Abstract
Hepatocellular carcinoma (HCC) remains to be one of the top causing cancer-related deaths today. The majority of HCC cases are reported to be the result of chronic hepatitis B virus (HBV) infection. Current treatments for HBV-related HCC revolve around the use of drugs to inhibit viral replication, as a high level of viral load and antigen in circulation often presents a poor patient prognosis. However, existing therapies are inefficient in the complete eradication of HBV, often resulting in tumour recurrence. The involvement of microRNAs (miRNAs) in important processes in HBV-related HCC makes it an important player in the progression of HCC in chronic hepatitis B infected patients. In this review, we discuss the key aspects of HBV infection and the important viral products that may regulate cancer-related processes via their interaction with miRNAs or their closely related protein machinery. Conversely, we also look at how miRNAs may go about regulating the virus, especially in vital processes like viral replication. Apart from miRNAs acting as either oncogenes or tumour-suppressors, we also look at how miRNAs may function as biomarkers that may possibly serve as better candidates than those currently employed in the diagnosis of HBV infection or HBV-related HCC. A summary of the roles of miRNAs in HBV-related HCC will hopefully lead to a gain in understanding of the pathogenesis process and pave the way for new insights in medical therapy.
Keywords: Hepatitis B virus, Hepatitis B virus X protein, Hepatocellular carcinoma, Mechanisms, MicroRNAs, Profiling
Core tip: Hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC) is a complex cancer of the liver stemming from the long term infection of the virus. MiRNAs are found to be involved in a multitude of events, ranging from the diagnosis, pathogenesis as well as possibly in the treatment of this cancer. This review explores the current knowledge in understanding the role of miRNAs in HBV-related HCC, and how it is able to regulate, or be regulated, such that important cellular processes may be affected.
INTRODUCTION
Hepatocellular carcinoma (HCC) is currently third leading cause of cancer-related deaths in the world, with mortality rates reaching up to 500000 deaths per annum. Among all cancers, patients with HCC display the shortest survival times, with most dying within 12 mo of developing the tumour[1]. There are many risk factors for HCC including cirrhosis, chronic active hepatitis (CAH), heavy use of alcohol and chemical contamination by aflatoxins and arsenic in foods and water respectively. Other physiological factors like obesity, gender and age may also pose as a risk in developing HCC. Among these risk factors, chronic hepatitis B virus (HBV) infection remains to be the most significant etiological factor of HCC[2]. Its impact on human health may be illustrated with the following statistics: (1) A total of 2 billion people are estimated to have been infected with HBV before[3]; (2) Current chronic HBV carrier count averages at 240 million people worldwide[4]; (3) More than half of all patients with HCC are chronic carriers of HBV[2]; and (4) The risk of developing HCC is elevated by approximately 30 times in HBsAg carriers as compared to uninfected patients[5,6]. Typically, patients with HBV-related HCC first get infected with the virus, suffer from subsequent damage of liver due to the body’s immune response against the virus, develop cirrhosis and finally HCC.
HBV LIFE CYCLE
Structure of genome
HBV belongs to the Hepadnaviridae group of DNA viruses that preferably replicates within hepatocytes. It is noncytopathic; cytotoxicity in HBV infection results from the body’s immune response against the virus, mainly due to the activity of HBV-specific T cells. The structure of the HBV genome is simple and compact. The 3.2 kb HBV genome is a relaxed, circular, and partially double-stranded DNA. There are 4 known open reading frames (ORFs) within the HBV genome that encode viral surface proteins, the e (HBeAg), core (HBcAg) and surface (HBsAg) antigens, the terminal protein (TP) and a viral reverse DNA polymerase, as well as a small about 17 kDa protein known as hepatitis B virus X protein (HBx). Another viral protein, HBSP, is found to be encoded by a spliced viral transcript[7]. The production of viral transcripts is controlled by 4 promoters and 2 enhancers.
HBV genome integration and replication
While its mechanism of entry into hepatocytes is poorly understood and believed to involve the N-terminus of the viral’s large envelope protein[8], its subsequent infection and replication is better studied. Upon entry into the cells, the virus nucleocapsid is released into the cytoplasm and transported to the nucleus via microtubules. Importins α and β aid in transporting the nucleocapsid through the nuclear membrane and into the nucleus, where the nucleocapsid then disintegrates and the HBV genome is released[9]. Once in the nucleus, the HBV genome undergoes one of two mechanisms. The first is the integration of the viral genome into the host chromosome, which is seen in about 80% of HBV-related HCC[10,11]. It has been shown that HBV causes higher rates of chromosomal alterations as compared to other HCC risk factors[12]. This may result in a loss of tumour suppressor gene functions and/or activate tumour-promoting genes, with some studies showing an activation of the mitotic cell cycle, p53 mutations and activation of the AKT pathway[13]. It is also thought that this integration occurs in damaged host genome harbouring break-points and is not required for viral DNA replication. Apart from the direct integration of HBV genome into the chromosomes, chronic inflammation of the immune system due to viral presence results in increased production of reactive oxygen species (ROS), which can cause further DNA damage and gene mutations[14,15]. The second is its conversion by viral and cellular enzymes into covalently closed circular DNA (cccDNA), which forms the template on which the host RNA polymerase II acts on to produce viral RNA transcripts. The viral mRNAs are then transported into the cytoplasm where they are translated into viral proteins, which play a role in viral replication and HCC development. Among the viral transcripts transcribed, the longest (3.5 kb) transcript is known as the pregenomic RNA, because it serves as the template for viral genome replication. This mRNA strand is packaged along with a viral-encoded DNA polymerase and core proteins to make up the inner core of the new viral capsid. Following reverse transcription of the mRNA strand, a new stand of viral DNA genome is produced within the capsid. A final coat with surface lipoproteins completes the replication process, and the virus may then be either exported out of the cells to infect other cells or return to the nucleus to act as a source of cccDNA for further transcription and replication[16].
HBV viral products
Current therapies in HBV-related HCC aim at repressing viral replication by using drugs (e.g., IFN-α) in activating antiviral defence mechanisms in the body, thus reducing the chances of progression to liver cirrhosis and HCC[17]. The main disadvantage though, of these treatment options, is its ineffectiveness in complete cccDNA elimination in the nucleus of infected hepatocytes[18], resulting in viral rebound upon removal of anti-viral therapy and poor prognosis of patients infected with HBV. The implication of this is that HBV replication and its viral products, both stemming from the cccDNA, play important roles in disease progression and recurrence. Hence, the study of the HBV replication and its viral products is an important step in understanding the mechanisms in which HCC may be developed from HBV infection. The main products of the viral transcripts crucial in HCC are the various antigens and viral proteins. The HBV DNA and viral antigens are important in viral detection during screening while the well-known HBx protein directly plays a part in HBV to HCC progression by dysregulating important cancer-related pathways.
HBx protein
The HBx protein, though small in size, plays a large role in the malignant transformation of chronic hepatitis B (CHB)-infected cells. It may be found in both the cell cytoplasm and nucleus, and is involved in a myriad of cellular activities. Some studies have found that it plays a role in HBV transcription and replication by enhancing the activities of viral promoters and enhancers. In vitro studies show that HBx stimulates viral replication via transactivation[19,20], by interacting with DNA damage binding protein 1 (DDB1) and Src tyrosine kinase protein complex[21,22], by increasing cytosolic calcium ion concentration[23,24] and by directly being recruited to the cccDNA and involved in its transcription[25]. HBV cell lines and mouse livers that were deficient in the X gene had retarded viral replication, and HBx expression was able to restore HBV replication to that of the wild-type levels[21,26].
Apart from its participation in HBV genome replication, HBx may also be involved in the transformation of cells. Till now, it is still uncertain whether HBx is overall, an oncoprotein or a tumour suppressor protein, because of its ability to partake in multiple pathways and activations that result in different cellular phenotypes. It is known to exhibit pleiotropy in important cancer-related pathways like apoptosis, cell division, stress response, protein degradation, inflammation and immune response[27,28]. Some mechanisms in which HBx acts on may be through its interactions with cellular promoters and enhancers of signalling pathways containing important binding sites for NF-κB, activator protein 1 (AP-1), AP-2, CCAAT-enhancer-binding protein (c-EBP), or of cell proliferation genes such as IL-8, TNF, transforming growth factor (TGF) beta and epidermal growth factor receptor (EGRF), or of cellular proliferation signalling pathways like Ras/Raf mitogen-activated protein kinase (MAPK), Src kinases, cjun N-terminal kinase, JAK1/STAT and protein kinase[27,28]. There are also many other important proteins that HBx has interactions with, like tumour suppressor p53[29], proteasomes[22,30], heat shock proteins[31,32], vascular endothelial growth factor (VEGF) and hypoxia inducible factor-1 (HIF-1)[33,34]. It may also be able to regulate the levels of matrix metalloproteinase, a protein highly involved in cell migration[35,36]. Hence, HBx could be seen as a multi-functional protein and may exert non-specific, opposing phenotypic effects on a cell. These in vitro experiments studying the effects of HBx in cell culture highlight the potential mechanisms in which HBx acts upon in infected cells.
These results, however, may not be truly illustrative of the actual impact in vivo. In vitro cell culture experiments tend to employ a higher expression level of HBx for a clearer elucidation of its functions. In chronically infected livers however, HBx expression is found to be kept at low levels[37]. Nevertheless, because of the wide spectrum of activities it is involved in, a high expression level of HBx may not be required for observable changes in cellular phenotype. In infected mouse models, the levels of HBx protein varies, depending on the different genetic makeup and various promoters of the models used. Both apoptosis and proliferation are activated in these models, while simultaneously sensitizing hepatocytes to malignant transformation[38-42]. Infected mice livers also suffer from an impaired regeneration or early cell cycle entry following partial hepatectomy[43-45]. Not all in vivo mouse models are consistent with the outcomes of HBx expression in hepatocytes. Some develop HCC as a result of the high expression of HBx[46], while others with a different genetic background did not, although slight changes in histology are observed in them[47]. This could mean that the outcome of HBx expression in livers vary, depending on the genetic background of the host hepatocyte. Regardless, any deviation from normal, wild-type hepatocyte functioning may potentially contribute to tumorigenesis. Hence, the impact of a small protein like HBx cannot be neglected in HBV infections.
