Abstract
Colorectal cancer (CRC) is one of the most common cancers worldwide, with 5%-15% of CRC patients eventually developing lung metastasis (LM). Despite doubts about the role of locoregional therapy in the management of systemic disease, many surgeons have performed pulmonary metastasectomy (PM) for CRC in properly selected patients. However, the use of pulmonary metastasectomy remains controversial due to the lack of randomized controlled studies. This article reviews the results of surgical treatment of pulmonary metastases for CRC, focusing on (1) current treatment guidelines and surgical techniques of PM in patients with LM from CRC; (2) outcomes of PM and its prognostic factors; and (3) controversial issues in PM, focusing on repeated metastasectomy, bilateral multiple metastases, and combined liver and lung metastasectomy.
Keywords: Colorectal cancer, Pulmonary metastasectomy, Surgery, Metastases
Core tip: Pulmonary metastasectomy is now the accepted treatment of choice in the multimodal management of metastatic colorectal cancer. There is no absolute contraindication for pulmonary metastasectomy as long as complete resection can be achieved. However, there are still many questions regarding the proper indication of pulmonary metastasectomy, for example: How many times can pulmonary metastasectomy be performed for recurrent pulmonary metastases? How many nodules can be resected safely and effectively in patients with recurrent and multiple metastatic nodules? This article reviews the different therapeutic strategies for patients with pulmonary metastases of colorectal cancer, with a particular focus on these questions.
INTRODUCTION
Colorectal cancer (CRC) is one of the most common cancers worldwide[1]. Although mortality from CRC has decreased by almost 35% from 1990 to 2007, possibly due to earlier diagnoses through screening and improved treatment modalities, CRC remains the second leading cause of cancer death in the United States[2,3]. Recurrence is the primary reason for treatment failure, and approximately 50% of patients undergoing resection of CRC develop metastases[4-6]. The most common sites of metastasis from CRC are the liver and lungs, with 5%-15% of CRC patients eventually developing lung metastasis (LM)[4-8].
As in other conditions with a diagnosis of stage IV disease, LM in CRC indicates widespread hematogenous dissemination of cancer. If untreated, patients with LM have a median survival of less than 10 mo and a 5-year survival rate of less than 5%[9,10]. When used for hepatic metastases of CRC, chemotherapy in combination with surgery may prolong survival time or downsize the lesions to render them resectable in patients who were previously considered inoperable[11]. However, there is still controversy over the optimal management strategy. Despite doubts about the effect of locoregional therapy in the management of systemic disease, many surgeons have performed surgical resection of LM in properly selected patients[12-17]. Numerous studies have suggested that resecting LM offers a better chance of survival compared with the expected 5-year survival rate of less than 10% for stage IV CRC managed with palliative chemotherapy[18,19]. Thus, pulmonary metastasectomy (PM) has become widely accepted as a potentially curative treatment option for LM from CRC[20].
However, the role of surgery in patients with LM remains controversial due to the lack of prospective randomized controlled trials comparing PM with a control of either medical therapy or observation[21]. Retrospective and non-comparative studies include only candidates selected as suitable for PM and exclude those who cannot be offered PM. The encouraging outcomes of PM to date might therefore be attributed to the fact that selected candidates already have beneficial prognostic factors, typically resectability, rather than the benefit of surgical resection per se[22], and it is therefore unknown whether the reported good results are due to surgery or selection[23]. Nonetheless, the fact that there is insufficient evidence supporting PM in the total patient population with stage IV CRC does not necessarily mean that the opportunity of surgery should be denied for selected patients who could benefit from PM. Moreover, since chemotherapy alone is not quite reliable in controlling LM in many cases, the best way to improve treatment outcomes is to carry out PM more aggressively in patients who are most likely to benefit from PM.
No one would argue about offering PM when a solitary, slowly growing, and isolated LM is detected after a long disease-free interval. Conversely, it would be unreasonable to recommend PM when numerous metastatic lesions are extensively widespread in both lungs and extrathoracic organs within several months after surgical resection of CRC. Between these extremes however, there are always borderline cases with a combination of favorable and unfavorable features in which the indication of PM is uncertain, such as recurrent LM after first PM, bilateral multiple metastases, or synchronous liver and lung metastases. This review discusses the accumulated knowledge regarding: (1) current treatment guidelines and surgical technique of PM in patients with LM from CRC; (2) outcomes of PM and its prognostic factors; and (3) controversial issues regarding PM, focusing on repeated metastasectomy, bilateral multiple metastases, and combined liver and lung metastasectomy.
TREATMENT GUIDELINE
Since Blalock first described pulmonary resection for metastases from CRC[24], PM has become the mainstay of treatment for LM in a highly selected subset of patients. In 1965, Thomford et al[25] suggested the criteria for resection of LM as follows: (1) patient is low risk for surgical intervention; (2) primary site is controlled; (3) no other extrapulmonary metastases exist, or if present, it can be controlled by surgery or another treatment modality; and (4) pulmonary metastases are thought be completely resectable. These criteria have been generally accepted by most surgeons and must be met when considering PM[26].
However, these guidelines have inherent limitations due to not being based on the results of randomized controlled trials. In addition to the rarity of LM patients, ethical dilemmas about withholding surgery in an ideal candidate make it difficult to conduct randomized trials. Instead, the rationale for PM is supported by other lines of evidence, such as multi-institutional prospective data registries and systematic review of non-randomized or non-comparative studies. The International Registry of Lung Metastases was established in 1991 by the European Society of Thoracic Surgeons (ESTS)[12]. Said registry is a large prospective study encompassing a total of 5206 patients who underwent surgery at 18 sites in Europe, the United States, and Canada[12]. With a 5-year survival of 36% in patients undergoing complete resection, this study made a substantial contribution to the consideration of PM as an effective therapy[12]. Several authors have reported the results of systematic reviews in order to overcome the limitations of previously published retrospective series based on small numbers of patients[14-17]. Pfannschmidt et al[14] conducted a systematic review of 1870 patients from 20 published case series, summarizing the available evidence for the effectiveness of PM with an emphasis on prognostic factors that influence survival. Recently, Gonzalez et al[17] performed a more extensive meta-analysis of series published between 2000 and 2011 that included 2925 patients from 25 studies.
Based on these efforts to seek a higher level of evidence from previously reported studies, new indications for PM in metastatic CRC patients have been established by the National Comprehensive Cancer Network (NCCN) as follows[3]: (1) complete resection based on the anatomic location and extent of disease with maintenance of adequate function is required; (2) the primary tumor must have been resected for cure; (3) resectable extrapulmonary metastases do not preclude resection; (4) re-resection can be considered in selected patients; and (5) patients with resectable synchronous metastases can be resected synchronously or using a staged approach.
CURRENT PRACTICE
Preoperative imaging tests
According to the NCCN, the Association of Coloproctology of Great Britain and Ireland, and the Danish Colorectal Cancer Group[3,27,28], the initial staging procedure should include preoperative chest computerized tomography (CT). The use of CT is justified by its higher overall sensitivity than chest X-ray and higher sensitivity for LM less than 1 cm in diameter than positron emission tomography (PET)[29,30]. As well in terms of a PET/CT scan, these are below the level of routine chest CT detection, especially for sub-centimeter lesions; a PET/CT scan is not routinely indicated as a baseline for preoperative workup[3,31]. A PET/CT scan is considerable only if prior anatomic imaging indicates the presence of potentially surgically curable M1 disease, with the purpose to evaluate for unrecognized metastatic disease that would preclude the possibility of surgical management[3].
Application of an appropriate CT acquisition technique is a prerequisite for the optimal evaluation of pulmonary metastasis. This includes, most importantly, the use of thin-section (1-2 mm) image reconstruction, in addition to appropriate exposure factors. The high sensitivity of CT scans is not accompanied by an equally high specificity; therefore this staging procedure reveals lung lesions of uncertain nature in up to one-third of patients, with some of these indeterminate nodules representing metastatic disease[30,32,33]. Nordholm-Carstensen et al[34] recently performed a systematic review of 12 studies that included the prevalence of indeterminate pulmonary nodules and specific radiological and clinical characteristics that predict their malignancy at initial staging chest CT in patients with colorectal cancer. Among a total of 5873 patients, 9% had indeterminate pulmonary nodules at chest CT, of which 10.8% (95%CI: 10.3%-11.2%) turned out to be colorectal cancer metastases at follow-up[34]. Generally, regional lymph node metastasis and multiple numbers of indeterminate pulmonary nodules were predictive of malignancy, whereas calcification of the nodules indicated benign lesions. However, nodules that are truly metastatic disease cannot be definitively identified by specific radiological characteristics, therefore the demonstration of indeterminate pulmonary nodules should not delay curatively intended surgery[34].
Regarding the follow-up duration for indeterminate pulmonary nodules in patients with CRC, at least two consecutive annual studies are suggested as a minimum requirement to document stability. According to the NCCN guidelines 2014 Ver. 1.0[3], a follow-up every 3-6 mo for the initial 2 years and every 6-12 mo for an additional 3 years in cases with metastatic disease, or three annual follow-ups for the initial 2 years in cases with no metastasis, is recommended for surveillance of metastasis[35]. However, the optimal follow-up duration for surveillance of pulmonary metastasis has yet to be definitely determined.
