Abstract
Objective
To determine if BPA is found in maternal circulation of pregnant women in a U.S. population and is related to gestational length and birthweight..
Methods
Circulating levels of BPA were quantified by high performance liquid chromatography-tandem mass spectrometry at delivery in 40 Southeastern Michigan mothers and correlated with gestational length and offspring birthweight.
Results
Maternal levels of unconjugated BPA ranged between 0.5 to 22.3 ng/mL in Southeastern Michigan mothers. There was no correlation between BPA concentrations and gestational length or birth weight of offspring.
Conclusions
This is the first study to document measurable levels of BPA in maternal blood of a U.S. population. Long-term follow-up studies of offspring are needed to validate or refute concerns over human fetal exposure to synthetic exogenous steroids.
Keywords: Endocrine disrupting chemicals, pregnancy
Introduction
Environmental endocrine disrupting chemicals (EDCs) are hormonally active compounds that interfere with the normal functioning of the endocrine system1. Exposure to EDCs appears to be a significant and contentious public health issue2. In recent years, attention has focused on human exposure to bisphenol-A (BPA), a widely used industrial plasticizer. Every year, over 6 billion pounds of BPA are used in the manufacture of epoxy resins and polycarbonate plastics used in a wide variety of domestic products3. BPA accounts for most estrogenic activity in landfill leachates4. BPA are used in the manufacture of epoxy resins and polycarbonate plastics that are used in dental fillings, plastic food and commercially available water containers, baby bottles, and food wrap, as well as in the lining of beverage and food cans, thus presenting a large number of possibilities for human exposure5–8. Small quantities of BPA have been detected in river water and sediments9–11, and more recently, in indoor air and dust12.
There is long-standing evidence that BPA can bind to the estrogen receptor and induce estrogen receptor-mediated gene expression13–15 although recent evidence suggests that BPA can also bind to the thyroid receptor and affect thyroid hormone signaling16. BPA does not bind to plasma estrogen binding proteins that normally limit the bioavailability of estradiol17. Accumulation of BPA appears to occur in pregnant adult females3, more likely in fat and other tissues7,18,19. BPA is particularly potent during fetal and neonatal development because the liver has limited capacity to deactivate BPA in fetuses and newborns, especially in humans17.
It is becoming increasingly evident that inappropriate exposure to sex steroids/steroid mimics have an impact on fetal growth and organ differentiation20,21. For instance, fetal exposure to excess prenatal testosterone, an estrogen precursor, from days 30–90 of gestation (term 147 days), resulted in intrauterine growth restriction (IUGR) and low birth weight offspring in sheep22–24. On the contrary, prenatal treatment with dihydrotestosterone, a non-aromatizable androgen, did not reduce birth weight (Padmanabhan V, unpublished). Combined with the fact that in utero exposure to diethylstilbestrol (DES), an estrogenic agent, is associated with IUGR25 and prenatal BPA treatment leads to low birth weight offspring in sheep26 these findings support the hypothesis that increased estrogen signaling during inopportune times of fetal development can lead to IUGR. Because BPA can bind estrogen receptors13,15, continued exposure to this compound during gestation is likely to have an impact on the developmental trajectory of the fetus.
The widespread presence of BPA and increased susceptibility of the developing fetus to estrogen mimics necessitates comprehensive evaluation of exposure levels in pregnant women. Studies from industrialized nations such as Germany and Japan, suggest that measurable levels of BPA are found in maternal circulation27–29. Similar studies have not been undertaken with pregnant women in the US. The only available information in the U.S. population relates to urinary levels of BPA metabolites in the general population30,31. A recent CDC survey found higher BPA metabolites (inactive) in the urine of 6–9 year old girls with BMI <85th percentile compared to those with BMI >85th percentile32. Urinary measures provide an index as to whether humans are exposed to BPA but does not indicate circulating levels. Assuming environmental exposure levels are similar, high levels of urinary metabolites would imply lower circulating levels of parent compound and vice versa. Considering the susceptibility of fetuses to endocrine disrupting chemicals, it is important to gain an understanding of maternal circulating levels of BPA in the U.S. Therefore, the objective of this study was to determine if BPA is found in the circulation of pregnant women in a U.S. population.
