Abstract
Chronic hepatitis B (CHB) virus infection is a global public health problem, affecting more than 400 million people worldwide. The clinical spectrum is wide, ranging from a subclinical inactive carrier state, to progressive chronic hepatitis, cirrhosis, decompensation, and hepatocellular carcinoma. However, complications of hepatitis B virus (HBV)-related chronic liver disease may be reduced by viral suppression. Current international guidelines recommend first-line treatment of CHB infection with pegylated interferon, entecavir, or tenofovir, but the optimal treatment for an individual patient is controversial. The indications for treatment are contentious, and increasing evidence suggests that HBV genotyping, as well as serial on-treatment measurements of hepatitis B surface antigen and HBV DNA kinetics should be used to predict antiviral treatment response. The likelihood of achieving a sustained virological response is also increased by extending treatment duration, and using combination therapy. Hence the paradigm for treatment of CHB is constantly evolving. This article summarizes the different indications for treatment, and systematically reviews the evidence for the efficacy of various antiviral agents. It further discusses the shortcomings of current guidelines, use of rescue therapy in drug-resistant strains of HBV, and highlights the promising clinical trials for emerging therapies in the pipeline. This concise overview presents an updated practical approach to guide the clinical management of CHB.
Keywords: Chronic hepatitis B virus infection, National institute for health and care excellence, Treatment guidelines, Interferon, Pegylated interferon, Nucleos(t)ide analogues, Antiviral resistance, Rescue therapy, Clinical trials
Core tip: This article summarizes the different indications for treatment, and systematically reviews the evidence for the efficacy of various antiviral agents. It further discusses the shortcomings of current guidelines, use of rescue therapy in drug-resistant strains of hepatitis B virus, and highlights the promising clinical trials for emerging therapies in the pipeline. This concise overview presents an updated practical approach to guide the clinical management of chronic hepatitis B.
INTRODUCTION
An estimated 400 million people worldwide have chronic hepatitis B virus (HBV) infection, and more than 750000 deaths are attributed annually to HBV-related complications[1,2]. HBV carriers are not only predisposed to developing liver cirrhosis and hepatic decompensation, but also have a 100-fold increased risk of developing hepatocellular carcinoma (HCC)[3,4]. Hence early diagnosis and treatment of chronic hepatitis B (CHB) infection is crucial for reducing morbidity and mortality.
Management is guided by recommendations from the American Association for the Society of Liver Disease[5], Asian Pacific Association for the Study of Liver (APASL)[6], European Association for the Study of Liver[7], and the National Institute for Health and Care Excellence (NICE)[8]. Broadly, there are two different treatment strategies for patients with CHB infection: therapies of finite duration using immunomodulators such as standard or pegylated interferon-α, as well as long-term treatment with the nucleos(t)ide analogues lamivudine, adefovir dipivoxil, entecavir, telbivudine, or tenofovir. Increasing rates of resistance to antiviral therapy however necessitates consideration of combination therapy, and research into novel treatments. The objective of this review is to provide an update on major advances in the field, addressing controversial areas of uncertainty to aid clinicians in selecting an appropriate therapeutic strategy.
LIFE CYCLE AND NATURAL HISTORY OF HBV
HBV is a small, partially double-stranded DNA virus that belongs to the family Hepadnaviridae[9]. The virion is comprised of a core particle containing the viral genome, nucleocapsid protein and polymerase, as well as a lipoprotein envelope composed of viral antigens. Broadly, HBV is classified into four serotypes (adr, adw, ayr and ayw) based on antigenic determinants of the hepatitis B surface antigen (HBsAg), and eight genotypes (A to H) based on its nucleotide sequence. The genotypes have distinct geographic distributions, and an increasing body of evidence suggests it may also influence disease severity and response to treatment (Table 1)[10].
Table 1.
Geographic distribution of hepatitis B virus genotypes
| Genotypes | Geographic distribution | Tendency of chronicity | Clinical outcome |
| A | Europe, United States | Higher | Better |
| B | Eastern Asia | Lower | Better |
| C | Eastern Asia | Higher | Worse |
| D | Southern Europe, North Africa, Middle East, Indian Sub-Continent | Lower | Worse |
| E | Sub-Saharan Africa | - | - |
| F | South America | - | - |
| G | Europe, United States | - | - |
| H | Central America | - | - |
The replication cycle of HBV begins with viral entry into hepatocytes, mediated by the binding of the pre-S1 region on the virion envelope to the cellular sodium taurocholate cotransporting polypeptide[11]. The virion is then uncoated, and transported into the nucleus. From a drug discovery point of view, two key events occur. One is the formation of covalently closed circular DNA (cccDNA) through covalent ligation[12]. This DNA intermediate is responsible for viral persistence, and is highly resistant to antiviral therapy. The second key event is viral genome replication by reverse transcription via pre-genomic RNA. The reverse transcriptase is inherently error prone, and is ultimately responsible for the emergence of nucleos(t)ide-resistant HBV quasispecies. The mature nucleocapsids may subsequently be recycled into the nucleus to mediate viral persistence, or secreted through exocytosis as Dane particles to infect other hepatocytes (Figure 1)[13]. A greater understanding of the viral cycle of HBV will enable new therapeutic strategies.
Figure 1.
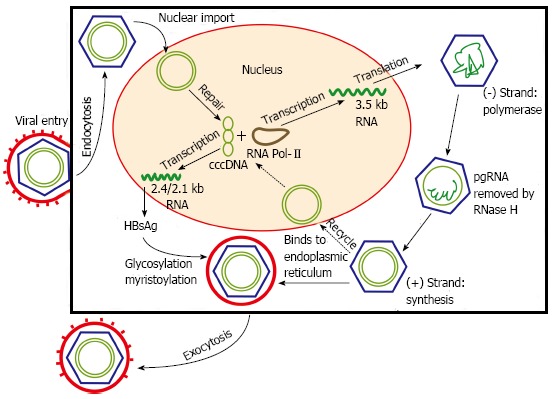
Hepatitis B viral replication cycle. The hepatitis B virus virion enters the hepatocyte via endocytosis. Viral nucleocapsids are uncoated and transported into the nucleus, where viral DNA is transformed into covalently closed circular DNA (cccDNA). Replication subsequently occurs through reverse transcription. The mature nucleocapsids are responsible for mediating viral persistence, and may be released to infect neighbouring hepatocytes. HBsAg: Hepatitis B surface antigen.
The natural history of CHB infection is dynamic, involving a complex interplay between the virus and the host immune system. Broadly, there are four stages of variable duration. The initial phase of infection is characterized by immune tolerance. Consequently, serum alanine transaminase (ALT) is normal and liver disease is minimal despite a high level of HBV DNA replication. Individuals, however, are e-antigen (HBeAg) positive and highly infectious. This phase is short when infection is acquired as an adult, but may persist for decades in patients infected perinatally. Nevertheless, tolerance is eventually lost. In the following immune clearance phase, the lysis of infected hepatocytes causes hepatitis, as evidenced by liver necroinflammation and fibrosis, as well as elevations in serum HBV DNA and ALT. The annual rate of HBeAg seroconversion and clearance is between 10% to 20%, and is dependent on factors including HBV genotype, and individuals’ age at acute infection[14,15]. Where 80% to 90% of infants infected will develop chronic infections, less than 5% of otherwise healthy adults who are infected will fail to spontaneously resolve an acute infection[16]. Since repeated exacerbations may occur before viral clearance, the cumulative risk of developing cirrhosis and HCC is increased. Following seroconversion, there is a decrease in viral replication, and remission of inflammation as evidenced by normalization of serum ALT. In contrast to the inactive carrier state, individuals with HBeAg-negative CHB continue to have moderate levels of HBV replication, and active liver disease. This stage may develop immediately after seroconversion, or following several years in the inactive carrier state. It is important to distinguish between inactive carriers and individuals with HBeAg-negative CHB, because the former has a favourable long-term outcome, whilst the latter is less responsive to treatment and associated with progressive liver disease[17]. Consequently, an understanding of the phases in chronic HBV infection is needed to risk stratify patients, and identify those whom would benefit from treatment.
IMMUNE SYSTEM IN PATHOGENESIS OF INFECTION
In patients with acute self-limiting HBV infection, the production of interferon gamma (IFN-γ) triggers the activation of natural killer cells, and induction of a robust T cell response. HBV-infected hepatocytes are subsequently cleared by CD8+ T cells through cytolytic, and non-cytolytic mechanisms[18]. Conversely, a weak and transient virus-specific T cell response is observed in patients with chronic HBV infection[19]. Potential causes of intrinsic T cell defects include up-regulation of Bcl2-interacting mediator (BIM), which causes deletion of HBV-specific CD8+ T cells[20]. T cell tolerance and exhaustion is perpetuated by an excess of co-inhibitory signals, including CTLA-4 and PD-L1[21], with the activity of remaining CD8+ T cells further suppressed by high levels of HBV DNA, microRNA-146a, and immunosuppressive cytokines such as interleukin-10[22,23]. More controversially, a tolerogenic liver environment, excessive immunosuppression by regulatory T cells[24], and dysfunction of dendritic cells[25] have been implicated in the impaired host immune response.
However, repeated attempts by the host immune system to control the infection causes hepatic injury. Natural killer cells may contribute to hepatocellular inflammation through expression of TNF-related apoptosis-inducing ligand[26], and induce apoptosis through the release of granzymes and perforins[27]. Recent research has also identified dense non-antigen specific T cell infiltration as a cause of hepatocyte lysis[28]. Critically, HBV is not directly cytopathic. Hence modulation of the host immune response may enable viral clearance in chronic HBV infection.
TREATMENT CRITERIA - WHO SHOULD WE TREAT?
The primary goal of CHB treatment is to reduce the risk of developing chronic liver disease associated complications. Longitudinal studies in large cohorts of chronically infected patients have revealed a 15% to 40% cumulative lifetime risk of developing cirrhosis, and amongst patients with established cirrhosis, an annual incidence rate of HCC between 2% to 5%[4]. Consequently, every patient would be a candidate for therapy if the virus could be eradicated. However, current therapeutic options only achieve a functional cure through viral suppression, and are associated with numerous adverse effects. Hence the decision on when to initiate treatment is controversial.
The indications for treatment are based on a combination of three criteria: levels of serum HBV DNA, serum ALT, and the severity of liver disease[7]. There is no significant distinction made between HBeAg positive and HBeAg negative infection. Current guidelines from NICE in the United Kingdom recommend the use of transient elastography as an initial test to assess the severity of liver disease, and need for treatment (Figure 2)[8]. For adults with a transient elastography score ≥ 11, there is a high likelihood of hepatic fibrosis, and treatment should be commenced irrespective of viral load to prevent further deterioration of liver function[29]. Similarly, all patients with a HBV DNA level > 20000 IU/mL are offered treatment due to a strong correlation between high viremia, cirrhosis, and HCC[30,31]. Cohort studies have also demonstrated a poorer prognosis in patients who had a prolonged immune clearance phase, with early clearance of HBeAg bringing about a 2.2-fold decrease in mortality[32]. Hence treatment is recommended for most individuals with a high HBV DNA and an elevated serum ALT.
Figure 2.
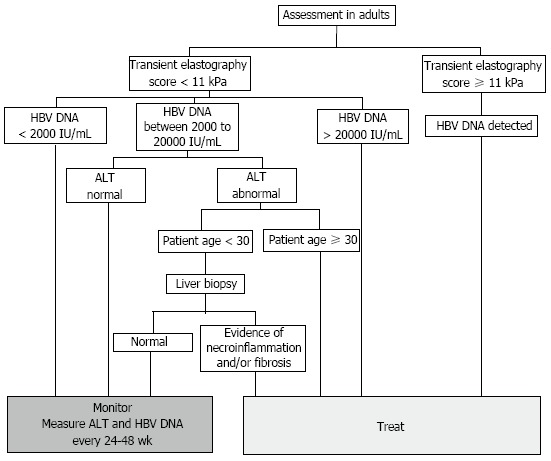
National Institute for Health and Care Excellence algorithm for initiation of treatment in chronic hepatitis B infection. Current indications for treatment are based on a combination of levels of serum hepatitis B virus (HBV) DNA, serum alanine transaminase (ALT), and the severity of liver disease. Specifically, patients with a transient elastography score ≥ 11 kPa are likely to have cirrhosis and confirmation by liver biopsy is not needed. Abnormal ALT, measured by two consecutive tests conducted 3 mo apart, is defined as ≥ 30 IU/mL in males, and ≥ 19 IU/mL in females.