Surgical technique
As in other surgical techniques for primary malignant tumors, the most important aspect that can never be compromised in PM is completeness of resection[36]. At the same time, healthy lung parenchyma should also be preserved as much as possible[36]. These essential considerations should be kept in mind when planning PM in patients with LM from CRC. To achieve better outcomes of surgical treatment while adhering to this principle, several important issues need to be addressed, including surgical approach, minimally invasive surgery, extent of resection, and lymph node dissection.
Surgical approach: For unilateral lesions, anterior or posterolateral thoracotomy has been the most commonly used approach in PM[13]. Thoracotomy offers reasonable access to all areas of the hemithorax for performing wedge and other anatomic resections, along with systematic mediastinal and hilar lymph node dissection[37]. Some surgeons suggested that a median sternotomy is the best approach to ensure a more complete resection for all lesions, even unilateral ones, as it can provide access to bilateral hemithoracic cavities through a single incision[38,39]. However, a study by Roth et al[40] comparing median sternotomy with unilateral thoracotomy demonstrated no survival advantage of the bilateral approach through median sternotomy. Moreover, a major limitation of median sternotomy is poor exposure of lesions located at the posterior aspect of the lung. Saito et al[41] reported that the survival of patients undergoing simultaneous bilateral PM by median sternotomy was significantly lower than that of those undergoing unilateral or bilateral sequential PM, and postulated that remaining lesions after median sternotomy could be the reason for the poor prognosis of patients with bilateral LM. It is difficult to determine the optimal timing of surgery involving simultaneous or sequential bilateral thoracotomies. Careful patient selection is required when considering simultaneous bilateral thoracotomies because this approach may substantially affect respiration in the immediate postoperative period. Pfannschmidt et al[37] showed that the surgical method did not influence long-term survival when all metastases were completely resected.
Open approach vs video-assisted thoracic surgery: As a minimally invasive surgery, video-assisted thoracic surgery (VATS) is now the method of choice for the treatment of stage I non-small cell lung cancer[42]. Several surgeons have advocated the use of the VATS technique for PM, arguing that it offers a decrease in postoperative morbidity, pain, and duration of hospital stay[43,44]. However, most surgeons still prefer the open approach, since it allows for manual palpation of the entire lung parenchyma, thus enabling detection of additional smaller lesions that are rarely found by thoracoscopic instruments[13]. Many authors have expressed concern about the risk of missing small metastatic lesions during VATS[45-47]. Another potential concern is whether a safe resection margin can be adequately obtained through VATS[48]. Resection of lesions located deep in the lung parenchyma with a safe surgical margin may be technically demanding given the limited exposure in VATS. Even though they are advocates of VATS for PM, Chao et al[49] admitted that they found a closer resection margin in the VATS group.
The use of helical CT has been shown to improve detection of smaller pulmonary nodules during VATS when compared with conventional CT[50-52]. If a lesion is too small to be detected on high-resolution CT, manual palpation is also almost impossible. Therefore, surgeons in favor of VATS for PM argue that there is no need for manual palpation through thoracotomy for these tiny nodules. Nakajima et al[53] found that more than 40% of pulmonary nodules 5 mm or smaller at CT in patients with suspicious LM were not pulmonary metastases, which implies that thorough bimanual palpation might potentially overestimate the number of pulmonary nodules[48]. Given that repeated metastasectomy does not adversely affect postoperative morbidity and survival, even lesions that are missed at initial PM can be re-resected safely and effectively[54,55]. Because LM is a systemic disease, better compliance with adjuvant therapy after VATS should also be considered[56]. More importantly, Onaitis et al[57] reported no difference in recurrence-free survival between VATS and thoracotomy for PM, suggesting that resection of very small nodules may not significantly contribute to recurrence-free survival even if thoracoscopic palpation is inferior to that performed through thoracotomy. Despite its limitations, VATS is being applied to PM more and more frequently based on the benefits of minimal invasiveness[48,49,51-53,57]. Although the fact that VATS is a less invasive technique is undeniable, completeness of resection cannot be sacrificed in exchange for reduced invasiveness. However, if complete resection can be guaranteed then there is no reason to avoid VATS for PM. More evidence supporting the benefit of VATS for CRC patients with LM is required.
Extent of resection: Lobectomy is the standard procedure for patients with early-stage non-small cell lung cancer, whereas wedge resection is the preferred procedure for LM, especially those located in the periphery of the lung. Wedge resection can be justified only if there is a safe resection margin around the metastatic lesion. It is therefore necessary to establish how wide the safe resection margin should be. To prevent local recurrence, Rusch recommended a margin of normal lung tissue of least 0.5-1.0 cm in all directions, thus removing a conical-shaped wedge of lung parenchyma circumferentially around the nodule[36]. On the other hand, lobectomy can be a reasonable option for cases in which wedge resection is not feasible, such as lesions located deep in the hilum or multiple lesions localized within a single lobe. However, if multiple lesions are scattered over more than one lobe or throughout both lungs, it is difficult, and even harmful, to perform a lobectomy for every single lesion. Moreover, considering the potential for recurrent LM after initial PM, wedge resection would be helpful in preserving the lung parenchyma as much as possible in case further metastasectomies are needed in the event of relapse. Surprisingly, Yedibela et al[58] and Lin et al[59] reported significantly better survival on multivariate analysis for patients undergoing lobectomy for LM. Except for these reports, however, most authors have shown that there are no significant differences in treatment outcomes between wedge resection and lobectomy. Given the high risk of postoperative morbidity and mortality, pneumonectomy is usually not indicated, except in extremely exceptional cases[36,60].
Lymph node dissection: The role of mediastinal lymph node dissection (MLND) in PM remains controversial. Systematic MLND offers accurate staging and thus provides a better understanding of the extent of disease. Given that the reported incidence of mediastinal lymph node metastasis ranges from 10% to 33%[37,61-66], a significant number of metastasectomies performed without MLND but considered complete resection may actually have been incomplete[64]. The presence of mediastinal lymph node metastases suggests more aggressive features of LM, and consequently has a significantly unfavorable influence on long-term survival. Welter et al[66] reported that 5-year survival rates with or without lymph node metastases were 19% and 42%, respectively (P = 0.02). Therefore, proponents of routine MLND argue that PM is not worthwhile in patients with mediastinal lymph node metastases, with palliative chemotherapy being recommended in such cases instead[37,61-66]. Although some authors demonstrated that there were long-term survivors after MLND despite their metastatic nodal involvement[67], a therapeutic effect of MLND has not been fully proven. Thus, many surgeons still do not routinely perform MLND in their clinical practice. A recent survey among members of the ESTS found that only 13% of responding surgeons routinely performed complete mediastinal lymphadenectomy, and approximately 32% performed neither lymph node sampling nor dissection[13]. To clarify the role of MLND in patients with LM of CRC, the following questions need to be addressed: Should MLND be performed regardless of the probability of mediastinal lymph node metastases based on imaging studies at the expense of potentially increased complication rates, or should MLND be applied selectively according to the location, number, and size of lesions in patients with LM of CRC? When mediastinal lymph node involvement is unexpectedly found during PM, should PM with MLND be performed or should the procedure be stopped? If MLND reveals the presence of mediastinal lymph node metastases, should systemic chemotherapy be recommended? Prospective, randomized studies are required to answer these questions.
Perioperative chemotherapy
The treatment options for advanced colorectal cancer have been almost exclusively based on 5-fluorouracil (5-FU) for more than three decades[68]. Until the early 1990s, 5-FU, often modulated by leucovorin, was the only effective chemotherapy available, although it resulted in meaningful responses in only a small minority of treated patients. The recent integration of oxaliplatin and irinotecan for the management of advanced CRC patients has significantly extended median overall survival[68,69]. Currently, combination chemotherapy including 5-FU, leucovorin, and oxaliplatin (FOLFOX) or 5-FU, leucovorin, and irinotecan (FOLFIRI) is the most widely used regimen, and the addition of molecular targeting agents, such as cetuximab or bevacizumab, has resulted in further significant increases in response rate and overall survival[70,71]. In CRC patients with potentially resectable liver metastases, perioperative combination chemotherapy with the FOLFOX regimen is recommended, as it improves progression-free survival at 3 years by 7%-8%. In patients with surgically resectable CRC with liver metastases, perioperative chemotherapy is frequently given for 3 mo before and 3 mo after surgical resection of the metastases. However, the duration of chemotherapy or the timing of metastasectomy can vary among patients. If no preoperative chemotherapy has been administered, postoperative chemotherapy with FOLFOX is recommended. Currently, there is a lack of robust evidence that the addition of a biological, such as bevacizumab or cetuximab, improves the outcome in CRC patients with resected metastases compared with chemotherapy alone.
Initially unresectable liver metastases can be converted to resectable disease after downsizing the tumor with chemotherapy. Standard combination chemotherapy regimens comprising FOLFIRI or FOLFOX have been reported to facilitate resection in 7%-40% of patients with initially unresectable metastases, depending upon the initial selection of patients. Nevertheless, approximately 80% of these patients will experience recurrence within 2 years of resection. Recently, the combination of FOLFOX or FOLFIRI chemotherapy plus cetuximab has resulted in higher resection rates in liver-limited unresectable CRCs that are wild-type for KRAS.
