Abstract
Gossypol is a phenolic compound produced by pigment glands in cotton stems, leaves, seeds, and flower buds (Gossypium spp.). Cottonseed meal is a by-product of cotton that is used for animal feeding because it is rich in oil and proteins. However, gossypol toxicity limits cottonseed use in animal feed. High concentrations of free gossypol may be responsible for acute clinical signs of gossypol poisoning which include respiratory distress, impaired body weight gain, anorexia, weakness, apathy, and death after several days. However, the most common toxic effects is the impairment of male and female reproduction. Another important toxic effect of gossypol is its interference with immune function, reducing an animal's resistance to infections and impairing the efficiency of vaccines. Preventive procedures to limit gossypol toxicity involve treatment of the cottonseed product to reduce the concentration of free gossypol with the most common treatment being exposure to heat. However, free gossypol can be released from the bound form during digestion. Agronomic selection has produced cotton varieties devoid of glands producing gossypol, but these varieties are not normally grown because they are less productive and are more vulnerable to attacks by insects.
1. Introduction
Cotton (Gossypium spp.) is an arborous plant from the Malvaceae family. It is one of the earliest plants that were cultivated by man and it has been used for over 4,000 years. It is primarily cultivated for fiber used in the textile industry and the oil from the cotton seed [1]. The genus Gossypium spp. includes many species distributed throughout the world, but only four species are grown for cotton fiber: Gossypium hirsutum L., Gossypium barbadense L., Gossypium arboreum L., and Gossypium herbaceum L. The most economically important cotton species is G. hirsutum, which is grown to produce 90% of the world's cotton [2]. Cotton fiber and oil production generate byproducts rich in fat from oil and protein which are used for animal feeding. However, this plant contains a toxic compound, gossypol [1].
2. Chemistry of Gossypol
Gossypol is a phenolic compound that was first isolated in 1899. The name is derived from the plant genus scientific name (Gossypium) combined with the ending “ol” from phenol [1]. Gossypol has a 518.55 Dalton molecular weight, has a yellow pigment, is crystalline, is insoluble in water and hexane, is soluble in acetone, chloroform, ether, and methyl ethyl ketone (butanone), and is partly soluble in crude vegetable oils. The chemical formula is C30H30O8, and the chemical structural formula is 2,2′-bis(8-formyl-1,6,7-trihydroxy-5-isopropyl-3-methylnaphthalene) (Figure 1) [1, 3, 4].
Figure 1.
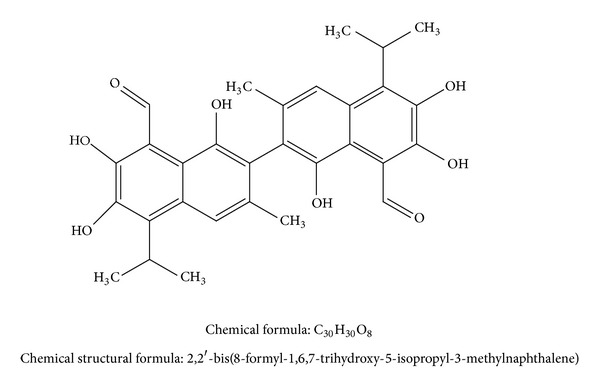
Chemical structure, formula, and structural formula of gossypol.
Gossypol is produced by pigment glands in cotton stems, leaves, seeds, and flower buds. The pigment glands are small black spots distributed throughout the cotton plant but their greatest concentration is in the seeds [1, 4–6]. The seed of G. barbadense may contain up to 34 g of gossypol/kg [7]. Gossypol promotes several toxic effects in vertebrates but provides the cotton plant with resistance to pests [1, 4–6]. The pigment glands produce additional phenolic pigments (at least 14), but they are at concentrations well below the concentration of gossypol and thus have little toxicological significance [1].
Gossypol is a mixture of two enantiomers, (−) and (+) gossypol [1, 8–11]. The (−) gossypol enantiomer is more slowly eliminated [12], although it is the most biologically active form. Consequently, it is more toxic than the (+) gossypol [11, 13]. The Gossypium species produces both enantiomers in varying proportions, which is genetically determined [1, 9, 10, 14]. For example, the (−) gossypol proportion ranges from 33.8 to 47.0% in the seeds of upland variety (G. hirsutum) [15, 16] and from 24.9 to 68.9% in the seeds of G. barbadense [7].
Two gossypol forms have been observed, free and bound [6]. The bound form is produced via covalent bonds between gossypol and the free epsilon-amino groups from lysine and arginine [1, 17, 18] through the browning or Maillard reaction [1]. However, this reaction reduces the availability of amino acids for absorption by the animal with lysine being the most affected [18].
Total gossypol production is influenced by several factors, including weather conditions and cotton species. Considering weather conditions, gossypol production is positively correlated with the rainfall rate and negatively correlated with temperature [19]. Regarding variation among cotton species, G. barbadense has higher gossypol concentrations than G. hirsutum. On the other hand, cotton storage slightly decreases the free gossypol content [1].
3. Gossypol in Cotton Products
The free gossypol content in whole cotton seeds varies among the many cotton varieties [6, 20]; gossypol concentrations range from 0.02 to 6.64% [21]. Cottonseed may contain concentrations greater than 14,000 mg/kg of total gossypol and 7,000 mg/kg of free gossypol [6]. However, after oil extraction from the seeds, up to 0.6% is available following solvent extraction, but approximately 0.06% is available, if the extraction process involves mechanical pressure and heat treatment [22].
In addition to its harmful effects, gossypol and its derivatives have potential therapeutic use. These compounds showed in vitro action against some viruses such as human immunodeficiency virus [23, 24] and H5N1 influenza virus [24, 25] and several bacteria and yeasts [26–29]. Gossypol is a promising treatment for leukemia [30], lymphoma [31], colon carcinoma [32], breast cancer [33, 34], myoma [35], prostate cancer [36], and other malignancies [37–43]. Furthermore, it was used in China, in 1970, to treat uterine fibroids, endometriosis, and uterine bleeding in women [35].
4. Toxicokinetics
The gossypol absorption rate is inversely proportional to the amount of iron in the diet [71], and dietary supplementation with ferrous sulfate inactivates free gossypol [72]. In ruminants, microbial fermentation in the rumen binds dietary free gossypol with proteins [73], but it is not known whether the bound form can be absorbed by the intestines or the microorganisms can release free gossypol from the bound form. The absorbed gossypol accumulates in the liver [74] and kidneys [75]. The primary gossypol excretion route is through bile; it is then eliminated through feces after conjugation with glucuronides and sulfates [76]. In rats dosed orally with 5 mg of both racemic forms of gossypol, 70.4% of (+) and 80.2% of (−) gossypol were excreted in the feces within five days, whereas 2.30% of (+) and 2.79% of (−) gossypol were excreted in the urine [77]. Small amounts of gossypol are also excreted in expired air [1]. Little to no gossypol is excreted in the milk [74]. The half-lives (t 1/2) of total (+) and (−) gossypol in rats following a single intravenous dose were estimated as 25.26 hours and 10.53 hours, respectively [77].
5. Gossypol Poisoning
Cottonseed includes sufficiently high gossypol concentrations to produce acute poisoning. However, there are cumulative effects of dietary gossypol and toxicity which can occur following an ingestion period of one to three months [1, 78–81]. Gossypol poisoning has been reported in many species, including broiler chicks [82], pigs [71], dogs [83, 84], sheep [85], and goats [86]. Monogastric animals, such as pigs, birds, fish, and rodents, are more susceptible to gossypol toxicity than ruminants [5, 6, 20, 87]. Moreover, young ruminants are more sensitive to gossypol compared with adult ruminants [1] because gossypol is not bound during ruminal fermentation, as it occurs in animals with fully functional rumens. However, if the gossypol intake overwhelms the ruminal detoxification capacity, free gossypol may be absorbed at hazardous concentrations even in adult ruminant animals [88].
General signs of acute toxicity are similar among animal species and include respiratory distress, impaired body weight gain, anorexia, weakness, apathy, and death after several days [1, 6, 80, 85, 89–93]. Heart failure was reported in calves [90, 94], lambs [85], and dogs [79].
The postmortem findings in ruminants include pulmonary edema, yellowish liquid in the chest and peritoneal cavities, gastroenteritis, centrilobular liver necrosis, and hypertrophic cardiac fiber degeneration. In calves, the major pathologic findings are ascites, visceral edema, acute centrilobular hepatocyte necrosis, kidney damage, and cardiovascular lesions. Increased pneumonia has also been observed, likely due to an increased sensitivity to secondary infections [85, 90–92].
Pigs may present reduced weight gain, anorexia, respiratory distress, cardiac insufficiency, coughing, and exercise intolerance. Necropsy findings include fluid accumulation in the body cavities; edema and congestion in the liver, lung, and spleen; and cardiac hypertrophy with degenerated muscle fiber [71].