The exception is for patients under 30 years of age with a normal liver biopsy. Whilst nucleos(t)ide therapy accelerates seroconversion, the risk of HBV reactivation is higher after nucleos(t)ide analogue induced seroconversion, as evidenced by an annual incidence of 12.0% compared to 2.9% following spontaneous seroconversion (P = 0.004)[33]. Hence close monitoring of young, non-cirrhotic, and compensated HBeAg positive patients may be more appropriate than immediate nucleos(t)ide analogue therapy. Treatment is also not recommended for patients who are in the immunotolerant (HBV DNA < 20000 IU/mL, normal serum ALT) or inactive carrier phase (HBV DNA < 2000 IU/mL, serum ALT normal) due to minimal liver disease[34]. Instead, patients should be monitored every 6 mo to diagnose a break in immune tolerance or reactivation of viral replication.
In the future, NICE guidelines should be revised to include levels of HBsAg as an indication for, and predictor of treatment response. In HBeAg negative patients with a low viral load (< 2000 IU/mL), multivariate analyses have showed that high levels of HBsAg (≥ 1000 IU/mL) increase the likelihood of developing cirrhosis and HCC[35,36]. Significantly, the adjusted hazard ratio for HCC in patients with levels of HBsAg ≥ 1000 IU/mL vs < 1000 IU/mL was 13.7[36]. Thus HBsAg is an independent predictor of outcome in patients with a low viral load. Emerging evidence also suggests increased mortality from liver disease in patients with ALT levels on the upper limit of normal[37]. Consequently, further research is needed to improve risk stratification, and identification of patients who would benefit from treatment.
TREATMENT OPTIONS - WHAT WEAPONS ARE IN OUR ARSENAL?
There are currently seven drugs approved for the treatment of CHB infection in Europe and the United States (Table 2). Broadly, nucleos(t)ide analogues have few side effects, and effectively suppress HBV DNA levels to cause clinical and histological improvements. However, long-term treatment is required to maintain virological control. Conversely, pegylated interferon (PEG-IFN) has a finite duration of treatment and is more likely to produce a sustained virological response. Its use, however, is limited by high costs and numerous associated side effects. Hence treatment must be optimized for each individual.
Table 2.
Comparison of antiviral agents for chronic hepatitis B
| Antiviral agents |
Immunomodulators |
Nucleos(t)ide analogues |
||||||
| IFN-α | PEG-IFN-α | Thymosin | Lamivudine | Adefovir | Entecavir | Telbivudine | Tenofovir | |
| Route | SC | SC | Oral | Oral | Oral | Oral | Oral | Oral |
| Dose | 5-10 MIU tiw | 180 μg qw | 1.6 mg biw | 100 mg od | 10 mg od | 0.5-1 mg od | 600 mg od | 300 mg od |
| Year approved | 1992 | 2005 | Asia only | 1998 | 2002 | 2005 | 2006 | 2008 |
| Antiviral effects | ||||||||
| HBV DNA | 37 | 30 | 42 | 36-40 | 21 | 67 | 60 | 76 |
| HBsAg clearance | ++ | ++ | N/A | - | - | + | - | - |
| HBeAg seroconversion | 20-40 | 27 | 40 | 18-20 | 12 | 21 | 22 | 21 |
| ALT normalization | 39 | 42 | 62-77 | 48 | 68 | 77 | 68 | |
| Histological improvement | 38 | N/A | 56-62 | 53 | 72 | 65 | 74 | |
| Side effects | Many | Many | Negligible | Negligible | Nephrotoxicity | Negligible | Negligible | Nephrotoxicity |
| Contraindications | Numerous | Numerous | Uncommon | Uncommon | Uncommon | Uncommon | Uncommon | Uncommon |
| Drug resistance (treatment-naïve patients) | ||||||||
| 1 yr | None, but non-response | 24 | None | 0 | 4 | 0 | ||
| 2 yr | 38 | 3 | 0.2 | 25 | 0 | |||
| > 5 yr | 80 | 29 | 1 | N/A | 0 | |||
| Drug resistance (LAM resistant patients) | ||||||||
| 2 yr | None, but non-response | N/A | 25 | 9 | N/A | 0 | ||
| 4 yr | N/A | N/A | 39 | N/A | 0 | |||
PEG-IFN: Pegylated interferon; SC: Subcutaneous; tiw: Three times a week; qw: Once a week; biw: Twice a week; od: Once daily; ALT: Alanine transaminase; ETV: Entecavir; LAM: Lamivudine; ADV: Adefovir; TBV: Telbivudine; TDF: Tenofovir disoproxil fumarate; N/A: Not applicable.
PEG-IFN
IFNs are cytokines which interfere with viral replication in host cells by inhibiting viral DNA synthesis, and enhancing the cellular immune response against HBV-infected hepatocytes. Its half-life and drug efficacy may be improved through pegylation. A study of a 24-wk course of weekly PEG-IFN-α-2a showed a higher combined response rate (HBeAg loss, HBV DNA suppression, and ALT normalization) than that achieved by conventional IFN-α-2a (24% vs 12%, P = 0.036), with no significant difference with respect to frequency and severity of adverse events[38]. Hence standard IFN is no longer used where PEG-IFN is available.
PEG-IFN not only offers a finite duration of therapy, but is also superior to lamivudine on the basis of HBeAg seroconversion, HBV DNA suppression, and HBsAg seroconversion in both HBeAg-positive and HBeAg-negative CHB[39,40]. At 6-mo post-treatment, HBeAg seroconversion was observed in 32% of patients treated with PEG-IFN, compared with 19% of patients on lamivudine therapy[40,41]. HBsAg seroconversion was also achieved in 16 patients on PEG-IFN therapy, as opposed to zero in the group receiving lamivudine alone (P = 0.001)[40]. Hence PEG-IFN induces a more durable virological response[42]. However, the response to IFN is relatively low, and its use is associated with numerous adverse phenomena including depression, paraesthesia, myelosuppression, and other influenza-like symptoms such as fatigue, headaches, and weight loss. Thus HBV genotype and pre-treatment HBV DNA levels must be used to determine the likelihood of patients deriving benefit from PEG-IFN treatment.
Entecavir
Entecavir is a guanosine analogue with potent activity against HBV through inhibition of DNA polymerase which has been evaluated in two large double-blinded phase III clinical trials involving 715 HBeAg positive and 648 HBeAg negative nucleos(t)ide-naïve patients. Entecavir administered at a dose of 0.5 mg orally once daily was shown to be superior to lamivudine and adefovir[43-45]. Specifically, 80% and 87% of entecavir-treated vs 39% and 79% of lamivudine-treated patients achieved undetectable HBV DNA and ALT normalization through 96 wk respectively[46]. Long term follow-up of the same cohort revealed that an additional 23% achieved HBeAg seroconversion, and 1.4% lost HBsAg after 5 years of continuous entecavir therapy[47]. More importantly, entecavir-mediated viral suppression decreased the risk of decompensation, HCC, and death during a median of 20 mo of follow-up[48]. Long-term use of entecavir is not associated with any serious adverse effects, and development of resistance is rare in nucleos(t)ide-naïve patients[49]. Hence meta-analyses recommend entecavir and tenofovir as optimal antiviral agents in treatment-naïve individuals[50].
Tenofovir disoproxil fumarate
Tenofovir is the newest antiviral agent licensed for the treatment of CHB. In a phase III clinical trial, tenofovir at a daily dose of 300 mg for 48 wk induced viral suppression with HBV DNA less than 400 copies/mL in 76% of HBeAg positive and 93% of HBeAg negative patients, as opposed to only 68% and 14% of patients treated with adefovir respectively[51]. 8% of HBeAg positive patients had loss of HBsAg after 3 years of tenofovir monotherapy[52]. Critically, follow-up of the same cohort after 4.5 years revealed that 87% had histological improvement, 51% had regression of fibrosis, and that 74% of patients with cirrhosis at baseline were no longer cirrhotic[53]. Virological breakthrough was infrequent, and there have been no reports of tenofovir resistance after 6 years of therapy[54].
Alternative treatments
Other pharmacological agents effective in CHB infection include thymosin-α1, an immunomodulating agent which augments the host Th1 immune response[55]. In a meta-analysis including 353 patients from five randomized trials, the odds ratio for a virological response to thymosin-α1 over placebo at the end of treatment, 6 mo post-treatment, and 12 mo post-treatment were 0.56 (0.2-1.52), 1.67 (0.83-3.37), and 2.67 (1.25-5.68) respectively, with virological response increasing over time after cessation of thymosin treatment[56]. Subsequent clinical trials demonstrated that the odds of ALT normalization and negative HBV DNA at the end of follow-up (12 mo) was three-fold higher in the thymosin-α1 than the IFN-α group[57]. Hence thymosin α1 is approved in 35 different countries worldwide, and is recommended by the APASL as an option in treatment naïve patients[6].
Oxymatrine, an alkaloid extracted from Sophora alopecuraides L, has also been shown to effectively and safely suppress HBV replication[58]. It acts not only as an immunomodulator[59], but also induces cytochrome P450 to enhance degradation of HBV mRNA to inhibit viral replication[58]. In a randomized double-blind placebo-controlled trial, normalization of serum ALT and HBeAg seroconversion occurred in 83% and 40% of patients respectively - an efficacy comparable to that of IFN-α[60]. Nevertheless, trials have been small, and larger studies are needed.
CURRENT TREATMENT GUIDELINES - WHERE DO THEY FALL SHORT?
In adults with compensated liver disease, current guidelines from the NICE advise first-line treatment with 48 wk of PEG-IFN-α-2a, because it results in the highest rate of off-treatment sustained response. Following 24 wk of therapy, HBV DNA should be measured to predict the likelihood of a sustained virological response. In patients with a suboptimal response, defined as a decrease in HBV DNA of less than 2 log10 IU/mL after 24 wk of therapy, and patients who do not undergo HBeAg seroconversion or a decrease in serum HBsAg after 48 wk of therapy, second line treatment with tenofovir should be offered. For HBeAg negative patients with detectable HBV DNA after 48 wk of treatment, consider switching from tenofovir to entecavir as third line treatment. For HBeAg positive patients where HBV DNA remains detectable at 96 wk with no history of lamivudine resistance, add lamivudine to tenofovir. Otherwise, consider combination therapy with entecavir and tenofovir (Table 3).
Table 3.
National Institute for Health and Care Excellence treatment guidelines
| Guidelines | HBeAg positive | HBeAg negative | Decompensated |
| 1st line | 48-wk of PEG-IFN-α-2a | 48-wk of PEG-IFN-α-2a | ETV or TDF (LAM resistance) |
| 2nd line | TDF or ETV (TDF contraindication) | ETV or TDF | |
| 3rd line | LAM + TDFor ETV + TDF (LAM resistance) | ETV or TDF |
In adults with compensated liver disease, current guidelines advise first-line treatment with 48 wk of PEG-IFN-α-2a. If PEG-IFN-α-2a is contraindicated, tenofovir or entecavir should be trialed. PEG-IFN: Pegylated interferon; HBeAg: Hepatitis B e antigen; ETV: Entecavir; LAM: Lamivudine; TDF: Tenofovir disoproxil fumarate.
