Abstract
Objective
To evaluate the efficacy, tolerability, and safety of once-daily 1200 mg and 2400 mg SPN-804 (Oxtellar XR™, Supernus Pharmaceuticals), an extended-release tablet formulation of oxcarbazepine (OXC), added to 1-3 concomitant antiepileptic drugs (AEDs) in adults with refractory partial-onset seizures, with or without secondary generalization.
Methods
The Prospective, Randomized Study of OXC XR in Subjects with Partial Epilepsy Refractory (PROSPER) study was a multinational, randomized, double-blind, parallel-group Phase 3 study. The primary efficacy endpoint was median percent reduction from baseline in monthly (28-day) seizure frequency for the 16-week double-blind treatment period in the intent-to-treat (ITT) population with analyzable seizure data. Other efficacy analyses included proportion of patients with ≥ 50% seizure reduction, proportion of patients seizure free, and the relationship between clinical response and plasma concentration.
Results
Median percent reduction was -28.7% for placebo, −38.2% (P = 0.08 vs placebo) for once-daily SPN-804 1200 mg, and −42.9% (P = 0.003) for SPN-804 2400 mg. Responder rates were 28.1%, 36.1% (P = 0.08), and 40.7% (P = 0.02); 16-week seizure-free rates in a pragmatic ITT analysis were 3.3%, 4.9% (P = 0.59), and 11.4% (P = 0.008), respectively. When data were analyzed separately for study site clusters, a post hoc analysis demonstrated that both SPN-804 dosages were significantly superior to placebo in median percent seizure reduction (placebo: −13.3%; 1200 mg: −34.5%, P = 0.02; 2400 mg: −52.7%, P = 0.006) in the North American study site cluster. A concentration–response analysis also supported a clinically meaningful effect for 1200 mg. Adverse event types reflected the drug's established profile. Adverse event frequency was consistent with a pharmacokinetic profile in which SPN-804 produces lower peak plasma concentrations vs immediate-release OXC. Once-daily dosing was not associated with any new safety signals.
Conclusions
Adjunctive once-daily SPN-804 improved seizure control in patients with inadequately controlled partial-onset seizures. Adverse event occurrence and discontinuations due to adverse events suggest improved tolerability vs previously published data with immediate-release OXC.
Keywords: extended-release oxcarbazepine, partial-onset seizures, adjunctive therapy, refractory epilepsy
Introduction
Oxcarbazepine (OXC) is a 10-keto analogue of carbamazepine (CBZ) – the antiepileptic drug (AED) that has served as the benchmark for efficacy in partial-onset seizures. Although the efficacy of OXC appears to mirror that of CBZ 1, OXC has a markedly different metabolic and pharmacokinetic profile that simplifies its use clinically. As opposed to oxidation, OXC undergoes rapid and almost complete reductive metabolism to the active metabolite 10-monohydroxy derivative (MHD; licarbazepine). Because it is the predominant moiety in plasma, MHD is largely responsible for the drug's clinical activity. Oxcarbazepine is converted presystemically to S- and R-licarbazepine enantiomers (80% : 20%) that are virtually indistinguishable in terms of their biological activity 2. Reductive metabolism of OXC avoids the generation of reactive epoxides and reduces the potential for interactions with AEDs that induce the cytochrome P450 system. Pharmacokinetics of MHD are linear and dose proportional from 300 to 2400 mg 3. Efficacy as adjunctive therapy is likewise dose related for dosages ranging from 600 to 2400 mg/day 4.
