Abstract
Background and Purpose
HeADDFIRST was a randomized pilot study to obtain information necessary to design a Phase III trial to evaluate the benefit of surgical decompression for brain swelling from large supratentorial cerebral hemispheric infarction (LSCHI).
Methods
All stroke patients were screened for eligibility [age 18–75, NIHSS ≥ 18 with Item 1a < 2 (responsive to minor stimulation), and CT demonstrating unilateral, complete MCA territory infarction by specific imaging criteria]. All enrolled patients were treated using a standardized medical treatment protocol. Those with both ≥ 4 mm of pineal shift and deterioration in level of arousal or ≥ 7.5 mm of anteroseptal shift within 96 hours of stroke onset were randomized to continued Medical Treatment Only (MTO) or Medical Treatment plus Surgery (MTS). Death at 21 days was the primary outcome measure.
Results
Among 4,909 screened patients, only 66 (1.3%) were eligible for HeADDFIRST. Forty patients were enrolled, and 26 developed the requisite brain swelling for randomization. All who failed to meet randomization criteria were alive at 21 days. Mortality at 21 and 180 days was 40% (4/10) in the MTO and 21% (3/14) and 36% (5/14) in the MTS arms, respectively.
Conclusions
HeADDFIRST randomization criteria effectively distinguished low from high risk of death from LSCHI. Lower mortality in the MTO group than in other published trials suggests a possible benefit to standardizing medical management. These results can inform the interpretation of recently completed European trials regarding patient selection and medical management.
Keywords: stroke, cerebral edema, craniectomy, standardized medical management
Introduction
Large supratentorial cerebral hemispheric infarctions can cause life-threatening brain swelling, frequently leading to death within the first week.1,2 The well-recognized and widely published high mortality with infarctions of this magnitude has led to increasing acceptance of the potential merits of surgical decompression in selected patients with hemicraniectomy and durotomy.
HeADDFIRST was a NINDS-sponsored pilot clinical trial designed to provide the information necessary to design and implement a Phase III study to evaluate the benefit of surgical decompression for life-threatening swelling from large supratentorial cerebral hemispheric infarction. The primary aims were: (1) to estimate the proportions of stroke patients who would be eligible and of eligible patients who would deteriorate sufficiently to become randomizable (described below) using well-defined criteria appropriate for a Phase III trial, (2) to estimate the proportion of eligible patients whose families would consent to participate in a randomized trial, and to determine whether treatment assignment can be maintained without cross-over, (3) to estimate the distribution of a variety of functional and quality of life outcomes at 21, 90, and 180 days in each treatment group, and (4) to determine whether outcome assessments can be conducted in a blinded fashion.
Since the completion of HeADDFIRST, there have been several published randomized clinical trials on the topic.3–5 However, several unique aspects of the design and conduct of HeADDFIRST allow the results to critically supplement the findings of these important studies.
Methods
Participating Center Selection
Twenty centers in North America participated in HeADDFIRST, each with its own neurologist investigator. Only investigators (neurologists and neurosurgeons) who were comfortable with randomizing all eligible patients without bias were included, and only medical centers that could guarantee screening and considering all eligible patients were included. All principal investigators were neurologists, and the care organization of each participating center required a neurologist with expertise in neurocritical care to be the primary managing physician throughout the hospitalization. The study protocol was approved by the Institutional Review Board at each participating center and at the Data Coordinating Center (DCC) located at the University of Chicago. A Performance and Safety Monitoring Board (PSMB) was constituted by NINDS to review study accrual and patient safety every six months.
Investigator Preparation and Protocol Development
Prior to study initiation, all medical and surgical investigators participated in a conference during which the study design and protocols were discussed and all treatment protocols were finalized. While there was significant controversy and variability among the investigators about what constituted best medical and surgical management, all investigators agreed to adhere to the developed protocols for the purposes of HeADDFIRST. This was true for both the medical and surgical treatment protocols. All participants (neurologists and neurosurgeons) were tested to evaluate their uniform understanding of the study design, relevant criteria, scan interpretation, and treatment protocols.
Overview of Study
All ischemic stroke patients were screened for study eligibility (inclusion/exclusion criteria). Eligible patients who consented to participate were then registered in HeADDFIRST, a Standardized Medical Management Protocol (SMMP) was initiated, and registered patients were monitored clinically using serial CT scans at specified times from stroke onset. Registered patients who deteriorated sufficiently to meet randomization criteria were randomized at that point to continued Medical Treatment Only (MTO) or Medical Treatment plus Surgery (MTS). Registered patients who did not meet randomization criteria were managed according to the same SMMP as those who met randomization criteria. Functional and quality-of-life outcomes were evaluated at 21, 90, and 180 days post-randomization. Details of the study protocol omitted below are described in an online supplement.