In contrast to liver metastasis, few data is available comparing survival between patients undergoing PM with and without adjuvant or neoadjuvant chemotherapy for LM only. Most retrospective studies of PM have not analyzed the prognostic significance of perioperative chemotherapy in patients undergoing PM for LM. Several authors favored the use of perioperative chemotherapy for patients undergoing PM for LM[57,66], but these suggestions were not based on convincing evidence due to a lack of randomized controlled trials on the role of perioperative chemotherapy in patients undergoing PM for metastatic CRC. Whether the results of perioperative chemotherapy in patients undergoing metastasectomy for liver metastases can be applied to those undergoing PM for LM remains unknown. Therefore, whether patients undergoing PM for LM of CRC will benefit from perioperative chemotherapy requires further evaluation.
TREATMENT OUTCOMES AND PROGNOSTIC FACTORS
Since most studies are retrospective reviews of single-center reports of relatively small numbers of patients over a time period of 15 to 20 years, there is considerable variation in overall survival rates and prognostic factors as a result of heterogeneous selection criteria, diverse patterns of clinical practice, and differences in variables analyzed[14-17]. The reported 5-year survival rates of PM ranged from 27% to 68%[14-17]. Treatment outcomes and related prognostic factors are summarized in Table 1.
Table 1.
Summary of survival outcomes and prognostic factors in recently published studies
| Ref. | Year | Patients (n) | 5-yr survival | Px factor |
| Zink et al[82] | 2001 | 110 | 32.6% | Number of mets, CEA, size of mets |
| Rena et al[79] | 2002 | 80 | 41.1% | DFI, number of mets, CEA |
| Saito et al[41] | 2002 | 165 | 39.6% | LN mets, CEA |
| Pfannschmidt et al[37] | 2003 | 167 | 32.4% | LN mets, CEA, number of mets |
| Inoue et al[61] | 2004 | 128 | 45.3% | Primary tumor stage, distribution of mets |
| Melloni et al[64] | 2006 | 81 | 42.0% | Primary tumor stage, complete resection |
| Yedibela et al[58] | 2006 | 153 | 37.0% | Number of mets, DFI, transfusion |
| Welter et al[66] | 2007 | 169 | 39.1% | DFI, number of mets, LN mets |
| Lin et al[59] | 2009 | 63 | 43.9% | DFI, type of resection |
| Onaitis et al[57] | 2009 | 378 | 78.0%1 | Age, sex, DFI, number of mets |
| Watanabe et al[91] | 2009 | 113 | 67.8% | CEA, lymphatic invasion |
| Landes et al[101] | 2010 | 40 | 43.4% | Prior history of liver mets |
| Riquet et al[67] | 2010 | 127 | 41.0% | Complete resection |
| Zabaleta et al[104] | 2011 | 84 | 54.0% | Prior history of liver mets, LN mets, DFI, number of mets |
Indicates 3-year survival rate. mets: Metastasis. LN: Lymph node; DFI: Disease-free interval; CEA: Carcinoembryonic antigen.
Several authors have conducted systematic reviews or meta-analyses to summarize the treatment outcomes of published retrospective studies[14-17]. Pfannschmidt et al[14] reported a systematic review of 17 studies involving 1684 patients who underwent PM for metastatic CRC in which the 5-year survival rates ranged between 41% and 56% (median 48%). Recently, in a meta-analysis of 1112 metastasectomies performed in 927 patients between 1983 and 2008, Salah et al[16] found an overall 5-year survival rate of 54.3% following initial PM. These numbers are encouraging when compared with the disappointing outcomes of chemotherapy[18,19].
Many authors have also performed prognostic factor analyses, which will be helpful in establishing valuable treatment guidelines for PM by identifying patients most likely to benefit from PM. Completeness of resection has consistently been reported to affect survival after PM for metastatic CRC, as it is the most important prerequisite of metastasectomy[12]. Age, sex, and pathologic features of the primary CRC have been more rarely reported as significant factors[14-17]. There are conflicting data regarding other prognostic factors, although the most commonly suggested prognostic factors are: (1) disease-free interval; (2) number of LM; (3) size of the largest LM; (4) distribution of LM; (5) preoperative serum carcinoembryonic antigen (CEA) level; and (6) lymph node metastasis.
Disease-free interval
A prolonged disease-free interval (DFI) between CRC resection and PM is associated with a favorable treatment outcome[17]. In contrast, a short DFI represents early dissemination of metastatic disease, which implies more aggressive tumor biology[17]. At the extreme end of a short DFI is synchronous LM. Although it has been recommended that patients with resectable synchronous LM can be resected synchronously or by using a staged approach, survival after PM for synchronous LM is reported to be poorer than for metachronous LM[3]. Onaitis et al[57] reported that a DFI of less than 1 year was an independent predictor of recurrence after PM. They also showed that none of the patients with three or more lesions and a DFI of less than 1 year were cured by surgery, suggesting that medical management alone should be considered for these patients. However, not all investigators showed that a short DFI correlated with a poor prognosis after PM[14,72-78]. In their systematic review, Pfannschmidt et al[14] did not find DFI to be a significant prognostic factor. Despite the clinical relevance of DFI between CRC resection and PM, only six studies reported that short DFI was a poor prognostic factor[57-59,79-81]. Although various cut-off values for defining the short DFI might have affected these differences, it is still difficult to explain this inconsistency. Based on current findings, it appears that a short DFI might be a poor prognostic factor, but is not an absolute contraindication for PM.
Number of metastasis
Many authors reported that multiplicity of LM was a poor prognostic factor[37,57,73-75,82]. Multiple lesions may increase the likelihood of widespread undetected tiny nodules throughout the lung or even in other distant organs. Pfannschmidt et al[37] demonstrated that patients with a solitary LM had significantly better survival than those with multiple LM. On the contrary, some authors did not find a significant relationship between prognosis and number of LM[6,41,61,72]. Inoue et al[61] found no significant difference in survival between patients with solitary and multiple lesions, and suggested that occult micrometastases might have existed at the time of PM in patients considered to have a solitary lesion, resulting in incomplete resection. Advances in CT imaging techniques can help us decide whether to recommend PM through preoperative identification of the exact number of LM. Therefore, a solitary LM described before the development of these advanced techniques might not have truly been solitary. Most authors would agree that a larger number of lesions are associated with a poor prognosis, but the cut-off value for denying PM for patients with multiple LM is undetermined[20]. The presence of more than one metastatic lesion is not always a contraindication for PM, and it seems unfair to deny PM for patients with two to four lesions[17].
Size of the largest metastatic nodule
Although some authors showed that the size of LM was an independent prognostic factor[83,84], most studies have found no significant relationship between survival and the size of LM[73,85]. Similar to the number of LM, a larger tumor may indicate a greater possibility that other undetectable metastatic lesions are present.
Distribution of metastasis
Conflicting data exist as to whether the distribution of metastasis affects survival after PM in metastatic CRC patients. Inoue et al[31] reported that unilateral location of LM was an independent predictor of longer survival and Chen et al[86] reported no long-term survivors with bilateral LM. McCormack et al[77] showed no significant difference in survival with respect to bilaterality of LM, suggesting that patients with bilateral lesions may benefit from PM in addition to those with ipsilateral multiple lesions. Riquet et al[67] reported that 5-year survival rates of patients undergoing complete bilateral metastasectomies tended to be comparable to those observed in cases of complete unilateral metastasectomy (68% vs 35.5%; P = 0.09). In this context, bilateral lesions do not seem to be a contraindication for PM if they can be completely resected.
Preoperative CEA
CEA is an antigen expressed on the apical surface of colonic epithelial cells that is involved in intracellular recognition and adhesion of tumor cells to host cells[87,88]. Serum CEA level is an indication of the total tumor mass and the ability of tumor cells to express CEA[89]. Elevated serum level of CEA has consistently been found to be an independent negative prognostic factor[14,37,41,72,73,75,79,82,84,89,90]; patients with a high serum CEA level had 5-year survival rates ranging from 0% to 53%, compared with 23% to 80% for those with normal CEA levels[20]. Sakamoto et al[72] demonstrated that the preoperative CEA level was the only significant prognostic factor. However, not all investigators found that a high serum CEA level is associated with poor prognoses[59,91]. Watanabe et al[91] reported that the 5-year survival rate in patients with a serum CEA concentration of 5 ng/mL or greater was 53%, suggesting that an elevated CEA level, although related to unfavorable outcomes, is not a contraindication to PM. However, in patients with multiple bilateral LM an increased serum CEA can play a pivotal role in determining operability. Close monitoring of CEA level after PM would also be critical in the management of metastatic CRC patients[17].
Lymph node metastasis
Hilar and mediastinal lymph node metastasis is also consistently reported to have a negative impact on survival outcomes, and there is little doubt about its importance as a prognostic factor for LM of CRC. In the meta-analysis performed by Gonzalez et al[17], hilar and/or mediastinal lymph node involvement was a prognostic factor of poor outcomes. They suggested that PET/CT scans need to be performed before PM in order to identify potential mediastinal lymph node metastasis and, when suspected, histologic assessment such as mediastinoscopy should be considered[17]. As discussed above, the role of MLND remains unclear.