Anemia is often observed in animals fed cottonseed. In fact, gossypol is a highly reactive compound that readily binds to minerals and amino acids. Binding with iron forms a gossypol-iron complex, which inhibits the absorption of this metal. The consequent iron deficiency affects erythropoiesis. Furthermore, gossypol promotes increased erythrocyte fragility [57, 74, 87, 95]. Gossypol also stimulates the eryptosis (apoptosis-like erythrocyte death) by increasing cytosolic Ca2+ activity resulting in cell membrane scrambling and contraction, which contributes to anemia [96].
Gossypol also affects thyroidal metabolism [68, 97–100]. Some studies with male [98] and female [99] rats showed decreased blood concentrations of T4 and T3 after dosing with gossypol. On the other hand, gossypol dosing resulted in increased T3 serum concentrations without affecting T4 in rats [97] and sheep [68]. The histopathological evaluation of thyroid glands from male rats dosed with gossypol revealed follicular degeneration and atrophy [98]. The thyrotropic cells in the pituitary gland, which are specialized for TSH synthesis and secretion, showed hypertrophy, hyperplasia, and degranulation after gossypol dosing in rats [100].
Certain clinical signs of gossypol poisoning have been attributed to reduced antioxidants in tissues and increased reactive oxygen species formation, which produces lipid peroxidation [101–104]. At high concentrations, gossypol also impairs energy generation from oxidative metabolism by interfering with enzymatic activity in the mitochondrial electron transport chain and oxidative phosphorylation [105–107]. Furthermore, gossypol decreases the contraction force of the heart and the extent of contraction of cardiac fibers [108].
6. Liver Damage
In addition to such effects, gossypol is hepatotoxic (Table 1) [11, 44–47, 71, 109, 110]. Ascites and hepatocyte degeneration (strong cytoplasmic eosinophilia and nuclear pyknosis) were observed in rats that received a single intraperitoneal gossypol dose of 25 mg/kg BW [45] or 30 mg/kg BW [46]. Rats that received lower gossypol doses (15 mg/kg/day for four weeks or 30 mg/kg/day for two weeks) showed morphological changes in the liver, as observed through electron microscopy, which were characterized by mitochondrial vacuolation, an enlarged endoplasmatic reticulum, an expanded perinuclear space, and collagen fiber proliferation in the perisinusoidal space [109]. Chickens fed a diet with 0.1% free gossypol for 21 days had increased plasma gamma glutamyltransferase activity and liver lipidosis [44]. Broilers that received a diet with 0.4% total gossypol for 20 days had greater liver weights [11].
Table 1.
Experimental studies showing liver damage induced by gossypol.
| Animals | Gossypol dose | Route of administration | Duration of treatment | Reference |
|---|---|---|---|---|
| Broiler | 0.4% of total gossypol in food | Oral | 20 days | [11] |
| Chickens | 0.1% of free gossypol in food | Oral | 21 days | [44] |
| Rats | 25 mg/kg BW | Intraperitoneal | Single dose | [45] |
| Rats | 30 mg/kg BW | Intraperitoneal | Single dose | [46] |
| Rats | 5, 10 and 20 mg/kg BW | Intraperitoneal | 10 days | [47] |
7. Reproductive Effects
Gossypol affects male and female gametogenesis and promotes embryo lesions [81]. In the 1950s, China underwent a sharp drop in the birthrate in many rural areas where humans were consuming cottonseed oil containing gossypol. This observation was initially associated with male infertility caused by gossypol in the cottonseed oil that they were consuming. Gossypol has been investigated for use as a male contraceptive in a number of experimental studies [1, 81, 111–115].
The gossypol toxicity for male reproduction (Table 2) was reported in several studies showing that it inhibits spermatogenesis, which decreases the sperm count and spermatozoid motility and viability [20, 47–51, 53, 55, 102, 116–120]. The male antifertility effect is dose and time dependent; in effective doses, gossypol causes infertility by inhibiting sperm motility, decreasing sperm concentrations, inducing specific mitochondrial injury to the sperm tail, and damaging the germinal epithelium [20]. However, such effects are reversible when gossypol is no longer ingested [52]. Furthermore, gossypol administration to male rats did not interfere in the embryonic and fetal development of untreated dam offspring [121].
Table 2.
Selected experimental studies describing effects of gossypol on male reproduction.
| Animals | Gossypol dose | Effects | Reference |
|---|---|---|---|
| Hamsters | 10 mg/kg BW/day | Degeneration of spermatocytes | [48] |
| Rats | 20 mg/kg BW/day | Degeneration of spermatocytes | [48] |
| Mice | 40 mg/kg BW/day | No degeneration | [48] |
| Rats | 25 mg/kg BW/day | Decreased spermatogenesis, Sertoli cell, and seminiferous tubules damage | [49] |
| Rats | 10 mg/kg BW/day | Tubular degeneration, reduced testosterone concentrations, and involutions of ventral prostate and seminal vesicles | [50] |
| Rats | 5, 10 and 20 mg/kg BW/day | Decreased sperm count and motility, increased abnormal sperm count, and reduced serum levels of testosterone, LH, and FSH | [47] |
| Bulls | 16.4 mg/kg BW/day | Reduced sperm production and motility and increased proportion of sperm midpiece abnormalities | [51] |
| Bulls | 8 mg/kg BW/day | Primary and secondary sperm abnormalities and increased number of sperm with proximal droplets | [52] |
The deleterious effects on male reproduction have not been observed for all animals fed cottonseed meal. In adult male goats [122] and sheep [123] fed a diet with 0.5 kg/animal/day cottonseed meal for 120 consecutive days, no detrimental effects on semen volume, sperm concentration, motility, and morphology.
The gossypol-mediated spermatozoid disturbance mechanism includes the inhibition of release and utilization of ATP by the sperm cells [124]. Another effect of gossypol is the reduction of cellular and microtubular β-tubular content in spermatocytes and spermatids [125]. Furthermore, gossypol inhibits calcium influx [126, 127] and Mg-ATPase and Ca-Mg-ATPase activity in spermatozoid plasmatic membranes [126]. Abnormal spermatozoids are produced because gossypol produces ultrastructural alterations in the nuclear membrane, endoplasmic reticulum, and mitochondria [119, 128–130]. In cultivated Sertoli cells from piglets, gossypol also decreases cellular oxidase activity and damages the DNA [131]. Reduced nuclear expression of androgen receptors was observed in Leydig cells, Sertoli cells, and myoid cells from rats fed gossypol-rich cottonseed flour [132].
Gossypol also affects female reproduction (Table 3), and ruminant females tolerate higher dietary gossypol concentrations than nonruminant females [20, 54, 118, 133], probably due to the ruminal detoxification. Female exposure to gossypol has been associated with interference with the estrous cycle, pregnancy, and early embryonic development [20, 57, 81]. Gossypol interfered with rodent estrous cycles [54, 134] and pig granulosa cell function [135]. Furthermore, ovaries from heifers fed cottonseed meal had fewer large follicles (>5 mm) than heifers fed soybean meal [57]. Gossypol affected in vitro ovarian steroidogenesis [136, 137] as well as bovine oocyte cumulus expansion and nuclear maturation [137].
Table 3.
Selected experimental studies describing effects of gossypol on female reproduction.
| Animals | Gossypol dose | Effects | Reference |
|---|---|---|---|
| Rats | 5 mg/kg BW/day | Longer diestrus | [53] |
| Rats | 25 mg/kg/day | Lower levels of estradiol-17β | [54] |
| Rats | 20 mg/kg/day | Irregular and longer estrous cycles, prolonged time for mating, decreased pregnancy rate, and reduced number of viable embryos | [55] |
| Heifers | ~51 mg/kg BW/day | No interference on cycling, first service conception rate, and ovarian morphology | [56] |
| Heifers | 5 g of free gossypol/animal/day | Reduced number of ovarian follicles >5 mm | [57] |
Previous studies have shown that gossypol interferes with embryonic development [118, 138–141]. In fact, gossypol may reach the uterine fluids through the maternal circulation [141]. A gossypol-mediated embryotoxic effect has been observed in in vitro [118, 138, 140–142] and in vivo [57, 139, 141, 143] studies. The early pregnancy loss promoted by gossypol is not due exclusively to direct damage to embryos but also to interference with implantation of the embryo [139]. However, this compound significantly reduced the fetal body weight in pregnant mice, but no fetal abnormalities were observed [144].
The probable mechanism for gossypol embryotoxicity is through direct embryonic cytotoxicity [20, 143]. This cytotoxic effect might be promoted by (1) generation of reactive oxygen species inducing oxidative stress [102, 104, 145], (2) intercellular communication disruption [146], (3) apoptosis induction [32, 147–152], or (4) interference with ionic transport in membranes, which increases intracellular calcium [153].
8. Immunotoxicity
Gossypol may cause a reduced number of leukocytes and primarily lymphocytes, which affects the immunocompetence of the organism [154]. In vivo and in vitro mouse experiments also demonstrated that gossypol has immunosuppressive activity [155], which operates by affecting lymphocytes through inhibiting proliferation and inducing apoptosis [155, 156]. Mice that received gossypol had significantly decreased numbers of lymphocytes in the thymus and mesenteric lymph nodes [157], in the total spleen cell population [144], and in the capacity of blood and lymphatic cells to produce antibodies after sheep erythrocyte immunization [144, 157]. Furthermore, the spleen and lymph nodes from mice receiving gossypol had decreased CD4+ thymocyte populations and increased CD8+ lymphocyte populations [157].
