Abstract
Aberrant expression of glycoconjugates occurs during malignant transformation of cancer cells. Overexpression of sialoglycoconjugates in particular may play an important role in the progression, i.e., invasion or metastasis, of cancer. Various types of sialoglycoconjugates have been investigated to clarify their biological significance and clinical utility in diagnosing and treating colorectal cancer. This review focuses specifically on expression of mucin (MUC) 1 and it suggests that MUC1 with the specific structure of a sialo-oligosaccharide has biological significance in determining the metastatic potential of colorectal cancer cells and clinicopathological utility in evaluating the effectiveness of treatments and the prognosis for patients with colorectal cancer. Further studies are expected to contribute to the expanded use of cancer-associated sialoglycoconjugates in cancer diagnosis and therapy.
Keywords: Sialoglycoconjugates, Sialic acid, Mucin 1, Colorectal cancer, Invasion, Metastasis
Core tip: Many types of cancer-associated sialoglycoconjugates are produced during oncogenesis or in various stages of malignant transformation. Aberrant expression of sialoglycoconjugates has been evaluated in many histochemical and molecular biological studies. An overview of the current knowledge is crucial to understand its clinicopathological utility in diagnosing and treating colorectal cancer. The biological significance of sialoglycoconjugates in the progression of cancer is also discussed. This review may contribute to expanding the use of cancer-associated sialoglycoconjugates in cancer diagnosis and therapy.
INTRODUCTION
Expression of various substances changes drastically during oncogenesis and the subsequent stages of cancer progression. The expression profile of components on the cell membrane in particular has a significant role in the proliferation and migration of cancer cells. Detecting cancer-associated molecules is an effective way to predict the prognosis for cancer patients.
Sialoglycoconjugates with a molecular structure containing sialo-oligosaccharides are expressed in many types of cells, where they participate in various biological events, e.g., cell adhesion and recognition[1]. The overexpression of sialoglycoconjugates, however, has been detected in cancer cells or tissues and may correlate with cancer behavior[2-4]. Many histochemical studies using colorectal cancer tissues have shown that elevated expression of sialoglycoconjugates was related to a worse prognosis for patients. Furthermore, molecular biological studies have revealed that those overexpressed sialoglycoconjugates had specific structures of sialo-oligosaccharides. The present article reviews the clinicopathological utility of sialoglycoconjugates in diagnosing and treating colorectal cancer and its metastatic foci, and discusses the biological significance of sialoglycoconjugates in the progression of cancer.
MOLECULAR CHARACTERISTICS AND BIOLOGICAL SIGNIFICANCE OF SIALOGLYCOCONJUGATES IN COLORECTAL CANCER
Sialo-oligosaccharides as a whole
There are three types of linkages between sialic acid and terminal galactose (Gal) or N-acetylgalactosamine (GalNAc) residues, but expression of glycoconjugates with α2,3- or α2,6-sialylated oligosaccharides in particular has often been investigated in relation to colorectal cancer. The sialic acid-binding lectins Maackia amurensis leukoagglutinin (MAL) and Sambucus nigra agglutinin (SNA) were used to respectively detect the expression of α2,3- and α2,6-sialoglycoconjugates[5,6]. Overexpression of sialoglycoconjugates recognized by those lectins was frequently detected in colorectal cancer tissue compared to surrounding non-cancerous tissue[7-9]. Various sialyltransferases contribute to creation of cancer-associated sialo-oligosaccharides, and some types of sialyltransferases such as β-galactoside α2,6-sialyltransferase (ST6Gal I) and α2,3-sialyltransferase (ST3Gal I) enhance their activity in colorectal cancer tissues[10-13]. Results of those studies suggested that overexpression of sialoglycoconjugates via activation of sialyltransferases occurs during the malignant transformation of cancer. Overexpression of sialoglycoconjugates has two patterns: (1) upregulating modification of sialo-oligosaccharides to glycoconjugates; and (2) upregulating expression of sialoglycoconjugates themselves. Alteration of the sialic acid moieties of glycoconjugates induces biological behavior of cancer cells, such as activation of motility and resistance to cell death and drugs (Figure 1). Several reports have shown that upregulation of α2,6 sialylation, and especially the sialylation of β1 integrins, is associated with the adhesion, migration, and invasion of colorectal cancer cells[14-16]. Swindall et al[17] revealed that the death receptor Fas is a substrate of ST6Gal I and that α2,6 sialylation of Fas confers protection against Fas-mediated apoptosis. ST6Gal I also catalyzed the sialylation of epidermal growth factor receptor (EGFR) and the loss of this sialylation by ST6Gal I knockdown increased the anti-cancer effect of the EGFR kinase inhibitor gefitinib[18]. In light of these findings, activation of sialyltransferases in colorectal cancer cells may change the function of various glycoconjugates and trigger events in cancer progression. Many previous studies also investigated altered expression of sialoglycoconjugates themselves. These sialoglycoconjugates have been designated cancer-associated molecules. Major cancer-associated sialo-oligosaccharides and sialoglycoconjugates that have been studied in relation to colorectal cancer are described later.
Figure 1.
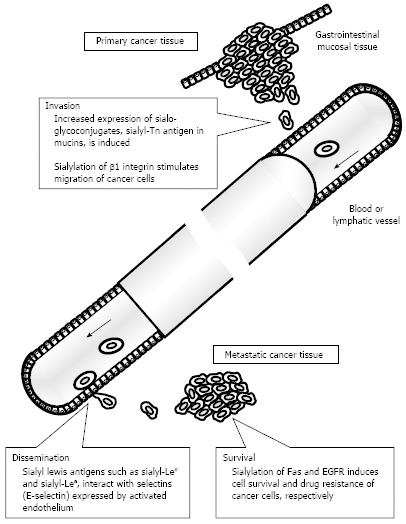
Relationship between altered expression of sialoglycoconjugates and various steps of metastasis. EGFR: Epidermal growth factor receptor.
Cancer-associated sialyl-Lewis and sialyl-Tn antigens
Specific types of sialo-oligosaccharides and sialoglycoconjugates have garnered the attention of efforts to understand the biological and clinical significance of colorectal cancer behavior. Sialyl-Lewis-related antigens such as sialyl-Lewis x (sialyl-Lex) and sialyl-Lewis a (sialyl-Lea) antigens are the sialo-oligosaccharide complexes most often investigated in relation to colorectal cancer. The sialyl-Lewis structure consists of an N-acetylglucosamine (GlcNAc)-Gal backbone with an α2,3-linked sialic acid bound to Gal and fucose bound to GlcNAc. Elevated levels of sialyl-Lewis-related antigens are induced by down-regulation of ST6GalNAc VI, which transfers α2,6-linked sialic acid to GlcNAc to synthesize a disialyl Lewis structure[19,20]. This disialyl-Lewis structure-containing oligo-saccharide is expressed in normal tissue, so down-regulation of ST6GalNAc VI followed by formation of a sialyl-Lewis structure may be an important event during the malignant transformation of colorectal cancer. Furthermore, enhanced fucosyltransferase activity also leads to the stimulation of a cancer-associated sialyl-Lewis structure[21,22]. However, several studies have noted that expression of fucosyltransferases was not significantly enhanced in colorectal cancer tissues compared to adjacent normal tissue[23,24]. Although findings for colorectal cancer tissues are not consistent, the systematic regulation of glycosyltransferases plays an important role in expression of sialyl-Lewis-related antigens.
Another cancer-associated sialo-oligosaccharide antigen is sialyl-Tn antigen. This antigen is slightly expressed in normal epithelial tissue and therefore is highly specific as a cancer-associated molecule. The structure of sialyl-Tn consists of a terminal sialic acid α2,6-linked to GalNAc that is bound to the serine or threonine residue of a protein. Studies have suggested that the major regulator of sialyl-Tn antigen expression is ST6GalNAc I[25,26]. However, Vázquez-Martín et al[27] found that there was no correlation between ST6GalNAc I activity and the histological level of expression of sialyl-Tn antigen in colorectal cancer tissues. In addition, another sialyltransferase, ST6GalNAc II, synthesizes sialyl-Tn antigen in vitro and may be related to expression of sialyl-Tn antigen, along with ST6GalNAc I, in colorectal cancer[28]. A systematic mechanism is thought to induce the overexpression of sialyl-Tn antigen in colorectal cancer tissue.