Patient Screening
All ischemic stroke patients admitted to each participating center were screened for four criteria: unilateral middle cerebral artery (MCA) stroke, 18–75 years old, National Institutes of Health Stroke Scale (NIHSS) score of ≥18, and responsive to minor stimulation (NIHSS Item 1a <2). Those who met these four criteria, satisfied the neuroimaging criterion of either hypodensity involving ≥50% of the MCA territory on a CT performed <5 hours after the stroke onset,6 or hypodensity involving the complete MCA territory on a CT performed <48 hours after stroke onset,1 and who met no exclusion criteria (Table 1) were deemed eligible, and those patients (and/or their surrogates) were approached for consent.
Table 1.
Exclusion Criteria
| Deterioration to randomizable condition prior to admission to the participating hospital |
| Confluent parenchymal hematoma |
| Subdural hematoma |
| Subarachnoid hemorrhage |
| PTT > 40 seconds |
| INR > 1.4 |
| Platelet count < 100 k/µL prior to correction with blood products |
| Pre-existing illness limiting life expectancy to less than 6 months |
| Pre-existing disability with modified Rankin > 2 |
| Pre-existing or concurrent brain injury with associated deficits in addition to principal stroke |
| Current participation in another clinical trial |
Consent, Registration, Randomization
Upon receipt of written informed consent, patients were registered into HeADDFIRST, admitted to a neuromonitoring or intensive care unit, and the SMMP and monitoring began. Registered patients who deteriorated sufficiently within 96 hours of stroke onset became randomizable, based on meeting one of the following criteria: (a) horizontal anterior septum pellucidum shift from the midline of at least 7.5 mm with unchanged or worse neurological examination,7 and/or (b) ≥4 mm of horizontal pineal shift from the midline8 with depression of arousability to the level of effortful awakening with immediate subsequent sleepiness or worse (NIHSS Item 1a ≥2). Patients were randomized either to continued MTO using the SMMP or to MTS using an ipsilateral standardized hemicraniectomy and durotomy performed within four hours of meeting randomization criteria.
Standardized Medical Management Protocol (SMMP)
All registered patients were cared for in a specialized neuromonitoring unit (intermediate or intensive care) with a consensus developed SMMP with the formal agreement of all investigators for required adherence to the protocol (not simply recommended) after in person training and an examination that assessed an understanding of the protocols and required adherence.
Randomization triggered transfer to a neurological intensive care unit (if not already in one) with placement of an arterial line, central venous catheter and an ipsilateral parenchymal intracranial pressure (ICP) monitor. A comprehensive protocol specified detailed procedures for airway management, ventilator settings, blood pressure control and agents, fluid and electrolyte management, gastrointestinal and nutritional management, hematological monitoring and management, intracranial pressure monitoring, sedation, use of mannitol, anticonvulsants, prophylaxis against deep-vein thrombosis, and rehabilitation. (Full details of the protocol are contained in the online supplement.)
Standardized Surgical Management Protocol (SHD)
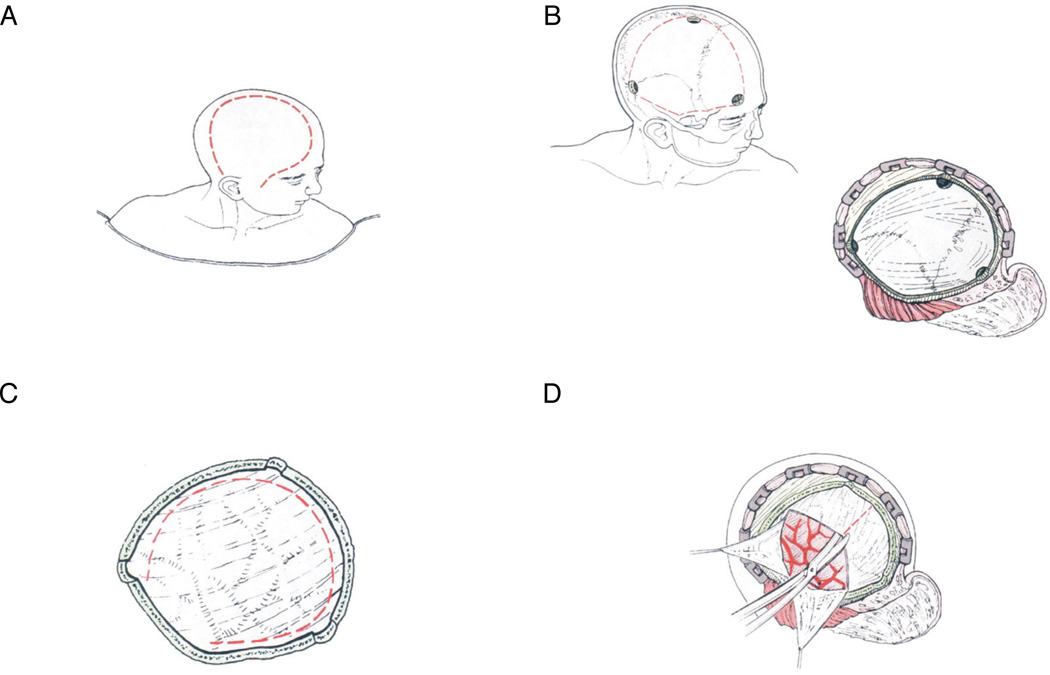
In patients who were randomized to surgical treatment, the SHD required rapid initiation of surgery, with a target of ≤4 hours from meeting criteria for randomization, in addition to continued compliance with the SMMP. The minimal surgical decompression boundaries were anteriorly from the floor of the anterior cranial fossa at the mid-pupillary line, posteriorly to 4 cm posterior to the external auditory canal, superiorly to 1 cm lateral to the superior sagittal sinus, and inferiorly to the floor of the middle cranial fossa (Figure 1). All patients underwent a durotomy (circumferential or cruciate) with dural grafting recommended but not required. No brain amputation was allowed in any case. Perioperative antibiotics (unspecified) were required for the first 24 hours after surgery. Post-operative dressings were non-compressive. Ventricular drains could not be used. All randomized patients required an ipsilateral parenchymal ICP monitor, frontally located. All bone flaps were saved in a bone bank, frozen in an antibiotic solution to be replaced within 3 months or earlier for either safety, medical, or cosmetic indications.
