Abstract
Irritable bowel syndrome (IBS) is a functional intestinal disease characterized by abdominal pain or discomfort and altered bowel habits. It has drawn great attention because of its high prevalence, reoccurring symptoms, and severe influence on patients’ lives. Many clinical studies have demonstrated the efficacy of acupuncture-moxibustion in treating IBS. Increasing attention has been paid to research regarding the action mechanisms of acupuncture-moxibustion for IBS, and the adoption of modern techniques has achieved some progress. This article reviews the latest advances among action mechanism studies from the perspectives of gastrointestinal motility, visceral hypersensitivity, the brain-gut axis, the neuroendocrine system, and the immune system. It is shown that acupuncture-moxibustion can effectively regulate the above items, and thus, this treatment should have a high efficacy in the treatment of IBS. This article also identifies existing problems in current mechanism research and raises several ideas for future studies. Further revelations regarding these action mechanisms will promote the application of acupuncture-moxibustion in treating IBS.
Keywords: Irritable bowel syndrome, Acupuncture-moxibustion, Mechanism study, Gastrointestinal motility, Visceral hypersensitivity, Brain-gut axis
Core tip: This is a review of the latest advances made towards identifying the action mechanisms of acupuncture-moxibustion in treating irritable bowel syndrome (IBS). How does this ancient therapy affect gastrointestinal motility, visceral hypersensitivity, the brain-gut axis, the neuroendocrine system, the immune system, and other factors involved in the pathogenesis of IBS? This paper details answers to these questions.
INTRODUCTION
Irritable bowel syndrome (IBS) is a common chronic functional gastrointestinal condition that is majorly characterized by abdominal pain, bloating, and disturbed defecation and is coupled with psychological conditions. Falling under the scope of “abdominal pain”, “diarrhea”, or “constipation” in traditional Chinese medicine (TCM), it exists worldwide with a relatively high prevalence[1-3]. Because its recurrent symptoms severely affect the quality of life (QoL) of patients, driving them to consult extensive medical resources, IBS has drawn great global attention. A great amount of epidemiological research from various countries at different times has revealed that the prevalence and distributing characteristics of IBS vary according to different countries, regions, and populations. According to the Rome III diagnostic criteria, the global IBS prevalence is between 5% and 20%[4,5].
The pathogenesis of IBS remains unknown. However, during recent years, pathophysiological research has increasingly indicated that multiple factors, such as genetic factors, psychological factors, diet, infections, immunity, and the brain-gut axis, can combine in a complex manner, leading to the visceral hypersensitivity and gastrointestinal dysmotility that are manifested by corresponding symptoms[6-9].
Acupuncture-moxibustion is a crucial part of TCM, comprising both acupuncture and moxibustion methods. As external treatments of TCM, acupuncture and moxibustion act by stimulating acupoints to unblock the meridians and collaterals, regulating the function of qi and blood, supporting health and expelling pathogens. When acupuncture needles are inserted into acupoints, needling manipulations, such as twirling and lifting-thrusting needles, are usually adopted to treat diseases. After the acupoints are targeted, moxibustion allows for the further treatment of diseases by the thermal stimulation generated by ignited moxa. Thus far, a large number of studies have proven the efficacy of acupuncture-moxibustion in attenuating the symptoms of IBS without producing obvious adverse effects[10]. Meta-analyses have also revealed that the therapeutic efficacy of acupuncture plus moxibustion is better than that of Western medications for IBS[11-14]. One clinical study also showed that acupuncture enhanced and extended the prescribed regimen’s efficacy in treating IBS[15]. As its therapeutic efficacy has been confirmed, the action mechanisms of this traditional therapy have become the focus of much current research.
REGULATION OF GASTROINTESTINAL MOTILITY
The gastrointestinal dysmotility in IBS can be further classified into four groups based on their clinical features: spastic colon syndrome, functional diarrhea, diarrhea-predominant spastic colon syndrome, and midgut dysmotility[16]. Stress reactions can either enhance or attenuate dysmotility as well as subsequent symptoms. The pathophysiology of IBS involves dysmotility of both the colon and the small intestine, and the migratory motor complex (MMC) cycles have been reported to be shorter in diarrhea-predominant IBS (D-IBS) patients but longer in constipation-predominant IBS (C-IBS) patients[17].
In a clinical study, 10 patients with D-IBS conforming to the Rome III diagnostic criteria received acupuncture and were observed for the changes in their borborygmus frequency and colonic peristalsis after acupuncture intervention. Compared with 10 healthy controls, the borborygmus frequency and colonic peristalsis were significantly higher before acupuncture (P < 0.01). However, after the acupuncture treatment, the two metrics of the IBS patients had been downregulated (P < 0.05). These results indicate that acupuncture can immediately regulate colonic peristalsis in D-IBS patients[18]. An electrocolonogram (ECOM) revealed that acupuncture at Zusanli (ST 36) (Figure 1A) was able to produce a virtuous bidirectional regulation of the ECOM in IBS cases of different TCM syndromes. Before treatment, IBS patients with splenic deficiency due to dampness had a decreased frequency of peak (Fp) in the sigmoid colon, suggesting that the tension of sigmoid colon should be low. The amplitude of peak (Ap), Fp, and the average zero-crossing frequency (Fz) increased after acupuncture, revealing that acupuncture at ST 36 can enhance colon contraction. In contrast, Ap, Fp, and Fz were abnormally high in IBS patients due to liver-intestine qi stagnation before acupuncture, suggesting that the sigmoid colon was hyperactive and the intestine wall was extremely contracted. After acupuncture, Ap, Fp, Fz dropped significantly, revealing that acupuncture at ST 36 downregulated colonic motility[19].
Figure 1.

Acupoints of human. A: Acupoint Zusanli (ST 36); B: Acupoint Neiguan (PC 6); C: Acupoint Tianshu (ST 25).
In animal experiments, IBS rat models were designed to observe the effect of electroacupuncture on intestinal dysmotility. Bilateral ST 36 and Shangjuxu (ST 37) (Figure 2A) were treated with electroacupuncture by selecting sparse-intense waves [100 Hz/2 Hz; 1, 2, 3 mA (increased by every 10 min)]. Each session lasted 30 min, and sham electroacupuncture was adopted in the controls. Compared with normal controls before treatment, the colonic peristalsis was significantly higher in adult IBS rats (P < 0.05). After 30 min of electroacupuncture treatment, the colonic peristalsis of the IBS rats had dropped (P < 0.05), while the IBS rats in the sham electroacupuncture group showed no obvious changes in colonic peristalsis (P > 0.05). The above study showed that IBS rat models had an abnormally increased intestinal motility that was significantly suppressed by electroacupuncture[20]. Another experiment revealed that herb-partitioned moxibustion can enhance gastric emptying and small intestinal propulsion in rats with functional gastrointestinal disorders (FGIDs) due to liver depression and spleen deficiency[21].
Figure 2.
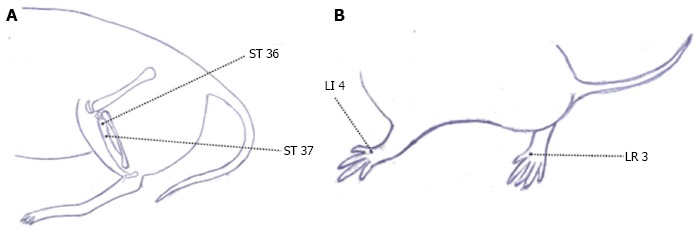
Acupoints of rat. A: Acupoint Zusanli (ST 36) and Shangjuxu (ST 37); B: Acupoint Taichong (LR 3) and Hegu (LI 4).
