Abstract
Although roles of phosphatase and tensin homolog deleted on chromosome 10 (PTEN) in regulating cell proliferation are well established, its function in immune responses remains to be fully appreciated. In the current study, we analyzed myeloid-specific PTEN function in regulating tissue inflammatory immune response in a murine liver partial warm ischemia model. Myeloid-specific PTEN knock-out (KO) resulted in liver protections from ischemia reperfusion injury (IRI) by deviating local innate immune response against IR toward the regulatory type: expressions of pro-inflammatory genes were selectively decreased and anti-inflammatory IL-10 was simultaneously increased in IR livers of PTEN KO mice, as compared with those of wild-type (WT) mice. PI3 kinase inhibitor and IL-10 neutralizing Abs, but not exogenous LPS, recreated liver IRI in these KO mice. At the cellular level, Kupffer cells, as well as peritoneal macrophages, isolated from KO mice expressed higher levels of M2 markers, and produced lower TNF-α and higher IL-10 in response to TLR ligands, than their WT counterparts. They had enhanced Stat3 and Stat6, but diminished Stat1 signaling pathway activations in response to TLR4 stimulation. Inactivation of KCs by gadolinium chloride enhanced pro-inflammatory immune activation and increased IRI in livers of myeloid PTEN KO mice. Thus, myeloid PTEN deficiency protects livers from IRI by facilitating M2 macrophage differentiation.
Introduction
TLR4 activation has been identified in recent years as the key initiating step of liver inflammatory immune response against IR (1-3). As both pro- and anti-inflammatory gene programs are triggered downstream of the TLR4 engagement via multiple intracellular signaling pathways (4, 5), the question of whether we could manipulate TLR signaling pathways to curtail its tissue damaging pro-inflammatory property is of high interest to identify potential therapeutic targets. The PI3K-Akt signaling pathway has been shown as an endogenous gate-keeping system to prevent excessive innate immune responses (6). Mice deficient of PI3K regulatory subunit showed enhanced Th1 response, due to increased IL-12 production from DCs (7). More recent studies have revealed that glycogen synthase kinase 3β (Gsk3β) represents a key target of this negative regulatory pathway of TLR responses (8, 9). As a constitutively active kinase, Gsk3β is inactivated upon innate immune stimulation in macrophages by Akt, and Gsk3 inhibition results in diminished NF-kB-driven pro-inflammatory, but increased IL-10, gene expression (8, 10). We have shown that active Gsk3β is critical for the liver pro-inflammatory immune response against IR, as its inhibitor SB216367 was able to shift the liver immune response toward an IL-10 dominated regulatory type and protected livers from IRI (11).
As the PI3K-Akt-Gsk3β signaling pathway is involved in multiple aspects of cellular functions in different cell types, including proliferation, differentiation, apoptosis and chemotaxis (12, 13), the precise definition of its immune regulatory function in vivo in a complex organ, such as liver, will require cell-type specific analysis. In particular, the immune response against IR is triggered by tissue damages via DAMPs, any regulatory mechanisms of parenchymal cell death will have indirect immunological impacts. Thus, non-cell-selective targeting approaches of this signaling pathway, such as chemical inhibitors of PI3K or PTEN siRNA, will not differentiate cellular mechanisms of their immune regulatory effects in vivo. In the current study, we utilized Cre-LoxP system to create myeloid PTEN KO mice to study specifically PI3K activation in myeloid cells in liver IRI. PTEN is a dual-specificity protein/lipid phosphatase and functions as a major negative regulator of the PI3K/Akt signaling pathway. PTEN was original identified as a tumor suppressor gene and loss of its function stimulates cell growth and survival (14). Its inhibition with small molecule inhibitor has been wide used in infarction models to ameliorate cardiomyocyte/neuron apoptosis and cell death (15-20). PTEN knock-down with its specific siRNA has also been tested in liver IR model recently (21, 22), with an implication of immune regulation. However, only correlative conclusions can be draw from these studies, due to issues of target-cell specificities, incomplete gene inhibition/downregulation and off-target effects of chemical inhibitors and siRNAs. Our myeloid-specific KO model enabled us for the first time to determine specifically whether PTEN was directly involved in liver innate immune activation against IR.
Materials and Methods
Animals
PTEN-LoxP (generous gift from Dr. Hong Wu, UCLA) and the myeloid-specific Cre mice (Lyz2-Cre, The Jackson Laboratory, Bar Harbor, ME) were used to create myeloid specific PTEN KO mice. Briefly, homozygous PTENloxP/loxP mice were first bred with homozygous Lyz2-Cre mice, the heterozygous offspring (for both PTEN and Cre) was back-crossed with homozygous PTEN loxP/loxP mice. Mouse genotyping was performed by using a standard protocol with primers described in JAX Genotyping protocols database. Animals were housed in the UCLA animal facility under specific pathogen-free conditions, and received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institute of Health.
Mouse liver IRI model
PTEN normal and deficient mice (6-8 weeks old) were used in experiments. As described previously (23), mice anesthetized with sodium pentobarbital (60 mg/kg i.p) were injected with heparin (100 mg/Kg), and an atraumatic clip was used to interrupt the arterial and portal venous blood supply to the cephalad lobes of the liver. After 90 min of partial hepatic warm ischemia, the clip was removed, initiating hepatic reperfusion. Mice were sacrificed after various time of reperfusion (0, 6, 24 hrs.). Sham controls underwent the same procedure, but without vascular occlusion. LPS (1μg/mouse, LPS-EK, Invivogen, San Diego, CA) or Anti-IL-10 (JES5-2A5) or control Ig (300μg/mouse, BioXCell, West Lebanon, NH) were administered prior to the liver reperfusion via portal vein. Other treatments include Wortmanin (WM, at 1μg/g in 200μl, i.p. Tocris Bioscience) 1h, Gadolinium chloride (GdCl3, at10μg/g, in 200μl DMSO, i.p. Sigma, St. Louis, MO) 16h, prior to the onset of liver ischemia.
Serum alanine aminotransferase (sALT) levels were measured with an auto analyzer by ANTECH Diagnostics (Los Angeles, CA). Part of liver specimens were fixed in 10% buffered formalin and embedded in paraffin. Liver sections (4 m) were stained with hematoxylin and eosin (HE). The severity of liver IRI was graded blindly using Suzuki's criteria on a scale from 0 to 4. No necrosis, congestion/ centrilobular ballooning is given a score of 0, while severe congestion and >60% lobular necrosis is given a score of 4 (24).
Liver macrophages and neutrophils were detected using primary rat anti-mouse F4/80 (AbD Serotec, Raleigh, NC) and Ly6G (BD Biosciences, San Jose, CA) mAb, respectively. The secondary, biotinylated goat anti-rat IgG (Vector, Burlingame, CA) was incubated with immunoperoxidase (ABC Kit, Vector).
