Abstract
Occult hepatitis B virus (HBV) infection (OBI) is a challenging pathobiological and clinical issue that has been widely debated for several decades. By definition, OBI is characterized by the persistence of HBV DNA in the liver tissue (and in some cases also in the serum) in the absence of circulating HBV surface antigen (HBsAg). Many epidemiological and molecular studies have indicated that OBI is an important risk factor for hepatocellular carcinoma (HCC) development. OBI may exert direct pro-oncogenic effects through the activation of the same oncogenic mechanisms that are activated in the course of an HBsAg-positive infection. Indeed, in OBI as in HBV-positive infection, HBV DNA can persist in the hepatocytes both integrated into the host genome as well as free episome, and may maintain the capacity to produce proteins-mainly X protein and truncated preS-S protein - provided with potential transforming properties. Furthermore, OBI may indirectly favor HCC development. It has been shown that the persistence of very low viral replicative activity during OBI may induce mild liver necro-inflammation continuing for life, and substantial clinical evidence indicates that OBI can accelerate the progression of liver disease towards cirrhosis that is considered the most important risk factor for HCC development.
Keywords: Hepatitis B virus, Hepatitis B virus surface antigen, Hepatitis B virus DNA, HBx protein, Hepatocellular carcinoma, Occult hepatitis B virus infection
Core tip: Accumulating evidence indicates that occult hepatitis B virus (HBV) infection (OBI) is an important risk factor for hepatocellular carcinoma (HCC) development both in hepatitis C virus (HCV)-infected and HCV-negative patients with chronic liver disease. Data form humans and animal models have shown that OBI may contribute to hepatocellular transformation through the same direct and indirect mechanisms that subtend HCC development in overt HBV infection. In this review, we aimed at revising the current epidemiological, clinical and molecular data linking OBI to HCC development.
INTRODUCTION
Around 2 billion people in the world show evidence of past or present infection with hepatitis B virus (HBV). Despite the availability of a very efficacious and safe vaccine, HBV infection is still a major public health problem, with an estimate of about 400 million chronic HBV carriers, many of whom suffering from severe and progressive forms of liver disease and being at high risk of hepatocellular carcinoma (HCC) development[1,2]. HCC is the fifth most prevalent cancer and the third leading cause of tumor-related deaths worldwide, and recent epidemiological data have shown that its incidence is continuously increasing worldwide[3,4]. HBV is a major etiological agent of HCC, and chronic HBV infection is estimated to be the cause of about 55%-60% of all HCC in the world. Strong epidemiological evidence has demonstrated the causative role for HBV in liver carcinogenesis: geographic areas of high HBV endemicity are also those with the highest incidence rates for liver cancer, the risk of developing HCC is up to 100-fold greater among HBV carriers compared with uninfected subjects, programs of universal infant vaccination have been shown to be effective in reducing the incidence rate of HCC in young children[5-10]. All these demonstrations together with the evidence provided by molecular studies have led to HBV being categorized, along with tobacco smoking, in ‘‘group 1’’ human carcinogens, classifying it among the most potent environmental carcinogens[11].
Several extensive studies evaluating viral and host factors associated with an increased risk of HCC development have identified high serum HBV DNA levels and the most severe forms of chronic liver damage (i.e., cirrhosis) as major factors implicated in the process of hepatic tumorigenesis[12-14]. However, important evidence indicates that the risk of HCC development may remain elevated also in individuals who-despite the persistence of viral genomes in the liver-are negative for circulating HBV surface antigen (HBsAg) and show absence or very low levels of serum viral DNA (Table 1)[15,16]. This peculiar condition is termed occult HBV infection (OBI). In fact, according to the statements produced at the international expert meeting held in Taormina (Italy) in 2008, the current consensus definition of OBI is ‘‘presence of HBV DNA in the liver (with detectable or undetectable HBV DNA in the serum) of individuals testing hepatitis B surface antigen (HBsAg) negative by currently available assays”[15].
Table 1.
Selection of studies reporting the association between occult hepatitis B virus infection and hepatocellular carcinoma
| Ref. | Geographic area | Aetiology |
| Shafritz et al[65] | South Africa | Unknown |
| Brechot et al[103] | France | Unknown |
| Paterlini et al[104] | South Africa, Italy, France and Japan | Unknown |
| Sheu et al[66] | Taiwan | HCV/cryptogenic |
| Paterlini et al[68] | France | HCV/cryptogenic |
| Yu et al[70] | United States | HCV/cryptogenic |
| Koike et al[72] | Japan | HCV |
| Kubo et al[73] | Japan | HCV |
| Huo et al[71] | Taiwan | HCV/cryptogenic |
| Pollicino et al[52] | Italy | HCV/cryptogenic |
| Yotsuyanagi et al[75] | Japan | Alcoholic |
| Donato et al[76] | Italy | HCV/alcoholic |
| Squadrito et al[105] | Italy | HCV |
| Ikeda et al[106] | Japan | HCV |
| Miura et al[107] | Japan | HCV |
| Obika et al[108] | Japan | HCV |
| Shetty et al[79] | United States | HCV |
| Matsuoka et al[109] | Japan | HCV |
| Kew et al[110] | South Africa | Unknown |
| Ikeda et al[111] | Japan | Alcoholic/cryptogenic |
| Chen et al[112] | Taiwan | Cryptogenic |
| Tamori et al[113] | Japan | HCV |
| Wong et al[50] | China | Cryptogenic |
| Kondo et al[114] | Japan | Cryptogenic |
| Pollicino et al[100] | Italy | Haemochromatosis |
| Squadrito et al[22] | Italy | HCV |
| Shi et al[86] | Meta-analysis | |
HCV: Hepatitis C virus.
Recognized since the 1970s[17,18], OBI started to be extensively studied only since the end of the 90s, when a landmark study was published providing new important insight into both its virological aspects and its potential clinical impact[19]. Since then, much evidence has been accumulated showing that OBI may have several important pathological and clinical implications[20-22]. In particular, OBI may have relevance in immunocompromised patients (because of the risk of HBV reactivation with consequent (re)development of HBV-related liver disease), in liver transplanted patients when the donor is OBI positive (because the recipient may develop typical hepatitis B if correct pre-emptive therapy is not performed), and in the field of transfusion medicine (because the residual risk of HBV transmission by blood transfusion may be related to the presence of OBI in the donor)[20,23-31]. Moreover, OBI has been suggested to favor the development of cirrhosis and a large body of evidence indicates that the pro-oncogenic activities of the HBsAg positive (namely, overt) HBV infection are maintained also in cases with OBI[16,20,21,31-35]. The focus of this review is an analysis of the epidemiology and molecular data that provide evidence for the association between occult HBV infection and HCC.
VIROLOGY OF THE OCCULT HBV INFECTION
HBV is the prototypic member of the Hepadnaviridae, a family of hepatotropic DNA viruses infecting selected mammalian (orthohepadnaviruses) and avian (avihepadnaviruses) hosts, and sharing similar genomic organization, organ tropisms, and a peculiar replication cycle. The HBV genome consists of a partially double-stranded relaxed circular DNA (rcDNA) of approximately 3200 nucleotides containing four partially overlapping open-reading frames (ORFs): the pre-S/S, pre-C/C, P, and X ORF[36,37].