However, the paradigm of treatment for CHB is constantly evolving. Growing evidence suggests that the response rate to IFN therapy may vary amongst HBV genotypes. Studies have shown that the rates of HBeAg loss are significantly higher in patients with genotype B compared to genotype C[61,62]. The response to IFN therapy is also higher amongst patients with genotype A as opposed to those with genotype D, in both HBeAg positive (46% vs 24%), and HBeAg negative CHB (59% vs 29%)[63]. Moreover, numerous trials have demonstrated that PEG-IFN-α-2b is the best therapy for achieving HBsAg clearance and a sustained virological response in patients with genotype A[64,65]. Therefore, contrary to recommendations from NICE, we believe that routine genotyping is essential for all patients in whom IFN therapy is considered. The likelihood of achieving a sustained response should be predicted based on HBV genotype, ALT, and HBV DNA levels (Table 4)[66]. We advise that patients should only receive PEG-IFN as first-line treatment if the baseline probability of sustained virological response is greater than 30%, for only in this patient subgroup do the potential benefits outweigh the costs[66].
Table 4.
Recommendations for the use of pegylated interferon as initial antiviral therapy
| HBV genotype | General recommendations for HBeAg positive patients |
| A | Either high ALT (≥ 2 × ULN) or low HBV DNA levels (< 9 log10 copies/mL) |
| B and C | Both high ALT (≥ 2 × ULN) and low HBV DNA levels (< 9 log10 copies/mL) |
| D | Not recommended |
HBV: Hepatitis B virus; HBeAg: Hepatitis B e antigen; ALT: Alanine transaminase; ULN: Upper limit of normal.
More recently, studies have shown that patients’ IL28B genotypes are significantly associated with the likelihood of HBeAg seroconversion, and HBsAg loss. In HBeAg negative patients, a retrospective analysis found that a sustained virological response with HBsAg seroconversion was more likely in the rs12979860 CC (vs CT/TT) genotype carriers (29% vs 13%, P < 0.039)[67]. Other multi-centre studies in HBeAg positive patients have demonstrated a favourable outcome, with an adjusted odds ratio for seroconversion following PEG-IFN treatment of 3.16 for AA vs AA/GG genotype carriers at rs12980275[68,69]. Hence genome sequencing for IL-28 polymorphisms may provide additional information on the probability of response to IFN therapy.
On-treatment monitoring of HBsAg kinetics may further optimize PEG-IFN treatment by identifying non-responders at an early stage. Broadly, serum HBsAg levels correlate with intrahepatic cccDNA, and low cccDNA levels are predictive of a sustained virological response. Retrospective analyses have demonstrated that 57% of patients with HBsAg levels < 1500 IU/mL after 12 wk of treatment achieved HBeAg seroconversion 6 mo post-treatment[70]. This is significantly higher than in patients with HBsAg 1500-20000 IU/mL or > 20000 IU/mL, where HBeAg seroconversion was attained in only 32% and 16% of cases respectively (P < 0.0001)[70]. The predictive power of early HBsAg levels was further emphasized by retrospective analysis of patients enrolled in the Neptune study, where no patients with HBsAg levels > 20000 IU/mL at week 12 on-treatment achieved HBeAg seroconversion[71]. Similarly in HBeAg negative patients, the PARC study found that a decrease in HBsAg of < 2 log10 IU/mL at week 12 was invariably associated with treatment failure[72]. Consequently, the cost-effectiveness of PEG-IFN therapy may be improved by using a response-guided approach based on HBsAg kinetics at 12 wk of treatment.
The recommended duration of PEG-IFN therapy should also be prolonged. Results from a recent clinical trial demonstrated that treatment extension from 48 to 96 wk improved clinical outcome, significantly increasing rates of viral suppression, ALT normalization, and HBsAg clearance[73]. Furthermore, discontinuation rates did not increase, implying that a prolonged course of treatment was equally well tolerated. Consequently, guidelines should be updated to recommend extension of PEG-IFN treatment to 96 wk.
In patients where IFN therapy is contraindicated, or where there was a suboptimal response, treatment with entecavir or tenofovir is recommended by numerous meta-analyses and international guidelines due to its high efficacy, high barrier to resistance, and favourable safety profile[50,74]. However, there is little evidence for the use of lamivudine and tenofovir combination therapy in treatment refractory patients. In a population of human immunodeficiency virus (HIV) co-infected patients, combination therapy with lamivudine and tenofovir did not increase the probability of HBV DNA suppression[75]. There is more evidence for the use of combination therapy in patients harbouring patterns of viral resistance. In a population with mixed patterns of nucleos(t)ide analogue resistance, the majority of patients treated with entecavir and tenofovir achieved undetectable HBV DNA levels[76]. However, tenofovir monotherapy is also highly effective in multi-drug resistant strains of HBV[77]. Consequently, further studies are needed to determine whether combination therapy offers any advantage over monotherapy alone.
The management guidelines differ for patients with decompensated liver disease. Notably, entecavir should be offered as first-line treatment instead of PEG-IFN, due to the high risk of serious infections and hepatic failure. PEG-IFN is also contraindicated in pregnancy due to teratogenic effects arising from inhibition of cell proliferation. Instead, the category B antiviral drugs lamivudine or tenofovir should be used to reduce vertical transmission[78]. Both topics have been extensively reviewed elsewhere.
COMBINATION THERAPY - IS TWO BETTER THAN ONE?
Combination therapy offers the potential of achieving long-term viral suppression whilst avoiding drug resistance, and has been shown to be beneficial in patients with HIV and chronic HCV. Consequently, numerous combinations of antivirals have been trialed in the treatment of CHB (Table 5). Current guidelines recommend first-line treatment of CHB/HIV co-infection with emtricitabine and tenofovir combination therapy[79]. However, the use of combination therapy de novo, or in cases where there is suboptimal response or drug resistance is more controversial and subject to ongoing studies.
Table 5.
Comparison of de novo combination therapy and monotherapy
| Combination therapy | Monotherapy | HBeAg seroconversion | HBV DNA | HBsAg clearance | Histological improvement | Drug resistance |
| LAM + ADV | LAM | = | = | N/A | = | ↓ |
| LAM + ADV | ETV | N/A | = | N/A | N/A | N/A |
| ETV + TDF | ETV | = | = | N/A | = | = |
| LAM + PEG-IFN | LAM | ↑ | ↓ | ↑ | ↑ | ↓ |
| ADV + PEG-IFN | PEG-IFN | N/A | ↓ | ↑ | N/A | N/A |
| ETV + PEG-IFN | ETV | ↑ | ↓ | ↑ | N/A | N/A |
In several randomized controlled trials, combination therapy with nucleos(t)ide analogues and PEG-IFN are superior to existing management options with regards to viral load, HBeAg seroconversion, and HBsAg clearance. HBeAg: Hepatitis B e antigen; HBsAg: Hepatitis B surface antigen; PEG-IFN: Pegylated interferon; ETV: Entecavir; LAM: Lamivudine; ADV: Adefovir; TBV: Telbivudine; TDF: Tenofovir disoproxil fumarate; N/A: Not applicable.
At present, there is no evidence supporting de novo combination therapy with nucleos(t)ide analogues that have a high barrier of resistance. A large randomized-controlled trial found the antiviral efficacy of entecavir monotherapy to be comparable to that of entecavir plus tenofovir combination therapy in a mixed-population of nucleos(t)ide-naïve patients[80]. Where entecavir or tenofovir is not available as first-line treatment, the combination of lamivudine and adefovir may be used with similar efficacy[81].
The combination of nucleos(t)ide analogues with PEG-IFN therapy is a more promising strategy. In phase III clinical trials, the combination of PEG-IFN and lamivudine induced a significantly greater decline in HBV DNA, as well as a higher rate of HBeAg and HBsAg seroconversion than lamivudine monotherapy[40,82]. In addition, there was a higher rate of sustained virological response, and lower rate of resistance when lamivudine was administered together with PEG-IFN, presumably because IFN is effective against HBV resistant mutants. Evidence also demonstrates that the de novo use of adefovir and PEG-IFN combination therapy lead to a greater decline in HBV DNA, HBsAg seroconversion, and intrahepatic cccDNA than PEG-IFN alone[83]. More importantly, a recent multi-centered randomized controlled study found that the use of entecavir, a nucleos(t)ide analogue recommended for first-line use by international guidelines, in combination with PEG-IFN-α-2a, increased clearance of HBeAg and HBsAg[84]. Hence combination therapy with nucleos(t)ide analogues and PEG-IFN may be an option for management in treatment-naïve patients.
In cases of inadequate HBV DNA suppression (> 2000 IU/mL), combination therapy may also be used to salvage treatment. One recent study demonstrated that the addition of lamivudine for 24 wk in patients with suboptimal response to adefovir monotherapy induced clearance of HBV DNA and HBeAg in approximately 35% of patients[85]. However, the addition of adefovir to entecavir, which has an inherently high barrier of resistance, did not increase virological response[86]. Given that all nucleos(t)ide analogues share the same viral target, the advantage of lamivudine and adefovir combination therapy is likely to be a consequence of reduced drug resistance. This hypothesis is supported by a 3-year study of 145 lamivudine-resistant patients, where treatment with the combination of adefovir and lamivudine induced clearance of HBV DNA in 80% of patients[87]. Moreover, all patients remained free of virological or clinical breakthroughs, with the 1-, 2-, 3- and 4-year cumulative rates of drug resistance at 1%, 2%, 4%, and 4% respectively[87]. Thus combination therapy is useful for rescuing therapy in patients with suboptimal treatment response or drug resistance.
DRUG RESISTANCE - THE NEED FOR RESCUE THERAPY
HBV reverse transcriptase is an error-prone enzyme - its low fidelity, and lack of proofreading activity leads to a mutation rate 10-fold higher than that of other DNA viruses[88]. This fact, together with its high rate of replication (1012 virions/d), may result in the selection of HBV quasispecies containing mutations conferring drug-resistance.
Currently, effective monotherapy has been made po-ssible with entecavir and tenofovir - two agents with high efficacy and low rates of resistance. Earlier use of less potent nucleos(t)ide analogues, however, has led to the emergence of multi-drug resistant strains of HBV. A summary of common mutation patterns, and the best available rescue treatment strategies is summarized in Table 6.
Table 6.
Antiviral resistance patterns and rescue therapy
| Resistance | Mutation patterns | Treatment adaptation |
| LAM | M204V/I + L180M M204I M204V + L180M + V173L M204I + L180I Q215S + M204I/V + M204V I169T + V173L + L180M + M204V A181T T184S + M204I/V + L180M M204S + L180M | Switch to TDF or Switch to ETV + ADV combination therapy or Add ADV if TDF or ETV unavailable |
| ADV | A181V/T N236T A181V/T + N236T | Switch to TDF and add ETV |
| ADV + LAM | A181V/T + N236T L80V/I | Switch to TDF and add ETV |
| TBV | M204I/V ± L180M L80I/V ± L180M A181T/V | Switch to or add TDF |
| ETV | L180M + M204V/I ± I169T ± M250V L180M + M204V/I ± T184G ± S202I/G | Switch to or add TDF |
| TDF | No known mutations | N/A |
Common mutation patterns, and rescue therapies suggested by the American Association for the Society of Liver Disease, Asian Pacific Association for the Study of Liver; European Association for the Study of Liver and National Institute for Health and Care Excellence. LAM: Lamivudine; ETV: Entecavir; ADV: Adefovir; TBV: Telbivudine; TDF: Tenofovir disoproxil fumarate.
Presently, lamivudine resistance poses a major problem in the management of patients with CHB infection. Approximately 80% of patients develop lamivudine resistance after 5 years of therapy[89]. One retrospective review of various rescue strategies on patients with lamivudine resistance showed that switching to adefovir and entecavir combination therapy was the most effective, with 100% normalization in ALT, suppression of viral load, and no development of resistance[90]. In contrast, a switch to entecavir monotherapy was associated with drug resistance in 9% of patients after 2 years of therapy[91]. If costs prohibit the use of entecavir, add-on adefovir or oxymatrine may be considered as an alternative[90,92].