MPO assay
Liver myeloperoxidase (MPO) activities were measured. Briefly, the frozen tissue was thawed and placed in iced 0.5% hexadecyltrimethyl-ammonium bromide and 50 mM potassium phosphate buffer solution (pH = 5.0). Each sample was homogenized and centrifuged at 15,000 rpm for 15 min at 4°C. Supernatants were mixed with hydrogen peroxide-sodium acetate and tetramethyl-benzidine solutions. The change in absorbance was measured by spectrophotometry at 655 nm. One unit of MPO activity was defined as the quantity of enzyme degrading 1μmol peroxide/min at 25°C/g of tissue.
Cell cultures
Cells were derived from PTEN-loxP+/+ (Cre-ptenL/L) mice as PTEN normal/WT; or Lyz2-Cre+/− PTEN-loxP+/+ (Cre+ptenL/L) mice as PTEN deficient/KO after euthanasia (pentobarbital 100mg/Kg, i.p.).
Peritoneal macrophages were generated by injection of 1mL 2% Bio-Gel P-100 (BioRad, Hercules, CA) into the mouse peritoneal cavity, followed by peritoneal lavage with sterile PBS 4 days later.
KCs were isolated as follows: livers were perfused in situ via the portal vein with calcium- and magnesium-free HBSS supplemented with 2% heat-inactivated FBS, followed by 0.27% collagenase IV (Sigma, St Louis, MO). Perfused livers were dissected, and teased through 70μm nylon mesh cell strainers (BD Biosciences, San Diego, CA). Non-parenchymal cells (NPCs) were separated from hepatocytes by centrifuging at 50g ×2min for 3 times. NPCs were then suspended in HBSS and layered onto a 50%/25% two-step percoll gradient (Sigma, St Louis, MO) in a 50-ml conical centrifuge tube and centrifuged at 1800g, 4°C for 15 minutes. KCs in the middle layer were collected and allowed to attach onto cell culture plates in DMEM with 10% FBS, 10mM HEPES, 2mM GlutaMax, 100U/ml penicillin and 100ug/ml streptomycinfor for 15 minutes at 37°C. Non-adherent cells were removed by replacing the culture medium. The purity of KCs was 80% assessed by immunofluorescence staining for F4/80.
Spleen macrophages were isolated by using immunomagnetic CD11b positive selection kits (STEMCELL Tech. Inc., Vancouver, BC, Canada) according to the manufacture manual. Macrophages or KCs were cultured overnight prior to the start of stimulations with TLR ligands: 100ng/ml LPS-EK, or 1 ug/ml LAM-MS, or 1ug/ml Poly I:C (InvivoGen, San Diego, CA). Cells or culture supernatants were harvested after various time of stimulation for RNA, protein and ELISA assays.
Quantitative RT-PCR
Total RNA (2.0μg) was reverse-transcribed into cDNA using SuperScriptTM III First-Strand Synthesis System (Invitrogen, Carlsbad, CA). Quantitative-PCR was performed using the DNA Engine with Chromo 4 Detector (MJ Research, Waltham, MA). In a final reaction volume of 20 μl, the following were added: 1×SuperMix (Platinum SYBR Green qPCR Kit, Invitrogen, Carlsbad, CA), cDNA and 0.5 mM of each primer. Amplification conditions were: 50°C (2 min), 95°C (5 min) followed by 50 cycles of 95 °C (15 s), 60 °C (30 s). Primers used to amplify a specific mouse gene fragments are the same as described previously (25).
Western Blots
Tissue or cellular proteins were extracted with ice cold lysis buffer (1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 10% glycerol, 137mM sodium chloride, 20mM Tris, pH 7.4). Proteins (20 μg) were subjected to 12% SDS-PAGE electrophoresis and transferred to PVDF nitrocellulose membrane. Antibodies against PTEN, p-AKT, stat3, foxo1, β-actin (Cell Signaling Technology, San Diego, CA), and HO-1 (Stressgen Biotech, Victoria, BC, Canada) were used for Western blot analysis.
ELISA
Cytokines (TNF-α, IL-6, IL-12p40 and IL-10) secretion in cell culture supernatants or serum was measured by ELISA, according to the manufacturer's protocols (eBioscience, San Diego, CA).
Statistical Analysis
Results are shown as mean±SD. Statistical analyses were performed using unpaired Student's t test with p<0.05 (two tailed) considered as significant.
Results
Myeloid PTEN deficiency protects livers from IRI
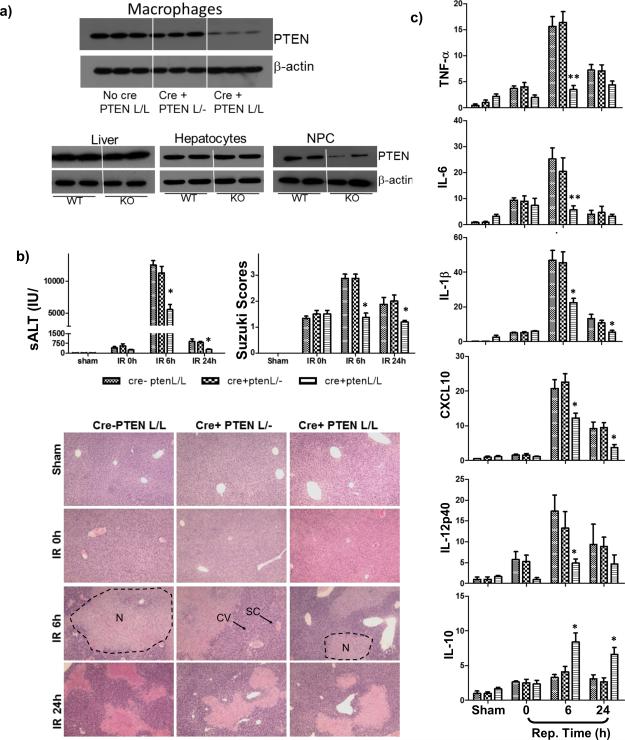
Three different PTEN mice, including PTEN-loxP+/+ (Cre-ptenL/L), Lyz2-Cre+/− PTEN-loxP+/−(Cre+ptenL/-), and Lyz2-Cre+/− PTEN-loxP+/+ (Cre+ptenL/L) which had normal, haploid- or complete-deficient PTEN in myeloid cells, as documented by Western blots of PTEN of peritoneal macrophages (Fig.1a, top panel), were used in our liver IRI experiments. Although total liver tissue levels of PTEN were similar between WT and myeloid PTEN KO mice, selective PTEN deficiency in liver non-parenchymal, vs. parenchymal cell populations was confirmed (Fig.1a, lower panel). Unlike its tumor suppressor function, myeloid PTEN regulated the development of liver IRI in haploid sufficient manner: only PTEN complete, but not haploid, deficiency protected mice from liver IRI, as shown by lower sALT levels and better preserved liver architectures (S/E staining) with lower Suzuki scores, at both 6 and 24h of reperfusion in Cre+ptenL/L, but not Cre+ptenL/- or Cre-ptenL/L mice (Fig.1b). Indeed, IR livers in PTEN KO mice revealed less edema, minimal sinusoidal congestion and cytoplasmic vacuolization, and decreased hepatocellular necrosis, as compared those in control animals. Liver pro-inflammatory immune response against IR was also regulated similarly by myeloid PTEN, as shown by lower inductions of pro-inflammatory genes, including TNF-a, IL-6, IL-1b, CXCL10 and IL-12p40, and higher induction of anti-inflammatory IL-10 genes only in livers of complete, but not haploid, PTEN KO mice (Fig.1c).