HBV transcription is under the control of the following regulatory regions: enhancer II/basal core promoter, pre-S1 promoter, pre-S2/S promoter, and enhancer I/X promoter, respectively. The viral translational products include the three viral surface proteins (large, middle, and small, the latter corresponding to HBsAg), the core antigen (HBcAg), the soluble “e” antigen (HBeAg), the viral polymerase (that act as reverse transcriptase, DNA polymerase, RNase H, and terminal protein for priming), and the X protein that is essential for virus replication and capable of transactivating the expression of numerous cellular and viral genes[38]. The life cycle of HBV can be schematically summarized as follows: (1) a noncell-type specific primary attachment followed by an irreversible binding of the virus to an unidentified hepatocyte-specific receptor; (2) release of the core nucleocapsid containing the rcDNA into the cytoplasm and its transport along microtubules to the nuclear membrane; (3) release of rcDNA into the nucleoplasm, where it is “repaired” and converted into a covalently closed circular DNA (cccDNA), which after being associated with histone and non-histone proteins is organized into a chromatin-like structure as a viral minichromosome; (4) transcription of cccDNA by the host RNA polymerase II into genomic and subgenomic viral mRNAs; (5) nuclear export and translation in the cytoplasm of HBV transcripts into the viral envelope, core, “e”, polymerase, and X proteins; (6) selective packaging of the pregenomic RNA (pgRNA) into progeny capsids where it is reverse transcribed by the co-packaged P protein into new rcDNA genomes; and (7) assembly of replicating core with viral surface proteins in the endoplasmic reticulum and subsequent release of mature virions or alternatively recycling of progeny nucleocapsids into the nucleus to replenish the cccDNA pool[39].
The stability of viral cccDNA minichromosomes along with the long half-life of hepatocytes ensure that HBV infection, once established, may continue indefinitely over time[40,41], and possibly also in cases that experienced HBsAg seroconversion. Indeed, the molecular basis of occult HBV infection is related to the long-lasting persistence of HBV cccDNA as a stable chromatinized free episome in the nucleus of the infected hepatocytes[21,42,43]. OBI is considered a phase of the HBV natural history[44], but the mechanisms leading to its occurrence remain relatively poorly understood. Nevertheless, some recent studies have provided new important information, thus clarifying several aspects of this intriguing feature of the HBV infection.
OBI is significantly associated with the presence of anti-HBV antibodies (namely, anti-HBc and anti-HBs antibodies) directed against the viral core antigen and HBsAg, respectively. However, more than 20% of occult-infected individuals are negative for all HBV serum markers. Thus, it is possible to distinguish seropositive (anti-HBc and/or anti-HBs positive) and seronegative OBI (anti-HBc and anti-HBs negative individuals) (Figure 1)[15,20,21]. In seropositive OBI, HBsAg seroclearance may have occurred either soon after the resolution of acute hepatitis or after years or decades of overt chronic HBV infection, whereas the seronegative OBI cases might have either progressively lost all HBV serum markers or might have been HBV-negative since the beginning of the infection, similarly to what has been described in the woodchuck animal model when infected with the hepadnavirus “woodchuck hepatitis virus” (WHV)[45]. OBI, therefore, may take shape with different possible virological and immunological profiles.
Figure 1.
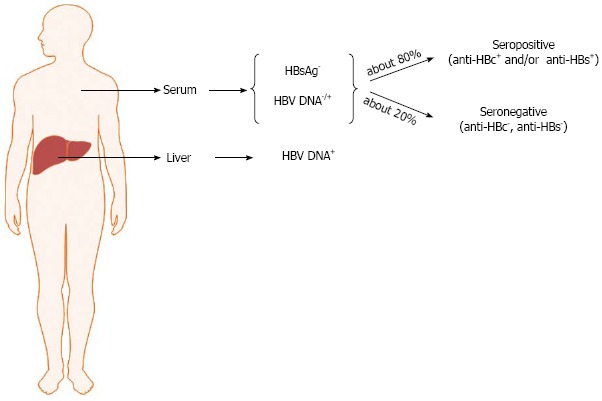
Schematic representation of hepatitis B virus profiles in occult hepatitis B virus infection. HBV: Hepatitis B virus.
The absence of detectable HBsAg despite the presence of episomal free HBV genomes at intrahepatic level can be due in a small number of cases to HBV genomic variability. HBV mutants linked to occult infection include (1) those producing antigenically modified HBsAg that is not recognized by commercially available detection assays; (2) those showing defective replication activity because of mutations in the Pol gene (possibly selected under the pressure of antiviral therapy); and (3) those showing an impaired synthesis of S proteins because of mutations in the S promoter genomic regions[21,33]. Strong evidence from numerous studies indicates that in most cases “occult” HBV genomes are replication-competent and that their genetic heterogeneity is comparable with those of HBV isolates from individuals with “overt” (HBsAg-positive) HBV infection[46]. Therefore, it is generally accepted that OBI status is largely due to a strong suppression of HBV replication and gene expression[15].
Various mechanisms could be involved in the induction and maintenance of HBV suppression, and the host immune response together with epigenetic factors appear to play a major role[21,33].
Since the 1970s, numerous clinical studies have clearly demonstrated that all the conditions inducing immunosuppression, such as hematologic malignancies, chemo- or immunotherapies and so on, can cause the reactivation of OBI with the reappearance of the typical serological profile of overt active HBV infection[17,20,21]. Though indirect, this is quite overwhelming proof of the role played by host immune surveillance in OBI development. The involvement of the immune system is also confirmed by the data showing that a long-lasting memory T cell response against HBV antigens is still present several years after recovery from acute hepatitis B. It is, indeed, possible that during the occult infection HBV may maintain the capacity to synthesize minute amounts of antigens, which are not revealed by available technical approaches but that are sufficient enough to elicit an HBV-specific T cell response[47,48]. Indeed, in addition to HBV cccDNA molecules[49-51], all viral mRNAs may be detected and quantified in the liver of occult infected individuals[50,52].
Various studies have provided evidence indicating the presence of potent, and multispecific HBV-specific T cell responses in individuals with OBI[53,54]. In particular, one study demonstrated that OBI patients with and without anti-HBc antibodies show a different profile of HBV-specific T cell responses. Although in seronegative OBI patients circulating HBV-specific T cells could be detected at frequencies comparable with seropositive OBI subjects, in vitro expansion and IFN-γ production by HBV-specific T cells was much weaker than in OBI-seropositive individuals[54]. On the basis of the evidence obtained in the woodchuck animal model, it was hypothesized that such differences in seropositive and seronegative OBIs might reflect different modalities of HBV transmission. Indeed, in woodchucks, exposure to low WHV doses (containing less than 103 virions) may lead to a persistent infection in the absence of viral serum markers. Of interest, this so-called woodchuck “primary” occult infection does not confer protective immunity, thus indicating that only infection with a higher dose of inoculum can elicit an efficient memory T cell response[55].
Recent evidence has shown that epigenetic mechanisms may play an important role in the regulation of HBV replication and transcription[40]. In fact, a number of studies have uncovered the importance of viral chromatin dynamic changes (in histone composition and modification) in the regulation of virus replication and gene expression, particularly during the different stages of productive or latent infection and reactivation from latency, highlighting the possibility that epigenetic control may play a role in the outcome of infections[56]. As a note, the transcriptional activity of DNA viruses such as Epstein-Barr virus and herpes viruses appears to be under strict epigenetic control during the latency status[57,58].
HBV cccDNA molecules are harbored in the nucleus of infected hepatocytes as stable minichromosomes packaged into nucleosomal arrays by histone and nonhistone proteins, and similarly to host cell chromatin are subjected to the same enzymatic activities involved in chromatin remodelling. In vivo and in vitro data have provided evidence showing that HBV replication is regulated by the acetylation status of viral cccDNA-bound H3/H4 histones. Interestingly, recruitment of histone deacetylases onto the cccDNA was correlated with low HBV replication in vitro and with low viremia in vivo[59]. Consistent with this finding, class I and class III histone deacetylase inhibitors trichostatin, valproate, and nicotinamide induced an evident increase of both cccDNA-bound acetylated H4 and HBV replication[59]. It is worth mentioning that valproate, which is used clinically as an anticonvulsant and in the treatment of bipolar syndromes, has been shown to reactivate HBV in infected patients[60,61]. In addition to the posttranslational modifications of histones, methylation of CpG-rich regions (CpG islands) in the HBV DNA appear to significantly contribute to the regulation of HBV replication and gene expression[33,40,62,63].