In patients with multi-drug resistant HBV, studies have demonstrated that tenofovir monotherapy is able to induce long-term viral suppression. A prospective multi-centre open-label study of 60 patients who previously failed serial lamivudine and adefovir switch or add-on therapy showed that 4 years of continuous tenofovir resulted in 63% (38/60) patients achieving viral suppression by intention-to-treat analysis[77]. However, in patients with HIV co-infection, HBV strains with rtL180M, rtA194T, and rtM204V mutations displayed a 10-fold increase in the IC50 value for tenofovir as compared with wildtype strains[93]. An early switch to combination therapy with tenofovir and emtricitabine, or tenofovir and entecavir, has been proven to be effective at inducing viral suppression in this difficult-to-treat population[76]. Moreover, patients should be investigated for drug compliance - recent studies have suggested that up to 40% of patients may not be fully adherent to their treatment regime, significantly influencing rates of viral suppression[94]. Consequently, all patients should be monitored closely for compliance, and development of resistance during antiviral therapy.
DEFINING TREATMENT ENDPOINTS - WHEN DO WE STOP?
The ideal treatment endpoint is clearance of cccDNA from hepatocytes, but eradication of HBV is rarely achieved with currently available treatment. Instead, various biochemical, virological, and histological markers including the suppression of HBV DNA, HBeAg seroconversion, loss of HBsAg, ALT normalization, and improvement of inflammation or fibrosis on liver biopsy are used as surrogate endpoints to measure the efficacy of antiviral therapy (Table 7).
Table 7.
Treatment endpoints in clinical use
| Treatment | Description |
| Biochemical | Normalization of serum ALT |
| Virological | |
| HBeAg-positive | Loss of HBeAg, Anti-HBe antibodies, serum HBV-DNA < 2000 IU/mL |
| HBeAg-negative | Serum HBV-DNA < 2000 IU/mL |
| Complete | Biochemical and virological response with loss of serum HBsAg |
| Histological | Decrease in necroinflammatory activity without worsening in fibrosis |
ALT: Alanine transaminase; HBV: Hepatitis B virus; HBeAg: Hepatitis B e antigen; HBsAg: Hepatitis B surface antigen.
In HBeAg positive patients without cirrhosis, current NICE guidelines suggest stopping nucleos(t)ide analogue therapy 12 mo after HBeAg seroconversion. Whilst spontaneous or treatment-induced HBeAg seroconversion is associated with improved survival, it may not be a sufficient therapeutic endpoint in Asian populations, in which risks for disease progression are strongly related to viremia independent of HBeAg status[95]. Hence complete suppression of HBV DNA may be a better endpoint.
In HBeAg negative patients without cirrhosis, the ideal treatment endpoint is considerably less clear. It is possible that nucleos(t)ide analogue therapy may be safely withdrawn after achieving complete HBV DNA suppression. However, studies have shown that 90% of patients relapse within a year of discontinuing nucleos(t)ide analogue therapy[96]. The rate of relapse may be reduced by prolonging antiviral consolidation therapy[97], or by continuing treatment indefinitely until HBsAg seroconversion is achieved. Interferon- and nucleos(t)ide analogue therapy-induced HBsAg seroclearance is durable after treatment discontinuation, with less than 5% of patients experiencing either virological or biochemical relapse[98]. Hence current NICE guidelines recommend stopping nucleos(t)ide treatment 12 mo after achieving both undetectable HBV DNA and HBsAg seroconversion in non-cirrhotic patients. But given only 5% of HBeAg negative patients clear HBsAg after 5 years of continuous therapy, life-long treatment is often required. Further research is needed to establish a safe and realistic endpoint for CHB therapy.
FUTURE TREATMENTS - IS A CURE ON THE HORIZON?
Current treatments for CHB infection based on nucleos(t)ide analogues effectively suppress viral replication to induce a functional cure. However, drug resistance is common, and life-long therapy is frequently required to prevent disease recurrence. Treatment with IFN-α is more likely to produce a sustained virological response, but is associated with numerous side effects, and is contraindicated in many patient populations. We believe that improvements in drug delivery systems, and development of drugs with different mechanisms of action will prevent resistance and improve viral clearance.
From a drug discovery point of view, rational drug targets might include several steps of the HBV viral cycle, such as viral entry, formation of cccDNA, viral genome integration, capsid assembly, viral envelopment, and exocytosis. In particular, the clearance of cccDNA, which mediates viral persistence in hepatocytes, is critical for preventing viral reactivation and disease recurrence following cessation of antiviral therapy. Since maintenance of the cccDNA pool results from a weak HBV-specific immune response, research should also focus on the development of immunomodulators. A number of new drug delivery systems and antiviral agents are currently under development (Table 8).
Table 8.
Emerging pipeline drugs for chronic hepatitis B virus infection
| Drug name | Mechanism of action | Status |
| Nucleos(t)ide analogues | ||
| Clevudine | Inhibits DNA polymerase | Partial approval |
| Emtricitabine | Inhibits DNA polymerase | FDA approved for HIV |
| Nucleos(t)ide analogue prodrugs | ||
| Amdoxovir | Inhibits DNA polymerase | II (for HIV) |
| LB80380 | Inhibits DNA polymerase | IIb |
| Famciclovir | Inhibits DNA polymerase | Abandoned |
| Pradefovir | Inhibits DNA polymerase | Abandoned |
| Tenofovir alafenamide (GS 7340) | Inhibits DNA polymerase | II/III |
| MIV-210 | Inhibits DNA polymerase | Abandoned |
| Non-nucleos(t)ide antivirals | ||
| NOV-205 (BAM 205) | Unknown | Approved in Russia |
| Myrcludex-B | Inhibits viral entry | Ib/IIa |
| Bay 41-4109 | Inhibits viral core formation | I |
| GLS4 | Inhibits HBV viral core assembly | Pre-clinical |
| Rep 9 AC | Blocks HBsAg release | II |
| NVR-1221 | Capsid inhibitor | Pre-clinical |
| Immunomodulators | ||
| Pegylated interferon lambda | Cytokine modulating innate/adaptive immune response | I |
| GS-9620 | TLR-7 agonist | Pre-clinical |
| Nitazoxanide | Unknown | II |
| EHT899 | Immune enhancer | II |
| Therapeutic vaccines | ||
| HBV core antigen vaccine | Enhance T cell response | I |
| HBV-EPV | Immunogenic | Withdrawn |
| ePA-44 | Immunogenic | II |
| HI-8 HBV | Stimulates IFN-γ producing T cells | II |
| Others | ||
| β-thujaplicinol | Blocks viral ribonuclease H activity | Pre-clinical |
| ARC520 | RNA interference | I |
| Herbal bushen formula | Down-regulate CD4+ and CD25+ T cells | TCM |
HBV: Hepatitis B virus; TCM: Traditional chinese medicine; HBsAg: Hepatitis B surface antigen; HIV: Human immunodeficiency virus; FDA: Food and Drug Administration; IFN: Interferon.
Improvements in drug delivery systems
The efficacy of existing antiviral therapies is partly dependent on their pharmacokinetics. Hence implementation of liver-targeting drug delivery systems may improve drug efficacy by overcoming the development of resistance, and tolerability by promoting selective accumulation in the liver to limit systemic side effects. Dextran is a complex polysaccharide which may be used as a carrier molecule for tissue-specific delivery of drugs. Recently, it was reported that lamivudine-dextran conjugates selectively accumulate in hepatocytes and Kupffer cells, resulting in a seven-fold higher concentration compared to controls[99]. The inhibitory effects of adefovir loaded into nanoparticles on HBsAg, HBeAg, and serum HBV DNA levels in vitro were also significantly enhanced[100]. Nevertheless, these drugs do not eradicate cccDNA, which perpetuates infection. Consequently, liver-targeting drug delivery systems must be used in conjunction with new anti-HBV drug candidates to improve viral clearance.
New drugs to current targets
Emtricitabine is a nucleos(t)ide analogue approved for the treatment of HIV with clinical activity against HBV. It significantly suppresses viral replication and improves liver histology compared to placebo, but is not used as a monotherapy due to high rates of resistance[101]. Instead, dual therapy with tenofovir and emtricitabine was found to produce high rates of HBeAg seroconversion and HBsAg loss, improving clinical outcomes for patients with decompensated HBV cirrhosis[102]. It is also the treatment of choice in patients with HIV co-infection[103]. However, further studies are needed to establish whether tenofovir plus emtricitabine offers any advantage over tenofovir monotherapy in treatment-naïve patients (Table 8).
LB80380 is a nucleos(t)ide analogue prodrug which has potent anti-HBV activity in treatment-naïve patients[104], as well as in vitro activity against mutant strains resistant to lamivudine, adefovir, entecavir, and telbivudine[105]. Additionally, 90 mg and 150 mg of LB80380 daily were found to be non-inferior to entecavir 0.5 mg daily at 48-wk in a phase IIb study of treatment-naïve CHB patients[106]. Results from a 96-wk extension study (NCT01242787) are awaited (Table 9). With its high potency, effectiveness in lamivudine-resistant patients[107], and good safety profile, LB80380 is a potential alternative agent for the treatment of CHB.
Table 9.
A partial list of ongoing clinical trials
| Phase | Trial identifier | Design | Drugs | Enrollment | Expected end date |
| I | NCT01872065 | Double-blind, randomized | ARC520 | 44 | October 2013 |
| NCT01590641 | Double-blind, randomized | GS-9620 | 48 | September 2013 | |
| II | NCT00524173 | Open-label, randomized | TDF vs TDF + emtricitabine | 100 | January 2015 |
| NCT01204762 | Double-blind, randomized | IFN-λ + ETV | 170 | July 2017 | |
| NCT01242787 | Open-label, randomized | LB80380 | 115 | September 2012 | |
| III | NCT01595685 | Open-label, randomized | TBV vs ETV | 184 | December 2014 |
| NCT01369199 | Open-label, randomized | 8 wk ETV followed by 40 wkPEG-IFN-α-2a + ETV | 250 | May 2016 | |
| IV | NCT01804387 | Open-label, randomized | TBV + ADV vs LAM + ADV | 60 | May 2014 |
| NCT01906580 | Open-label, randomized | PEG-IFN-α-2a vs ETV | 105 | July 2014 |
Full details of the clinical trials may be found on the United States National Institutes of Health. Available from: URL: http://www.clinicaltrials.gov. LAM: Lamivudine; ETV: Entecavir; ADV: Adefovir; TBV: Telbivudine; TDF: Tenofovir disoproxil fumarate; PEG-IFN: Pegylated interferon.
Clevudine is another nucleos(t)ide analogue which has potent antiviral activity against HBV[108]. Initial studies demonstrated that 24 wk of clevudine at 30 mg once daily achieved statistically significant differences in viral suppression and durability compared to placebo[109]. Clevudine was also found to be superior to lamivudine in suppressing HBV replication[110], but its efficacy is no higher than that of entecavir[111]. Long-term use is further limited by development of drug resistance[112], and drug-induced toxic myopathy due to depletion of mitochondrial DNA[113]. Clevudine is currently approved for treatment of CHB in South Korea and the Philippines, but given the safety concerns and availability of more effective alternatives, clevudine is unlikely to have a role in treatment where entecavir and tenofovir are available. Thus new therapeutic approaches are needed.
Emerging targets for HBV treatment
In developing novel anti-HBV therapies, many approaches including pharmacophore-based antiviral drug design and traditional high-throughput screening have been used. A number of lead compounds with new mechanisms of action are currently being evaluated in vitro, in animal models of CHB, and in clinical trials (Table 8).