Sialo-oligosaccharides play a significant biological role in cancer progression, and especially in cancer invasion and metastasis (Figure 1). Sialyl-Lex and sialyl-Lea antigens function as a ligand of selectins. Sialyl-Lex antigen expressed on leukocytes interacts with selectins expressed on activated vascular endothelium, inducing the rolling of leukocytes and contributing to recruitment of leukocytes to inflammatory lesions[29]. The interaction of sialyl-Lex and sialyl-Lea antigens with E-selectin also contributes to the adhesion of cancer cells to the vascular endothelium[30,31]. Cancer cell lines expressing sialyl-Lex and/or sialyl-Lea antigens adhered to human umbilical vein endothelial cells, and this interaction was inhibited by use of anti-E-selectin antibody or benzyl N-acetyl-α-D-galactosaminide[31]. In addition, this effect depended on the expressed level of sialyl-Lex antigen in colorectal cancer cells. Sialylation of glycoprotein CD44 and mucin may be altered in case of overexpression of sialyl-Tn antigen. CD44 is a transmembrane protein related to cell adhesion, and its splice variant (CD44v) carrying sialyl-Tn antigen was upregulated in colorectal cancer cells and was related to enhanced metastatic potential[32]. Sialo-mucins, and especially MUC1, also carry sialyl-Tn antigen in their extracellular highly glycosylated domains, and overexpression of sialyl-Tn antigen is related to worse tumor behavior, as described later. In other molecules, integrin β1, MUC1, and osteopontin may be sialyl-Tn antigen carriers and contribute to the stimulation of cell migration, but their biological significance in colorectal cancer is still a subject of debate[4]. Although additional evidence should be accumulated from biochemical and molecular biological studies, the upregulated modification of sialyl-Tn antigens may disable the primary function of those proteins and induce an invasive phenotype in colorectal cancer cells. Histochemical analysis indicated that tissues from colorectal metastases had a significant increase in sialyl-Tn and sialyl-Lex expression compared to primary tumor tissues[33]. Alteration of the level of expression of glycoconjugates with those sialo-oligosaccharides occurs during the process of metastasis and is perpetuated in cancer cells in metastatic foci. This biological phenomenon can be utilized to develop sensitive methods for detecting metastatic tissues.
Carcinoembryonic antigen
Carcinoembryonic antigen (CEA) was first identified by Gold and Freedman and its expression was detected in colon cancer and other cancers of digestive organs as well as fetal digestive system tissues[34,35]. A subsequent study indicated that CEA was expressed in normal body fluids and mucosal tissues and had a variety of cross-reacting forms that induced variable expression patterns[36]. Sanders et al[37] showed that Lex and sialyl-Lex were coexpressed on CEA-related glycoproteins, thus suggesting that CEA is a transmembrane sialoglycoconjugate. CEA expressed on the cell surface was found to act as a homotypic intercellular adhesion molecule, and CEA may contribute to colon carcinogenesis by inhibiting differentiation of colonic epithelium[38]. Alteration of the expression and distribution of CEA may have an important role in the metastasis of colon cancer to the liver[39]. Colorectal cancer cells with elevated expression of CEA by transfection of cDNA had an enhanced potential for metastasis to the liver in nude mice[40]. In addition, circulating CEA, which is elevated in patients with various gastrointestinal cancers, was recognized by a receptor expressed on the liver cell surface[41]. This biological phenomenon may lead to the induction of a receptor for circulating cancer cells that express CEA-related glycoproteins, enhancing the metastatic potential of cancer cells.
Cancer-associated mucin
Mucins are expressed in gastrointestinal tissues, including the large intestine[42]. Mucins are categorized as transmembrane (MUC1, MUC3A, MUC3B, MUC4, MUC12, MUC13, MUC15, MUC16, and MUC17) or secreted (MUC2, MUC5AC, MUC5B, MUC6, MUC7, and MUC19). The level of each mucin’s expression or secretion differs in different types of tissues and organs. Colorectal tissue mainly contains several types of secreted mucins (MUC2, MUC5AC) and transmembrane mucins (MUC1, MUC3, MUC4, and MUC12), and alteration of their expression may be related to the malignancy of colorectal cancer. MUC2 and MUC5AC are clustered at the same chromosomal locus (11p15.5), and their expression may be regulated by a common mechanism[43]. In colorectal cancer cells, however, repression of mucin transcription is controlled by the epigenetic mechanism of methylation[44,45]. MUC2 expression decreases in differentiated colorectal adenocarcinoma tissues but not in mucinous carcinoma tissues, whereas MUC5AC expression increases in differentiated colorectal adenocarcinoma but decreases in mucinous carcinoma tissues[46]. A study of Muc2-deficient mice showed that decreased expression of MUC2 induced tumor formation in the small intestine and the colon[47]. MUC5AC is not expressed in normal colonic epithelium whereas de novo expression was frequently detected in adenoma and carcinoma tissues of the colon[48,49]. As a transmembrane mucin, MUC1 has often been investigated to clarify its significance in the progression, i.e., invasion or metastasis, of colorectal cancer. MUC1 has a variable number of tandem repeats of the 20 amino acids in its extracellular domain. This tandem repeat sequence contains a number of O-linked glycosylation sites, and the level of glycosylation of those tandem repeats is a characteristic of MUC1[50]. There are various antibodies that can recognize different types of MUC1 molecules (Table 1). Antibodies recognizing the core peptide sequence can detect poorly glycosylated MUC1 while antibodies recognizing glycosides on the tandem repeat domain can detect highly glycosylated MUC1[51-56]. Various forms of MUC1 with different glycoforms are expressed in colonic epithelium and tumor cells, and those glycoforms may change during disease progression. The present review has focused on alteration of sialo-oligosaccharide expression. There are several antibodies recognizing sialylated MUC1, such as monoclonal antibody MY.1E12 and KL-6. Monoclonal antibody MY.1E12 was established by immunizing mice with human milk fat globules and was found to recognize α2,3-sialylated O-linked oligosaccharides[55,57]. The latter monoclonal antibody, KL-6 antibody, also recognizes MUC1 and was obtained by Kohno et al[58,59] from a hybridoma established from the splenocytes of a BALB/c mouse immunized with a human pulmonary adenocarcinoma cell line, VMRC-LCR. The epitope structure of KL-6 antibody is still being studied. A chemical study by Ohyabu et al[60] showed that the minimal antigenic structure recognized by KL-6 antibody was a heptapeptide sequence (PDTRPAP) with the sialo-oligosaccharide Neu5Acα2,3Galβ1,3GalNAcα. A recent study by Seko et al[61] showed that the epitope of KL-6 antibody has a 3’-sialylated, 6’-sulfated lacto-N-neotetraose (LNnT) and 3’-sialylated, 6’-sulfated core 1 structure. A previous study by the current authors confirmed this relationship between expression of KL-6 mucin and metastatic potential through analysis using colorectal cancer cell lines[62]. A cytochemical assay detected KL-6 mucin in the surrounding membrane and cytoplasm of cell lines with a high metastatic potential but not in cell lines with a low metastatic potential. Thus, this study indicated that expression of KL-6 mucin affected cancer cell morphology and metastatic potential. The clinicopathological significance of the expression of KL-6 mucin in the surrounding membrane and/or cytoplasm, which may be an important indicator of the liver metastasis of colorectal carcinoma, is discussed later.
Table 1.