Materials and Methods
Maternal sample collection
The study involved the use of maternal blood samples collected at the time of delivery from 40 pregnant mothers as part of standard clinical hospital procedures. Blood samples were collected beginning August 4, 2006 and continued until November 2, 2006 from pregnant women delivering at the University of Michigan Hospital in Ann Arbor, MI. Maternal blood samples were drawn by venipuncture directly into a vacutainer tube, which contained EDTA. After procuring blood samples from the hospital, samples were placed in a cooler and transported to the research laboratory. Maternal blood samples were centrifuged at 3000 RPM and plasma was transferred to a glass tube and placed in −80°C freezer until time for BPA measurement. Human blood samples were handled according to the guidelines for safe laboratory practices. Identical collection and processing protocol were followed for all subjects. Information on first visit weight, height, weight at time of delivery, gestational length and birth weight were obtained for each subject. Confidentiality was maintained by substituting a subject identification number in place of name and patient identification thus eliminating any link to the patient. All materials collected from a given subject and computer data were identified with this number. The University of Michigan’s Institutional Review Board reviewed and approved the research protocol.
Because BPA is present in plastics and can leach into the blood, validations were also carried out to determine the degree of BPA contamination resulting from contact with plastics during collection. These include 1) comparison of BPA levels in plasma samples transferred with glass pipettes and plastic transfer pipettes, 2) recovery of BPA from blood, plasma and serum. These validations were carried out using sheep blood.
Transfer with glass or Pasteur pipettes
To determine if transferring plasma with plastic transfer pipettes resulted in leaching of BPA into the sample, 10 mL blood samples were collected by venipuncture from three female sheep, and transferred to EDTA tubes containing 112 µL of a stock solution of BPA (stock concentration: 0.45 mg/mL; Aldrich #239658, 99+% pure; Sigma-Aldrich, St. Louis, MO) in absolute ethanol (Aaper Alcohol and Chemical Co, Shelbyville, KY) to yield a final concentration of 5 ng/ml. Syringes and needles used for procurement of blood samples were from Becton Dickinson (Franklin Lakes NJ). The blood was then split in half using glass pipettes and samples spun down. The resulting plasma was aspirated using either a glass (Fisher #13-678-7C flint glass pipettes; Fisher Scientific, Pittsburgh PA) or polyethylene plastic (Fisher #12-711-7 Polyethylene) pipette into glass tubes and stored until BPA measurement.
Recovery of BPA from whole blood, plasma or serum
Blood samples were collected from female sheep by venipuncture into glass tubes to which 5, 10 and 20 ng/mL BPA was added. Five mL from each BPA-spiked sample was then transferred to; 1) a heparinized glass tube and spun down for plasma 2) a glass tube with no additives, allowed to clot for 2 hours at room temperature and then spun down to recover serum and 3) a glass tube with no additives added, left as whole blood. Blood, plasma and serum samples were frozen and stored at –20° C. The initial BPA spiking process took less than a minute to avoid complications from clotting. Recovery of BPA from whole blood, serum and plasma were then compared.
BPA measurements
BPA levels in samples were quantified using a high-performance liquid chromatography (HPLC) coupled with API 2000 electrospray triple-quadruple mass spectrometer (ESI-MS/MS). Briefly, a 0.8–1 mL aliquot of blood was transferred into a 15 mL polypropylene tube and 10 µL of 1-ppm butylphenol was added as an internal standard. Samples were extracted twice with 5 mL of ethyl ether by shaking in an orbital shaker for 30 min. The ethyl ether (2 × 5 mL) was pooled and evaporated to dryness under a gentle stream of nitrogen. The sample extract was then reconstituted with 0.5 mL of methanol. Standards of BPA were prepared in methanol at concentrations ranging from 0.2 to 100 ng/mL for calibration.
Analyte separation and detection were carried out using an Agilent 1100 series HPLC interfaced with an Applied Biosystems API 2000 electrospray MS/MS (Applied Biosystems, Foster City, CA. A 10 µL of the extract was injected onto an analytical column (Betasil® C18, 100 × 2.1 mm column; Thermo Electron Corporation, Waltham, MA), which was connected to a Javelin® guard column (Betasil® C18, 20 × 2.1 mm). The mobile phase was comprised of methanol and water at a gradient starting from 25% methanol to 99% methanol in 4 min and held for 10 min before it was reversed to initial condition. The flow rate and the column temperature were 300 µL/min and 25°C respectively. The MS/MS was operated in the electrospray negative ion mode. Instrumental parameters were optimized to transmit the [M-H]− ion before fragmentation to one or more product ions. For BPA, cone voltage and collision energies were 30 V and 25 V, respectively. Capillary voltage was 4.5 KV, and desolvation temperature was 400° C. Data were acquired using multiple reaction monitoring (MRM) for the transitions of 227>212.