The above studies all illustrate that acupuncture-moxibustion has positive regulatory effects on gastrointestinal dysmotility, constituting one of the most crucial mechanisms of acupuncture-moxibustion in treating IBS.
REGULATION OF VISCERAL HYPERSENSITIVITY
Visceral hypersensitivity refers to the decreased pain threshold of inner organs and more intense experience of stimuli. IBS patients of various subtypes and healthy volunteers underwent rectal noxious stimulation with an air balloon and a water balloon. IBS patients were found to have significantly lower thresholds for abdominal discomfort than healthy volunteers, and the hypersensitivity subgroups had significantly lower thresholds than the normosensitive subgroups[22,23]. Another study discovered that hypersensitive IBS patients had more severe gastric conditions than normosensitive patients[24].
A clinical study on D-IBS found that transcutaneous electrical acustimulation (TEAS) at Neiguan (PC 6) (Figure 1B) and ST 36 significantly increased the threshold for the rectal sensation of gas, the desire to defecate, the sensation of pain, improved rectal perception, and attenuated visceral hypersensitivity[25]. D-IBS patients usually experienced the urge to defecate and a decreased pain threshold. Short-term transcutaneous electrical nerve stimulation (TENS) was able to increase the threshold of rectal perception. After a 2-mo TENS intervention, the threshold of rectal perception had obviously increased in the D-IBS patients, while the defecation frequency and pain intensity had obviously decreased. The psychological scores dropped to a normal level[26]. In animal experiments, the abdominal withdrawal reflex (AWR) and abdominal myoelectric activity (AMA) were adopted to evaluate intestinal sensitivity. Studies on electroacupuncture or moxibustion as IBS interventions all showed that the acupuncture-moxibustion effectively alleviated visceral hypersensitivity in IBS rats[27-30].
REGULATION OF THE BRAIN-GUT AXIS AND THE NEUROENDOCRINE SYSTEM
The role of the brain-gut axis has drawn great attention regarding the pathogenesis of IBS. Because the inducing factors of IBS, such as gastrointestinal dysmotility, visceral hypersensitivity, infection, and mental conditions, are all included in this system, the potential for breakthroughs in studying the pathogenesis of IBS is considerable. The brain-gut axis is a complex, bidirectional signaling system between the central nervous system (CNS) and the gastrointestinal system. The regulation of gastrointestinal function via the brain-gut axis is called brain-gut interaction.
Regulation of brain-gut peptides
Brain-gut interaction is realized by multiple neural transmitters that reside in the endocrine cells of the CNS, enteric nervous system (ENS) and the gastrointestinal tract. They work as both neurotransmitters and hormones and are termed brain-gut peptides. Brain-gut peptides work extensively to regulate gastrointestinal activities and are thus closely related to IBS. The major excitatory neurotransmitters include histamine, 5-HT, substance P (SP), calcitonin gene-related peptide (CGRP), and corticotropin-releasing factor-related peptide (CRF). The major inhibitory neurotransmitters include cholecystokinin (CCK), NO, norepinephrine (NE), and vasoactive peptide (VIP)[31].
Approximately 95% of 5-HT arise from the enterochromaffin cells (EC) of the intestinal mucosa, which are involved in the regulation of intestinal movement and perception. Changes in the 5-HT signal system are among the pathophysiological feature of IBS[32]. A clinical study was conducted to observe the change in colonic mucosal 5-HT among D-IBS patients and to assess the efficacy of herb-partitioned moxibustion. The results showed that IBS patients had a significantly increased expression of 5-HT in the colonic mucosa, while herb-partitioned moxibustion simultaneously improved IBS symptoms and downregulated the level of 5-HT[33]. Meanwhile, herb-partitioned moxibustion has been shown to downregulate the concentration of serum 5-HT in IBS patients[34]. Laboratory studies[35,36] also discovered that electroacupuncture enhanced the pain threshold of rats with chronic visceral hypersensitivity (CVH), downregulated the abnormally increased 5-HT level in colons, and enhanced the expression of 5-HT4 receptor and serotonin transporter (SERT), although the 5-HT3 receptor was insignificantly influenced. Therefore, we can conclude that electroacupuncture improves visceral hypersensitivity and stress-induced colonic dysfunction via the 5-HT signal system. Another study also showed that both herb-partitioned moxibustion and suspended moxibustion can increase the pain threshold and relieve hypersensitivity in CVH rats[37].
CRF and its receptor both play important roles in the onset and development of IBS and may work synergistically via the CNS and peripheral systems[38]. As a key factor in the pathogenesis of IBS[39], CRF can induce repeated defecation in rats and mice, although this activity can be blocked by its antagonist[40]. CRF also alters the rectal perceptions of human beings[41]. Anxiety, depression, altered colonic movement, and visceral algesthesia are involved in the CRF/CRF1 signal pathway in the brain, whereas the activation of central and peripheral CRF2 receptor can inhibit this pathway[42]. We discovered that electroacupuncture effectively downregulated the hypothalamic CRF concentrations of CVH IBS rats[43]. The TENS-related experiment also confirmed that TENS can downregulate the hyper-expression of CRF in the PVN of rats[44].
Moreover, the concentrations of other brain-gut peptides, e.g., SP, VIP, and neuropeptide Y (NPY), are also changed in IBS patients, and these changes all play crucial roles in the pathogenesis of IBS[45]. It has been reported that electroacupuncture can regulate the secretion of colonic SP, SP receptor, and VIP in CVH IBS models[46]. It has also been found that acupuncture at ST 37 can reduce the concentrations of serum motilin (MTL) and somatostatin (SS) in CVH IBS rats[47]. A D-IBS model was developed by chronic mild restraint stress plus neonatal maternal separation and gastric administration of Fan Xie Ye (Folium Sennae) to observe the effect of acupuncture at bilateral ST 36 and Taichong (LR 3) (Figure 2B). The results showed that acupuncture at ST 36 and LR 3 inhibited the production of SS, SP, and VIP while increasing the level of NPY, which may be related to the efficacy of acupuncture in treating IBS[48,49]. Moreover, the effect of acupuncture on brain-gut peptides may be specific. A study observed the effect of electroacupuncture at ST 36 and Hegu (LI 4) (Figure 2B) on colonic NPY and SS of IBS rats prepared by the rectal administration of acetic acid (AA). The results showed that, after electroacupuncture, the hypothalamic NPY increased significantly in both electroacupuncture groups, while the colonic NPY level showed no obvious change. The colonic SS decreased in both electroacupuncture groups after intervention, while the plasma SS level only dropped significantly in the electroacupuncture group of ST 36, not in the LI 4 group[50].
The above results indicate that acupuncture-moxibustion can improve intestinal motility and visceral sensitivity by regulating brain-gut peptide levels in the CNS, intestines, and blood.
Regulation of nervous system
Effect on brain activation: IBS patients all experience visceral hypersensitivity of different levels. Over the recent years, studies on CVH IBS have proven that the abnormal activities of pain processing systems, including the anterior cingulate cortex (ACC), prefrontal cortex, insular cortex, thalamus, dorsal pons, and periaqueductal gray matter, are more or less associated with the IBS pathogenesis[51-53].
