. 2008;135:1258–1266. doi: 10.1053/j.gastro.2008.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattchow DA, Porter AJ, Brookes SJ, Costa M. The polarity of neurochemically defined myenteric neurons in the human colon. Gastroenterology. 1997;113:497–506. doi: 10.1053/gast.1997.v113.pm9247469. [DOI] [PubMed] [Google Scholar]
- Wattchow D, Brookes S, Murphy E, Carbone S, de Fontgalland D, Costa M. Regional variation in the neurochemical coding of the myenteric plexus of the human colon and changes in patients with slow transit constipation. Neurogastroenterol Motil. 2008;20:1298–1305. doi: 10.1111/j.1365-2982.2008.01165.x. [DOI] [PubMed] [Google Scholar]
- Wedel T, Roblick U, Gleiss J, Schiedeck T, Bruch HP, Kuhnel W, Krammer HJ. Organization of the enteric nervous system in the human colon demonstrated by wholemount immunohistochemistry with special reference to the submucous plexus. Ann Anat. 1999;181:327–337. doi: 10.1016/S0940-9602(99)80122-8. [DOI] [PubMed] [Google Scholar]
- Yogo K, Onoma M, Ozaki K, Koto M, Itoh Z, Omura S, Takanashi H. Effects of oral mitemcinal (GM-611), erythromycin, EM-574 and cisapride on gastric emptying in conscious rhesus monkeys. Dig Dis Sci. 2008;53:912–918. doi: 10.1007/s10620-007-9951-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.