HBV ANTIGENS IN VIRAL DETECTION
The three HBV antigens are characteristic of the various phases of CHB infection. There are five stages of CHB infection: (1) Immune tolerant phase; (2) Immune active phase; (3) Inactive HBV carrier state; (4) HBeAg-negative CHB; and (5) HBsAg-negative phase[18,48]. Current assays for the detection of HBV infection involve serum or blood tests that detect these viral antigens or the antibodies produced by the host. The typical early indications of CHB infection are that of HBeAg-positivity, high viral load and a varied level of serum alanine aminotransferase (ALT). Subsequent entry into the immune active phase results in a reduction of serum viral load, increased ALT levels and liver damage that may lead to fibrosis. Liver senescence may also occur, where hepatocytes show no signs of proliferation or apoptosis. This is believed to be one of the defence mechanisms of cells in preventing malignant transformation into carcinoma[49,50]. Following the immune active phase is the inactive HBV carrier phase where seroconversion to anti-HBe antibodies occurs. At this stage, clinical remission may follow, which is characterized by a low viral load and normal ALT levels. Over time, fibrosis and inflammation may be relieved[48,51,52] and patients may have a good prognosis[53]. However, if viral reactivation occurs, patients enter the HBeAg-negative CHB stage where ALT levels and viral load may rise again while not expressing the e antigen[54], leading to an increase in liver fibrosis and a high risk of developing cirrhosis and HCC. Out of these patients, those with a loss of HBsAg tend to have a positive outcome.
Despite the characterization and presence of the viral antigens in blood serum in the various stages of HBV progression, none of these can really serve as a biomarker or indicator for the prognosis of the infection. Although it is broadly accepted that the persistence of HBeAg and blood plasma viral load are highly correlated with the development of cirrhosis and HCC, the highly variable clearance rates tend to make predicting of disease outcomes difficult. Studies have also shown that HBV DNA may still be detectable in the blood, even years after recovery from acute hepatitis[55]. In the case of occult infections, patients with HBV-related HCC do not have any detectable levels of HBsAg in blood serum but do have low levels of HBV DNA in the serum and insertions in cellular DNA. Some HBsAg-negative HCC patients have HBV DNA present in tumour or adjacent non-tumour liver tissue, while other patients have anti-HBc as the only biomarker of HBV infection. The erratic expression of these viral antigens and HBV DNA in serum, as well as their weak prognostic assessment of disease progression, are evidences of them being inadequate biomarkers of HBV detection and subsequent cirrhosis or HCC development. A new standard of biomarkers need to be found that shortens time to diagnosis and more accurately predicts the course of disease outcome, such that suitable treatments may be administered.
MICRORNAS IN HBV-RELATED HCC
MicroRNAs (miRNAs) are single-stranded, non-coding RNA strands of 19-25 nucleotides in length. They are increasingly found to be involved in many regulatory processes such as cellular proliferation, differentiation, cell cycle regulation, angiogenesis, metabolism, regulation of immune response and apoptosis[56,57]. They perform their function by targeting cellular mRNAs at their 3’UTR, resulting in their degradation or a repression of their translation into proteins. Either host or viral miRNAs may be involved in pathogenesis of HBV-related HCC. It is therefore unsurprising, that miRNAs are found to be dysregulated in cancer, and miRNA signatures are found to be associated with the various cancers[13,58-60]. In HCC, miRNAs have also been shown to correlate with the extent of histological tumour differentiation[61]. Not only that, but miRNA profiling in neoplasms are believed to be more accurate in tumour diagnosis as compared to mRNA profiling[59]. The current lack of adequate, specific, and non-invasive biomarkers calls for the need to develop a new diagnostic technique in HBV-related HCC cases. Recently, an aberrant expression of plasma miRNAs was illustrated in adults with CHB as compared to healthy controls[62-64]. This could open up a new avenue in biomarker establishment that could possibly serve as an accurate tool in HBV-related HCC diagnosis and outcome prediction.
CHB AND HBV-RELATED HCC SERUM MIRNA PROFILING
Similar to the current non-invasive detection of HBV antigens, the human serum is also found to contain miRNAs derived from various tissues and organs. However, unlike the antigens, the levels of these miRNAs are stable because they do not directly depend on the body’s immune response. This makes them a better biomarker candidate of CHB infection and HBV-related HCC than antigens or antibodies, especially in occult HBV infections where patients are negative for HBsAg[65]. The expression of these serum miRNAs may be used to profile various diseases[66-69]. One such study has shown a notable alteration of miRNA expression in HBV as compared to control serum, identifying 13 miRNAs that are significantly upregulated in HBV serum. Importantly, it was also noted that there was no significant difference in these miRNA expression levels between CHB and asymptomatic HBV carriers. In addition, these 13 miRNAs were able to differentiate between HBV-positive HCC, HBV, HCV and control cases in patients with no signs of liver cirrhosis, among which miR-375 and miR-92a were shown to be of significance. Liver cirrhosis may be consequential of HBV infection. Some patients however, carry HBV but do not or have not developed cirrhosis. The ability of miRNAs to distinguish such cases may be useful in the early detection of HBV. MiR-25 and let-7f levels in the serum are found to be upregulated in HBV-positive HCC but are unchanged or downregulated in CHB cases[64]. This may suggest that certain miRNAs are involved in the direct transformation of HBV into HBV-positive HCC, independent of HBV intervention. These findings highlight the extensive effects miRNAs are able to exert in diseases, themselves acting as biomarkers or as a direct cause of malignant transformation.
MIRNAS DYSREGULATED IN A MULTISTEP HBV-RELATED HCC DEVELOPMENT
Not only are miRNAs dysregulated in the blood serum, but also in the liver tissues. HBV-related HCC typically develops in a stepwise manner, starting from CHB-infection, progressing on to liver cirrhosis and/or low/high grade dysplastic nodule (LGDN/HGDN), and finally to HCC[70,71]. Certain miRNAs in liver tissues have been identified to be dysregulated in a similar fashion, varying and accumulating according to the level of disease development. One study found that miR-145 and miR-199b was frequently downregulated in LGDNs with little signs of allelic loss[72], suggesting that miRNA dysregulation may possibly occur earlier than genetic alterations during the start of transformation. The progression to small HCC from LGDN saw an accumulation in dysregulated miRNAs, because on top of the downregulation of miR-145 and miR-199b, miR-224 was also found to be overexpressed in small HCCs and not in LGDNs. These miRNA expressions in HGDN cases were huddled in the middle of that of LGDN and small HCCs. These results suggest that the downregulation of miRNAs like miR-145 and miR-199b may be indicative of early pre-malignant DNs, while the progressive upregulation of miR-224 could imply further malignant transformation onto HCC[73]. The down-regulation of miR-145 has been shown to be involved in the immortalization and of non-tumorigenic cells, and its overexpression is able to inhibit proliferation and cell migration. MiRNAs are also able to distinguish between malignant and benign tumours, and in one study it was found that miR-200c and miR-203 were underexpressed in benign tumours while miR-21, miR-222 and miR-10b were overexpressed in HCC[74].
PLASMA MIRNAS DIFFERENTIATE HBEAG-POSITIVE AND HBEAG-NEGATIVE CHB CASES
Even within CHB patients with no signs of HCC, there are various phases of the infection characterized by the presence (or absence) of certain viral antigens and host antibodies, as aforementioned. Not only are miRNAs able to differentiate between the different phases of HCC transformation (i.e., CHB infection to DN to HCC), plasma miRNAs have also shown to be able to distinguish the phases within CHB infection. One study identified a panel of 16 miRNAs that are found to be significantly upregulated in children with CHB who are positive for HBeAg as compared to their negative HBeAg counterparts[75]. Among these 16 miRNAs identified by this study, miR-122 and miR-194 have been validated by another study to be associated with the presence of HBeAg[76]. All 16 miRNAs also formed a positive correlation with the amount of viral load in the serum, another important risk factor of CHB progression into cirrhosis or HCC[77,78]. The measure of ALT is a marker for liver damage. However, there was also no correlation found between ALT and these 16 miRNAs, which may suggest that circulating miRNAs may be more sensitive biomarkers of liver injury. These findings provide a link between the abundance of certain miRNAs found circulating in blood serum and the immunological stages of CHB infection, either by miRNAs acting as biomarkers, or as a direct link to disease progression by enhancing viral replication. A pathway analysis on the processes affected by these 16 miRNAs showed their heavy involvement in signalling pathways or cancer specific pathways, possibly another means in which their dysregulation could lead to tumorigenesis. Table 1 lists some commonly dysregulated miRNAs in both serum and tumour tissues of CHB-infected and HBV-HCC patients.
Table 1.
Examples of dysregulated miRNAs in hepatitis B virus-infected or hepatitis B virus-related hepatocellular carcinoma patients at various locations as compared to healthy or non-tumorous controls
| Locations | Regulation1 | List of miRNAs |
| HBV serum/plasma | Up | miR-375[64], miR-92a[64], miR-10a[64], miR-223[64], miR-423[64], miR-23b/a[64], miR-342-3p[64], miR-99a[64,75], miR-122a[64], miR-125b[64,75], miR-150[64], let-7c[64], miR-100[75], miR-122[75], miR-122*[75], miR-192[75], miR-192*[75], miR-193b[75], miR-194[75], miR-215[75], miR-365[75], miR-455-5p[75], miR-455-3p[75], miR-483-3p[75], miR-885-5p[75], miR-1247[75] |
| Down | - | |
| HBV-HCC serum/plasma | Up | miR-122[81], miR-192[81], miR-194[81], miR-21[81], miR-23b[81], miR-801[81] |
| Down | miR-223[81], miR-132[104] | |
| Dysplastic nodules | Up | miR-224[73] |
| Down | miR-145[73], miR-199b[73] | |
| HCC tumours | Up | miR-224[74], miR-27a[112], miR-501[110] |
| Down | miR-422b[74], miR-122a[74], miR-132[104], miR-148a[108], let-7a[107], miR-101[102] |
Compared to healthy or non-tumorous controls. HBV: Hepatitis B virus; HCC: Hepatocellular carcinoma.