In the only placebo-controlled study in adults with immediate-release OXC (OXC-IR) as adjunctive therapy, 67% of patients assigned to 2400 mg/day OXC-IR discontinued due to adverse events 4, demonstrating that poor tolerability may limit the maximum clinically relevant dosage to 1200 mg/day in most patients. As noted in U.S. product labeling for OXC-IR, ‘daily doses above 1200 mg/day show somewhat greater effectiveness in controlled trials, but most patients were not able to tolerate the 2400 mg/day dose, primarily because of CNS effects' 3. The OXC-IR study reported a significant correlation between mean trough MHD concentrations and the incidences of the most common adverse effects, that is, dizziness, diplopia, somnolence, vomiting, nausea. Adverse effects have also been shown to correlate with peak plasma concentrations, particularly MHD concentrations ≥30 μg/ml 5,6. Although these adverse effects may be transient, occurring twice daily within 2–3 h after dose administration with b.i.d. OXC-IR dosing, they nonetheless can persist throughout the dosing interval 6. Adverse effects with OXC-IR administration, including intermittent events associated with peak plasma MHD concentrations, can often be ameliorated by administering the same total daily OXC-IR dosage more frequently in smaller doses, for example, by switching from b.i.d. to t.i.d. administration 6. These observations strongly suggest the potential for improved tolerability with an extended-release (ER) OXC formulation that reduces peak plasma MHD concentrations with less frequent dosing.
SPN-804 (Oxtellar XR™, Supernus Pharmaceuticals) is a novel extended-release OXC tablet using a matrix delivery technology to overcome the low, pH-dependent solubility of OXC in aqueous solutions. A dissolving drug-polymer core releases OXC continuously over a 24-hour dosing interval to produce a smoother plasma concentration profile. The MHD concentration peaks 7 h after dosing, is sustained for an extended period, and gradually declines over the remainder of the dosing interval, beginning approximately 12 h after a dose. With once-daily SPN-804 1200 mg, MHD exposures and peak MHD concentrations are ∼19% lower and trough MHD concentration is ∼16% lower than with OXC-IR 600 mg b.i.d 7. The PROSPER study was designed as a placebo-controlled Phase 3 study to evaluate the efficacy, tolerability, and safety of once-daily SPN-804 1200 mg and 2400 mg as adjunctive therapy in adults with inadequately controlled partial-onset seizures. Dosages were selected based on a similarly designed Phase 3 dose-ranging study of OXC-IR b.i.d. as adjunctive therapy in adults with refractory partial-onset seizures receiving one to three concomitant AEDs 4.
Methods
Study design
This was a multinational, multicenter, randomized, double-blind, parallel-group, placebo-controlled Phase 3 study (ClinicalTrials.gov identifier: NCT00-772603) conducted between November 2008 and April 2010 at 88 sites in eight countries in North America (United States, n = 25; Mexico, n = 13; Canada, n = 2) and Eastern Europe/Russia (Poland, n = 18; Russia, n = 16; Bulgaria, n = 10; Croatia, n = 6; Romania, n = 5). The study was designed with an 8-week prospective observation phase to establish baseline seizure frequency, which was followed by a double-blind treatment period comprised of a 4-week titration phase, 12-week maintenance phase, and 3-week interval for blinded conversion to open-label treatment or tapering to baseline therapy.
For the double-blind period, patients were randomized 1:1:1 to one of three treatment groups with study drug administered once daily as placebo, SPN-804 1200 mg, or SPN-804 2400 mg. Randomization was managed by a centralized interactive voice response system, with the vendor using a pseudo-random number generator to produce study drug kit numbers for the randomization schedule. At each visit, study sites accessed the voice response system for instructions on the individual kit number to use. Study drug blinding was maintained with identical 600-mg SPN-804 or placebo tablets and packaging in blister cards. During the double-blind treatment period, patients and all personnel involved with the study's conduct or interpretation remained blinded to study drug codes. Personnel involved in plasma assays for evaluating population pharmacokinetics did not have access to the randomization schedule and were not otherwise involved in the study's conduct.
Study drug was administered under fasting conditions as four tablets once daily. During titration, the dose was increased weekly in 600-mg increments. Dosages of concomitant AEDs and vagal nerve stimulator (VNS) settings remained constant throughout the study. After week 4, study drug could be down-titrated if needed by discarding the last tablet on each blister pack row, thereby blindly reducing the dose only in the SPN-804 2400-mg group (reduced to 1800 mg/day). After maintaining the target dose for 12 weeks, patients who chose to enroll in the open-label follow-on study were titrated to once-daily SPN-804 1200 mg in a blinded fashion; all other patients were tapered off study medication before the final study visit. During the 16-week blinded treatment phase for data analysis, study visits were scheduled for days 1 (end of baseline/randomization), 29 (end of titration), 57, 85, and 113 (end of maintenance phase).