Figure 1.
Complete myeloid PTEN KO protected livers from IRI. PTEN-loxP+/+ (No Cre), Lyz-Cre+/− PTEN-loxP+/− (Cre+PTEN L/-), and Lyz-Cre+/− PTEN-loxP+/+ (Cre+PTEN L/L) mice were used in liver IR experiments as described in Material and Methods. (a) Western blots of PTEN in peritoneal macrophages, as well as in liver tissues, hepatocytes and NPCs, isolated from WT or myeloid PTEN KO mice. (b) Serum ALT, Suzuki scores of liver histological analysis (H/E stain) in different groups of mice subjected to either sham operation or 90m ischemia, followed by 0, 6, 24h reperfusion were shown. Typical areas with edema (E), sinusoidal congestion (SC), cytoplasmic vacuolization (CV) and hepatocellular necrosis (N) were indicated. (c) Quantitative RT-PCR analysis of inflammatory gene expressions in sham-operated and ischemic livers after various length of reperfusion time. The ratios of target gene vs. HPRT were plotted against different experimental groups. Bar labels are the same as in (b). Representative results of 2 different experiments. N=3-4/group. *p<0.05, **p<0.01.
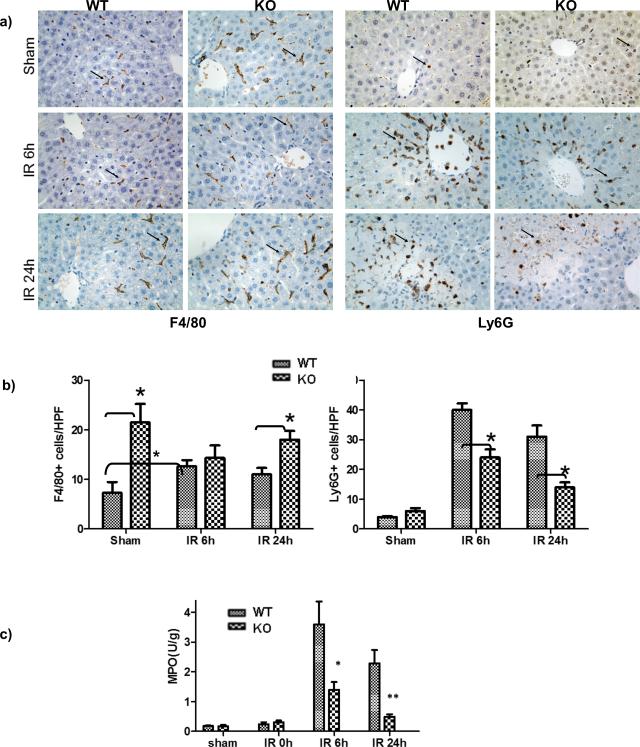
As PTEN is a tumor suppressor gene and regulates cell proliferation, we compared myeloid cell compositions between WT and myeloid PTEN KO mice. Increased numbers of monocytes and neutrophils were found in peripheral blood (data not shown) and livers (F4/80+ or Ly6G+ cells in sham) of KO animals, as compared with WT controls. However, their inflammatory infiltration into livers in response to IR was diminished. In sharp contrast to the significant increases of PTEN normal cells, PTEN deficient F4/80+ cells were not increased (or showed a trend of decrease) in IR livers at 6h post reperfusion; PTEN deficient Ly6G+ cells were increased at significantly less degree in IR livers than normal cells at both 6 and 24h post reperfusion (Fig.2a, b). Liver MPO activities were also reduced in livers of KO mice at both 6 and 24h of reperfusion (Fig.2c). Thus, myeloid PTEN deficiency protected livers from IRI. This was due possibly to impairment of myeloid cell functions, rather than cell depletions in KO mice.
Figure 2.
Myeloid PTEN KO diminished liver myeloid cell infiltration and activation in IR. Sham and ischemic liver tissues from PTEN-loxP+/+ (WT) and Lyz-Cre+/− PTEN-loxP+/+ (KO) mice were harvested after 6 and 24h reperfusion. (a) Macrophage and neutrophil infiltrations were analyzed by immunohistological staining with antibodies against F4/80 and Ly6G, respectively in sham, IR livers at 6 and 24h post reperfusion. Arrows indicate positive cells. (b) F4/80+ or Ly6G+ cells were quantitated by counting numbers of positive cess/area. (c) Myeloperoxiase (MPO) activities were also measured in these liver tissues. Representative results of 2 different experiments. N=3-4/group. *p<0.05, **p<0.01.
The PI3 kinase activation-dependent IL-10 induction is responsible for the liver protection in myeloid PTEN KO mice
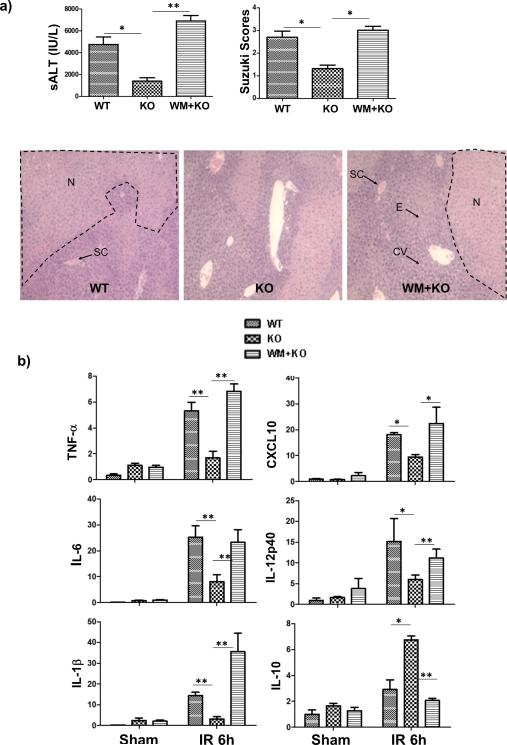
As PTEN could function via both PI3K-dependent and -independent pathways, we determined whether the liver protection from IR in myeloid PTEN deficient mice was dependent on PI3K activation by using a PI3K inhibitor, wortmanin. Indeed, injections of the inhibitor restored liver IRI in the KO mice, as evidenced by increased sALT levels and worse damaged liver histological architectures (H/E staining) with higher Suzuki scores (Fig.3a). No significant increases of liver IRI were observed in WT mice treated with wortmanin (data not shown), as reported previously in our own study (11). did not In addition, liver pro-inflammatory immune response against IR was recreated that intrahepatic TNF-a, IL-1b, IL-6, IL-12p40, and CXCL10 transcription levels were increased, while IL-10 was decreased in the inhibitor-treated myeloid PTEN KO mice (Fig.3b). Thus, PI3K activation was critical for liver immune regulatory immune response and protection from IRI in myeloid PTEN deficient mice.
