Abstract
There is wide variation in the management of coagulation and blood transfusion practice in liver transplantation. The use of blood products intraoperatively is declining and transfusion free transplantations take place ever more frequently. Allogenic blood products have been shown to increase morbidity and mortality. Primary haemostasis, coagulation and fibrinolysis are altered by liver disease. This, combined with intraoperative disturbances of coagulation, increases the risk of bleeding. Meanwhile, the rebalancing of coagulation homeostasis can put patients at risk of hypercoagulability and thrombosis. The application of the principles of patient blood management to transplantation can reduce the risk of transfusion. This includes: preoperative recognition and treatment of anaemia, reduction of perioperative blood loss and the use of restrictive haemoglobin based transfusion triggers. The use of point of care coagulation monitoring using whole blood viscoelastic testing provides a picture of the complete coagulation process by which to guide and direct coagulation management. Pharmacological methods to reduce blood loss include the use of anti-fibrinolytic drugs to reduce fibrinolysis, and rarely, the use of recombinant factor VIIa. Factor concentrates are increasingly used; fibrinogen concentrates to improve clot strength and stability, and prothrombin complex concentrates to improve thrombin generation. Non-pharmacological methods to reduce blood loss include surgical utilisation of the piggyback technique and maintenance of a low central venous pressure. The use of intraoperative cell salvage and normovolaemic haemodilution reduces allogenic blood transfusion. Further research into methods of decreasing blood loss and alternatives to blood transfusion remains necessary to continue to improve outcomes after transplantation.
Keywords: Liver disease, Transplantation, Coagulation, Transfusion, Patient blood management, Thromboelastography, Cell salvage
Core tip: Liver transplantation was historically associated with major blood loss. Over the years, improvements in both surgical and anaesthetic management have made transfusion free transplantation an increasingly attainable reality. Research into the complex nature of the coagulopathy of liver disease, has led to the concept that the haemostatic profile is “re-balanced” in these patients, and that stable patients do not have an inherent bleeding diathesis, but rather a reduced reserve, and can be readily tipped towards a bleeding or thrombotic tendency. This review article discusses the various approaches that can be taken to adopt the principles of patient blood management in these patients.
INTRODUCTION
There is wide variation between individual centres’ approach to the management of coagulation and blood transfusion for orthotopic liver transplantation (OLT). Transfusion free transplantation is a goal that is difficult to achieve, yet has been shown to be increasingly attainable over recent years. Some studies have reported rates as high as 79.6% of transplants being undertaken without transfusion[1]. However, these results have been difficult to replicate in other centres. Surgical technique and transfusion triggers vary between institutions, as do methods of monitoring coagulation during transplantation.
It is well known that coagulopathy and catastrophic bleeding can accompany OLT. Strategies to manage the coagulopathy and bleeding have been designed to reduce the necessity to transfuse blood during these operations[2]. These include pharmacological and non-pharmacological methods, as well as coagulation monitoring and treatment protocols.
Avoidance of blood transfusion has survival benefits for patients, but also cost implications for the healthcare system, as well as preserving this limited and precious resource only for when it is truly necessary. Patient blood management (PBM) is defined as the timely application of evidence-based medical and surgical concepts designed to maintain haemoglobin (Hb) concentrations, optimise haemostasis and minimise blood loss in an effort to improve patient outcome[3]. The World Health Organisation has adopted this paradigm to improve transfusion safety[4].
The first pillar of PBM is the recognition and treatment of anaemia, with the optimisation of red cell mass. Anaemia is common in chronic liver disease. It is multifactorial in its aetiology, including: folate deficiency, hypersplenism, haemodilution, haemolysis, bone marrow suppression due to viruses or ethanol, renal insufficiency and variceal bleeding[5].
The second pillar of PBM is to minimise blood loss and bleeding. This article shall discuss the identification of those most at risk of bleeding and the various strategies that can be employed to minimise that risk including anaesthetic blood conserving strategies, the use of pharmacological agents, autologous blood options and surgical technique.
The third pillar involves the harnessing and optimisation of tolerance of anaemia. This includes the use of restrictive transfusion strategies, which have been shown to have no detrimental impact upon outcome in critically ill patients. A threshold Hb concentration for transfusion of 70 g/L has been advocated from several trials and between 80-90 g/L for those at a higher risk of adverse effects of anaemia[6,7]. Optimisation of cardiac output, ventilation and oxygenation can help to improve the tolerance of lower concentrations of Hb.
CONSEQUENCES OF TRANSFUSION
The use of blood products during OLT increases morbidity and mortality[1]. Multiple studies have shown the intraoperative transfusion of red blood cells (RBCs) to be a major predictor of post-operative mortality[1,8,9]. Massive transfusion (greater than six units of RBCs) during surgery has been shown to reduce survival rates amongst patients - at six months 63.8% vs 83.3% and at five years 34.5% vs 49.2%[2]. However, transfusion requirements have also been considered a surrogate for sicker, higher risk patients, and more complex surgery, potentially confounding its role in outcome[10].
The generic risks of blood transfusion remain high. These include: transfusion related immunomodulation (TRIM) due to accumulation of non-specific soluble immune mediators in stored blood; transfusion associated circulatory overload causing acute left ventricular or congestive cardiac failure; transfusion associated acute lung injury (TRALI); haemolytic transfusion reactions (both immediate and delayed), acute non-haemolytic transfusion reactions (febrile, allergic or both in nature), transfusion associated dyspnoea, post transfusion purpura, transfusion-associated graft versus host disease and transfusion transmitted infection (bacterial, viral and prion) (Table 1).
Table 1.
Risks of transfusion
| General risks | |
| Transfusion related immunomodulation | Accumulation of immune mediators in stored blood |
| Transfusion associated circulatory overload | Acute left ventricular failure or congestive cardiac failure |
| Transfusion related acute lung injury | Capillary leak and neutrophil extravasation and activation caused by: Immune mediated: Donor antibodies react with recipient white blood cells, forming leukoagglutinate that become trapped in the lung Non-immune mediated: Endothelium suffers initial insult (e.g., sepsis, surgery or trauma), attracting neutrophils that are activated by biologically active compounds in stored blood |
| Haemolytic transfusion reactions | Immediate: Donor membrane antigens react with antibodies against these present in recipient plasma |
| Delayed: Alloimmunised recipient with specific antibodies respond to re-exposure to antigen positive red blood cells | |
| Acute non haemolytic transfusion reactions | Febrile: Donor leucocyte antigens react with recipient white cell antibodies |
| Allergic: Soluble donor antigens react in an already sensitised recipient | |
| Post-transfusion purpura | Previous sensitisation produces antibodies which attach donor platelet antigens and additionally destroy their own platelets |
| Transfusion associated graft vs host disease | Donor lymphocytes proliferate within immunocompromised recipient, attacking host cells as ‘‘foreign’’ |
| Infection | Bacterial 1:2000-1:500000 |
| Viral: hepatitis B 1:450000; hepatitis C 1:32000000; HIV 1:5000000; human T-cell leukaemia virus 1:12500000 | |
HIV: Human immunodeficiency virus; RBC: Red blood cells.
The exact mechanism linking transfusion and poor outcomes after OLT is unknown. TRIM has been linked to reduced rejection rates in renal transplantation, but is implicated in increased cancer recurrence and bacterial infection. After OLT, increased rates of infection and hepatic artery thrombosis have been associated with RBC transfusions[11]. The risk of infection increases in a dose dependent manner by 7% per unit of RBCs transfused[12]. Residual amounts of donor leucocytes in the transfused blood, human leucocyte antigen peptides, bioactive lipids and preservation related changes to RBCs are implicated in poor outcome[13]. Universal leukoreduction of blood has helped to reduce this risk.
Higher intraoperative RBC transfusion requirements are associated with higher re-intervention rates, despite being matched for preoperative Child-Pugh classification and clotting profile. In general increased transfusion requirements for bleeding lead to higher proportions of patients requiring re-exploration for bleeding and evacuation of haematoma, with increased rates of anastomotic leakage. Thereafter patients who undergo re-intervention have three times higher mortality than those who do not have re-interventions[14]. Length of stay has been widely demonstrated to increase with RBC transfusion[12].
All blood products [RBCs, fresh frozen plasma (FFP) and platelets] have been shown to be negatively associated with graft survival at 1 and 5 years by univariate analysis[15]. FFP and platelets are associated with higher levels of TRALI compared with RBCs post OLT. However, the rates of TRALI are much lower in post OLT patients[12].