SPECIAL CONSIDERATIONS
Repeated metastasectomy
Tumor recurrence is always a possibility in patients with LM from CRC, even after successful PM. The reported recurrence rate after PM is as high as 68%, with the remaining lung being the most common site of recurrence[31,92]. Recurrent metastatic nodules that develop after complete resection of initial LM represent residual lesions that were too small to be detected by high-resolution CT and/or manual palpation at the time of initial PM. This means that PM might not be truly complete even when all the metastatic nodules are believed to be completely resected. More importantly, this rate of recurrence also suggests the presence of occult micrometastases disseminated throughout extrapulmonary distant organs in addition to the remaining lungs. Accordingly, there is an intrinsically high chance of relapse after repeated PM. Moreover, reoperation may increase the risk of postoperative morbidity compared with the first operation because patients are less able to tolerate repeated operations due to limited residual lung capacity, and pleural adhesions from a previous operation may complicate subsequent surgery. For these reasons, most surgeons have been reluctant to perform repeated PM for recurrent LM and thus there is no clear consensus on optimal treatment strategies and the role of repeated PM for recurrent LM.
However, several authors, including our group, have tried repeated PM despite its unfavorable aspects, and reported that PM can be performed multiple times safely and effectively[54,55,59,73,93,94]. Welter et al[54] showed that among 175 patients who underwent PM for LM of CRC, 33 had PM up to three times with no postoperative mortality and a 5-year survival rate of 53.8% with a median survival of 72.6 mo. Lin et al[59] even reported that the 5-year survival rate of repeated PM was 85.7%, albeit in only eight cases. Our group also demonstrated excellent outcomes of repeated PM[55]. Among 202 patients who received PM for LM of CRC at our institution between 1995 and 2007, 48 underwent second metastasectomy. Among these 48 patients, 28 developed recurrence again, with 10 of those receiving a third metastasectomy. Overall and disease-free 5-year survival rates for second metastasectomies were 79% and 49%, respectively, and overall 5-year survival rate for third metastasectomies was 78%. Recently, Salah et al[95] conducted a pooled analysis of seven published retrospective series that included 148 patients undergoing repeated PM. They reported that the 5-year survival rate of repeated PM was 57.9%, indicating that repeated PM offers an excellent chance for long-term survival.
However, it should be noted that the favorable outcomes of repeated PM might have been due to the fact that the candidates for repeated PM were highly selected, as for first PM. Patients eligible for repeated PM may have better prognostic factors than those who are ineligible, irrespective of the potential benefits of resection of recurrent LM. The survival benefit might therefore be attributed to patient selection rather than repeated PM per se. Since a randomized controlled trial is difficult to conduct for first PM, let alone repeated PM, and there is no way to prove the therapeutic effect of repeated PM compared with observation or chemotherapy. Considering that the only therapeutic option for recurrent LM is repeated PM, the best way to improve survival is to determine which patients are most likely to benefit from repeated PM. Chen et al[94] found that patients with a DFI greater than 1 year after the first metastasectomy showed significantly better overall survival, whereas Welter et al[54] reported that the number of metastases was an important prognostic factor. Our group also tried to determine the prognostic factors, but multivariate analysis revealed no independent prognostic factor even though preoperative CEA level was a significant factor in univariate analysis[55]. Given the smaller number of patients undergoing repeated PM relative to initial PM, the prognostic factors are not consistently reproducible, and there is still debate about the best candidates for repeated PM[95]. Nonetheless, when patients with recurrent LM after PM have multiple favorable prognostic factors such as prolonged DFI, solitary recurrent LM, and normal CEA level, repeated PM can be a valuable alternative to palliative chemotherapy.
Several points need to be considered when considering repeated PM. First, meticulous restaging is crucial to rule out the presence of extrapulmonary metastases[95]. PET/CT is known to be a useful and sensitive restaging modality to identify hilar or mediastinal lymph node metastasis, as well as extrapulmonary metastases[96]. If a PET/CT scan suggests thoracic lymph node involvement, mediastinoscopy should be considered[95]. Second, as we emphasized in our series, it is very important to attempt to preserve as much of the lung parenchyma as possible, as future repeated PM may be necessary[55]. Parenchyma-saving procedures, such as wedge resection, precision excision, and segmentectomy rather than lobectomy, will allow for further resection in cases of future relapse[55]. Third, vigilant surveillance based on a regular follow-up schedule will be helpful for the early detection of tumor recurrence. In our institution, chest CT with serum CEA and liver function testing are routinely performed postoperatively every 3 mo for the first year, and then on a 6-mo basis for at least 2 years and annually thereafter[55]. These efforts provide a chance to repeat PM, especially in the most suitable candidates.
Multiple metastatic nodules
As discussed above, the presence of multiple metastatic nodules is a strong predictor of poor survival[37,57,73-76,82]. Onaitis et al[57] reported that the most potent predictor of recurrence after PM for metastatic CRC was the number of metastases. This can be explained by the notion that patients with multiple metastatic nodules are more likely to have occult micrometastases in the lungs or extrapulmonary organs at the time of PM, which eventually lead to recurrence.
However, some investigators reported that the prognoses of patients who underwent PM despite multiple nodules were not as poor as originally predicted, which suggests that even patients with multiple metastatic nodules might benefit from PM[97,98]. Welter et al[66] found a median survival of 58 mo in patients with a solitary LM and 33 mo in those with up to 10 metastases, which still seems to be a good outcome. An analysis of the International Registry of Lung Metastases revealed that the 5-year survival rates gradually decreased as the number of metastatic nodules increased from 43% (single) to 34% (two or three) and 27% (four or more), although the study included various histologic types of primary tumor[12]. Even in patients who had 10 or more metastases resected (n = 342), survival reached 26% at 5 years with a median survival of 26 mo[12]. In our institution, the 5-year survival rates were 65%, 50%, and 33% for single, two or three, and four or more lesions, respectively (unpublished data, Figure 1). Patients with five or more lesions also showed acceptable survival outcomes, with a 5-year survival rate of 31%.
Figure 1.
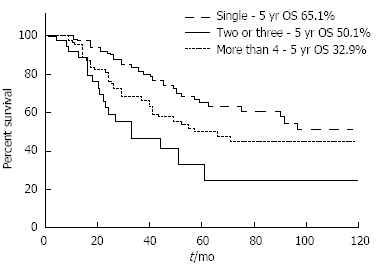
Kaplan-Meier survival curve according to the number of metastases.
These encouraging outcomes in patients with multiple nodules might also be related to patient selection. PM would not have been recommended to patients with multiple lesions if they had additional unfavorable factors such as a short DFI, increased serum CEA, or a history of liver metastasectomy. The fact that patients received PM for multiple metastatic lesions indicates a good preoperative status, both oncologically and physiologically. Although long-term survivors have been seen among patients for whom surgeons usually hesitate to offer PM, no one can deny that survival rates progressively decline as the number of LM increases. This raises some important questions: Is there an absolute limit on the number of LM beyond which we should never recommend PM? How many nodules can be considered a reasonable indication for PM in patients with LM of CRC? It is still difficult to answer these questions in the absence of studies that specifically address these issues.
It should also be noted that patients with four or five lesions and those with more than ten lesions should not be treated as the same category (i.e., multiplicity). Although the exact cut off has not been determined, we believe that patients with less than a certain number of metastases could benefit from PM despite the fact that they have multiple LM. If a patient is found to have LM from CRC but has no other unfavorable prognostic factors, it is unreasonable to deny PM for this patient just because of multiplicity of LM. The acceptable cut off number for not recommending PM needs to be further defined in the future.
Combined liver and lung metastasectomy
With recent advances in the management of liver metastases in CRC patients, the prognosis of CRC patients treated for liver metastases is improving, with a 5-year survival rate of nearly 50%[99]. Almost two-thirds of patients who underwent hepatic metastasectomy (HM) developed extrahepatic metastases, with the lung being the most common site, and such patients could be candidates for PM[15,100,101]. However, there is no clear consensus on the optimal management in patients presenting with both liver and lung metastases, and the role of PM in these patients remains controversial. It also remains unknown whether patients undergoing both HM and PM have similar survival outcomes to those undergoing PM only.
Although some authors reported that a previous HM correlated with an increased risk of death in patients undergoing PM[101-104], most studies have found that a history of liver metastasis at the time of PM is not a significant factor affecting survival, and that outcomes after combined HM and PM were similar to those after HM alone or PM alone, with reported 5-year survival rates ranging from 11% to 61%[17,105-115]. Based on these findings, most investigators suggest that a history of resected liver metastases should not be regarded as a contraindication to PM.
Although based on a small number of cases, our previous molecular study showed that pulmonary metastases were significantly diverged from hepatic metastases indistinguishable from primary colorectal cancers, which may also support such an aggressive approach[116].
Therefore, provided liver metastases are completed resected, there is no reason to deny PM when patients have no unfavorable prognostic factors other than a history of previous liver metastases. Some authors indicated that this treatment strategy can also be applied to patients with synchronous liver and lung metastases[89,114].