OCCULT HBV AND HCC CLINICAL EVIDENCE
Most findings implicate OBI as an important risk factor accelerating the progression of liver disease and the development of cirrhosis and HCC. A large number of studies conducted since the early 1980s have demonstrated a high rate of HBV infection persistence in HBsAg negative patients with HCC, many of whom were also negative for all HBV serum markers (Table 1)[50,52,64-76]. Despite this body of evidence, however, whether occult HBV could directly induce or contribute to liver damage remains still a major and largely debated topic. The pathobiological significance of HBV-DNA persistence as OBI likely resides in the maintenance of chronic HBV infection through a very low replication rate, and there is evidence indicating that the strongly reduced replication activity could be accompanied by histological signs of mild hepatic inflammation[21,42]. In particular, available data show that the persistence of OBI for up to several decades after the resolution of an HBV-related acute hepatitis in individuals who did not show any clinical or biochemical evidence of liver disease can be associated with histological patterns of a mild necroinflammation in the liver[77,78]. These observations are in accordance with studies on the woodchuck animal model showing that woodchucks convalescing from acute WHV hepatitis show persistence over time of minute amounts of replicating virus associated with mild hepatic necroinflammation continuing for life[45,55].
With the discovery of hepatitis C virus (HCV) and the development of sensitive molecular approaches for HCV and HBV detection since the 1990s, it was possible to unveil a hidden virologic scenario: a high proportion of anti-HCV positive infections were also HBsAg-negative HBV DNA-positive. In particular, occult HBV has been detected in about one-third of HBsAg-negative HCV infected individuals in the Mediterranean Basin and in more than 50% in Far East Asian countries[20,21]. Of interest, some studies from the United States on patients of Caucasian origin undergoing liver transplantation for HCV-related cirrhosis showed that 50% of these subjects were OBI-positive[79]. Furthermore, in the last twenty years, epidemiological studies from different geographic areas have shown that OBI is associated with the most severe forms of liver disease in HCV-infected patients, thus suggesting that occult HBV might favor or accelerate the progression of HCV-related chronic liver disease (CLD)[20-22]. In this respect, interestingly, some reports have shown an association between phases of increased levels of ALT and reappearance of detectable amounts of HBV DNA in the sera of patients with chronic hepatitis C (CHC) and combined OBI, indicating an active role of transient reactivation of HBV replication in liver cell damage[80,81]. These data led to formulate the hypothesis that OBI per se is innocuous, but when other causative agents of liver injury coexist (i.e., HCV infection, alcohol abuse, etc.), the minimal lesions produced by the presence of the occult virus might contribute in making worse the course of the liver disease over time[20,21,82]. Consistent with this, several studies-mostly from Europe and Asia-have found a higher prevalence of OBI in patients with HCV-associated HCC when compared with HCV infected patients that had not developed HCC. In most of these studies, the prevalence of OBI in HCV patients with HCC was as high as 60%-70%, confirming that OBI represents an important risk factor for HCC development in CHC patients and supporting the hypothesis of a synergistic interaction between OBI and HCV in promoting HCC[20-22,42,52,83]. Of note, data from a very recent study have shown that OBI may also lead patients with HCV-associated HCC to have shorter disease-free as well as shorter overall postoperative survival compared with OBI-negative HCV infected patients[84]. Among non-HCV infected patients, a large proportion of subjects with alcohol-related HCC were found to be OBI positive[75], and it is plausible to hypothesize that the hepatocarcinogenic risk increases synergistically also in these patients, just as alcohol is proven to interact with overt HBV infection in causing liver damage in CHB patients[85]. However, although a recent meta-analysis confirmed that OBI increases the risk of HCC development in both HCV and non-HCV infected patients[86], other studies performed in North America did not find such an association[87]. Moreover, a wide variation in the prevalence of OBI has been found in the different case series studied up to date[20,21,42]. These discrepancies appear to be mostly dependent on the lack of methodological uniformity among the different studies, and on the high variability-in terms of sensitivity and specificity-of the technical approaches utilized for OBI detection[15]. Not all the studies, for instance, extended the detection of HBV DNA to both tumor and non-tumor liver tissues in the HCC cases analyzed. Indeed, there is evidence of a significant difference in the detection rate of HBV DNA between tumor and non-tumor tissues from subjects with OBI, thus a certain number of OBI positive cases were missed if only tumor or non-tumor liver tissue was used for the analysis[50,52]. In addition and of great importance, most of the available data was not obtained by applying the currently accepted criteria for the identification of OBI, which are based on the positive detection of HBV DNA in at least two different HBV genomic regions, among at least 3 out of the 4 genomic regions that should be analyzed by nested polymerase chain reaction (PCR)[15]. Of note, data from an observational cohort study have very recently been published, evaluating the clinical evolution of CHC patients according to their OBI status. These patients were followed-up for a median time of 11 years and showed statistically significant differences according to OBI positive/negative status in terms of clinical outcomes. In particular, it was found that HCC development occurred more frequently in OBI positive than OBI negative patients (35% OBI positive vs 8.7% OBI negative patients, respectively, P < 0.01). In addition, OBI positive patients developed more frequently decompensated cirrhosis, and had a cumulative survival rate significantly shorter than OBI-negative subjects[22]. Interestingly, several reports have shown that OBI may be associated with the progression of liver fibrosis and HCC development also in cases with cryptogenic liver disease[50,88]. These data seem to be in contrast with the above mentioned hypothesis that occult HBV might be unable to induce severe liver damage by itself. However, it has to be considered that patients with OBI may overlap with those who would previously have been identified as having recovered from an acute or a chronic HBV infection, and that after HBsAg clearance, the outcomes of OBI may vary significantly depending on the duration of active HBV infection, the extent of liver damage that had occurred before HBsAg clearance, and the interval between the time of HBsAg clearance and that of OBI diagnosis[20,42,89]. In this context, a recent population-based cohort study carried on for more than two decades on Taiwanese mothers screened for HBV infection at each delivery is of great relevance. This study demonstrated that the risk of HCC development was significantly higher in women with persistent HBsAg-positive status; however, among the HBsAg-negative mothers, those who underwent HBsAg sero-clearance during follow-up had a significantly higher risk of HCC development compared to persistently HBsAg-negative women. In fact, this study indicates that HBV maintains its pro-oncogenic role also in the occult status and even in women that are known to be much less prone to develop liver cancer than men[90].
PATHOGENETIC MECHANISMS IMPLICATED IN OCCULT HBV-RELATED HCC
Complex and multifactorial pathogenetic mechanisms underlie HCC development, and much evidence indicates that many of these mechanisms would be implicated in HBV-induced hepatocarcinogenesis[91]. At present, carcinogenesis associated with chronic HBV infection is viewed as a process that includes both direct and indirect mechanisms that might act synergistically. The indirect mechanisms involve liver injury caused by chronic necroinflammation, which provokes repeated cycles of apoptosis, necrosis and compensatory regeneration, and promotes the progression towards cirrhosis (which is a preneoplastic lesion). The direct carcinogenic mechanisms are mainly related to the ability of HBV to integrate into the host genome. Several HBV sequences have been found in tumoral liver tissues, including HBX and truncated pre-S2/S genomic sequences. These viral integrants can produce proteins that have transforming properties. Indeed, both HBx and carboxy-terminally truncated pre-S or S polypeptides are able to alter host gene expression and cellular phenotypes. They may promote growth factor-independent proliferation, resistance to growth inhibition, tissue invasion and metastasis, angiogenesis, reprogramming of energy metabolism, and resistance to apoptosis[16,92,93]. Concerning the occult infection, if it is considered that (1) HBV DNA can persist in the hepatocytes both integrated into the host genome and as free episome, in the form of a mini-chromosome; (2) the virus may maintain the capacity to replicate, to transcribe, and to synthesize proteins, though at very low levels[49-52,69,94]; and (3) the occult virus may induce a mild but continuous status of chronic necroinflammation, thus favoring the progression toward cirrhosis[77,78], then it is reasonable to hypothesize that OBI can contribute to hepatocellular transformation through the same direct and indirect mechanisms that subtend HCC development in overt HBV infection.