The interference of gossypol with lymphocytes influence immune function as observed in a number of studies [157–160]. After inoculation with Brucella abortus smooth strain 99 (S99), specific anti-Brucella antibody production was impaired in lambs [159] and calves [160] fed cottonseed meal. Mice treated with gossypol had decreased IgM and IgG production after sheep erythrocyte immunization [157]. Men treated with gossypol as a male contraceptive showed reduced IgG titers which could be associated with altered lymphocytes [158].
In vitro murine macrophage proliferation was inhibited by gossypol [157]. Furthermore, rat peritoneal macrophages incubated with gossypol inhibited arachidonic acid metabolism and prostaglandin E2 production [161]. On the other hand, macrophage chemotaxis induced by Edwardsiella ictaluri challenge was increased in channel catfish (Ictalurus punctatus) fed cottonseed [162] or receiving gossypol [163], but catfish were unaffected by gossypol in another study [27]. Gossypol also increased serum lysozyme activity in channel catfish following an E. ictaluri challenge [27, 163].
9. Preventive Procedures
The preventive procedures at this time involve the treatment of cottonseed products to decrease the concentrations of free gossypol through the use of heat and pressure in the processing of these products (Table 4). Agronomic selection has produced cotton varieties devoid of glands producing gossypol [164], but these varieties are less grown because they are not as productive and are more vulnerable to attacks by insects [1]. One alternative is the selection and use of cotton varieties containing a relatively high (+) to (−) gossypol enantiomer ratio [13]. The directive 2002/32 of the European Union (2002L0032 - EN - 26.02.2013 - 017.001) states that the maximum free gossypol concentrations for cottonseed are 5,000 ppm and 1,200 ppm for cottonseed meal or cake and, for complete feeding stuffs, are 20 ppm for laying hens and piglets, 60 ppm for rabbits and pigs, 100 ppm for poultry and calves, and 500 ppm for cattle, sheep, and goats.
Table 4.
Preventive procedures for reducing gossypol toxicity.
| Procedures | Reference |
|---|---|
| Heat treatment Roasting extrusion |
[58] [58, 59] |
| Irradiation Gamma irradiation Electron beam irradiation |
[156–158] [60, 61] |
| Fungal fermentation Aspergillus niger Aspergillus oryzae Candida tropicalis Saccharomyces cerevisiae Geotrichum candidum |
[161–163] [62] [63–66] [63, 64] [67] |
| Nutritional supplementation Ferric sulfate Sodium selenite Vitamin E |
[1] [68] [69, 70] |
Processing including heat treatment [58, 165] and extrusion process [59] can reduce free gossypol concentrations in cottonseed. However, it is possible that the conjugate formed can release free gossypol during digestion. In fact, cows fed diets containing whole cottonseed with similar total gossypol concentrations but different free gossypol concentrations had similar total plasma gossypol [59]. Furthermore, even though the extrusion process reduced free gossypol concentration but not the total gossypol concentration; broiler chicks fed extruded cottonseed meal or feed-grade cottonseed meal showed decreased body weight gain, increased feed intake, and inefficient feed conversion rate [166].
Radiation treatment using gamma [60, 167, 168] or electron beam irradiation [60, 61] may reduce free gossypol concentrations. In fact, gossypol irradiation reduced in vitro prooxidative activity and embryotoxicity in mice [168]. The mechanism for gossypol destruction through radiation is unknown, but it has been speculated that gossypol molecule aggregation, gossypol cross-linking with other molecules, and gossypol molecule fragmentation or breakdown may produce such destruction [61]. On the other hand, ammoniation, which is a procedure that is used to reduce aflatoxin content of food, increased cottonseed meal toxicity in dairy cattle [169].
Some fungus may reduce free gossypol concentrations in cottonseed meal by fermentation, including Aspergillus niger [63, 64, 170], Aspergillus oryzae [62], Candida tropicalis [63–66], Saccharomyces cerevisiae [63, 64], and Geotrichum candidum [67]. The use of fermented cottonseed meal to feed animals seems to be safe [62, 171]. However, while these microorganisms could be used to reduce free gossypol concentration in cottonseed meal, they are not currently commercially available.
Supplementation with ferric sulfate reduces free gossypol concentrations in food due to ferric sulfate binding with reactive groups from gossypol, which forms a conjugate. The recommendation for supplementation is 1 mol of gossypol for each mol of iron, which could increase the maximum concentration of gossypol from 50 to 150 ppm for laying birds and from 100 ppm to 400 ppm for pigs and poultry [1]. Additional nutrients may be used for dietary supplementation to reduce gossypol availability. Supplementing the diet with 1 mg of sodium selenite per day in adult sheep reduced the gossypol toxicity affecting semen quality [68]. Dietary vitamin E supplementation at 4000 IU/bull/day also reversed the negative effects of gossypol on sperm production and semen quality of bulls [69] and reversed the increased erythrocyte osmotic fragility in heifers [70] promoted by feeding cottonseed meal.
Gossypol was produced as a conjugate with bovine serum albumin for vaccines. This conjugate induces antibody production against gossypol in rats, but the immunized animals were more sensitive to the acute hepatotoxic effect of gossypol [46].
10. Conclusions and Future Research Directions
The ingestion of gossypol present in cottonseed and its products (cakes and meal) may promote clinical poisoning, liver damage, male and female reproductive toxicity, and immunological impairment. The acute poisoning is not currently a significant problem but the reproductive damage causes serious economic losses to the livestock industry. Even though the male reproductive toxicity is well known, there is a need for more studies to understand the female reproductive damage promoted by gossypol. The immunotoxicity of gossypol is far from being completely elucidated, but it impacts animals by reducing their resistance to infections and by impairing the efficiency of vaccines. Extensive research is needed to develop more efficient and inexpensive technologies to reduce gossypol toxicity.
Acknowledgment
This work received support for language editing by the Pró-Reitoria de Pesquisa of the Universidade Federal de Minas Gerais (Edital PRP-UFMG 03/2013).
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Soto-Blanco B. Gossipol e fatores antinutricionais da soja. In: Spinosa HS, Górniak SL, Neto JP, editors. Toxicologia Aplicada à MedicIna VeterInária. Barueri, Brazil: Manole; 2008. pp. 531–545. [Google Scholar]
- 2.Borém A, Freire EC, Cesar J, Penna V, Vianna PA. Considerations about cotton gene escape in Brazil: a review. Crop Breeding and Applied Biotechnology. 2003;3(4):315–332. [Google Scholar]
- 3.Abou-Donia MB. Physiological effects and metabolism of gossypol. Residue Reviews. 1976;61:125–160. doi: 10.1007/978-1-4613-9401-3_5. [DOI] [PubMed] [Google Scholar]
- 4.Rogers GM, Poore MH, Paschal JC. Feeding cotton products to cattle. Veterinary Clinics of North America: Food Animal Practice. 2002;18(2):267–294. doi: 10.1016/s0749-0720(02)00020-8. [DOI] [PubMed] [Google Scholar]
- 5.Kenar JA. Reaction chemistry of gossypol and its derivatives. Journal of the American Oil Chemists’ Society. 2006;83(4):269–302. [Google Scholar]
- 6.Alexander J, Benford D, Cockburn A, et al. Gossypol as undesirable substance in animal feed. EFSA Journal. 2008;908:1–55. [Google Scholar]
- 7.Percy RG, Calhoun MC, Kim HL. Seed gossypol variation within Gossypium barbadense L. cotton. Crop Science. 1996;36(1):193–197. [Google Scholar]