FUTURE DIRECTION
Ideally, a randomized controlled trial is needed to answer the question of whether patients with LM from CRC could benefit from PM, compared with observation or chemotherapy, and which patients are most likely to benefit the most. However, as discussed above, it is not feasible to conduct a randomized trial to address this question. Nonetheless, a trial on pulmonary metastasectomy in colorectal cancer (PulMiCC) is currently open to recruitment to examine whether surgical resection of LM from CRC lengthens survival, and its critically important results are expected to be released in the near future[117]. In addition to this trial, additional prospective studies investigating various aspects of PM should be carried out to gather more convincing evidence. Before we can draw robust conclusions from current and future trials, it is vitally important to perform PM based on the comprehensive knowledge and reliable experience of surgeons. Moreover, great effort should be made to select patients who are most likely to benefit from PM. Continued application of PM will provide valuable information to address the questions of how many times PM can be performed for recurrent LM and how many nodules can be resected safely and effectively, which will in turn expand the indication of PM in challenging cases such as recurrent LM and multiple metastatic nodules.
Footnotes
P- Reviewers: Takabe K, Wang JW S- Editor: Ma YJ L- Editor: Rutherford A E- Editor: Ma S
References
- 1.Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA Cancer J Clin. 2009;59:366–378. doi: 10.3322/caac.20038. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.NCCN Clinical Practice Guidelines in Oncology Version 1.2014: Colon cancer. Accessed August 31, 2013. Available from: http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf.
- 4.August DA, Ottow RT, Sugarbaker PH. Clinical perspective of human colorectal cancer metastasis. Cancer Metastasis Rev. 1984;3:303–324. doi: 10.1007/BF00051457. [DOI] [PubMed] [Google Scholar]
- 5.Brister SJ, de Varennes B, Gordon PH, Sheiner NM, Pym J. Contemporary operative management of pulmonary metastases of colorectal origin. Dis Colon Rectum. 1988;31:786–792. doi: 10.1007/BF02560108. [DOI] [PubMed] [Google Scholar]
- 6.McCormack PM, Burt ME, Bains MS, Martini N, Rusch VW, Ginsberg RJ. Lung resection for colorectal metastases. 10-year results. Arch Surg. 1992;127:1403–1406. doi: 10.1001/archsurg.1992.01420120037006. [DOI] [PubMed] [Google Scholar]
- 7.Kievit J. Follow-up of patients with colorectal cancer: numbers needed to test and treat. Eur J Cancer. 2002;38:986–999. doi: 10.1016/s0959-8049(02)00061-8. [DOI] [PubMed] [Google Scholar]
- 8.Desch CE, Benson AB, Somerfield MR, Flynn PJ, Krause C, Loprinzi CL, Minsky BD, Pfister DG, Virgo KS, Petrelli NJ. Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2005;23:8512–8519. doi: 10.1200/JCO.2005.04.0063. [DOI] [PubMed] [Google Scholar]
- 9.Poon MA, O’Connell MJ, Moertel CG, Wieand HS, Cullinan SA, Everson LK, Krook JE, Mailliard JA, Laurie JA, Tschetter LK. Biochemical modulation of fluorouracil: evidence of significant improvement of survival and quality of life in patients with advanced colorectal carcinoma. J Clin Oncol. 1989;7:1407–1418. doi: 10.1200/JCO.1989.7.10.1407. [DOI] [PubMed] [Google Scholar]
- 10.Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Engl J Med. 2005;352:476–487. doi: 10.1056/NEJMra040958. [DOI] [PubMed] [Google Scholar]
- 11.Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, Giacchetti S, Paule B, Kunstlinger F, Ghémard O, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240:644–57; discussion 657-8. doi: 10.1097/01.sla.0000141198.92114.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. The International Registry of Lung Metastases. J Thorac Cardiovasc Surg. 1997;113:37–49. doi: 10.1016/s0022-5223(97)70397-0. [DOI] [PubMed] [Google Scholar]
- 13.Internullo E, Cassivi SD, Van Raemdonck D, Friedel G, Treasure T. Pulmonary metastasectomy: a survey of current practice amongst members of the European Society of Thoracic Surgeons. J Thorac Oncol. 2008;3:1257–1266. doi: 10.1097/JTO.0b013e31818bd9da. [DOI] [PubMed] [Google Scholar]
- 14.Pfannschmidt J, Dienemann H, Hoffmann H. Surgical resection of pulmonary metastases from colorectal cancer: a systematic review of published series. Ann Thorac Surg. 2007;84:324–338. doi: 10.1016/j.athoracsur.2007.02.093. [DOI] [PubMed] [Google Scholar]
- 15.Fiorentino F, Hunt I, Teoh K, Treasure T, Utley M. Pulmonary metastasectomy in colorectal cancer: a systematic review and quantitative synthesis. J R Soc Med. 2010;103:60–66. doi: 10.1258/jrsm.2009.090299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salah S, Watanabe K, Welter S, Park JS, Park JW, Zabaleta J, Ardissone F, Kim J, Riquet M, Nojiri K, et al. Colorectal cancer pulmonary oligometastases: pooled analysis and construction of a clinical lung metastasectomy prognostic model. Ann Oncol. 2012;23:2649–2655. doi: 10.1093/annonc/mds100. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez M, Poncet A, Combescure C, Robert J, Ris HB, Gervaz P. Risk factors for survival after lung metastasectomy in colorectal cancer patients: a systematic review and meta-analysis. Ann Surg Oncol. 2013;20:572–579. doi: 10.1245/s10434-012-2726-3. [DOI] [PubMed] [Google Scholar]
- 18.O'Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004;96:1420–1425. doi: 10.1093/jnci/djh275. [DOI] [PubMed] [Google Scholar]
- 19.Sanoff HK, Sargent DJ, Campbell ME, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Goldberg RM. Five-year data and prognostic factor analysis of oxaliplatin and irinotecan combinations for advanced colorectal cancer: N9741. J Clin Oncol. 2008;26:5721–5727. doi: 10.1200/JCO.2008.17.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez M, Ris HB, Krueger T, Gervaz P. Colorectal cancer and thoracic surgeons: close encounters of the third kind. Expert Rev Anticancer Ther. 2012;12:495–503. doi: 10.1586/era.12.21. [DOI] [PubMed] [Google Scholar]
- 21.Treasure T. Pulmonary metastasectomy for colorectal cancer: weak evidence and no randomised trials. Eur J Cardiothorac Surg. 2008;33:300–302. doi: 10.1016/j.ejcts.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 22.Primrose J, Treasure T, Fiorentino F. Lung metastasectomy in colorectal cancer: is this surgery effective in prolonging life? Respirology. 2010;15:742–746. doi: 10.1111/j.1440-1843.2010.01759.x. [DOI] [PubMed] [Google Scholar]
- 23.Treasure T, Fallowfield L, Lees B, Farewell V. Pulmonary metastasectomy in colorectal cancer: the PulMiCC trial. Thorax. 2012;67:185–187. doi: 10.1136/thoraxjnl-2011-200015. [DOI] [PubMed] [Google Scholar]
- 24.Blalock A. Recent advances in surgery. N Engl J Med. 1944;231:261–267. [Google Scholar]
- 25.Thomford NR, Woolner LB, Clagett OT. The surgical treatment of metastatic tumors in the lungs. J Thorac Cardiovasc Surg. 1965;49:357–363. [PubMed] [Google Scholar]
- 26.Erhunmwunsee L, D’Amico TA. Surgical management of pulmonary metastases. Ann Thorac Surg. 2009;88:2052–2060. doi: 10.1016/j.athoracsur.2009.08.033. [DOI] [PubMed] [Google Scholar]
- 27.Group. TDCC. Guidelines for the Management of Colorectal Cancer. Available from: http://dccg.dk/03_Publikation/Retningslinier2009revOKT2010.pdf. 2010.
- 28.Erhunmwunsee L Ireland. TAoCoGBa. Guidelines for the Management of Colorectal Cancer. Available from: http://www.acpgbi.org.uk/wp-content/uploads/2007-CC-Management-Guidelines.pdf. 2007.