Figure 1.
HeADDFIRST Standardized Hemicraniectomy and Durotomy (SHD) Procedure. A: Incision; B: Simplified diagram to delineate craniotomy burr hole positioning and boundaries; C: Example of circumferential durotomy; D: Example of cruciate durotomy.
Outcome Assessment and Blinding
The primary endpoint was survival at 21 days following stroke onset. Secondary endpoints included the following: Modified Rankin Scale, NIHSS, Glasgow Outcome Scale, and Barthel Index Score. In addition, each randomized patient was evaluated by a trained blinded examiner at each participating center using the same functional outcome assessments at 90 and 180 days following onset. During the exam, patients wore a specially designed cap intended to mask any signs of surgery, and family and/or caregivers were instructed not to discuss the patient’s acute management with the examiner. After the exam, the examiner completed a questionnaire in which he or she was asked to guess the patient’s treatment assignment and to report on any conversation or other factors that might have revealed the patient’s treatment assignment. Finally, patients and their families were asked to complete the SF-36, Perceived Quality of Life Scale, and Caregiving Burden Scale at 90 and 180 days.
Randomization Procedures and Sample Size
In order to ensure that registration and randomization could be conducted quickly and efficiently, the Data Coordinating Center designed a web-based registration and randomization system. This permitted centers to register patients online, and, once registered, to obtain a treatment assignment immediately upon deterioration to randomizability. DCC staff and the Principal Investigator were notified immediately (via both pager and email) upon registration or randomization, and provided 24-hour support in case of problems accessing the site.
Randomization was performed in blocks of size four within each center and separately by hemispheric side (left or right). In addition, assignments were further restricted to guarantee that both treatments would be assigned within the first three patients enrolled at each center. The method of randomization was known only to the study statisticians (RT and PS).
The planned sample size was 75 randomized patients, which would have permitted us to estimate the mortality rate in each treatment group with a standard error of approximately 8%. This would have provided sufficient precision to verify that the mortality rate in the non-surgical group was high enough to warrant proposing a substantial benefit of surgery.
Monitoring for Adherence to Protocols
Every center was visited by the Principal Investigator (JF) after the first patient was randomized, and while the patient was still hospitalized. The chart orders were scrutinized for adherence to the key elements of the protocol as delineated in the supplementary data. Deviations were divided into minor and major. Minor deviations were related management variance that related to minor medication substitutions. Major deviations related to variances of neurological specific and critical care treatments such as the use of mannitol, fluid and electrolyte management, ICP-directed treatment decisions, hemodynamic management, and airway and ventilation management.
Data Analysis
Descriptive statistics (median, 25th and 75th percentiles, or frequency counts) were used to summarize the demographics, co-morbidities, and disease characteristics of the study groups. Fisher’s exact test was used to evaluate differences in categorical measures between groups.9 Confidence intervals (CI) for mortality rates in each treatment group were calculated using exact binomial methods10 and a confidence interval for the difference in mortality rates was calculated using the normal approximation.11 Statistical significance was defined as p < 0.05. Analyses and data management were performed using Stata.12
Results
From March 2000 to September 2002, 4,909 stroke patients were screened at 20 sites, representing all of the stroke patients admitted to the participating centers during the study period. Of these, 73% were between ages of 18 and 75 years, and of these, 32% had unilateral MCA strokes. Of those with the unilateral MCA strokes, only 18% had a NIHSS score >= 18, and of those, 56% had an item 1a < 2.