Figure 3.
PI3 kinase activation protects livers from IRI in myeloid PTEN KO mice. PTEN-loxP+/+ (WT) and Lyz-Cre+/− PTEN-loxP+/+ (KO) mice were used in our liver IR experiment as described in Material and Methods. A separate group of KO mice treated PI3K inhibitor, wortmannin (WM), prior to the start of liver ischemia was also included. (a) Liver IRI was evaluated at 6h post reperfusion by measuring sALT, liver histological analysis (H/E stain) scored by Suzuki Standard. Typical areas with edema (E), sinusoidal congestion (SC), cytoplasmic vacuolization (CV) and hepatocellular necrosis (N) were indicated. (b) Liver immune response against IR was determined by quantitative RT-PCR analysis of inflammatory gene expressions in sham-operated and IR livers. The ratios of target gene expression levels vs. housekeeping gene HPRT were plotted against different experimental groups. Representative results of 2 different experiments. N=3-4/group. *p<0.05, **p<0.01.
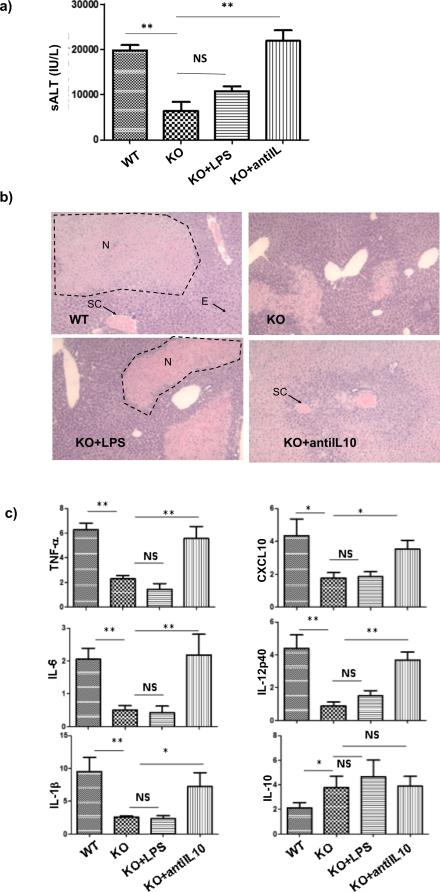
To test the functional significance of IL-10 in liver protection by myeloid PTEN deficiency, we administered anti-IL-10 Abs prior to the start of liver ischemia. In addition, LPS was injected into a separate group of KO mice to determine whether lacks of TLR activation played a role in the defective inflammatory immune response in PTEN deficient mice in response to IR. Liver injuries and immune responses were measured at 6h post reperfusion. Our results clearly showed that anti-IL-10, but not LPS, restored liver IRI and pro-inflammatory immune activation. Significant increases in sALT levels and worsening of liver histopathology (H/E staining) with higher Suzuki scores were observed in PTEN KO mice treated with anti-IL-10, but not LPS (Fig.4a, b). Liver cytokine/chemokine inductions, including TNF-a, IL-6, IL-1b, IL-14p40 and CXCL10 as measured by qRT-PCR, were also increased in the same group of treated KO mice (Fig.4c). IL-10 gene inductions were not much different by treatments. Thus, myeloid PTEN deficiency protected livers from IRI by an IL-10-mediated immune regulatory mechanism.
Figure 4.
Myeloid PTEN KO protected livers against IRI via an IL-10-dependent mechanism. Anti-IL-10 Abs or LPS were administered in KO mice after liver ischemia and prior to the start of reperfusion via portal vein, as described in the material and methods. WT and KO mice without any treatments were also included as controls. Liver IRI was evaluated at 6h post reperfusion by measuring sALT (a) and liver histological analysis (b. H/E stain: Typical areas with edema (E), sinusoidal congestion (SC), cytoplasmic vacuolization (CV) and hepatocellular necrosis (N) were indicated). Liver immune response against IR was determined by quantitative RT-PCR analysis of inflammatory gene expressions in IR livers (c). The ratios of target gene expression levels vs. housekeeping gene HPRT were plotted against different experimental groups. Representative results of 2 different experiments. N=3-4/group. *p<0.05, **p<0.01, NS: no significant difference.
PTEN regulated macrophage differentiation
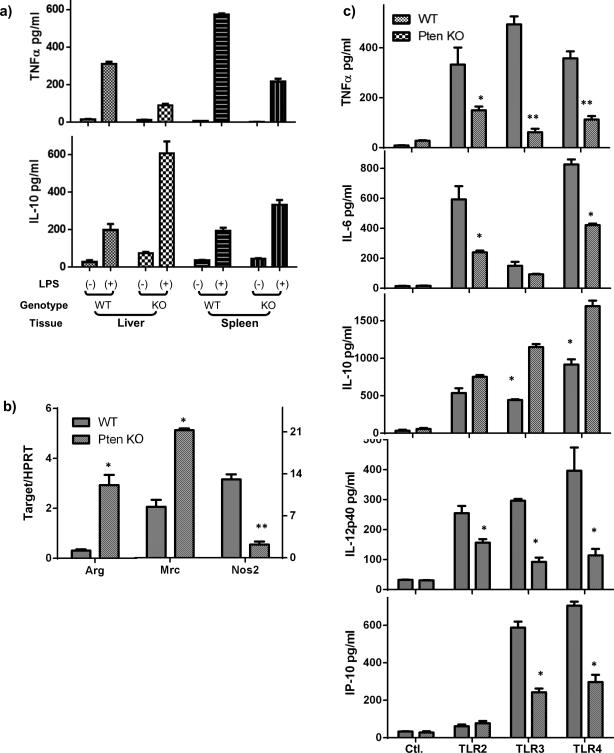
As KCs represent the major responding cells in liver innate immune activation by IR, we determined whether myeloid PTEN KO affected their functions. KCs and splenic macrophages were isolated from livers of both control and KO mice and stimulated in vitro with LPS. ELISA results showed that PTEN KO KCs and spleen macrophages produced significantly less TNF-α and more IL-10 in response to TLR stimulation, as compared with those from WT controls (Fig.5a).
Figure 5.