- 29.Arulampalam TH, Costa DC, Loizidou M, Visvikis D, Ell PJ, Taylor I. Positron emission tomography and colorectal cancer. Br J Surg. 2001;88:176–189. doi: 10.1046/j.1365-2168.2001.01657.x. [DOI] [PubMed] [Google Scholar]
- 30.McIntosh J, Sylvester PA, Virjee J, Callaway M, Thomas MG. Pulmonary staging in colorectal cancer--is computerised tomography the answer? Ann R Coll Surg Engl. 2005;87:331–333. doi: 10.1308/003588405X60579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inoue M, Ohta M, Iuchi K, Matsumura A, Ideguchi K, Yasumitsu T, Nakagawa K, Fukuhara K, Maeda H, Takeda S, et al. Benefits of surgery for patients with pulmonary metastases from colorectal carcinoma. Ann Thorac Surg. 2004;78:238–244. doi: 10.1016/j.athoracsur.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 32.Brent A, Talbot R, Coyne J, Nash G. Should indeterminate lung lesions reported on staging CT scans influence the management of patients with colorectal cancer? Colorectal Dis. 2007;9:816–818. doi: 10.1111/j.1463-1318.2007.01229.x. [DOI] [PubMed] [Google Scholar]
- 33.Kronawitter U, Kemeny NE, Heelan R, Fata F, Fong Y. Evaluation of chest computed tomography in the staging of patients with potentially resectable liver metastases from colorectal carcinoma. Cancer. 1999;86:229–235. [PubMed] [Google Scholar]
- 34.Nordholm-Carstensen A, Wille-Jørgensen PA, Jorgensen LN, Harling H. Indeterminate pulmonary nodules at colorectal cancer staging: a systematic review of predictive parameters for malignancy. Ann Surg Oncol. 2013;20:4022–4030. doi: 10.1245/s10434-013-3062-y. [DOI] [PubMed] [Google Scholar]
- 35.Calandrino R, Ardu V, Corletto D, del Vecchio A, Origgi D, Signorotto P, Spinelli A, Tosi G, Bolognesi A, Cariati M, et al. Evaluation of second cancer induction risk by CT follow-up in oncological long-surviving patients. Health Phys. 2013;104:1–8. doi: 10.1097/HP.0b013e3182690c8e. [DOI] [PubMed] [Google Scholar]
- 36.Rusch VW. Pulmonary metastasectomy. Current indications. Chest. 1995;107:322S–331S. doi: 10.1378/chest.107.6_supplement.322s. [DOI] [PubMed] [Google Scholar]
- 37.Pfannschmidt J, Muley T, Hoffmann H, Dienemann H. Prognostic factors and survival after complete resection of pulmonary metastases from colorectal carcinoma: experiences in 167 patients. J Thorac Cardiovasc Surg. 2003;126:732–739. doi: 10.1016/s0022-5223(03)00587-7. [DOI] [PubMed] [Google Scholar]
- 38.Johnston MR. Median sternotomy for resection of pulmonary metastases. J Thorac Cardiovasc Surg. 1983;85:516–522. [PubMed] [Google Scholar]
- 39.van der Veen AH, van Geel AN, Hop WC, Wiggers T. Median sternotomy: the preferred incision for resection of lung metastases. Eur J Surg. 1998;164:507–512. doi: 10.1080/110241598750005859. [DOI] [PubMed] [Google Scholar]
- 40.Roth JA, Beech DJ, Putnam JB, Pollock RE, Patel SR, Fidler IJ, Benjamin RS. Treatment of the patient with lung metastases. Curr Probl Surg. 1996;33:881–952. doi: 10.1016/s0011-3840(96)80003-7. [DOI] [PubMed] [Google Scholar]
- 41.Saito Y, Omiya H, Kohno K, Kobayashi T, Itoi K, Teramachi M, Sasaki M, Suzuki H, Takao H, Nakade M. Pulmonary metastasectomy for 165 patients with colorectal carcinoma: A prognostic assessment. J Thorac Cardiovasc Surg. 2002;124:1007–1013. doi: 10.1067/mtc.2002.125165. [DOI] [PubMed] [Google Scholar]
- 42.McKenna RJ, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg. 2006;81:421–45; discussion 421-45;. doi: 10.1016/j.athoracsur.2005.07.078. [DOI] [PubMed] [Google Scholar]
- 43.Landreneau RJ, De Giacomo T, Mack MJ, Hazelrigg SR, Ferson PF, Keenan RJ, Luketich JD, Yim AP, Coloni GF. Therapeutic video-assisted thoracoscopic surgical resection of colorectal pulmonary metastases. Eur J Cardiothorac Surg. 2000;18:671–66; discussion 676-677. doi: 10.1016/s1010-7940(00)00580-7. [DOI] [PubMed] [Google Scholar]
- 44.Lin JC, Wiechmann RJ, Szwerc MF, Hazelrigg SR, Ferson PF, Naunheim KS, Keenan RJ, Yim AP, Rendina E, DeGiacomo T, et al. Diagnostic and therapeutic video-assisted thoracic surgery resection of pulmonary metastases. Surgery. 1999;126:636–41; discussion 641-2. [PubMed] [Google Scholar]
- 45.McCormack PM, Bains MS, Begg CB, Burt ME, Downey RJ, Panicek DM, Rusch VW, Zakowski M, Ginsberg RJ. Role of video-assisted thoracic surgery in the treatment of pulmonary metastases: results of a prospective trial. Ann Thorac Surg. 1996;62:213–216; discussion 216-7. doi: 10.1016/0003-4975(96)00253-6. [DOI] [PubMed] [Google Scholar]
- 46.Liu HP, Lin PJ, Hsieh MJ, Chang JP, Chang CH. Application of thoracoscopy for lung metastases. Chest. 1995;107:266–268. doi: 10.1378/chest.107.1.266. [DOI] [PubMed] [Google Scholar]
- 47.Mutsaerts EL, Zoetmulder FA, Meijer S, Baas P, Hart AA, Rutgers EJ. Outcome of thoracoscopic pulmonary metastasectomy evaluated by confirmatory thoracotomy. Ann Thorac Surg. 2001;72:230–233. doi: 10.1016/s0003-4975(01)02629-7. [DOI] [PubMed] [Google Scholar]
- 48.Nakajima J, Murakawa T, Fukami T, Takamoto S. Is thoracoscopic surgery justified to treat pulmonary metastasis from colorectal cancer? Interact Cardiovasc Thorac Surg. 2008;7:212–216; discussion 216-7. doi: 10.1510/icvts.2007.167239. [DOI] [PubMed] [Google Scholar]
- 49.Chao YK, Chang HC, Wu YC, Liu YH, Hsieh MJ, Chiang JM, Liu HP. Management of lung metastases from colorectal cancer: video-assisted thoracoscopic surgery versus thoracotomy--a case-matched study. Thorac Cardiovasc Surg. 2012;60:398–404. doi: 10.1055/s-0031-1295574. [DOI] [PubMed] [Google Scholar]
- 50.Kang MC, Kang CH, Lee HJ, Goo JM, Kim YT, Kim JH. Accuracy of 16-channel multi-detector row chest computed tomography with thin sections in the detection of metastatic pulmonary nodules. Eur J Cardiothorac Surg. 2008;33:473–479. doi: 10.1016/j.ejcts.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 51.Nakas A, Klimatsidas MN, Entwisle J, Martin-Ucar AE, Waller DA. Video-assisted versus open pulmonary metastasectomy: the surgeon’s finger or the radiologist’s eye? Eur J Cardiothorac Surg. 2009;36:469–474. doi: 10.1016/j.ejcts.2009.03.050. [DOI] [PubMed] [Google Scholar]
- 52.Carballo M, Maish MS, Jaroszewski DE, Holmes CE. Video-assisted thoracic surgery (VATS) as a safe alternative for the resection of pulmonary metastases: a retrospective cohort study. J Cardiothorac Surg. 2009;4:13. doi: 10.1186/1749-8090-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakajima J, Murakawa T, Fukami T, Sano A, Sugiura M, Takamoto S. Is finger palpation at operation indispensable for pulmonary metastasectomy in colorectal cancer? Ann Thorac Surg. 2007;84:1680–1684. doi: 10.1016/j.athoracsur.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 54.Welter S, Jacobs J, Krbek T, Krebs B, Stamatis G. Long-term survival after repeated resection of pulmonary metastases from colorectal cancer. Ann Thorac Surg. 2007;84:203–210. doi: 10.1016/j.athoracsur.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 55.Park JS, Kim HK, Choi YS, Kim K, Shim YM, Jo J, Lee WY, Chun HK, Park YS, Kang WK, et al. Outcomes after repeated resection for recurrent pulmonary metastases from colorectal cancer. Ann Oncol. 2010;21:1285–1289. doi: 10.1093/annonc/mdp475. [DOI] [PubMed] [Google Scholar]
- 56.Petersen RP, Pham D, Burfeind WR, Hanish SI, Toloza EM, Harpole DH, D’Amico TA. Thoracoscopic lobectomy facilitates the delivery of chemotherapy after resection for lung cancer. Ann Thorac Surg. 2007;83:1245–129; discussion 1250. doi: 10.1016/j.athoracsur.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 57.Onaitis MW, Petersen RP, Haney JC, Saltz L, Park B, Flores R, Rizk N, Bains MS, Dycoco J, D’Amico TA, et al. Prognostic factors for recurrence after pulmonary resection of colorectal cancer metastases. Ann Thorac Surg. 2009;87:1684–1688. doi: 10.1016/j.athoracsur.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 58.Yedibela S, Klein P, Feuchter K, Hoffmann M, Meyer T, Papadopoulos T, Göhl J, Hohenberger W. Surgical management of pulmonary metastases from colorectal cancer in 153 patients. Ann Surg Oncol. 2006;13:1538–1544. doi: 10.1245/s10434-006-9100-2. [DOI] [PubMed] [Google Scholar]