Quality assurance and quality control parameters include validation of the method by spiking BPA into blood matrices and passing through the entire analytical procedure to calculate recoveries of BPA through the analytical method. A procedural blank, containing milli-Q water in place of plasma, was analyzed with every set of 10 samples to check for interferences or laboratory contamination. The limit of detection was 0.5 ng/mL and this was calculated as twice that of the valid "lowest acceptable calibration standard". A curve point was deemed valid if 1) it was back calculated to be within 30% of the theoretical value when evaluated versus the 1/× weighted curve, and 2) the peak area of the standard was at least two times greater than the surrogate matrix blank. Trace levels of BPA present in blanks (<0.1 ng) were subtracted from sample values for determining the concentrations in samples. Reported concentrations were not corrected for the recoveries of butylphenol, the internal standard. Quantification was based on an external calibration curve prepared by injecting 10 mL of 0.2, 0.5, 1, 5, 10, 50 and 100 ng/mL standards.
Statistical analyses
Recovery of BPA with glass and plastic pipette were compared using paired T test. Recovery of BPA from blood, serum and plasma were also compared by paired T test. Regression analyses were performed between maternal BPA measures and body weight gain, gestational length and birth weight.
Results
Figure 1 shows the elution profile of BPA following HPLC separation of standards and samples. Detection limit of BPA was 0.5 ng/mL. Recovery of BPA from sheep samples transferred with plastic transfer and glass pipette averaged 85.9±5.3 and 83.4±3.7%, respectively, and did not differ statistically. Recovery of BPA from sheep whole blood, serum, and plasma were 72.8±3.9, 88.8±9.8 and 83.1±12.8%, respectively. While there were no statistical differences, recovery from whole blood tended to be numerically lower.
Fig. 1.
HPLC-MS/MS chromatograms showing procedural blank, BPA standard (1 ppb and 50 ppb), a serum sample spiked with BPA (20 ppb), and a maternal blood sample. Multiple reaction monitoring for the transitions of 227>212 was used to acquire BPA data.
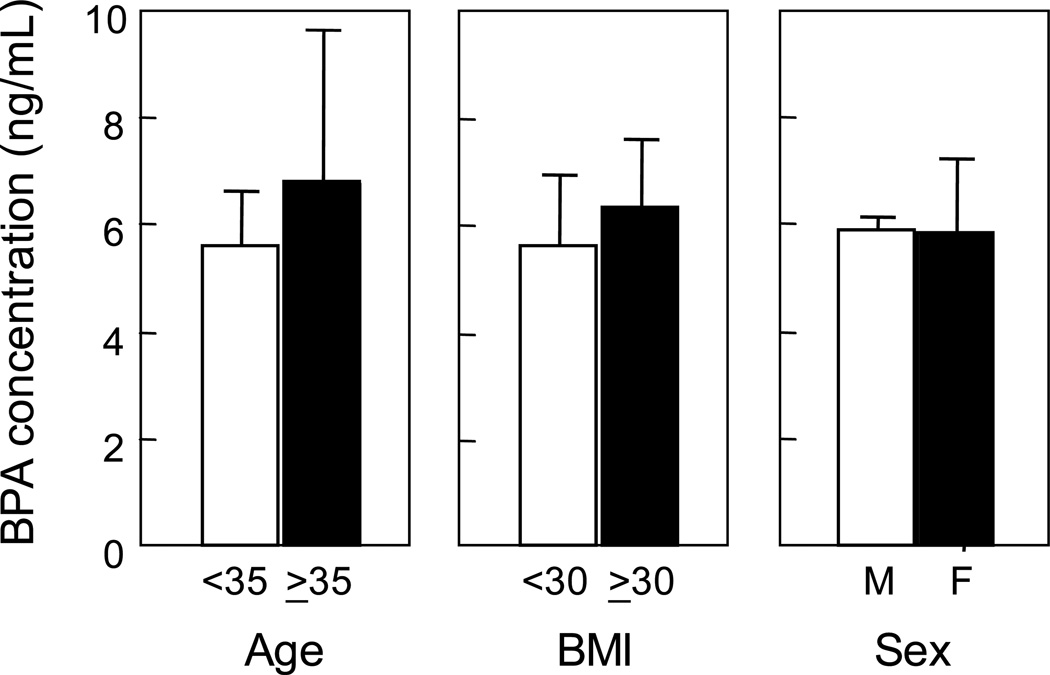
Table1 summarizes details of the human study population and the range of BPA concentration of maternal circulation at the time of delivery. The study population varied in age, BMI, weight gain during pregnancy, gestational length and offspring birth weight. Circulating BPA concentrations at the time of delivery ranged from near detection (0.5 ng/mL) to 22.3 ng/mL (Table 1). Figure 2 summarizes circulating BPA levels relative to age of women (<35 or ≥35 years), BMI (<30 or ≥30) and sex of the offspring. Maternal circulating levels of BPA did not differ by age, BMI or sex of the offspring. Figure 3 summarizes the mean gestational length and offspring weight relative to maternal circulating levels of BPA (≤5 or >5 ng/mL). There were no differences in gestational length or offspring birth weight relative to maternal BPA levels. Regression analyses also revealed no association between circulating BPA levels with any of the variables studied.