Functional magnetic resonance imaging (fMRI) has realized its capacity for non-invasive study on the effect of acupuncture-moxibustion on brain activation in IBS patients. By using PET to observe the functional changes of the visceral sensory center under rectal distension in D-IBS patients and the effect of electroacupuncture at Tianshu (ST 25) (Figure 1C) on the visceral sensory center, the study revealed that IBS patients had an increased glucose metabolism in the bilateral superior temporal gyri, right middle occipital gyrus, right superior frontal gyrus, and bilateral middle frontal gyri, but not in the visceral sensory center. Rectal distension enhanced the glucose metabolism in the prefrontal cortex, left cingulate gyrus, anterior and posterior central gyri, and temporal gyrus and also activated part of the visceral sensory center, including the anterior cingulate gyrus. Electroacupuncture at ST 25 significantly downregulated the glucose metabolism in the left cingulate gyrus, right insula, right parahippocampal gyrus, precuneus, and right caudate nucleus[54]. SPECT was adopted to observe the effects of eye acupuncture on cerebral blood flow in D-IBS patients. It was discovered that the blood flow in bilateral thalamus dropped significantly after eye acupuncture, indicating that a reduction of the blood flow in specific brain regions was involved in the action mechanisms of eye acupuncture in treating D-IBS[55]. Researchers once used fMRI to observe the effect of electroacupuncture and sham electroacupuncture on brain activities in IBS patients in different sessions (pre-intervention, during intervention, and post-intervention). A blank control group was set up for comparative study. Of the participants who underwent rectal distention, there was no regional difference in brain activation between the electroacupuncture and sham electroacupuncture groups at baseline. In the electroacupuncture group, increased brain activation from the baseline was observed in the perigenual cingulate cortex, the bilateral prefrontal cortex and the temporal lobes, while high activation was noted at the right insula and bilateral somatosensory cortex during intervention. In the sham electroacupuncture group, increased brain activity was observed only in the anterior cingulate cortex, bilateral prefrontal cortex and left somatosensory cortex. The electroacupuncture group had significantly higher brain activation in the right insula and thalamus than the sham electroacupuncture group. After intervention, the brain activation in the electroacupuncture group significantly dropped in all regions; however, in the sham electroacupuncture group, the brain activation decreased in the temporal lobes and anterior cingulate gyrus but increased in the prefrontal cortex. No significant difference in brain activation was detected between the two groups. Of the participants who did not receive a rectal distension, there was no significant difference in the brain activation between the electroacupuncture and sham electroacupuncture groups, regardless of the different sessions[56].
The locus ceruleus is a nucleus located on the brain stem and plays an important role in producing norepinephrine. It is also closely related to stress reactions. When observing the effects of electroacupuncture at ST 37 on the neuronal discharge of the locus ceruleus in rats with acute restraint stress undergoing noxious rectal distension, scholars found that acupuncture at ST 37 inhibited the neuronal discharge that was activated by noxious rectal distension (P < 0.01). This result indicates that electroacupuncture may regulate rat colonic functions by downregulating the activation of neurons in the locus ceruleus[57]. A novel study observed the excitability of visceral sensory neurons in the rostral ventromedial medulla (RVM) and the N-methyl-D-aspartate receptor 1 (NR1) in IBS rats, before and after electroacupuncture at ST 36 and ST 37. It was noted that electroacupuncture significantly inhibited the hyper-excitability of the neurons and the expression of NR1 in RVM. This result suggests that the inhibition of NR1 should be an important factor in reducing the hyper-excitability of the neurons in RVM, as well as one of the action mechanisms of acupuncture in alleviating visceral hyperalgesia[58].
Regulation of neuronal excitability in spinal cord: The dorsal horn of the spinal cord is a crucial contributor to visceral and somatic perception. Thick myelinated primary afferent fibers (Aα, β) and thin myelinated/unmyelinated primary afferent fibers (Aδ and C), projection neurons (T), and inhibitory interneurons in the substantia gelatinosa form a neural web for the segmental regulation of the spinal cord. In IBS, the increased response of dorsal horn cells to the current afferent impulses and old under-threshold afferent impulses leads to an increased response to non-noxious stimulation, enlarged perception region, and decreased activation threshold. An action mechanism study on acupuncture-moxibustion for alleviating visceral hyperalgesia adopted colorectal distension (CRD) as the noxious stimulation. The study found that CRD activated the convergent neurons in the dorsal horn; the mechanical stimulation to contra-lateral body surface and hand acupuncture at ST 36 inhibited this noxious response. Thus, acupuncture and noxious stimulation are believed to meet and interact on the level of the spinal cord, and acupuncture can inhibit the neuronal activation induced by noxious stimulation in an action involving the spinal cord at even higher levels[59]. C-Fos is a proto-oncogene that is the human homolog of the retroviral oncogene v-fos. The expression of the c-fos protein is strengthened during neuronal activation, and its production is considered the biological marker for the activation of nociceptive neurons[60]. Compared to normal rats, CVH IBS rats had significantly more activated c-fos neurons in the superficial laminae (SDH, laminae I and II), nucleus proprius (NP, laminae III and IV), and neck of the dorsal horn (NECK, laminae V and VI) in the spinal segments of L6-S2 and in the neck of the dorsal horn of T13-L2 (P < 0.05). Electroacupuncture significantly downregulated the number of activated neurons of c-fos in the SDH, NP, and NECK of L6-S2 and in the sub-region of NECK of T13-L2 (P < 0.05). Sham electroacupuncture produced no notable effects on the expression of c-fos protein. Therefore, electroacupuncture can significantly inhibit the hyper-excitability of visceral sensory neurons in the dorsal horns of IBS rats, which qualifies as one of the action mechanisms of acupuncture in attenuating chronic visceral hyperalgesia[61].
In addition, the N-methyl-D-aspartic acid (NMDA) receptor (NR) also participates in the sustainment of functional chronic visceral hyperalgesia. As an excitatory neurotransmitter receptor in the CNS of mammals, the NMDA receptor mediates the excitability of glutamic acid and other endogenous acidic amino acids. A hetero-oligomer composed of NR1 and NR2, NMDAR is widely distributed in the brain, spinal cord, and peripheral nervous system. NR1 is an essential component, while NR2 modulates the functional feature of the whole receptor[62]. The phosphorylation site of the NMDA receptor is located on serine-896, a sub-unit of NR1. Its phosphorylation is involved in the regulatory effect of orphanin on pain perception in the spinal cord, and the activated receptor plays a specific role in the development of visceral hyperalgesia[63]. Zhou et al[64] prepared IBS CVH models using neonatal SD rats at the age of 9 d. The study showed that the rat models had significantly higher expressions of NR1 mRNA in the lumbar enlargement of the spinal cord than normal controls (P < 0.05). While pre-treatment with electroacupuncture downregulated these expressions to a normal level, sham-electroacupuncture had no obvious effect. These results indicate that electroacupuncture could realize its efficacy in treating IBS CVH by regulating the expressions of NR1 mRNA in the spinal cord. Tian et al[65] conducted a controlled study by giving electroacupuncture and sham electroacupuncture to rat models of IBS CVH, discovering that electroacupuncture downregulated the phosphorylation of NR1 in rats’ L4-5. Hence, the effect of electroacupuncture in alleviating CVH should be related to the downregulation of the phosphorylation of the NMDA receptor in the spinal cord.