COAGULOPATHY
Bleeding during OLT is multifactorial, due both to surgical trauma and to haemostatic defects. The coagulopathy of chronic liver disease is present pre-operatively and further disturbance of coagulation can occur intraoperatively, resulting in bleeding complications, but also thrombotic events.
In chronic liver disease, all procoagulant factors are decreased, except factor VIII and Von Willebrand factor (vWF), which increase. Levels of the endogenous anticoagulant factors, antithrombin and protein C fall, as well as nitric oxide and prostacyclin increasing. Levels of tissue plasminogen activator (t-PA) and plasminogen activator inhibitor (PAI-1) re-equilibrate[16]. This rebalancing of procoagulant and anticoagulant factors require coagulation tests that demonstrate the net result of these changes to give a true picture of the coagulation system in liver disease. Prothrombin time (PT) tests do not utilise thrombomodulin, a transmembrane protein on vascular endothelial cells that down regulates thrombin generation. This is the main physiological activator of protein C. By failing to measure the anticoagulant factors’ effect on thrombin generation, and just the effect of procoagulant factors, this balance of coagulation is inaccurately measured by PT, thereby misrepresenting the risk of haemorrhage[17].
Platelet function is also affected by liver disease and thrombocytopaenia is common. It is multifactorial in aetiology, including hypersplenism secondary to portal hypertension, decreased thrombopoietin synthesis, immune complex associated platelet clearance and reticuloendothelial destruction[18].
The increased level of vWF in liver disease promotes platelet aggregation to the endothelium. ADAMTS-13 (vWF cleaving protease), which limits vWF function, is reduced in liver disease; thus up regulating vWF activity, increasing platelet activity. This has been demonstrated to preserve thrombin generation in platelet counts as low as 60 × 109/L, equivalent to the lower limit of normal in healthy subjects[17].
Platelet function was traditionally assessed by bleeding time (BT). There is a poor association between platelet count and BT however, and a prolonged BT can be seen in patients with platelet counts > 100 × 109/L and vice versa[19]. Since platelet activation is not diminished but may actually be increased in some patients with cirrhosis, it is possible that BT prolongation in these patients is also a result of the changes in vasoreactivity and /or arterial dysfunction, which are well documented in cirrhosis. Platelet function tests such as the platelet function analyser (PFA-100) or multiplate may give a more objective measure, but data in this group of patients is lacking.
During the dissection phase of the transplant, excessive bleeding is substantially related to the degree of difficulty experienced during the surgical dissection, and the presence of portal hypertension, with large dilated collateral vessels. Surgical bleeding results from this coagulopathy, increased portal hypertension and oesophageal-gastric venous distension caused by compression and vascular clamping[16].
During the anhepatic phase there is reduced coagulation factor synthesis and clearance. Enhanced fibrinolytic activity occurs due mainly to lack of tPA clearance whilst levels of PAI-1 remain relatively unchanged, increasing the likelihood of fibrinolysis developing.
During reperfusion, profound coagulation abnormalities are common, due to the ‘‘heparin like effect’’ (HLE)[20], platelet entrapment in the sinusoids of the donor liver, a global reduction of all coagulation factors, decreased PAI-1 and antifibrinolytic factors, with simultaneous generation of tPA. Fibrinolysis is normally carefully balanced by pro- and anti-fibrinolytic factors, of which levels are also disturbed by liver disease. Fibrinolysis is rarely seen in acute liver failure (ALF) due to high levels of PAI-1. Post reperfusion, some patients have accelerated release of t-PA from the graft endothelium, causing hyperfibrinolysis[21]. Usually hyperfibrinolysis resolves within an hour post reperfusion, but if the graft is marginal or functions poorly it may persist. The use of anti-fibrinolytic drugs can improve this state. Post operatively, thrombocytopaenia, due to consumption of platelets in the new liver, is counteracted by their activation, risking hypercoagulability[22].
Thus, primary haemostasis, coagulation and fibrinolysis are altered by liver disease. Previously it was thought that the balance lay towards a bleeding tendency as evidenced by abnormal conventional tests of anticoagulation. However, the low levels of pro-coagulant factors are to some extent “re-balanced” by the reduced levels of anticoagulant factors, so that thrombin generation remains normal or even enhanced, which can lead to a prothrombotic state in some patients[23]. In addition, thrombocytopenia is partially compensated for by the high levels of vWF with reduced ADAMTS-13. It has been demonstrated that over 15% of OLT recipients are hypercoagulable on baseline viscoelastic testing in theatre[24]. This effect is significantly increased in patients with cholestatic disease such as primary biliary cirrhosis and primary sclerosing cholangitis (Table 2).
Table 2.
Alterations in both anti- and pro- haemostatic dynamics in liver disease
| Haemostasis | Anti-haemostatic | Pro-haemostatic |
| Primary haemostasis (platelet-vessel wall interaction) | Thrombocytopaenia | Elevated levels of Von Willebrand factor |
| Platelet function defects | Decreased levels of ADAMTS-13 | |
| Increased nitric oxide and prostacyclin | Platelet hyper reactivity | |
| Secondary haemostasis (thrombin generation and inhibition) | Low levels of factors II, V, VII, IX, X, XI | Increased factor VIII |
| Vitamin K deficiency | Decreased protein C, S, AT-III, alpha 2 macroglobulin, heparin co-factor II | |
| Fibrinolysis | Low levels of alpha 2 anti-plasmin, factor XIII, thrombin activatable fibrinolysis inhibitor | Low levels of plasminogen |
| Elevated tissue plasminogen activator | High levels of plasminogen activator inhibitor | |
| Dysfibrinogenaemia |
Given the reductions of elements on both sides, there is a lower haemostatic reserve, leading the balance to be easily tipped towards bleeding or thrombosis (Table 3).
Table 3.
Coagulopathy during transplantation
| Stage | Coagulation abnormalities increasing bleeding | Other risk factors for bleeding | TEG |
| Dissection | Thrombocytopaenia Platelet function defects Increased nitric oxide and prostacyclin Low levels of factors II, V, VII, IX, X, XI Vitamin K deficiency Low levels of alpha 2 anti-plasmin, factor XIII, thrombin activatable fibrinolysis inhibitor Elevated t-PA Dysfibrinogenaemia | Surgical technical difficulty Portal hypertension Oesophago-gastric venous distension secondary to compression and vascular clamping | Prolonged R time Decreased alpha-angle Reduced MA |
| Anhepatic | Reduced coagulation factor synthesis Reduced clearance of t-PA | Duration greater than 45 min | Increased lysis |
| Reperfusion | ‘‘Heparin like effect’’ Platelet entrapment in sinusoids of donor liver Reduction of all coagulation factors Decreased PAI-1 Decreased antifibrinolytic factors Hyper-fibrinolysis | Acidosis Hypothermia | Virtually ‘‘flat’’ native trace with prolonged R time and significantly reduced MA Heparinase trace required Lysis |
| Post reperfusion | Accelerated t-PA release Thrombocytopaenia (balanced by increased activation) | Delayed graft function | MA reduced |
TEG: Thromboelastography; MA: Maximum amplitude; t-PA: Tissue plasminogen activator; PAI-1: Plasminogen activator inhibitor.
RISK FACTORS FOR TRANSFUSION
Pre-operative risk factors for transfusion
Several variables have been identified as predictors of tranσfusion during OLT. These include recipient factors, donor organ and surgical variables. Some authors have debated the predictive ability of preoperative variables to correctly predict transfusion[25], even in homogenous populations, recommending that individual centres evaluate centre-specific risk factors for transfusion[26,27]. However, truly predictive models of blood transfusion requirements are difficult to create[28]. One such model is the McKlusky risk index for massive transfusion consisting of seven variables: age > 40 years, Hb concentration < 100 g/L, INR > 2.0, platelet count < 70 × 109, creatinine (> 100 μmol/L for females, > 120 μmol/L for males) and albumin < 24 g/L; as well as repeat transplantation[29].
The severity of the patient’s liver disease has frequently been associated with increased risk of blood transfusion[8,16,30]. However, one series, with a mean model of end-stage liver disease (MELD) score of 22 (± 10) showed no significant difference in transfusion rates between patients with high scores. It should be noted that the mean transfusion requirement in that population was only 0.5 (± 1.3 units RBC), and therefore may not be relevant to other transplant centres that have a higher mean blood loss[31]. Nonetheless, a MELD score of 25 or above indicates severe disease, and clinicians should be alert to the increased risk of transfusion in these patients. As a component of the MELD score it is unsurprising that bilirubin has been identified in one study as a direct predictor of transfusion[8].