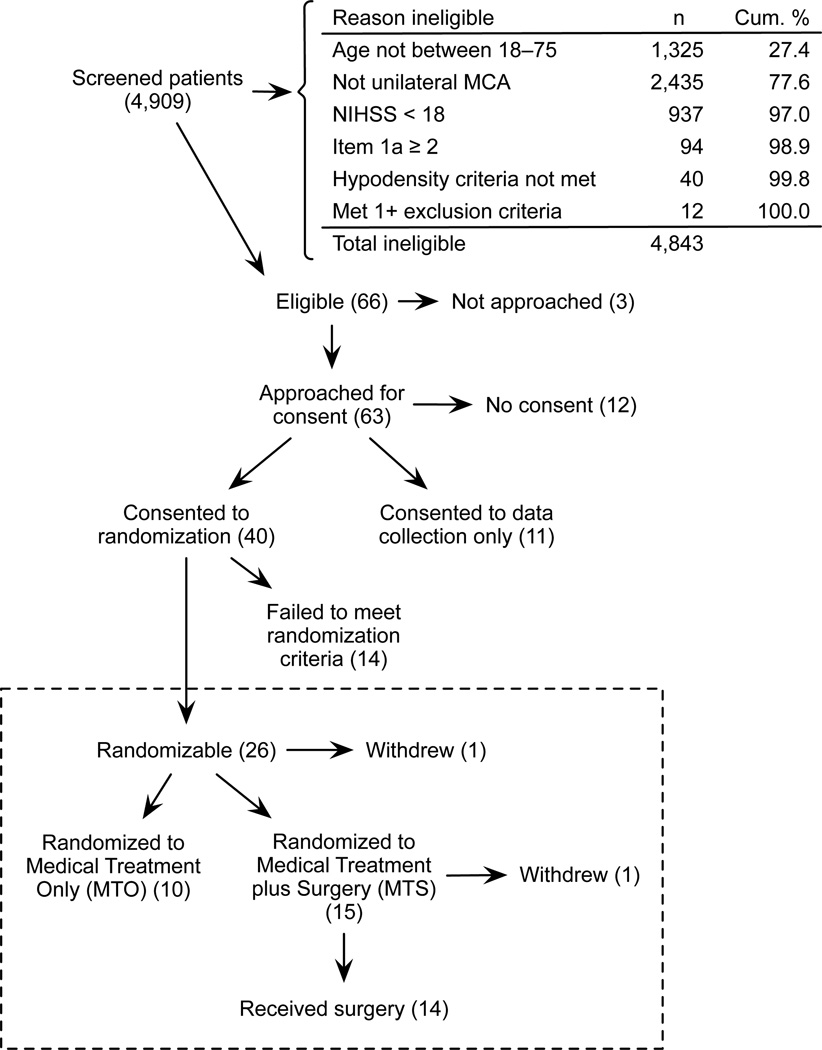
118 of the 4909 screened (2.4%) met all 4 screening criteria. Of these, 66 met the remaining eligibility criteria for participation in HeADDFIRST (Figure 2). Among the excluded 52, 40 failed to meet the hypodensity criteria, and another 12 met the hypodensity criteria but also met one or more of the exclusion criteria. After two years, we determined together with the Pilot Safety Monitoring Board that we had achieved our aims for the pilot study, and accrual was terminated.
Figure 2.
HeADDFIRST Study Flow Diagram.
Families for three of the eligible patients were not approached for participation because the patient and/or family had already decided to limit care (1), the family was not available (1), or for other reasons (1). Of the 63 who were approached, 40 (from 16 sites) consented to participate in the randomized portion of the trial. Common reasons for declining included making a decision to limit care (3), not wanting to participate in a research trial (6), not wanting treatment determined by randomization (6), wanting surgery (1), and not wanting surgery (5). Among the 23 who declined to participate in the randomized portion of the trial, 11 gave consent for data collection only.
Twenty-six patients progressed to meet the criteria for randomization, one of whom was not randomized due to the physician’s decision to limit treatment. The remaining 25 were randomized to MTO (10) or MTS (15). One of the patients who suffered a dominant hemispheric stroke and was randomized to surgery was withdrawn because his wife, after reflection, had come to believe that surgical treatment would be excessive given his likely future level of disability.
Table 2 shows the demographic and disease characteristics and co-morbidities of each study group. The data-collection-only group was similar in composition to the groups participating in the randomized portion of the trial. Median time from stroke onset to meeting randomization criteria was 52.5 hours in the medical group and 53.8 hours in the surgical group, with 75th percentiles of 64.4 and 80.4, respectively. Stroke characteristics and outcomes for individual randomized patients are provided in Table 3.
Table 2.