PTEN deficiency results in M2 macrophage development. (a) Cytokine productions from KCs and splenic macrophages from WT and myeloid PTEN KO mice. Liver and spleen macrophages were isolated from either WT or KO mice, as described in the material and methods, and cultured in vitro with or without LPS stimulation for 24h. TNF-α and IL-10 levels in culture supernatants were measured by ELISA. (b) Peritoneal macrophages were isolated from WT and PTEN KO mice and M1/M2 marker gene expressions were measured by qRT-PCR. Note, left Y axis for Arg., right Y axis for Mrc and Nos2. (c) Inflammatory cytokine/chemokine productions by peritoneal macrophages. Cells were cultured in vitro for 24h before TLR stimulations. Cytokines in culture supernatants after 24h stimulation were measured by ELISA. Representative results of 2 different experiments. N=3/group. *p<0.05, **p<0.01.
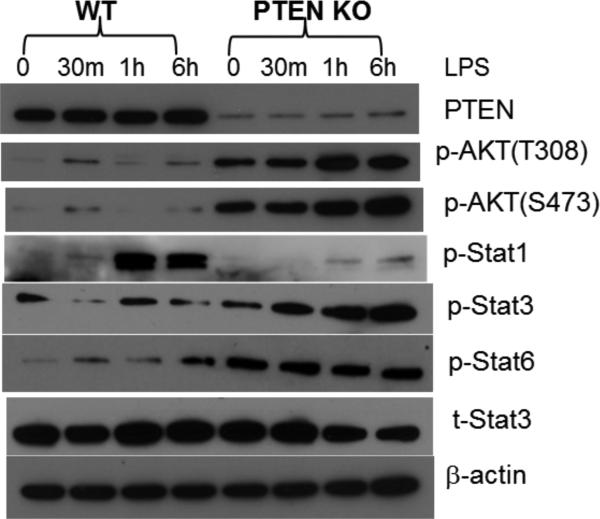
Macrophages can be differentiated into two distinctive types, classical pro-inflammatory M1, or alternative immune regulatory M2. To determine whether PTEN KO resulted in the development of M2 macrophages, we measured expressions of macrophage differentiation markers in KCs and peritoneal macrophages by quantitative RT-PCR. Clearly, PTEN KO cells expressed constitutively higher levels of Arginase 1, Mrc1, but lower level of Nos2 gene (Fig.5b). Functionally, these KO macrophages produced significantly less TNF-a, IL-6, IL-12p40 and CXCL10, but more IL-10, than WT cells in response to LPS (Fig.5c). Additionally, their responses to other TLR ligands also became less pro-inflammatory: less TNF-α and IL-6 against TLR2 ligand; and less TNF-a, IL-12p40, CXCL10, but more IL-10 against TLR3 ligand (Fig.5c). Furthermore, Western blot results showed that these KO cells responded to LPS stimulation with reduced activation (phosphorylation) of Stat1, but enhanced activation of Stat3 and Stat 6 (Fig.6). Consistent with its role in limiting PI3 kinase activation, PTEN KO resulted in its constitutive activation, as Akt was phosphorylated at both S473 and T308 sites prior to TLR stimulation. Akt phosphorylation was further elevated after stimulation (Fig.6). Thus, PTEN regulates macrophage differentiation and its deficiency results in the development of M2 macrophages.
Figure 6.
Macrophage intracellular signaling pathway activations. Peritoneal macrophages were stimulated with LPS for 0, or 30m, or 1h, or 6h, and cell lysates were prepared and analyzed by Western blots to determine intracellular signaling molecule activation (phosphorylation). Representative results of 2 different experiments.
PTEN deficient KCs protected livers against IRI
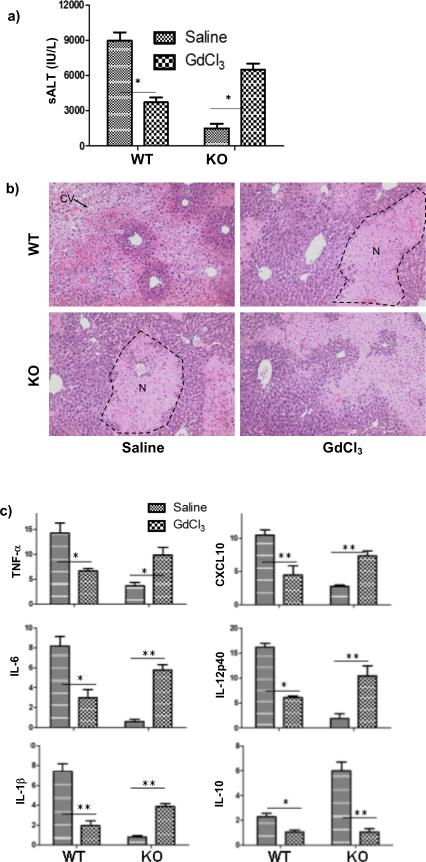
To test potential immune regulatory functions of PTEN KO macrophage in vivo in response to IR, GdCl3 was used to inactivate these cells prior to the start of liver ischemia. Consistent with previous reports, GdCl3-treated WT mice had decreased levels of sALT with better preserved liver architectures (HE staining) (Fig.7a, b). Liver inflammatory gene inductions, including TNF-a, IL-1b, IL-6, IL-12b, CXCL10 and IL-10, were also decreased as compared with their vehicle-treated controls (Fig.7c). In contrast, GdCl3-treated KO mice had increased levels of sALT and liver pro-inflammatory gene expressions, as compared with their controls. The IL-10 gene level was, however, downregulated by the treatment in KO mice. Thus, unlike its protective effect in WT mice, GdCl3 treatment resulted in increased liver IRI and enhanced tissue pro-inflammatory immune activation in myeloid PTEN KO mice, indicating that PTEN KO macrophages exerted anti-inflammatory functions in vivo in the pathogenesis of liver IRI.
Figure 7.
PTEN KO macrophages protect livers from IRI. Saline or GdCl3 were administered 24h prior to the start of liver ischemia, as described in the material and methods. Liver IRI was evaluated at 6h post reperfusion by measuring sALT (a) and liver histological analysis (b. H/E stain: Typical areas with edema (E), sinusoidal congestion (SC), cytoplasmic vacuolization (CV) and hepatocellular necrosis (N) were indicated). Liver immune response against IR was determined by quantitative RT-PCR analysis of inflammatory gene expressions in IR livers (c). The ratios of target gene expression levels vs. housekeeping gene HPRT were plotted against different experimental groups. Representative results of 2 different experiments. N=3-4/group. *p<0.05, **p<0.01.
Discussion
Our current study provides strong evidence that PTEN is involved directly in liver innate immune response against IR by regulating macrophage differentiation and activation. Myeloid PTEN deficiency results in the development of M2 type macrophages, which respond to IR-induced innate immune stimulation by producing a regulatory inflammatory response with higher levels of IL-10, but lower levels of TNF-a, IL-6 and CXCL10, which protects livers from IRI. These findings establish an innate immune regulatory role of PTEN in vivo in a clinical relevant disease model and provide us rationale of selectively targeting this signaling pathway as a novel therapeutic strategy to ameliorate tissue inflammatory diseases.