The use of sensitive nucleic acid amplification techniques has, in the last decade, made it possible to reveal important molecular and patho-biological aspects of OBI. Indeed, early molecular studies had been performed using DNA hybridization techniques that-because of the very low copy number of HBV DNA per liver cell, typical of OBI-were not sensitive enough to allow the identification of entire full-length HBV genome or other forms of HBV replicative intermediates in hepatic tissue of OBI-positive individuals[42]. More recently, It has been clearly demonstrated that both HBV cccDNA and all viral transcripts-including the pgRNA replicative intermediate and the HBx RNA-are detectable in both tumor and non-tumor tissues of HCC patients with OBI[50-52,69,94]. In addition, by applying real-time PCR quantification techniques it has been possible to more precisely estimate the intrahepatic amounts of total HBV DNA, cccDNA and pgRNA, and also to calculate the pgRNA/cccDNA ratio, which reflects the persistence of viral transcriptional/replicative activity in the liver of these patients[50]. Interestingly, intrahepatic total HBV DNA and cccDNA as well as HBV replicative activity in some HCC patients with OBI proved to be not significantly different than in some HBeAg-negative CHB patients with HCC[49,50]. This evidence suggests that the low replicative level of HBV in the liver tissues of some OBI-positive patients might already be enough to predispose to HCC development.
These data have been widely confirmed in animal models prone to infection by other hepadnaviruses. In fact, in the woodchuck HCC model, a large proportion of animals infected by WHV developed HCC despite the WHV surface antigen seroconversion and the appearance of the corresponding antibody[95,96]. Furthermore, WHV DNA was detected in tumor tissue from these animals and-similarly to what has been observed in humans-the amounts of viral DNA molecules per cell number was much less than in WHsAg positive woodchucks[95,96]. Ground squirrels also represent an interesting model, since once infected with the ground squirrel hepatitis virus (GSHV) these animals are at high risk of developing HCC even after the apparent clearance of the virus, and GSHV DNA has been identified in HCCs developing in completely seronegative animals[97,98].
A further argument supporting the direct role of HBV in liver cancer arises from the detection of HBV DNA in HCCs from non-cirrhotic HBsAg negative individuals[69,74,99-101]. In this respect, the case of a young man with HFE haemochromatosis, seronegative for hepatitis B and C infections, who developed HCC in the absence of cirrhosis has been recently reported. OBI evaluation in this patient, through the analyses of both tumor and non-tumor liver tissues by nested-PCR amplification, revealed the presence of X gene sequences only in tumor DNA extracts. By applying HBV-Alu PCR, it was possible to detect an HBV integration involving a deleted X gene with an intact enhancer-II/basal core promoter region. The study of viral-host junction revealed that the HBV integrant was located upstream of the partitioning-defective-6-homolog-gamma gene (PARD6G) (Figure 2A) and real time-PCR quantification demonstrated that PARD6G was overexpressed in tumor compared to non-tumor liver tissues, suggesting that occult HBV in this patient might have led to a sequel of cellular events that determined the development of HCC even in the absence of cirrhosis[100]. Actually, a previous study had already described cellular gene cis-activation by HBV integrants in tumor cells from non-cirrhotic patients with HCC. In particular, the HBx-related cis-activation of the SERCA1 encoding gene in an HBsAg negative, anti-HBc positive individual was described (Figure 2B)[94]. Apart from cis-acting mechanisms, the large number of integration events described to date support trans-activation as a major mechanism in HCC. Indeed, the products translated from the integrated templates seem to more commonly affect host gene expression by trans-acting mechanisms. In this respect, the expression of the HBx transcriptional transactivator has been frequently reported in both tumor and non-tumor liver tissues of OBI-positive individuals[50,52,69,74,102].
Figure 2.
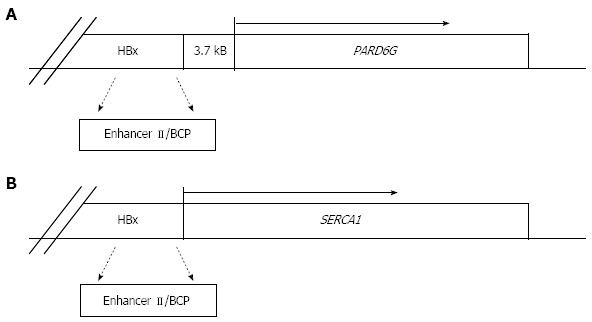
Schematic representation of hepatitis B virus DNA integration in the cellular genome of two different occult hepatitis B virus infected patients. A: Described by Pollicino et al[100]; B: Described by Gozuacik et al[94]. HBx: Hepatitis B virus X gene; BCP: Basal core promoter; PARD6G: Partitioning-defective-6-homolog-gamma; SERCA1: Sarco(endo)plasmic reticulum Ca2+-ATPase 1.
CONCLUSION
The availability of highly sensitive molecular methods has made it possible to unveil several virological features of OBI, to show its worldwide diffusion, and to reveal its possible involvement in different clinical settings. Relevant evidence indicates that HBV persistence as an OBI represents an important risk factor for HCC development. In fact, although several aspects still need to be elucidated, available data strongly indicate that the long-term persistence of OBI might exert an indirect oncogenic role (by chronically sustaining a mild necroinflammation that may contribute to the development of cirrhosis and liver cancer) as well as a direct oncogenic effect through both its integration into the host genome and the maintained synthesis of proteins-as X protein-with transforming properties.