Baseline and Pre-randomization Characteristics
| MTO | MTS | RC not met | Data only | |
|---|---|---|---|---|
| N* | 10 | 14 | 14 | 11 |
| Background Characteristics† | ||||
| Age | 57.9 (45.4–65.8) | 52.3 (45.5–59.0) | 61.7 (45.0–72.0) | 62.9 (54.1–69.8) |
| Age 60+ (%) | 50 | 21 | 54 | 55 |
| Male (%) | 60 | 64 | 46 | 64 |
| History of stroke (%) | 10 | 7 | 8 | 0 |
| Hypertension (%) | 70 | 57 | 62 | 73 |
| CAD (%) | 10 | 14 | 15 | 18 |
| Arrhythmia (%) | 40 | 7 | 23 | 27 |
| Diabetes (%) | 30 | 7 | 15 | 0 |
| Current smoker (%) | 60 | 71 | 31 | 45 |
| Dominant hemisphere (%) | 50 | 36 | 50 | 55 |
| Registration | ||||
| NIHSS | 19.0 (18.8–21.3) | 21.5 (18.8–23.3) | 19.5 (18.0–21.3) | 20.0 (18.0–22.0) |
| Pineal Shift (mm)‡ | 0.0 (0.0–2.0) | 0.0 (0.0–2.0) | 0.0 (0.0–0.0) N=12 | 0.0 (0.0–3.0) |
| Septal Shift (mm)‡ | 0.0 (0.0–6.0) | 0.0 (0.0–5.0) | 0.0 (0.0–0.0) N=12 | 2.0 (0.0–5.0) |
| Volume (cc) ‡ | 163.0 (137.0–230.0) | 198.0 (183.0–214.0) | 150.0 (126.5–206.0) N=13 | 172.0 (126.0–227.0) |
| Randomization | ||||
| Randomizable (%) | 100 | 100 | - | - |
| Onset to meeting RC (hrs) | 52.5 (29.5–64.4) | 53.8 (27.7–80.4) | - | - |
| <24 hrs (%) | 10 | 21 | ||
| <48 hrs (%) | 40 | 43 | ||
| NIHSS | 21.5 (19.5–23.8) | 23.0 (20.5–27.5) | - | - |
| Pineal Shift (mm) | 6.0 (4.8–6.5) | 5.5 (4.0–8.0) | - | - |
| Septal Shift (mm) | 9.5 (7.8–11.0) | 9.0 (8.0–10.3) | - | - |
Numbers in table are median (25%-75%) unless otherwise stated. MTO = Medical Treatment Only, MTS = Medical Treatment + Surgery, RC = Randomization Criteria, CAD = Coronary Artery Disease, NIHSS = NIH Stroke Scale
One subject was not randomized due to MD preference and another was randomized to surgery but did not receive it. These subjects are not included in the table.
One subject in the RC not met group was missing all background characteristics except for whether it was a dominant hemispheric stroke.
Three MTO subjects and one MTS subject were excluded from these summaries since their eligibility CT scan was also used as their randomization CT scan.
Table 3.
Clinical Characteristics and Outcomes of Randomized Patients
| Imaging at Randomization |
modified Rankin score |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study Subject |
Group | NIHSS at Randomization |
Hemisphere | Pineal Shift (mm) |
Septal Shift (mm) |
Onset to Meeting RC (hrs) |
Meeting RC to Surgery (hrs) |
Survival from Stroke Onset (days) |
Day 21 |
Day 90 |
Day 180 |
| 1 | MTO | 23 | L | 5 | 5 | 20.4 | NA | * | 5 | 4 | 3 |
| 2 | MTO | 18 | R | 5 | 11 | 65.3 | NA | * | 5 | 4 | 4 |
| 3 | MTO | 21 | R | 6 | 12 | 30.7 | NA | * | 5 | 4 | 4 |
| 4 | MTO | 22 | L | 6 | 9 | 64.1 | NA | * | 4 | 4 | 5 |
| 5 | MTO | 22 | L | 6 | 7 | 65.8 | NA | * | 4 | 3 | 3 |
| 6 | MTO | 17 | R | 8 | 10 | 50.9 | NA | * | 4 | 4 | 3 |
| 7 | MTO | 28 | R | 4 | 10 | 40.8 | NA | 3 | 6 | 6 | 6 |
| 8 | MTO | 21 | R | 6 | 9 | 25.8 | NA | 4 | 6 | 6 | 6 |
| 9 | MTO | 26 | L | 8 | 8 | 54.0 | NA | 6 | 6 | 6 | 6 |
| 10 | MTO | 20 | L | 3 | 11 | 59.0 | NA | 4 | 6 | 6 | 6 |
| 11 | MTS | 19 | R | 6 | 11 | 77.4 | 3.1 | * | 4 | 3 | 4 |
| 12 | MTS | 23 | R | 10 | 11 | 50.3 | 5.2 | * | 4 | 2 | 1 |
| 13 | MTS | 19 | R | 3 | 7 | 16.7 | 1.5 | * | 4 | 4 | 4 |
| 14 | MTS | 19 | R | 5 | 9 | 57.2 | 3.0 | * | 3 | 3 | 2 |
| 15 | MTS | 21 | R | 5 | 6 | 68.2 | 3.1 | * | 4 | 4 | 4 |
| 16 | MTS | 25 | L | 8 | 10 | 90.7 | 5.3 | * | 5 | 5 | 4 |
| 17 | MTS | 27 | L | 4 | 8 | 38.8 | 2.4 | * | 5 | 4 | 4 |
| 18 | MTS | 23 | R | 2 | 8 | 43.1 | 3.5 | * | 5 | 3 | 3 |
| 19 | MTS | 29 | L | 5 | 10 | 23.2 | 1.3 | * | 4 | 4 | 3 |
| 20 | MTS | 37 | R | 7 | 10 | 16.7 | 7.0 | 2 | 6 | 6 | 6 |
| 21 | MTS | 21 | R | 8 | 11 | 90.8 | 2.7 | 13 | 6 | 6 | 6 |
| 22 | MTS | 24 | L | 8 | 8 | 89.5 | 1.8 | 34 | 5 | 6 | 6 |
| 23 | MTS | 33 | L | 6 | 9 | 59.3 | 4.2 | 4 | 6 | 6 | 6 |
| 24 | MTS | 21 | R | 4 | 8 | 29.2 | 4.5 | 56 | 5 | 6 | 6 |
NIHSS = NIH Stroke Scale, RC = Randomization Criteria, MTO = Medical Treatment Only, MTS = Medical Treatment + Surgery, NA = Not Applicable
Alive at 180 days
One center had a single major violation in one patient that involved the use of mannitol off protocol in a MTO patient on one occasion thereby triggering a warning and planned site visit for the next registered patient which never occurred. Seven patients at 4 centers had minor violations related to formulary differences at the respective centers.