In many studies, Hb concentration has been shown to predict transfusion requirements[16,27-29,31,32]. Anaemic patients remain at the greatest risk of blood transfusion. Thrombocytopaenia can also increase the risk of transfusion[33]. In one study, platelet count was removed from a predictive model on multivariate regression, but PT was included as a predictive variable[28]. A low baseline fibrinogen concentration can contribute to bleeding risk[16,30]. The quantity of fibrin degradation products has also been ascribed to an increased risk of transfusion[27]. Given that the maximum amplitude (MA)/maximum clot firmness (MCF) on viscoelastic tests is reflective of the clot strength resulting from the interaction of platelets and fibrinogen, those patients with a low MA (< 40 mm) on baseline are a high risk for bleeding[34]. A low MA and low baseline fibrinogen concentration increases the risk of fibrinolysis[35].
Recipient age is implicated in predicting transfusion[28-30]. Those recipients over 50 form the highest risk group. Donor age has also been implicated as a predictive risk for massive transfusion[2].
Intraoperative risk factors for transfusion
Factors that increase the technical difficulty of the operation include previous abdominal surgery, which has been identified an independent risk factor of blood transfusion[27], as well as increasing the duration of surgery, which in itself can be a predictor of transfusion[30]. Inflammatory adhesions can develop following previous surgery, increasing the bleeding risk. Another variable influencing the duration of surgery is the experience of the transplantation team, which has a direct effect upon blood loss during surgery, particularly during the dissection phase. Factors that increase the technical difficulty of the operation, such as portal vein thrombosis, increase the risk of bleeding[36].
A hyperdynamic circulation and portal hypertension are significant contributors to the bleeding risk. Collateral vessels that develop secondary to portal hypertension, including from the surface of the liver, can be injured, increasing bleeding. Patients with cirrhosis and portal hypertension have an altered response to volume loading. Blood pools in the splanchnic circulation, responding to volume loading by a greater magnitude than in the central and arterial circulation. The resultant increase in portal venous pressure from volume loading can lead to increased bleeding. Cardiac output is less responsive to volume loading than in healthy individuals[1,37].
Haemodilution during the operation secondary to fluid replacement with crystalloid and colloid solutions, and the preservation solution from the donor liver, further reduce the plasma concentration of clotting factors. As the coagulation factors are already at reduced levels, a dilutional coagulopathy can develop much more rapidly than in the healthy individual. Cautious fluid replacement is essential to both minimise haemodilution of coagulation factors and to reduce portal venous pressure increases. Strategies to reduce portal pressure have included the use of phlebotomy to reduce central venous pressure and portal pressure, using vasopressors to maintain mean arterial blood pressure[1,10].
Surgical bleeding can be brisk secondary to vascular injury, or diffuse, continuous microvascular bleeding. Damaged endothelial cells release t-PA, increasing fibrinolysis[1,8,9,16]. Portal vein hypoplasia and decreased donor liver size, making the surgery more technically challenging have been associated with transfusion requirements. Raw edges of partial liver grafts are left exposed, therefore at risk of postoperative bleeding.
Acidosis, hypothermia, hypocalcaemia, and citrate toxicity can all contribute to the coagulopathy intraoperatively. The length of cold ischaemic time can affect short-term graft dysfunction. Inadequate graft-recipient body weight ratio and poor graft preservation increase bleeding tendency. Poor quality grafts proportionally increase the risk of primary non-function according to the degree of steatosis. Dysfunctional grafts lead to a delayed production and restoration of clotting factors, prolonging massive bleeding. Marginal grafts, such as those donated after cardiac death (DCD) increase the risk of fibrinolysis post reperfusion[1,38,39].
Renal failure
Acute renal failure (ARF) is common in patients with cirrhosis, and occurs in over half of patients with ALF[40]. This can be part of multi-organ failure in the critically ill, the hepatorenal syndrome or secondary to complications of cirrhosis such as bacterial peritonitis, sepsis, hypovolaemia secondary to diuretics or gastrointestinal bleeding, and nephrotoxic drugs[41]. In patients referred for OLT, 10%-25% have acute kidney injury (AKI), and 12%-70% develop AKI in the post operative period[42].
Uraemia is associated with bleeding, particularly due to platelet dysfunction due to decreased platelet aggregation and adhesion. This can be related to intrinsic defects within the platelet, including glycoprotein IIb/IIIa dysfunction, or due to extrinsic factors. Extrinsic factors include uraemic toxins and increased nitric oxide synthesis. Renal replacement therapy (RRT) can improve platelet function by removing uraemic toxins.
It has been shown that a considerable proportion of patients with both acute and chronic liver failure are hypercoagulable despite deranged laboratory clotting times and thrombocytopaenia. Passage of blood through continuous RRT (CRRT) circuits leads to thrombin formation and platelet activation, predominantly due to leucocyte and platelet activation. A combination of increased tissue factor and microparticle release, endothelial activation and a reduction in natural anticoagulants leads to clotting of CRRT circuits in ALF. The duration of CRRT circuit life is unrelated to worsening laboratory clotting parameters or thrombocytopaenia in liver failure, and anticoagulation of these circuits should be implemented to improve their lifespan[43]. Anticoagulation can be implemented with prostacyclin, heparin, or citrate.
Heparinisation of patients receiving CRRT, particularly in OLT has been associated with increased bleeding. Prostacyclin is an alternative, however it can cause vasodilatation and hypotension. Regional anticoagulation with citrated circuits has been shown to be feasible and safe in patients undergoing OLT, despite the risk of failure to metabolise citrate by the liver[44].
MEASURES TO REDUCE BLEEDING AND TRANSFUSION
Preoperative optimisation of red cell mass
Experience from liver transplantation in Jehovah’s Witness patients who refuse to receive blood products due to religious beliefs has provided evidence of the benefit of pre-operative management to reduce blood transfusion. Strategies have included the use of recombinant human erythropoietin (rEPO), iron and folic acid to increase the red cell mass.
The use of iron has been found beneficial in patients with iron deficiency microcytic anaemia, and those with functional iron deficiency, who in spite of total body iron stores being normal, have a reduction of iron available for metabolic processes[16,45].
Erythropoietin is produced in the kidney, stimulating erythrogenesis. Erythropoiesis is seen within 3 d, and the equivalent of 1 unit of blood produced in 7 d, and five units within 28 d. This can be associated with functional iron deficiency and iron supplementation is recommended for patients undergoing rEPO therapy[17,45]. One centre used rEPO 20000 U subcutaneously twice a week or 40000 U once a week preoperatively until the haematocrit reached 45% in Jehovah’s Witness patients awaiting OLT[46]. However, this treatment is accompanied by a significant risk of thrombotic events and must be undertaken with great caution.
Anaemia itself can increase the risk of bleeding. Anaemia can worsen the hyper dynamic circulation, as found in portal hypertension, thus increasing the bleeding risk. It has been shown that RBCs have an active role in thrombin generation. RBCs release adenosine diphosphate, which promotes platelet aggregation and stimulates platelet synthesis of thromboxane-A2, a platelet activator, thus reducing platelet activation in anaemia[5,17].
Management of coagulopathy
The management of bleeding and coagulopathy varies greatly between different centres. This is partially due to patient population and surgical technique and experience. However, the methods used to monitor coagulopathy, or the failure to do so, the thresholds for transfusion, particularly FFP and the use of cell salvage and anti-fibrinolytic therapy account for much of the variation[47,48]. Given the coagulopathy expected during OLT, and the detrimental outcomes of transfusion, the management of this coagulopathy to limit blood loss is central.
Conventional coagulation tests (CCTs) such as activated partial thromboplastin time (APTT), prothrombin time/international normalised ratio (PT/INR) poorly reflect the whole coagulation system of the blood, unable to assess the procoagulant-anticoagulant balance. The platelet count is quantitative, unable to detect platelet function, or dysfunction. CCTs are unable to detect fibrinolysis or give an indication of clot stability, nor can they generally detect hypercoagulability[16,49].
PT/INR and APTT are sensitive to deficiencies of pro-coagulant factors, but not the concomitant reduction of anti-coagulant factors found in liver disease. In addition, these tests are based upon plasma alone; so do not reflect the complete interaction between platelets, vascular endothelium, and fibrinolytic factors producing haemostasis. CCTs are also limited by the length of time from sampling to providing a result to the clinician. Bedside point-of-care testing (POCT) PT/INR measurements can be made using devices such as the Haemochron® (ITC, Edison, United States).