The study was performed in accordance with the International Conference on Harmonization Good Clinical Practice guidelines, the Declaration of Helsinki, European Medicines Agency requirements, and the US Code of Federal Regulations. The protocol, amendments, and informed consent were reviewed and approved by the appropriate regulatory authorities in each country and Institutional Review Board or Independent Ethics Committee at each site. Patients provided written informed consent before study participation.
Patients
Patients aged 18–65 years with partial-onset seizures with or without secondary generalization according to the 1981 International League Against Epilepsy Classification of Epileptic Seizures 8 were enrolled. Seizure descriptions for the first three patients screened by each study site were submitted for the Epilepsy Study Consortium review in order to verify seizure classification; review of information for additional patients was required if initial classifications were incorrect. Patients had to have an average of at least three seizures monthly (28 days) during the 8-week screening period despite ongoing treatment with stable doses of one to three AEDs with/without adjunctive VNS. Partial-onset seizures without discognitive features (simple partial) had to have a motor component to be countable (See Appendix S1).
Outcome measures
Efficacy
Efficacy was assessed using seizure counts from patient-maintained seizure diaries as well as Patient Global Impression of Change (PGIC) and the Quality of Life in Epilepsy (QOLIE-31) questionnaires. Seizure frequency was calculated as the total number of seizures reported in the seizure diary for the study phase divided by the number of days on which patients recorded data, multiplied by 28. The primary endpoint was the median percent change from baseline in monthly (28-day) seizure frequency for the 16-week treatment period in an intent-to-treat population with analyzable seizure data. Secondary endpoints included responder rate (proportion of patients with ≥ 50% seizure frequency reduction), proportion of patients seizure free, and changes in PGIC and QOLIE-31 scores. Data for patients who discontinued early were included in analyses up to their discontinuation, using data recorded through their last visits in the blinded treatment phase.
Tolerability and safety
Assessments included monitoring of treatment-emergent adverse events coded using the Medical Dictionary for Regulatory Affairs (MedDRA), neurologic/physical examinations, clinical laboratory values, vital signs, and ECG tracings.
Pharmacokinetics and concentration–response analysis
Blood samples were drawn at maintenance phase visits to determine OXC and MHD concentrations for population pharmacokinetic (PK) analysis with nonlinear mixed-effects modeling (NONMEM). Samples were obtained at separate visits for trough (predose), 1, 2, 4, and 7 h post-dose concentrations. OXC and MHD concentrations were determined with a validated liquid chromatography tandem mass spectrometry assay with a quantification limit of 0.005 and 0.05 μg/ml, respectively. Pharmacokinetic variables derived from the population PK analysis included apparent oral clearance (CL/F), maximum (Cmax), and minimum/trough (Cmin) concentrations for OXC and MHD. Data from the population PK analysis were applied to seizure frequency data to evaluate the concentration–response relationship in subgroups defined by Cmin plasma MHD concentrations (≥10 vs <10 μg/ml) estimated from population PK analysis.
Statistical analysis
Sample size calculation
Sample size was calculated using the methods proposed by Noether et al. 9 for nonparametric tests (i.e., Wilcoxon rank-sum test), based on the observed differences for placebo vs OXC-IR in a similarly designed study 4. Calculations suggested that 120 patients per treatment group would provide over 80% power to detect a difference of 24–32% between placebo and once-daily SPN-804 1200 mg and over 95% power to detect a difference of 31–42% between placebo and once-daily SPN-804 2400 mg, each at a two-sided 0.025 level for an overall type 1 error rate of 0.05.
Analysis sets
Efficacy was assessed in the intent-to-treat population with analyzable seizure data, that is, all randomized patients with seizure data for ≥21 consecutive days in the baseline phase who received at least one dose of study drug, had at least one on-treatment visit, and ≥14 consecutive days of seizure diary data after study drug receipt. For patients who discontinued early, seizure data through the last visit during the blinded treatment phase were used for determining seizure frequency. The safety population was all randomized patients who had received at least one dose of study drug. Patients assigned to SPN-804 2400 mg who were down-titrated to 1800 mg/day were included in the 2400-mg group for all analyses.