Table 1.
Subject characteristics and maternal BPA concentrations (Mean±SEM and range)
| Variable | Mean±SEM | Range |
|---|---|---|
| Age (yrs) | 28.9±0.8 | 20 – 37 |
| BMI at first visit | 29.5±0.9 | 19.3 – 41.8 |
| Gestational length (weeks) | 38.6±0.3 | 31.0 – 42.1 |
| Weight gain during pregnancy (kg) | 10.7±0.9 | −6.3 – 21.3 |
| Birth weight (kg) | 3.3±0.1 | 1.3 – 4.2 |
| BPA concentrations (ng/mL) | 5.9±0.94 | 0.5 – 22.3 |
Fig. 2.
Maternal levels of BPA (mean ±SEM) in Southeastern Michigan relative to age, BMI and sex of the offspring.
Fig. 3.
Gestational length and offspring weight (mean ±SEM) relative to maternal BPA levels of Southeastern Michigan
Discussion
This is the first study to document measurable levels of BPA in maternal blood of a U.S. population. The soft extraction procedure used in this study suggests that levels measured in plasma are free BPA rather than from conjugated BPA metabolites. Furthermore, sample collection, storage, and analytical conditions used in this study do not support hydrolysis of any conjugated forms of BPA in blood. The highest circulating levels found in pregnant women from Southeast Michigan are half as much as those achieved during early gestation in our recent sheep study26 that resulted in low birth weight offspring and adult reproductive deficits, raising public concern over human fetal exposure to synthetic exogenous steroids.
The scientific dogma is that BPA cannot be a biologically important pollutant since the body metabolizes and excretes it relatively quickly. Dekant et al.33 found that ingested BPA was metabolized completely to BPA monoglucuronide and was excreted into urine within 24 hours in humans. Biotransformation studies carried out in rodent models indicate BPA is extensively metabolized to biologically inactive glucuronide (57–98 %) and up to 4% form sulfate conjugates, which are excreted via urine and feces leaving 1 to 12 % active (unconjugated) BPA14,34,35. Our findings, similar to studies conducted in Germany and Japan27–29, provide evidence supporting the existence of significant levels of unconjugated BPA in the circulation of pregnant women in Southeastern Michigan. Maternal levels found in our study in the U.S. are comparable to those reported in studies from other industrialized countries27–29. For instance, BPA levels in maternal circulation of German women ranged between 0.3 and 18.9 ng/mL (median 3.1 ng/mL)28.
Prior to determining the biological significance of the levels BPA found in maternal circulation, it was important to address how accurate these measures are. Considering that all the samples were acquired from one site and processed similarly by the same individuals, the range of maternal BPA concentrations achieved (below detection to >20 ng/mL) suggest that leaching of BPA during handling, was likely to be at levels below the detection limit (0.5 ng/mL). Recovery studies carried out with sheep blood also provide further evidence that leaching of BPA from plastic syringe during sampling and transfer was minimal. BPA levels in HPLC blanks were also below the detection limit. While it is conceivable that low levels of BPA may have leached into the sample, this contribution is likely to be less than 0.5 ng/mL (detection limit) because background from blanks were consistently low and subtracted from concentrations measured in samples. Taken together, these findings suggest that the levels of BPA measured in circulation of pregnant women living in Southeastern Michigan are relatively accurate.