Antibodies against mucin 1 recognizing various epitopes
| Antibody | Epitope | Antigen |
| DF3 | Core peptide sequence (DTRPAPGS) in the extracellular tandem repeat | Poorly glycosylated MUC1 |
| NCL-MUC-1-CORE | Core peptide sequence (GVTSAPDTRPAP) in the extracellular tandem repeat | Poorly glycosylated MUC1 |
| MY.1E12 | O-linked Sialyl T1 antigen in the extracellular tandem repeat | Sialylated MUC1 |
| NCL-MUC-1-GP | Carbohydrate in the extracellular tandem repeat | Sialylated MUC1 |
| KL-6 | Core peptide sequence (PDTRPAP) with O-linked Sialyl T1 antigen in the extracellular tandem repeat | Sialylated MUC1 |
| 3'-sialylated, 6'-sulfated LNnT2 and 3'-sialylated, 6-sulfated T antigen3 | ||
| HMFG-1 | Core peptide sequence (PDTR) in the extracellular tandem repeat | Highly-glycosylated MUC1 |
Sialyl T: Neu5Acα2,3Galβ1,3GalNAc;
3'-Sialylated, 6'-sulfated LNnT: Neu5Acα2-3(SO3−-6)Galβ1-4GlcNAcβ1-3Galβ1-4Glc;
3'-Sialylated, 6'-sulfated T: Neu5Acα2-3Galβ1-3(SO3−-6)GalNAc. MUC1: Mucin 1.
CLINICAL UTILITY OF SIALOGLYCOCONJUGATES IN DIAGNOSING AND TREATING COLORECTAL CANCER
Cancer-associated molecules have been used to detect cancer and diagnose cancer behavior[63,64]. Molecules secreted by cancer cells into a patient’s blood in particular are effective as serological markers to screen for cancer and predict patient outcomes[63,65]. Furthermore, the levels of those molecules in serum may be related to cancer behavior and patient prognosis. Clinicopathological analysis of levels of serological and histological markers has suggested that marker expression plays a functional role in cancer progression, such as cancer cell invasion and metastasis[66-71].
Sialoglycoconjugates as a whole
Lectin-immunohistochemical studies have revealed aberrant expression of sialoglycoconjugates in colorectal cancer tissue. Compared to α2,3-linked sialoglycoconjugates (recognized by MAL lectin), α2,6-linked sialoglycoconjugates (recognized by SNA lectin) are significantly related to a worse prognosis for patients with colorectal cancer[7,8]. In a previous study, the current authors detected overexpression of α2,3- and α2,6-linked sialoglycoconjugates in colorectal cancer tissues and found that this overexpression was associated with worse patient survival[9]. Elevated expression of α2,6-linked sialoglycoconjugates was detected in cancerous tissue with lymphatic vessel and venous invasion, lymph node metastasis, and a more advanced tumor stage. Metastatic lymph node tissues also exhibited overexpression of those sialoglycoconjugates. The histological significance of expression profiles of those sialoglycoconjugates is still a subject of debate. An elevated level of total sialic acid was also detected in serum from patients with colorectal cancer[72,73]. In a study of patients with distant metastasis of colorectal cancer, an elevated level of total sialic acid in plasma decreased significantly as a result of 5-fluorouracil administration, eventually dropping below normal levels[73]. Although the correlation between overexpression in cancerous tissue and elevated levels in serum is still not clear, sialoglycoconjugates may be used to screen for patients with metastasis and evaluate the effectiveness of treatments.
Sialoglycoconjugates with cancer-associated sialo-oligosaccharides
Because sialoglycoconjugates have various moieties of the oligosaccharide structure, specific molecules of sialoglycoconjugates have been used as diagnostic markers with a high level of sensitivity. Antibodies against various sialo-oligosaccharides, such as sialyl-Lex, sialyl-Lea, and sialyl-Tn antigens, have been used in histochemical and serological analyses of colorectal cancer. Several histochemical studies suggested that the overexpression of sialyl-Lex or sialyl-Lea antigen can be used to evaluate worse tumor behavior, such as cancer cell invasion and metastasis, as well as worse patient survival[74-77]. Histochemical expression of sialyl-Tn antigen was also analyzed and may be related to worse patient survival[78]. However, other studies have found no significant association between sialyl-Tn antigen expression and prognosis for patients with colorectal cancer[79]. Nakagoe et al[80] compared the clinicopathological significance of those three antigens and found that patients with overexpression of sialyl-Lex antigen had a shorter disease-free survival time than those with low levels of expression of sialyl-Lex antigen. In another study, the same authors found that overexpression of sialyl-Lex antigen was significantly related to a worse overall survival rate[81]. Although studies have described varying clinicopathological significance, the histochemical expression of those antigens, especially sialyl-Lex antigen, is considered an effective way to predict recurrence and worse patient survival. Numerous serological studies of sialo-oligosaccharide antigens have been conducted, and those studies have cited their clinical utility as markers of colorectal cancer. Elevated levels of serum sialyl-Lex, sialyl-Lea, and sialyl-Tn antigen may be useful markers for predicting the metastasis of colorectal cancer along with serum CEA levels[82]. Carbohydrate antigen (CA) 19-9 (identical to sialyl-Lea) and CEA have frequently been studied to clarify their effectiveness as markers to determine the prognosis for patients with colorectal cancer. These antigens could possibly be used to predict the recurrence of colorectal cancer. Many studies have suggested that patients with elevated preoperative serum levels of CEA and/or CA19-9 frequently experience recurrence[83-86]. The combined evaluation of CEA and CA19-9 is recommended for better sensitivity[87,88]. If the preoperative levels of CEA and/or CA19-9 are elevated, a decrease in the postoperative levels of these antigens could be used to evaluate the effectiveness of surgery. Furthermore, measurement of these antigen levels in serum during chemotherapy in patients with colorectal liver metastases helps to evaluate the effectiveness of treatment[89-91]. Recurrent elevation of antigen levels is significantly related to recurrence or progression of disease. According to studies, monitoring serum levels of CEA and CA19-9 perioperatively or during treatment such as chemotherapy may be a method to detect recurrence or measure therapeutic efficacy[92].
Cancer-associated sialo-mucin, MUC1
The structure and level of expression of various sialoglycoconjugates change during cancer formation and progression. Several types of mucins have been studied exhaustively to understand their clinical utility. Decreased expression of MUC2, a secreted mucin, was frequently detected in colorectal adenocarcinoma tissues and significantly related to recurrence and worse survival[93,94]. MUC1, a transmembrane mucin, has often been investigated as a cancer-associated mucin antigen while a few studies have analyzed the pathological utility of other antigens. Shanmugam et al[95] immunohistochemically analyzed MUC4 in colorectal cancer tissue and found that a high level of MUC4 expression was significantly related to shorter survival for patients with colorectal cancer, especially for those with early stages of cancer. The present review has focused on expression of MUC1 in colorectal cancer and its clinicopathological significance. A number of studies have examined MUC1 expression in colorectal cancer tissue and analyzed its pathological significance and clinical utility[96-98]. Baldus et al[99] histochemically analyzed MUC1 (detected using antibodies recognizing the tandem repeat peptide), MUC2, sialyl-Lex, and sialyl-Lea antigens in colorectal cancer tissues. They found that strong immunoreactivity for MUC1 was correlated with an advanced tumor stage and the presence of distant metastasis and they posited that MUC1 may be an independent predictor of survival.