Regulation of the ENS: The ENS plays an important role in the pathogenesis of IBS. The ENS is embedded throughout the intestine wall, from the mucosa to the serosa. Its sensory neurons report on the mechanical and chemical conditions, while the motor neurons control peristalsis and secretion[66]. ENS is composed of submucosal and myenteric plexuses. The submucosal plexuses are located in the submucosa, with motor neurons producing acetylcholine (ACh) and VIP; the myenteric plexuses are located between the inner and outer layers of the muscularis externa and contain excitatory neurons (transmitted by ACh, SP, etc.) and inhibitory neurons (transmitted by VIP, NO, etc.). The ENS is mainly in charge of peristalsis and secretions, as well as the regulation of visceral sensitivity. The reduction of neurons in the submucosal and myenteric plexuses has been discovered to be the plausible common pathogenic factor of D-IBS and C-IBS[67,68]. Electroacupuncture can increase the number of neurons in the myenteric plexuses of C-IBS rats[69], but the effect of acupuncture-moxibustion on the ENS neurons of D-IBS rats requires further study. ENS also regulates certain gastrointestinal activities via neurotransmitters, such as 5-HT, Ach, norepinephrine (NE), ATP, and multiple neuropeptides. Acupuncture-moxibustion produces a positive regulatory effect on the intestinal secretion of 5-HT, SP[36], SPR, VIP[46], SS[48], NPY[49], and AchE[70]; therefore, this procedure is able to correct the gastrointestinal dysmotility and visceral hypersensitivity.
Regulation of intestinal endocrine cells
EC is the predominant endocrine cell in the intestine. With the largest number and broadest distribution, ECs work to synthesize and contain 5-HT and produce peptides such as SP. Many studies[71,72] have shown that IBS patients can significantly increase the number of active ECs. C-IBS rat models were developed by the gastric administration of normal saline at 0 °C-4 °C to observe the effects of electroacupuncture on the activation of colonic EC. The mean optical density (MOD) of the colonic EC has also been found to be significantly increased in the model rats (P < 0.05), and electroacupuncture has been shown to downregulate this increased MOD (P < 0.05). These results indicate that the correction of the abnormal status of the colonic EC may be one of the action mechanisms of electroacupuncture in treating C-IBS[73]. A study with IBS rat models developed by restraint and stress also showed that IBS rats increased the expression of the colonic EC, while eye acupuncture effectively downregulated these levels[74].
REGULATION OF IMMUNOLOGICAL FUNCTION
The activation of the immune system is deeply involved in IBS[75] and is majorly manifested by the abnormal expression of immune cells and active substances, e.g., T-lymphocytes and the subgroups, immunoglobulins, inflammatory cytokines, etc. A study determined the concentrations of serum IgM, IgG, IgA, C3 and C4, as well as the subgroups of T-lymphocytes in IBS patients, before and after herb-partitioned moxibustion. The results showed that IBS patients had significantly higher serum IgM concentrations than the normal controls, while the IgG, IgA, C3, and C4 concentrations were statistically equal between the two groups. The IgM levels remarkably decreased after herb-partitioned moxibustion, while the concentrations of the other items remained the same. The lymphocyte transformation of the IBS patients was significantly lower than that of the normal controls, and moxibustion restored the lymphocyte transformation to approach normal levels. Herb-partitioned moxibustion also significantly increased the T8+ in peripheral blood (P < 0.01), and the abnormal ratio of T4+/T8+ was effectively corrected. These results indicate that herb-partitioned moxibustion can correct the abnormal immune dysfunction of IBS[76]. IL-18, IL-23, and TNF-α are pro-inflammatory mediators. It has been reported that acupuncture-moxibustion can downregulate the expressions of serum IL-18, IL-23, and TNF-α in elderly IBS patients[77].
As a key cell in inducing intestinal dysfunction and paresthesia, the mast cell (MC) plays a crucial role in the immunopathological changes of IBS. The activated intestinal mucosal MC participates in the formation of visceral hypersensitivity in IBS, which constitutes a key portion of the pathogenesis of IBS[78]. It was found that CVH IBS rats had significantly lower visceral pain thresholds and an increased number of colonic mucosal MC compared to normal controls. Concurrently, electroacupuncture markedly reduced the number of MC, indicating that electroacupuncture can effectively regulate the production of colonic MC[27,46].
INTESTINAL FLORA IMBALANCE
The composition of the intestinal flora varies in individuals. IBS patients have been shown to lack Lactobacilli and Bifidobacterium[79]. Other scholars have found that the over-growth of small intestinal bacteria may also represent an important factor in inducing IBS[80]. Little research has reported on acupuncture-moxibustion interventions for IBS. However, Wang et al[81] found that the prevalence of Lactobacilli and Bifidobacterium increased in rats with ulcerative colitis (UC) after herb-partitioned moxibustion, indicating that moxibustion can produce certain regulatory effects on the intestinal flora. However, further evidence supporting this indication is required.
OTHER ACTION MECHANISMS
Aquaporins (AQP) are integral membrane proteins from a larger family of major intrinsic proteins (MIP) that form pores in the membranes of biological cells. AQP3, AQP8, and AQP4 are broadly resident in human intestinal tissues, where they work to regulate the absorption and secretion of the intestines. By developing diarrhea-predominant IBS rat models with chronic stress and restraints, scholars found that IBS rats had significantly lower expressions of colonic AQP8 and AQP3 mRNA and protein. However, as eye acupuncture significantly increased the expressions of colonic AQP8 and AQP3 mRNA and protein, we believe that fluid transportation disturbances mediated by AQP8 and AQP3 could possibly be an important factor in developing diarrhea-predominant IBS models. The action mechanisms of eye acupuncture for IBS may involve the regulation of the expressions of colonic AQP8 and AQP3[82,83].
Due to the shortage of free radical scavengers in the blood of IBS patients, gastrointestinal nerves are easily damaged by free radicals. Scholars have investigated the relationship between free radicals and IBS, as well as the effects of acupuncture-moxibustion treatment, by determining the levels of spasm superoxide dismutase (SOD), malonyldialdehyde (MDA), and NO in IBS patients. The results showed that acupuncture-moxibustion is effective in treating IBS. Spasm SOD was significantly increased, while MDA and NO were significantly decreased after acupuncture-moxibustion. These data suggest that acupuncture-moxibustion can enhance the antioxidant capacity of the body, eliminating the accumulated free radicals and maintaining the stability of the intracellular environment and the functional status of the body[84].
CONCLUSION
Despite the high prevalence and vast influence of IBS, its pathophysiological action mechanisms have not been clearly understood. None of the theories put forth in the past, including intestinal dysmotility, visceral hypersensitivity, interaction of brain-gut, and dysimmunity, can fully explain the pathogenesis of IBS, as this entity is likely due to the interactive contributions of multiple factors. This article has reviewed the action mechanisms of acupuncture-moxibustion for IBS regarding the regulation of gastrointestinal motility, visceral hypersensitivity, brain-gut axis, neuroendocrine, and immunity and suggests that the treatment efficacy should be closely related to the regulation of the above aspects (Figure 3). Based on the current research, we can see that the study of the mechanisms used by acupuncture-moxibustion to treat IBS has been thoroughly pursued. The particular accomplishments of this research have provided scientific evidence to elucidate IBS’s action mechanisms.