Footnotes
P- Reviewers: Hann HWL, Peng CY S- Editor: Zhai HH L- Editor: A E- Editor: Zhang DN
References
- 1.Zanetti AR, Van Damme P, Shouval D. The global impact of vaccination against hepatitis B: a historical overview. Vaccine. 2008;26:6266–6273. doi: 10.1016/j.vaccine.2008.09.056. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127:S27–S34. doi: 10.1053/j.gastro.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 5.Beasley RP, Hwang LY, Lin CC, Chien CS. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981;2:1129–1133. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- 6.Sherman M. Risk of hepatocellular carcinoma in hepatitis B and prevention through treatment. Cleve Clin J Med. 2009;76 Suppl 3:S6–S9. doi: 10.3949/ccjm.76.s3.02. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen VT, Law MG, Dore GJ. Hepatitis B-related hepatocellular carcinoma: epidemiological characteristics and disease burden. J Viral Hepat. 2009;16:453–463. doi: 10.1111/j.1365-2893.2009.01117.x. [DOI] [PubMed] [Google Scholar]
- 8.Lee CL, Hsieh KS, Ko YC. Trends in the incidence of hepatocellular carcinoma in boys and girls in Taiwan after large-scale hepatitis B vaccination. Cancer Epidemiol Biomarkers Prev. 2003;12:57–59. [PubMed] [Google Scholar]
- 9.Chang MH. Cancer prevention by vaccination against hepatitis B. Recent Results Cancer Res. 2009;181:85–94. doi: 10.1007/978-3-540-69297-3_10. [DOI] [PubMed] [Google Scholar]
- 10.Lee MS, Kim DH, Kim H, Lee HS, Kim CY, Park TS, Yoo KY, Park BJ, Ahn YO. Hepatitis B vaccination and reduced risk of primary liver cancer among male adults: a cohort study in Korea. Int J Epidemiol. 1998;27:316–319. doi: 10.1093/ije/27.2.316. [DOI] [PubMed] [Google Scholar]
- 11.Lee MS . IARC. Tobacco smoke and involuntary smoking. In: IARC Monograph., editor. Lyon, France: International Agency for Research on Cancer; 2004. [Google Scholar]
- 12.Yang HI, Yuen MF, Chan HL, Han KH, Chen PJ, Kim DY, Ahn SH, Chen CJ, Wong VW, Seto WK. Risk estimation for hepatocellular carcinoma in chronic hepatitis B (REACH-B): development and validation of a predictive score. Lancet Oncol. 2011;12:568–574. doi: 10.1016/S1470-2045(11)70077-8. [DOI] [PubMed] [Google Scholar]
- 13.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 14.Di Bisceglie AM. Hepatitis B and hepatocellular carcinoma. Hepatology. 2009;49:S56–S60. doi: 10.1002/hep.22962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raimondo G, Allain JP, Brunetto MR, Buendia MA, Chen DS, Colombo M, Craxì A, Donato F, Ferrari C, Gaeta GB, et al. Statements from the Taormina expert meeting on occult hepatitis B virus infection. J Hepatol. 2008;49:652–657. doi: 10.1016/j.jhep.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 16.Pollicino T, Saitta C, Raimondo G. Hepatocellular carcinoma: the point of view of the hepatitis B virus. Carcinogenesis. 2011;32:1122–1132. doi: 10.1093/carcin/bgr108. [DOI] [PubMed] [Google Scholar]
- 17.Wands JR, Chura CM, Roll FJ, Maddrey WC. Serial studies of hepatitis-associated antigen and antibody in patients receiving antitumor chemotherapy for myeloproliferative and lymphoproliferative disorders. Gastroenterology. 1975;68:105–112. [PubMed] [Google Scholar]
- 18.Hoofnagle JH, Seeff LB, Bales ZB, Zimmerman HJ. Type B hepatitis after transfusion with blood containing antibody to hepatitis B core antigen. N Engl J Med. 1978;298:1379–1383. doi: 10.1056/NEJM197806222982502. [DOI] [PubMed] [Google Scholar]
- 19.Cacciola I, Pollicino T, Squadrito G, Cerenzia G, Orlando ME, Raimondo G. Occult hepatitis B virus infection in patients with chronic hepatitis C liver disease. N Engl J Med. 1999;341:22–26. doi: 10.1056/NEJM199907013410104. [DOI] [PubMed] [Google Scholar]
- 20.Torbenson M, Thomas DL. Occult hepatitis B. Lancet Infect Dis. 2002;2:479–486. doi: 10.1016/s1473-3099(02)00345-6. [DOI] [PubMed] [Google Scholar]
- 21.Raimondo G, Caccamo G, Filomia R, Pollicino T. Occult HBV infection. Semin Immunopathol. 2013;35:39–52. doi: 10.1007/s00281-012-0327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Squadrito G, Cacciola I, Alibrandi A, Pollicino T, Raimondo G. Impact of occult hepatitis B virus infection on the outcome of chronic hepatitis C. J Hepatol. 2013;59:696–700. doi: 10.1016/j.jhep.2013.05.043. [DOI] [PubMed] [Google Scholar]
- 23.Marzano A, Angelucci E, Andreone P, Brunetto M, Bruno R, Burra P, Caraceni P, Daniele B, Di Marco V, Fabrizi F, et al. Prophylaxis and treatment of hepatitis B in immunocompromised patients. Dig Liver Dis. 2007;39:397–408. doi: 10.1016/j.dld.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 24.Hollinger FB. Hepatitis B virus infection and transfusion medicine: science and the occult. Transfusion. 2008;48:1001–1026. doi: 10.1111/j.1537-2995.2008.01701.x. [DOI] [PubMed] [Google Scholar]
- 25.Cheung CK, Lo CM, Man K, Lau GK. Occult hepatitis B virus infection of donor and recipient origin after liver transplantation despite nucleoside analogue prophylaxis. Liver Transpl. 2010;16:1314–1323. doi: 10.1002/lt.22169. [DOI] [PubMed] [Google Scholar]
- 26.Cholongitas E, Papatheodoridis GV, Burroughs AK. Liver grafts from anti-hepatitis B core positive donors: a systematic review. J Hepatol. 2010;52:272–279. doi: 10.1016/j.jhep.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Allain JP, Cox L. Challenges in hepatitis B detection among blood donors. Curr Opin Hematol. 2011;18:461–466. doi: 10.1097/MOH.0b013e32834bac10. [DOI] [PubMed] [Google Scholar]
- 28.Hu KQ. Occult hepatitis B virus infection and its clinical implications. J Viral Hepat. 2002;9:243–257. doi: 10.1046/j.1365-2893.2002.00344.x. [DOI] [PubMed] [Google Scholar]
- 29.Raimondo G, Pollicino T, Cacciola I, Squadrito G. Occult hepatitis B virus infection. J Hepatol. 2007;46:160–170. doi: 10.1016/j.jhep.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Lalazar G, Rund D, Shouval D. Screening, prevention and treatment of viral hepatitis B reactivation in patients with haematological malignancies. Br J Haematol. 2007;136:699–712. doi: 10.1111/j.1365-2141.2006.06465.x. [DOI] [PubMed] [Google Scholar]
- 31.Larrubia JR. Occult hepatitis B virus infection: a complex entity with relevant clinical implications. World J Gastroenterol. 2011;17:1529–1530. doi: 10.3748/wjg.v17.i12.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de la Fuente RA, Gutiérrez ML, Garcia-Samaniego J, Fernández-Rodriguez C, Lledó JL, Castellano G. Pathogenesis of occult chronic hepatitis B virus infection. World J Gastroenterol. 2011;17:1543–1548. doi: 10.3748/wjg.v17.i12.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samal J, Kandpal M, Vivekanandan P. Molecular mechanisms underlying occult hepatitis B virus infection. Clin Microbiol Rev. 2012;25:142–163. doi: 10.1128/CMR.00018-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernandez-Rodriguez CM, Gutierrez ML, Lledó JL, Casas ML. Influence of occult hepatitis B virus infection in chronic hepatitis C outcomes. World J Gastroenterol. 2011;17:1558–1562. doi: 10.3748/wjg.v17.i12.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lledó JL, Fernández C, Gutiérrez ML, Ocaña S. Management of occult hepatitis B virus infection: an update for the clinician. World J Gastroenterol. 2011;17:1563–1568. doi: 10.3748/wjg.v17.i12.