However, some reports have noted that aberrant expression of MUC1 is more prevalent in advanced stages of colorectal cancer tissue but is not significantly related to various clinicopathological features[100]. Studies have differing views on the clinicopathological significance of MUC1 expression overall in cancerous tissue. MUC1 has been localized in cancer cells and tissue. Stromal MUC1/MY.1E12 (detected using the monoclonal antibody MY.1E12) was frequently detected in advanced-stage cancer tissue and was significantly related to the presence of distant metastasis[101]. Expression of MUC1/KL-6 (detected using the monoclonal antibody KL-6) at the deepest site of invasion by colorectal cancer was significantly associated with the presence of lymphatic or venous invasion, lymph node and distal metastasis, and an advanced Duke’s stage[102]. According to these studies, specific types of MUC1 expression could be used clinicopathologically to evaluate the behavior of colorectal cancer. Moreover, recent studies by the current authors and their colleagues have analyzed the clinicopathology of MUC1/KL-6 in colorectal cancer and liver metastases[103-105]. Localization of MUC1/KL-6 in the surrounding membrane and/or cytoplasm of colorectal cancer cells was significantly related to the presence of lymphatic vessel invasion, venous invasion, lymph node metastasis, and an advanced TNM stage as well as worse overall survival[103]. Although further studies should be conducted to determine its specificity, detection of subcellular localization of MUC1/KL-6 might help to evaluate cancer behavior and the prognosis for patients. In addition, analysis of colorectal metastasis to the liver indicated that the surrounding membrane and/or cytoplasm of cancer cells had immunoreactivity for MUC1/KL-6 while the surrounding non-cancerous liver tissue had no immunoreactivity for MUC1/KL-6[105]. This result suggested that MUC1/KL-6 is effective in sensitive detection of liver metastasis. Although further studies should be conducted with larger samples, MUC1/KL-6 may possibly be used to detect the foci of liver metastasis of colorectal cancer.
CONCLUSION
Many types of cancer-associated sialoglycoconjugates are produced during oncogenesis or in various stages of malignant transformation. Each of these sialoglycoconjugates has a characteristic role, such as cancer cell invasion or metastasis, that contributes to the progression of cancer. That said, these sialoglycoconjugates can also be used to evaluate cancer behavior, and clinicians have used several sialoglycoconjugates as markers to evaluate the effectiveness of treatments or detect the recurrence of cancer (Figure 2). Further verification of the utility of sialoglycoconjugates will encourage their continued use and highlight their clinical utility. Furthermore, use of sialoglycoconjugates in combination with unique imaging techniques such as molecular fluorescent imaging may lead to establishment of novel methods for diagnosing cancer with a high level of sensitivity. Use of cancer-associated sialoglycoconjugates is expected to expand in the future.
Figure 2.
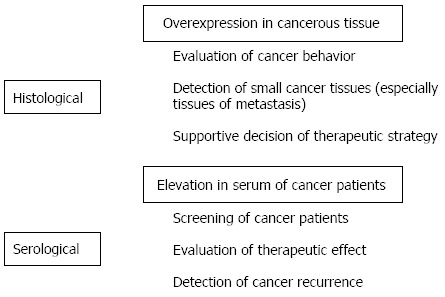
Clinical utility of sialoglycoconjugates as histological and serological markers in colorectal cancer.
Footnotes
P- Reviewers: Caboclo JLF, Tiberio GAM S- Editor: Gou SX L- Editor: Wang TQ E- Editor: Zhang DN
References
- 1.Varki A. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature. 2007;446:1023–1029. doi: 10.1038/nature05816. [DOI] [PubMed] [Google Scholar]
- 2.Fogel M, Altevogt P, Schirrmacher V. Metastatic potential severely altered by changes in tumor cell adhesiveness and cell-surface sialylation. J Exp Med. 1983;157:371–376. doi: 10.1084/jem.157.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hakomori S. Tumor malignancy defined by aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer Res. 1996;56:5309–5318. [PubMed] [Google Scholar]
- 4.Schultz MJ, Swindall AF, Bellis SL. Regulation of the metastatic cell phenotype by sialylated glycans. Cancer Metastasis Rev. 2012;31:501–518. doi: 10.1007/s10555-012-9359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang WC, Cummings RD. The immobilized leukoagglutinin from the seeds of Maackia amurensis binds with high affinity to complex-type Asn-linked oligosaccharides containing terminal sialic acid-linked alpha-2,3 to penultimate galactose residues. J Biol Chem. 1988;263:4576–4585. [PubMed] [Google Scholar]
- 6.Shibuya N, Goldstein IJ, Broekaert WF, Nsimba-Lubaki M, Peeters B, Peumans WJ. The elderberry (Sambucus nigra L.) bark lectin recognizes the Neu5Ac(alpha 2-6)Gal/GalNAc sequence. J Biol Chem. 1987;262:1596–1601. [PubMed] [Google Scholar]
- 7.Vierbuchen MJ, Fruechtnicht W, Brackrock S, Krause KT, Zienkiewicz TJ. Quantitative lectin-histochemical and immunohistochemical studies on the occurrence of alpha(2,3)- and alpha(2,6)-linked sialic acid residues in colorectal carcinomas. Relation to clinicopathologic features. Cancer. 1995;76:727–735. doi: 10.1002/1097-0142(19950901)76:5<727::aid-cncr2820760504>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 8.Fernández-Rodríguez J, Feijoo-Carnero C, Merino-Trigo A, Páez de la Cadena M, Rodríguez-Berrocal FJ, de Carlos A, Butrón M, Martínez-Zorzano VS. Immunohistochemical analysis of sialic acid and fucose composition in human colorectal adenocarcinoma. Tumour Biol. 2000;21:153–164. doi: 10.1159/000030122. [DOI] [PubMed] [Google Scholar]
- 9.Inagaki Y, Tang W, Guo Q, Kokudo N, Sugawara Y, Karako H, Konishi T, Nakata M, Nagawa H, Makuuchi M. Sialoglycoconjugate expression in primary colorectal cancer and metastatic lymph node tissues. Hepatogastroenterology. 2007;54:53–57. [PubMed] [Google Scholar]
- 10.Dall’Olio F, Chiricolo M, Ceccarelli C, Minni F, Marrano D, Santini D. Beta-galactoside alpha2,6 sialyltransferase in human colon cancer: contribution of multiple transcripts to regulation of enzyme activity and reactivity with Sambucus nigra agglutinin. Int J Cancer. 2000;88:58–65. doi: 10.1002/1097-0215(20001001)88:1<58::aid-ijc9>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 11.Chiricolo M, Malagolini N, Bonfiglioli S, Dall’Olio F. Phenotypic changes induced by expression of beta-galactoside alpha2,6 sialyltransferase I in the human colon cancer cell line SW948. Glycobiology. 2006;16:146–154. doi: 10.1093/glycob/cwj045. [DOI] [PubMed] [Google Scholar]
- 12.Vázquez-Martín C, Gil-Martín E, Fernández-Briera A. Elevation of ST6Gal I activity in malignant and transitional tissue in human colorectal cancer. Oncology. 2005;69:436–444. doi: 10.1159/000089999. [DOI] [PubMed] [Google Scholar]