Myeloid PTEN deficient mice have been used previously in studies of its role in innate immune response. In vitro, it was shown to negatively regulate Fcγ receptor, but support TLR4, signaling, that TNF-a production was increased in response to Fcγ receptor stimulation, but decreased against LPS in PTEN KO macrophages, as compared to WT controls (26). These PTEN KO macrophages also showed enhanced phagocytic ability. In a murine pneumococcal pneumonia model, myeloid PTEN deficiency dampened pulmonary inflammation, reduced neutrophil infiltration, but augmented phagocytosis, which led to decreased tissue injury and improved survival (27). Studies of neutrophils, the other type of myeloid cells, revealed that PTEN suppressed neutrophil chemotaxis both in vitro and in vivo (28-30). In a neutropenia-associated pneumonia model, PTEN deficient neutrophils, as well as macrophages, were in fact accumulated more in inflamed lungs which resulted in elevated productions of pro-inflammatory cytokines and chemokines (29), which is very different from our finding in liver inflammatory injury model. Thus, how myeloid PTEN regulates immune responses could be varied drastically, probably due to differences in cellular and molecular mechanisms of disease pathogenesis.
Our results show that IR-induced liver inflammation was alleviated in myeloid PTEN KO mice. Despite that both macrophages and neutrophils cells were present at higher levels in sham livers of these KO animals, they failed to respond to IR. Liver macrophages are the major responding cells to IR and responsible for triggering tissue inflammation, leading to neutrophil activation and infiltration. The inhibition of macrophage pro-inflammatory response by PTEN KO would result in diminished neutrophil infiltration/activation. In fact, we found that PTEN KO macrophages became anti-inflammatory M2 type, which actively secreted IL-10 in response to innate TLR stimulation. Thus, neutrophils might have been inactivated by the regulatory immune response generated by PTEN KO macrophages. This was supported by our finding that both GdCl3, which inactivates KCs/macrophages, and anti-IL-10 Abs reversed the nature of liver immune response and recreate IRI in myeloid PTEN KO mice. In both cases, local innate immune cells, DCs and neutrophils, were free from the KC/IL-10-mediated immune regulation and became activated in response to IR.
As the key negative regulator of the pro-survival PI3K-Akt signaling pathways, PTEN has been studied extensively in brain and myocardial ischemia reperfusion injury models. A transient PTEN downregulation has been demonstrated as a physiological response against ischemia in these tissues. Post-translational modifications of PTEN, including proteolytic degradation, phosphorylation, and ubiquitination-mediated nuclear relocation were responsible for the reduction of its lipid phosphatase activities, which correlated with PI3K activation (15, 31, 32). PI3K/Akt activation has been closely associated with ischemia preconditioning, which represents one the most effective protective procedures against IRI. Thus, pharmacological inhibitors of PTEN, such as bisperoxovanadium (bpv), may functionally simulate ischemia preconditioning. Indeed, multiple studies have shown that PTEN inhibition protected brains/hearts from stroke/infarction (16, 18, 20, 33-35). Inhibition of caspase 3 activation and promotion of mTOR activation have been associated with the beneficial effect of bpv (16, 36). In vitro, PI3K activation was critical for hepatocyte preconditioning against hypoxia/reperfusion (37), which could be mimicked by bpv and translated into in vivo protection against liver IRI (38). More recently, studies using siRNA to knockdown PTEN were shown to promote Akt/beta-catenin/Foxo1 signaling in livers, leading to local enhancement of anti-apoptotic gene induction (21). Although liver inflammatory immune response was also reduced, it might not be a direct effect in liver innate immune cells, but rather an indirect one by reducing DAMP release from necrotic parenchymal cells which are protected from IR-induced cell death by PTEN inhibition/downregulation. Interestingly, we have observed that myeloid PTEN deficiency does not impact liver total PTEN level and only complete PTEN KO in macrophages showed significant immune regulatory effect. These indicate to us that hepatocyte PTEN is dominant quantitatively in the liver. Only cell-specific genetic approaches would allow us dissent PTEN functions in liver innate immune cells without interferences from hepatocytes. Thus, our study is the first to document immune regulatory function of PTEN in a tissue ischemia reperfusion model.
PTEN regulates liver immune response against IR in a “haploid-sufficient” manner: significant effects were observed only in total myeloid PTEN deficient animals. This is in sharp contrast to its tumor suppressor function that loss of heterozygosity or partial inhibition of its expression/activity is sufficient to promote carcinogenesis. Hepatocyte-specific PTEN KO induces hepatomegaly, hepatocellular adenoma and hepatocellular carcinoma with aging, and promote the development of steatohepatitis and fibrosis (39, 40). In our myeloid PTEN deficient mice, cell numbers of both neutrophils and monocytes were increased in peripheral blood and liver tissues, consistent with its role in cell proliferation. However, functions of these cells were altered. Tissue macrophages from both liver and spleen, as well as those from peritoneal, were all defective in terms of their pro-inflammatory immune activation by TLR ligands. DCs from the same animals, which are PTEN normal, remain capable of mounting similar innate immune responses as those from WT animals (data not shown). This is supported by the result from our in vivo KC depletion experiment, in which liver inflammation and injury was recreated in PTEN KO mice after the GdCl3 treatment.
As PTEN negatively regulates PI3 kinase activation, its deficiency results in the constitutive activation of this kinase pathway, as documented by higher levels of Akt phosphorylation. Our result showed that PI3K activation was critical for the liver protection phenotype in PTEN KO mice, which actually facilitated the differentiation of M2-type macrophages. M2 macrophages have been shown to protect kidneys, brains and hindlimb from ischemia injuries (41-43) by promoting either the resolution of tissue inflammation or healing/recovery. Our study extended its role in liver IRI. Mechanistically, the PI3K-Akt-mTOR pathway has been shown to regulate macrophage differentiation (44, 45), via partially miR-155 upregulation. It remains to be determined in PTEN KO cells the molecular details of M2 differentiation program.
In summary, our data documents an innate immune regulatory role of PTEN in liver inflammatory response against IR. Its genetic inactivation in macrophages results in their differentiation into anti-inflammatory M2 type, which protects livers from IRI via an IL-10 dependent mechanism.