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nassal M. Hepatitis B viruses: reverse transcription a different way. Virus Res. 2008;134:235–249. doi: 10.1016/j.virusres.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 37.Seeger C, Mason WS. Hepatitis B virus biology. Microbiol Mol Biol Rev. 2000;64:51–68. doi: 10.1128/mmbr.64.1.51-68.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beck J, Nassal M. Hepatitis B virus replication. World J Gastroenterol. 2007;13:48–64. doi: 10.3748/wjg.v13.i1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Urban S, Schulze A, Dandri M, Petersen J. The replication cycle of hepatitis B virus. J Hepatol. 2010;52:282–284. doi: 10.1016/j.jhep.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 40.Levrero M, Pollicino T, Petersen J, Belloni L, Raimondo G, Dandri M. Control of cccDNA function in hepatitis B virus infection. J Hepatol. 2009;51:581–592. doi: 10.1016/j.jhep.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 41.Zoulim F. New insight on hepatitis B virus persistence from the study of intrahepatic viral cccDNA. J Hepatol. 2005;42:302–308. doi: 10.1016/j.jhep.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 42.Bréchot C, Thiers V, Kremsdorf D, Nalpas B, Pol S, Paterlini-Bréchot P. Persistent hepatitis B virus infection in subjects without hepatitis B surface antigen: clinically significant or purely “occult”? Hepatology. 2001;34:194–203. doi: 10.1053/jhep.2001.25172. [DOI] [PubMed] [Google Scholar]
- 43.Mason AL, Xu L, Guo L, Kuhns M, Perrillo RP. Molecular basis for persistent hepatitis B virus infection in the liver after clearance of serum hepatitis B surface antigen. Hepatology. 1998;27:1736–1742. doi: 10.1002/hep.510270638. [DOI] [PubMed] [Google Scholar]
- 44.European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–185. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 45.Mulrooney-Cousins PM, Michalak TI. Persistent occult hepatitis B virus infection: experimental findings and clinical implications. World J Gastroenterol. 2007;13:5682–5686. doi: 10.3748/wjg.v13.i43.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pollicino T, Raffa G, Costantino L, Lisa A, Campello C, Squadrito G, Levrero M, Raimondo G. Molecular and functional analysis of occult hepatitis B virus isolates from patients with hepatocellular carcinoma. Hepatology. 2007;45:277–285. doi: 10.1002/hep.21529. [DOI] [PubMed] [Google Scholar]
- 47.Rehermann B, Ferrari C, Pasquinelli C, Chisari FV. The hepatitis B virus persists for decades after patients’ recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nat Med. 1996;2:1104–1108. doi: 10.1038/nm1096-1104. [DOI] [PubMed] [Google Scholar]
- 48.Penna A, Artini M, Cavalli A, Levrero M, Bertoletti A, Pilli M, Chisari FV, Rehermann B, Del Prete G, Fiaccadori F, et al. Long-lasting memory T cell responses following self-limited acute hepatitis B. J Clin Invest. 1996;98:1185–1194. doi: 10.1172/JCI118902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Werle-Lapostolle B, Bowden S, Locarnini S, Wursthorn K, Petersen J, Lau G, Trepo C, Marcellin P, Goodman Z, Delaney WE, et al. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology. 2004;126:1750–1758. doi: 10.1053/j.gastro.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 50.Wong DK, Huang FY, Lai CL, Poon RT, Seto WK, Fung J, Hung IF, Yuen MF. Occult hepatitis B infection and HBV replicative activity in patients with cryptogenic cause of hepatocellular carcinoma. Hepatology. 2011;54:829–836. doi: 10.1002/hep.24551. [DOI] [PubMed] [Google Scholar]
- 51.Wong DK, Yuen MF, Poon RT, Yuen JC, Fung J, Lai CL. Quantification of hepatitis B virus covalently closed circular DNA in patients with hepatocellular carcinoma. J Hepatol. 2006;45:553–559. doi: 10.1016/j.jhep.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 52.Pollicino T, Squadrito G, Cerenzia G, Cacciola I, Raffa G, Craxi A, Farinati F, Missale G, Smedile A, Tiribelli C, et al. Hepatitis B virus maintains its pro-oncogenic properties in the case of occult HBV infection. Gastroenterology. 2004;126:102–110. doi: 10.1053/j.gastro.2003.10.048. [DOI] [PubMed] [Google Scholar]
- 53.Bes M, Vargas V, Piron M, Casamitjana N, Esteban JI, Vilanova N, Pinacho A, Quer J, Puig L, Guardia J, et al. T cell responses and viral variability in blood donation candidates with occult hepatitis B infection. J Hepatol. 2012;56:765–774. doi: 10.1016/j.jhep.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 54.Zerbini A, Pilli M, Boni C, Fisicaro P, Penna A, Di Vincenzo P, Giuberti T, Orlandini A, Raffa G, Pollicino T, et al. The characteristics of the cell-mediated immune response identify different profiles of occult hepatitis B virus infection. Gastroenterology. 2008;134:1470–1481. doi: 10.1053/j.gastro.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 55.Coffin CS, Pham TN, Mulrooney PM, Churchill ND, Michalak TI. Persistence of isolated antibodies to woodchuck hepatitis virus core antigen is indicative of occult infection. Hepatology. 2004;40:1053–1061. doi: 10.1002/hep.20419. [DOI] [PubMed] [Google Scholar]
- 56.Lieberman PM. Chromatin organization and virus gene expression. J Cell Physiol. 2008;216:295–302. doi: 10.1002/jcp.21421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takacs M, Banati F, Koroknai A, Segesdi J, Salamon D, Wolf H, Niller HH, Minarovits J. Epigenetic regulation of latent Epstein-Barr virus promoters. Biochim Biophys Acta. 2010;1799:228–235. doi: 10.1016/j.bbagrm.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 58.Bloom DC, Giordani NV, Kwiatkowski DL. Epigenetic regulation of latent HSV-1 gene expression. Biochim Biophys Acta. 2010;1799:246–256. doi: 10.1016/j.bbagrm.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pollicino T, Belloni L, Raffa G, Pediconi N, Squadrito G, Raimondo G, Levrero M. Hepatitis B virus replication is regulated by the acetylation status of hepatitis B virus cccDNA-bound H3 and H4 histones. Gastroenterology. 2006;130:823–837. doi: 10.1053/j.gastro.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 60.Ritchie D, Piekarz RL, Blombery P, Karai LJ, Pittaluga S, Jaffe ES, Raffeld M, Janik JE, Prince HM, Bates SE. Reactivation of DNA viruses in association with histone deacetylase inhibitor therapy: a case series report. Haematologica. 2009;94:1618–1622. doi: 10.3324/haematol.2009.008607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grewal J, Dellinger CA, Yung WK. Fatal reactivation of hepatitis B with temozolomide. N Engl J Med. 2007;356:1591–1592. doi: 10.1056/NEJMc063696. [DOI] [PubMed] [Google Scholar]
- 62.Vivekanandan P, Thomas D, Torbenson M. Methylation regulates hepatitis B viral protein expression. J Infect Dis. 2009;199:1286–1291. doi: 10.1086/597614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaur P, Paliwal A, Durantel D, Hainaut P, Scoazec JY, Zoulim F, Chemin I, Herceg Z. DNA methylation of hepatitis B virus (HBV) genome associated with the development of hepatocellular carcinoma and occult HBV infection. J Infect Dis. 2010;202:700–704. doi: 10.1086/655398. [DOI] [PubMed] [Google Scholar]
- 64.Ruiz J, Sangro B, Cuende JI, Beloqui O, Riezu-Boj JI, Herrero JI, Prieto J. Hepatitis B and C viral infections in patients with hepatocellular carcinoma. Hepatology. 1992;16:637–641. doi: 10.1002/hep.1840160305. [DOI] [PubMed] [Google Scholar]