- 8.Hron RJ, Sr., Kim HL, Calhoun MC, Fisher GS. Determination of (+)-, (−)-, and total gossypol in cottonseed by high-performance liquid chromatography. Journal of the American Oil Chemists’ Society. 1999;76(11):1351–1355. [Google Scholar]
- 9.Lordelo MM, Davis AJ, Calhoun MC, Dowd MK, Dale NM. Relative toxicity of gossypol enantiomers in broilers. Poultry Science. 2005;84(9):1376–1382. doi: 10.1093/ps/84.9.1376. [DOI] [PubMed] [Google Scholar]
- 10.Lordelo MM, Calhoun MC, Dale NM, Dowd MK, Davis AJ. Relative toxicity of gossypol enantiomers in laying and broiler breeder hens. Poultry Science. 2007;86(3):582–590. doi: 10.1093/ps/86.3.582. [DOI] [PubMed] [Google Scholar]
- 11.Kakani R, Gamboa DA, Calhoun MC, Haq AU, Bailey CA. Relative toxicity of cottonseed gossypol enantiomers in broilers. Open Toxicology Journal. 2010;4:26–31. [Google Scholar]
- 12.Wu D-F, Yu Y-W, Tang Z-M, Wang M-Z. Pharmacokinetic of (±)-, (+)-, and (−)-gossypol in humans and dogs. Clinical Pharmacology and Therapeutics. 1986;39(6):613–618. doi: 10.1038/clpt.1986.108. [DOI] [PubMed] [Google Scholar]
- 13.Bailey CA, Stipanovic RD, Ziehr MS, et al. Cottonseed with a high (+)- to (−)-gossypol enantiomer ratio favorable to broiler production. Journal of Agricultural and Food Chemistry. 2000;48(11):5692–5695. doi: 10.1021/jf000211n. [DOI] [PubMed] [Google Scholar]
- 14.Scheffler JA, Romano GB. Breeding and genetics: modifying gossypol in cotton (Gossypium hirsutum L.): a cost effective method for small seed samples. Journal of Cotton Science. 2008;12(3):202–209. [Google Scholar]
- 15.Calhoun MC, Kuhlmann SK, Balwin BC. Assessing the gossypol status of cattle fed cotton feed products; 1995; Proceedings of the Pacific Northwest Animal Nutrition Conference; pp. 147A–158A. [Google Scholar]
- 16.Stipanovic RD, Puckhaber LS, Liu J, Bell AA. Total and percent atropisomers of Gossypol and Gossypol-6-methyl ether in seeds from Pima cottons and accessions of Gossypium barbadense L. Journal of Agricultural and Food Chemistry. 2009;57(2):566–571. doi: 10.1021/jf802756e. [DOI] [PubMed] [Google Scholar]
- 17.Bressani R, Jarquín R, Elías LG. Free and total gossypol, epsilon-amino lysine, and biological evaluation of cottonseed meals and flours in Central America. Journal of Agricultural and Food Chemistry. 1964;12(3):278–282. [Google Scholar]
- 18.Fernandez SR, Zhang Y, Parsons CM. Dietary formulation with cottonseed meal on a total amino acid versus a digestible amino acid basis. Poultry Science. 1995;74(7):1168–1179. doi: 10.3382/ps.0741168. [DOI] [PubMed] [Google Scholar]
- 19.Pons WA, Jr., Hoffpauir CL, Hopper TH. Gossypol in cottonseed: influence of variety of cottonseed and environment. Journal of Agricultural and Food Chemistry. 1953;1(18):1115–1118. [Google Scholar]
- 20.Randel RD, Chase CC, Jr., Wyse SJ. Effects of gossypol and cottonseed products on reproduction of mammals. Journal of Animal Science. 1992;70(5):1628–1638. doi: 10.2527/1992.7051628x. [DOI] [PubMed] [Google Scholar]
- 21.Price WD, Lovell RA, McChesney DG. Naturally occurring toxins in feedstuffs: center for veterinary medicine perspective. Journal of Animal Science. 1993;71(9):2556–2562. doi: 10.2527/1993.7192556x. [DOI] [PubMed] [Google Scholar]
- 22.Nicholson SS. Cottonseed toxicity. In: Gupta RC, editor. VeterInary Toxicology: Basic and Clinical Principles. 2nd edition. London, UK: Academic Press; 2012. pp. 1161–1165. [Google Scholar]
- 23.Polsky B, Segal SJ, Baron PA, Gold JWM, Ueno H, Armstrong D. Inactivation of human immunodeficiency virus in vitro by gossypol. Contraception. 1989;39(6):579–587. doi: 10.1016/0010-7824(89)90034-6. [DOI] [PubMed] [Google Scholar]
- 24.Yang J, Zhang F, Li J, et al. Synthesis and antiviral activities of novel gossypol derivatives. Bioorganic and Medicinal Chemistry Letters. 2012;22(3):1415–1420. doi: 10.1016/j.bmcl.2011.12.076. [DOI] [PubMed] [Google Scholar]
- 25.Yang J, Chen G, Li LL, et al. Synthesis and anti-H5N1 activity of chiral gossypol derivatives and its analogs implicated by a viral entry blocking mechanism. Bioorganic & Medicinal Chemistry Letters. 2013;23(9):2619–2623. doi: 10.1016/j.bmcl.2013.02.101. [DOI] [PubMed] [Google Scholar]
- 26.Margalith P. Inhibitory effect of gossypol on microorganisms. Applied Microbiology. 1967;15(4):952–953. doi: 10.1128/am.15.4.952-953.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yildirim-Aksoy P, Lim C, Dowd MK, Wan PJ, Klesius PH, Shoemaker C. In vitro inhibitory effect of gossypol from gossypol-acetic acid, and (+)- and (−)-isomers of gossypol on the growth of Edwardsiella ictaluri . Journal of Applied Microbiology. 2004;97(1):87–92. doi: 10.1111/j.1365-2672.2004.02273.x. [DOI] [PubMed] [Google Scholar]
- 28.Turco E, Vizzuso C, Franceschini S, Ragazzi A, Stefanini FM. The in vitro effect of gossypol and its interaction with salts on conidial germination and viability of Fusarium oxysporum sp. vasinfectum isolates. Journal of Applied Microbiology. 2007;103(6):2370–2381. doi: 10.1111/j.1365-2672.2007.03503.x. [DOI] [PubMed] [Google Scholar]
- 29.Anna Y, Medentsev AG, Krupyanko VI. Gossypol inhibits electron transport and stimulates ROS generation in yarrowia lipolytica mitochondria. Open Biochemistry Journal. 2012;6:11–15. doi: 10.2174/1874091X01206010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balakrishnan K, Wierda WG, Keating MJ, Gandhi V. Gossypol, a BH3 mimetic, induces apoptosis in chronic lymphocytic leukemia cells. Blood. 2008;112(5):1971–1980. doi: 10.1182/blood-2007-12-126946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson PWM. New targets for lymphoma treatment. Annals of Oncology. 2008;19(4):iv56–iv59. doi: 10.1093/annonc/mdn198. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Wang J, Wong SCH, et al. Cytotoxic effect of gossypol on colon carcinoma cells. Life Sciences. 2000;67(22):2663–2671. doi: 10.1016/s0024-3205(00)00857-2. [DOI] [PubMed] [Google Scholar]
- 33.van Poznak C, Seidman AD, Reidenberg MM, et al. Oral gossypol in the treatment of patients with refractory metastatic breast cancer: a phase I/II clinical trial. Breast Cancer Research and Treatment. 2001;66(3):239–248. doi: 10.1023/a:1010686204736. [DOI] [PubMed] [Google Scholar]
- 34.Ye W, Chang H-L, Wang L-S, et al. Modulation of multidrug resistance gene expression in human breast cancer cells by (−)-gossypol-enriched cottonseed oil. Anticancer Research. 2007;27(1):107–116. [PubMed] [Google Scholar]
- 35.Han M-L, Wang Y-F, Tang M-Y, et al. Gossypol in the treatment of endometriosis and uterine myoma. Contributions to Gynecology and Obstetrics. 1987;16:268–270. [PubMed] [Google Scholar]
- 36.Jiang J, Slivova V, Jedinak A, Sliva D. Gossypol inhibits growth, invasiveness, and angiogenesis in human prostate cancer cells by modulating NF-κB/AP-1 dependent- and independent-signaling. Clinical and Experimental Metastasis. 2012;29(2):165–178. doi: 10.1007/s10585-011-9439-z. [DOI] [PubMed] [Google Scholar]
- 37.Tuszynski GP, Cossu G. Differential cytotoxic effect of gossypol on human melanoma, colon carcinoma, and other tissue culture cell lines. Cancer Research. 1984;44(2):768–771. [PubMed] [Google Scholar]
- 38.Wu Y-W, Chik CL, Knazek RA. An in vitro and in vivo study of antitumor effects of gossypol on human SW-13 adrenocortical carcinoma. Cancer Research. 1989;49(14):3754–3758. [PubMed] [Google Scholar]
- 39.Badawy SZA, Souid A-K, Cuenca V, Montalto N, Shue F. Gossypol inhibits proliferation of endometrioma cells in culture. Asian Journal of Andrology. 2007;9(3):388–393. doi: 10.1111/j.1745-7262.2007.00168.x. [DOI] [PubMed] [Google Scholar]
- 40.Ko C-H, Shen S-C, Yang L-Y, Lin C-W, Chen Y-C. Gossypol reduction of tumor growth through ROS-dependent mitochondria pathway in human colorectal carcinoma cells. International Journal of Cancer. 2007;121(8):1670–1679. doi: 10.1002/ijc.22910. [DOI] [PubMed] [Google Scholar]