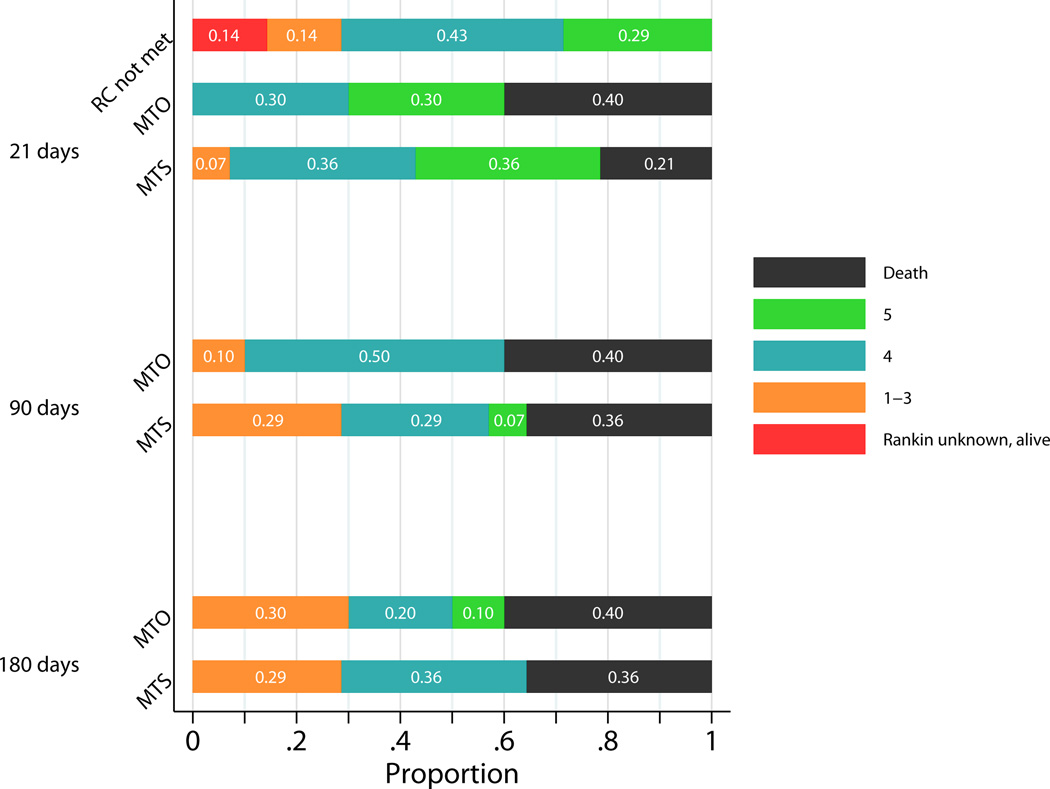
Twenty-one day mortality was 40% (90% CI (15%, 70%)) in the medical group and 21% (90% CI (6%, 47%)) in the surgical group (difference = 19%, 90% CI (−13%, 50%)). At 180 days, the mortality remained unchanged in the medical group and had risen to 36% in the surgical group (Figure 3). In contrast, among patients whose stroke did not progress sufficiently to meet criteria for randomization, none died within the first 21 days. The causes of death as reported on death certificates in the medical group were: uncontrolled intracranial hypertension, brain stem compression, cerebral infarction, and left middle cerebral artery stroke. The causes of death in the surgical group were: massive right hemispheric stroke with malignant cerebral edema, intractable intracranial hypertension, cardiac arrhythmia (possibly secondary to sepsis), progression of disease, and withdrawal of support. Three deaths in randomized patients were due to medical complications and/or to a decrease in treatment aggressiveness. No deaths were due to surgical complications.
Figure 3.
HeADDFIRST Modified Rankin Scores at 21, 90 and 180 Days.
The blinded examiners at 90 and 180 days were queried regarding the effectiveness of the blind. Of the 9 patients in the surgical group and 6 patients in the medical group who had blinded exams, the examiner knew the treatment received with certainty in only 2 cases (both in the surgical group) based on physical clues and revealing conversations.