- 13.Taniguchi A, Yoshikawa I, Matsumoto K. Genomic structure and transcriptional regulation of human Galbeta1,3GalNAc alpha2,3-sialyltransferase (hST3Gal I) gene. Glycobiology. 2001;11:241–247. doi: 10.1093/glycob/11.3.241. [DOI] [PubMed] [Google Scholar]
- 14.Seales EC, Jurado GA, Brunson BA, Wakefield JK, Frost AR, Bellis SL. Hypersialylation of beta1 integrins, observed in colon adenocarcinoma, may contribute to cancer progression by up-regulating cell motility. Cancer Res. 2005;65:4645–4652. doi: 10.1158/0008-5472.CAN-04-3117. [DOI] [PubMed] [Google Scholar]
- 15.Shaikh FM, Seales EC, Clem WC, Hennessy KM, Zhuo Y, Bellis SL. Tumor cell migration and invasion are regulated by expression of variant integrin glycoforms. Exp Cell Res. 2008;314:2941–2950. doi: 10.1016/j.yexcr.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee M, Park JJ, Ko YG, Lee YS. Cleavage of ST6Gal I by radiation-induced BACE1 inhibits golgi-anchored ST6Gal I-mediated sialylation of integrin β1 and migration in colon cancer cells. Radiat Oncol. 2012;7:47. doi: 10.1186/1748-717X-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swindall AF, Bellis SL. Sialylation of the Fas death receptor by ST6Gal-I provides protection against Fas-mediated apoptosis in colon carcinoma cells. J Biol Chem. 2011;286:22982–22990. doi: 10.1074/jbc.M110.211375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park JJ, Yi JY, Jin YB, Lee YJ, Lee JS, Lee YS, Ko YG, Lee M. Sialylation of epidermal growth factor receptor regulates receptor activity and chemosensitivity to gefitinib in colon cancer cells. Biochem Pharmacol. 2012;83:849–857. doi: 10.1016/j.bcp.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Tsuchida A, Okajima T, Furukawa K, Ando T, Ishida H, Yoshida A, Nakamura Y, Kannagi R, Kiso M, Furukawa K. Synthesis of disialyl Lewis a (Le(a)) structure in colon cancer cell lines by a sialyltransferase, ST6GalNAc VI, responsible for the synthesis of alpha-series gangliosides. J Biol Chem. 2003;278:22787–22794. doi: 10.1074/jbc.M211034200. [DOI] [PubMed] [Google Scholar]
- 20.Miyazaki K, Ohmori K, Izawa M, Koike T, Kumamoto K, Furukawa K, Ando T, Kiso M, Yamaji T, Hashimoto Y, et al. Loss of disialyl Lewis(a), the ligand for lymphocyte inhibitory receptor sialic acid-binding immunoglobulin-like lectin-7 (Siglec-7) associated with increased sialyl Lewis(a) expression on human colon cancers. Cancer Res. 2004;64:4498–4505. doi: 10.1158/0008-5472.CAN-03-3614. [DOI] [PubMed] [Google Scholar]
- 21.Majuri ML, Niemelä R, Tiisala S, Renkonen O, Renkonen R. Expression and function of alpha 2,3-sialyl- and alpha 1,3/1,4-fucosyltransferases in colon adenocarcinoma cell lines: role in synthesis of E-selectin counter-receptors. Int J Cancer. 1995;63:551–559. doi: 10.1002/ijc.2910630416. [DOI] [PubMed] [Google Scholar]
- 22.Malagolini N, Santini D, Chiricolo M, Dall’Olio F. Biosynthesis and expression of the Sda and sialyl Lewis x antigens in normal and cancer colon. Glycobiology. 2007;17:688–697. doi: 10.1093/glycob/cwm040. [DOI] [PubMed] [Google Scholar]
- 23.Hanski C, Klussmann E, Wang J, Böhm C, Ogorek D, Hanski ML, Krüger-Krasagakes S, Eberle J, Schmitt-Gräff A, Riecken EO. Fucosyltransferase III and sialyl-Le(x) expression correlate in cultured colon carcinoma cells but not in colon carcinoma tissue. Glycoconj J. 1996;13:727–733. doi: 10.1007/BF00702336. [DOI] [PubMed] [Google Scholar]
- 24.Ito H, Hiraiwa N, Sawada-Kasugai M, Akamatsu S, Tachikawa T, Kasai Y, Akiyama S, Ito K, Takagi H, Kannagi R. Altered mRNA expression of specific molecular species of fucosyl- and sialyl-transferases in human colorectal cancer tissues. Int J Cancer. 1997;71:556–564. doi: 10.1002/(sici)1097-0215(19970516)71:4<556::aid-ijc9>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 25.Marcos NT, Bennett EP, Gomes J, Magalhaes A, Gomes C, David L, Dar I, Jeanneau C, DeFrees S, Krustrup D, et al. ST6GalNAc-I controls expression of sialyl-Tn antigen in gastrointestinal tissues. Front Biosci (Elite Ed) 2011;3:1443–1455. doi: 10.2741/e345. [DOI] [PubMed] [Google Scholar]
- 26.Marcos NT, Pinho S, Grandela C, Cruz A, Samyn-Petit B, Harduin-Lepers A, Almeida R, Silva F, Morais V, Costa J, et al. Role of the human ST6GalNAc-I and ST6GalNAc-II in the synthesis of the cancer-associated sialyl-Tn antigen. Cancer Res. 2004;64:7050–7057. doi: 10.1158/0008-5472.CAN-04-1921. [DOI] [PubMed] [Google Scholar]
- 27.Vázquez-Martín C, Cuevas E, Gil-Martín E, Fernández-Briera A. Correlation analysis between tumor-associated antigen sialyl-Tn expression and ST6GalNAc I activity in human colon adenocarcinoma. Oncology. 2004;67:159–165. doi: 10.1159/000081003. [DOI] [PubMed] [Google Scholar]
- 28.Kono M, Tsuda T, Ogata S, Takashima S, Liu H, Hamamoto T, Itzkowitz SH, Nishimura S, Tsuji S. Redefined substrate specificity of ST6GalNAc II: a second candidate sialyl-Tn synthase. Biochem Biophys Res Commun. 2000;272:94–97. doi: 10.1006/bbrc.2000.2745. [DOI] [PubMed] [Google Scholar]
- 29.Kannagi R. Regulatory roles of carbohydrate ligands for selectins in the homing of lymphocytes. Curr Opin Struct Biol. 2002;12:599–608. doi: 10.1016/s0959-440x(02)00365-2. [DOI] [PubMed] [Google Scholar]
- 30.Takada A, Ohmori K, Yoneda T, Tsuyuoka K, Hasegawa A, Kiso M, Kannagi R. Contribution of carbohydrate antigens sialyl Lewis A and sialyl Lewis X to adhesion of human cancer cells to vascular endothelium. Cancer Res. 1993;53:354–361. [PubMed] [Google Scholar]
- 31.Izumi Y, Taniuchi Y, Tsuji T, Smith CW, Nakamori S, Fidler IJ, Irimura T. Characterization of human colon carcinoma variant cells selected for sialyl Lex carbohydrate antigen: liver colonization and adhesion to vascular endothelial cells. Exp Cell Res. 1995;216:215–221. doi: 10.1006/excr.1995.1027. [DOI] [PubMed] [Google Scholar]
- 32.Singh R, Campbell BJ, Yu LG, Fernig DG, Milton JD, Goodlad RA, FitzGerald AJ, Rhodes JM. Cell surface-expressed Thomsen-Friedenreich antigen in colon cancer is predominantly carried on high molecular weight splice variants of CD44. Glycobiology. 2001;11:587–592. doi: 10.1093/glycob/11.7.587. [DOI] [PubMed] [Google Scholar]
- 33.Bresalier RS, Ho SB, Schoeppner HL, Kim YS, Sleisenger MH, Brodt P, Byrd JC. Enhanced sialylation of mucin-associated carbohydrate structures in human colon cancer metastasis. Gastroenterology. 1996;110:1354–1367. doi: 10.1053/gast.1996.v110.pm8613039. [DOI] [PubMed] [Google Scholar]