- 41.Chien C-C, Ko C-H, Shen S-C, Yang L-Y, Chen Y-C. The role of COX-2/PGE2 in gossypol-induced apoptosis of colorectal carcinoma cells. Journal of Cellular Physiology. 2012;227(8):3128–3137. doi: 10.1002/jcp.23067. [DOI] [PubMed] [Google Scholar]
- 42.Hsiao W-T, Tsai M-D, Jow G-M, Tien L-T, Lee YJ. Involvement of Smac, p53, and caspase pathways in induction of apoptosis by gossypol in human retinoblastoma cells. Molecular Vision. 2012;18:2033–2042. [PMC free article] [PubMed] [Google Scholar]
- 43.Wong FY, Liem N, Xie C, et al. Combination therapy with gossypol reveals synergism against gemcitabine resistance in cancer cells with high BCL-2 expression. PLoS ONE. 2012;7(12) doi: 10.1371/journal.pone.0050786.e50786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blevins S, Siegel PB, Blodgett DJ, Ehrich M, Saunders GK, Lewis RM. Effects of silymarin on gossypol toxicosis in divergent lines of chickens. Poultry Science. 2010;89(9):1878–1886. doi: 10.3382/ps.2010-00768. [DOI] [PubMed] [Google Scholar]
- 45.Deoras DP, Young-Curtis P, Dalvi RR, Tippett FE. Effect of gossypol on hepatic and serum γ-glutamyltransferase activity in rats. Veterinary Research Communications. 1997;21(5):317–323. doi: 10.1023/a:1005856119553. [DOI] [PubMed] [Google Scholar]
- 46.Fonseca NBS, Gadelha ICN, Oloris SCS, Soto-Blanco B. Effectiveness of albumin-conjugated gossypol as an immunogen to prevent gossypol-associated acute hepatotoxicity in rats. Food and Chemical Toxicology. 2013;56:149–153. doi: 10.1016/j.fct.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 47.El-Sharaky AS, Newairy AA, Elguindy NM, Elwafa AA. Spermatotoxicity, biochemical changes and histological alteration induced by gossypol in testicular and hepatic tissues of male rats. Food and Chemical Toxicology. 2010;48(12):3354–3361. doi: 10.1016/j.fct.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 48.Hahn DW, Rusticus C, Probst A. Antifertility and endocrine activities of gossypol in rodents. Contraception. 1981;24(1):97–105. doi: 10.1016/0010-7824(81)90072-x. [DOI] [PubMed] [Google Scholar]
- 49.Heywood R, Lloyd GK, Majeed SK, Gopinath C. The toxicity of gossypol to the male rat. Toxicology. 1986;40(3):279–284. doi: 10.1016/0300-483x(86)90060-0. [DOI] [PubMed] [Google Scholar]
- 50.Gåfvels M, Wang J, Bergh A, Damber JE, Selstam G. Toxic effects of the antifertility agent gossypol in male rats. Toxicology. 1984;32(4):325–333. doi: 10.1016/0300-483x(84)90084-2. [DOI] [PubMed] [Google Scholar]
- 51.Chenoweth PJ, Risco CA, Larsen RE, Velez J, Tran T, Chase CC., Jr. Effects of dietary gossypol on aspects of semen quality, sperm morphology and sperm production in young Brahman bulls. Theriogenology. 1994;42(1):1–13. doi: 10.1016/0093-691x(94)90657-5. [DOI] [PubMed] [Google Scholar]
- 52.Hassan ME, Smith GW, Ott RS, et al. Reversibility of the reproductive toxicity of gossypol in peripubertal bulls. Theriogenology. 2004;61(6):1171–1179. doi: 10.1016/j.theriogenology.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 53.Gu Y, Anderson NO. Effects of gossypol on the estrous cycle and ovarian weight in the rat. Contraception. 1985;32(5):491–496. doi: 10.1016/0010-7824(85)90019-8. [DOI] [PubMed] [Google Scholar]
- 54.Lin YC, Fukaya T, Rikihisa Y, Walton A. Gossypol in female fertility control: ovum implantation and early pregnancy inhibited in rats. Life Sciences. 1985;37(1):39–47. doi: 10.1016/0024-3205(85)90623-x. [DOI] [PubMed] [Google Scholar]
- 55.Lagerlöf RK, Tone JN. The effect of gossypol acetic acid on female reproduction. Drug and Chemical Toxicology. 1985;8(6):469–482. doi: 10.3109/01480548509041070. [DOI] [PubMed] [Google Scholar]
- 56.Gambill MD, Humphrey WD. Effects of diets containing gossypol on ovarian histology, function and fertility in prepubertal beef heifers. Theriogenology. 1993;40(3):585–593. doi: 10.1016/0093-691x(93)90411-w. [DOI] [PubMed] [Google Scholar]
- 57.Randel RD, Willard ST, Wyse SJ, French LN. Effects of diets containing free gossypol on follicular development, embryo recovery and corpus luteum function in brangus heifers treated with bFSH. Theriogenology. 1996;45(5):911–922. doi: 10.1016/0093-691x(96)00021-0. [DOI] [PubMed] [Google Scholar]
- 58.Arieli A. Whole cottonseed in dairy cattle feeding: a review. Animal Feed Science and Technology. 1998;72(1-2):97–110. [Google Scholar]
- 59.Noftsger SM, Hopkins BA, Diaz DE, Brownie C, Whitlow LW. Effect of whole and expanded-expelled cottonseed on milk yield and blood gossypol. Journal of Dairy Science. 2000;83(11):2539–2547. doi: 10.3168/jds.S0022-0302(00)75146-0. [DOI] [PubMed] [Google Scholar]
- 60.Shawrang P, Mansouri MH, Sadeghi AA, Ziaie F. Evaluation and comparison of gamma- and electron beam irradiation effects on total and free gossypol of cottonseed meal. Radiation Physics and Chemistry. 2011;80(6):761–762. [Google Scholar]
- 61.Ebrahimi-Mahmoudabad SR, Taghinejad-Roudbaneh M. Investigation of electron beam irradiation effects on anti-nutritional factors, chemical composition and digestion kinetics of whole cottonseed, soybean and canola seeds. Radiation Physics and Chemistry. 2011;80(12):1441–1447. [Google Scholar]
- 62.Lim S-J, Lee K-J. A microbial fermentation of soybean and cottonseed meal increases antioxidant activity and gossypol detoxification in diets for nile tilapia, oreochromis niloticus. Journal of the World Aquaculture Society. 2011;42(4):494–503. [Google Scholar]
- 63.Zhang W-J, Xu Z-R, Sun J-Y, Yang X. Effect of selected fungi on the reduction of gossypol levels and nutritional value during solid substrate fermentation of cottonseed meal. Journal of Zhejiang University B: Science. 2006;7(9):690–695. doi: 10.1631/jzus.2006.B0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang W-J, Xu Z-R, Zhao S-H, Sun J-Y, Yang X. Development of a microbial fermentation process for detoxification of gossypol in cottonseed meal. Animal Feed Science and Technology. 2007;135(1-2):176–186. [Google Scholar]
- 65.Weng X-Y, Sun J-Y. Kinetics of biodegradation of free gossypol by Candida tropicalis in solid-state fermentation. Biochemical Engineering Journal. 2006;32(3):226–232. [Google Scholar]
- 66.Zhang W-J, Xu Z-R, Zhao S-H, Jiang J-F, Wang Y-B, Yan X-H. Optimization of process parameters for reduction of gossypol levels in cottonseed meal by Candida tropicalis ZD-3 during solid substrate fermentation. Toxicon. 2006;48(2):221–226. doi: 10.1016/j.toxicon.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 67.Sun Z-T, Liu C, Du J-H. Optimisation of fermentation medium for the detoxification of free gossypol in cottonseed powder by Geotrichum candidum G07 in solid-state fermentation with response surface methodology. Annals of Microbiology. 2008;58(4):683–690. [Google Scholar]
- 68.EL-Mokadem MY, Taha TA, Samak MA, Yassen AM. Alleviation of reproductive toxicity of gossypol using selenium supplementation in rams. Journal of Animal Science. 2012;90(9):3274–3285. doi: 10.2527/jas.2011-4545. [DOI] [PubMed] [Google Scholar]
- 69.Velasquez-Pereira J, Chenoweth PJ, McDowell LR, et al. Reproductive effects of feeding gossypol and vitamin E to bulls. Journal of Animal Science. 1998;76(11):2894–2904. doi: 10.2527/1998.76112894x. [DOI] [PubMed] [Google Scholar]
- 70.Velasquez-Pereira J, Aréchiga CF, McDowell LR, et al. Effects of gossypol from cottonseed meal and dietary vitamin E on the reproductive characteristics of superovulated beef heifers. Journal of Animal Science. 2002;80(9):2485–2492. doi: 10.2527/2002.8092485x. [DOI] [PubMed] [Google Scholar]
- 71.Haschek WM, Beasley VR, Buck WB, Finnell JH. Cottonseed meal (gossypol) toxicosis in a swine herd. Journal of the American Veterinary Medical Association. 1989;195(5):613–615. [PubMed] [Google Scholar]