Figure 3.
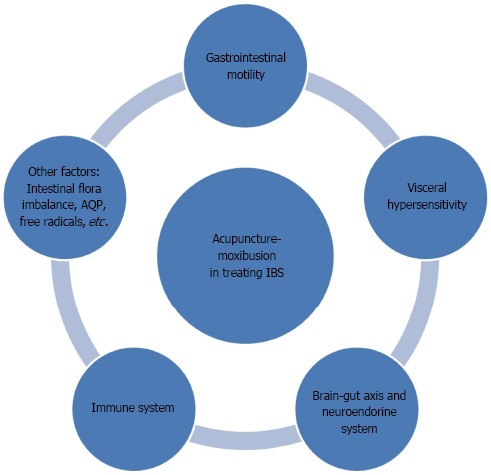
Multiple regulating channels of acupuncture-moxibustion in treating irritable bowel syndrome. IBS: Irritable bowel syndrome; AQP: Aquaporins.
As acupuncture-moxibustion treats the body as a whole system through acupoints, its action mechanisms for the treatment of IBS could involve multiple segments, layers, and targets. Indeed, the current literature supports this perspective. These mechanisms were studied from various disciplinary perspectives and the variety of acupuncture-moxibustion methods makes it impossible to study systemic and comprehensive issues related to the action mechanisms of this method. Meanwhile, separate from medications, acupuncture-moxibustion is efficacious because it stimulates acupoints on the surface of body. Current studies have mainly focused on the regulation and mechanisms of the target organs after acupuncture-moxibustion has stimulated the appropriate acupoints. However, the precise action mechanisms of acupuncture-moxibustion used to stimulate the acupoints and the pathways through which IBS can be managed remain unknown. These questions still require further research to properly address them. Furthermore, both acupuncture and moxibustion, either in combination or alone, are effective in attenuating the symptoms of IBS. Is there actually any difference between these two treatments in activating acupoints and intervening in IBS? Although acupuncture and moxibustion both act through acupoints, they stimulate the acupoints in different ways, acupuncture through mechanical stimulation and moxibustion through thermal stimulation. Each of these questions should be kept in mind during further investigations. Moreover, we lack a universally approved IBS animal model[85]. The current models cannot fully reflect the clinical manifestations and pathogenic mechanisms of IBS. The use of different animal models and varying acupuncture-moxibustion methods has also caused the current research to be open to doubt and difficult to replicate. The ideal IBS animal model must integrate all of the associated factors of IBS, including its physiology, cognition, emotion, behavior, etc.[86,87].
By clarifying of the etiology and pathogenesis of IBS, establishing an ideal IBS animal model, standardizing an acupuncture-moxibustion intervention, and applying modern medical research methods and results, we should be able to better study the action mechanisms of acupuncture-moxibustion in the treatment of IBS and in the activation of acupoints, which will also help promote the application of acupuncture-moxibustion in the treatment of IBS.
Footnotes
Supported by National Natural Science Foundation of China, No. 81273843, No. 81072879; National Key Basic Research Program of China, No. 2009CB522900
P- Reviewers: Cheng SX, Dillon JF, Xiao B S- Editor: Wen LL L- Editor: A E- Editor: Ma S
References
- 1.Philpott H, Gibson P, Thien F. Irritable bowel syndrome - An inflammatory disease involving mast cells. Asia Pac Allergy. 2011;1:36–42. doi: 10.5415/apallergy.2011.1.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almansa C, Díaz-Rubio M, Rey E. The burden and management of patients with IBS: Results from a survey in spanish gastroenterologists. Rev Esp Enferm Dig. 2011;103:570–575. doi: 10.4321/s1130-01082011001100003. [DOI] [PubMed] [Google Scholar]
- 3.Faresjö Å, Grodzinsky E, Hallert C, Timpka T. Patients with irritable bowel syndrome are more burdened by co-morbidity and worry about serious diseases than healthy controls--eight years follow-up of IBS patients in primary care. BMC Public Health. 2013;13:832. doi: 10.1186/1471-2458-13-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang L, Lacy BE, Spiegel BM. An Evidence-based Approach to Therapy in IBS-D: A Case Study Compendium. Gastroenterol Hepatol (N Y) 2010;6:1–12. [PMC free article] [PubMed] [Google Scholar]
- 5.He WR, Zhang FC, Liang LX. The epidemiology of irritable bowel syndrome. Weichangbingxue He Ganbingxue Zazhi. 2012;21:83–85. [Google Scholar]
- 6.Camilleri M. Peripheral mechanisms in irritable bowel syndrome. N Engl J Med. 2013;368:578–579. doi: 10.1056/NEJMc1214185. [DOI] [PubMed] [Google Scholar]
- 7.Matricon J, Meleine M, Gelot A, Piche T, Dapoigny M, Muller E, Ardid D. Review article: Associations between immune activation, intestinal permeability and the irritable bowel syndrome. Aliment Pharmacol Ther. 2012;36:1009–1031. doi: 10.1111/apt.12080. [DOI] [PubMed] [Google Scholar]
- 8.Spiller R, Lam C. An Update on Post-infectious Irritable Bowel Syndrome: Role of Genetics, Immune Activation, Serotonin and Altered Microbiome. J Neurogastroenterol Motil. 2012;18:258–268. doi: 10.5056/jnm.2012.18.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee H, Park JH, Park DI, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI, Chae SW. Mucosal mast cell count is associated with intestinal permeability in patients with diarrhea predominant irritable bowel syndrome. J Neurogastroenterol Motil. 2013;19:244–250. doi: 10.5056/jnm.2013.19.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anastasi JK, McMahon DJ, Kim GH. Symptom management for irritable bowel syndrome: a pilot randomized controlled trial of acupuncture/moxibustion. Gastroenterol Nurs. 2009;32:243–255. doi: 10.1097/SGA.0b013e3181b2c920. [DOI] [PubMed] [Google Scholar]
- 11.Pei LX, Zhang XC, Sun JH, Geng H, Wu XL. [Meta analysis of acupuncture-moxibustion in treatment of irritable bowel syndrome] Zhongguo Zhen Jiu. 2012;32:957–960. [PubMed] [Google Scholar]
- 12.Zhao C, Mu JP, Cui YH, Yang L, Ma XP, Qi L. Meta-analysis of acupuncture-moxibustion in treatment of irritable bowel syndrome. Zhonghua Zhongyiyao Xuekan. 2010;28:961–963. [Google Scholar]