Primary efficacy analysis
For seizure frequency analysis, monthly (28-day) seizure frequency was calculated for baseline and 16-week double-blind treatment periods. Median percent seizure frequency change was analyzed with Wilcoxon rank-sum test to test the hypothesis of equal median percent seizure frequency change from baseline between SPN-804 2400 mg and placebo and SPN-804 1200 mg and placebo. To preserve the overall type I error rate at 0.050, a step-up Hochberg procedure 10 was used for the pairwise comparison of each SPN-804 group against placebo. If both observed P-values were <0.050 in favor of SPN-804 treatment groups, then differences for both groups were declared significant. If the observed P-value was >0.050 for only one SPN-804 treatment group, then the other SPN-804 was significantly superior to placebo only if P < 0.025. The Hodges–Lehmann point estimate and associated 95% confidence interval (95% CI) for median treatment differences between placebo and each SPN-804 group were determined. To assess the sensitivity of the primary efficacy results to the effects of dropouts and incomplete seizure diary data, three analyses were conducted: primary efficacy analysis in patients who completed double-blind treatment without premature discontinuation (completer analysis); mixed-model repeated-measures analysis in the overall ITT population; and impact of study site cluster (post hoc grouping of study site clusters: North America and Eastern Europe/Russia).
Secondary efficacy variables
Responder rates were analyzed using a logistic regression model, with treatment group, country/study site cluster, age, gender, and baseline seizure frequency as explanatory variables. Responder rate effect sizes for SPN-804 vs placebo were estimated with odds ratio and 95% CI. A pragmatic ITT analysis was used for determining seizure-free rates, with the overall ITT population as the denominator. In this analysis, patients were only considered seizure free if they completed double-blind treatment; patients who discontinued early were considered non-responders 11. Fisher's exact test was used for pairwise comparisons of seizure-free rates. Between-group differences in PGIC score changes were compared using Wilcoxon rank-sum test. Analysis of covariance (ANCOVA) assessed between-group differences in QOLIE-31 subscale scores, total score, and health status score, with treatment group, age, gender, study site cluster/country, and pretreatment score as explanatory variables.
Concentration–response analysis
Differences in median percent seizure frequency reduction over 16 weeks of double-blind treatment vs baseline in subgroups defined by Cmin (≥10 vs < 10 μg/ml) were analyzed using Wilcoxon rank-sum test.
Results
Patient disposition and characteristics
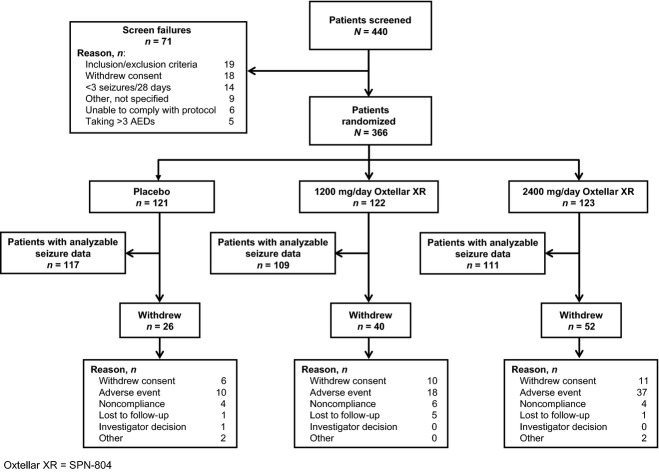
Of 440 patients screened, 366 patients were randomized to once-daily placebo (n = 121), SPN-804 1200 mg (n = 122), and SPN-804 2400 mg (n = 123). Analyzable seizure data for the primary efficacy analysis were available for 96.7% (n = 117), 89.3% (n = 109), and 90.3% (n = 111), respectively (Fig. 1). Of the 111 patients in the 2400-mg group with analyzable seizure data, 26 were patients in whom SPN-804 was down-titrated to 1800 mg/day. Overall, 67.8% of patients completed 16 weeks, with 21.5% of placebo patients discontinuing prematurely compared with 32.8% and 42.3% in the 1200-mg and 2400-mg SPN-804 groups, respectively. The most common reason for study withdrawal was adverse events, accounting for the higher discontinuation rates in the active treatment groups. Based on pill counts, 96.0% (placebo), 93.2% (1200 mg), and 95.1% (2400 mg) of patients were adherent with once-daily dosing.