The discovery of compounds which directly target cccDNA has been one of the major challenges to curing CHB infection. In an infected hepatocyte, HBV replication is regulated by the acetylation or methylation of histone proteins which surround the cccDNA minichromosome. The hSirt1/2 activator MC2791 and the JMJD3 inhibitor MC3119 suppressed both HBV replication and cccDNA transcription, providing a proof of concept that epigenetic modifiers may mediate persistent cccDNA silencing[114]. More recent data presented at the 2013 International Liver Congress suggests that antibody mediated stimulation of the lymphtoxin beta receptor in cell culture models exerts a strong and dose-dependent anti-HBV effect, including cccDNA in cells where HBV infection was established. In addition, zinc finger proteins designed to target the HBV enhancer region[115], and various small-molecule compounds including CCC-0975 and CCC-0346[116] have been identified as inhibitors of cccDNA formation in vitro. Other replicative intermediates may also be targeted in CHB therapy. ARC-520 is a liver-tropic cholesterol-conjugated small interfering RNA which targets conserved HBV sequences to knockdown expression of replicative intermediates. In a chimpanzee with CHB infection, a low dose of ARC-520 induced rapid and multi-log repression of viral RNA, DNA, and key viral antigens including HBsAg and HBeAg with long duration of effect[117]. Collectively, this preliminary data suggests that targeting replicative intermediates such as cccDNA may form the basis of a cure for CHB infection.
Another promising therapeutic approach is to inhibit HBV entry into the hepatocyte, as this not only prevents cell-to-cell spread in established infection, but can replace HBIg to prevent infection. Myrcludex-B is a synthetic lipopeptide derived from the HBV envelope protein which inactivates the HBV pre-S1 receptor at picomolar concentrations[118]. In immunodeficient chimeric mice reconstituted with human hepatocytes (uPA/SCID-mice), myrcludex-B not only prevented viral spreading in between hepatocytes, but also reduced levels of HBV DNA, HBsAg and cccDNA compared to untreated mice[119]. Results from a phase Ib/IIa clinical trial are expected at the end of 2013. Initial data for REP 9AC’, a nucleic acid-based polymer which clears serum HBsAg by blocking HBV subviral particle formation and release, is also promising. A proof-of-concept study in 8 patients demonstrated that REP 9AC’ reduced serum HBV DNA, inducing HBsAg seroclearance and development of anti-HBs to sustain virological response over 24 mo of follow-up off treatment. REP 9AC’ also potentiated the immunostimulatory effects of PEG-IFN-α, generating anti-HBs titres comparable to those seen in healthy individuals with a strong vaccine response, raising the possibility of durable immunological control[120].
The immune system is inherently capable of controlling HBV infection, as evidenced by the 90% of adults who resolve acute HBV infection, and by the resolution of CHB following bone marrow transplantation from an immune donor[121]. An increasing emphasis is thus placed on developing immunomodulatory therapy. Immunotherapeutic strategies for CHB include exogenous administration of cytokines with antiviral activity, and stimulation of the host T cell immune response. However, the antiviral effects of therapeutic vaccines have been disappointing. S and pre-S antigen vaccines, as well as T cell specific vaccines have all failed to achieve significant viral clearance[122,123]. Greater success has been found with IFN-λ and GS-9620. IFN-λ has potent anti-HBV activity in vitro and in transgenic mice[124,125], and was shown in a phase IIb trial to cause significantly greater suppression of HBV DNA and HBsAg than IFN-λ[126]. Critically, it also has improved tolerability due to the limited distribution of λ receptors outside the liver[127]. GS-9620 is an orally bioavailable small-molecule which activates Toll-like receptor 7 signalling. Administration of GS-9620 thrice weekly for 4 wk at 1 mg/kg, followed by 2 mg/kg, resulted in a prolonged 2.2 log unit reduction in HBV DNA in all three chronically infected chimpanzees[128]. Clinical trials for both IFN-λ and GS-9620 are currently on going (Tables 8 and 9).
CONCLUSION
Patients with minimal disease should not be treated. Conversely, patients at risk of developing complications such as cirrhosis and HCC should receive antiviral therapy. In patients with predictive factors of favourable response, a finite course of PEG-IFN should be trialed as first-line treatment. However, the majority of patients still require life-long treatment with nucleos(t)ide analogues, which is associated with substantial costs and a high risk for developing antiviral resistance. In our opinion, further studies are required to identify mechanisms by which the low viral load in the persistent carrier state may be eliminated. New strategies will likely favour an immunomodulatory approach, and or involve eradication of the pool of cccDNA from infected hepatocytes.
Footnotes
Supported by Collaborative Research Fund (CUHK3/CRF/12R; HKU3/CRF11R) of the Research Grant Council Hong Kong; National Basic Research Program of China, 973 Program, No. 2013CB531401; CUHK Focused Investments Scheme B to HY Lan; and Theme-based Research Scheme of the Hong Kong Research Grants Council, No. T12-403-11
P- Reviewers: Huerta-Franco MR, Karayiannis P, Peter LL S- Editor: Ma YJ L- Editor: A E- Editor: Wu HL
References
- 1.McMahon BJ. Epidemiology and natural history of hepatitis B. Semin Liver Dis. 2005;25 Suppl 1:3–8. doi: 10.1055/s-2005-915644. [DOI] [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liaw YF, Tai DI, Chu CM, Chen TJ. The development of cirrhosis in patients with chronic type B hepatitis: a prospective study. Hepatology. 1988;8:493–496. doi: 10.1002/hep.1840080310. [DOI] [PubMed] [Google Scholar]
- 4.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35–S50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–662. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 6.Liaw YF, Kao JH, Piratvisuth T, Chan HLY, Chien RN, Liu CJ, Gane E, Locarnini S, Lim SG, Han KH, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012;3:531–561. doi: 10.1007/s12072-012-9365-4. [DOI] [PubMed] [Google Scholar]
- 7.European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–185. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Sarri G, Westby M, Bermingham S, Hill-Cawthorne G, Thomas H. Diagnosis and management of chronic hepatitis B in children, young people, and adults: summary of NICE guidance. BMJ. 2013;346:f3893. doi: 10.1136/bmj.f3893. [DOI] [PubMed] [Google Scholar]
- 9.Robinson WS, Lutwick LI. The virus of hepatitis, type B (first of two parts) N Engl J Med. 1976;295:1168–1175. doi: 10.1056/NEJM197611182952105. [DOI] [PubMed] [Google Scholar]
- 10.Fung SK, Lok AS. Hepatitis B virus genotypes: do they play a role in the outcome of HBV infection? Hepatology. 2004;40:790–792. doi: 10.1002/hep.1840400407. [DOI] [PubMed] [Google Scholar]
- 11.Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H, et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012;1:e00049. doi: 10.7554/eLife.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tuttleman JS, Pourcel C, Summers J. Formation of the pool of covalently closed circular viral DNA in hepadnavirus-infected cells. Cell. 1986;47:451–460. doi: 10.1016/0092-8674(86)90602-1. [DOI] [PubMed] [Google Scholar]
- 13.Roingeard P, Lu SL, Sureau C, Freschlin M, Arbeille B, Essex M, Romet-Lemonne JL. Immunocytochemical and electron microscopic study of hepatitis B virus antigen and complete particle production in hepatitis B virus DNA transfected HepG2 cells. Hepatology. 1990;11:277–285. doi: 10.1002/hep.1840110219. [DOI] [PubMed] [Google Scholar]
- 14.Liaw YF, Chu CM, Lin DY, Sheen IS, Yang CY, Huang MJ. Age-specific prevalence and significance of hepatitis B e antigen and antibody in chronic hepatitis B virus infection in Taiwan: a comparison among asymptomatic carriers, chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma. J Med Virol. 1984;13:385–391. doi: 10.1002/jmv.1890130410. [DOI] [PubMed] [Google Scholar]
- 15.Yuen MF, Sablon E, Yuan HJ, Wong DK, Hui CK, Wong BC, Chan AO, Lai CL. Significance of hepatitis B genotype in acute exacerbation, HBeAg seroconversion, cirrhosis-related complications, and hepatocellular carcinoma. Hepatology. 2003;37:562–567. doi: 10.1053/jhep.2003.50098. [DOI] [PubMed] [Google Scholar]
- 16.Beasley RP, Hwang LY, Lee GC, Lan CC, Roan CH, Huang FY, Chen CL. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet. 1983;2:1099–1102. doi: 10.1016/s0140-6736(83)90624-4. [DOI] [PubMed] [Google Scholar]
- 17.Ganem D, Prince AM. Hepatitis B virus infection--natural history and clinical consequences. N Engl J Med. 2004;350:1118–1129. doi: 10.1056/NEJMra031087. [DOI] [PubMed] [Google Scholar]
- 18.Guidotti LG, Ishikawa T, Hobbs MV, Matzke B, Schreiber R, Chisari FV. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- 19.Jung MC, Spengler U, Schraut W, Hoffmann R, Zachoval R, Eisenburg J, Eichenlaub D, Riethmüller G, Paumgartner G, Ziegler-Heitbrock HW. Hepatitis B virus antigen-specific T-cell activation in patients with acute and chronic hepatitis B. J Hepatol. 1991;13:310–317. doi: 10.1016/0168-8278(91)90074-l. [DOI] [PubMed] [Google Scholar]
- 20.Lopes AR, Kellam P, Das A, Dunn C, Kwan A, Turner J, Peppa D, Gilson RJ, Gehring A, Bertoletti A, et al. Bim-mediated deletion of antigen-specific CD8 T cells in patients unable to control HBV infection. J Clin Invest. 2008;118:1835–1845. doi: 10.1172/JCI33402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schurich A, Khanna P, Lopes AR, Han KJ, Peppa D, Micco L, Nebbia G, Kennedy PT, Geretti AM, Dusheiko G, et al. Role of the coinhibitory receptor cytotoxic T lymphocyte antigen-4 on apoptosis-Prone CD8 T cells in persistent hepatitis B virus infection. Hepatology. 2011;53:1494–1503. doi: 10.1002/hep.24249. [DOI] [PubMed] [Google Scholar]
- 22.Sobao Y, Tomiyama H, Sugi K, Tokunaga M, Ueno T, Saito S, Fujiyama S, Morimoto M, Tanaka K, Takiguchi M. The role of hepatitis B virus-specific memory CD8 T cells in the control of viral replication. J Hepatol. 2002;36:105–115. doi: 10.1016/s0168-8278(01)00264-1. [DOI] [PubMed] [Google Scholar]