- 13.Manheimer E, Wieland LS, Cheng K, Li SM, Shen X, Berman BM, Lao L. Acupuncture for irritable bowel syndrome: systematic review and meta-analysis. Am J Gastroenterol. 2012;107:835–47; quiz 848. doi: 10.1038/ajg.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manheimer E, Cheng K, Wieland LS, Min LS, Shen X, Berman BM, Lao L. Acupuncture for treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2012;5:CD005111. doi: 10.1002/14651858.CD005111.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacPherson H, Tilbrook H, Bland JM, Bloor K, Brabyn S, Cox H, Kang’ombe AR, Man MS, Stuardi T, Torgerson D, et al. Acupuncture for irritable bowel syndrome: primary care based pragmatic randomised controlled trial. BMC Gastroenterol. 2012;12:150. doi: 10.1186/1471-230X-12-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cole SJ, Duncan HD, Claydon AH, Austin D, Bowling TE, Silk DB. Distal colonic motor activity in four subgroups of patients with irritable bowel syndrome. Dig Dis Sci. 2002;47:345–355. doi: 10.1023/a:1013722122622. [DOI] [PubMed] [Google Scholar]
- 17.Eshraghian A, Eshraghian H. Interstitial cells of Cajal: a novel hypothesis for the pathophysiology of irritable bowel syndrome. Can J Gastroenterol. 2011;25:277–279. doi: 10.1155/2011/478370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou EH, Ding GH, Wu HG, Qi L, Liu HR, Wang XM, Ma XP. Immediate effect of acupuncture on colon motility in patients with irritable bowel syndrome. Zhonghua Zhongyiyao Xuekan. 2011;29:293–296. [Google Scholar]
- 19.Chen YP. Effects of acupuncture on irritable bowel syndrome patients with different TCM syndromes in electrocolonogram and brain-gut peptide. Guangzhou, China: Thesis Collection Doctors Guangzhou University Traditional Chinese Medicine; 2000. [Google Scholar]
- 20.Wang ZJ, Li WM. [Effects of electroacupuncture on disorder of intestinal motility in a rat model of irritable bowel syndrome] Zhong Xi Yi Jie He Xue Bao. 2010;8:883–887. doi: 10.3736/jcim20100913. [DOI] [PubMed] [Google Scholar]
- 21.Liu WA, Chang XR, Yu BS, Liu M, Zhang HF, Yue ZH. The influence of herbal-cake-separated-moxibustion on gastrointestinal hormone and gastrointestinal motility in functional gastrointestinal disorders’ rats with syndrome of liver stagnation and spleen deficiency. Shijie Huaren Xiaohua Zazhi. 2013;21:1002–1007. [Google Scholar]
- 22.Kuiken SD, Lindeboom R, Tytgat GN, Boeckxstaens GE. Relationship between symptoms and hypersensitivity to rectal distension in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2005;22:157–164. doi: 10.1111/j.1365-2036.2005.02524.x. [DOI] [PubMed] [Google Scholar]
- 23.Jiang M, Tang H, Liu ZY, Zhang YX, Fu BY. Relationship between visceral hypersensitivity and clinical symptoms in irritable bowel syndrome. Shijie Huaren Xiaohua Zazhi. 2005;13:561–564. [Google Scholar]
- 24.Posserud I, Syrous A, Lindström L, Tack J, Abrahamsson H, Simrén M. Altered rectal perception in irritable bowel syndrome is associated with symptom severity. Gastroenterology. 2007;133:1113–1123. doi: 10.1053/j.gastro.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 25.Xing J, Larive B, Mekhail N, Soffer E. Transcutaneous electrical acustimulation can reduce visceral perception in patients with the irritable bowel syndrome: a pilot study. Altern Ther Health Med. 2004;10:38–42. [PubMed] [Google Scholar]
- 26.Xiao WB, Liu YL. Rectal hypersensitivity reduced by acupoint TENS in patients with diarrhea-predominant irritable bowel syndrome: a pilot study. Dig Dis Sci. 2004;49:312–319. doi: 10.1023/b:ddas.0000017458.55517.33. [DOI] [PubMed] [Google Scholar]
- 27.Wu HG, Liu HR, Zhang ZA, Zhou EH, Wang XM, Jiang B, Shi Z, Zhou CL, Qi L, Ma XP. Electro-acupuncture relieves visceral sensitivity and decreases hypothalamic corticotropin-releasing hormone levels in a rat model of irritable bowel syndrome. Neurosci Lett. 2009;465:235–237. doi: 10.1016/j.neulet.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 28.Wu JC, Ziea ET, Lao L, Lam EF, Chan CS, Liang AY, Chu SL, Yew DT, Berman BM, Sung JJ. Effect of electroacupuncture on visceral hyperalgesia, serotonin and fos expression in an animal model of irritable bowel syndrome. J Neurogastroenterol Motil. 2010;16:306–314. doi: 10.5056/jnm.2010.16.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang JJ, Xiong HL, Chu D, Cheng PF, Qian W, Liu S. Effect and mechanism of electroacupuncture at ST-36 on visceral hypersensitivity in rats. Weichangbing Xue. 2010;15:665–668. [Google Scholar]
- 30.Qi L, Liu HR, Yi T, Wu LY, Liu XR, Zhao C, Shi Y, Ma XP, Wu HG. Warming Moxibustion Relieves Chronic Visceral Hyperalgesia in Rats: Relations to Spinal Dynorphin and Orphanin-FQ System. Evid Based Complement Alternat Med. 2013;2013:920675. doi: 10.1155/2013/920675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Kim DY, Camilleri M. Serotonin: a mediator of the brain-gut connection. Am J Gastroenterol. 2000;95:2698–2709. doi: 10.1111/j.1572-0241.2000.03177.x. [DOI] [PubMed] [Google Scholar]
- 33.Liu HR, Hua XG, Yang Y, Wu HG. Clinical study on the treatment of diarrhea-predominant IBS with herb-partitioned moxibustion and the expression of colonic mucosal 5-HT. Liaoning Zhongyi Zazhi. 2006;33:984–985. [Google Scholar]
- 34.Liu X, Guo G, Ma YX, Liu CZ, Gao SZ. Effect of navel treatment on serum brain-gut peptide level in the irritable bowel syndrome patients with spleen deficiency. Shandong Zhongyiyao Daxue Xuebao. 2013;28:262–264. [Google Scholar]
- 35.Liu HR, Wang XM, Zhou EH, Shi Y, Li N, Yuan LS, Wu HG. Acupuncture at both ST25 and ST37 improves the pain threshold of chronic visceral hypersensitivity rats. Neurochem Res. 2009;34:1914–1918. doi: 10.1007/s11064-009-9972-1. [DOI] [PubMed] [Google Scholar]
- 36.Tian XY, Bian ZX, Hu XG, Zhang XJ, Liu L, Zhang H. Electro-acupuncture attenuates stress-induced defecation in rats with chronic visceral hypersensitivity via serotonergic pathway. Brain Res. 2006;1088:101–108. doi: 10.1016/j.brainres.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 37.Zhou EH, Liu HR, Wu HG, Shi Y, Wang XM, Yao LQ, Zhong YS, Yang Y. Herb-partition moxibustion relieves chronic visceral hyperalgesia and 5-HT concentration in colon mucosa of rats. Neurol Res. 2009;31:734–737. doi: 10.1179/174313209X382313. [DOI] [PubMed] [Google Scholar]