MIRNA PANEL IN THE CLINICAL DIAGNOSIS AND PROGNOSIS OF HBV-RELATED HCC?
The frequent finding of dysregulated miRNAs in HBV-related HCC, coupled with the insensitivity and inaccuracy of using the current alpha-fetoprotein (AFP) as a serum biomarker for HCC[79,80], has sparked one group to conduct a search with a large pool of participants to identify a stable panel of serum miRNAs that may be potentially useful in the diagnosis of HBV-related HCC. Their study revealed a panel of 7 miRNAs that demonstrated a high accuracy in the diagnosis of HCC: miR-122, miR-192, miR-21, miR-223, miR-26a and miR-801. This panel is able to successfully distinguish HCC from healthy, CHB and liver cirrhosis cases[81], and 4 out of the 7 miRNAs have been validated by other studies to be frequently associated and dysregulated with HCC[74,82-84]. Another group has demonstrated the use of miRNAs in predicting the patient outcome of surgical treatment of HBV-related HCCs. As cancer recurrence (due to metastasis or in the liver) is one of the main setbacks of surgical resection, the group studied the ability of miRNAs to identify HBV-related HCC patients prone to recurrence. Among the miRNAs identified, miR-29a-5p is successfully validated and shown to be upregulated in patients’ formalin fixed, paraffin-embedded tissue (FFPE) with early tumour recurrence as compared to those without[85]. Patients may be therefore selected for various follow-up treatments based on their specific conditions. These new findings, if further validated, may provide much clinical value to the diagnosis and treatment of HBV-related HCC.
MIRNAS AT THE MOLECULAR LEVEL: MECHANISMS AND PATHWAYS IN HBV-RELATED HCC
MiRNAs as biomarkers could be a prelude to their functionally important role in the tumorigenesis process, by having a direct effect in the pathogenesis or the treatment of HBV-related HCC. As biomarkers, they are believed to “leak” out of cells during necrosis or apoptosis, or are secreted out as a survival mechanism of cancer cells. As effectors of disease progression, many studies have been done that illustrate the various roles of miRNAs in important processes and pathways in HBV-related HCC. They are found to be involved in viral replication, latency, epigenetic modulation, interacting with viral products or indirectly activating/deactivating important cancer-related pathways.
MIRNAS AND THEIR INTERACTIONS WITH THE HBV GENOME
Regulating replication through direct interaction with the HBV genome
Many miRNAs have been identified to affect viral replication, either by direct interaction with the HBV genome or indirectly regulating important transcription factors. In cases where miRNAs regulates HBV replication through direct targeting of the HBV genome, one study employed a bioinformatics approach in elucidating possible miRNAs capable of direct binding to the viral DNA. Cellular miR-199a-3p and miR-210 were found to possess putative binding sites in the HBV pre-S1/pre-S2/S region. Further in vitro studies showed a positive binding between miR-199a-3p and the HBsAg encoded region, as well as between miR-210 and the pre-S1 region of the HBV genome. The results of these bindings led to a reduction in HBsAg mRNA and protein levels, and a suppression of HBV replication respectively, without affecting cellular proliferation[86]. These 2 miRNAs are found to be upregulated in HepG2.2.15 as compared to HepG2 cells. The low viral load that is often maintained in CHB-infected patients may be attributed to the overexpression of these 2 miRNAs, possibly contributing to HBV dormancy and a chronic viral infection. Another important miRNA that is found to directly bind to the HBV transcript is miR-122. It is significant as it is the most abundant miRNA in the liver, making its role in HBV replication rather considerable. It directly binds to HBV RNA at the coding regions for viral polymerase and 3’UTR of the core protein, consequentially suppressing HBV replication[87]. It also targets and regulates cyclin G(1) activity and delays entry into the viral cell cycle, therefore indirectly repressing replication[88]. This again, may lead to viral latency and a chronic viral infection. A separate study on miR-122 further validated its negative regulation on viral and antigen expressions by measuring both viral load and antigen expression upon miR-122 transfection, without any significant effect on cell proliferation. They however, also showed that miR-122 negatively regulates heme oxygenase-1 (HO-1) protein expression, a mechanism that activates an opposing pathway as this results in an increase in viral expression[89]. Putting these results of miR-122 together, it may suggest that miR-122 may overall suppress viral replication. However, the extent of this suppression may be attenuated due to the conflicting pathways activated by miR-122.
Not only do cellular miRNAs interact with the HBV genome, it is also believed that viral miRNAs can bind to and regulate their own gene expression. Viral miRNAs have been identified in Epstein-Barr virus infected cells. One study aimed to elucidate the presence of viral miRNAs transcribed by the HBV genome. Using bioinformatics and a computational approach, they found that only 1 putative pre-miRNA that could possibly be encoded by HBV. From there, they generated the putative mature miRNA sequence, which was subsequently detected and validated in blood cells mock-infected with HBV. According to its sequence, the viral miRNA is predicted to complementarily bind to the viral polymerase gene, although no experiments had been carried out to validate this[90]. However, if this is the case, it could mean that the viral miRNA may regulate its own replication via this viral miRNA, possibly leading to latency and a long-term infection in patients.
Regulating replication through epigenetics
In cases where miRNAs indirectly regulate HBV replication, one study showed that miR-1 acts as a positive regulator of the viral replication by targeting histone deacetylase 4 (HDAC4) and increasing the expression of nuclear receptor farnesoid X receptor (FXR)[91]. By directly binding and reducing HDAC4 expression, changes in promoter activity may result. Coupled with the increase in FXR expression by miR-1, HBV RNA levels and antigens were found to increase in culture supernatants of HepG2.2.15 cells due to the increase in transcription at the HBV core promoter. FXR has been shown to increase the synthesis of HBV pregenomic RNA and RI[92]. Other similar studies have been conducted. Where miRNAs repress HBV replication, one study identified miR-141 to effectively repress HBV replication as well as HBsAg/HBeAg expression in HepG2 cells transfected with pHBV1.3 plasmid. Again, cell proliferation was not affected, thereby attributing the effect of miR-141 directly on viral replication. MiR-141 was found to target 3 binding sites in the 3’UTR of PPARA mRNA, reducing both the PPARA mRNA and protein levels[93]. PPARA is a transcription factor with many binding sites at the promoters and enhancers of the HBV genome[94]. Its targeting by miR-141 could have led to a reduction of promoter transcription activities. Other studies have found that PPARA is one of the essential transcription factors required for HBV pregenomic RNA transcription and replication[95].
HBV viral proteins regulate cellular miRNA expression levels
Not only do miRNAs regulate viral processes, but they themselves may also be regulated by viral proteins. The HBx protein is notorious for its extensive role in the many aspects of viral infection, not just in viral replication. It is found associated with miRNAs in many studies. Metastasis, a source of cancer recurrence, is often found linked to miRNAs as they are able to directly regulate cell migration and invasion. Specifically in HBx-transfected hepatoma cells and in transgenic mouse models, the level of miR-29a is found to be overexpressed as compared to control cells and wild type mice respectively. HBx could therefore, upregulate the expression of miR-29a. MiR-29a positively correlates with metastasis potential and its overexpression results in increased migration ability of cells, due to its targeting of phosphatase and tensin homolog (PTEN) at both the mRNA and protein level. The downregulation of PTEN increases phosphorylation of Akt at ser473[96], activating a pathway leading to increased cell migration[97,98]. An elevated level of miR-29a is also found in MHCC-97H cells, which possess a high metastatic potential[99].
The HBx protein may also exert its oncogenic effect via its indirect role in epigenetic modification through miRNAs. One study found that HBx-expressing HepG2 cells, as well as HepG2.2.15 cells, both had a significantly lower miR-101 expression level than control HepG2 cells that do not express the HBx protein. The study also showed that miR-101 directly binds to and targets the 3’UTR of DNA methyltransferase 3A (DNMT3A) mRNA, and is able to decrease both its mRNA and protein levels. The DNMT family is known to catalyse the addition of a methyl group to the 5’-CpG dinucleotide of the cytosine ring, leading to epigenetic gene silencing and is one of the causes of many cancers, including that of the liver[100,101]. The inverse relationship of DNMT3A and miR-101 was further validated by the lowered mRNA expression levels and increased methylation in the promoter regions of 6 tumour suppressive genes (TSG) when miR-101 was inhibited[102]. The 6 TSGs: glutathione S-transferase pi 1 (GSTP1), Ras association domain family 1A (RASSF1), cyclin-dependent kinase inhibitor 2A (CDKN2A), adenomatous polyposis coli (APC), RUNX3 and PRDM2, have been found to be one of the earliest genes to be silenced during HCC transformation[103]. Their lowered expression levels upon miR-101 inhibition may have possibly been partly due to the relief in DNMT3A targeting by miR-101 when miR-101 was inhibited. HBx, therefore, may enhance tumorigenesis by lowering miR-101 expression levels, ultimately leading to the epigenetic silencing of TSGs. Instead of miRNAs altering the epigenetic mechanisms in cells, another study[104] found the converse to be true as well. Specifically, HBx induced DNA methylation of the promoter region of miR-132, leading to the repression of its expression. MiR-132 is downregulated in HepG2.2.15 and HBV-related HCC tissue samples compared to HepG2 cells and the adjacent noncancerous liver tissues respectively. MiR-132 decreases cell proliferation, possibly through the inactivation of the Akt-signalling pathway. The serum levels of miR-132 was also found to correlate with that of the tumour tissues, making miR-132 a non-invasive biomarker candidate of HBV-related HCC diagnosis. These findings demonstrate the multiple roles miRNAs may be involved in, in a single process in this case in the epigenetic modulation of HBV-related HCC.