How are the levels of BPA seen in maternal blood related to fetal exposure levels and well-being? While we did not measure BPA in fetal circulation, other studies suggest that the fetus is likely to be exposed to similar (if not higher) levels of BPA as the mother. Evidence exists in support of the passage of BPA across the placenta28, (high levels of BPA were also reported in human amniotic fluid28,30) and in umbilical cord blood27,28. Because most biotransformation enzymes such as uradinediphosphate-glucuronosyltransferase are not produced until after birth36 and the rate of BPA clearance from the fetal circulation is slower than from the maternal circulation37, any toxic effects of BPA are likely to be amplified in the fetus. Animal studies have provided evidence that fetal exposure to low levels of BPA can influence development, growth and reproduction3,38–41. To what extent do these studies relate to any threat posed in humans is a contentious topic3,42,43. We recently found reduced birth weight and adult reproductive deficits in sheep following sustained maternal exposure to BPA26 (at twice the levels found in human maternal circulation27–29 (also this study).
Lack of association between maternal levels of BPA at term and gestational length/birth weight in this study fails to address whether BPA, at levels found in human maternal circulation, poses risks to fetal well being. A limitation of this study is that measures of BPA were obtained at birth and not early/mid gestation, a time when organ differentiation occurs and the fetus is vulnerable to insults. Other studies have found differences in amniotic fluid levels of BPA with higher levels found in samples obtained during early than late pregnancy27. Second, conventional birth weight standards appear not to be sufficient for assessing fetal growth restriction in preterm infants44. As a result, offspring weight range seemingly appropriate for gestational age may have abnormal body composition and thus not provide an index of risk. Third, postnatal consequences for the offspring born to these women are yet to be determined. Fourth, the sample size of 40 is small and the role of potentially confounding variables such as economic status and race were not considered in this study and may be an important variable. Fifth, the BPA levels reported here represent the freely available form in blood; glucuronide conjugates were not measured in this study. A prospective study with a larger cohort that relates BPA levels across gestation with various fetal measures and postnatal developmental measures is needed to truly address this question.
Our study also found no association between BMI at first prenatal visit and BPA assessment at birth. As mentioned previously, a recent CDC survey found higher BPA metabolites (inactive) in 6–9 year old girls with BMI <85th percentile compared to those with BMI >85th percentile32. Assuming environmental exposure levels are similar between these BMI groups, this would imply the reverse in circulation, namely high levels of parent (active) BPA levels in girls with BMI>85th percentile. In the absence of assessment of BPA levels at the first prenatal visit, the findings from this study cannot address the impact of obesity on circulating BPA levels.
Could racial disparities in exposure to BPA contribute to well-documented disparities in pregnancy outcome between African Americans and Whites45 in the U.S.? Minority communities are disproportionately exposed to environmental pollutants through residential segregation and other social structural factors46–48. BPA accounts for most estrogenic activity that leaches from landfills into the surrounding ecosystem, neighborhoods where African Americans predominate4. African-American communities also have fewer sources of fresh food and a higher proportion of fast food restaurants. This could potentially contribute to greater exposure to BPA49,50. This is a question that warrants further investigation.
In conclusion, our findings document the presence of BPA in maternal circulation in samples from Southeastern Michigan at levels close to what is shown to be detrimental to reproductive, metabolic and behavioral health in animal models3,38–41. Long-term follow up studies are required to determine whether BPA at levels found in human maternal circulation have an impact on the developmental trajectory of the offspring.
Acknowledgement
We thank Dr. Mohan Manikkam and Mr. James S Lee for assistance with the processing of human blood samples and procurement of sheep blood samples, respectively. This study was supported by P20RR020682 from the National Institute of Health.