- 65.Shafritz DA, Shouval D, Sherman HI, Hadziyannis SJ, Kew MC. Integration of hepatitis B virus DNA into the genome of liver cells in chronic liver disease and hepatocellular carcinoma. Studies in percutaneous liver biopsies and post-mortem tissue specimens. N Engl J Med. 1981;305:1067–1073. doi: 10.1056/NEJM198110293051807. [DOI] [PubMed] [Google Scholar]
- 66.Sheu JC, Huang GT, Shih LN, Lee WC, Chou HC, Wang JT, Lee PH, Lai MY, Wang CY, Yang PM. Hepatitis C and B viruses in hepatitis B surface antigen-negative hepatocellular carcinoma. Gastroenterology. 1992;103:1322–1327. doi: 10.1016/0016-5085(92)91523-7. [DOI] [PubMed] [Google Scholar]
- 67.Liang TJ, Jeffers LJ, Reddy KR, De Medina M, Parker IT, Cheinquer H, Idrovo V, Rabassa A, Schiff ER. Viral pathogenesis of hepatocellular carcinoma in the United States. Hepatology. 1993;18:1326–1333. [PubMed] [Google Scholar]
- 68.Paterlini P, Driss F, Nalpas B, Pisi E, Franco D, Berthelot P, Bréchot C. Persistence of hepatitis B and hepatitis C viral genomes in primary liver cancers from HBsAg-negative patients: a study of a low-endemic area. Hepatology. 1993;17:20–29. [PubMed] [Google Scholar]
- 69.Paterlini P, Poussin K, Kew M, Franco D, Brechot C. Selective accumulation of the X transcript of hepatitis B virus in patients negative for hepatitis B surface antigen with hepatocellular carcinoma. Hepatology. 1995;21:313–321. [PubMed] [Google Scholar]
- 70.Yu MC, Yuan JM, Ross RK, Govindarajan S. Presence of antibodies to the hepatitis B surface antigen is associated with an excess risk for hepatocellular carcinoma among non-Asians in Los Angeles County, California. Hepatology. 1997;25:226–228. doi: 10.1002/hep.510250141. [DOI] [PubMed] [Google Scholar]
- 71.Huo TI, Wu JC, Lee PC, Chau GY, Lui WY, Tsay SH, Ting LT, Chang FY, Lee SD. Sero-clearance of hepatitis B surface antigen in chronic carriers does not necessarily imply a good prognosis. Hepatology. 1998;28:231–236. doi: 10.1002/hep.510280130. [DOI] [PubMed] [Google Scholar]
- 72.Koike K, Kobayashi M, Gondo M, Hayashi I, Osuga T, Takada S. Hepatitis B virus DNA is frequently found in liver biopsy samples from hepatitis C virus-infected chronic hepatitis patients. J Med Virol. 1998;54:249–255. [PubMed] [Google Scholar]
- 73.Kubo S, Nishiguchi S, Tamori A, Hirohashi K, Kinoshita H, Kuroki T. Development of hepatocellular carcinoma in patients with HCV infection, with or without past HBV infection, and relationship to age at the time of transfusion. Vox Sang. 1998;74:129. doi: 10.1046/j.1423-0410.1998.7420129.x. [DOI] [PubMed] [Google Scholar]
- 74.Poussin K, Dienes H, Sirma H, Urban S, Beaugrand M, Franco D, Schirmacher P, Bréchot C, Paterlini Bréchot P. Expression of mutated hepatitis B virus X genes in human hepatocellular carcinomas. Int J Cancer. 1999;80:497–505. doi: 10.1002/(sici)1097-0215(19990209)80:4<497::aid-ijc3>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 75.Yotsuyanagi H, Hashidume K, Suzuki M, Maeyama S, Takayama T, Uchikoshi T. Role of hepatitis B virus in hepatocarcinogenesis in alcoholics. Alcohol Clin Exp Res. 2004;28:181S–185S. doi: 10.1097/01.alc.0000134413.68962.ac. [DOI] [PubMed] [Google Scholar]
- 76.Donato F, Gelatti U, Limina RM, Fattovich G. Southern Europe as an example of interaction between various environmental factors: a systematic review of the epidemiologic evidence. Oncogene. 2006;25:3756–3770. doi: 10.1038/sj.onc.1209557. [DOI] [PubMed] [Google Scholar]
- 77.Bläckberg J, Kidd-Ljunggren K. Occult hepatitis B virus after acute self-limited infection persisting for 30 years without sequence variation. J Hepatol. 2000;33:992–997. doi: 10.1016/s0168-8278(00)80134-8. [DOI] [PubMed] [Google Scholar]
- 78.Yuki N, Nagaoka T, Yamashiro M, Mochizuki K, Kaneko A, Yamamoto K, Omura M, Hikiji K, Kato M. Long-term histologic and virologic outcomes of acute self-limited hepatitis B. Hepatology. 2003;37:1172–1179. doi: 10.1053/jhep.2003.50171. [DOI] [PubMed] [Google Scholar]
- 79.Shetty K, Hussain M, Nei L, Reddy KR, Lok AS. Prevalence and significance of occult hepatitis B in a liver transplant population with chronic hepatitis C. Liver Transpl. 2008;14:534–540. doi: 10.1002/lt.21284. [DOI] [PubMed] [Google Scholar]
- 80.Kannangai R, Vivekanandan P, Netski D, Mehta S, Kirk GD, Thomas DL, Torbenson M. Liver enzyme flares and occult hepatitis B in persons with chronic hepatitis C infection. J Clin Virol. 2007;39:101–105. doi: 10.1016/j.jcv.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 81.Chemin I, Guillaud O, Queyron PC, Trépo C. Close monitoring of serum HBV DNA levels and liver enzymes levels is most useful in the management of patients with occult HBV infection. J Hepatol. 2009;51:824–825. doi: 10.1016/j.jhep.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 82.Raimondo G, Pollicino T, Squadrito G. What is the clinical impact of occult hepatitis B virus infection? Lancet. 2005;365:638–640. doi: 10.1016/S0140-6736(05)17961-6. [DOI] [PubMed] [Google Scholar]
- 83.Koike K, Shimotouno K, Okada S, Okamoto H, Hayashi N, Ueda K, Kaneko S, Koike K, Yokosuka O, Chiba T, et al. Survey of hepatitis B virus co-infection in hepatitis C virus-infected patients suffering from chronic hepatitis and hepatocellular carcinoma in Japan. Jpn J Cancer Res. 1999;90:1270–1272. doi: 10.1111/j.1349-7006.1999.tb00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chang ML, Lin YJ, Chang CJ, Yeh C, Chen TC, Yeh TS, Lee WC, Yeh CT. Occult and Overt HBV Co-Infections Independently Predict Postoperative Prognosis in HCV-Associated Hepatocellular Carcinoma. PLoS One. 2013;8:e64891. doi: 10.1371/journal.pone.0064891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chung HT, Lai CL, Wu PC, Lok AS. Synergism of chronic alcoholism and hepatitis B infection in liver disease. J Gastroenterol Hepatol. 1989;4:11–16. doi: 10.1111/j.1440-1746.1989.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 86.Shi Y, Wu YH, Wu W, Zhang WJ, Yang J, Chen Z. Association between occult hepatitis B infection and the risk of hepatocellular carcinoma: a meta-analysis. Liver Int. 2012;32:231–240. doi: 10.1111/j.1478-3231.2011.02481.x. [DOI] [PubMed] [Google Scholar]
- 87.Lok AS, Everhart JE, Di Bisceglie AM, Kim HY, Hussain M, Morgan TR. Occult and previous hepatitis B virus infection are not associated with hepatocellular carcinoma in United States patients with chronic hepatitis C. Hepatology. 2011;54:434–442. doi: 10.1002/hep.24257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chemin I, Trépo C. Clinical impact of occult HBV infections. J Clin Virol. 2005;34 Suppl 1:S15–S21. doi: 10.1016/s1386-6532(05)80005-8. [DOI] [PubMed] [Google Scholar]
- 89.Chen YC, Sheen IS, Chu CM, Liaw YF. Prognosis following spontaneous HBsAg seroclearance in chronic hepatitis B patients with or without concurrent infection. Gastroenterology. 2002;123:1084–1089. doi: 10.1053/gast.2002.36026. [DOI] [PubMed] [Google Scholar]
- 90.Fwu CW, Chien YC, Kirk GD, Nelson KE, You SL, Kuo HS, Feinleib M, Chen CJ. Hepatitis B virus infection and hepatocellular carcinoma among parous Taiwanese women: nationwide cohort study. J Natl Cancer Inst. 2009;101:1019–1027. doi: 10.1093/jnci/djp146. [DOI] [PubMed] [Google Scholar]