Another mechanism in which HBx acts as an oncogene is its ability to regulate cell proliferation. One study found that HBx negatively regulates let-7a, a miRNA often downregulated in many cancers, including the liver[105]. Let-7 miRNAs have been shown to be involved in cellular differentiation and proliferation[106], and increasing the expression of let-7a significantly decreases proliferation in both HepG2 and SNU-182 cells. The converse was seen when let-7a was inhibited. Let-7a is subsequently shown to target STAT3, a protein mediating the JAK/STAT pathway, therefore very much involved in cell proliferation. Despite its regulation of proliferation through let-7a and STAT3, HBx expression was not shown to significantly enhance proliferation in HBx-infected cells as compared to control cells. However, this was explained by the simultaneous activation of both proliferation and apoptosis in HBx-infected cells, because when apoptosis was inhibited, HBx-infected cells were seen to show a significantly higher cell proliferation than control cells[107]. Another group saw HBx expression decreasing the level of miR-148a. They identified HPIP as a target of miR-148a and showed that miR-148a (HPIP) negatively (positively) regulates mTOR through the AKT/ERK/FOXO4/ATF5 pathway. mTOR is a protein kinase that leads to cell proliferation, migration and invasion. They also managed to elucidate the mechanism of HBx-miR-148a regulation. HBx was found to interact with p53, a crucial transcription factor and tumour suppressor that is recruited to the miR-148a promoter. The interaction of HBx with p53 thus reduces the availability of p53 transcription of miR-148a. As expected, miR-148a overexpression suppressed cell proliferation, migration and epithelial-mesenchymal transition (EMT). The expression levels of miR-148a (HPIP) are found to be lower (higher) in patients with HBV-related HCC as compared to those with HCC but without HBV infection[108]. A similar study[109] reported opposing results, where they found that HBx enhances miR-148a levels, and inhibiting miR-148a reduced cell proliferation and migration in HBx-expressing hepatoma cells. This difference in observation could have been due to the different liver cancer cell lines used, experimental strategies and population size. This contradiction illustrates the complexity of liver cancer and emphasizes the need for more robust and accurate studies/models of the disease.
Other separate independent studies further describe the role of miRNAs in HBV-related HCC: MiR-501 has been shown to be overexpressed in HepG2 cells with high levels of HBV replication. It is found to increase HBV replication and HBsAg levels by targeting HBXIP, a protein that negatively regulates HBx activity and therefore represses viral replication[110]. MiR-125a-5p has been shown to interact with HBV surface antigen and interfere with its expression[111]. MiR-27a is significantly upregulated in HBV-infected HCC patients and positively correlates with cell proliferation, migration, invasion and cell cycle entry without any effect on apoptosis. Unlike some other miRNAs, the levels of miR-27a in blood plasma and liver tumour tissues do not correlate with each other. A low level of miR-27a expression is reported in the plasma in HBV-infected HCC patients compared to control groups while the inverse was seen in tissue samples[112]. This portrays the variation of miRNA levels at the different locations of the body. Therefore, the choice of biomarkers should be location-dependent.
Regulation of miRNA-related machinery by viral proteins
The regulation of miRNA-related machinery is an indirect method HBV deploys to control the levels of miRNA expression. One study found that Argonaute2 (AGO2) co-localizes with HBcAg and HBsAg in T23 cells expressing these viral proteins. They were found to conglomerate at organelles like the ER, endosomes and Golgi complex. AGO2 is a component of the RNA-induced silencing complex (RISC) and plays a role in the binding of miRNAs to their mRNA targets. As AGO2 is being sequestered by HBcAg and HBsAg, their function in the cells as gene regulators may be lost. Not only that, the miRNAs that are bound to the AGO2 may also lose their targeting ability. Indeed, when AGO2 expression was downregulated, HBV replication was seen to be suppressed[113]. This opens up the possibility of HBV controlling the general miRNA profile in the cell by managing the levels of “functional” AGO2-miRNA and their cellular location. It is believed that the low levels of HBV genome aids in its persistence in CHB-infected patients, and this may be one of the mechanisms of achieving it.
Apart from AGO2, HBV is also able to decrease the expression of Drosha, an RNase III enzyme that processes the biogenesis of miRNAs, through HBx protein. Drosha mRNA and protein levels were both expressed at a lower level in HepG2.2.15 than HepG2 cells. HBx also inhibited the Drosha promoter activity, although the mechanism of this inhibition was not successfully elucidated in the study. They however, speculate that HBx performs this by phosphorylating transcription factor SP1, causing it to bind to the Drosha promoter site and ultimately downregulating Drosha expression[114]. The implication of inhibiting Drosha expression is that HBx may cause an overall downregulation of miRNAs, affecting the entire miRNA profile in the cell. Another independent study[59] reported a general downregulation of miRNAs in tumour tissues compared to healthy tissues. This again, could contribute to its pleiotropic tendencies, making its functional classification in cancers more complex.
HBV replication and miRNAs in interferon-mediated anti-viral defence
The role of miRNAs, by now, can be seen as being very extensive. Not only are they implicated in molecular pathogenesis, they are also very much involved in the effectiveness (or lack of) of HBV therapy. Recent studies have demonstrated the role of miRNAs in interferon (IFN)-mediated anti-viral defence. They have been found to act by targeting viral RNA or by regulating the innate immune signal pathway through the modulation of IFN-β protein expression[115-120]. One study on HBV replication elucidated over 200 differentially expressed miRNAs in HBV expressing HepG2 cells (i.e., HepG2.2.15) and HepG2 cells. A further investigation on their response to IFN-α2b treatment showed fewer miRNAs activated upon IFN treatment in HepG2.2.15 cells as compared to HepG2 cells. Furthermore, a pathway search showed an activation of inflammation, cell cycle, IFN-γ, interleukin and β cell activation when HepG2 cells were treated with IFN-therapy due to the change in HepG2 miRNA profile upon treatment. HepG2.2.15 cells, on the other hand, only showed a dysregulation of 5 miRNAs, among which only 1 (i.e., miR-98) had some involvement in apoptosis and inflammation. This suggests that HBV replication may impair the IFN-mediated anti-viral miRNA response in HepG2 cells, further extending the effects of HBV replication and miRNAs not just on disease progression but also on current IFN treatment[121]. Table 2 lists some examples of miRNAs and their targets in HBV-related HCC.
Table 2.
Examples of miRNAs involved in chronic hepatitis B infection or hepatitis B virus-related hepatocellular carcinoma
| miRNA | Target gene | Process(es) affected |
| miR-1 | HDAC4 | HBV replication |
| E2F5 | Cell proliferation; cell cycle | |
| miR-199a-3p, miR-210 | HBV genome | HBV replication and antigen production |
| miR-501 | HBXIP | HBV replication |
| miR-141 | PPARA | HBV replication |
| miR-29a | PTEN | Cell migration |
| miR-101 | DNMT3A | DNA methylation and gene silencing |
| let-7 | STAT3 | Cell proliferation |
| miR-148a | HPIP | Cell proliferation; migration; EMT |
Their validated miRNA targets and subsequent effects on cells are listed accordingly. HBV: Hepatitis B virus; EMT: Epithelial-mesenchymal transition.
Artificial miRNAs in anti-viral defence strategy?
The current lack of effectiveness in HBV-related HCC treatments (IFN-related or not) presents a need to establish better treatment options. One alternative could be the use of artificial miRNAs in targeting the HBV genome. One study developed 3 artificial plasmid miRNAs targeting various regions of the S region of the genome. The transfection of these into HepG2.2.15 cells resulted in a significant reduction of viral mRNA, up to 83% decrease in HBV S mRNA expression levels. HBsAg and HBeAg protein levels, as well as HBV DNA levels were also significantly decreased in the culture supernatant and within the cells respectively. The 3 artificial miRNAs had varying levels of effectiveness. Among them, HBV-S608 proved to be the most potent in repressing viral replication[122]. The use of artificial miRNAs may potentially improve the effectiveness of HBV-related HCC therapies, because of its relative insensitivity to the interferon system and little cytotoxicity induced in the host cell. As seen in the study, the use of synthetic miRNAs to target viral genome could be a good option. However, more work needs to be done to formulate an optimal plasmid capable of effective HBV targeting in HBV-infected cells.
TECHNIQUES IN MIRNA CANCER RESEARCH
A brief summary of techniques often encountered in miRNA research may be useful in the design and planning of experiments. Most studies make use of microarrays in establishing differentially expressed miRNAs between two or more samples of cells/tissues/serum. A general panel of miRNAs may be used or selected miRNAs involved in processes important in the area of study may be chosen to streamline the scope of the work. Following the identification of dysregulated miRNAs, their validation is usually confirmed by a qPCR or northern blot analysis. Subsequent work may revolve around the identification of their target mRNAs and/or a functional analysis of their effects in cells. Functional analysis of miRNAs in HBV-related HCC typically covers measuring viral antigen detection and expression levels, HBV DNA expression levels, cell proliferation and apoptosis, cell migration and invasion, EMT progression, cell cycle, or any other processes important in cancer.
In the identification of direct mRNA targets, a bioinformatics analysis and/or computational approach is usually used in predicting putative mRNA targets. Common programs such as TargetScan, miRanda, miRTar etc., are often used in combination and overlapping targets are chosen for further experimental validation. Subsequently, surface plasmon resonance (SPR) and luciferase assays may be carried out to detect RNA interactions in vitro and in vivo respectively. To further validate the binding, target mRNA and/or protein levels may be measured to study the level of target regulation, whether at the mRNA or protein level. Pathway analysis may also be investigated to see which pathways are activated, and the miRNA may be “categorized” as an oncogene or tumour suppressor, depending on the pathways activated or deactivated by it. Why miRNAs are temporarily “categorized”, is because miRNAs are known to affect a myriad of cellular pathways, sometimes even conflicting somewhat like HBx. Also, their overall effect depends on cell type and stages of cell differentiation etc., hence their individual classification as an oncogene or tumour suppressor may not be obvious just by studying their effect on a single process or pathway.