The alterations of haemostasis require global assessments of coagulation, such as thrombin generation assays, and also whole blood testing, such as thromboelastography (TEG) and rotational thromboelastometry (ROTEM). These POCT devices offer a rapid diagnostic bedside test to aid the clinician in directing therapeutic interventions. It has been shown that POCT based coagulation management can reduce perioperative blood loss and blood product transfusion rates[22]. The use of perioperative coagulation monitoring using TEG/ROTEM for targeted management of coagulopathy in OLT now forms part of the European Society of Anaesthesia (ESA) guidelines for the management of massive bleeding[36,38].
TEG and ROTEM assess the viscoelastic properties of whole blood, giving a global assessment of haemostatic function from clot formation through to clot retraction and fibrinolysis. Different reagents and activators are available, for TEG and ROTEM including kaolin, the standard activator for TEG assays. Other reagents include platelet-mapping assays, and platelet inhibitors that allow the fibrinogen component of clot strength to be measured individually. Heparinase containing cups reverse heparin and heparinoids, allowing detection of the HLE and the action of administered heparin, including low molecular weight heparin. Aprotinin and calcium containing cuvettes allow the repaid detection of fibrinolysis to guide antifibrinolytic agent usage[19,50] (Figure 1, Table 4).
Figure 1.
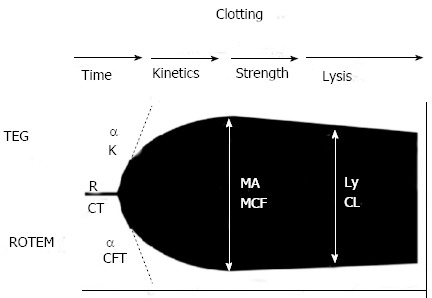
Standard tracing of viscoelastic tests - thromboelastography/rotational thromboelastometry. TEG: Thromboelastography; ROTEM: Rotational thromboelastometry; MA: Maximum amplitude; MCF: Maximum clot firmness; R: Reaction time; CT: Clotting time; CFT: Clot formation time.
Table 4.
Parameters of viscoelastic tests - thromboelastography/rotational thromboelastometry
| Parameter | TEG® | ROTEM® | Description |
| Clotting time | R | CT | Relates to concentration of soluble clotting factors in the plasma, the period of initial fibrin formation (time to reach 2 mm amplitude on the tracing) |
| Clot kinetics | K (K value) (min) | CFT | Measure the kinetics of clot formation |
| Measures the speed to reach a specific level of clot strength (period for amplitude to increase from 2 to 20 mm) | |||
| Alpha angle (angle in degrees) (°) | Alpha | Measures the rate of clot formation, reflects rate of fibrin build up and cross-linking (angle between a tangent to the tracing at 2 mm amplitude and the horizontal midline) | |
| Clot strength | MA (mm) | MCF | Represents the ultimate strength of the clot (platelets and fibrin), maximum dynamic properties of fibrin and platelet bonding via GPIIb/IIIa receptors (greatest vertical width of tracing) |
| Clot stability | LY30 | CLI | Relates to clot stability and lysis, measures the rate of amplitude reduction from MA at 30 min (in per cent) |
TEG: Thromboelastography; ROTEM: Rotational thromboelastometry; R: Reaction time; CT: Clotting time; CFT: Clot formation time; MCF: Maximum clot firmness; CLI: Clot lysis index; MA: Maximum amplitude; MCF: Maximum clot firmness; LY30: Lysis at 30 min as a ratio of MA.
As yet, the need for clear consensus guidelines to guide transfusion practice, coagulation monitoring and optimal transfusion triggers for OLT has not been met[51]. TEG based transfusion algorithms have been reported since Kang et al[52] in 1985[26]. Since then, TEG/ROTEM based algorithms guiding blood product transfusions have evolved[35]. TEG/ROTEM based transfusion and coagulation management algorithms reduce transfusion requirements and costs by optimising the management of bleeding, differentiating between micro-vascular and surgical bleeding, enabling haemostatic therapy to be finely targeted. The TEG/ROTEM can also be used to monitor for fibrinolysis and to guide anti-fibrinolytic therapy. A small, randomised prospective study in OLT patients showed a significant reduction in transfusion in the TEG monitored group, most notably in the use of FFP. The trigger threshold for FFP was reached much more frequently using conventional INR values compared to R values on TEG[32].
It should be noted however, that transfusion trigger thresholds described for viscoelastic tests have not been validated and large controlled clinical trials comparing strategies of coagulation management and cut off values for transfusion of blood product components are still needed.
Pharmacological methods
A systematic review of 33 trials investigated: pharmacological interventions, blood substitutes, TEG and cardiovascular interventions[53]. Only aprotinin, recombinant factor VIIa and TEG potentially reduced blood loss and transfusion requirements. However, all trials showed a high risk of bias and risk of random errors and the authors concluded that further trials were necessary to potentially lower blood loss and transfusion requirements.
Antifibrinolytics
Antifibrinolytic therapy reduces blood loss and transfusion[54]. Antifibrinolytic drugs are recommended for the treatment of fibrinolysis evidenced by microvascular oozing or TEG/ROTEM clot lysis measurement, (CLI < 15). The timing and degree of fibrinolysis is relevant since lesser degrees of fibrinolysis post reperfusion may resolve spontaneously. In one retrospective review of over 600 transplants, 60% developed hyperfibrinolysis, yet only 40% required antifibrinolytic therapy[35]. In that series, prophylactic antifibrinolytic therapy was only administered to patients with a significantly reduced MCF (< 30 m) at baseline. In the early years of OLT, routine use of prophylactic antifibrinolytic agents was common, since the mortality associated with massive blood loss was high, and the risk associated with antifibrinolytic drugs was small in comparison. Now that massive haemorrhage is less frequent, antifibrinolytics are no longer recommended for routine prophylaxis[38].
Prediction of hyperfibrinolysis is difficult since it may only occur in the post reperfusion stage, being dependent to a great extent on the quality of the donor liver, and not predictable from the preoperative condition of the recipient.
Aprotinin has been shown to potentially reduce blood loss and transfusion requirements[53]. However, it is no longer available having been withdrawn from the market due to safety concerns. Lysine analogues, such as tranexamic acid have been shown to have a lower risk of death when compared to aprotinin[38].
Tranexamic acid competitively inhibits the activation of plasminogen to plasmin, preventing plasmin from degrading fibrin. E-aminocaproic acid (EACA) is also used widely. Meta analyses have shown both tranexamic acid and aprotinin to reduce RBC transfusion during OLT[55]. Various dosing regimens have been used, and it is unknown what the lowest effective dose is. Currently tranexamic acid is usually given in 1-2 g increments. The response to antifibrinolytic agents should be monitored using TEG/ROTEM to guide further doses.
Fibrinogen concentrates
Hypofibrinogenaemia has been shown to influence blood product requirements. A baseline MA of < 35 mm at the beginning of transplant and measured fibrin degradation products > 48 mg/L has been demonstrated to lead to hyper-fibrinolysis in 100% of patients[56]. When haemodilution and massive bleeding occur, fibrinogen is the first factor to reach critical levels[57].
The use of fibrinogen concentrate for significant bleeding if accompanied by (at least suspected) low fibrinogen concentrations or function is advocated. Fibrinogen concentrates have been shown to optimise coagulation, reduce perioperative bleeding and significantly reduce transfusion. A fibrinogen concentration above 2 g/L has been shown to be the minimum concentration at which clot formation normalises[58]. A concentration of < 1.5-2 g/L increases haemorrhagic tendency, so this, or signs of functional fibrinogen deficit on TEG or ROTEM should be triggers for use[38]. Fibrinogen supplementation may also compensate for defects in fibrin cross polymerisation and also for low platelet counts, helping to reduce bleeding. Noval-Padillo et al[34] conducted an observational study comparing fibrinogen concentrate administration to maintain an acceptable MCF with a historical cohort from the previous year at that centre. In the study group, 45% of patients received fibrinogen. Transfusion of RBCs, FFP and platelets was reduced by over 50% and OLT without transfusion rose from 3.5% to 20%.
Fibrinogen concentrates rather than cryoprecipitate provide standardised fibrinogen doses, which can be quickly reconstituted. The risk of pathogen transmission and immune mediated complications are reduced, and cryoprecipitate is now only indicated when there is a lack of available fibrinogen concentrate for the treatment of bleeding and hypofibrinogenaemia[16,38].