- 34.Gold P, Freedman SO. Demonstration of tumor-specific antigens in human colonic carcinomata by immunological tolerance and absorption techniques. J Exp Med. 1965;121:439–462. doi: 10.1084/jem.121.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gold P, Freedman SO. Specific carcinoembryonic antigens of the human digestive system. J Exp Med. 1965;122:467–481. doi: 10.1084/jem.122.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson J, Zimmermann W. The carcinoembryonic antigen gene family: structure, expression and evolution. Tumour Biol. 1988;9:63–83. doi: 10.1159/000217547. [DOI] [PubMed] [Google Scholar]
- 37.Sanders DS, Stocks SC, Milne DM, Milne GA, Hopwood D, Kerr MA. Membranous expression of carcinoembryonic antigen (CEA) in the normal cervical squamous mucosa. J Pathol. 1992;167:77–82. doi: 10.1002/path.1711670113. [DOI] [PubMed] [Google Scholar]
- 38.Ilantzis C, Jothy S, Alpert LC, Draber P, Stanners CP. Cell-surface levels of human carcinoembryonic antigen are inversely correlated with colonocyte differentiation in colon carcinogenesis. Lab Invest. 1997;76:703–716. [PubMed] [Google Scholar]
- 39.Johnson JP. Cell adhesion molecules of the immunoglobulin supergene family and their role in malignant transformation and progression to metastatic disease. Cancer Metastasis Rev. 1991;10:11–22. doi: 10.1007/BF00046840. [DOI] [PubMed] [Google Scholar]
- 40.Hashino J, Fukuda Y, Oikawa S, Nakazato H, Nakanishi T. Metastatic potential of human colorectal carcinoma SW1222 cells transfected with cDNA encoding carcinoembryonic antigen. Clin Exp Metastasis. 1994;12:324–328. doi: 10.1007/BF01753839. [DOI] [PubMed] [Google Scholar]
- 41.Byrn RA, Medrek P, Thomas P, Jeanloz RW, Zamcheck N. Effect of heterogeneity of carcinoembryonic antigen on liver cell membrane binding and its kinetics of removal from circulation. Cancer Res. 1985;45:3137–3142. [PubMed] [Google Scholar]
- 42.Yamashita S, Tanaka N, Takahashi M, Hata S, Nomura Y, Ooe K, Suzuki Y. Clinicopathological subclassification of biliary cystic tumors: Report of 4 cases with a review of the literature. Intractable Rare Dis Res. 2013;2:63–68. doi: 10.5582/irdr.2013.v2.2.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Seuningen I, Pigny P, Perrais M, Porchet N, Aubert JP. Transcriptional regulation of the 11p15 mucin genes. Towards new biological tools in human therapy, in inflammatory diseases and cancer? Front Biosci. 2001;6:D1216–D1234. doi: 10.2741/seuning. [DOI] [PubMed] [Google Scholar]
- 44.Hanski C, Riede E, Gratchev A, Foss HD, Böhm C, Klussmann E, Hummel M, Mann B, Buhr HJ, Stein H, et al. MUC2 gene suppression in human colorectal carcinomas and their metastases: in vitro evidence of the modulatory role of DNA methylation. Lab Invest. 1997;77:685–695. [PubMed] [Google Scholar]
- 45.Walsh MD, Clendenning M, Williamson E, Pearson SA, Walters RJ, Nagler B, Packenas D, Win AK, Hopper JL, Jenkins MA, et al. Expression of MUC2, MUC5AC, MUC5B, and MUC6 mucins in colorectal cancers and their association with the CpG island methylator phenotype. Mod Pathol. 2013;26:1642–1656. doi: 10.1038/modpathol.2013.101. [DOI] [PubMed] [Google Scholar]
- 46.Imai Y, Yamagishi H, Fukuda K, Ono Y, Inoue T, Ueda Y. Differential mucin phenotypes and their significance in a variation of colorectal carcinoma. World J Gastroenterol. 2013;19:3957–3968. doi: 10.3748/wjg.v19.i25.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, Kucherlapati R, Lipkin M, Yang K, Augenlicht L. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295:1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- 48.Bartman AE, Sanderson SJ, Ewing SL, Niehans GA, Wiehr CL, Evans MK, Ho SB. Aberrant expression of MUC5AC and MUC6 gastric mucin genes in colorectal polyps. Int J Cancer. 1999;80:210–218. doi: 10.1002/(sici)1097-0215(19990118)80:2<210::aid-ijc9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 49.Nollet S, Forgue-Lafitte ME, Kirkham P, Bara J. Mapping of two new epitopes on the apomucin encoded by MUC5AC gene: expression in normal GI tract and colon tumors. Int J Cancer. 2002;99:336–343. doi: 10.1002/ijc.10335. [DOI] [PubMed] [Google Scholar]
- 50.Gendler SJ, Lancaster CA, Taylor-Papadimitriou J, Duhig T, Peat N, Burchell J, Pemberton L, Lalani EN, Wilson D. Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. J Biol Chem. 1990;265:15286–15293. [PubMed] [Google Scholar]
- 51.Taylor-Papadimitriou J, Peterson JA, Arklie J, Burchell J, Ceriani RL, Bodmer WF. Monoclonal antibodies to epithelium-specific components of the human milk fat globule membrane: production and reaction with cells in culture. Int J Cancer. 1981;28:17–21. doi: 10.1002/ijc.2910280104. [DOI] [PubMed] [Google Scholar]
- 52.Hull SR, Bright A, Carraway KL, Abe M, Hayes DF, Kufe DW. Oligosaccharide differences in the DF3 sialomucin antigen from normal human milk and the BT-20 human breast carcinoma cell line. Cancer Commun. 1989;1:261–267. [PubMed] [Google Scholar]
- 53.Burchell J, Taylor-Papadimitriou J, Boshell M, Gendler S, Duhig T. A short sequence, within the amino acid tandem repeat of a cancer-associated mucin, contains immunodominant epitopes. Int J Cancer. 1989;44:691–696. doi: 10.1002/ijc.2910440423. [DOI] [PubMed] [Google Scholar]
- 54.Dohi DF, Sutton RC, Frazier ML, Nakamori S, McIsaac AM, Irimura T. Regulation of sialomucin production in colon carcinoma cells. J Biol Chem. 1993;268:10133–10138. [PubMed] [Google Scholar]
- 55.Yamamoto M, Bhavanandan VP, Nakamori S, Irimura T. A novel monoclonal antibody specific for sialylated MUC1 mucin. Jpn J Cancer Res. 1996;87:488–496. doi: 10.1111/j.1349-7006.1996.tb00250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsumoto-Takasaki A, Horie J, Sakai K, Furui Y, Sato R, Kawakami H, Toma K, Takayanagi A, Shimizu N, Fujita-Yamaguchi Y. Isolation and characterization of anti-T-antigen single chain antibodies from a phage library. Biosci Trends. 2009;3:87–95. [PubMed] [Google Scholar]
- 57.Takeuchi H, Kato K, Denda-Nagai K, Hanisch FG, Clausen H, Irimura T. The epitope recognized by the unique anti-MUC1 monoclonal antibody MY.1E12 involves sialyl alpha 2-3galactosyl beta 1-3N-acetylgalactosaminide linked to a distinct threonine residue in the MUC1 tandem repeat. J Immunol Methods. 2002;270:199–209. doi: 10.1016/s0022-1759(02)00298-3. [DOI] [PubMed] [Google Scholar]
- 58.Kohno N, Akiyama M, Kyoizumi S, Hakoda M, Kobuke K, Yamakido M. Detection of soluble tumor-associated antigens in sera and effusions using novel monoclonal antibodies, KL-3 and KL-6, against lung adenocarcinoma. Jpn J Clin Oncol. 1988;18:203–216. [PubMed] [Google Scholar]
- 59.Kohno N. Serum marker KL-6/MUC1 for the diagnosis and management of interstitial pneumonitis. J Med Invest. 1999;46:151–158. [PubMed] [Google Scholar]