- 91.Arzumanyan A, Reis HM, Feitelson MA. Pathogenic mechanisms in HBV- and HCV-associated hepatocellular carcinoma. Nat Rev Cancer. 2013;13:123–135. doi: 10.1038/nrc3449. [DOI] [PubMed] [Google Scholar]
- 92.Tan YJ. Hepatitis B virus infection and the risk of hepatocellular carcinoma. World J Gastroenterol. 2011;17:4853–4857. doi: 10.3748/wjg.v17.i44.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fallot G, Neuveut C, Buendia MA. Diverse roles of hepatitis B virus in liver cancer. Curr Opin Virol. 2012;2:467–473. doi: 10.1016/j.coviro.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 94.Gozuacik D, Murakami Y, Saigo K, Chami M, Mugnier C, Lagorce D, Okanoue T, Urashima T, Bréchot C, Paterlini-Bréchot P. Identification of human cancer-related genes by naturally occurring Hepatitis B Virus DNA tagging. Oncogene. 2001;20:6233–6240. doi: 10.1038/sj.onc.1204835. [DOI] [PubMed] [Google Scholar]
- 95.Korba BE, Wells FV, Baldwin B, Cote PJ, Tennant BC, Popper H, Gerin JL. Hepatocellular carcinoma in woodchuck hepatitis virus-infected woodchucks: presence of viral DNA in tumor tissue from chronic carriers and animals serologically recovered from acute infections. Hepatology. 1989;9:461–470. doi: 10.1002/hep.1840090321. [DOI] [PubMed] [Google Scholar]
- 96.Michalak TI, Pardoe IU, Coffin CS, Churchill ND, Freake DS, Smith P, Trelegan CL. Occult lifelong persistence of infectious hepadnavirus and residual liver inflammation in woodchucks convalescent from acute viral hepatitis. Hepatology. 1999;29:928–938. doi: 10.1002/hep.510290329. [DOI] [PubMed] [Google Scholar]
- 97.Transy C, Fourel G, Robinson WS, Tiollais P, Marion PL, Buendia MA. Frequent amplification of c-myc in ground squirrel liver tumors associated with past or ongoing infection with a hepadnavirus. Proc Natl Acad Sci U S A. 1992;89:3874–3878. doi: 10.1073/pnas.89.9.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Marion PI. Molecular Biology of Hepatitis B Virus. Boca Raton, FL: CRC Press; 1991. [Google Scholar]
- 99.Corradini E, Ferrara F, Pollicino T, Vegetti A, Abbati GL, Losi L, Raimondo G, Pietrangelo A. Disease progression and liver cancer in the ferroportin disease. Gut. 2007;56:1030–1032. doi: 10.1136/gut.2007.122549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pollicino T, Vegetti A, Saitta C, Ferrara F, Corradini E, Raffa G, Pietrangelo A, Raimondo G. Hepatitis B virus DNA integration in tumour tissue of a non-cirrhotic HFE-haemochromatosis patient with hepatocellular carcinoma. J Hepatol. 2013;58:190–193. doi: 10.1016/j.jhep.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 101.Pontisso P, Morsica G, Ruvoletto MG, Barzon M, Perilongo G, Basso G, Cecchetto G, Chemello L, Alberti A. Latent hepatitis B virus infection in childhood hepatocellular carcinoma. Analysis by polymerase chain reaction. Cancer. 1992;69:2731–2735. doi: 10.1002/1097-0142(19920601)69:11<2731::aid-cncr2820691117>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 102.Tamori A, Nishiguchi S, Kubo S, Koh N, Moriyama Y, Fujimoto S, Takeda T, Shiomi S, Hirohashi K, Kinoshita H, et al. Possible contribution to hepatocarcinogenesis of X transcript of hepatitis B virus in Japanese patients with hepatitis C virus. Hepatology. 1999;29:1429–1434. doi: 10.1002/hep.510290520. [DOI] [PubMed] [Google Scholar]
- 103.Bréchot C, Hadchouel M, Scotto J, Fonck M, Potet F, Vyas GN, Tiollais P. State of hepatitis B virus DNA in hepatocytes of patients with hepatitis B surface antigen-positive and -negative liver diseases. Proc Natl Acad Sci U S A. 1981;78:3906–3910. doi: 10.1073/pnas.78.6.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Paterlini P, Gerken G, Nakajima E, Terre S, D’Errico A, Grigioni W, Nalpas B, Franco D, Wands J, Kew M. Polymerase chain reaction to detect hepatitis B virus DNA and RNA sequences in primary liver cancers from patients negative for hepatitis B surface antigen. N Engl J Med. 1990;323:80–85. doi: 10.1056/NEJM199007123230202. [DOI] [PubMed] [Google Scholar]
- 105.Squadrito G, Pollicino T, Cacciola I, Caccamo G, Villari D, La Masa T, Restuccia T, Cucinotta E, Scisca C, Magazzu D, et al. Occult hepatitis B virus infection is associated with the development of hepatocellular carcinoma in chronic hepatitis C patients. Cancer. 2006;106:1326–1330. doi: 10.1002/cncr.21702. [DOI] [PubMed] [Google Scholar]
- 106.Ikeda K, Marusawa H, Osaki Y, Nakamura T, Kitajima N, Yamashita Y, Kudo M, Sato T, Chiba T. Antibody to hepatitis B core antigen and risk for hepatitis C-related hepatocellular carcinoma: a prospective study. Ann Intern Med. 2007;146:649–656. doi: 10.7326/0003-4819-146-9-200705010-00008. [DOI] [PubMed] [Google Scholar]
- 107.Miura Y, Shibuya A, Adachi S, Takeuchi A, Tsuchihashi T, Nakazawa T, Saigenji K. Occult hepatitis B virus infection as a risk factor for hepatocellular carcinoma in patients with chronic hepatitis C in whom viral eradication fails. Hepatol Res. 2008;38:546–556. doi: 10.1111/j.1872-034X.2007.00316.x. [DOI] [PubMed] [Google Scholar]
- 108.Obika M, Shinji T, Fujioka S, Terada R, Ryuko H, Lwin AA, Shiraha H, Koide N. Hepatitis B virus DNA in liver tissue and risk for hepatocarcinogenesis in patients with hepatitis C virus-related chronic liver disease. A prospective study. Intervirology. 2008;51:59–68. doi: 10.1159/000121363. [DOI] [PubMed] [Google Scholar]
- 109.Matsuoka S, Nirei K, Tamura A, Nakamura H, Matsumura H, Oshiro S, Arakawa Y, Yamagami H, Tanaka N, Moriyama M. Influence of occult hepatitis B virus coinfection on the incidence of fibrosis and hepatocellular carcinoma in chronic hepatitis C. Intervirology. 2008;51:352–361. doi: 10.1159/000187720. [DOI] [PubMed] [Google Scholar]
- 110.Kew MC, Welschinger R, Viana R. Occult hepatitis B virus infection in Southern African blacks with hepatocellular carcinoma. J Gastroenterol Hepatol. 2008;23:1426–1430. doi: 10.1111/j.1440-1746.2008.05481.x. [DOI] [PubMed] [Google Scholar]
- 111.Ikeda K, Kobayashi M, Someya T, Saitoh S, Hosaka T, Akuta N, Suzuki F, Suzuki Y, Arase Y, Kumada H. Occult hepatitis B virus infection increases hepatocellular carcinogenesis by eight times in patients with non-B, non-C liver cirrhosis: a cohort study. J Viral Hepat. 2009;16:437–443. doi: 10.1111/j.1365-2893.2009.01085.x. [DOI] [PubMed] [Google Scholar]
- 112.Chen CH, Changchien CS, Lee CM, Tung WC, Hung CH, Hu TH, Wang JH, Wang JC, Lu SN. A study on sequence variations in pre-S/surface, X and enhancer II/core promoter/precore regions of occult hepatitis B virus in non-B, non-C hepatocellular carcinoma patients in Taiwan. Int J Cancer. 2009;125:621–629. doi: 10.1002/ijc.24416. [DOI] [PubMed] [Google Scholar]
- 113.Tamori A, Hayashi T, Shinzaki M, Kobayashi S, Iwai S, Enomoto M, Morikawa H, Sakaguchi H, Shiomi S, Takemura S, et al. Frequent detection of hepatitis B virus DNA in hepatocellular carcinoma of patients with sustained virologic response for hepatitis C virus. J Med Virol. 2009;81:1009–1014. doi: 10.1002/jmv.21488. [DOI] [PubMed] [Google Scholar]
- 114.Kondo R, Nakashima O, Sata M, Imazeki F, Yokosuka O, Tanikawa K, Kage M, Yano H; The Liver Cancer Study Group of Kyushu. Pathological characteristics of patients who develop hepatocellular carcinoma with negative results of both serous hepatitis B surface antigen and hepatitis C virus antibody. Hepatol Res. 2013:Epub ahead of print. doi: 10.1111/hepr.12219. [DOI] [PubMed] [Google Scholar]