Recombinant factor VIIa
Recombinant factor VIIa (rFVIIa) improves haemostasis by directly activating factor X, precipitating the conversion of prothrombin to thrombin to form a haemostatic clot. rFVIIa binds to the surface of activated platelets at sites of vascular injury, increasing localised thrombin generation. Meta-analysis and systematic reviews of the use of rFVIIa in hepatic surgery (including transplantation) failed to show a benefit in the number of blood transfusions yet showed a significant increase in the incidence of arterial thrombotic events[59-61]. The ESA guidelines for massive bleeding in visceral and transplant surgery echo this with recommendation against the prophylactic use of rFVIIa, reserving its use only as rescue therapy for uncontrolled bleeding[38].
Prothrombin complex concentrate
Prothrombin Complex Concentrates (PCC) are purified coagulation concentrates from pooled plasma, after removal of antithrombin and factor XI, with approximately twenty-five times higher concentrations of clotting factors. They allow the correction of coagulation using small volumes. PCCs undergo viral reduction or elimination. Most contain the vitamin K dependent clotting factors II, VII, IX and X, and the coagulation inhibitors protein C and S. Reported risks include thromboembolic events such as venous thromboembolism, acute myocardial infarction and disseminated intravascular coagulation. However, the formulation of currently available PCCs differ from those previously available, which did not routinely contain anticoagulants, as well as routinely being used in haemophilia patients. Current evidence suggests that even in high-risk patients, PCCs are safe and that thromboembolic events are rare[62]. A randomised controlled trial (the PROTON trial) studying PCCs effect on RBC transfusion requirements in OLT is currently in progress[63].
FFP is often transfused in order to correct a deranged INR. The exponential relationship of coagulation factors on PT/INR is not always appreciated. It has been shown that FFP is unable to contribute sufficient coagulation factors to correct PT/INR by 50% in most cases, even for mildly prolonged PT/INRs[64]. The INR varies between laboratories in patients with liver disease, making a defined cut-off of risk difficult. The R/CT on TEG/ROTEM may therefore be a better reflection of true bleeding potential than INR. Prolongation of the R/CT in the absence of excess anticoagulants is only usually seen once the procoagulant levels are less than the haemostatic level of 30%[65].
There is no evidence for administering prophylactic FFP based upon INR[19]. Mild to moderate INR elevation should not be corrected with FFP and in ALF INR should not be corrected before invasive procedures except for intracranial pressure monitor insertion[38].
Protamine and the heparin like effect
The appearance of a heparin effect on TEG at reperfusion was first described by Kang et al[52]. A marked HLE on the TEG at the time of reperfusion is common and is due to both exogenous heparins administered to the donor, and the release of endogenous heparinoids from the vascular endothelium and activated macrophages due to ischaemia/reperfusion injury. It does not appear to contribute significantly to bleeding risk and is usually a temporary phenomenon unless graft function is poor[66]. Reversal with protamine is rarely indicated. Native TEG is extremely sensitive to heparin and endogenous heparin can be detected in some patients even prior to reperfusion[67]. It is recommended to run a heparinase modified TEG in parallel with the native TEG during OLT. With the more activated Kaolin TEG and INTEM (intrinsic ROTEM), it is unusual to identify a significant heparin effect, except at reperfusion and an additional heparinase TEG or HEPTEM (heparinase modified thromboelastometry) test is helpful to distinguish between a coagulation factor deficiency and a heparin produced prolongation of the R or CT. An empirical dose of 50 mg of protamine is often administered if the decision to treat this is made.
Platelets
Intraoperative platelet transfusions have been identified as a strong independent risk factor for survival after OLT, with a greater hazard ratio than RBCs transfused, particularly for TRALI[15]. Platelets contain many cytokines and vasoactive and inflammatory mediators, which are released upon activation after reperfusion. Animal models have demonstrated platelets to be involved in the pathogenesis of reperfusion injury of the liver graft by inducing endothelial cell apoptosis[36]. It has been shown that thrombin generation is maintained in liver disease despite quantitative thrombocytopaenia. Thus, care must be exercised when transfusing platelets to avoid hypercoagulability and thrombosis.
Clot strength (MA/MCF) is a composite reflection of platelet-fibrinogen interaction. Rather than combining the total effect of changes in all coagulation parameters, CCTs provide single values in isolation, not always predictive of a bleeding tendency. Viscoelastic tests demonstrate that even in the presence of a low platelet count, adequate clot strength is still achieved if fibrinogen levels are at the upper end of normal, or raised. A low platelet count combined with a low fibrinogen always leads to a reduced MA/MCF and is strongly associated with an increased bleeding tendency[68].
Non-pharmacological measures
Strategies including acute normovolaemic haemodilution and the use of autologous cell salvage alongside appropriate surgical and anaesthetic management have been employed to reduce allogenic blood transfusion requirements for OLT. Restrictive fluid replacement during the dissection phase, maintaining decreased venous pressure in the surgical field lowers intravascular volume in the portal collaterals. This is contrary to the historical practice of fluid loading to maintain arterial blood pressure and renal perfusion.
Surgical technique
The piggyback technique is recognised to reduce blood transfusion requirements[37]. The piggyback technique permits a hypovolaemic state to be better tolerated. This involves anastomosing the donor retro hepatic vena cava directly to the recipient inferior vena cava instead of two end-to-end anastomoses in the classic technique. Reduced dissection of the peri-caval anatomy can also reduce bleeding from portal collaterals in those with portal hypertension. This is said to improve physiological variables of temperature, cardiac output, tissue perfusion, gas exchange, acid-base statues and fluid-electrolyte balance[16].
Low central venous pressure
In hepatic resection surgery a low central venous pressure (CVP) and restrictive fluid administration are shown to decrease bleeding. A CVP of less than 5 cm H2O is advocated to reduce blood loss from hepatic veins. However, the risks of a low CVP include cardiovascular instability and air embolism[69]. For OLT, a low CVP is thought to reduce bleeding and transfusion by a similar mechanism, reducing portal venous bleeding[53]. Indeed Massicotte et al[1] state that their low transfusion rates and post-operative Hb concentrations are made possible by keeping the CVP low.
However, caval clamping coupled with hypovolaemia can precipitate severe haemodynamic instability. Hypovolaemia presents other risks to the patient associated with adverse outcomes including ARF, air embolism and systemic tissue hypoperfusion. Thus maintenance of low venous pressures must be balanced against adequate perfusion of the organs. The use of terlipressin has been investigated, demonstrating reduce portal venous pressure whilst maintaining renal perfusion[70]. However, further studies are required to evaluate its safety in terms of splanchnic perfusion and post transplantation portal venous blood flow. Other methods to minimise pre-load and potentially reduce the portal pressure include using lower tidal volumes (6-8 mL/kg) and avoiding positive end-expiratory pressure[71].
Intraoperative cell salvage
The use of autologous cell salvaged blood can reduce the need for allogenic blood transfusion. A prospective survey involving 150 consecutive OLT’s demonstrated that cell salvage saved 21 g/L of Hb, or two units of RBCs[72].
RBCs are salvaged from the operative field using a double lumen suction device, adding anticoagulants to the aspirated blood. Citrate anticoagulation is used to avoid heparin accumulation. This blood is stored in a reservoir, before being centrifuged to separate out the components. The RBCs are washed and filtered, removing biochemical debris including free Hb, platelets, white blood cells, plasma and heparin. RBCs can haemolyse during suctioning into the cell saver, haemolysing due to shear stress. The salvaged RBCs are suspended in normal saline, with a haematocrit of 50%-70% for re-transfusion, and 200 mL of cell-salvaged RBCs equate to 1 unit of blood. Since the re-transfused washed RBCs from cell salvage provide no plasma, clotting factors or platelets, this must be factored in when assessing the need for replacement haemostatic therapy.
Transfusion of potentially harmful fat-micro emboli, free Hb, denatured proteins and platelet-leukocyte microaggreagates have been postulated as causes of disseminated intravascular coagulopathy (DIC), acute respiratory distress syndrome and ARF in some case reports. However, other studies have failed to show a significant increase in these complications[45].
Cell salvage has been controversial in OLT due to conflicting results of some historical studies. Many of these results are from older trials and are disputed in the current literature[72]. Higher rates of blood loss and increased cost have been reported[10]. Cell salvage can be set up in a ‘‘stand-by’’ manner to reduce costs; using only a double lumen suction catheter, with anticoagulant solution and a sterile reservoir, with centrifugation only if sufficient blood is collected. Increased blood loss been ascribed to the release of fibrinolytic compounds from RBCs in the collected blood or from the transplanted liver that are not washed out by the cell saver[36].