Discussion
While large supratentorial cerebral infarctions involving at least the majority of the MCA territory are known to be associated with life-threatening cerebral edema (“space-occupying middle cerebral artery territory infarction”= SOMCATI), their actual risk of death is controversial. The majority of recent publications suggest that medical management for SOMCATI without surgical decompression is associated with a 70–80% mortality. This conclusion is predominantly derived from surgical decompression series or retrospective reviews without mandatory compliance (or monitoring of compliance) with contemporary standardized medical and/or surgical treatment protocols. Often it is presumed that either medical management is uniform or that differences between physicians in medical management do not importantly impact outcome; a notion we have challenged previously.2
Since the treatment protocols in HeADDFIRST were standardized and adherence was required, our results can provide unique insights into the potential relevance of the SMMP used here to the outcome of SOMCATI. The HeADDFIRST randomization criteria defined an extent of cerebral edema of SOMCATI that was associated with increased mortality (regardless of treatment) relative to the registered patients who did not achieve randomization criteria (29% vs. 0%, p=0.03). These data suggest that the HeADDFIRST randomization criteria effectively distinguished between those at high and low risk of 21-day mortality from SOMCATI.
The HeADDFIRST mortality for those randomized to MTO was 40% at 21 days, while the mortality for all 35 patients treated by the SMMP without hemicraniectomy (including those registered without deterioration, randomized to MTO, and registered to data collection only) was only 17% at 21 days. Randomization in HeADDFIRST required more significant mass effect than that required for treatment assignment in the three randomized European trials (DECIMAL, DESTINY, and HAMLET). In addition, many of the HeADDFIRST patients who were registered but never met randomization criteria (none of whom died) would have been randomized in the European trials. Supplementary Table I shows that many of the patients in this group would have fulfilled the inclusion criteria for randomization in the three European trials with a 12-month mortality in the pooled meta-analysis of conservative treatment of 71%13 and 76% (16 of 21) in another non-randomized prospective study.14
The lower mortality of the HeADDFIRST conservatively treated patients may be related to the fact that HeADDFIRST inclusion criteria allowed older patients than the randomized European trials. (Supplementary Table I) The mean age (years) of the randomized patients in HeADDFIRST, DECIMAL, DESTINY, and HAMLET was 54.6, 43.4, 44.6, and 48.2, respectively. Older patients have more brain atrophy and are well-recognized to tolerate their brain swelling better than younger patients.2 Even with the older patient group, however, the lower mortality of the HeADDFIRST MTO patients relative to the other trials may be related to other factors. This is, in part, supported by the reported 76.2% conservative treatment mortality of one prospective non-randomized trial that also included older patients ≥ 70 years old with a mean age of 58.4 years.14 None of the European trials required adherence to a SMMP. It is therefore plausible that required adherence to the HeADDFIRST standardized treatment protocols is responsible for the better survival in its nonsurgical patients, in contrast to the notion that differences in the execution of best medical management with a recommended protocol makes no difference to the outcome.
The mortality for the HeADDFIRST MTS patients was 21% at 21 days compared to the 40% MTO mortality. While this illustrates a favorable trend toward improved survival with surgical decompression it did not reach statistical significance (p=0.39), due to the small sample size of this pilot clinical trial. Furthermore, any beneficial trend was diminished by 90 days due to additional deaths at day 34 (progression of disease) and day 56 (withdrawal of support) in the MTS group. Although HeADDFIRST only followed patients for 180 days, subsequent studies should include a longer follow-up, since some of the previously published trials of hemicraniectomy have shown that patients can continue to improve after this period.
Notably, HeADDFIRST allowed randomization of patients up to 96 hours after stroke onset which was the same as HAMLET but significantly longer than DECIMAL (30 hours) and DESTINY (36 hours). Studies that allowed later randomization (with the time of randomization being linked to magnitude of mass effect and clinical deterioration as in HeADDFIRST) should be associated with higher mortality, a finding that could not be verified by our study.
HeADDFIRST also demonstrated that masking treatment for purposes of blinded outcome evaluation was feasible and should be incorporated in future randomized trials, as was successfully accomplished in the subsequent HAMLET study.3
The recent series of randomized, controlled studies on surgical decompression for SOMCATI including HeADDFIRST have implications for management of patients with large supratentorial hemispheric infarctions. The European trials provide definitive evidence of the potential life-saving benefit of early hemicraniectomy and durotomy in selected younger patients, thereby allowing this procedure to become an integral part of the stroke management armamentarium. HeADDFIRST was completed more than 10 years ago, but there have not been any major leaps forward in medical management of patients with SOMCATI that would diminish the significance of its results today in many key areas. The SMMP and required adherence in HeADDFIRST may have contributed to its lower mortality, emphasizing that the specifics of medical management may matter and potentially alter patient outcomes. Furthermore, the HeADDFIRST randomization criteria differentiated between early survivors and non-survivors with SOMCATI when patients are managed using the HeADDFIRST SMMP, and this can provide some useful clinical guidance regarding patient selection for the procedure and design of future trials relevant to the management of SOMCATI.