- 23.Wang S, Zhang X, Ju Y, Zhao B, Yan X, Hu J, Shi L, Yang L, Ma Z, Chen L, et al. MicroRNA-146a feedback suppresses T cell immune function by targeting Stat1 in patients with chronic hepatitis B. J Immunol. 2013;191:293–301. doi: 10.4049/jimmunol.1202100. [DOI] [PubMed] [Google Scholar]
- 24.Stoop JN, van der Molen RG, Baan CC, van der Laan LJ, Kuipers EJ, Kusters JG, Janssen HL. Regulatory T cells contribute to the impaired immune response in patients with chronic hepatitis B virus infection. Hepatology. 2005;41:771–778. doi: 10.1002/hep.20649. [DOI] [PubMed] [Google Scholar]
- 25.Op den Brouw ML, Binda RS, van Roosmalen MH, Protzer U, Janssen HL, van der Molen RG, Woltman AM. Hepatitis B virus surface antigen impairs myeloid dendritic cell function: a possible immune escape mechanism of hepatitis B virus. Immunology. 2009;126:280–289. doi: 10.1111/j.1365-2567.2008.02896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunn C, Brunetto M, Reynolds G, Christophides T, Kennedy PT, Lampertico P, Das A, Lopes AR, Borrow P, Williams K, et al. Cytokines induced during chronic hepatitis B virus infection promote a pathway for NK cell-mediated liver damage. J Exp Med. 2007;204:667–680. doi: 10.1084/jem.20061287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kägi D, Vignaux F, Ledermann B, Bürki K, Depraetere V, Nagata S, Hengartner H, Golstein P. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994;265:528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- 28.Maini MK, Boni C, Lee CK, Larrubia JR, Reignat S, Ogg GS, King AS, Herberg J, Gilson R, Alisa A, et al. The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J Exp Med. 2000;191:1269–1280. doi: 10.1084/jem.191.8.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48:835–847. doi: 10.1016/j.jhep.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678–686. doi: 10.1053/j.gastro.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 31.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 32.de Jongh FE, Janssen HL, de Man RA, Hop WC, Schalm SW, van Blankenstein M. Survival and prognostic indicators in hepatitis B surface antigen-positive cirrhosis of the liver. Gastroenterology. 1992;103:1630–1635. doi: 10.1016/0016-5085(92)91188-a. [DOI] [PubMed] [Google Scholar]
- 33.Tseng TC, Liu CJ, Su TH, Yang HC, Wang CC, Chen CL, Kuo SF, Liu CH, Chen PJ, Chen DS, et al. Young chronic hepatitis B patients with nucleos(t)ide analogue-induced hepatitis B e antigen seroconversion have a higher risk of HBV reactivation. J Infect Dis. 2012;206:1521–1531. doi: 10.1093/infdis/jis569. [DOI] [PubMed] [Google Scholar]
- 34.Papatheodoridis GV, Manolakopoulos S, Liaw YF, Lok A. Follow-up and indications for liver biopsy in HBeAg-negative chronic hepatitis B virus infection with persistently normal ALT: a systematic review. J Hepatol. 2012;57:196–202. doi: 10.1016/j.jhep.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 35.Tseng TC, Liu CJ, Yang HC, Su TH, Wang CC, Chen CL, Hsu CA, Kuo SF, Liu CH, Chen PJ, et al. Serum hepatitis B surface antigen levels help predict disease progression in patients with low hepatitis B virus loads. Hepatology. 2013;57:441–450. doi: 10.1002/hep.26041. [DOI] [PubMed] [Google Scholar]
- 36.Tseng TC, Liu CJ, Yang HC, Su TH, Wang CC, Chen CL, Kuo SF, Liu CH, Chen PJ, Chen DS, et al. High levels of hepatitis B surface antigen increase risk of hepatocellular carcinoma in patients with low HBV load. Gastroenterology. 2012;142:1140–1149.e3; quiz e13-14. doi: 10.1053/j.gastro.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Kim HC, Nam CM, Jee SH, Han KH, Oh DK, Suh I. Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. BMJ. 2004;328:983. doi: 10.1136/bmj.38050.593634.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooksley WG, Piratvisuth T, Lee SD, Mahachai V, Chao YC, Tanwandee T, Chutaputti A, Chang WY, Zahm FE, Pluck N. Peginterferon alpha-2a (40 kDa): an advance in the treatment of hepatitis B e antigen-positive chronic hepatitis B. J Viral Hepat. 2003;10:298–305. doi: 10.1046/j.1365-2893.2003.00450.x. [DOI] [PubMed] [Google Scholar]
- 39.Marcellin P, Lau GK, Bonino F, Farci P, Hadziyannis S, Jin R, Lu ZM, Piratvisuth T, Germanidis G, Yurdaydin C, et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2004;351:1206–1217. doi: 10.1056/NEJMoa040431. [DOI] [PubMed] [Google Scholar]
- 40.Lau GK, Piratvisuth T, Luo KX, Marcellin P, Thongsawat S, Cooksley G, Gane E, Fried MW, Chow WC, Paik SW, et al. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005;352:2682–2695. doi: 10.1056/NEJMoa043470. [DOI] [PubMed] [Google Scholar]
- 41.Janssen HL, van Zonneveld M, Senturk H, Zeuzem S, Akarca US, Cakaloglu Y, Simon C, So TM, Gerken G, de Man RA, Niesters HG, Zondervan P, Hansen B, Schalm SW. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet. 2005;365:123–129. doi: 10.1016/S0140-6736(05)17701-0. [DOI] [PubMed] [Google Scholar]
- 42.van Nunen AB, Hansen BE, Suh DJ, Löhr HF, Chemello L, Fontaine H, Heathcote J, Song BC, Janssen HL, de Man RA, et al. Durability of HBeAg seroconversion following antiviral therapy for chronic hepatitis B: relation to type of therapy and pretreatment serum hepatitis B virus DNA and alanine aminotransferase. Gut. 2003;52:420–424. doi: 10.1136/gut.52.3.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lai CL, Shouval D, Lok AS, Chang TT, Cheinquer H, Goodman Z, DeHertogh D, Wilber R, Zink RC, Cross A, et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2006;354:1011–1020. doi: 10.1056/NEJMoa051287. [DOI] [PubMed] [Google Scholar]
- 44.Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC, Lok AS, Han KH, Goodman Z, Zhu J, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354:1001–1010. doi: 10.1056/NEJMoa051285. [DOI] [PubMed] [Google Scholar]
- 45.Leung N, Peng CY, Hann HW, Sollano J, Lao-Tan J, Hsu CW, Lesmana L, Yuen MF, Jeffers L, Sherman M, et al. Early hepatitis B virus DNA reduction in hepatitis B e antigen-positive patients with chronic hepatitis B: A randomized international study of entecavir versus adefovir. Hepatology. 2009;49:72–79. doi: 10.1002/hep.22658. [DOI] [PubMed] [Google Scholar]
- 46.Gish RG, Lok AS, Chang TT, de Man RA, Gadano A, Sollano J, Han KH, Chao YC, Lee SD, Harris M, et al. Entecavir therapy for up to 96 weeks in patients with HBeAg-positive chronic hepatitis B. Gastroenterology. 2007;133:1437–1444. doi: 10.1053/j.gastro.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 47.Chang TT, Lai CL, Kew Yoon S, Lee SS, Coelho HS, Carrilho FJ, Poordad F, Halota W, Horsmans Y, Tsai N, et al. Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2010;51:422–430. doi: 10.1002/hep.23327. [DOI] [PubMed] [Google Scholar]
- 48.Zoutendijk R, Reijnders JG, Zoulim F, Brown A, Mutimer DJ, Deterding K, Hofmann WP, Petersen J, Fasano M, Buti M, et al. Virological response to entecavir is associated with a better clinical outcome in chronic hepatitis B patients with cirrhosis. Gut. 2013;62:760–765. doi: 10.1136/gutjnl-2012-302024. [DOI] [PubMed] [Google Scholar]
- 49.Tenney DJ, Rose RE, Baldick CJ, Pokornowski KA, Eggers BJ, Fang J, Wichroski MJ, Xu D, Yang J, Wilber RB, et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naïve patients is rare through 5 years of therapy. Hepatology. 2009;49:1503–1514. doi: 10.1002/hep.22841. [DOI] [PubMed] [Google Scholar]
- 50.Woo G, Tomlinson G, Nishikawa Y, Kowgier M, Sherman M, Wong DK, Pham B, Ungar WJ, Einarson TR, Heathcote EJ, et al. Tenofovir and entecavir are the most effective antiviral agents for chronic hepatitis B: a systematic review and Bayesian meta-analyses. Gastroenterology. 2010;139:1218–1229. doi: 10.1053/j.gastro.2010.06.042. [DOI] [PubMed] [Google Scholar]
- 51.Marcellin P, Heathcote EJ, Buti M, Gane E, de Man RA, Krastev Z, Germanidis G, Lee SS, Flisiak R, Kaita K, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359:2442–2455. doi: 10.1056/NEJMoa0802878. [DOI] [PubMed] [Google Scholar]
- 52.Heathcote EJ, Marcellin P, Buti M, Gane E, De Man RA, Krastev Z, Germanidis G, Lee SS, Flisiak R, Kaita K, et al. Three-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B. Gastroenterology. 2011;140:132–143. doi: 10.1053/j.gastro.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 53.Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, Washington MK, Germanidis G, Flaherty JF, Schall RA, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468–475. doi: 10.1016/S0140-6736(12)61425-1. [DOI] [PubMed] [Google Scholar]
- 54.Kitrinos KM, Corsa A, Liu Y, Flaherty J, Snow-Lampart A, Marcellin P, Borroto-Esoda K, Miller MD. No detectable resistance to tenofovir disoproxil fumarate after 6 years of therapy in patients with chronic hepatitis B. Hepatology. 2014;59:434–442. doi: 10.1002/hep.26686. [DOI] [PubMed] [Google Scholar]
- 55.Low TL, Goldstein AL. Thymosins: structure, function and therapeutic applications. Thymus. 1984;6:27–42. [PubMed] [Google Scholar]
- 56.Chan HL, Tang JL, Tam W, Sung JJ. The efficacy of thymosin in the treatment of chronic hepatitis B virus infection: a meta-analysis. Aliment Pharmacol Ther. 2001;15:1899–1905. doi: 10.1046/j.1365-2036.2001.01135.x. [DOI] [PubMed] [Google Scholar]
- 57.You J, Zhuang L, Cheng HY, Yan SM, Yu L, Huang JH, Tang BZ, Huang ML, Ma YL, Chongsuvivatwong V, et al. Efficacy of thymosin alpha-1 and interferon alpha in treatment of chronic viral hepatitis B: a randomized controlled study. World J Gastroenterol. 2006;12:6715–6721. doi: 10.3748/wjg.v12.i41.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen XS, Wang GJ, Cai X, Yu HY, Hu YP. Inhibition of hepatitis B virus by oxymatrine in vivo. World J Gastroenterol. 2001;7:49–52. doi: 10.3748/wjg.v7.i1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dong Y, Xi H, Yu Y, Wang Q, Jiang K, Li L. Effects of oxymatrine on the serum levels of T helper cell 1 and 2 cytokines and the expression of the S gene in hepatitis B virus S gene transgenic mice: a study on the anti-hepatitis B virus mechanism of oxymatrine. J Gastroenterol Hepatol. 2002;17:1299–1306. doi: 10.1046/j.1440-1746.2002.02885.x. [DOI] [PubMed] [Google Scholar]
- 60.Lu LG, Zeng MD, Mao YM, Li JQ, Wan MB, Li CZ, Chen CW, Fu QC, Wang JY, She WM, et al. Oxymatrine therapy for chronic hepatitis B: a randomized double-blind and placebo-controlled multi-center trial. World J Gastroenterol. 2003;9:2480–2483. doi: 10.3748/wjg.v9.i11.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kao JH, Wu NH, Chen PJ, Lai MY, Chen DS. Hepatitis B genotypes and the response to interferon therapy. J Hepatol. 2000;33:998–1002. doi: 10.1016/s0168-8278(00)80135-x. [DOI] [PubMed] [Google Scholar]