- 38.Zhang L, Zhou H, Lv B, Chu L, Li M. Role of CRF on 5-HT signaling pathway and visceral hypersensitivity in rats. Weichangbing Xue. 2011;16:534–538. [Google Scholar]
- 39.Bravo JA, Dinan TG, Cryan JF. Alterations in the central CRF system of two different rat models of comorbid depression and functional gastrointestinal disorders. Int J Neuropsychopharmacol. 2011;14:666–683. doi: 10.1017/S1461145710000994. [DOI] [PubMed] [Google Scholar]
- 40.Hirata T, Funatsu T, Keto Y, Nakata M, Sasamata M. Pharmacological profile of ramosetron, a novel therapeutic agent for IBS. Inflammopharmacology. 2007;15:5–9. doi: 10.1007/s10787-006-1537-1. [DOI] [PubMed] [Google Scholar]
- 41.Nozu T, Kudaira M. Corticotropin-releasing factor induces rectal hypersensitivity after repetitive painful rectal distention in healthy humans. J Gastroenterol. 2006;41:740–744. doi: 10.1007/s00535-006-1848-4. [DOI] [PubMed] [Google Scholar]
- 42.Skórzewska A, Lehner M, Hamed A, Wisłowska-Stanek A, Turzyńska D, Sobolewska A, Płaźnik A. The effect of CRF2 receptor antagonists on rat conditioned fear responses and c-Fos and CRF expression in the brain limbic structures. Behav Brain Res. 2011;221:155–165. doi: 10.1016/j.bbr.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 43.Ma XP, Tan LY, Yang Y, Wu HG, Jiang B, Liu HR, Yang L. Effect of electro-acupuncture on substance P, its receptor and corticotropin-releasing hormone in rats with irritable bowel syndrome. World J Gastroenterol. 2009;15:5211–5217. doi: 10.3748/wjg.15.5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshimoto S, Babygirija R, Dobner A, Ludwig K, Takahashi T. Anti-stress effects of transcutaneous electrical nerve stimulation (TENS) on colonic motility in rats. Dig Dis Sci. 2012;57:1213–1221. doi: 10.1007/s10620-012-2040-8. [DOI] [PubMed] [Google Scholar]
- 45.Lin L, Zhao ZQ, Yan Y, Lin Z, Zhang HJ. Relation between irritable bowel syndrome and gastrointestinal peptides. Zhonghua Xiaohua Zazhi. 2003;23:501–502. [Google Scholar]
- 46.Wu HG, Jiang B, Zhou EH, Shi Z, Shi DR, Cui YH, Kou ST, Liu HR. Regulatory mechanism of electroacupuncture in irritable bowel syndrome: preventing MC activation and decreasing SP VIP secretion. Dig Dis Sci. 2008;53:1644–1651. doi: 10.1007/s10620-007-0062-4. [DOI] [PubMed] [Google Scholar]
- 47.Wang W, Zhang Y, Lü EJ, Gao ZX, Zhao JR. Effect of acupuncture at ST 37 on serum gastrointestinal hormones in rat models of IBS chronic visceral hyperalgesia. Gansu Zhongyi Xueyuan Xuebao. 2011;28:5–7. [Google Scholar]
- 48.Liu MR, Xiao RF, Peng ZP, Zuo HN, Zhu K, Wang SM. [Effect of acupuncture at “Zusanli” (ST 36 and “Taichong” (LR 3) on gastrointestinal hormone levels in rats with diarrhea type irritable bowel syndrome] Zhen Ci Yan Jiu. 2012;37:363–368. [PubMed] [Google Scholar]
- 49.Liu MR, Xiao RF, Zuo HN. Effect of acupuncture at ST 36 and LR 3 on NPY and VIP levels in diarrhea type IBS rat models. Jiangsu Zhongyiyao. 2013;45:69–71. [Google Scholar]
- 50.Zhu WL, Li Y, Wei HF, Ren XX, Sun J, Zhang LF, Zhu J. Effect of electro-acupuncture at different acupoints on neuropeptide and somatostatin in rat brain with irritable bowel syndrome. Chin J Integr Med. 2012;18:288–292. doi: 10.1007/s11655-011-0795-y. [DOI] [PubMed] [Google Scholar]
- 51.Silverman DH, Munakata JA, Ennes H, Mandelkern MA, Hoh CK, Mayer EA. Regional cerebral activity in normal and pathological perception of visceral pain. Gastroenterology. 1997;112:64–72. doi: 10.1016/s0016-5085(97)70220-8. [DOI] [PubMed] [Google Scholar]
- 52.Mertz H, Morgan V, Tanner G, Pickens D, Price R, Shyr Y, Kessler R. Regional cerebral activation in irritable bowel syndrome and control subjects with painful and nonpainful rectal distention. Gastroenterology. 2000;118:842–848. doi: 10.1016/s0016-5085(00)70170-3. [DOI] [PubMed] [Google Scholar]
- 53.Bonaz B, Baciu M, Papillon E, Bost R, Gueddah N, Le Bas JF, Fournet J, Segebarth C. Central processing of rectal pain in patients with irritable bowel syndrome: an fMRI study. Am J Gastroenterol. 2002;97:654–661. doi: 10.1111/j.1572-0241.2002.05545.x. [DOI] [PubMed] [Google Scholar]
- 54.Liu HR, Zuo CT, Zhao TP, Wang XL, Wu HG, Ma XP. PET brain imaging study on electroacupuncture at ST 25 for irritable bowel syndrome. Hangzhou, China: National Symposium of Clinical and Scientific Study on Acupuncture and Moxibustion & Study Progress of Spinal Diseases; 2005. pp. 108–115. [Google Scholar]
- 55.Guo MN, Wang PQ. Study on the effect of eye acupuncture on cerebral blood flow in patients with diarrhea-predominant irritable bowel syndrome. Shanghai Zhenjiu Zazhi. 2012;31:540–541. [Google Scholar]
- 56.Chu WC, Wu JC, Yew DT, Zhang L, Shi L, Yeung DK, Wang D, Tong RK, Chan Y, Lao L, et al. Does acupuncture therapy alter activation of neural pathway for pain perception in irritable bowel syndrome?: a comparative study of true and sham acupuncture using functional magnetic resonance imaging. J Neurogastroenterol Motil. 2012;18:305–316. doi: 10.5056/jnm.2012.18.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu QY. Regulatory effect of electroacupuncture at ST 37 on colonic function of rats of acute restraint stress and its central mechanism. Wuhan, China: Thesis Doctors Hubei University Traditional Chinese Medicine; 2008. [Google Scholar]
- 58.Qi DB, Li WM. Effects of electroacupuncture on expression of c-fos protein and N-methyl-D-aspartate receptor 1 in the rostral ventromedial medulla of rats with chronic visceral hyperalgesia. Zhong Xi Yi Jie He Xue Bao. 2012;10:416–423. doi: 10.3736/jcim20120410. [DOI] [PubMed] [Google Scholar]
- 59.Rong PJ, Zhu B, Huang QF, Gao XY, Ben H, Li YH. [Acupuncture inhibiting responses of spinal dorsal dorsal horn neurons induced by noxious dilation rectum and colon] Zhongguo Zhen Jiu. 2005;25:645–650. [PubMed] [Google Scholar]
- 60.Mönnikes H, Rüter J, König M, Grote C, Kobelt P, Klapp BF, Arnold R, Wiedenmann B, Tebbe JJ. Differential induction of c-fos expression in brain nuclei by noxious and non-noxious colonic distension: role of afferent C-fibers and 5-HT3 receptors. Brain Res. 2003;966:253–264. doi: 10.1016/s0006-8993(02)04197-5. [DOI] [PubMed] [Google Scholar]