- 72.Barraza ML, Coppock CE, Brooks KN, Wilks DL, Saunders RG, Latimer GW., Jr. Iron sulfate and feed pelleting to detoxify free gossypol in cottonseed diets for dairy cattle. Journal of Dairy Science. 1991;74(10):3457–3467. doi: 10.3168/jds.S0022-0302(91)78536-6. [DOI] [PubMed] [Google Scholar]
- 73.Schneider IC, Ames ML, Rasmussen MA, Reilly PJ. Fermentation of cottonseed and other feedstuffs in cattle rumen fluid. Journal of Agricultural and Food Chemistry. 2002;50(8):2267–2273. doi: 10.1021/jf010783n. [DOI] [PubMed] [Google Scholar]
- 74.Lindsey TO, Hawkins GE, Guthrie LD. Physiological responses of lactating cows to gossypol from cottonseed meal rations. Journal of Dairy Science. 1980;63(4):562–573. doi: 10.3168/jds.S0022-0302(80)82972-9. [DOI] [PubMed] [Google Scholar]
- 75.Kim HL, Calhoun MC, Stipanovic RD. Accumulation of gossypol enantiomers in ovine tissues. Comparative Biochemistry and Physiology B: Biochemistry and Molecular Biology. 1996;113(2):417–420. doi: 10.1016/0305-0491(95)02061-6. [DOI] [PubMed] [Google Scholar]
- 76.Abou-Donia MB, Othman MA, Obih P. Interspecies comparison of pharmacokinetic profile and bioavailability of (±)-gossypol in male Fischer-344 rats and male B6C3F mice. Toxicology. 1989;55(1-2):37–51. doi: 10.1016/0300-483x(89)90173-x. [DOI] [PubMed] [Google Scholar]
- 77.Chen QQ, Chen H, Lei HP. Comparative study on the metabolism of optical gossypol in rats. Journal of Ethnopharmacology. 1987;20(1):31–37. doi: 10.1016/0378-8741(87)90116-4. [DOI] [PubMed] [Google Scholar]
- 78.Eagle E. Effect of repeated doses of gossypol on the dog. Archives of Biochemistry. 1950;26(1):68–71. [Google Scholar]
- 79.Patton CS, Legendre AM, Gompf RE, Walker MA. Heart failure caused by gossypol poisoning in two dogs. Journal of the American Veterinary Medical Association. 1985;187(6):625–627. [PubMed] [Google Scholar]
- 80.Kerr LA. Gossypol toxicosis in cattle. Compendium on Continuing Education for the Practising Veterinarian. 1989;11(9):1139–1146. [Google Scholar]
- 81.Gadelha ICN, do Nascimento Rangel AH, Silva AR, Soto-Blanco B. Efeitos do gossipol na reprodução animal. Acta Veterinaria Brasilica. 2011;5(2):129–135. [Google Scholar]
- 82.Henry MH, Pesti GM, Brown TP. Pathology and histopathology of gossypol toxicity in broiler chicks. Avian Diseases. 2001;45(3):598–604. [PubMed] [Google Scholar]
- 83.West JL. Lesions of gossypol poisoning in the dog. Journal of the American Veterinary Medical Association. 1940;96:74–76. [Google Scholar]
- 84.Uzal FA, Puschner B, Tahara JM, Nordhausen RW. Gossypol toxicosis in a dog consequent to ingestion of cottonseed bedding. Journal of Veterinary Diagnostic Investigation. 2005;17(6):626–629. doi: 10.1177/104063870501700622. [DOI] [PubMed] [Google Scholar]
- 85.Morgan S, Stair EL, Martin T, Edwards WC, Morgan GL. Clinical, clinicopathologic, pathologic, and toxicologic alterations associated with gossypol toxicosis in feeder lambs. American Journal of Veterinary Research. 1988;49(4):493–499. [PubMed] [Google Scholar]
- 86.East NE, Anderson M, Lowenstine LJ. Apparent gossypol-induced toxicosis in adult dairy goats. Journal of the American Veterinary Medical Association. 1994;204(4):642–643. [PubMed] [Google Scholar]
- 87.Zhang W-J, Xu Z-R, Pan X-L, Yan X-H, Wang Y-B. Advances in gossypol toxicity and processing effects of whole cottonseed in dairy cows feeding. Livestock Science. 2007;111(1-2):1–9. [Google Scholar]
- 88.Willard ST, Neuendorff DA, Lewis AW, Randel RD. Effects of free gossypol in the diet of pregnant and postpartum Brahman cows on calf development and cow performance. Journal of Animal Science. 1995;73(2):496–507. doi: 10.2527/1995.732496x. [DOI] [PubMed] [Google Scholar]
- 89.Rogers PAM, Henaghan TP, Wheeler B. Gossypol poisoning in young calves. Irish Veterinary Journal. 1975;29(1):9–13. [Google Scholar]
- 90.Holmberg CA, Weaver LD, Gutterbock WM, Genes J, Montgomery P. Pathological and toxicological studies of calves fed a high concentration cotton seed meal diet. Veterinary Pathology. 1988;25(2):147–153. doi: 10.1177/030098588802500207. [DOI] [PubMed] [Google Scholar]
- 91.Risco CA, Holmberg CA, Kutches A. Effect of graded concentrations of gossypol on calf performance: toxicological and pathological considerations. Journal of Dairy Science. 1992;75(10):2787–2798. doi: 10.3168/jds.S0022-0302(92)78042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zelski RZ, Rothwell JT, Moore RE, Kennedy DJ. Gossypol toxicity in preruminant calves. Australian Veterinary Journal. 1995;72(10):394–398. doi: 10.1111/j.1751-0813.1995.tb06180.x. [DOI] [PubMed] [Google Scholar]
- 93.Fombad RB, Bryant MJ. An evaluation of the use of cottonseed cake in the diet of growing pigs. Tropical Animal Health and Production. 2004;36(3):295–305. doi: 10.1023/b:trop.0000016828.48326.59. [DOI] [PubMed] [Google Scholar]
- 94.Hudson LM, Kerr LA, Maslin WR. Gossypol toxicosis in a herd of beef calves. Journal of the American Veterinary Medical Association. 1988;192(9):1303–1305. [PubMed] [Google Scholar]
- 95.Mena H, Santos JEP, Huber JT, Tarazon M, Calhoun MC. The effects of varying gossypol intake from whole cottonseed and cottonseed meal on lactation and blood parameters in lactating dairy cows. Journal of Dairy Science. 2004;87(8):2506–2518. doi: 10.3168/jds.S0022-0302(04)73375-5. [DOI] [PubMed] [Google Scholar]
- 96.Zbidah M, Lupescu A, Shaik N, Lang F. Gossypol-induced suicidal erythrocyte death. Toxicology. 2012;302(2-3):101–105. doi: 10.1016/j.tox.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 97.Tang F, Wong PYD. Serum potassium and aldosterone levels in gossypol-treated rats. International Journal of Andrology. 1984;7(2):149–153. doi: 10.1111/j.1365-2605.1984.tb00770.x. [DOI] [PubMed] [Google Scholar]
- 98.Rikihisa Y, Lin YC. Effect of gossypol on the thyroid in young rats. Journal of Comparative Pathology. 1989;100(4):411–417. doi: 10.1016/0021-9975(89)90006-6. [DOI] [PubMed] [Google Scholar]
- 99.Lin YC, Chitcharoenthum M, Rikihisa Y. Effect of gossypol on thyroid hormones in young female rats. Contraception. 1990;41(4):431–440. doi: 10.1016/0010-7824(90)90042-t. [DOI] [PubMed] [Google Scholar]
- 100.Udoh P, Patil DR, Deshpande MK. Histopathological and biochemical effects of gossypol acetate on pituitary-gonadal axis of male albino rats. Contraception. 1992;45(5):493–509. doi: 10.1016/0010-7824(92)90162-m. [DOI] [PubMed] [Google Scholar]
- 101.Janero DR, Burghardt B. Protection of rat myocardial phospholipid against peroxidative injury through superoxide-(xanthine oxidase)-dependent, iron-promoted fenton chemistry by the male contraceptive gossypol. Biochemical Pharmacology. 1988;37(17):3335–3342. doi: 10.1016/0006-2952(88)90647-8. [DOI] [PubMed] [Google Scholar]
- 102.Fornes MW, Barbieri AM, Burgos MH. Sperm motility loss induced by gossypol: relation with OH. scavengers, motile stimulators and malondialdehyde production. Biochemical and Biophysical Research Communications. 1993;195(3):1289–1293. doi: 10.1006/bbrc.1993.2183. [DOI] [PubMed] [Google Scholar]
- 103.de Peyster A, Quintanilha A, Packer L, Smith MT. Oxygen radical formation induced by gossypol in rat liver microsomes and human sperm. Biochemical and Biophysical Research Communications. 1984;118(2):573–579. doi: 10.1016/0006-291x(84)91341-x. [DOI] [PubMed] [Google Scholar]
- 104.Kovaci P. Mechanism of drug and toxic actions of gossypol: focus on reactive oxygen species and electron transfer. Current Medicinal Chemistry. 2003;10(24):2711–2718. doi: 10.2174/0929867033456369. [DOI] [PubMed] [Google Scholar]
- 105.Meksongsee LA, Clawson AJ, Smith FH. The in vivo effect of gossypol on cytochrome oxidase, succinoxidase, and succinic dehydrogenase in animal tissues. Journal of Agricultural and Food Chemistry. 1970;18(5):917–920. doi: 10.1021/jf60171a043. [DOI] [PubMed] [Google Scholar]