Figure 1.
Study disposition for patients assigned to placebo or Oxtellar XR (SPN-804).
Treatment groups were generally well balanced in terms of baseline demographics and disease characteristics (Table 1). Overall, the mean age was 38.9 years; 55.2% of patients were women. The mean number of years since epilepsy diagnosis was 20.8 years; median monthly seizure frequency at baseline was 6.5 seizures despite ongoing treatment with two or three concomitant AEDs in 67.2% of patients.
Table 1.
Demographics and baseline characteristics (safety population)
| Characteristics | Placebo (N = 121) | Oxtellar XR (SPN-804), mg/day | |
|---|---|---|---|
| 1200 (N = 122) | 2400 (N = 123) | ||
| Age (years), mean ± SD | 39.1 ± 12.5 | 39.1 ± 11.5 | 38.5 ± 11.6 |
| Female, n (%) | 67 (55.4) | 71 (58.2) | 64 (52.0) |
| Race, n (%) | |||
| White | 107 (88.4) | 104 (85.2) | 105 (85.4) |
| Black | 1 (0.8) | 5 (4.1) | 1 (0.8) |
| Other | 13 (10.7) | 13 (10.7) | 17 (13.8) |
| Epilepsy duration (years), mean ± SD | 21.2 ± 13.9 | 21.3 ± 14.5 | 19.8 ± 13.0 |
| Baseline seizure frequency (seizures/28 days), median | 7.0 | 6.0 | 6.0 |
| Concomitant AEDs, n (%) | |||
| 1 AED | 43 (35.5) | 36 (29.5) | 40 (32.5) |
| 2 AEDs | 61 (50.4) | 68 (55.7) | 67 (54.5) |
| 3 AEDs | 17 (14.0) | 18 (14.8) | 16* (13.0) |
| Valproate | 49 (37.2) | 55 (45.1) | 62 (50.4) |
| Carbamazepine | 44 (36.4) | 53 (43.4) | 49 (39.8) |
| Lamotrigine | 37 (30.6) | 31 (25.4) | 34 (27.6) |
| Levetiracetam | 27 (22.3) | 20 (16.4) | 28 (22.8) |
| Topiramate | 21 (17.3) | 23 (18.8) | 23 (18.7) |
| Phenytoin | 4 (3.3) | 3 (2.5) | 2 (1.6) |
| Other | 26 (21.5) | 29 (23.8) | 18 (14.6) |
N, total number of patients; n, number of patients with analyzable data; SD, standard deviation; AEDs, antiepileptic drugs.
One patient assigned to SPN-804 2400 mg was receiving 4 AEDs.
Efficacy
The median percent seizure frequency change over the 16-week double-blind treatment period was −28.7% with placebo vs −38.3% (P = 0.08) for once-daily 1200-mg and −42.9% (P = 0.003) in the 2400-mg group (Fig. 2). The estimated treatment difference favoring SPN-804 over placebo was −10.3% (95% CI: −22.3, 1.2) for 1200-mg and −18.3% (95% CI: −30.4, −5.8) for 2400-mg group. Secondary endpoints generally mirrored the primary efficacy analysis. Responder rates, that is, proportion of patients with ≥50% seizure frequency reduction (Fig. 2), were placebo, 28.1%; 1200 mg, 36.1% (P = 0.08); and 40.7% (P = 0.02) of those receiving 2400 mg. The odds ratio comparing the responder rates was 1.67 (95% CI: 0.95, 2.94) for SPN-804 1200 mg vs placebo and 1.98 (95% CI: 1.13, 3.50) when comparing 2400 mg and placebo. For the prespecified pragmatic ITT analysis of seizure freedom, 3.3% of the placebo group, 4.9% (P = 0.53) in the 1200-mg group, and 11.4% (P = 0.008) in the 2400-mg group were seizure free for the entire 16-week blinded treatment period.
Figure 2.