- 62.Wai CT, Chu CJ, Hussain M, Lok AS. HBV genotype B is associated with better response to interferon therapy in HBeAg(+) chronic hepatitis than genotype C. Hepatology. 2002;36:1425–1430. doi: 10.1053/jhep.2002.37139. [DOI] [PubMed] [Google Scholar]
- 63.Erhardt A, Blondin D, Hauck K, Sagir A, Kohnle T, Heintges T, Häussinger D. Response to interferon alfa is hepatitis B virus genotype dependent: genotype A is more sensitive to interferon than genotype D. Gut. 2005;54:1009–1013. doi: 10.1136/gut.2004.060327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Flink HJ, van Zonneveld M, Hansen BE, de Man RA, Schalm SW, Janssen HL. Treatment with Peg-interferon alpha-2b for HBeAg-positive chronic hepatitis B: HBsAg loss is associated with HBV genotype. Am J Gastroenterol. 2006;101:297–303. doi: 10.1111/j.1572-0241.2006.00418.x. [DOI] [PubMed] [Google Scholar]
- 65.Buster EH, Flink HJ, Cakaloglu Y, Simon K, Trojan J, Tabak F, So TM, Feinman SV, Mach T, Akarca US, Schutten M, Tielemans W, van Vuuren AJ, Hansen BE, Janssen HL. Sustained HBeAg and HBsAg loss after long-term follow-up of HBeAg-positive patients treated with peginterferon alpha-2b. Gastroenterology. 2008;135:459–467. doi: 10.1053/j.gastro.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 66.Buster EH, Hansen BE, Lau GK, Piratvisuth T, Zeuzem S, Steyerberg EW, Janssen HL. Factors that predict response of patients with hepatitis B e antigen-positive chronic hepatitis B to peginterferon-alfa. Gastroenterology. 2009;137:2002–2009. doi: 10.1053/j.gastro.2009.08.061. [DOI] [PubMed] [Google Scholar]
- 67.Lampertico P, Viganò M, Cheroni C, Facchetti F, Invernizzi F, Valveri V, Soffredini R, Abrignani S, De Francesco R, Colombo M. IL28B polymorphisms predict interferon-related hepatitis B surface antigen seroclearance in genotype D hepatitis B e antigen-negative patients with chronic hepatitis B. Hepatology. 2013;57:890–896. doi: 10.1002/hep.25749. [DOI] [PubMed] [Google Scholar]
- 68.Sonneveld MJ, Wong VW, Woltman AM, Wong GL, Cakaloglu Y, Zeuzem S, Buster EH, Uitterlinden AG, Hansen BE, Chan HL, et al. Polymorphisms near IL28B and serologic response to peginterferon in HBeAg-positive patients with chronic hepatitis B. Gastroenterology. 2012;142:513–520.e1. doi: 10.1053/j.gastro.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 69.Wu X, Xin Z, Zhu X, Pan L, Li Z, Li H, Liu Y. Evaluation of susceptibility locus for response to interferon-α based therapy in chronic hepatitis B patients in Chinese. Antiviral Res. 2012;93:297–300. doi: 10.1016/j.antiviral.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 70.Piratvisuth T, Marcellin P, Popescu M, Kapprell HP, Rothe V, Lu ZM Hepatitis B surface antigen: association with sustained response to peginterferon alfa-2a in hepatitis B e antigen-positive patients. Hepatol Int. 2011:Jun 24; Epub ahead of print. doi: 10.1007/s12072-011-9280-0. [DOI] [PubMed] [Google Scholar]
- 71.Liaw YF, Jia JD, Chan HL, Han KH, Tanwandee T, Chuang WL, Tan DM, Chen XY, Gane E, Piratvisuth T, et al. Shorter durations and lower doses of peginterferon alfa-2a are associated with inferior hepatitis B e antigen seroconversion rates in hepatitis B virus genotypes B or C. Hepatology. 2011;54:1591–1599. doi: 10.1002/hep.24555. [DOI] [PubMed] [Google Scholar]
- 72.Rijckborst V, Hansen BE, Cakaloglu Y, Ferenci P, Tabak F, Akdogan M, Simon K, Akarca US, Flisiak R, Verhey E, Van Vuuren AJ, Boucher CA, ter Borg MJ, Janssen HL. Early on-treatment prediction of response to peginterferon alfa-2a for HBeAg-negative chronic hepatitis B using HBsAg and HBV DNA levels. Hepatology. 2010;52:454–461. doi: 10.1002/hep.23722. [DOI] [PubMed] [Google Scholar]
- 73.Lampertico P, Viganò M, Di Costanzo GG, Sagnelli E, Fasano M, Di Marco V, Boninsegna S, Farci P, Fargion S, Giuberti T, et al. Randomised study comparing 48 and 96 weeks peginterferon α-2a therapy in genotype D HBeAg-negative chronic hepatitis B. Gut. 2013;62:290–298. doi: 10.1136/gutjnl-2011-301430. [DOI] [PubMed] [Google Scholar]
- 74.Wiens A, Lenzi L, Venson R, Correr CJ, Rotta I, Pedroso ML, Pontarolo R. Comparative efficacy of oral nucleoside or nucleotide analog monotherapy used in chronic hepatitis B: a mixed-treatment comparison meta-analysis. Pharmacotherapy. 2013;33:144–151. doi: 10.1002/phar.1188. [DOI] [PubMed] [Google Scholar]
- 75.Schmutz G, Nelson M, Lutz T, Sheldon J, Bruno R, von Boemmel F, Hoffmann C, Rockstroh J, Stoehr A, Wolf E, et al. Combination of tenofovir and lamivudine versus tenofovir after lamivudine failure for therapy of hepatitis B in HIV-coinfection. AIDS. 2006;20:1951–1954. doi: 10.1097/01.aids.0000247116.89455.5d. [DOI] [PubMed] [Google Scholar]
- 76.Petersen J, Ratziu V, Buti M, Janssen HL, Brown A, Lampertico P, Schollmeyer J, Zoulim F, Wedemeyer H, Sterneck M, et al. Entecavir plus tenofovir combination as rescue therapy in pre-treated chronic hepatitis B patients: an international multicenter cohort study. J Hepatol. 2012;56:520–526. doi: 10.1016/j.jhep.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 77.Lim LY, Patterson S, George J, Strasser SI, Lee AU, Sievert W, Nicoll AJ, Roberts SK, Desmond P V, Bowden S, et al. Tenofovir Rescue Therapy Achieves Long-Term Suppression of HBV Replication in Patients with Multi-Drug Resistant HBV: 4 Year Follow-Up of the TDF109 Cohort. Hepatology. 2012;56:368A. [Google Scholar]
- 78.Guclu E, Karabay O. Choice of drugs in the treatment of chronic hepatitis B in pregnancy. World J Gastroenterol. 2013;19:1671–1672. doi: 10.3748/wjg.v19.i10.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dore GJ, Cooper DA, Pozniak AL, DeJesus E, Zhong L, Miller MD, Lu B, Cheng AK. Efficacy of tenofovir disoproxil fumarate in antiretroviral therapy-naive and -experienced patients coinfected with HIV-1 and hepatitis B virus. J Infect Dis. 2004;189:1185–1192. doi: 10.1086/380398. [DOI] [PubMed] [Google Scholar]
- 80.Lok AS, Trinh H, Carosi G, Akarca US, Gadano A, Habersetzer F, Sievert W, Wong D, Lovegren M, Cohen D, Llamoso C. Efficacy of entecavir with or without tenofovir disoproxil fumarate for nucleos(t)ide-naïve patients with chronic hepatitis B. Gastroenterology. 2012;143:619–628.e1. doi: 10.1053/j.gastro.2012.05.037. [DOI] [PubMed] [Google Scholar]
- 81.Wang LC, Chen EQ, Cao J, Liu L, Zheng L, Li DJ, Xu L, Lei XZ, Liu C, Tang H. De novo combination of lamivudine and adefovir versus entecavir monotherapy for the treatment of naïve HBeAg-negative chronic hepatitis B patients. Hepatol Int. 2011;5:671–676. doi: 10.1007/s12072-010-9243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barbaro G, Zechini F, Pellicelli AM, Francavilla R, Scotto G, Bacca D, Bruno M, Babudieri S, Annese M, Matarazzo F, et al. Long-term efficacy of interferon alpha-2b and lamivudine in combination compared to lamivudine monotherapy in patients with chronic hepatitis B. An Italian multicenter, randomized trial. J Hepatol. 2001;35:406–411. doi: 10.1016/s0168-8278(01)00145-3. [DOI] [PubMed] [Google Scholar]
- 83.Wursthorn K, Lutgehetmann M, Dandri M, Volz T, Buggisch P, Zollner B, Longerich T, Schirmacher P, Metzler F, Zankel M, et al. Peginterferon alpha-2b plus adefovir induce strong cccDNA decline and HBsAg reduction in patients with chronic hepatitis B. Hepatology. 2006;44:675–684. doi: 10.1002/hep.21282. [DOI] [PubMed] [Google Scholar]
- 84.Sonneveld MJ, Xie Q, Zhang NP, Zhang Q, Fehmi T, Streinu-cercel A, Wang J, Idilman R, de Niet A, Diculescu M, et al. Adding peginterferon alfa-2a to entecavir increases HBsAg decline and HBeAg clearance - first results from a global randomized trial (ARES study) Hepatology. 2012;56:S199A. [Google Scholar]
- 85.Wang LC, Chen EQ, Cao J, Liu L, Wang JR, Lei BJ, Tang H. Combination of Lamivudine and adefovir therapy in HBeAg-positive chronic hepatitis B patients with poor response to adefovir monotherapy. J Viral Hepat. 2010;17:178–184. doi: 10.1111/j.1365-2893.2009.01164.x. [DOI] [PubMed] [Google Scholar]
- 86.Kim SS, Cheong JY, Lee D, Lee MH, Hong SP, Kim SO, Cho SW. Adefovir-based combination therapy with entecavir or lamivudine for patients with entecavir-refractory chronic hepatitis B. J Med Virol. 2012;84:18–25. doi: 10.1002/jmv.22227. [DOI] [PubMed] [Google Scholar]
- 87.Lampertico P, Viganò M, Manenti E, Iavarone M, Sablon E, Colombo M. Low resistance to adefovir combined with lamivudine: a 3-year study of 145 lamivudine-resistant hepatitis B patients. Gastroenterology. 2007;133:1445–1451. doi: 10.1053/j.gastro.2007.08.079. [DOI] [PubMed] [Google Scholar]
- 88.Wei X, Peterson DL. Expression, purification, and characterization of an active RNase H domain of the hepatitis B viral polymerase. J Biol Chem. 1996;271:32617–32622. doi: 10.1074/jbc.271.51.32617. [DOI] [PubMed] [Google Scholar]
- 89.Lok AS, Lai CL, Leung N, Yao GB, Cui ZY, Schiff ER, Dienstag JL, Heathcote EJ, Little NR, Griffiths DA, et al. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology. 2003;125:1714–1722. doi: 10.1053/j.gastro.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 90.Zhao P, Wang C, Huang L, Xu D, Li T. Comparison of rescue strategies in lamivudine-resistant patients with chronic hepatitis B. Antiviral Res. 2012;96:100–104. doi: 10.1016/j.antiviral.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 91.Tenney DJ, Rose RE, Baldick CJ, Levine SM, Pokornowski KA, Walsh AW, Fang J, Yu CF, Zhang S, Mazzucco CE, et al. Two-year assessment of entecavir resistance in Lamivudine-refractory hepatitis B virus patients reveals different clinical outcomes depending on the resistance substitutions present. Antimicrob Agents Chemother. 2007;51:902–911. doi: 10.1128/AAC.00833-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang YP, Zhao W, Xue R, Zhou ZX, Liu F, Han YX, Ren G, Peng ZG, Cen S, Chen HS, et al. Oxymatrine inhibits hepatitis B infection with an advantage of overcoming drug-resistance. Antiviral Res. 2011;89:227–231. doi: 10.1016/j.antiviral.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 93.Sheldon J, Camino N, Rodés B, Bartholomeusz A, Kuiper M, Tacke F, Núñez M, Mauss S, Lutz T, Klausen G, et al. Selection of hepatitis B virus polymerase mutations in HIV-coinfected patients treated with tenofovir. Antivir Ther. 2005;10:727–734. [PubMed] [Google Scholar]
- 94.Pol S, Sogni P. [Treatment of chronic hepatitis B: adherence and safety] Gastroenterol Clin Biol. 2010;34 Suppl 2:S142–S148. doi: 10.1016/S0399-8320(10)70034-8. [DOI] [PubMed] [Google Scholar]