CONCLUSION
HBV-related HCC is a cancer with two individually complex parts intertwined together: HBV and HCC. It is currently a dire medical problem that is the cause of many deaths in spite of the advance in technology. This is partially due to its complicated nature involving both viral and host cell components. HBV alone has many parts to it: Genome, cccDNA and replication machinery, mRNA transcripts, protein products and possibly viral miRNAs. These could all be involved in the replication of the virus, its integration into host chromosomes, as well as interfering with important cellular pathways that may lead to chronic infection and possibly HCC. These may themselves also be potential targets in HCC therapy. Liver transplantation or surgical resections are more likely to produce a better patient prognosis. However, the often late diagnosis of the cancer makes these options unavailable to patients, therefore requiring the need for other types of suitable treatment like chemotherapy. The problem with those treatments is that they produce a lot of toxicity and also evoke the immune response. The existing IFN therapy is unable to fully eliminate the cccDNA in HBV-infected cells, possibly an outcome that leads to cancer recurrence.
The involvement of miRNAs in HBV-related HCC is very extensive. As they are stably present in the blood serum and plasma, they may act as non-invasive biomarkers for the diagnosis of HBV-infection and even for its progression into cirrhosis, DNs and HCCs. One other advantage miRNAs have over current biomarkers like the various viral antigens and DNA is that they are stable and not as immune-dependent. MiRNA profiles may be derived directly from liver tumour tissues, however it must be noted that the miRNA profile in these tissues may differ from that in the serum/plasma. Not only that, but it has been shown that there is a significantly higher concentration of miRNAs found in the serum as compared to plasma[123]. This could indicate some difference, possibly, even between serum vs plasma miRNA. The miRNAs present in serum/plasma is believed to be excreted from the hepatocytes, either as part of a signalling cascade in aiding viral replication and infection or as the body’s means of preventing viral growth and further spread. It is important that an accurate, stable panel of miRNAs be designed such that it is representative of the diagnosis status of the patient, or even one that has prognostic value. For this to be effectively established, the time of miRNA profiling, in what disease stage, from which location the miRNA profile is obtained, and even the HBV genotype may be important factors to consider that could give rise to different miRNA profiles.
Apart from miRNAs being biomarkers, they may be important players in the pathogenesis of HBV-related HCC or take part in its eradication. MiRNAs may be from host cells or transcribed by the virus. Till now, not much of HBV miRNA is elucidated, but they potentially may exist and interfere in important cellular processes in cancer progression or in chronic HBV infection. On the other hand, the vast and ever-increasing amount of miRNAs being discovered in human cells, accompanied by their multiple non-specific targeting of mRNAs, enable them to be involved in a multitude of processes. Not surprisingly, they have been frequently reported to take part in HBV-related HCC progression. The different components of HBV may interact with miRNAs. One important aspect where miRNAs are involved in HBV to HCC progression is in viral replication. Cellular miRNAs may suppress HBV replication by directly binding to the genome or by altering replication epigenetically. Interactions between cellular miRNAs and transcription factors or epigenetic machinery like HDAC are some examples of how miRNAs indirectly regulate viral replication. Moving away from the HBV genome, miRNAs may also interact with viral proteins, especially with HBx. HBx affects a whole list of processes, and hence their association with miRNAs is expected. It is still unclear, exactly how HBx is able to regulate the levels of miRNAs. It could be at the transcriptional level, where miRNA transcription is either enhanced or inhibited by HBx. Perhaps miRNAs may be transcribed but also quickly degraded or sequestered by HBx. More studies are required to further elucidate the mechanisms of this interaction. However, it is observed that the presence of HBx is able to alter the expression of many miRNAs, such that important cancer processes are activated. Cell proliferation, viral replication, migration and invasion, are a few of the many processes that could be activated by HBx through its miRNA regulation. Not only does HBx regulate miRNAs, it also is found to regulate Drosha, an important enzyme involved in miRNA biogenesis. By affecting the expression levels of Drosha, HBx is able to control the miRNA profile of a cell at a general level, instead of just single, individual miRNAs each time. In addition, viral antigens may also bind to and sequester AGO2, another enzyme involved in the miRNA targeting of mRNAs. This may result in a quarantine of miRNAs along with AGO2, leading to their loss of function in a cell.
These examples illustrate the various independent ways where miRNAs may be involved in HBV-related HCC. Figure 1 shows a summary of the main aspects of HBV-related HCC where miRNAs may play an important role in the infection and tumorigenesis process. The pleiotropic properties of both HBx and miRNAs make the cancer much more complex to understand and treat. However, studies have also shown that miRNAs themselves may possess some anti-viral and anti-HCC effects. Their application as medical biomarkers could be the first step in improving current diagnostic methods used in HBV-related HCC, by either resulting in its earlier detection and/or greater prognostic value. Their further understanding would be required before they may become of any therapeutic value.
Figure 1.
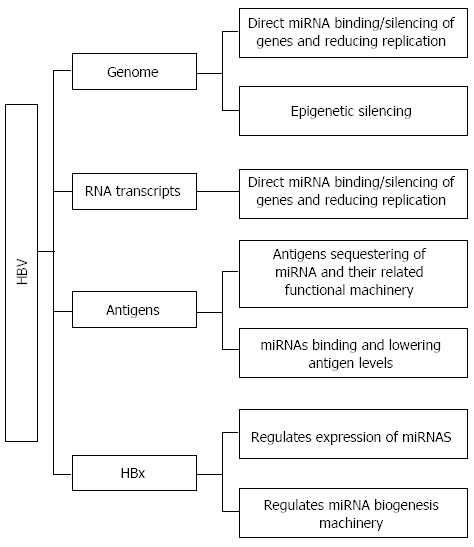
General overview of how miRNAs may associate with hepatitis B virus, apart from acting as biomarkers in chronic hepatitis B-infected or hepatitis B virus-related hepatocellular carcinoma patients. Hepatitis B virus (HBV) consists of 4 main components that may interact with miRNAs: The genome, RNA transcripts, the various antigens and the pleiotropic hepatitis B virus X (HBx) protein. MiRNAs may bind to the genome, resulting in the silencing of tumour-suppressor genes, or this could be achieved epigenetically through miRNAs’ modulation of histone deacetylase etc. The binding of miRNAs to HBV DNA or RNA transcripts may also regulate HBV replication, a mechanism that may lead to latency and chronic hepatitis B infection. Important protein products like the various antigens and HBx could also regulate miRNA activity. The antigens are found to localize with miRNAs and Argonaute2, which may result in their sequestering and loss of function. MiRNAs, conversely, are found to target and reduce the expression levels of circulating antigens. HBx protein affects miRNAs at both an individual level, by regulating specific miRNAs’ expression, and also at a general miRNA level, by decreasing the level of Drosha, an enzyme involved in the biogenesis of miRNAs. As miRNAs are able to regulate mRNA and subsequently protein levels, the effect of HBV on the miRNA profile is considerable, implying its potentially substantial modulation of important cellular activities.