Re-transfused blood can be potentially harmful due to the presence of other substances aspirated from the surgical field including bacteria and malignant cells. Bacterial contamination can arise from blood borne bacteria, from the skin upon aspiration, or from blood from the bile duct. Aspirated blood should not be returned to the patient once the bile duct anastomosis is underway intraoperatively. However, studies have shown that despite aspiration of microbiologically contaminated blood, there is no increase in positive blood cultures or postoperative infection, despite the washing phase being unable to eliminate all bacteria[45].
Malignant cells are not washed from cell-salvaged blood and cell salvage has been contraindicated in the presence of malignant disease due to the theoretical risk of metastasis. However, studies of patients with malignant disease who received autologous transfusions did not increase recurrence rates whilst reducing allogenic blood requirements. Given the risk of allogenic blood transfusion worsening outcome after cancer surgery, including immunomodulation, cell salvage is used in some cancer surgeries, including OLT for hepatocellular carcinoma. This risk is greatly reduced by suctioning blood from non-ruptured tumours during the operation. Leukocyte depletion filters are used to reduce the number of malignant cells in the recovered blood[73]. ESA guidelines suggest that the decision to use salvaged blood potentially contaminated with bacteria or malignant cells should be on a case by case basis[38].
Intraoperative normovolaemic haemodilution
Intraoperative normovolaemic haemodilution involves venesection of the patient, and volume replaced with other fluids. The venesected blood is then re-transfused intra and post operatively as required, usually post reperfusion. This preserves the integrity of the venesected RBCs and clotting factors, and provides a reserve of whole blood to be transfused as required. Contraindications include coronary heart disease, significant anaemia and severe pulmonary hypertension[36]. In practice, most patients are too anaemic to make venesection a viable option.
CONCLUSION
The transfusion practices of liver transplantation have been transformed since the early experiences of this operation[74]. Transplantation without transfusion has become an increasingly attainable goal. Our knowledge of the coagulopathy of liver disease has been redrawn over recent years. Liver transplantation presents a significant risk of coagulopathy and bleeding. This is multifactorial in nature, with recipient and donor factors, surgical and anaesthetic techniques playing a role. The benefits of effectively monitoring and treating coagulopathy and using techniques to reduce bleeding have been demonstrated to reduce transfusion requirements and improve outcomes after OLT. This has been aided by the wider availability of POCT to monitor and correct severe coagulopathy with factor concentrates instead of blood products.
Transfusion and coagulation management in liver transplantation needs to adopt the three pillars of patient blood management to improve upon its achievements thus far. Preoperative optimisation of red cell mass can reduce transfusion. Peri-operative coagulation management using restrictive transfusion strategies and clotting factor algorithms based upon TEG have been shown to be beneficial. The ESA guidelines on the management of severe bleeding advocate fluid restriction, phlebotomy, vasopressors and transfusion protocols to achieve this[38].
It should not be forgotten that blood transfusion can be lifesaving and preparations made to give blood and blood products when required during all transplantations. The known and unknown risks of transfusion are extensive and varied. If practice can be improved to avoid unnecessary transfusions, outcomes from liver transplantation will continue to improve.
Footnotes
P- Reviewers: Frenette C, Juntermanns B S- Editor: Zhai HH L- Editor: A E- Editor: Wu HL
References
- 1.Massicotte L, Denault AY, Beaulieu D, Thibeault L, Hevesi Z, Nozza A, Lapointe R, Roy A. Transfusion rate for 500 consecutive liver transplantations: experience of one liver transplantation center. Transplantation. 2012;93:1276–1281. doi: 10.1097/TP.0b013e318250fc25. [DOI] [PubMed] [Google Scholar]
- 2.Boin IF, Leonardi MI, Luzo AC, Cardoso AR, Caruy CA, Leonardi LS. Intraoperative massive transfusion decreases survival after liver transplantation. Transplant Proc. 2008;40:789–791. doi: 10.1016/j.transproceed.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 3.Shander A, Hofmann A, Isbister J, Van Aken H. Patient blood management--the new frontier. Best Pract Res Clin Anaesthesiol. 2013;27:5–10. doi: 10.1016/j.bpa.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Spahn DR, Theusinger OM, Hofmann A. Patient blood management is a win-win: a wake-up call. Br J Anaesth. 2012;108:889–892. doi: 10.1093/bja/aes166. [DOI] [PubMed] [Google Scholar]
- 5.Thachil J. Anemia--the overlooked factor in bleeding related to liver disease. J Hepatol. 2011;54:593–594; author reply 594-595. doi: 10.1016/j.jhep.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 6.Hébert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 7.McIntyre L, Tinmouth AT, Fergusson DA. Blood component transfusion in critically ill patients. Curr Opin Crit Care. 2013;19:326–333. doi: 10.1097/MCC.0b013e3283632e56. [DOI] [PubMed] [Google Scholar]
- 8.Rana A, Petrowsky H, Hong JC, Agopian VG, Kaldas FM, Farmer D, Yersiz H, Hiatt JR, Busuttil RW. Blood transfusion requirement during liver transplantation is an important risk factor for mortality. J Am Coll Surg. 2013;216:902–907. doi: 10.1016/j.jamcollsurg.2012.12.047. [DOI] [PubMed] [Google Scholar]
- 9.Xia VW, Du B, Braunfeld M, Neelakanta G, Hu KQ, Nourmand H, Levin P, Enriquez R, Hiatt JR, Ghobrial RM, et al. Preoperative characteristics and intraoperative transfusion and vasopressor requirements in patients with low vs. high MELD scores. Liver Transpl. 2006;12:614–620. doi: 10.1002/lt.20679. [DOI] [PubMed] [Google Scholar]
- 10.de Boer MT, Molenaar IQ, Hendriks HG, Slooff MJ, Porte RJ. Minimizing blood loss in liver transplantation: progress through research and evolution of techniques. Dig Surg. 2005;22:265–275. doi: 10.1159/000088056. [DOI] [PubMed] [Google Scholar]
- 11.Dunn LK, Thiele RH, Ma JZ, Sawyer RG, Nemergut EC. Duration of red blood cell storage and outcomes following orthotopic liver transplantation. Liver Transpl. 2012;18:475–481. doi: 10.1002/lt.23379. [DOI] [PubMed] [Google Scholar]
- 12.Benson AB, Burton JR, Austin GL, Biggins SW, Zimmerman MA, Kam I, Mandell S, Silliman CC, Rosen H, Moss M. Differential effects of plasma and red blood cell transfusions on acute lung injury and infection risk following liver transplantation. Liver Transpl. 2011;17:149–158. doi: 10.1002/lt.22212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azevedo LD, Stucchi RS, Ataíde EC, Boin IF. Assessment of causes of early death after twenty years of liver transplantation. Transplant Proc. 2013;45:1116–1118. doi: 10.1016/j.transproceed.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Hendriks HG, van der Meer J, de Wolf JT, Peeters PM, Porte RJ, de Jong K, Lip H, Post WJ, Slooff MJ. Intraoperative blood transfusion requirement is the main determinant of early surgical re-intervention after orthotopic liver transplantation. Transpl Int. 2005;17:673–679. doi: 10.1007/s00147-004-0793-5. [DOI] [PubMed] [Google Scholar]
- 15.de Boer MT, Christensen MC, Asmussen M, van der Hilst CS, Hendriks HG, Slooff MJ, Porte RJ. The impact of intraoperative transfusion of platelets and red blood cells on survival after liver transplantation. Anesth Analg. 2008;106:32–44. doi: 10.1213/01.ane.0000289638.26666.ed. [DOI] [PubMed] [Google Scholar]