References
- 1.Damstra T, Barlow S, Bergman A, Kavlock R, Van der Kraak G. Global assessment of the state-of-the-science of endocrine disruptors. International Programme on Chemical Safety (IPCS) Geneva, Switzerland: World Health organization; 2002. [Google Scholar]
- 2.Gierthy JF. Testing for Endocrine Disruption: How Much is Enough? Toxicol Sci. 2002;68:1–3. doi: 10.1093/toxsci/68.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Welshons WV, Nagel SC, vom Saal FS. Large Effects from Small Exposures. III. Endocrine Mechanisms Mediating Effects of Bisphenol A at Levels of Human Exposure. Endocrinology. 2006;147:s56–s69. doi: 10.1210/en.2005-1159. [DOI] [PubMed] [Google Scholar]
- 4.vom Saal FS, Huges C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ Health Perspect. 2005;113:926–933. doi: 10.1289/ehp.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brotons JA, Olea-Serrano MF, Villalobos M, Pedraza V, Olea N. Xenoestrogens released from lacquer coatings in food cans. Environ Health Perspect. 1995;103:608–612. doi: 10.1289/ehp.95103608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuo H-W, Ding W-H. Trace determination of bisphenol A and phytoestrogens in infant formula powders by gas chromatography-mass spectrometry. J Chromatogr A. 2004;1027:67–74. doi: 10.1016/j.chroma.2003.08.084. [DOI] [PubMed] [Google Scholar]
- 7.Munguia-Lopez EM, Gerardo-Lugo S, Peralta E, Bolumen S, Soto-Valdez H. Migration of bisphenol A (BPA) from can coatings into a fatty-food simulant and tuna fish. Food Addit Contam. 2005;22:892–898. doi: 10.1080/02652030500163674. [DOI] [PubMed] [Google Scholar]
- 8.Thomson BM, Grounds PR. Bisphenol A in canned foods in New Zealand: an exposure assessment. Food Addit Contam. 2005;22:65–72. doi: 10.1080/02652030400027920. [DOI] [PubMed] [Google Scholar]
- 9.Boyd GR, Palmeri JM, Zhang S, Grimm DA. Pharmaceuticals and personal care products (PPCPs) and endocrine disrupting chemicals (EDCs) in stormwater canals and Bayou St. John in New Orleans, Louisiana, USA. Sci Total Environ. 2004;333:137–148. doi: 10.1016/j.scitotenv.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 10.Khim JS, Lee KT, Villeneuve DL, Kannan K, Giesy JP, Koh CH. In vitro bioassay determination of dioxin-like and estrogenic activity in sediment and water from Ulsan Bay and its vicinity, Korea. Arch Environ Contam Toxicol. 2001;40:151–160. doi: 10.1007/s002440010158. [DOI] [PubMed] [Google Scholar]
- 11.Loos R, Hanke G, Eisenreich SJ. Multi-component analysis of polar water pollutants using sequential solid-phase extraction followed by LC-ESI-MS. J Environ Monit. 2003;5:384–394. doi: 10.1039/b300440f. [DOI] [PubMed] [Google Scholar]
- 12.Rudel RA, Brody JG, Spengler JD, Vallarino J, Geno PW, Sun G, Yau A. Identification of selected hormonally active agents and animal mammary carcinogens in commercial and residential air and dust samples. J Air Waste Manag Assoc. 2001;51:499–513. doi: 10.1080/10473289.2001.10464292. [DOI] [PubMed] [Google Scholar]
- 13.Dodds E, Lawson W. Molecular structure in relation to oestrogenic activity. Compounds without a phenanthrene nucleus. Proc R Soc Lond B. 1938;125:222–232. [Google Scholar]
- 14.Matthews JB, Twomey K, Zacharewski TR. In vitro and in vivo interactions of bisphenol A and its metabolite, bisphenol A glucuronide, with estrogen receptors alpha and beta. Chem Res Toxicol. 2001;14:149–157. doi: 10.1021/tx0001833. [DOI] [PubMed] [Google Scholar]
- 15.Wetherill YB, Akingbemi B, Kenno J, McLachlan JA, Nadal A, Sonnenschein C, Watson CS, Zoeller RT, Belcher SM. In Vitro molecular mechanisms of bisphenol A action. Reprod Tox. 2007 doi: 10.1016/j.reprotox.2007.05.010. print copy in press. [DOI] [PubMed] [Google Scholar]
- 16.Zoeller RT, Bansal R, Parris C. Bisphenol-A, an Environmental Contaminant that Acts as a Thyroid Hormone Receptor Antagonist in Vitro, Increases Serum Thyroxine, and Alters RC3/Neurogranin Expression in the Developing Rat Brain. Endocrinology. 2005;146:607–612. doi: 10.1210/en.2004-1018. [DOI] [PubMed] [Google Scholar]