Footnotes
P- Reviewers: Chun YH, Corrales FJ, Grizzi F, Shi Q S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 3.Ganem D, Prince AM. Hepatitis B virus infection--natural history and clinical consequences. N Engl J Med. 2004;350:1118–1129. doi: 10.1056/NEJMra031087. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Hepatitis B. 2013 July. Available from: http://www.who.int/mediacentre/factsheets/fs204/en/
- 5.Hassan MM, Hwang LY, Hatten CJ, Swaim M, Li D, Abbruzzese JL, Beasley P, Patt YZ. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002;36:1206–1213. doi: 10.1053/jhep.2002.36780. [DOI] [PubMed] [Google Scholar]
- 6.Sun CA, Wu DM, Lin CC, Lu SN, You SL, Wang LY, Wu MH, Chen CJ. Incidence and cofactors of hepatitis C virus-related hepatocellular carcinoma: a prospective study of 12,008 men in Taiwan. Am J Epidemiol. 2003;157:674–682. doi: 10.1093/aje/kwg041. [DOI] [PubMed] [Google Scholar]
- 7.Soussan P, Garreau F, Zylberberg H, Ferray C, Brechot C, Kremsdorf D. In vivo expression of a new hepatitis B virus protein encoded by a spliced RNA. J Clin Invest. 2000;105:55–60. doi: 10.1172/JCI8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lepère-Douard C, Trotard M, Le Seyec J, Gripon P. The first transmembrane domain of the hepatitis B virus large envelope protein is crucial for infectivity. J Virol. 2009;83:11819–11829. doi: 10.1128/JVI.01026-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rabe B, Vlachou A, Panté N, Helenius A, Kann M. Nuclear import of hepatitis B virus capsids and release of the viral genome. Proc Natl Acad Sci USA. 2003;100:9849–9854. doi: 10.1073/pnas.1730940100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bréchot C, Gozuacik D, Murakami Y, Paterlini-Bréchot P. Molecular bases for the development of hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC) Semin Cancer Biol. 2000;10:211–231. doi: 10.1006/scbi.2000.0321. [DOI] [PubMed] [Google Scholar]
- 11.Brechot C, Pourcel C, Louise A, Rain B, Tiollais P. Presence of integrated hepatitis B virus DNA sequences in cellular DNA of human hepatocellular carcinoma. Nature. 1980;286:533–535. doi: 10.1038/286533a0. [DOI] [PubMed] [Google Scholar]
- 12.Marchio A, Pineau P, Meddeb M, Terris B, Tiollais P, Bernheim A, Dejean A. Distinct chromosomal abnormality pattern in primary liver cancer of non-B, non-C patients. Oncogene. 2000;19:3733–3738. doi: 10.1038/sj.onc.1203713. [DOI] [PubMed] [Google Scholar]
- 13.Boyault S, Rickman DS, de Reyniès A, Balabaud C, Rebouissou S, Jeannot E, Hérault A, Saric J, Belghiti J, Franco D, et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology. 2007;45:42–52. doi: 10.1002/hep.21467. [DOI] [PubMed] [Google Scholar]
- 14.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 15.Safadi R, Friedman SL. Hepatic fibrosis--role of hepatic stellate cell activation. MedGenMed. 2002;4:27. [PubMed] [Google Scholar]
- 16.Lu X, Block T. Study of the early steps of the Hepatitis B Virus life cycle. Int J Med Sci. 2004;1:21–33. doi: 10.7150/ijms.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grimm D, Thimme R, Blum HE. HBV life cycle and novel drug targets. Hepatol Int. 2011;5:644–653. doi: 10.1007/s12072-011-9261-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.European Association For The Study Of The Liver. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227–242. doi: 10.1016/j.jhep.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Tang H, Oishi N, Kaneko S, Murakami S. Molecular functions and biological roles of hepatitis B virus x protein. Cancer Sci. 2006;97:977–983. doi: 10.1111/j.1349-7006.2006.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melegari M, Wolf SK, Schneider RJ. Hepatitis B virus DNA replication is coordinated by core protein serine phosphorylation and HBx expression. J Virol. 2005;79:9810–9820. doi: 10.1128/JVI.79.15.9810-9820.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leupin O, Bontron S, Schaeffer C, Strubin M. Hepatitis B virus X protein stimulates viral genome replication via a DDB1-dependent pathway distinct from that leading to cell death. J Virol. 2005;79:4238–4245. doi: 10.1128/JVI.79.7.4238-4245.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Z, Protzer U, Hu Z, Jacob J, Liang TJ. Inhibition of cellular proteasome activities enhances hepadnavirus replication in an HBX-dependent manner. J Virol. 2004;78:4566–4572. doi: 10.1128/JVI.78.9.4566-4572.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan C, Guo H, Zheng M, Chen Y, Huang W. Involvement of mitochondrial permeability transition in hepatitis B virus replication. Virus Res. 2009;145:307–311. doi: 10.1016/j.virusres.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 24.McClain SL, Clippinger AJ, Lizzano R, Bouchard MJ. Hepatitis B virus replication is associated with an HBx-dependent mitochondrion-regulated increase in cytosolic calcium levels. J Virol. 2007;81:12061–12065. doi: 10.1128/JVI.00740-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levrero M, Pollicino T, Petersen J, Belloni L, Raimondo G, Dandri M. Control of cccDNA function in hepatitis B virus infection. J Hepatol. 2009;51:581–592. doi: 10.1016/j.jhep.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 26.Tang H, Delgermaa L, Huang F, Oishi N, Liu L, He F, Zhao L, Murakami S. The transcriptional transactivation function of HBx protein is important for its augmentation role in hepatitis B virus replication. J Virol. 2005;79:5548–5556. doi: 10.1128/JVI.79.9.5548-5556.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen DH, Ludgate L, Hu J. Hepatitis B virus-cell interactions and pathogenesis. J Cell Physiol. 2008;216:289–294. doi: 10.1002/jcp.21416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X, Zhang H, Ye L. Effects of hepatitis B virus X protein on the development of liver cancer. J Lab Clin Med. 2006;147:58–66. doi: 10.1016/j.lab.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Bergametti F, Prigent S, Luber B, Benoit A, Tiollais P, Sarasin A, Transy C. The proapoptotic effect of hepatitis B virus HBx protein correlates with its transactivation activity in stably transfected cell lines. Oncogene. 1999;18:2860–2871. doi: 10.1038/sj.onc.1202643. [DOI] [PubMed] [Google Scholar]
- 30.Sirma H, Weil R, Rosmorduc O, Urban S, Israël A, Kremsdorf D, Bréchot C. Cytosol is the prime compartment of hepatitis B virus X protein where it colocalizes with the proteasome. Oncogene. 1998;16:2051–2063. doi: 10.1038/sj.onc.1201737. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka Y, Kanai F, Kawakami T, Tateishi K, Ijichi H, Kawabe T, Arakawa Y, Kawakami T, Nishimura T, Shirakata Y, et al. Interaction of the hepatitis B virus X protein (HBx) with heat shock protein 60 enhances HBx-mediated apoptosis. Biochem Biophys Res Commun. 2004;318:461–469. doi: 10.1016/j.bbrc.2004.04.046. [DOI] [PubMed] [Google Scholar]
- 32.Zhang SM, Sun DC, Lou S, Bo XC, Lu Z, Qian XH, Wang SQ. HBx protein of hepatitis B virus (HBV) can form complex with mitochondrial HSP60 and HSP70. Arch Virol. 2005;150:1579–1590. doi: 10.1007/s00705-005-0521-1. [DOI] [PubMed] [Google Scholar]
- 33.Yoo YG, Na TY, Seo HW, Seong JK, Park CK, Shin YK, Lee MO. Hepatitis B virus X protein induces the expression of MTA1 and HDAC1, which enhances hypoxia signaling in hepatocellular carcinoma cells. Oncogene. 2008;27:3405–3413. doi: 10.1038/sj.onc.1211000. [DOI] [PubMed] [Google Scholar]
- 34.Han HK, Han CY, Cheon EP, Lee J, Kang KW. Role of hypoxia-inducible factor-alpha in hepatitis-B-virus X protein-mediated MDR1 activation. Biochem Biophys Res Commun. 2007;357:567–573. doi: 10.1016/j.bbrc.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 35.Yu FL, Liu HJ, Lee JW, Liao MH, Shih WL. Hepatitis B virus X protein promotes cell migration by inducing matrix metalloproteinase-3. J Hepatol. 2005;42:520–527. doi: 10.1016/j.jhep.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 36.Ou DP, Tao YM, Tang FQ, Yang LY. The hepatitis B virus X protein promotes hepatocellular carcinoma metastasis by upregulation of matrix metalloproteinases. Int J Cancer. 2007;120:1208–1214. doi: 10.1002/ijc.22452. [DOI] [PubMed] [Google Scholar]
- 37.Su Q, Schröder CH, Hofmann WJ, Otto G, Pichlmayr R, Bannasch P. Expression of hepatitis B virus X protein in HBV-infected human livers and hepatocellular carcinomas. Hepatology. 1998;27:1109–1120. doi: 10.1002/hep.510270428. [DOI] [PubMed] [Google Scholar]
- 38.Slagle BL, Lee TH, Medina D, Finegold MJ, Butel JS. Increased sensitivity to the hepatocarcinogen diethylnitrosamine in transgenic mice carrying the hepatitis B virus X gene. Mol Carcinog. 1996;15:261–269. doi: 10.1002/(SICI)1098-2744(199604)15:4<261::AID-MC3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 39.Terradillos O, Billet O, Renard CA, Levy R, Molina T, Briand P, Buendia MA. The hepatitis B virus X gene potentiates c-myc-induced liver oncogenesis in transgenic mice. Oncogene. 1997;14:395–404. doi: 10.1038/sj.onc.1200850. [DOI] [PubMed] [Google Scholar]
- 40.Terradillos O, Pollicino T, Lecoeur H, Tripodi M, Gougeon ML, Tiollais P, Buendia MA. p53-independent apoptotic effects of the hepatitis B virus HBx protein in vivo and in vitro. Oncogene. 1998;17:2115–2123. doi: 10.1038/sj.onc.1202432. [DOI] [PubMed] [Google Scholar]
- 41.Madden CR, Finegold MJ, Slagle BL. Hepatitis B virus X protein acts as a tumor promoter in development of diethylnitrosamine-induced preneoplastic lesions. J Virol. 2001;75:3851–3858. doi: 10.1128/JVI.75.8.3851-3858.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Madden CR, Finegold MJ, Slagle BL. Altered DNA mutation spectrum in aflatoxin b1-treated transgenic mice that express the hepatitis B virus x protein. J Virol. 2002;76:11770–11774. doi: 10.1128/JVI.76.22.11770-11774.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu BK, Li CC, Chen HJ, Chang JL, Jeng KS, Chou CK, Hsu MT, Tsai TF. Blocking of G1/S transition and cell death in the regenerating liver of Hepatitis B virus X protein transgenic mice. Biochem Biophys Res Commun. 2006;340:916–928. doi: 10.1016/j.bbrc.2005.12.089. [DOI] [PubMed] [Google Scholar]