- 60.Ohyabu N, Hinou H, Matsushita T, Izumi R, Shimizu H, Kawamoto K, Numata Y, Togame H, Takemoto H, Kondo H, et al. An essential epitope of anti-MUC1 monoclonal antibody KL-6 revealed by focused glycopeptide library. J Am Chem Soc. 2009;131:17102–17109. doi: 10.1021/ja903361f. [DOI] [PubMed] [Google Scholar]
- 61.Seko A, Ohkura T, Ideo H, Yamashita K. Novel O-linked glycans containing 6’-sulfo-Gal/GalNAc of MUC1 secreted from human breast cancer YMB-S cells: possible carbohydrate epitopes of KL-6(MUC1) monoclonal antibody. Glycobiology. 2012;22:181–195. doi: 10.1093/glycob/cwr118. [DOI] [PubMed] [Google Scholar]
- 62.Guo Q, Tang W, Inagaki Y, Kokudo N, Sugawara Y, Karako H, Nakata M, Makuuchi M. Subcellular localization of KL-6 mucin in colorectal carcinoma cell lines: association with metastatic potential and cell morphology. Oncol Rep. 2007;17:1057–1060. [PubMed] [Google Scholar]
- 63.Gao J, Feng X, Inagaki Y, Song P, Kokudo N, Hasegawa K, Sugawara Y, Tang W. Des-γ-carboxy prothrombin and c-Met were concurrently and extensively expressed in hepatocellular carcinoma and associated with tumor recurrence. Biosci Trends. 2012;6:153–159. doi: 10.5582/bst.2012.v6.4.153. [DOI] [PubMed] [Google Scholar]
- 64.Song P, Feng X, Zhang K, Song T, Ma K, Kokudo N, Dong J, Yao L, Tang W. Screening for and surveillance of high-risk patients with HBV-related chronic liver disease: promoting the early detection of hepatocellular carcinoma in China. Biosci Trends. 2013;7:1–6. [PubMed] [Google Scholar]
- 65.Song PP, Gao JJ, Kokudo N, Dong JH, Tang W. “Knowledge into action” Exploration of an appropriate approach for constructing evidence-based clinical practice guidelines for hepatocellular carcinoma. Biosci Trends. 2012;6:147–152. doi: 10.5582/bst.2012.v6.3.147. [DOI] [PubMed] [Google Scholar]
- 66.Gao JJ, Inagaki Y, Xue X, Qu XJ, Tang W. c-Met: A potential therapeutic target for hepatocellular carcinoma. Drug Discov Ther. 2011;5:2–11. doi: 10.5582/ddt.2011.v5.1.2. [DOI] [PubMed] [Google Scholar]
- 67.Mei XD, Su H, Song J, Dong L. Prognostic significance of β-catenin expression in patients with non-small cell lung cancer: a meta-analysis. Biosci Trends. 2013;7:42–49. [PubMed] [Google Scholar]
- 68.Inagaki Y, Qi F, Gao J, Qu X, Hasegawa K, Sugawara Y, Tang W, Kokudo N. Effect of c-Met inhibitor SU11274 on hepatocellular carcinoma cell growth. Biosci Trends. 2011;5:52–56. doi: 10.5582/bst.2011.v5.2.52. [DOI] [PubMed] [Google Scholar]
- 69.Zamri N, Masuda N, Oura F, Yajima Y, Nakada H, Fujita-Yamaguchi Y. Effects of two monoclonal antibodies, MLS128 against Tn-antigen and 1H7 against insulin-like growth factor-I receptor, on the growth of colon cancer cells. Biosci Trends. 2012;6:303–312. [PubMed] [Google Scholar]
- 70.Chen W, Qiu J, Shen YM. Topoisomerase IIα, rather than IIβ, is a promising target in development of anti-cancer drugs. Drug Discov Ther. 2012;6:230–237. [PubMed] [Google Scholar]
- 71.Jaiswal PK, Goel A, Mittal RD. Association of p53 codon 248 (exon7) with urinary bladder cancer risk in the North Indian population. Biosci Trends. 2011;5:205–210. doi: 10.5582/bst.2011.v5.5.205. [DOI] [PubMed] [Google Scholar]
- 72.Feijoo C, Páez de la Cadena M, Rodríguez-Berrocal FJ, Martínez-Zorzano VS. Sialic acid levels in serum and tissue from colorectal cancer patients. Cancer Lett. 1997;112:155–160. doi: 10.1016/s0304-3835(96)04564-8. [DOI] [PubMed] [Google Scholar]
- 73.Painbeni T, Gamelin E, Cailleux A, Le Bouil A, Boisdron-Celle M, Daver A, Larra F, Allain P. Plasma sialic acid as a marker of the effect of the treatment on metastatic colorectal cancer. Eur J Cancer. 1997;33:2216–2220. doi: 10.1016/s0959-8049(97)00318-3. [DOI] [PubMed] [Google Scholar]
- 74.Nakamori S, Kameyama M, Imaoka S, Furukawa H, Ishikawa O, Sasaki Y, Kabuto T, Iwanaga T, Matsushita Y, Irimura T. Increased expression of sialyl Lewisx antigen correlates with poor survival in patients with colorectal carcinoma: clinicopathological and immunohistochemical study. Cancer Res. 1993;53:3632–3637. [PubMed] [Google Scholar]
- 75.Nakayama T, Watanabe M, Katsumata T, Teramoto T, Kitajima M. Expression of sialyl Lewis(a) as a new prognostic factor for patients with advanced colorectal carcinoma. Cancer. 1995;75:2051–2056. doi: 10.1002/1097-0142(19950415)75:8<2051::aid-cncr2820750804>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 76.Grabowski P, Mann B, Mansmann U, Lövin N, Foss HD, Berger G, Scherübl H, Riecken EO, Buhr HJ, Hanski C. Expression of SIALYL-Le(x) antigen defined by MAb AM-3 is an independent prognostic marker in colorectal carcinoma patients. Int J Cancer. 2000;88:281–286. [PubMed] [Google Scholar]
- 77.Portela SV, Martín CV, Romay LM, Cuevas E, Martín EG, Briera AF. sLea and sLex expression in colorectal cancer: implications for tumourigenesis and disease prognosis. Histol Histopathol. 2011;26:1305–1316. doi: 10.14670/HH-26.1305. [DOI] [PubMed] [Google Scholar]
- 78.Itzkowitz SH, Bloom EJ, Kokal WA, Modin G, Hakomori S, Kim YS. Sialosyl-Tn. A novel mucin antigen associated with prognosis in colorectal cancer patients. Cancer. 1990;66:1960–1966. doi: 10.1002/1097-0142(19901101)66:9<1960::aid-cncr2820660919>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 79.Lundin M, Nordling S, Roberts PJ, Lundin J, Carpelan-Holmström M, von Boguslawsky K, Haglund C. Sialyl Tn is a frequently expressed antigen in colorectal cancer: No correlation with patient prognosis. Oncology. 1999;57:70–76. doi: 10.1159/000012003. [DOI] [PubMed] [Google Scholar]
- 80.Nakagoe T, Fukushima K, Tanaka K, Sawai T, Tsuji T, Jibiki M, Nanashima A, Yamaguchi H, Yasutake T, Ayabe H, et al. Evaluation of sialyl Lewis(a), sialyl Lewis(x), and sialyl Tn antigens expression levels as predictors of recurrence after curative surgery in node-negative colorectal cancer patients. J Exp Clin Cancer Res. 2002;21:107–113. [PubMed] [Google Scholar]
- 81.Nakagoe T, Fukushima K, Hirota M, Kusano H, Ayabe H, Tomita M, Kamihira S. Immunohistochemical expression of sialyl Lex antigen in relation to survival of patients with colorectal carcinoma. Cancer. 1993;72:2323–2330. doi: 10.1002/1097-0142(19931015)72:8<2323::aid-cncr2820720807>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 82.Nakagoe T, Sawai T, Tsuji T, Jibiki M, Nanashima A, Yamaguchi H, Kurosaki N, Yasutake T, Ayabe H. Circulating sialyl Lewis(x), sialyl Lewis(a), and sialyl Tn antigens in colorectal cancer patients: multivariate analysis of predictive factors for serum antigen levels. J Gastroenterol. 2001;36:166–172. doi: 10.1007/s005350170124. [DOI] [PubMed] [Google Scholar]
- 83.Holubec L, Topolcan O, Pikner R, Pecen L, Vaclavickova J, Wirthova M, Molacek J, Stieber P, Holdenrieder S, Sen LH, et al. The significance of CEA, CA19-9 and CA72-4 in the detection of colorectal carcinoma recurrence. Anticancer Res. 2000;20:5237–5244. [PubMed] [Google Scholar]