- 17.Elsby R, Maggs JL, Ashby J, Park BK. Comparison of the modulatory effects of human and rat liver microsomal metabolism on the estrogenicity of bisphenol A: implications for extrapolation to humans. J Pharmacol Exp Ther. 2001;297:103–113. [PubMed] [Google Scholar]
- 18.Nunez A, Kannan K, Giesy J, Fang J, Clemens L. Effects of bisphenol A. on energy balance and accumulation in brown adipose tissue in rats. Chemosphere. 2001;42:917–922. doi: 10.1016/s0045-6535(00)00196-x. [DOI] [PubMed] [Google Scholar]
- 19.Takeuchi T, Tsutsumi O, Ikezuki Y, Takai Y, Taketani Y. Positive Relationship between Androgen and the Endocrine Disruptor, Bisphenol A, in Normal Women and Women with Ovarian Dysfunction. Endocr J. 2004;51:165–169. doi: 10.1507/endocrj.51.165. [DOI] [PubMed] [Google Scholar]
- 20.Abbott DH, Barnett DK, Bruns CM, Dumesic DA. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum Reprod Update. 2005;11:357–374. doi: 10.1093/humupd/dmi013. [DOI] [PubMed] [Google Scholar]
- 21.Padmanabhan V, Manikkam, Recabarren S, Foster DL. Prenatal testosterone programs reproductive and metabolic dysfunction in the female. Mol Cell Endocrinology. 2006;246:165–174. doi: 10.1016/j.mce.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 22.Crespi EJ, Steckler TL, MohanKumar PS, Padmanabhan V. Prenatal exposure to excess testosterone modifies the developmental trajectory of the insulin-like growth factor system in female sheep. J Physiol. 2006;572:119–130. doi: 10.1113/jphysiol.2005.103929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manikkam M, Crespi EJ, Doop DD, Herkimer C, Lee JS, Yu S, Brown MB, Foster DL, Padmanabhan V. Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology. 2004;145:790–798. doi: 10.1210/en.2003-0478. [DOI] [PubMed] [Google Scholar]
- 24.Steckler T, Wang J, Bartol F, Roy SK, Padmanabhan V. Fetal programming: prenatal testosterone treatment causes intrauterine growth retardation, reduces ovarian reserve and increases ovarian follicular recruitment. Endocrinology. 2005;146:3185–3193. doi: 10.1210/en.2004-1444. [DOI] [PubMed] [Google Scholar]
- 25.Giusti RM, Iwamoto K, Hatch EE. Diethylstilbestrol revisited: a review of the long-term health effects. Ann Intern Med. 1995;122:778–788. doi: 10.7326/0003-4819-122-10-199505150-00008. [DOI] [PubMed] [Google Scholar]
- 26.Savabieasfahani M, Kannan K, Astapova O, Evans N, Padmanabhan V. Developmental programming: differential effects of prenatal exposure to bisphenol-A or methoxychlor on reproductive function. Endocrinology. 2006;147:5956–5966. doi: 10.1210/en.2006-0805. [DOI] [PubMed] [Google Scholar]
- 27.Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod. 2002;17:2839–2841. doi: 10.1093/humrep/17.11.2839. [DOI] [PubMed] [Google Scholar]
- 28.Schonfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ Health Perspect. 2002;110:A703–A707. doi: 10.1289/ehp.110-1241091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamada H, Furuta I, Kato EH, Kataoka S, Usuki Y, Kobashi G, Sata F, Kishi R, Fujimoto S. Maternal serum and amniotic fluid bisphenol A concentrations in the early second trimester. Reprod Toxicol. 2002;16:735–739. doi: 10.1016/s0890-6238(02)00051-5. [DOI] [PubMed] [Google Scholar]
- 30.Brock JW, Yoshimura Y, Barr JR, Maggio VL, Graiser SR, Nakazawa H, Needham LL. Measurement of bisphenol A levels in human urine. J Expo Anal Environ Epidemiol. 2001;11:323–328. doi: 10.1038/sj.jea.7500174. [DOI] [PubMed] [Google Scholar]
- 31.Calafat A, Kuklenyik Z, Reidy J, Caudill S, Ekong J, Needham L. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect. 2005;113:391–395. doi: 10.1289/ehp.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolff MS, Teitelbaum SL, Windham G, Pinney SM, Britton JA, Chelimo C, Godbold J, Biro F, Kushi LH, Pfeffer CM, Caalafat AM. Pilot study of urinary biomarkers of phytoestrogens, phthalates, and phenols in girls. Environ Health Perspect. 2007;115:116–121. doi: 10.1289/ehp.9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dekant W, Lederer E, Wolf N, Colnot T, Volkel W. Toxicokinetics of bisphenol A in human subjects. Toxicol Sci. 2002;66(suppl):227. [Google Scholar]
- 34.Knaak JB, Sullivan LJ. Metabolism of bisphenol A in the rat. Toxicol Appl Pharmacol. 1966;8:175–184. doi: 10.1016/s0041-008x(66)80001-7. [DOI] [PubMed] [Google Scholar]