- 16.Sabate A, Dalmau A, Koo M, Aparicio I, Costa M, Contreras L. Coagulopathy management in liver transplantation. Transplant Proc. 2012;44:1523–1525. doi: 10.1016/j.transproceed.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med. 2011;365:147–156. doi: 10.1056/NEJMra1011170. [DOI] [PubMed] [Google Scholar]
- 18.Giannini EG, Savarino V. Thrombocytopenia in liver disease. Curr Opin Hematol. 2008;15:473–480. doi: 10.1097/MOH.0b013e32830a9746. [DOI] [PubMed] [Google Scholar]
- 19.Mallett SV, Chowdary P, Burroughs AK. Clinical utility of viscoelastic tests of coagulation in patients with liver disease. Liver Int. 2013;33:961–974. doi: 10.1111/liv.12158. [DOI] [PubMed] [Google Scholar]
- 20.Agarwal S, Senzolo M, Melikian C, Burroughs A, Mallett SV. The prevalence of a heparin-like effect shown on the thromboelastograph in patients undergoing liver transplantation. Liver Transpl. 2008;14:855–860. doi: 10.1002/lt.21437. [DOI] [PubMed] [Google Scholar]
- 21.Porte RJ, Bontempo FA, Knot EA, Lewis JH, Kang YG, Starzl TE. Systemic effects of tissue plasminogen activator-associated fibrinolysis and its relation to thrombin generation in orthotopic liver transplantation. Transplantation. 1989;47:978–984. doi: 10.1097/00007890-198906000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agarwal A, Sharma N, Vij V. Point-of-care coagulation monitoring during liver transplantation. Trends in Anaesth and Crit Care. 2013;3:42–48. [Google Scholar]
- 23.Gatt A, Riddell A, Calvaruso V, Tuddenham EG, Makris M, Burroughs AK. Enhanced thrombin generation in patients with cirrhosis-induced coagulopathy. J Thromb Haemost. 2010;8:1994–2000. doi: 10.1111/j.1538-7836.2010.03937.x. [DOI] [PubMed] [Google Scholar]
- 24.Krzanicki D, Sugavanam A, Mallett S. Intraoperative hypercoagulability during liver transplantation as demonstrated by thromboelastography. Liver Transpl. 2013;19:852–861. doi: 10.1002/lt.23668. [DOI] [PubMed] [Google Scholar]
- 25.Roullet S, Biais M, Millas E, Revel P, Quinart A, Sztark F. Risk factors for bleeding and transfusion during orthotopic liver transplantation. Ann Fr Anesth Reanim. 2011;30:349–352. doi: 10.1016/j.annfar.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Findlay JY, Rettke SR. Poor prediction of blood transfusion requirements in adult liver transplantations from preoperative variables. J Clin Anesth. 2000;12:319–323. doi: 10.1016/s0952-8180(00)00162-8. [DOI] [PubMed] [Google Scholar]
- 27.Steib A, Freys G, Lehmann C, Meyer C, Mahoudeau G. Intraoperative blood losses and transfusion requirements during adult liver transplantation remain difficult to predict. Can J Anaesth. 2001;48:1075–1079. doi: 10.1007/BF03020372. [DOI] [PubMed] [Google Scholar]
- 28.Araújo T, Cordeiro A, Proença P, Perdigoto R, Martins A, Barroso E. Predictive variables affecting transfusion requirements in orthotopic liver transplantation. Transplant Proc. 2010;42:1758–1759. doi: 10.1016/j.transproceed.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 29.McCluskey SA, Karkouti K, Wijeysundera DN, Kakizawa K, Ghannam M, Hamdy A, Grant D, Levy G. Derivation of a risk index for the prediction of massive blood transfusion in liver transplantation. Liver Transpl. 2006;12:1584–1593. doi: 10.1002/lt.20868. [DOI] [PubMed] [Google Scholar]
- 30.Mangus RS, Kinsella SB, Nobari MM, Fridell JA, Vianna RM, Ward ES, Nobari R, Tector AJ. Predictors of blood product use in orthotopic liver transplantation using the piggyback hepatectomy technique. Transplant Proc. 2007;39:3207–3213. doi: 10.1016/j.transproceed.2007.09.029. [DOI] [PubMed] [Google Scholar]
- 31.Massicotte L, Beaulieu D, Roy JD, Marleau D, Vandenbroucke F, Dagenais M, Lapointe R, Roy A. MELD score and blood product requirements during liver transplantation: no link. Transplantation. 2009;87:1689–1694. doi: 10.1097/TP.0b013e3181a5e5f1. [DOI] [PubMed] [Google Scholar]
- 32.Wang SC, Shieh JF, Chang KY, Chu YC, Liu CS, Loong CC, Chan KH, Mandell S, Tsou MY. Thromboelastography-guided transfusion decreases intraoperative blood transfusion during orthotopic liver transplantation: randomized clinical trial. Transplant Proc. 2010;42:2590–2593. doi: 10.1016/j.transproceed.2010.05.144. [DOI] [PubMed] [Google Scholar]
- 33.Deakin M, Gunson BK, Dunn JA, McMaster P, Tisone G, Warwick J, Buckels JA. Factors influencing blood transfusion during adult liver transplantation. Ann R Coll Surg Engl. 1993;75:339–344. [PMC free article] [PubMed] [Google Scholar]
- 34.Noval-Padillo JA, León-Justel A, Mellado-Miras P, Porras-Lopez F, Villegas-Duque D, Gomez-Bravo MA, Guerrero JM. Introduction of fibrinogen in the treatment of hemostatic disorders during orthotopic liver transplantation: implications in the use of allogenic blood. Transplant Proc. 2010;42:2973–2974. doi: 10.1016/j.transproceed.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Görlinger K. Coagulation management during liver transplantation. Hamostaseologie. 2006;26:S64–S76. [PubMed] [Google Scholar]
- 36.Feltracco P, Brezzi M, Barbieri S, Galligioni H, Milevoj M, Carollo C, Ori C. Blood loss, predictors of bleeding, transfusion practice and strategies of blood cell salvaging during liver transplantation. World J Hepatol. 2013;5:1–15. doi: 10.4254/wjh.v5.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ozier Y, Tsou MY. Changing trends in transfusion practice in liver transplantation. Curr Opin Organ Transplant. 2008;13:304–309. doi: 10.1097/MOT.0b013e3282faa0dd. [DOI] [PubMed] [Google Scholar]
- 38.Kozek-Langenecker SA, Afshari A, Albaladejo P, Santullano CA, De Robertis E, Filipescu DC, Fries D, Görlinger K, Haas T, Imberger G, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2013;30:270–382. doi: 10.1097/EJA.0b013e32835f4d5b. [DOI] [PubMed] [Google Scholar]
- 39.Broomhead RH, Patel S, Fernando B, O’Beirne J, Mallett S. Resource implications of expanding the use of donation after circulatory determination of death in liver transplantation. Liver Transpl. 2012;18:771–778. doi: 10.1002/lt.23406. [DOI] [PubMed] [Google Scholar]
- 40.Agarwal B, Gatt A, Riddell A, Wright G, Chowdary P, Jalan R, Burroughs AK, Davenport A. Hemostasis in patients with acute kidney injury secondary to acute liver failure. Kidney Int. 2013;84:158–163. doi: 10.1038/ki.2013.92. [DOI] [PubMed] [Google Scholar]
- 41.Betrosian AP, Agarwal B, Douzinas EE. Acute renal dysfunction in liver diseases. World J Gastroenterol. 2007;13:5552–5559. doi: 10.3748/wjg.v13.i42.5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matuszkiewicz-Rowińska J, Małyszko J, Wieliczko M. Renal support during liver transplantation: when to consider it? Transplant Proc. 2013;45:3157–3162. doi: 10.1016/j.transproceed.2013.08.071. [DOI] [PubMed] [Google Scholar]
- 43.Agarwal B, Shaw S, Shankar Hari M, Burroughs AK, Davenport A. Continuous renal replacement therapy (CRRT) in patients with liver disease: is circuit life different? J Hepatol. 2009;51:504–509. doi: 10.1016/j.jhep.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 44.Saner FH, Treckmann JW, Geis A, Lösch C, Witzke O, Canbay A, Herget-Rosenthal S, Kribben A, Paul A, Feldkamp T. Efficacy and safety of regional citrate anticoagulation in liver transplant patients requiring post-operative renal replacement therapy. Nephrol Dial Transplant. 2012;27:1651–1657. doi: 10.1093/ndt/gfr510. [DOI] [PubMed] [Google Scholar]
- 45.Darwish A. Liver transplant in Jehovah’s Witnesses patients. Curr Opin Organ Transplant. 2011;16:326–330. doi: 10.1097/MOT.0b013e328346dd36. [DOI] [PubMed] [Google Scholar]