- 59.Lin BR, Chang TC, Lee YC, Lee PH, Chang KJ, Liang JT. Pulmonary resection for colorectal cancer metastases: duration between cancer onset and lung metastasis as an important prognostic factor. Ann Surg Oncol. 2009;16:1026–1032. doi: 10.1245/s10434-008-0286-3. [DOI] [PubMed] [Google Scholar]
- 60.Shirouzu K, Isomoto H, Hayashi A, Nagamatsu Y, Kakegawa T. Surgical treatment for patients with pulmonary metastases after resection of primary colorectal carcinoma. Cancer. 1995;76:393–398. doi: 10.1002/1097-0142(19950801)76:3<393::aid-cncr2820760308>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 61.Inoue M, Kotake Y, Nakagawa K, Fujiwara K, Fukuhara K, Yasumitsu T. Surgery for pulmonary metastases from colorectal carcinoma. Ann Thorac Surg. 2000;70:380–383. doi: 10.1016/s0003-4975(00)01417-x. [DOI] [PubMed] [Google Scholar]
- 62.Loehe F, Kobinger S, Hatz RA, Helmberger T, Loehrs U, Fuerst H. Value of systematic mediastinal lymph node dissection during pulmonary metastasectomy. Ann Thorac Surg. 2001;72:225–229. doi: 10.1016/s0003-4975(01)02615-7. [DOI] [PubMed] [Google Scholar]
- 63.Kanemitsu Y, Kato T, Hirai T, Yasui K. Preoperative probability model for predicting overall survival after resection of pulmonary metastases from colorectal cancer. Br J Surg. 2004;91:112–120. doi: 10.1002/bjs.4370. [DOI] [PubMed] [Google Scholar]
- 64.Melloni G, Doglioni C, Bandiera A, Carretta A, Ciriaco P, Arrigoni G, Zannini P. Prognostic factors and analysis of microsatellite instability in resected pulmonary metastases from colorectal carcinoma. Ann Thorac Surg. 2006;81:2008–2013. doi: 10.1016/j.athoracsur.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 65.Szöke T, Kortner A, Neu R, Grosser C, Sziklavari Z, Wiebe K, Hofmann HS. Is the mediastinal lymphadenectomy during pulmonary metastasectomy of colorectal cancer necessary? Interact Cardiovasc Thorac Surg. 2010;10:694–698. doi: 10.1510/icvts.2009.213173. [DOI] [PubMed] [Google Scholar]
- 66.Welter S, Jacobs J, Krbek T, Poettgen C, Stamatis G. Prognostic impact of lymph node involvement in pulmonary metastases from colorectal cancer. Eur J Cardiothorac Surg. 2007;31:167–172. doi: 10.1016/j.ejcts.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 67.Riquet M, Foucault C, Cazes A, Mitry E, Dujon A, Le Pimpec Barthes F, Médioni J, Rougier P. Pulmonary resection for metastases of colorectal adenocarcinoma. Ann Thorac Surg. 2010;89:375–380. doi: 10.1016/j.athoracsur.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 68.de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 69.Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905–914. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 70.Van Cutsem E, Köhne CH, Láng I, Folprecht G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D, Tejpar S, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011–2019. doi: 10.1200/JCO.2010.33.5091. [DOI] [PubMed] [Google Scholar]
- 71.Bokemeyer C, Bondarenko I, Hartmann JT, de Braud F, Schuch G, Zubel A, Celik I, Schlichting M, Koralewski P. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol. 2011;22:1535–1546. doi: 10.1093/annonc/mdq632. [DOI] [PubMed] [Google Scholar]
- 72.Sakamoto T, Tsubota N, Iwanaga K, Yuki T, Matsuoka H, Yoshimura M. Pulmonary resection for metastases from colorectal cancer. Chest. 2001;119:1069–1072. doi: 10.1378/chest.119.4.1069. [DOI] [PubMed] [Google Scholar]
- 73.McAfee MK, Allen MS, Trastek VF, Ilstrup DM, Deschamps C, Pairolero PC. Colorectal lung metastases: results of surgical excision. Ann Thorac Surg. 1992;53:780–75; discussion 785-6. doi: 10.1016/0003-4975(92)91435-c. [DOI] [PubMed] [Google Scholar]
- 74.Okumura S, Kondo H, Tsuboi M, Nakayama H, Asamura H, Tsuchiya R, Naruke T. Pulmonary resection for metastatic colorectal cancer: experiences with 159 patients. J Thorac Cardiovasc Surg. 1996;112:867–874. doi: 10.1016/S0022-5223(96)70085-5. [DOI] [PubMed] [Google Scholar]
- 75.Girard P, Ducreux M, Baldeyrou P, Rougier P, Le Chevalier T, Bougaran J, Lasser P, Gayet B, Ruffié P, Grunenwald D. Surgery for lung metastases from colorectal cancer: analysis of prognostic factors. J Clin Oncol. 1996;14:2047–2053. doi: 10.1200/JCO.1996.14.7.2047. [DOI] [PubMed] [Google Scholar]
- 76.Yano T, Fukuyama Y, Yokoyama H, Tanaka Y, Miyagi J, Kuninaka S, Asoh H, Ichinose Y. Failure in resection of multiple pulmonary metastases from colorectal cancer. J Am Coll Surg. 1997;185:120–122. doi: 10.1016/s1072-7515(97)00032-x. [DOI] [PubMed] [Google Scholar]
- 77.McCormack PM, Ginsberg RJ. Current management of colorectal metastases to lung. Chest Surg Clin N Am. 1998;8:119–126. [PubMed] [Google Scholar]
- 78.Koga R, Yamamoto J, Saiura A, Yamaguchi T, Hata E, Sakamoto M. Surgical resection of pulmonary metastases from colorectal cancer: Four favourable prognostic factors. Jpn J Clin Oncol. 2006;36:643–648. doi: 10.1093/jjco/hyl076. [DOI] [PubMed] [Google Scholar]
- 79.Rena O, Casadio C, Viano F, Cristofori R, Ruffini E, Filosso PL, Maggi G. Pulmonary resection for metastases from colorectal cancer: factors influencing prognosis. Twenty-year experience. Eur J Cardiothorac Surg. 2002;21:906–912. doi: 10.1016/s1010-7940(02)00088-x. [DOI] [PubMed] [Google Scholar]
- 80.Takakura Y, Miyata Y, Okajima M, Okada M, Ohdan H. Short disease-free interval is a significant risk factor for intrapulmonary recurrence after resection of pulmonary metastases in colorectal cancer. Colorectal Dis. 2010;12:e68–e75. doi: 10.1111/j.1463-1318.2009.02070.x. [DOI] [PubMed] [Google Scholar]
- 81.Borasio P, Gisabella M, Billé A, Righi L, Longo M, Tampellini M, Ardissone F. Role of surgical resection in colorectal lung metastases: analysis of 137 patients. Int J Colorectal Dis. 2011;26:183–190. doi: 10.1007/s00384-010-1075-6. [DOI] [PubMed] [Google Scholar]
- 82.Zink S, Kayser G, Gabius HJ, Kayser K. Survival, disease-free interval, and associated tumor features in patients with colon/rectal carcinomas and their resected intra-pulmonary metastases. Eur J Cardiothorac Surg. 2001;19:908–913. doi: 10.1016/s1010-7940(01)00724-2. [DOI] [PubMed] [Google Scholar]
- 83.Vogelsang H, Haas S, Hierholzer C, Berger U, Siewert JR, Präuer H. Factors influencing survival after resection of pulmonary metastases from colorectal cancer. Br J Surg. 2004;91:1066–1071. doi: 10.1002/bjs.4602. [DOI] [PubMed] [Google Scholar]
- 84.Iizasa T, Suzuki M, Yoshida S, Motohashi S, Yasufuku K, Iyoda A, Shibuya K, Hiroshima K, Nakatani Y, Fujisawa T. Prediction of prognosis and surgical indications for pulmonary metastasectomy from colorectal cancer. Ann Thorac Surg. 2006;82:254–260. doi: 10.1016/j.athoracsur.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 85.Baron O, Amini M, Duveau D, Despins P, Sagan CA, Michaud JL. Surgical resection of pulmonary metastases from colorectal carcinoma. Five-year survival and main prognostic factors. Eur J Cardiothorac Surg. 1996;10:347–351. doi: 10.1016/s1010-7940(96)80093-5. [DOI] [PubMed] [Google Scholar]
- 86.Chen F, Hanaoka N, Sato K, Fujinaga T, Sonobe M, Shoji T, Sakai H, Miyahara R, Bando T, Okubo K, et al. Prognostic factors of pulmonary metastasectomy for colorectal carcinomas. World J Surg. 2009;33:505–511. doi: 10.1007/s00268-008-9875-3. [DOI] [PubMed] [Google Scholar]
- 87.Gutman M, Fidler IJ. Biology of human colon cancer metastasis. World J Surg. 1995;19:226–234. doi: 10.1007/BF00308631. [DOI] [PubMed] [Google Scholar]
- 88.Hammarström S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. 1999;9:67–81. doi: 10.1006/scbi.1998.0119. [DOI] [PubMed] [Google Scholar]
- 89.Regnard JF, Grunenwald D, Spaggiari L, Girard P, Elias D, Ducreux M, Baldeyrou P, Levasseur P. Surgical treatment of hepatic and pulmonary metastases from colorectal cancers. Ann Thorac Surg. 1998;66:214–28; discussion 218-9. doi: 10.1016/s0003-4975(98)00269-0. [DOI] [PubMed] [Google Scholar]