- 95.Fattovich G, Olivari N, Pasino M, D’Onofrio M, Martone E, Donato F. Long-term outcome of chronic hepatitis B in Caucasian patients: mortality after 25 years. Gut. 2008;57:84–90. doi: 10.1136/gut.2007.128496. [DOI] [PubMed] [Google Scholar]
- 96.Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, Marcellin P, Lim SG, Goodman Z, Ma J, et al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B. N Engl J Med. 2005;352:2673–2681. doi: 10.1056/NEJMoa042957. [DOI] [PubMed] [Google Scholar]
- 97.Jeng WJ, Sheen IS, Chen YC, Hsu CW, Chien RN, Chu CM, Liaw YF. Off-therapy durability of response to entecavir therapy in hepatitis B e antigen-negative chronic hepatitis B patients. Hepatology. 2013;58:1888–1896. doi: 10.1002/hep.26549. [DOI] [PubMed] [Google Scholar]
- 98.Invernizzi F, Lampertico P, Loglio A, Iavarone M, Viganò M, Facchetti F, Vezali E, Lunghi G, Colombo M. Nucleos(t)ide analogues can be safely discontinued in chronic hepatitis B patients achieving HBsAg seroclearance. Hepatology. 2012;56:368A. [Google Scholar]
- 99.Chimalakonda KC, Agarwal HK, Kumar A, Parang K, Mehvar R. Synthesis, analysis, in vitro characterization, and in vivo disposition of a lamivudine-dextran conjugate for selective antiviral delivery to the liver. Bioconjug Chem. 2007;18:2097–2108. doi: 10.1021/bc700193d. [DOI] [PubMed] [Google Scholar]
- 100.Zhang XG, Miao J, Li MW, Jiang SP, Hu FQ, Du YZ. Solid lipid nanoparticles loading adefovir dipivoxil for antiviral therapy. J Zhejiang Univ Sci B. 2008;9:506–510. doi: 10.1631/jzus.B0820047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lim SG, Ng TM, Kung N, Krastev Z, Volfova M, Husa P, Lee SS, Chan S, Shiffman ML, Washington MK, et al. A double-blind placebo-controlled study of emtricitabine in chronic hepatitis B. Arch Intern Med. 2006;166:49–56. doi: 10.1001/archinte.166.1.49. [DOI] [PubMed] [Google Scholar]
- 102.Liaw YF, Sheen IS, Lee CM, Akarca US, Papatheodoridis GV, Suet-Hing Wong F, Chang TT, Horban A, Wang C, Kwan P, Buti M, Prieto M, Berg T, Kitrinos K, Peschell K, Mondou E, Frederick D, Rousseau F, Schiff ER. Tenofovir disoproxil fumarate (TDF), emtricitabine/TDF, and entecavir in patients with decompensated chronic hepatitis B liver disease. Hepatology. 2011;53:62–72. doi: 10.1002/hep.23952. [DOI] [PubMed] [Google Scholar]
- 103.Kosi L, Reiberger T, Payer BA, Grabmeier-Pfistershammer K, Strassl R, Rieger A, Peck-Radosavljevic M. Five-year on-treatment efficacy of lamivudine-, tenofovir- and tenofovir + emtricitabine-based HAART in HBV-HIV-coinfected patients. J Viral Hepat. 2012;19:801–810. doi: 10.1111/j.1365-2893.2012.01601.x. [DOI] [PubMed] [Google Scholar]
- 104.Yuen MF, Kim J, Kim CR, Ngai V, Yuen JC, Min C, Kang HM, Shin BS, Yoo SD, Lai CL. A randomized placebo-controlled, dose-finding study of oral LB80380 in HBeAg-positive patients with chronic hepatitis B. Antivir Ther. 2006;11:977–983. [PubMed] [Google Scholar]
- 105.Min C, Kim C, Steffy K, Averett D, Locarnini S, Shaw T. The active metabolite of LB80380/ANA380, a novel nucleotide analog, exhibits activity in vitro against multiple clinically relevant hepatitis B virus mutants. J Hepatol. 2007;46:S159. [Google Scholar]
- 106.Lai CL, Ahn SH, Lee KS, Um SH, Cho M, Yoon SK, Lee JW, Park NH, Kweon YO, Sohn JH, et al. Phase IIb multicentred randomised trial of besifovir (LB80380) versus entecavir in Asian patients with chronic hepatitis B. Gut. 2014;63:996–1004. doi: 10.1136/gutjnl-2013-305138. [DOI] [PubMed] [Google Scholar]
- 107.Yuen MF, Han KH, Um SH, Yoon SK, Kim HR, Kim J, Kim CR, Lai CL. Antiviral activity and safety of LB80380 in hepatitis B e antigen-positive chronic hepatitis B patients with lamivudine-resistant disease. Hepatology. 2010;51:767–776. doi: 10.1002/hep.23462. [DOI] [PubMed] [Google Scholar]
- 108.Lee JS, Park ET, Kang SS, Gu ES, Kim JS, Jang DS, Lee KS, Lee JS, Park NH, Bae CH, et al. Clevudine demonstrates potent antiviral activity in naïve chronic hepatitis B patients. Intervirology. 2010;53:83–86. doi: 10.1159/000264197. [DOI] [PubMed] [Google Scholar]
- 109.Yoo BC, Kim JH, Chung YH, Lee KS, Paik SW, Ryu SH, Han BH, Han JY, Byun KS, Cho M, et al. Twenty-four-week clevudine therapy showed potent and sustained antiviral activity in HBeAg-positive chronic hepatitis B. Hepatology. 2007;45:1172–1178. doi: 10.1002/hep.21629. [DOI] [PubMed] [Google Scholar]
- 110.Lau GK, Leung N. Forty-eight weeks treatment with clevudine 30 mg qd versus lamivudine 100 mg qd for chronic hepatitis B infection: a double-blind randomized study. Korean J Hepatol. 2010;16:315–320. doi: 10.3350/kjhep.2010.16.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yoon EL, Yim HJ, Lee HJ, Lee YS, Kim JH, Jung ES, Kim JH, Seo YS, Yeon JE, Lee HS, et al. Comparison of clevudine and entecavir for treatment-naive patients with chronic hepatitis B virus infection: two-year follow-up data. J Clin Gastroenterol. 2011;45:893–899. doi: 10.1097/MCG.0b013e31821f8bdf. [DOI] [PubMed] [Google Scholar]
- 112.Kwon SY, Park YK, Ahn SH, Cho ES, Choe WH, Lee CH, Kim BK, Ko SY, Choi HS, Park ES, et al. Identification and characterization of clevudine-resistant mutants of hepatitis B virus isolated from chronic hepatitis B patients. J Virol. 2010;84:4494–4503. doi: 10.1128/JVI.02066-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Seok JI, Lee DK, Lee CH, Park MS, Kim SY, Kim HS, Jo HY, Lee CH, Kim DS. Long-term therapy with clevudine for chronic hepatitis B can be associated with myopathy characterized by depletion of mitochondrial DNA. Hepatology. 2009;49:2080–2086. doi: 10.1002/hep.22959. [DOI] [PubMed] [Google Scholar]
- 114.Pollicino T, Belloni L, Raffa G, Pediconi N, Squadrito G, Raimondo G, Levrero M. Hepatitis B virus replication is regulated by the acetylation status of hepatitis B virus cccDNA-bound H3 and H4 histones. Gastroenterology. 2006;130:823–837. doi: 10.1053/j.gastro.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 115.Zimmerman KA, Fischer KP, Joyce MA, Tyrrell DL. Zinc finger proteins designed to specifically target duck hepatitis B virus covalently closed circular DNA inhibit viral transcription in tissue culture. J Virol. 2008;82:8013–8021. doi: 10.1128/JVI.00366-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cai D, Mills C, Yu W, Yan R, Aldrich CE, Saputelli JR, Mason WS, Xu X, Guo JT, Block TM, et al. Identification of disubstituted sulfonamide compounds as specific inhibitors of hepatitis B virus covalently closed circular DNA formation. Antimicrob Agents Chemother. 2012;56:4277–4288. doi: 10.1128/AAC.00473-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wooddell CI, Rozema DB, Hossbach M, John M, Hamilton HL, Chu Q, Hegge JO, Klein JJ, Wakefield DH, Oropeza CE, et al. Hepatocyte-targeted RNAi therapeutics for the treatment of chronic hepatitis B virus infection. Mol Ther. 2013;21:973–985. doi: 10.1038/mt.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Petersen J, Dandri M, Mier W, Lütgehetmann M, Volz T, von Weizsäcker F, Haberkorn U, Fischer L, Pollok JM, Erbes B, et al. Prevention of hepatitis B virus infection in vivo by entry inhibitors derived from the large envelope protein. Nat Biotechnol. 2008;26:335–341. doi: 10.1038/nbt1389. [DOI] [PubMed] [Google Scholar]
- 119.Volz T, Allweiss L, Ben MBarek M, Warlich M, Lohse AW, Pollok JM, Alexandrov A, Urban S, Petersen J, Lütgehetmann M, et al. The entry inhibitor Myrcludex-B efficiently blocks intrahepatic virus spreading in humanized mice previously infected with hepatitis B virus. J Hepatol. 2013;58:861–867. doi: 10.1016/j.jhep.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 120.Al-Mahtab M, Bazinet M, Vaillant A. Establishment of a potent anti-HBsAg response and durable immunological control of viremia with short term immunotherapy after REP 9AC-induced HBsAg seroclearance in chronic HBV infection (Internet) J Hepatol. 2013;58:S316. [Google Scholar]
- 121.Ilan Y, Nagler A, Adler R, Tur-Kaspa R, Slavin S, Shouval D. Ablation of persistent hepatitis B by bone marrow transplantation from a hepatitis B-immune donor. Gastroenterology. 1993;104:1818–1821. doi: 10.1016/0016-5085(93)90664-x. [DOI] [PubMed] [Google Scholar]
- 122.Couillin I, Pol S, Mancini M, Driss F, Bréchot C, Tiollais P, Michel ML. Specific vaccine therapy in chronic hepatitis B: induction of T cell proliferative responses specific for envelope antigens. J Infect Dis. 1999;180:15–26. doi: 10.1086/314828. [DOI] [PubMed] [Google Scholar]
- 123.Heathcote J, McHutchison J, Lee S, Tong M, Benner K, Minuk G, Wright T, Fikes J, Livingston B, Sette A, et al. A pilot study of the CY-1899 T-cell vaccine in subjects chronically infected with hepatitis B virus. The CY1899 T Cell Vaccine Study Group. Hepatology. 1999;30:531–536. doi: 10.1002/hep.510300208. [DOI] [PubMed] [Google Scholar]
- 124.Hong SH, Cho O, Kim K, Shin HJ, Kotenko SV, Park S. Effect of interferon-lambda on replication of hepatitis B virus in human hepatoma cells. Virus Res. 2007;126:245–249. doi: 10.1016/j.virusres.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 125.Nakagawa S, Hirata Y, Kameyama T, Tokunaga Y, Nishito Y, Hirabayashi K, Yano J, Ochiya T, Tateno C, Tanaka Y, et al. Targeted induction of interferon-λ in humanized chimeric mouse liver abrogates hepatotropic virus infection. PLoS One. 2013;8:e59611. doi: 10.1371/journal.pone.0059611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chan H, Ahn S, Chang T, Peng C, Wong D, Coffin C, Lim S, Chen P, Janssen H, Marcellin P, Serfaty L, Zeuzem S, Hu W, Critelli L, Lopez-Talavera J, Cooney E. Peginterferon Lambda for the Treatment of HBeAg-Positive Chronic Hepatitis B: A Phase 2B Comparison with Peginterferon Alfa; APASL Liver Week; 2013 June 6-10; Singapore. 2013. [DOI] [PubMed] [Google Scholar]
- 127.Zeuzem S, Arora S, Bacon B, Box T, Charlton M, Diago M, Dieterich D, Mur RE, Everson G, Fallon M, et al. Peginterferon Lambda-1a (lambda) compared to peginterferon alfa-2a (alfa) in treatment-naive patients with HCV genotypes (G) 2 or 3: First SVR24 results from EMERGE phase IIB. J Hepatol. 2012;56 Supple:S5–S6. [Google Scholar]
- 128.Lanford RE, Guerra B, Chavez D, Giavedoni L, Hodara VL, Brasky KM, Fosdick A, Frey CR, Zheng J, Wolfgang G, et al. GS-9620, an oral agonist of Toll-like receptor-7, induces prolonged suppression of hepatitis B virus in chronically infected chimpanzees. Gastroenterology. 2013;144:1508–1517, 1517.e1-e10. doi: 10.1053/j.gastro.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