- 84.Nakagoe T, Sawai T, Tsuji T, Jibiki MA, Nanashima A, Yamaguchi H, Yasutake T, Ayabe H, Arisawa K. Preoperative serum level of CA19-9 predicts recurrence after curative surgery in node-negative colorectal cancer patients. Hepatogastroenterology. 2003;50:696–699. [PubMed] [Google Scholar]
- 85.Gasser M, Gerstlauer C, Grimm M, Bueter M, Lebedeva T, Lutz J, Maeder U, Ribas C, Ribas C, Nichiporuk E, et al. Comparative analysis of predictive biomarkers for therapeutical strategies in colorectal cancer. Ann Surg Oncol. 2007;14:1272–1284. doi: 10.1245/s10434-006-9155-0. [DOI] [PubMed] [Google Scholar]
- 86.Nakagoe T, Fukushima K, Hirota M, Kusano H, Ayabe H, Tomita M, Kamihira S. An immunohistochemical employer monoclonal antibodies against Le(a), sialyl Le(a), Le(x), and sialyl Le(x) antigens in primary colorectal, carcinomas and lymph node and hepatic lesions. J Gastroenterol. 1994;29:129–138. doi: 10.1007/BF02358673. [DOI] [PubMed] [Google Scholar]
- 87.Nakayama T, Watanabe M, Teramoto T, Kitajima M. Prognostic values of serum CA19-9 and CEA levels for colorectal cancer. Oncol Rep. 1997;4:819–822. doi: 10.3892/or.4.4.819. [DOI] [PubMed] [Google Scholar]
- 88.Forones NM, Tanaka M. CEA and CA 19-9 as prognostic indexes in colorectal cancer. Hepatogastroenterology. 1999;46:905–908. [PubMed] [Google Scholar]
- 89.Hanke B, Riedel C, Lampert S, Happich K, Martus P, Parsch H, Himmler B, Hohenberger W, Hahn EG, Wein A. CEA and CA 19-9 measurement as a monitoring parameter in metastatic colorectal cancer (CRC) under palliative first-line chemotherapy with weekly 24-hour infusion of high-dose 5-fluorouracil (5-FU) and folinic acid (FA) Ann Oncol. 2001;12:221–226. doi: 10.1023/a:1008378412533. [DOI] [PubMed] [Google Scholar]
- 90.de Haas RJ, Wicherts DA, Flores E, Ducreux M, Lévi F, Paule B, Azoulay D, Castaing D, Lemoine A, Adam R. Tumor marker evolution: comparison with imaging for assessment of response to chemotherapy in patients with colorectal liver metastases. Ann Surg Oncol. 2010;17:1010–1023. doi: 10.1245/s10434-009-0887-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Petrioli R, Licchetta A, Roviello G, Pascucci A, Francini E, Bargagli G, Conca R, Miano ST, Marzocca G, Francini G. CEA and CA19.9 as early predictors of progression in advanced/metastatic colorectal cancer patients receiving oxaliplatin-based chemotherapy and bevacizumab. Cancer Invest. 2012;30:65–71. doi: 10.3109/07357907.2011.629380. [DOI] [PubMed] [Google Scholar]
- 92.Kawamura YJ, Tokumitsu A, Mizokami K, Sasaki J, Tsujinaka S, Konishi F. First alert for recurrence during follow-up after potentially curative resection for colorectal carcinoma: CA 19-9 should be included in surveillance programs. Clin Colorectal Cancer. 2010;9:48–51. doi: 10.3816/CCC.2010.n.006. [DOI] [PubMed] [Google Scholar]
- 93.Elzagheid A, Emaetig F, Buhmeida A, Laato M, El-Faitori O, Syrjänen K, Collan Y, Pyrhönen S. Loss of MUC2 expression predicts disease recurrence and poor outcome in colorectal carcinoma. Tumour Biol. 2013;34:621–628. doi: 10.1007/s13277-012-0588-8. [DOI] [PubMed] [Google Scholar]
- 94.Kang H, Min BS, Lee KY, Kim NK, Kim SN, Choi J, Kim H. Loss of E-cadherin and MUC2 expressions correlated with poor survival in patients with stages II and III colorectal carcinoma. Ann Surg Oncol. 2011;18:711–719. doi: 10.1245/s10434-010-1338-z. [DOI] [PubMed] [Google Scholar]
- 95.Shanmugam C, Jhala NC, Katkoori VR, Wan W, Meleth S, Grizzle WE, Manne U. Prognostic value of mucin 4 expression in colorectal adenocarcinomas. Cancer. 2010;116:3577–3586. doi: 10.1002/cncr.25095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nakamori S, Ota DM, Cleary KR, Shirotani K, Irimura T. MUC1 mucin expression as a marker of progression and metastasis of human colorectal carcinoma. Gastroenterology. 1994;106:353–361. doi: 10.1016/0016-5085(94)90592-4. [DOI] [PubMed] [Google Scholar]
- 97.Baldus SE, Hanisch FG, Kotlarek GM, Zirbes TK, Thiele J, Isenberg J, Karsten UR, Devine PL, Dienes HP. Coexpression of MUC1 mucin peptide core and the Thomsen-Friedenreich antigen in colorectal neoplasms. Cancer. 1998;82:1019–1027. doi: 10.1002/(sici)1097-0142(19980315)82:6<1019::aid-cncr3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 98.Inagaki Y, Xu H, Nakata M, Seyama Y, Hasegawa K, Sugawara Y, Tang W, Kokudo N. Clinicopathology of sialomucin: MUC1, particularly KL-6 mucin, in gastrointestinal, hepatic and pancreatic cancers. Biosci Trends. 2009;3:220–232. [PubMed] [Google Scholar]
- 99.Baldus SE, Mönig SP, Hanisch FG, Zirbes TK, Flucke U, Oelert S, Zilkens G, Madejczik B, Thiele J, Schneider PM, et al. Comparative evaluation of the prognostic value of MUC1, MUC2, sialyl-Lewis(a) and sialyl-Lewis(x) antigens in colorectal adenocarcinoma. Histopathology. 2002;40:440–449. doi: 10.1046/j.1365-2559.2002.01389.x. [DOI] [PubMed] [Google Scholar]
- 100.Manne U, Weiss HL, Grizzle WE. Racial differences in the prognostic usefulness of MUC1 and MUC2 in colorectal adenocarcinomas. Clin Cancer Res. 2000;6:4017–4025. [PubMed] [Google Scholar]
- 101.Suzuki H, Shoda J, Kawamoto T, Shinozaki E, Miyahara N, Hotta S, Iizuka Y, Nakahara A, Tanaka N, Yanaka A, et al. Expression of MUC1 recognized by monoclonal antibody MY.1E12 is a useful biomarker for tumor aggressiveness of advanced colon carcinoma. Clin Exp Metastasis. 2004;21:321–329. doi: 10.1023/b:clin.0000046133.35133.cc. [DOI] [PubMed] [Google Scholar]
- 102.Hiraga Y, Tanaka S, Haruma K, Yoshihara M, Sumii K, Kajiyama G, Shimamoto F, Kohno N. Immunoreactive MUC1 expression at the deepest invasive portion correlates with prognosis of colorectal cancer. Oncology. 1998;55:307–319. doi: 10.1159/000011868. [DOI] [PubMed] [Google Scholar]
- 103.Guo Q, Tang W, Inagaki Y, Midorikawa Y, Kokudo N, Sugawara Y, Nakata M, Konishi T, Nagawa H, Makuuchi M. Clinical significance of subcellular localization of KL-6 mucin in primary colorectal adenocarcinoma and metastatic tissues. World J Gastroenterol. 2006;12:54–59. doi: 10.3748/wjg.v12.i1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang W, Tang W, Inagaki Y, Qiu M, Xu HL, Li X, Sugawara Y, Nagawa H, Nakata M, Kokudo N. Positive KL-6 mucin expression combined with decreased membranous beta-catenin expression indicates worse prognosis in colorectal carcinoma. Oncol Rep. 2008;20:1013–1019. [PubMed] [Google Scholar]
- 105.Zhang K, Tang W, Qu X, Guo Q, Inagaki Y, Seyama Y, Abe H, Gai R, Kokudo N, Sugawara Y, et al. KL-6 mucin in metastatic liver cancer tissues from primary colorectal carcinoma. Hepatogastroenterology. 2009;56:960–963. [PubMed] [Google Scholar]