- 90.Lee WS, Yun SH, Chun HK, Lee WY, Yun HR, Kim J, Kim K, Shim YM. Pulmonary resection for metastases from colorectal cancer: prognostic factors and survival. Int J Colorectal Dis. 2007;22:699–704. doi: 10.1007/s00384-006-0218-2. [DOI] [PubMed] [Google Scholar]
- 91.Watanabe K, Nagai K, Kobayashi A, Sugito M, Saito N. Factors influencing survival after complete resection of pulmonary metastases from colorectal cancer. Br J Surg. 2009;96:1058–1065. doi: 10.1002/bjs.6682. [DOI] [PubMed] [Google Scholar]
- 92.Mori M, Tomoda H, Ishida T, Kido A, Shimono R, Matsushima T, Kuwano H, Sugimachi K. Surgical resection of pulmonary metastases from colorectal adenocarcinoma. Special reference to repeated pulmonary resections. Arch Surg. 1991;126:1297–301; discussion 1302. doi: 10.1001/archsurg.1991.01410340139020. [DOI] [PubMed] [Google Scholar]
- 93.Jaklitsch MT, Mery CM, Lukanich JM, Richards WG, Bueno R, Swanson SJ, Mentzer SJ, Davis BD, Allred EN, Sugarbaker DJ. Sequential thoracic metastasectomy prolongs survival by re-establishing local control within the chest. J Thorac Cardiovasc Surg. 2001;121:657–667. doi: 10.1067/mtc.2001.112822. [DOI] [PubMed] [Google Scholar]
- 94.Chen F, Sakai H, Miyahara R, Bando T, Okubo K, Date H. Repeat resection of pulmonary metastasis is beneficial for patients with colorectal carcinoma. World J Surg. 2010;34:2373–2378. doi: 10.1007/s00268-010-0695-x. [DOI] [PubMed] [Google Scholar]
- 95.Salah S, Watanabe K, Park JS, Addasi A, Park JW, Zabaleta J, Ardissone F, Kim J, Riquet M, Nojiri K, et al. Repeated resection of colorectal cancer pulmonary oligometastases: pooled analysis and prognostic assessment. Ann Surg Oncol. 2013;20:1955–1961. doi: 10.1245/s10434-012-2860-y. [DOI] [PubMed] [Google Scholar]
- 96.Pastorino U, Veronesi G, Landoni C, Leon M, Picchio M, Solli PG, Leo F, Spaggiari L, Pelosi G, Bellomi M, et al. Fluorodeoxyglucose positron emission tomography improves preoperative staging of resectable lung metastasis. J Thorac Cardiovasc Surg. 2003;126:1906–1910. doi: 10.1016/s0022-5223(03)00211-3. [DOI] [PubMed] [Google Scholar]
- 97.Suemitsu R, Takeo S, Kusumoto E, Hamatake M, Ikejiri K, Saitsu H. Results of a pulmonary metastasectomy in patients with colorectal cancer. Surg Today. 2011;41:54–59. doi: 10.1007/s00595-009-4244-x. [DOI] [PubMed] [Google Scholar]
- 98.Mongil Poce R, Pagés Navarrete C, Ruiz Navarrete JA, Roca Fernández J, Arrabal Sánchez R, Benítez Doménech A, Fernández de Rota Avecilla A, Fernández Bermúdez JL. [Survival analysis of resection of lung metastases from colorectal cancer] Arch Bronconeumol. 2009;45:235–239. doi: 10.1016/j.arbres.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 99.Castaing D, Vibert E, Ricca L, Azoulay D, Adam R, Gayet B. Oncologic results of laparoscopic versus open hepatectomy for colorectal liver metastases in two specialized centers. Ann Surg. 2009;250:849–855. doi: 10.1097/SLA.0b013e3181bcaf63. [DOI] [PubMed] [Google Scholar]
- 100.Simmonds PC, Primrose JN, Colquitt JL, Garden OJ, Poston GJ, Rees M. Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer. 2006;94:982–999. doi: 10.1038/sj.bjc.6603033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Landes U, Robert J, Perneger T, Mentha G, Ott V, Morel P, Gervaz P. Predicting survival after pulmonary metastasectomy for colorectal cancer: previous liver metastases matter. BMC Surg. 2010;10:17. doi: 10.1186/1471-2482-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nagakura S, Shirai Y, Yamato Y, Yokoyama N, Suda T, Hatakeyama K. Simultaneous detection of colorectal carcinoma liver and lung metastases does not warrant resection. J Am Coll Surg. 2001;193:153–160. doi: 10.1016/s1072-7515(01)00970-x. [DOI] [PubMed] [Google Scholar]
- 103.Hwang MR, Park JW, Kim DY, Chang HJ, Kim SY, Choi HS, Kim MS, Zo JI, Oh JH. Early intrapulmonary recurrence after pulmonary metastasectomy related to colorectal cancer. Ann Thorac Surg. 2010;90:398–404. doi: 10.1016/j.athoracsur.2010.04.058. [DOI] [PubMed] [Google Scholar]
- 104.Zabaleta J, Aguinagalde B, Fuentes MG, Bazterargui N, Izquierdo JM, Hernández CJ, Enriquez-Navascués JM, Emparanza JI. Survival after lung metastasectomy for colorectal cancer: importance of previous liver metastasis as a prognostic factor. Eur J Surg Oncol. 2011;37:786–790. doi: 10.1016/j.ejso.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 105.Smith JW, Fortner JG, Burt M. Resection of hepatic and pulmonary metastases from colorectal cancer. Surg Oncol. 1992;1:399–404. doi: 10.1016/0960-7404(92)90042-j. [DOI] [PubMed] [Google Scholar]
- 106.Gough DB, Donohue JH, Trastek VA, Nagorney DM. Resection of hepatic and pulmonary metastases in patients with colorectal cancer. Br J Surg. 1994;81:94–96. doi: 10.1002/bjs.1800810134. [DOI] [PubMed] [Google Scholar]
- 107.Ambiru S, Miyazaki M, Ito H, Nakagawa K, Shimizu H, Kato A, Nakamura S, Omoto H, Nakajima N. Resection of hepatic and pulmonary metastases in patients with colorectal carcinoma. Cancer. 1998;82:274–278. doi: 10.1002/(sici)1097-0142(19980115)82:2<274::aid-cncr5>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 108.Murata S, Moriya Y, Akasu T, Fujita S, Sugihara K. Resection of both hepatic and pulmonary metastases in patients with colorectal carcinoma. Cancer. 1998;83:1086–1093. doi: 10.1002/(sici)1097-0142(19980915)83:6<1086::aid-cncr6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 109.Lehnert T, Knaebel HP, Dück M, Bülzebruck H, Herfarth C. Sequential hepatic and pulmonary resections for metastatic colorectal cancer. Br J Surg. 1999;86:241–243. doi: 10.1046/j.1365-2168.1999.01010.x. [DOI] [PubMed] [Google Scholar]
- 110.Robinson BJ, Rice TW, Strong SA, Rybicki LA, Blackstone EH. Is resection of pulmonary and hepatic metastases warranted in patients with colorectal cancer? J Thorac Cardiovasc Surg. 1999;117:66–75; discussion 75-6. doi: 10.1016/s0022-5223(99)70470-8. [DOI] [PubMed] [Google Scholar]
- 111.Headrick JR, Miller DL, Nagorney DM, Allen MS, Deschamps C, Trastek VF, Pairolero PC. Surgical treatment of hepatic and pulmonary metastases from colon cancer. Ann Thorac Surg. 2001;71:975–99; discussion 979-80. doi: 10.1016/s0003-4975(00)02522-4. [DOI] [PubMed] [Google Scholar]
- 112.Ike H, Shimada H, Togo S, Yamaguchi S, Ichikawa Y, Tanaka K. Sequential resection of lung metastasis following partial hepatectomy for colorectal cancer. Br J Surg. 2002;89:1164–1168. doi: 10.1046/j.1365-2168.2002.02174.x. [DOI] [PubMed] [Google Scholar]
- 113.Mineo TC, Ambrogi V, Tonini G, Bollero P, Roselli M, Mineo D, Nofroni I. Longterm results after resection of simultaneous and sequential lung and liver metastases from colorectal carcinoma. J Am Coll Surg. 2003;197:386–391. doi: 10.1016/S1072-7515(03)00387-9. [DOI] [PubMed] [Google Scholar]
- 114.Barlow AD, Nakas A, Pattenden C, Martin-Ucar AE, Dennison AR, Berry DP, Lloyd DM, Robertson GS, Waller DA. Surgical treatment of combined hepatic and pulmonary colorectal cancer metastases. Eur J Surg Oncol. 2009;35:307–312. doi: 10.1016/j.ejso.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 115.Joosten J, Bertholet J, Keemers-Gels M, Barendregt W, Ruers T. Pulmonary resection of colorectal metastases in patients with or without a history of hepatic metastases. Eur J Surg Oncol. 2008;34:895–899. doi: 10.1016/j.ejso.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 116.Kim SH, Choi SJ, Park JS, Lee J, Cho YB, Kang MW, Lee WY, Choi YS, Kim HK, Han J, et al. Tropism between hepatic and pulmonary metastases in colorectal cancers. Oncol Rep. 2012;28:459–464. doi: 10.3892/or.2012.1837. [DOI] [PubMed] [Google Scholar]
- 117.Treasure T, Fallowfield L, Lees B. Pulmonary metastasectomy in colorectal cancer: the PulMiCC trial. J Thorac Oncol. 2010;5:S203–S206. doi: 10.1097/JTO.0b013e3181dca239. [DOI] [PubMed] [Google Scholar]