Acknowledgments
Grant support: NIH Grants RO1 DK083408 (YZ), The Dumont Research Foundation; and Natural Science Foundation of China (Grant numbers: 81100270, 81070380, 30872390, 1310108001, 81210108017) (XW)
Abbreviations
- PTEN
phosphatase and tensin homolog deleted on chromosome 10
- IR
ischemia reperfusion
- IRI
ischemia reperfusion injury
- KC
Kupffer cell
- KO
knock-out
- WT
wild-type
- NPCs
Non-parenchymal cells
- PI3K
phosphoinositide 3 kinase
- sALT
serum alanine aminotransferase
- WT
wild type
References
- 1.Fiorini RN, Shafizadeh SF, Polito C, Rodwell DW, Cheng G, Evans Z, Wan C, Belden S, Haines JK, Birsner J, Lewin D, Wasiluk KR, Dunn DL, Schmidt MG, Chavin KD. Anti-endotoxin monoclonal antibodies are protective against hepatic ischemia/reperfusion injury in steatotic mice. Am J Transplant. 2004;4:1567–1573. doi: 10.1111/j.1600-6143.2004.00549.x. [DOI] [PubMed] [Google Scholar]
- 2.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA, Billiar TR. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhai Y, Shen XD, O'Connell R, Gao F, Lassman C, Busuttil RW, Cheng G, Kupiec-Weglinski JW. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J Immunol. 2004;173:7115–7119. doi: 10.4049/jimmunol.173.12.7115. [DOI] [PubMed] [Google Scholar]
- 4.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 5.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 6.Fukao T, Koyasu S. PI3K and negative regulation of TLR signaling. Trends Immunol. 2003;24:358–363. doi: 10.1016/s1471-4906(03)00139-x. [DOI] [PubMed] [Google Scholar]
- 7.Fukao T, Tanabe M, Terauchi Y, Ota T, Matsuda S, Asano T, Kadowaki T, Takeuchi T, Koyasu S. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat Immunol. 2002;3:875–881. doi: 10.1038/ni825. [DOI] [PubMed] [Google Scholar]
- 8.Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beurel E, Michalek SM, Jope RS. Innate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3). Trends Immunol. 2010;31:24–31. doi: 10.1016/j.it.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohtani M, Nagai S, Kondo S, Mizuno S, Nakamura K, Tanabe M, Takeuchi T, Matsuda S, Koyasu S. Mammalian target of rapamycin and glycogen synthase kinase 3 differentially regulate lipopolysaccharide-induced interleukin-12 production in dendritic cells. Blood. 2008;112:635–643. doi: 10.1182/blood-2008-02-137430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ren F, Duan Z, Cheng Q, Shen X, Gao F, Bai L, Liu J, Busuttil RW, Kupiec-Weglinski JW, Zhai Y. Inhibition of glycogen synthase kinase 3 beta ameliorates liver ischemia reperfusion injury by way of an interleukin-10-mediated immune regulatory mechanism. Hepatology. 2011;54:687–696. doi: 10.1002/hep.24419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hers I, Vincent EE, Tavare JM. Akt signalling in health and disease. Cell Signal. 2011;23:1515–1527. doi: 10.1016/j.cellsig.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Hemmings BA, Restuccia DF. PI3K-PKB/Akt pathway. Cold Spring Harb Perspect Biol. 2012;4:a011189. doi: 10.1101/cshperspect.a011189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol. 2012;13:283–296. doi: 10.1038/nrm3330. [DOI] [PubMed] [Google Scholar]
- 15.Cai Z, Semenza GL. PTEN activity is modulated during ischemia and reperfusion: involvement in the induction and decay of preconditioning. Circ Res. 2005;97:1351–1359. doi: 10.1161/01.RES.0000195656.52760.30. [DOI] [PubMed] [Google Scholar]
- 16.Keyes KT, Xu J, Long B, Zhang C, Hu Z, Ye Y. Pharmacological inhibition of PTEN limits myocardial infarct size and improves left ventricular function postinfarction. Am J Physiol Heart Circ Physiol. 2010;298:H1198–1208. doi: 10.1152/ajpheart.00915.2009. [DOI] [PubMed] [Google Scholar]
- 17.Li D, Qu Y, Mao M, Zhang X, Li J, Ferriero D, Mu D. Involvement of the PTEN-AKT-FOXO3a pathway in neuronal apoptosis in developing rat brain after hypoxia-ischemia. J Cereb Blood Flow Metab. 2009;29:1903–1913. doi: 10.1038/jcbfm.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mao L, Jia J, Zhou X, Xiao Y, Wang Y, Mao X, Zhen X, Guan Y, Alkayed NJ, Cheng J. Delayed administration of a PTEN inhibitor BPV improves functional recovery after experimental stroke. Neuroscience. 2013;231:272–281. doi: 10.1016/j.neuroscience.2012.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siddall HK, Warrell CE, Yellon DM, Mocanu MM. Ischemia-reperfusion injury and cardioprotection: investigating PTEN, the phosphatase that negatively regulates PI3K, using a congenital model of PTEN haploinsufficiency. Basic Res Cardiol. 2008;103:560–568. doi: 10.1007/s00395-008-0735-y. [DOI] [PubMed] [Google Scholar]
- 20.Zhao J, Qu Y, Wu J, Cao M, Ferriero DM, Zhang L, Mu D. PTEN inhibition prevents rat cortical neuron injury after hypoxia-ischemia. Neuroscience. 2013;238:242–251. doi: 10.1016/j.neuroscience.2013.02.046. [DOI] [PubMed] [Google Scholar]
- 21.Kamo N, Ke B, Busuttil RW, Kupiec-Weglinski JW. PTEN-mediated Akt/beta-catenin/Foxo1 signaling regulates innate immune responses in mouse liver ischemia/reperfusion injury. Hepatology. 2013;57:289–298. doi: 10.1002/hep.25958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ke B, Shen XD, Ji H, Kamo N, Gao F, Freitas MC, Busuttil RW, Kupiec-Weglinski JW. HO-1-STAT3 axis in mouse liver ischemia/reperfusion injury: regulation of TLR4 innate responses through PI3K/PTEN signaling. J Hepatol. 2012;56:359–366. doi: 10.1016/j.jhep.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen XD, Ke B, Zhai Y, Amersi F, Gao F, Anselmo DM, Busuttil RW, Kupiec-Weglinski JW. CD154-CD40 T-cell costimulation pathway is required in the mechanism of hepatic ischemia/reperfusion injury, and its blockade facilitates and depends on heme oxygenase-1 mediated cytoprotection. Transplantation. 2002;74:315–319. doi: 10.1097/00007890-200208150-00005. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, Cejalvo D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation. 1993;55:1265–1272. doi: 10.1097/00007890-199306000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Zhai Y, Shen XD, Gao F, Zhao A, Freitas MC, Lassman C, Luster AD, Busuttil RW, Kupiec-Weglinski JW. CXCL10 regulates liver innate immune response against ischemia and reperfusion injury. Hepatology. 2008;47:207–214. doi: 10.1002/hep.21986. [DOI] [PubMed] [Google Scholar]