- 106.Abou Donia MB, Dieckert JW. Gossypol: uncoupling of respiratory chain and oxidative phosphorylation. Life Sciences. 1974;14(10):1955–1963. doi: 10.1016/0024-3205(74)90412-3. [DOI] [PubMed] [Google Scholar]
- 107.Tso WW, Lee CS. Gossypol uncoupling of respiratory chain and oxidative phosphorylation in ejaculated boar spermatozoa. Contraception. 1982;25(6):649–655. doi: 10.1016/0010-7824(82)90066-x. [DOI] [PubMed] [Google Scholar]
- 108.Huang WM, Urthaler F. The direct negative inotropic effect of gossypol. Journal of Ethnopharmacology. 1986;17(1):31–36. doi: 10.1016/0378-8741(86)90071-1. [DOI] [PubMed] [Google Scholar]
- 109.Wang Y, Lei H-P. Hepatotoxicity of gossypol in rats. Journal of Ethnopharmacology. 1987;20(1):53–64. doi: 10.1016/0378-8741(87)90119-x. [DOI] [PubMed] [Google Scholar]
- 110.Manabe S, Nuber DC, Lin YC. Zone-specific hepatotoxicity of gossypol in perfused rat liver. Toxicon. 1991;29(6):787–790. doi: 10.1016/0041-0101(91)90071-x. [DOI] [PubMed] [Google Scholar]
- 111.Qian SZ, Wang ZG. Gossypol: a potential antifertility agent for males. Annual Review of Pharmacology and Toxicology. 1984;24:329–360. doi: 10.1146/annurev.pa.24.040184.001553. [DOI] [PubMed] [Google Scholar]
- 112.Yu Z-H, Chan HC. Gossypol as a male antifertility agent—why studies should have been continued. International Journal of Andrology. 1998;21(1):2–7. doi: 10.1046/j.1365-2605.1998.00091.x. [DOI] [PubMed] [Google Scholar]
- 113.Coutinho EM. Gossypol: a contraceptive for men. Contraception. 2002;65(4):259–263. doi: 10.1016/s0010-7824(02)00294-9. [DOI] [PubMed] [Google Scholar]
- 114.Dodou K. Investigations on gossypol: past and present developments. Expert Opinion on Investigational Drugs. 2005;14(11):1419–1434. doi: 10.1517/13543784.14.11.1419. [DOI] [PubMed] [Google Scholar]
- 115.Chang Q, Liu Z, Ma W-Z, et al. Drug synergistic antifertility effect of combined administration of low-dose gossypol with steroid hormones in rats. Chinese Medical Journal. 2011;124(11):1678–1682. [PubMed] [Google Scholar]
- 116.Chongthammakun S, Ekavipat C, Sanitwongse B, Pavasuthipaisit K. Effects of gossypol on human and monkey sperm motility in vitro. Contraception. 1986;34(3):323–331. doi: 10.1016/0010-7824(86)90013-2. [DOI] [PubMed] [Google Scholar]
- 117.Hong CY, Huang JJ, Wu P. The inhibitory effect of gossypol on human sperm motility: relationship with time, temperature and concentration. Human Toxicology. 1989;8(1):49–51. doi: 10.1177/096032718900800109. [DOI] [PubMed] [Google Scholar]
- 118.Brocas C, Rivera RM, Paula-Lopes FF, et al. Deleterious actions of gossypol on bovine spermatozoa, oocytes, and embryos. Biology of Reproduction. 1997;57(4):901–907. doi: 10.1095/biolreprod57.4.901. [DOI] [PubMed] [Google Scholar]
- 119.Chenoweth PJ, Chase CC, Jr., Risco CA, Larsen RE. Characterization of gossypol-induced sperm abnormalities in bulls. Theriogenology. 2000;53(5):1193–1203. doi: 10.1016/S0093-691X(00)00264-8. [DOI] [PubMed] [Google Scholar]
- 120.Yuan YY, Shi QX. Inhibition of hamster sperm acrosomal enzyme by gossypol is closely associated with the decrease in fertilization capacity. Contraception. 2000;62(4):203–209. doi: 10.1016/s0010-7824(00)00161-x. [DOI] [PubMed] [Google Scholar]
- 121.Beaudoin AR. A developmental toxicity evaluation of gossypol. Contraception. 1988;37(2):197–219. doi: 10.1016/0010-7824(88)90131-x. [DOI] [PubMed] [Google Scholar]
- 122.Nunes FDCR, de Araujo DAFV, Bezerra MB, Soto-Blanco B. Effects of gossypol present in cottonseed cake on the spermatogenesis of goats. Journal of Animal and Veterinary Advances. 2010;9(1):75–78. [Google Scholar]
- 123.Guedes FCB, Soto-Blanco B. Sperm quality of sheep fed cottonseed cake. Acta Scientiae Veterinariae. 2010;38(4):415–418. [Google Scholar]
- 124.Ueno H, Sahni MK, Segal SJ, Koide SS. Interaction of gossypol with sperm macromolecules and enzymes. Contraception. 1988;37(3):333–341. doi: 10.1016/0010-7824(88)90033-9. [DOI] [PubMed] [Google Scholar]
- 125.Teng CS. Reversible changes in the content of cellular and microtubular tubulin in spermatogenic cells after gossypol treatment. Contraception. 1997;55(1):41–46. doi: 10.1016/s0010-7824(96)00240-5. [DOI] [PubMed] [Google Scholar]
- 126.Breitbart H, Rubinstein S, Nass-Arden L. Effect of gossypol-acetic acid on calcium transport and ATPase activity in plasma membranes from ram and bull spermatozoa. International Journal of Andrology. 1984;7(5):439–447. doi: 10.1111/j.1365-2605.1984.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 127.Breitbart H, Mayevsky A, Nass-Arden L. Molecular mechanisms of gossypol action on sperm motility. International Journal of Biochemistry. 1989;21(10):1097–1102. doi: 10.1016/0020-711x(89)90049-9. [DOI] [PubMed] [Google Scholar]
- 128.Hoffer AP. Effects of gossypol on the seminiferous epithelium in the rat: a light and electron microscope study. Biology of Reproduction. 1983;28(4):1007–1020. doi: 10.1095/biolreprod28.4.1007. [DOI] [PubMed] [Google Scholar]
- 129.Arshami J, Ruttle JL. Effects of diets containing gossypol on spermatogenic tissues of young bulls. Theriogenology. 1988;30(3):507–516. doi: 10.1016/0093-691x(88)90200-2. [DOI] [PubMed] [Google Scholar]
- 130.Romualdo GS, Klinefelter GR, de Grava Kempinas W. Postweaning exposure to gossypol results in epididymis-specific effects throughout puberty and adulthood in rats. Journal of Andrology. 2002;23(2):220–228. [PubMed] [Google Scholar]
- 131.Zhang M, Yuan H, He Z, et al. DNA damage and decrease of cellular oxidase activity in piglet sertoli cells exposed to gossypol. African Journal of Biotechnology. 2011;10(14):2797–2802. doi: 10.1292/jvms.10-0236. [DOI] [PubMed] [Google Scholar]
- 132.Timurkaan N, Yilmaz F, Timurkaan S. Effects of cottonseed flour on immunohistochemical localization of androgen receptors (AR) in rat testes. Revue de Medecine Veterinaire. 2011;162(1):13–17. [Google Scholar]
- 133.Gray ML, Greene LW, Williams GL. Effects of dietary gossypol consumption on metabolic homeostasis and reproductive endocrine function in beef heifers and cows. Journal of Animal Science. 1993;71(11):3052–3059. doi: 10.2527/1993.71113052x. [DOI] [PubMed] [Google Scholar]
- 134.Adeyemo GO, Longe OG, Adejumo DO. The reproductive performance of breeder cocks fed cottonseed cake-based diets. International Journal of Poultry Science. 2007;6(2):140–144. [Google Scholar]
- 135.Basini G, Bussolati S, Baioni L, Grasselli F. Gossypol, a polyphenolic aldehyde from cotton plant, interferes with swine granulosa cell function. Domestic Animal Endocrinology. 2009;37(1):30–36. doi: 10.1016/j.domaniend.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 136.Gu Y, Lin YC, Rikihisa Y. Inhibitory effect of gossypol on steroidogenic pathways in cultured bovine luteal cells. Biochemical and Biophysical Research Communications. 1990;169(2):455–461. doi: 10.1016/0006-291x(90)90353-o. [DOI] [PubMed] [Google Scholar]
- 137.Lin YC, Coskun S, Sanbuissho A. Effects of gossypol on in vitro bovine oocyte maturation and steroidogenesis in bovine granulosa cells. Theriogenology. 1994;41(8):1601–1611. [Google Scholar]
- 138.Zirkle SM, Lin YC, Gwazdauskas FC, Canseco RS. Effect of gossypol on bovine embryo development during the preimplantation period. Theriogenology. 1988;30(3):575–582. doi: 10.1016/0093-691x(88)90207-5. [DOI] [PubMed] [Google Scholar]
- 139.Lin YC, Rajamahendran P, Rikihisa Y. Inhibition of rat embryo implantation in the gossypol-treated uterine horn. Theriogenology. 1991;35(4):769–777. doi: 10.1016/0093-691x(91)90418-d. [DOI] [PubMed] [Google Scholar]
- 140.Hernández-Cerón J, Jousan FD, Soto P, Hansen PJ. Timing of inhibitory actions of gossypol on cultured bovine embryos. Journal of Dairy Science. 2005;88(3):922–928. doi: 10.3168/jds.S0022-0302(05)72759-4. [DOI] [PubMed] [Google Scholar]