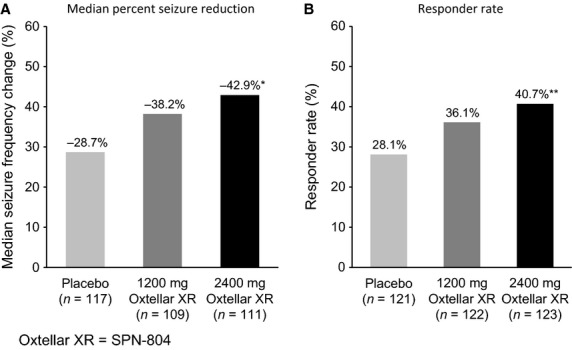
Oxtellar XR (SPN-804) vs placebo: Median percent reduction from baseline 28-day seizure frequency (A) and responder rate (B) for the 16-week double-blind treatment period in ITT population with analyzable data. *P = 0.003; **P = 0.02 vs placebo. ITT, intent-to-treat.
Sensitivity analyses using a completer and a mixed-model repeated-measures analysis did not change the primary statistical outcome that once-daily SPN-804 2400 mg was shown to be significantly superior to placebo, whereas 1200 mg was not. However, in a sensitivity analysis evaluating the potential influence of regional differences in this multinational trial, both SPN-804 dosage groups were significantly superior to placebo in median percent seizure reduction – placebo, −13.3%; 1200 mg, −34.5% (P = 0.02); 2400 mg, −52.7% (P = 0.006) among patients (n = 106) participating at North American study sites (Fig. 3). The estimated treatment difference favoring SPN-804 over placebo was −26.1% (95% CI: −47.9, −4.1) for 1200-mg and −35.3% (95% CI: −59.1, −12.5) for 2400-mg group. In contrast, with the substantially higher placebo effect (-33.2%) observed in patients (n = 231) from the Eastern Europe/Russia study site cluster (Fig. 3), neither SPN-804 dosage group was significantly superior to placebo (1200 mg: −38.4%, P = 0.60; 2400 mg: −41.2%, P = 0.13) in this subset.
Figure 3.
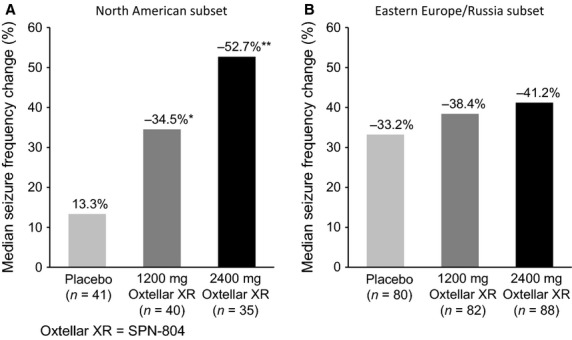
Oxtellar XR (SPN-804) vs placebo: Median percent reduction from baseline 28-day seizure frequency in the North American cluster of study sites (A) and Eastern Europe/Russia cluster (B) in the ITT population. *P = 0.02; **P = 0.006 vs placebo. ITT, intent-to-treat.
After 16 weeks of double-blind treatment, PGIC score changes were not significantly different for either SPN-804 dosage vs placebo. Mean total QOLIE-31 and subscale scores did not decrease from baseline during SPN-804 or placebo administration in any group. The only significant differences from placebo in QOLIE-31 score changes were significantly smaller increases in the QOLIE-31 subscales of Cognitive Functioning in the 1200-mg and Medication Effects in both SPN-804 groups (P < 0.01 vs placebo).
Tolerability and safety
The overall incidence of adverse events (Table 2) was similar in the placebo (55.4%) and SPN-804 1200-mg groups (56.6%) and was lower than in patients assigned to 2400 mg (69.1%). The incidences of specific adverse events were generally similar for the placebo and SPN-804 1200-mg groups. The most common adverse events were those related to the nervous system, occurring in 31.4% of placebo patients and 38.5% of those receiving 1200 mg, compared with 56.1% in the 2400-mg group. The most frequently reported adverse events – dizziness, nausea, somnolence, vomiting, headache, and diplopia – were typically dose related. Treatment-limiting adverse event rates were 8.3% and 14.8% in the placebo and 1200-mg groups, respectively; 30.1% in the 2400-mg group discontinued due to adverse events. In the active treatment groups, adverse events resulting in discontinuation occurred primarily during the forced-titration phase (1200 mg: 13/20, 65%; 2400 mg: 26/37, 70%). The fact that 23 patients were down-titrated to 1800 mg also suggests some difficulty with tolerability at 2400 mg.