- 26.Cao X, Wei G, Fang H, Guo J, Weinstein M, Marsh CB, Ostrowski MC, Tridandapani S. The inositol 3-phosphatase PTEN negatively regulates Fc gamma receptor signaling, but supports Toll-like receptor 4 signaling in murine peritoneal macrophages. J Immunol. 2004;172:4851–4857. doi: 10.4049/jimmunol.172.8.4851. [DOI] [PubMed] [Google Scholar]
- 27.Schabbauer G, Matt U, Gunzl P, Warszawska J, Furtner T, Hainzl E, Elbau I, Mesteri I, Doninger B, Binder BR, Knapp S. Myeloid PTEN promotes inflammation but impairs bactericidal activities during murine pneumococcal pneumonia. J Immunol. 2010;185:468–476. doi: 10.4049/jimmunol.0902221. [DOI] [PubMed] [Google Scholar]
- 28.Sarraj B, Massberg S, Li Y, Kasorn A, Subramanian K, Loison F, Silberstein LE, von Andrian U, Luo HR. Myeloid-specific deletion of tumor suppressor PTEN augments neutrophil transendothelial migration during inflammation. J Immunol. 2009;182:7190–7200. doi: 10.4049/jimmunol.0802562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Jia Y, Pichavant M, Loison F, Sarraj B, Kasorn A, You J, Robson BE, Umetsu DT, Mizgerd JP, Ye K, Luo HR. Targeted deletion of tumor suppressor PTEN augments neutrophil function and enhances host defense in neutropenia-associated pneumonia. Blood. 2009;113:4930–4941. doi: 10.1182/blood-2008-06-161414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subramanian KK, Jia Y, Zhu D, Simms BT, Jo H, Hattori H, You J, Mizgerd JP, Luo HR. Tumor suppressor PTEN is a physiologic suppressor of chemoattractant-mediated neutrophil functions. Blood. 2007;109:4028–4037. doi: 10.1182/blood-2006-10-055319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi JS, Park HJ, Kim HY, Kim SY, Lee JE, Choi YS, Chun MH, Chung JW, Lee MY. Phosphorylation of PTEN and Akt in astrocytes of the rat hippocampus following transient forebrain ischemia. Cell Tissue Res. 2005;319:359–366. doi: 10.1007/s00441-004-1033-0. [DOI] [PubMed] [Google Scholar]
- 32.Howitt J, Lackovic J, Low LH, Naguib A, Macintyre A, Goh CP, Callaway JK, Hammond V, Thomas T, Dixon M, Putz U, Silke J, Bartlett P, Yang B, Kumar S, Trotman LC, Tan SS. Ndfip1 regulates nuclear Pten import in vivo to promote neuronal survival following cerebral ischemia. J Cell Biol. 2012;196:29–36. doi: 10.1083/jcb.201105009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu J, Li J, Hu H, Liu P, Fang Y, Wu D. Rho-kinase inhibitor, fasudil, prevents neuronal apoptosis via the Akt activation and PTEN inactivation in the ischemic penumbra of rat brain. Cell Mol Neurobiol. 2012;32:1187–1197. doi: 10.1007/s10571-012-9845-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo JY, Ding J, Yuan F, Chen H, Chen SW, Tian HL. Dose-Dependent Protective Effect of Bisperoxovanadium against Acute Cerebral Ischemia in a Rat Model of Ischemia/Reperfusion Injury. Int J Mol Sci. 2013;14:12013–12022. doi: 10.3390/ijms140612013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu DN, Pei DS, Wang Q, Zhang GY. Down-regulation of PTEN by sodium orthovanadate inhibits ASK1 activation via PI3-K/Akt during cerebral ischemia in rat hippocampus. Neurosci Lett. 2006;404:98–102. doi: 10.1016/j.neulet.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 36.Shi GD, OuYang YP, Shi JG, Liu Y, Yuan W, Jia LS. PTEN deletion prevents ischemic brain injury by activating the mTOR signaling pathway. Biochem Biophys Res Commun. 2011;404:941–945. doi: 10.1016/j.bbrc.2010.12.085. [DOI] [PubMed] [Google Scholar]
- 37.Carini R, Grazia De Cesaris M, Splendore R, Baldanzi G, Nitti MP, Alchera E, Filigheddu N, Domenicotti C, Pronzato MA, Graziani A, Albano E. Role of phosphatidylinositol 3-kinase in the development of hepatocyte preconditioning. Gastroenterology. 2004;127:914–923. doi: 10.1053/j.gastro.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 38.Dal Ponte C, Alchera E, Follenzi A, Imarisio C, Prat M, Albano E, Carini R. Pharmacological postconditioning protects against hepatic ischemia/reperfusion injury. Liver Transpl. 2011;17:474–482. doi: 10.1002/lt.22256. [DOI] [PubMed] [Google Scholar]
- 39.Horie Y, Suzuki A, Kataoka E, Sasaki T, Hamada K, Sasaki J, Mizuno K, Hasegawa G, Kishimoto H, Iizuka M, Naito M, Enomoto K, Watanabe S, Mak TW, Nakano T. Hepatocyte-specific Pten deficiency results in steatohepatitis and hepatocellular carcinomas. J Clin Invest. 2004;113:1774–1783. doi: 10.1172/JCI20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stiles B, Wang Y, Stahl A, Bassilian S, Lee WP, Kim YJ, Sherwin R, Devaskar S, Lesche R, Magnuson MA, Wu H. Liver-specific deletion of negative regulator Pten results in fatty liver and insulin hypersensitivity [corrected]. Proc Natl Acad Sci U S A. 2004;101:2082–2087. doi: 10.1073/pnas.0308617100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang MZ, Yao B, Yang S, Jiang L, Wang S, Fan X, Yin H, Wong K, Miyazawa T, Chen J, Chang I, Singh A, Harris RC. CSF-1 signaling mediates recovery from acute kidney injury. J Clin Invest. 2012;122:4519–4532. doi: 10.1172/JCI60363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takeda Y, Costa S, Delamarre E, Roncal C, Leite de Oliveira R, Squadrito ML, Finisguerra V, Deschoemaeker S, Bruyere F, Wenes M, Hamm A, Serneels J, Magat J, Bhattacharyya T, Anisimov A, Jordan BF, Alitalo K, Maxwell P, Gallez B, Zhuang ZW, Saito Y, Simons M, De Palma M, Mazzone M. Macrophage skewing by Phd2 haplodeficiency prevents ischaemia by inducing arteriogenesis. Nature. 2011;479:122–126. doi: 10.1038/nature10507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, Ruhrberg C, Cantley LG. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol. 2011;22:317–326. doi: 10.1681/ASN.2009060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Byles V, Covarrubias AJ, Ben-Sahra I, Lamming DW, Sabatini DM, Manning BD, Horng T. The TSC-mTOR pathway regulates macrophage polarization. Nat Commun. 2013;4:2834. doi: 10.1038/ncomms3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arranz A, Doxaki C, Vergadi E, Martinez de la Torre Y, Vaporidi K, Lagoudaki ED, Ieronymaki E, Androulidaki A, Venihaki M, Margioris AN, Stathopoulos EN, Tsichlis PN, Tsatsanis C. Akt1 and Akt2 protein kinases differentially contribute to macrophage polarization. Proc Natl Acad Sci U S A. 2012;109:9517–9522. doi: 10.1073/pnas.1119038109. [DOI] [PMC free article] [PubMed] [Google Scholar]