- 141.Villaseñor M, Coscioni AC, Galvão KN, Chebel RC, Santos JEP. Gossypol disrupts embryo development in heifers. Journal of Dairy Science. 2008;91(8):3015–3024. doi: 10.3168/jds.2007-0939. [DOI] [PubMed] [Google Scholar]
- 142.Lin YC, Sanbuissho A, Coskun S, Rikihisa Y. Inhibition of in vitro fertilization and early embryonic development in hamsters by gossypol. Life Sciences. 1994;55(14):1139–1145. doi: 10.1016/0024-3205(94)00242-8. [DOI] [PubMed] [Google Scholar]
- 143.Li YF, Booth GM, Seegmiller RE. Evidence for embryotoxicity of gossypol in mice and chicks with no evidence of mutagenic activity in the ames test. Reproductive Toxicology. 1989;3(1):59–62. doi: 10.1016/0890-6238(89)90039-7. [DOI] [PubMed] [Google Scholar]
- 144.Sein GM. The embryotoxic and immunodepressive effects of gossypol. American Journal of Chinese Medicine. 1986;14(3-4):110–115. doi: 10.1142/S0192415X8600017X. [DOI] [PubMed] [Google Scholar]
- 145.Morales H, Tilquin P, Rees JF, Massip A, Dessy F, van Langendonckt A. Pyruvate prevents peroxide-induced injury of in vitro preimplantation bovine embryos. Molecular Reproduction and Development. 1999;52(2):149–157. doi: 10.1002/(SICI)1098-2795(199902)52:2<149::AID-MRD5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 146.Hervé J-C, Pluciennik F, Bastide B, et al. Contraceptive gossypol blocks cell-to-cell communication in human and rat cells. European Journal of Pharmacology. 1996;313(3):243–255. doi: 10.1016/0014-2999(96)00476-1. [DOI] [PubMed] [Google Scholar]
- 147.Yurtcu E, Ergun MA, Menevse A. Apoptotic effect of gossypol on human lymphocytes. Cell Biology International. 2003;27(9):791–794. doi: 10.1016/s1065-6995(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 148.Cui G-H, Xu Z-L, Yang Z-J, Xu Y-Y, Xue S-P. A combined regimen of gossypol plus methyltestosterone and ethinylestradiol as a contraceptive induces germ cell apoptosis and expression of its related genes in rats. Contraception. 2004;70(4):335–342. doi: 10.1016/j.contraception.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 149.Ergun MA, Konac E, Erbas D, Ekmekci A. Apoptosis and nitric oxide release induced by thalidomide, gossypol and dexamethasone in cultured human chronic myelogenous leukemic K-562 cells. Cell Biology International. 2004;28(3):237–242. doi: 10.1016/j.cellbi.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 150.Moon D-O, Kim M-O, Lee J-D, Kim G-Y. Gossypol suppresses NF-κB activity and NF-κB-related gene expression in human leukemia U937 cells. Cancer Letters. 2008;264(2):192–200. doi: 10.1016/j.canlet.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 151.Cengiz E, Karaca B, Kucukzeybek Y, et al. Overcoming drug resistance in hormone-and drug-refractory prostate cancer cell line, PC-3 by docetaxel and gossypol combination. Molecular Biology Reports. 2010;37(3):1269–1277. doi: 10.1007/s11033-009-9501-y. [DOI] [PubMed] [Google Scholar]
- 152.Moon D-O, Choi YH, Moon S-K, Kim W-J, Kim G-Y. Gossypol decreases tumor necrosis factor-α-induced intercellular adhesion molecule-1 expression via suppression of NF-κB activity. Food and Chemical Toxicology. 2011;49(4):999–1005. doi: 10.1016/j.fct.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 153.Cheng J-S, Lo Y-K, Yeh J-H, et al. Effect of gossypol on intracellular Ca2+ regulation in human hepatoma cells. Chinese Journal of Physiology. 2003;46(3):117–122. [PubMed] [Google Scholar]
- 154.Braga AP, MacIel MV, Guerra DGF, Maia ISAS, Oloris SCS, Soto-Blanco B. Extruded-expelled cottonseed meal decreases lymphocyte counts in male sheep. Revue de Medecine Veterinaire. 2012;163(3):147–152. [Google Scholar]
- 155.Xu W-B, Xu L-H, Lu H-S, et al. The immunosuppressive effect of gossypol in mice is mediated by inhibition of lymphocyte proliferation and by induction of cell apoptosis. Acta Pharmacologica Sinica. 2009;30(5):597–604. doi: 10.1038/aps.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Quintana PJE, de Peyster A, Klatzke S, Park HJ. Gossypol-induced DNA breaks in rat lymphocytes are secondary to cytotoxicity. Toxicology Letters. 2000;117(1-2):85–94. doi: 10.1016/s0378-4274(00)00244-7. [DOI] [PubMed] [Google Scholar]
- 157.Sijun D, Pawlak A, Poźniak B, et al. Effects of gossypol acetic acid on cellular and humoral immune response in non-immunized and SRBC-immunized mice. Central-European Journal of Immunology. 2012;37(1):11–19. [Google Scholar]
- 158.Xu D, Cai W-J, Zhu B-H, Dong C-J, Zheng Z-C, Gao Z-Q. Clinical safety of long-term administration of gossypol in 32 cases. Contraception. 1988;37(2):129–135. doi: 10.1016/0010-7824(88)90123-0. [DOI] [PubMed] [Google Scholar]
- 159.Nagalakshmi D, Sastry VRB, Agrawal DK, Katiyar RC. Haematological and immunological response in lambs fed on raw and variously processed cottonseed meal. Asian-Australasian Journal of Animal Sciences. 2001;14(1):21–29. [Google Scholar]
- 160.Pattanaik AK, Sastry VRB, Singh DK, Goswami TK, Mohanty DN. Effect of gossypol from cottonseed meal diets on some clinico-biochemical parameters and humoral immune response of crossbred calves fed barley or sorghum. Asian-Australasian Journal of Animal Sciences. 2003;16(9):1291–1296. [Google Scholar]
- 161.Ohuchi K, Watanabe M, Hirasawa N, Tsurufuji S, Ozeki T, Fujiki H. Inhibition by gossypol of tumor promoter-induced arachidonic acid metabolism in rat peritoneal macrophages. Biochimica et Biophysica Acta: Molecular Cell Research. 1988;971(1):85–91. doi: 10.1016/0167-4889(88)90164-4. [DOI] [PubMed] [Google Scholar]
- 162.Barros MM, Lim C, Klesius PH. Effect of soybean meal replacement by cottonseed meal and iron supplementation on growth, immune response and resistance of channel catfish (Ictalurus puctatus) to Edwardsiella ictaluri challenge. Aquaculture. 2002;207(3-4):263–279. [Google Scholar]
- 163.Yildirim M, Lim C, Wan PJ, Klesius PH. Growth performance and immune response of channel catfish (Ictalurus puctatus) fed diets containing graded levels of gossypol-acetic acid. Aquaculture. 2003;219(1–4):751–768. [Google Scholar]
- 164.Fisher GS, Frank AW, Cherry JP. Total gossypol content of glandless cottonseed. Journal of Agricultural and Food Chemistry. 1988;36(1):42–44. [Google Scholar]
- 165.Broderick GA, Craig WM. Effect of heat treatment on ruminal degradation and escape, and intestinal digestibility of cottonseed meal protein. Journal of Nutrition. 1980;110(12):2381–2389. doi: 10.1093/jn/110.12.2381. [DOI] [PubMed] [Google Scholar]
- 166.Henry MH, Pesti GM, Bakalli R, et al. The performance of broiler chicks fed diets containing extruded cottonseed meal supplemented with lysine. Poultry Science. 2001;80(6):762–768. doi: 10.1093/ps/80.6.762. [DOI] [PubMed] [Google Scholar]
- 167.Jaddou H, Al Hakim M, Al Adamy LZ, Mhaisen MT. Effect of gamma-radiation on gossypol in cottonseed meal. Journal of Food Science. 1983;48(3):988–989. [Google Scholar]
- 168.Jo C, Yook HS, Lee MS, et al. Irradiation effects on embryotoxicity and oxidative properties of gossypol dissolved in methanol. Food and Chemical Toxicology. 2003;41(10):1329–1336. doi: 10.1016/s0278-6915(03)00125-x. [DOI] [PubMed] [Google Scholar]
- 169.Smalley SA, Bicknell EJ. Gossypol toxicity in dairy cattle. Compendium on Continuing Education for the Practising Veterinarian. 1982;4(9):S378–S381. [Google Scholar]
- 170.Yang X, Sun J-Y, Guo J-L, Weng X-Y. Identification and proteomic analysis of a novel gossypol-degrading fungal strain. Journal of the Science of Food and Agriculture. 2012;92(4):943–951. doi: 10.1002/jsfa.4675. [DOI] [PubMed] [Google Scholar]
- 171.Sun H, Tang JW, Fang CL, et al. Molecular analysis of intestinal bacterial microbiota of broiler chickens fed diets containing fermented cottonseed meal. Poultry Science. 2013;92(2):392–401. doi: 10.3382/ps.2012-02533. [DOI] [PubMed] [Google Scholar]