Table 2.
Summary of treatment-emergent adverse events (safety population)
| Placebo (N = 121) | Oxtellar XR (SPN-804), mg/day | ||
|---|---|---|---|
| 1200 (N = 122) | 2400 (N = 123) | ||
| Any adverse event, n (%) | 67 (55.4) | 69 (56.6) | 85 (69.1) |
| Serious adverse events, n (%) | 7 (5.8) | 7 (5.7) | 10 (8.1) |
| Adverse events leading to discontinuation, n (%) | 10 (8.3) | 18 (14.8) | 37 (30.1) |
| Incidence, n (%) | |||
| Dizziness | 18 (14.9) | 24 (19.7) | 50 (40.7) |
| Vomiting | 11 (9.1) | 7 (5.7) | 19 (15.4) |
| Headache | 9 (7.4) | 10 (8.2) | 19 (15.4) |
| Somnolence | 11 (9.1) | 14 (11.5) | 17 (13.8) |
| Diplopia | 5 (4.1) | 12 (9.8) | 16 (13.0) |
| Nausea | 14 (11.6) | 14 (11.5) | 15 (12.2) |
| Asthenia | 1 (0.8) | 4 (3.3) | 9 (7.3) |
| Balance disorder | 6 (5.0) | 6 (4.9) | 8 (6.5) |
| Fatigue | 1 (0.8) | 7 (5.7) | 4 (3.3) |
N, total number of patients; n, number of patients.
Serious adverse events occurred in seven patients each in the placebo (5.8%) and SPN-804 1200-mg (5.7%) groups and in 10 (8.1%) in the 2400-mg group. Of the adverse events classified as serious, none were considered study drug-related in the SPN-804 1200-mg group and three were considered study drug-related in the placebo group (one subject each with erythematous rash, Stevens–Johnson syndrome, or dizziness). A fourth patient assigned to the placebo group was the only death in the study (death due to ovarian cancer diagnosed 2 days before randomization). Serious adverse events were considered study drug-related in six patients receiving SPN-804 2400 mg – symptomatic hyponatremia (n = 1), generalized rash (n = 1), dizziness (n = 1), vomiting (n = 1), and general drug intolerance (n = 2), which was characterized by other non-serious adverse events such as dizziness, diplopia, nausea, vomiting, abdominal pain, and/or headache. Study drug was discontinued in all patients with serious adverse events, although SPN-804 was initially down-titrated in one patient before being discontinued due to vertigo/dizziness.
Only one clinically significant laboratory, vital sign, or ECG abnormality was observed during the study. A shift from normal to lower serum sodium levels was observed in a relatively small subset of patients receiving active treatment (placebo, 1.7%; 1200 mg, 9.8%; 2400 mg, 6.5%). Serum sodium levels were < 130 mEq/l at any point during double-blind treatment in nine patients receiving SPN-804 (1200 mg, n = 4; 2400 mg, n = 5) and < 125 mEq/l in two patients (one in each SPN-804 group). One occurrence of a markedly abnormal sodium value resulted in symptomatic hyponatremia and was classified as a serious adverse event.
Pharmacokinetics and pharmacodynamics
Based on population PK analysis of plasma MHD concentrations, the mean (SD) Cmax of MHD was 17.9 (5.3) μg/ml in patients receiving once-daily SPN-804 1200-mg and 27.1 (8.7) μg/ml in the 2400-mg group; Cmin concentrations were 12.0 (4.3) μg/ml and 19.4 (7.8) μg/ml, respectively. Clearance was increased 31% in patients receiving carbamazepine, phenytoin, phenobarbital, or valproate as concomitant therapy, but was not increased further when more than one of these agents was given as co-therapy.