- 44.Tralhao JG, Roudier J, Morosan S, Giannini C, Tu H, Goulenok C, Carnot F, Zavala F, Joulin V, Kremsdorf D, et al. Paracrine in vivo inhibitory effects of hepatitis B virus X protein (HBx) on liver cell proliferation: an alternative mechanism of HBx-related pathogenesis. Proc Natl Acad Sci USA. 2002;99:6991–6996. doi: 10.1073/pnas.092657699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hodgson AJ, Keasler VV, Slagle BL. Premature cell cycle entry induced by hepatitis B virus regulatory HBx protein during compensatory liver regeneration. Cancer Res. 2008;68:10341–10348. doi: 10.1158/0008-5472.CAN-08-2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim CM, Koike K, Saito I, Miyamura T, Jay G. HBx gene of hepatitis B virus induces liver cancer in transgenic mice. Nature. 1991;351:317–320. doi: 10.1038/351317a0. [DOI] [PubMed] [Google Scholar]
- 47.Reifenberg K, Löhler J, Pudollek HP, Schmitteckert E, Spindler G, Köck J, Schlicht HJ. Long-term expression of the hepatitis B virus core-e- and X-proteins does not cause pathologic changes in transgenic mice. J Hepatol. 1997;26:119–130. doi: 10.1016/s0168-8278(97)80018-9. [DOI] [PubMed] [Google Scholar]
- 48.Hoofnagle JH, Doo E, Liang TJ, Fleischer R, Lok AS. Management of hepatitis B: summary of a clinical research workshop. Hepatology. 2007;45:1056–1075. doi: 10.1002/hep.21627. [DOI] [PubMed] [Google Scholar]
- 49.Ikeda H, Sasaki M, Sato Y, Harada K, Zen Y, Mitsui T, Nakanuma Y. Large cell change of hepatocytes in chronic viral hepatitis represents a senescent-related lesion. Hum Pathol. 2009;40:1774–1782. doi: 10.1016/j.humpath.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 50.Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–233. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 51.Realdi G, Alberti A, Rugge M, Bortolotti F, Rigoli AM, Tremolada F, Ruol A. Seroconversion from hepatitis B e antigen to anti-HBe in chronic hepatitis B virus infection. Gastroenterology. 1980;79:195–199. [PubMed] [Google Scholar]
- 52.Liaw YF, Chu CM, Su IJ, Huang MJ, Lin DY, Chang-Chien CS. Clinical and histological events preceding hepatitis B e antigen seroconversion in chronic type B hepatitis. Gastroenterology. 1983;84:216–219. [PubMed] [Google Scholar]
- 53.Martinot-Peignoux M, Boyer N, Colombat M, Akremi R, Pham BN, Ollivier S, Castelnau C, Valla D, Degott C, Marcellin P. Serum hepatitis B virus DNA levels and liver histology in inactive HBsAg carriers. J Hepatol. 2002;36:543–546. doi: 10.1016/s0168-8278(02)00004-1. [DOI] [PubMed] [Google Scholar]
- 54.Carman WF, Jacyna MR, Hadziyannis S, Karayiannis P, McGarvey MJ, Makris A, Thomas HC. Mutation preventing formation of hepatitis B e antigen in patients with chronic hepatitis B infection. Lancet. 1989;2:588–591. doi: 10.1016/s0140-6736(89)90713-7. [DOI] [PubMed] [Google Scholar]
- 55.Rehermann B, Ferrari C, Pasquinelli C, Chisari FV. The hepatitis B virus persists for decades after patients’ recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nat Med. 1996;2:1104–1108. doi: 10.1038/nm1096-1104. [DOI] [PubMed] [Google Scholar]
- 56.Kato M, Slack FJ. microRNAs: small molecules with big roles - C. elegans to human cancer. Biol Cell. 2008;100:71–81. doi: 10.1042/BC20070078. [DOI] [PubMed] [Google Scholar]
- 57.Ruan K, Fang X, Ouyang G. MicroRNAs: novel regulators in the hallmarks of human cancer. Cancer Lett. 2009;285:116–126. doi: 10.1016/j.canlet.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 58.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 59.Caldas C, Brenton JD. Sizing up miRNAs as cancer genes. Nat Med. 2005;11:712–714. doi: 10.1038/nm0705-712. [DOI] [PubMed] [Google Scholar]
- 60.Hwang HW, Mendell JT. MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br J Cancer. 2006;94:776–780. doi: 10.1038/sj.bjc.6603023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Murakami Y, Yasuda T, Saigo K, Urashima T, Toyoda H, Okanoue T, Shimotohno K. Comprehensive analysis of microRNA expression patterns in hepatocellular carcinoma and non-tumorous tissues. Oncogene. 2006;25:2537–2545. doi: 10.1038/sj.onc.1209283. [DOI] [PubMed] [Google Scholar]
- 62.Waidmann O, Bihrer V, Pleli T, Farnik H, Berger A, Zeuzem S, Kronenberger B, Piiper A. Serum microRNA-122 levels in different groups of patients with chronic hepatitis B virus infection. J Viral Hepat. 2012;19:e58–e65. doi: 10.1111/j.1365-2893.2011.01536.x. [DOI] [PubMed] [Google Scholar]
- 63.Qi P, Cheng SQ, Wang H, Li N, Chen YF, Gao CF. Serum microRNAs as biomarkers for hepatocellular carcinoma in Chinese patients with chronic hepatitis B virus infection. PLoS One. 2011;6:e28486. doi: 10.1371/journal.pone.0028486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li LM, Hu ZB, Zhou ZX, Chen X, Liu FY, Zhang JF, Shen HB, Zhang CY, Zen K. Serum microRNA profiles serve as novel biomarkers for HBV infection and diagnosis of HBV-positive hepatocarcinoma. Cancer Res. 2010;70:9798–9807. doi: 10.1158/0008-5472.CAN-10-1001. [DOI] [PubMed] [Google Scholar]
- 65.Liu CJ, Chen DS, Chen PJ. Epidemiology of HBV infection in Asian blood donors: emphasis on occult HBV infection and the role of NAT. J Clin Virol. 2006;36 Suppl 1:S33–S44. doi: 10.1016/s1386-6532(06)80007-7. [DOI] [PubMed] [Google Scholar]
- 66.Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 68.Resnick KE, Alder H, Hagan JP, Richardson DL, Croce CM, Cohn DE. The detection of differentially expressed microRNAs from the serum of ovarian cancer patients using a novel real-time PCR platform. Gynecol Oncol. 2009;112:55–59. doi: 10.1016/j.ygyno.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 69.Hu Z, Chen X, Zhao Y, Tian T, Jin G, Shu Y, Chen Y, Xu L, Zen K, Zhang C, et al. Serum microRNA signatures identified in a genome-wide serum microRNA expression profiling predict survival of non-small-cell lung cancer. J Clin Oncol. 2010;28:1721–1726. doi: 10.1200/JCO.2009.24.9342. [DOI] [PubMed] [Google Scholar]
- 70.Kudo M. Multistep human hepatocarcinogenesis: correlation of imaging with pathology. J Gastroenterol. 2009;44 Suppl 19:112–118. doi: 10.1007/s00535-008-2274-6. [DOI] [PubMed] [Google Scholar]
- 71.Nam SW, Park JY, Ramasamy A, Shevade S, Islam A, Long PM, Park CK, Park SE, Kim SY, Lee SH, et al. Molecular changes from dysplastic nodule to hepatocellular carcinoma through gene expression profiling. Hepatology. 2005;42:809–818. doi: 10.1002/hep.20878. [DOI] [PubMed] [Google Scholar]
- 72.Lee JM, Wong CM, Ng IO. Hepatitis B virus-associated multistep hepatocarcinogenesis: a stepwise increase in allelic alterations. Cancer Res. 2008;68:5988–5996. doi: 10.1158/0008-5472.CAN-08-0905. [DOI] [PubMed] [Google Scholar]
- 73.Gao P, Wong CC, Tung EK, Lee JM, Wong CM, Ng IO. Deregulation of microRNA expression occurs early and accumulates in early stages of HBV-associated multistep hepatocarcinogenesis. J Hepatol. 2011;54:1177–1184. doi: 10.1016/j.jhep.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 74.Ladeiro Y, Couchy G, Balabaud C, Bioulac-Sage P, Pelletier L, Rebouissou S, Zucman-Rossi J. MicroRNA profiling in hepatocellular tumors is associated with clinical features and oncogene/tumor suppressor gene mutations. Hepatology. 2008;47:1955–1963. doi: 10.1002/hep.22256. [DOI] [PubMed] [Google Scholar]
- 75.Winther TN, Bang-Berthelsen CH, Heiberg IL, Pociot F, Hogh B. Differential plasma microRNA profiles in HBeAg positive and HBeAg negative children with chronic hepatitis B. PLoS One. 2013;8:e58236. doi: 10.1371/journal.pone.0058236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ji F, Yang B, Peng X, Ding H, You H, Tien P. Circulating microRNAs in hepatitis B virus-infected patients. J Viral Hepat. 2011;18:e242–e251. doi: 10.1111/j.1365-2893.2011.01443.x. [DOI] [PubMed] [Google Scholar]
- 77.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 78.Ni YH, Chang MH, Chen PJ, Tsai KS, Hsu HY, Chen HL, Tsuei DJ, Chen DS. Viremia profiles in children with chronic hepatitis B virus infection and spontaneous e antigen seroconversion. Gastroenterology. 2007;132:2340–2345. doi: 10.1053/j.gastro.2007.03.111. [DOI] [PubMed] [Google Scholar]
- 79.Akeyama T, Koyama T, Kamada T. Alpha-fetoprotein in acute viral hepatitis. N Engl J Med. 1972;287:989. doi: 10.1056/nejm197211092871923. [DOI] [PubMed] [Google Scholar]
- 80.Di Bisceglie AM, Hoofnagle JH. Elevations in serum alpha-fetoprotein levels in patients with chronic hepatitis B. Cancer. 1989;64:2117–2120. doi: 10.1002/1097-0142(19891115)64:10<2117::aid-cncr2820641024>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 81.Zhou J, Yu L, Gao X, Hu J, Wang J, Dai Z, Wang JF, Zhang Z, Lu S, Huang X, et al. Plasma microRNA panel to diagnose hepatitis B virus-related hepatocellular carcinoma. J Clin Oncol. 2011;29:4781–4788. doi: 10.1200/JCO.2011.38.2697. [DOI] [PubMed] [Google Scholar]