Supplementary Material
Acknowledgments
We thank Judith Maratea for her administrative leadership and Melinda Drum, PhD, for her statistical contributions.
Sources of Funding
This study was funded by a grant from NINDS (R01 NS40229, Dr. Jeffrey Frank, PI).
HeADDFIRST Trialists
Albany Medical College (Dr. Gary Bernardini), The Cleveland Clinic Foundation (Dr. Derk Krieger and Dr. John Andrefsky), Columbia-Presbyterian Hospital (Dr. Mitchell Elkind), Detroit Receiving Hospital (Dr. William Coplin), Duke University Medical Center (Dr. Carmelo Graffagnino), Indiana University Medical Center (Dr. Jose Biller), OSF Saint Francis Medical Center (Dr. David Wang), St. Louis University Health Sciences Center (Dr. Salvador Cruz-Flores), Thomas Jefferson University (Dr. David Brock), University of Calgary (Dr. Andrew Demchuk), University of California Davis (Dr. Piero Verro), University of Chicago (Dr. Jeffrey Frank), University of Cincinnati (Dr. Daniel Woo), University Hospitals of Cleveland (Dr. Jose Suarez), University of Kentucky (Dr. Creed Pettigrew), University of Maryland (Dr. Marian LaMonte).
Footnotes
Conflict(s)-of-Interest/Disclosure(s)
Disclosures: None
References
- 1.Hacke W, Schwab S, Horn M, Spranger M, De Georgia M, von Kummer R. “Malignant” middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol. 1996;53:309–315. doi: 10.1001/archneur.1996.00550040037012. [DOI] [PubMed] [Google Scholar]
- 2.Frank JI. Large hemispheric infarction, deterioration, and intracranial pressure. Neurology. 1995;45:1286–1290. doi: 10.1212/wnl.45.7.1286. [DOI] [PubMed] [Google Scholar]
- 3.Hofmeijer J, Kappelle LJ, Algra A, Amelink GJ, van Gijn J, van der Worp HB. Surgical decompression for space-occupying cerebral infarction (the Hemicraniectomy After Middle Cerebral Artery infarction with Life-threatening Edema Trial [HAMLET]): a multicentre, open, randomised trial. Lancet Neurol. 2009;8:326–333. doi: 10.1016/S1474-4422(09)70047-X. [DOI] [PubMed] [Google Scholar]
- 4.Jüttler E, Schwab S, Schmiedek P, Unterberg A, Hennerici M, Woitzik J, et al. Decompressive Surgery for the Treatment of Malignant Infarction of the Middle Cerebral Artery (DESTINY): a randomized, controlled trial. Stroke. 2007;38:2518–2525. doi: 10.1161/STROKEAHA.107.485649. [DOI] [PubMed] [Google Scholar]
- 5.Vahedi K, Vicaut E, Mateo J, Kurtz A, Orabi M, Guichard J-P, et al. Sequential-design, multicenter, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL Trial) Stroke. 2007;38:2506–2517. doi: 10.1161/STROKEAHA.107.485235. [DOI] [PubMed] [Google Scholar]
- 6.Von Kummer R, Meyding-Lamadé U, Forsting M, Rosin L, Rieke K, Hacke W, et al. Sensitivity and prognostic value of early CT in occlusion of the middle cerebral artery trunk. Am J Neuroradiol. 1994;15:9–15. discussion 16–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Pullicino PM, Alexandrov AV, Shelton JA, Alexandrova NA, Smurawska LT, Norris JW. Mass effect and death from severe acute stroke. Neurology. 1997;49:1090–1095. doi: 10.1212/wnl.49.4.1090. [DOI] [PubMed] [Google Scholar]
- 8.Ropper AH. Lateral displacement of the brain and level of consciousness in patients with an acute hemispheral mass. N Engl J Med. 1986;314:953–958. doi: 10.1056/NEJM198604103141504. [DOI] [PubMed] [Google Scholar]
- 9.Agresti A. Categorical Data Analysis. New York: John Wiley & Sons; 1990. [Google Scholar]
- 10.Clopper C, Pearson E. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–413. [Google Scholar]
- 11.Fleiss JL. Statistical Methods for Rates and Proportions. 2nd ed. New York: John Wiley & Sons; 1981. [Google Scholar]
- 12.StataCorp. Stata: Release 12 Statistical Software. College Station, TX: StataCorp LP; 2011. [Google Scholar]
- 13.Vahedi K, Hofmeijer J, Juettler E, Vicaut E, George B, Algra A, et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol. 2007;6:215–222. doi: 10.1016/S1474-4422(07)70036-4. [DOI] [PubMed] [Google Scholar]
- 14.Rieke K, Schwab S, Krieger D, von Kummer R, Aschoff A, Schuchardt V, et al. Decompressive surgery in space-occupying hemispheric infarction: results of an open, prospective trial. Crit Care Med. 1995;23:1576–1870. doi: 10.1097/00003246-199509000-00019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.