- 61.Qi DB, Li WM. Effects of electroacupuncture on expression of c-fos protein in the spinal dorsal horn of rats with chronic visceral hyperalgesia. Zhong Xi Yi Jie He Xue Bao. 2012;10:1490–1496. doi: 10.3736/jcim20121224. [DOI] [PubMed] [Google Scholar]
- 62.Wei EQ. Pharmacology Frontier: Signal, Protein, Gene and Modern Pharmacology. Beijing: Science Press; 1999. pp. 120–136. [Google Scholar]
- 63.Gaudreau GA, Plourde V. Involvement of N-methyl-d-aspartate (NMDA) receptors in a rat model of visceral hypersensitivity. Behav Brain Res. 2004;150:185–189. doi: 10.1016/j.bbr.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 64.Zhou J, Li WM. Influence of electroacupuncture on expression of N-methyl-D-aspartate receptors in the spinal cord of irritable bowel syndrome of rat. Shanghai Zhenjiu Zazhi. 2008;27:38–40. [Google Scholar]
- 65.Tian SL, Wang XY, Ding GH. Repeated electro-acupuncture attenuates chronic visceral hypersensitivity and spinal cord NMDA receptor phosphorylation in a rat irritable bowel syndrome model. Life Sci. 2008;83:356–363. doi: 10.1016/j.lfs.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 66.Wouters MM, Boeckxstaens GE. Neuroimmune mechanisms in functional bowel disorders. Neth J Med. 2011;69:55–61. [PubMed] [Google Scholar]
- 67.Xu JR, Luo JY, Shang L, Kong WM. Effect of enteric neuron and enteric glia on irritable bowel syndrome subgroups and the interference tegaserod. Shenjing Jiepouxue Zazhi. 2006;22:643–647. [Google Scholar]
- 68.Xu JR, Luo JY, Shang L, Kong WM. The plasticity of the enteric submucosal plexus irritable bowel syndrome rats. Zhonghua Xiaohua Zazhi. 2007;27:107–110. [Google Scholar]
- 69.Zhu LT, Zhu Y. Plasticity of the enteric submucosal plexus in IBS-C rats by electroacupuncture. Chengdu, China: Eighth National Academic Conference, China Medical Association of Rehabilitation; 2011. p. 118. [Google Scholar]
- 70.Yang HY, Guo TT, Ma YN, Liu TY, Gao M. [Effects of 650 nm laser and moxibustion pretreatment on enteric nervous system and medullary visceral zone in rats with visceral traction pain] Zhongguo Zhen Jiu. 2010;30:745–751. [PubMed] [Google Scholar]
- 71.Li ZS, Zhan LX, Zou DW, Xu GM, Man XH, Ye XT. Morphological and functional alteration of serotonin-producing intestinal enterochromaffin cells in patients with irritable bowel syndrome. Zhonghua Xiaohua Zazhi. 2004;24:30–33. [Google Scholar]
- 72.Wheatcroft J, Wakelin D, Smith A, Mahoney CR, Mawe G, Spiller R. Enterochromaffin cell hyperplasia and decreased serotonin transporter in a mouse model of postinfectious bowel dysfunction. Neurogastroenterol Motil. 2005;17:863–870. doi: 10.1111/j.1365-2982.2005.00719.x. [DOI] [PubMed] [Google Scholar]
- 73.Jiang Y, Wu HG, Shi Z, Zhou EH, Zhao TP. Regulatory effect of electroacupuncture on intestinal sensitivity and motility of constipation-predominant IBS rats. Beijing, China: 20th Anniversary of World Acupuncture Association & World Academic Conference of Acupuncture and Moxibustion; 2007. pp. 84–85. [Google Scholar]
- 74.Song SY, Wang DS, Wang YJ, Zhao JR, Zhang FL. Effect of eye acupuncture on the expression of colonic enterochromaffin cell in IBS rats. Jiangsu Zhongyiyao. 2010;42:73–74. [Google Scholar]
- 75.Camilleri M, Lasch K, Zhou W. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;303:G775–G785. doi: 10.1152/ajpgi.00155.2012. [DOI] [PubMed] [Google Scholar]
- 76.Wu HG, Wang JH, Chen HP, Hua XG, Shi Z. Therapeutic efficacy of herb-partitioned moxibustion for irritable bowel syndrome and its immunological mechanism. Zhongguo Zhenjiu. 1996;16:43–45. [Google Scholar]
- 77.Zhang RH. Therapeutic efficacy of acupuncture-moxibustion for elderly irritable bowel syndrome and its effect on serum IL-18, IL-23, and TNF-α. Zhongguo Laonian Xue Zazhi. 2013;33:1435–1436. [Google Scholar]
- 78.Wang J, Liang ZX, Zhang ZX, Li GH, Qian W, Hou XH. Alterations of enteric mucosal mast cells in patients with irritable bowel syndrome. Shijie Huaren Xiaohua Zazhi. 2004;2:382–384. [Google Scholar]
- 79.El-Salhy M. Irritable bowel syndrome: diagnosis and pathogenesis. World J Gastroenterol. 2012;18:5151–5163. doi: 10.3748/wjg.v18.i37.5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Quigley EM, Craig OF. Irritable bowel syndrome; update on pathophysiology and management. Turk J Gastroenterol. 2012;23:313–322. doi: 10.4318/tjg.2012.0551. [DOI] [PubMed] [Google Scholar]
- 81.Wang XM, Lu Y, Wu LY, Yu SG, Zhao BX, Hu HY, Wu HG, Bao CH, Liu HR, Wang JH, et al. Moxibustion inhibits interleukin-12 and tumor necrosis factor alpha and modulates intestinal flora in rat with ulcerative colitis. World J Gastroenterol. 2012;18:6819–6828. doi: 10.3748/wjg.v18.i46.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang YJ, Liu HH, Liu XD, Chai JY, Zhao JR, Wang DS. Eye acupuncture therapy up-regulates aquaporin 3 expression in the colon of rats with diarrhea-predominant irritable bowel syndrome. Shijie Huaren Xiaohua Zazhi. 2011;19:899–904. [Google Scholar]
- 83.Liu HH, Liu XD, Wang YJ, Guan HQ, Chai JY, Zhao JR, Wang DS. [Effects of acupoint area and non-acupoint area of eye-acupuncture on expressions of VIP and AQP 8 in colonic tissues in rats with D-IBS] Zhongguo Zhen Jiu. 2012;32:919–924. [PubMed] [Google Scholar]
- 84.Xue Y, Tian XD. Clinical study on the relationship between free radical and irritable bowel syndrome and the effect of acupuncture. Chinese Archives Traditional Chinese Medicine. 2009;27:111–112. [Google Scholar]
- 85.Gilkin RJ. The spectrum of irritable bowel syndrome: A clinical review. Clin Ther. 2005;27:1696–1709. doi: 10.1016/j.clinthera.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 86.Liu HR, Xie JQ, Wu HG, Wang XL, Zhao C, Hua XG, Zhou S, Ma XP, Tan LY. Acupuncture and irritable bowel syndrome: a population-based epidemiological and clinical study. J Acupunct Tuina Sci. 2010;8:70–74. [Google Scholar]
- 87.Wang B, Han Z. Current research situation and progress in building animal model of irritable bowel syndrome. Guoji Xiaohuabing Zazhi. 2008;28:359–360, 367. [Google Scholar]