- 35.Pottenger LH, Domoradzki JY, Markham DA, Hansen SC, Cagen SZ, Waechter JM., Jr The relative bioavailability and metabolism of bisphenol A in rats is dependent upon the route of administration. Toxicol Sci. 2000;54:3–18. doi: 10.1093/toxsci/54.1.3. [DOI] [PubMed] [Google Scholar]
- 36.Coughtrie MW, Burchell B, Leakey JE, Hume R. The inadequacy of perinatal glucuronidation: immunoblot analysis of the developmental expression of individual UDP-glucuronosyltransferase isoenzymes in rat and human liver microsomes. Mol Pharmacol. 1988;34:729–735. [PubMed] [Google Scholar]
- 37.Takahashi O, Oishi S. Disposition of orally administered 2, 2-Bis(4-hydroxyphenyl) propane (Bisphenol A) in pregnant rats and the placental transfer to fetuses. Environ Health Perspect. 2000;108:931–935. doi: 10.1289/ehp.00108931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alonso-Magdalena P, Morimoto S, Ripoll C, Fuented E, Nadal A. The estrogenic effect of bisphenol A disrupts pancreatic beta-cell function in vivo and induces insulin resistance. Environ Health Perspect. 2006;114:106–112. doi: 10.1289/ehp.8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Durando K, kass L, Piva J, Sonnenschein C, Soto AM, Luque EH, Munoz-de-Toro M. Prenatal bisphenol A exposure induces preneoplastic lesions in the mammary gland in Wistar rats. Environ Health Perspect. 2007;115:80–86. doi: 10.1289/ehp.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kubo K, Arai O, Omura M, Watanabe R, Ogata R, Aou S. Low dose effects of bisphenol A on sexual differentiation of the brain and behavior in rats. Neurosci Res. 2003;45:345–356. doi: 10.1016/s0168-0102(02)00251-1. [DOI] [PubMed] [Google Scholar]
- 41.Timms BG, Howdeshell KL, Barton L, Bradley S, Richter CA, vom Saal FS. Estrogenic chemicals in plastic and oral contraceptives disrupt development of the fetal mouse prostate and urethra. Proc Natl Acad Sci USA. 2005;102:7014–7019. doi: 10.1073/pnas.0502544102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goodman JE, McConnell EE, Sipes IG, Witorsch RJ, Slayton Tm, Yu CJ, Lewis AS, Rhomberg LR. An updated weight of the evidence evaluation of reproductive and developmental effects of low doses of bisphenol A. Crit Rev Toxicol. 2006;36:387–457. doi: 10.1080/10408440600758317. [DOI] [PubMed] [Google Scholar]
- 43.Kamrin MA. The "low dose" hypothesis: validity and implications for human risk. Int J Toxicol. 2007;26:13–23. doi: 10.1080/10915810601117968. [DOI] [PubMed] [Google Scholar]
- 44.Cooke RW. Conventional birth weight standards obscure fetal growth restriction in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2007;92:F189–F192. doi: 10.1136/adc.2005.089698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoyert DL, Mathews TJ, Menacker F, Strobino DM, Guyer B. Annual summary of vital statistics: 2004. Pediatrics. 2006;117:168–183. doi: 10.1542/peds.2005-2587. [DOI] [PubMed] [Google Scholar]
- 46.Gee GC, Payne-Sturges DC. Environmental health disparities: A framework integrating psychosocial and environmental concepts. Environ Health Perspect. 2004;112:1645–1653. doi: 10.1289/ehp.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olden K, White SL. Health-related disparities: Influence of environmental factors. Med Clin North Am. 2005;89:721–738. doi: 10.1016/j.mcna.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 48.Silbergeld EK, Patrick TE. Environmental exposures, toxicologic mechanisms, and adverse pregnancy outcomes. Amer J Obstet & Gynecol. 2005;192(Suppl):S11–S21. doi: 10.1016/j.ajog.2004.06.117. [DOI] [PubMed] [Google Scholar]
- 49.Lewis LB, Sloane DC, Nascimento LM, Diamant Al, Guinyard JJ, Yancey AK, Flynn G. REACH African Americans' access to healthy food options in south los angeles restaurants. Am J Public Health. 2005;95:668–673. doi: 10.2105/AJPH.2004.050260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zenk SN, Schulz AJ, Israel BA, James SA, Bao S, Wilson ML. Fruit and vegetable access differs by community racial composition and socioeconomic position in Detroit, Michigan. Ethn Dis. 2006;16:275–280. [PubMed] [Google Scholar]