- 82.Coulouarn C, Factor VM, Andersen JB, Durkin ME, Thorgeirsson SS. Loss of miR-122 expression in liver cancer correlates with suppression of the hepatic phenotype and gain of metastatic properties. Oncogene. 2009;28:3526–3536. doi: 10.1038/onc.2009.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wong QW, Lung RW, Law PT, Lai PB, Chan KY, To KF, Wong N. MicroRNA-223 is commonly repressed in hepatocellular carcinoma and potentiates expression of Stathmin1. Gastroenterology. 2008;135:257–269. doi: 10.1053/j.gastro.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 84.Kota J, Chivukula RR, O’Donnell KA, Wentzel EA, Montgomery CL, Hwang HW, Chang TC, Vivekanandan P, Torbenson M, Clark KR, et al. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137:1005–1017. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhu HT, Dong QZ, Sheng YY, Wei JW, Wang G, Zhou HJ, Ren N, Jia HL, Ye QH, Qin LX. MicroRNA-29a-5p is a novel predictor for early recurrence of hepatitis B virus-related hepatocellular carcinoma after surgical resection. PLoS One. 2012;7:e52393. doi: 10.1371/journal.pone.0052393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang GL, Li YX, Zheng SQ, Liu M, Li X, Tang H. Suppression of hepatitis B virus replication by microRNA-199a-3p and microRNA-210. Antiviral Res. 2010;88:169–175. doi: 10.1016/j.antiviral.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 87.Chen Y, Shen A, Rider PJ, Yu Y, Wu K, Mu Y, Hao Q, Liu Y, Gong H, Zhu Y, et al. A liver-specific microRNA binds to a highly conserved RNA sequence of hepatitis B virus and negatively regulates viral gene expression and replication. FASEB J. 2011;25:4511–4521. doi: 10.1096/fj.11-187781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang S, Qiu L, Yan X, Jin W, Wang Y, Chen L, Wu E, Ye X, Gao GF, Wang F, et al. Loss of microRNA 122 expression in patients with hepatitis B enhances hepatitis B virus replication through cyclin G(1) -modulated P53 activity. Hepatology. 2012;55:730–741. doi: 10.1002/hep.24809. [DOI] [PubMed] [Google Scholar]
- 89.Qiu L, Fan H, Jin W, Zhao B, Wang Y, Ju Y, Chen L, Chen Y, Duan Z, Meng S. miR-122-induced down-regulation of HO-1 negatively affects miR-122-mediated suppression of HBV. Biochem Biophys Res Commun. 2010;398:771–777. doi: 10.1016/j.bbrc.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 90.Jin WB, Wu FL, Kong D, Guo AG. HBV-encoded microRNA candidate and its target. Comput Biol Chem. 2007;31:124–126. doi: 10.1016/j.compbiolchem.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 91.Zhang X, Zhang E, Ma Z, Pei R, Jiang M, Schlaak JF, Roggendorf M, Lu M. Modulation of hepatitis B virus replication and hepatocyte differentiation by MicroRNA-1. Hepatology. 2011;53:1476–1485. doi: 10.1002/hep.24195. [DOI] [PubMed] [Google Scholar]
- 92.Ramière C, Scholtès C, Diaz O, Icard V, Perrin-Cocon L, Trabaud MA, Lotteau V, André P. Transactivation of the hepatitis B virus core promoter by the nuclear receptor FXRalpha. J Virol. 2008;82:10832–10840. doi: 10.1128/JVI.00883-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hu W, Wang X, Ding X, Li Y, Zhang X, Xie P, Yang J, Wang S. MicroRNA-141 represses HBV replication by targeting PPARA. PLoS One. 2012;7:e34165. doi: 10.1371/journal.pone.0034165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Raney AK, Johnson JL, Palmer CN, McLachlan A. Members of the nuclear receptor superfamily regulate transcription from the hepatitis B virus nucleocapsid promoter. J Virol. 1997;71:1058–1071. doi: 10.1128/jvi.71.2.1058-1071.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tang H, McLachlan A. Transcriptional regulation of hepatitis B virus by nuclear hormone receptors is a critical determinant of viral tropism. Proc Natl Acad Sci USA. 2001;98:1841–1846. doi: 10.1073/pnas.041479698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kong G, Zhang J, Zhang S, Shan C, Ye L, Zhang X. Upregulated microRNA-29a by hepatitis B virus X protein enhances hepatoma cell migration by targeting PTEN in cell culture model. PLoS One. 2011;6:e19518. doi: 10.1371/journal.pone.0019518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dasari VR, Kaur K, Velpula KK, Gujrati M, Fassett D, Klopfenstein JD, Dinh DH, Rao JS. Upregulation of PTEN in glioma cells by cord blood mesenchymal stem cells inhibits migration via downregulation of the PI3K/Akt pathway. PLoS One. 2010;5:e10350. doi: 10.1371/journal.pone.0010350. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 98.Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 99.Li Y, Tang ZY, Ye SL, Liu YK, Chen J, Xue Q, Chen J, Gao DM, Bao WH. Establishment of cell clones with different metastatic potential from the metastatic hepatocellular carcinoma cell line MHCC97. World J Gastroenterol. 2001;7:630–636. doi: 10.3748/wjg.v7.i5.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Herceg Z. Epigenetics and cancer: towards an evaluation of the impact of environmental and dietary factors. Mutagenesis. 2007;22:91–103. doi: 10.1093/mutage/gel068. [DOI] [PubMed] [Google Scholar]
- 101.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 102.Wei X, Xiang T, Ren G, Tan C, Liu R, Xu X, Wu Z. miR-101 is down-regulated by the hepatitis B virus x protein and induces aberrant DNA methylation by targeting DNA methyltransferase 3A. Cell Signal. 2013;25:439–446. doi: 10.1016/j.cellsig.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 103.Formosa A, Lena AM, Markert EK, Cortelli S, Miano R, Mauriello A, Croce N, Vandesompele J, Mestdagh P, Finazzi-Agrò E, et al. DNA methylation silences miR-132 in prostate cancer. Oncogene. 2013;32:127–134. doi: 10.1038/onc.2012.14. [DOI] [PubMed] [Google Scholar]
- 104.Wei X, Tan C, Tang C, Ren G, Xiang T, Qiu Z, Liu R, Wu Z. Epigenetic repression of miR-132 expression by the hepatitis B virus x protein in hepatitis B virus-related hepatocellular carcinoma. Cell Signal. 2013;25:1037–1043. doi: 10.1016/j.cellsig.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 105.Wang Y, Lee CG. MicroRNA and cancer--focus on apoptosis. J Cell Mol Med. 2009;13:12–23. doi: 10.1111/j.1582-4934.2008.00510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Roush S, Slack FJ. The let-7 family of microRNAs. Trends Cell Biol. 2008;18:505–516. doi: 10.1016/j.tcb.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 107.Wang Y, Lu Y, Toh ST, Sung WK, Tan P, Chow P, Chung AY, Jooi LL, Lee CG. Lethal-7 is down-regulated by the hepatitis B virus x protein and targets signal transducer and activator of transcription 3. J Hepatol. 2010;53:57–66. doi: 10.1016/j.jhep.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 108.Xu X, Fan Z, Kang L, Han J, Jiang C, Zheng X, Zhu Z, Jiao H, Lin J, Jiang K, et al. Hepatitis B virus X protein represses miRNA-148a to enhance tumorigenesis. J Clin Invest. 2013;123:630–645. doi: 10.1172/JCI64265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yuan K, Lian Z, Sun B, Clayton MM, Ng IO, Feitelson MA. Role of miR-148a in hepatitis B associated hepatocellular carcinoma. PLoS One. 2012;7:e35331. doi: 10.1371/journal.pone.0035331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jin J, Tang S, Xia L, Du R, Xie H, Song J, Fan R, Bi Q, Chen Z, Yang G, et al. MicroRNA-501 promotes HBV replication by targeting HBXIP. Biochem Biophys Res Commun. 2013;430:1228–1233. doi: 10.1016/j.bbrc.2012.12.071. [DOI] [PubMed] [Google Scholar]
- 111.Potenza N, Papa U, Mosca N, Zerbini F, Nobile V, Russo A. Human microRNA hsa-miR-125a-5p interferes with expression of hepatitis B virus surface antigen. Nucleic Acids Res. 2011;39:5157–5163. doi: 10.1093/nar/gkr067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wu XJ, Li Y, Liu D, Zhao LD, Bai B, Xue MH. miR-27a as an oncogenic microRNA of hepatitis B virus- related hepatocellular carcinoma. Asian Pac J Cancer Prev. 2013;14:885–889. doi: 10.7314/apjcp.2013.14.2.885. [DOI] [PubMed] [Google Scholar]
- 113.Hayes CN, Akamatsu S, Tsuge M, Miki D, Akiyama R, Abe H, Ochi H, Hiraga N, Imamura M, Takahashi S, et al. Hepatitis B virus-specific miRNAs and Argonaute2 play a role in the viral life cycle. PLoS One. 2012;7:e47490. doi: 10.1371/journal.pone.0047490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ren M, Qin D, Li K, Qu J, Wang L, Wang Z, Huang A, Tang H. Correlation between hepatitis B virus protein and microRNA processor Drosha in cells expressing HBV. Antiviral Res. 2012;94:225–231. doi: 10.1016/j.antiviral.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 115.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 116.Lu LF, Liston A. MicroRNA in the immune system, microRNA as an immune system. Immunology. 2009;127:291–298. doi: 10.1111/j.1365-2567.2009.03092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nilsen TW. Mechanisms of microRNA-mediated gene regulation in animal cells. Trends Genet. 2007;23:243–249. doi: 10.1016/j.tig.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 118.Ghosh Z, Mallick B, Chakrabarti J. Cellular versus viral microRNAs in host-virus interaction. Nucleic Acids Res. 2009;37:1035–1048. doi: 10.1093/nar/gkn1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.David M. Interferons and microRNAs. J Interferon Cytokine Res. 2010;30:825–828. doi: 10.1089/jir.2010.0080. [DOI] [PubMed] [Google Scholar]
- 120.Pedersen IM, Cheng G, Wieland S, Volinia S, Croce CM, Chisari FV, David M. Interferon modulation of cellular microRNAs as an antiviral mechanism. Nature. 2007;449:919–922. doi: 10.1038/nature06205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pan XB, Ma H, Jin Q, Wei L. Characterization of microRNA expression profiles associated with hepatitis B virus replication and clearance in vivo and in vitro. J Gastroenterol Hepatol. 2012;27:805–812. doi: 10.1111/j.1440-1746.2011.06979.x. [DOI] [PubMed] [Google Scholar]
- 122.Gao YF, Yu L, Wei W, Li JB, Luo QL, Shen JL. Inhibition of hepatitis B virus gene expression and replication by artificial microRNA. World J Gastroenterol. 2008;14:4684–4689. doi: 10.3748/wjg.14.4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang K, Yuan Y, Cho JH, McClarty S, Baxter D, Galas DJ. Comparing the MicroRNA spectrum between serum and plasma. PLoS One. 2012;7:e41561. doi: 10.1371/journal.pone.0041561. [DOI] [PMC free article] [PubMed] [Google Scholar]