- 46.Jabbour N, Gagandeep S, Mateo R, Sher L, Genyk Y, Selby R. Transfusion free surgery: single institution experience of 27 consecutive liver transplants in Jehovah’s Witnesses. J Am Coll Surg. 2005;201:412–417. doi: 10.1016/j.jamcollsurg.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 47.Coakley M, Reddy K, Mackie I, Mallett S. Transfusion triggers in orthotopic liver transplantation: a comparison of the thromboelastometry analyzer, the thromboelastogram, and conventional coagulation tests. J Cardiothorac Vasc Anesth. 2006;20:548–553. doi: 10.1053/j.jvca.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 48.Schumann R. Intraoperative resource utilization in anesthesia for liver transplantation in the United States: a survey. Anesth Analg. 2003;97:21–28, table of contents. doi: 10.1213/01.ane.0000068483.91464.2b. [DOI] [PubMed] [Google Scholar]
- 49.Meybohm P, Zacharowski K, Weber CF. Point-of-care coagulation management in intensive care medicine. Crit Care. 2013;17:218. doi: 10.1186/cc12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Srivastava A, Kelleher A. Point-of-care coagulation testing. Contin Ed Anaesth, Crit Care Pain. 2013;13:12–16. [Google Scholar]
- 51.Lopez-Plaza I. Transfusion guidelines and liver transplantation: time for consensus. Liver Transpl. 2007;13:1630–1632. doi: 10.1002/lt.21225. [DOI] [PubMed] [Google Scholar]
- 52.Kang YG, Martin DJ, Marquez J, Lewis JH, Bontempo FA, Shaw BW, Starzl TE, Winter PM. Intraoperative changes in blood coagulation and thrombelastographic monitoring in liver transplantation. Anesth Analg. 1985;64:888–896. [PMC free article] [PubMed] [Google Scholar]
- 53.Gurusamy KS, Pissanou T, Pikhart H, Vaughan J, Burroughs AK, Davidson BR. Methods to decrease blood loss and transfusion requirements for liver transplantation. Cochrane Database Syst Rev. 2011;(12):CD009052. doi: 10.1002/14651858.CD009052.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Henry DA, Carless PA, Moxey AJ, O’Connell D, Stokes BJ, Fergusson DA, Ker K. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2011;(3):CD001886. doi: 10.1002/14651858.CD001886.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Molenaar IQ, Warnaar N, Groen H, Tenvergert EM, Slooff MJ, Porte RJ. Efficacy and safety of antifibrinolytic drugs in liver transplantation: a systematic review and meta-analysis. Am J Transplant. 2007;7:185–194. doi: 10.1111/j.1600-6143.2006.01591.x. [DOI] [PubMed] [Google Scholar]
- 56.Steib A, Gengenwin N, Freys G, Boudjema K, Levy S, Otteni JC. Predictive factors of hyperfibrinolytic activity during liver transplantation in cirrhotic patients. Br J Anaesth. 1994;73:645–648. doi: 10.1093/bja/73.5.645. [DOI] [PubMed] [Google Scholar]
- 57.Hiippala ST, Myllylä GJ, Vahtera EM. Hemostatic factors and replacement of major blood loss with plasma-poor red cell concentrates. Anesth Analg. 1995;81:360–365. doi: 10.1097/00000539-199508000-00026. [DOI] [PubMed] [Google Scholar]
- 58.Bolliger D, Szlam F, Molinaro RJ, Rahe-Meyer N, Levy JH, Tanaka KA. Finding the optimal concentration range for fibrinogen replacement after severe haemodilution: an in vitro model. Br J Anaesth. 2009;102:793–799. doi: 10.1093/bja/aep098. [DOI] [PubMed] [Google Scholar]
- 59.Chavez-Tapia NC, Alfaro-Lara R, Tellez-Avila F, Barrientos-Gutiérrez T, González-Chon O, Mendez-Sanchez N, Uribe M. Prophylactic activated recombinant factor VII in liver resection and liver transplantation: systematic review and meta-analysis. PLoS One. 2011;6:e22581. doi: 10.1371/journal.pone.0022581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yank V, Tuohy CV, Logan AC, Bravata DM, Staudenmayer K, Eisenhut R, Sundaram V, McMahon D, Olkin I, McDonald KM, et al. Systematic review: benefits and harms of in-hospital use of recombinant factor VIIa for off-label indications. Ann Intern Med. 2011;154:529–540. doi: 10.7326/0003-4819-154-8-201104190-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Levi M, Levy JH, Andersen HF, Truloff D. Safety of recombinant activated factor VII in randomized clinical trials. N Engl J Med. 2010;363:1791–1800. doi: 10.1056/NEJMoa1006221. [DOI] [PubMed] [Google Scholar]
- 62.Franchini M, Lippi G. Prothrombin complex concentrates: an update. Blood Transfus. 2010;8:149–154. doi: 10.2450/2010.0149-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arshad F, Ickx B, van Beem RT, Polak W, Grüne F, Nevens F, Ilmakunnas M, Koivusalo AM, Isoniemi H, Strengers PF, et al. Prothrombin complex concentrate in the reduction of blood loss during orthotopic liver transplantation: PROTON-trial. BMC Surg. 2013;13:22. doi: 10.1186/1471-2482-13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abdel-Wahab OI, Healy B, Dzik WH. Effect of fresh-frozen plasma transfusion on prothrombin time and bleeding in patients with mild coagulation abnormalities. Transfusion. 2006;46:1279–1285. doi: 10.1111/j.1537-2995.2006.00891.x. [DOI] [PubMed] [Google Scholar]
- 65.Fries D, Haas T, Klingler A, Streif W, Klima G, Martini J, Wagner-Berger H, Innerhofer P. Efficacy of fibrinogen and prothrombin complex concentrate used to reverse dilutional coagulopathy--a porcine model. Br J Anaesth. 2006;97:460–467. doi: 10.1093/bja/ael191. [DOI] [PubMed] [Google Scholar]
- 66.Harding SA, Mallett SV, Peachey TD, Cox DJ. Use of heparinase modified thrombelastography in liver transplantation. Br J Anaesth. 1997;78:175–179. doi: 10.1093/bja/78.2.175. [DOI] [PubMed] [Google Scholar]
- 67.Kettner SC, Gonano C, Seebach F, Sitzwohl C, Acimovic S, Stark J, Schellongowski A, Blaicher A, Felfernig M, Zimpfer M. Endogenous heparin-like substances significantly impair coagulation in patients undergoing orthotopic liver transplantation. Anesth Analg. 1998;86:691–695. doi: 10.1097/00000539-199804000-00002. [DOI] [PubMed] [Google Scholar]
- 68.Görlinger K, Dirkmann D, Hanke AA, Kamler M, Kottenberg E, Thielmann M, Jakob H, Peters J. First-line therapy with coagulation factor concentrates combined with point-of-care coagulation testing is associated with decreased allogeneic blood transfusion in cardiovascular surgery: a retrospective, single-center cohort study. Anesthesiology. 2011;115:1179–1191. doi: 10.1097/ALN.0b013e31823497dd. [DOI] [PubMed] [Google Scholar]
- 69.Hartog A, Mills G. Anaesthesia for hepatic resection surgery. Contin Ed Anaesth, Crit Care Pain. 2009;9:1–5. [Google Scholar]
- 70.Mukhtar A, Salah M, Aboulfetouh F, Obayah G, Samy M, Hassanien A, Bahaa M, Abdelaal A, Fathy M, Saeed H, et al. The use of terlipressin during living donor liver transplantation: Effects on systemic and splanchnic hemodynamics and renal function. Crit Care Med. 2011;39:1329–1334. doi: 10.1097/CCM.0b013e3182120842. [DOI] [PubMed] [Google Scholar]
- 71.Hannaman MJ, Hevesi ZG. Anesthesia care for liver transplantation. Transplant Rev (Orlando) 2011;25:36–43. doi: 10.1016/j.trre.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 72.Massicotte L, Thibeault L, Beaulieu D, Roy JD, Roy A. Evaluation of cell salvage autotransfusion utility during liver transplantation. HPB (Oxford) 2007;9:52–57. doi: 10.1080/13651820601090596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liang TB, Li DL, Liang L, Li JJ, Bai XL, Yu W, Wang WL, Shen Y, Zhang M, Zheng SS. Intraoperative blood salvage during liver transplantation in patients with hepatocellular carcinoma: efficiency of leukocyte depletion filters in the removal of tumor cells. Transplantation. 2008;85:863–869. doi: 10.1097/TP.0b013e3181671f2e. [DOI] [PubMed] [Google Scholar]
- 74.Starzl TE, Iwatsuki S, Van Thiel DH, Gartner JC, Zitelli BJ, Malatack JJ, Schade RR, Shaw BW, Hakala TR, Rosenthal JT, et al. Evolution of liver transplantation. Hepatology. 1982;2:614–636. doi: 10.1002/hep.1840020516. [DOI] [PMC free article] [PubMed] [Google Scholar]