In a concentration–response analysis, patients receiving SPN-804 were grouped according to estimated trough (Cmin) MHD concentrations. The breakpoint between subgroups (10 μg/ml) was determined by a sensitivity analysis. The overall median Cmin across SPN-804 groups was 13.9 μg/ml. Median percent seizure frequency reduction was significantly greater in the subgroup with Cmin ≥10 μg/ml MHD (n = 125, −50%, P = 0.02) compared with the <10 μg/ml subgroup (n = 41, −35.2%) or the placebo group (n = 117, −28.7%, P = 0.0004); the difference in median percent change for <10 μg/ml subgroup vs placebo was not significant (P = 0.57). The Cmin was ≥ 10 μg/ml in approximately 85% of patients in the SPN-804 2400-mg group and 66% in the 1200-mg group.
Discussion
The PROSPER study demonstrated the efficacy of SPN-804 (Oxtellar XR) as adjunctive therapy in adults with inadequately controlled partial-onset seizures, with or without secondary generalization, and demonstrated the potential for improved tolerability when compared with OXC-IR data 4.
Although it failed to separate statistically from placebo in the primary analysis, 1200 mg SPN-804 was shown to be an efficacious dose in the concentration–response analysis and has been approved by the U.S. Food & Drug Administration as a target dose when initiating therapy with SPN-804. The median percent seizure reduction with once-daily SPN-804 1200 mg and 2400 mg (−38.2% and −42.9%, respectively) in the PROSPER study was similar to that observed with 600 and 1200 mg b.i.d. OXC-IR (−40% and −50%, respectively) in a similarly designed study reported by Barcs et al. 4. However, the effect size in the PROSPER study was actually two- to threefold lower and coincided with an approximately fourfold higher placebo response. Because sample sizes for the PROSPER study were based on effect sizes observed in the previous OXC-IR dose-ranging study 4, the number of randomized patients in the PROSPER study may have been too low to demonstrate a significant effect favoring SPN-804 1200 mg over placebo in the overall treatment population. In the subset of patients participating at North American study sites, the placebo response was more consistent with that reported by Barcs et al. 4 The differences favoring SPN-804 over placebo in median percent seizure reduction were significant for both 1200 mg and 2400 mg and were consistent with the earlier OXC-IR study. The concentration–response analysis in the overall population further reinforced the clinical usefulness of once-daily SPN-804 1200 mg by showing that trough MHD concentrations achieved by most patients (66%) receiving SPN-804 1200 mg were associated with a significant clinical effect.
The reasons that separate similarly designed trials with the same active drug would have such different placebo responses are unclear. The unexpectedly high placebo effect in the PROSPER study may reflect a ‘placebo drift’ in AED adjunctive trials that has been especially notable over the last 5 years, with placebo responses increasing and effect sizes decreasing significantly over time 12. The phenomenon coincides with the globalization of clinical studies, that is, participation by study sites outside North America and western Europe where AED registration trials have traditionally been conducted. An exaggerated placebo effect in a recent study of perampanel as adjunctive therapy in partial-onset seizures prompted a subset analysis based on regional distribution of study sites in North, Central, and South America 13. As with the PROSPER study, a treatment effect favoring active drug was only significant in the North American subset, which had a lower placebo response than the overall population. Taken together, these findings demonstrate that regional differences within randomized controlled studies can have important influences on study outcomes. Although verification of seizure classification by the Epilepsy Study Consortium during initial patient screening in the PROSPER study presumably reduced variability of response due to uncertain seizure types, other factors could influence the variability. Pooling data from ∼360 placebo-treated patients in double-blind trials of lacosamide as adjunctive therapy in patients with refractory partial-onset seizures, Schmidt et al. 14 identified patient factors predictive of lower likelihood of placebo response, that is, history of ≥ 7 lifetime AEDs, baseline monthly seizure frequency ≥10 seizures, prior epilepsy surgery (not including VNS placement), age of epilepsy diagnosis at 6–20 years of age. Although the relatively small number of patients receiving placebo in the two regional clusters in this study (North America, n = 41; Eastern Europe/Russia, n = 80) precluded meaningful comparisons of patient subsets according to the predictive factors identified by Schmidt et al., the placebo effects observed in this study, as well as others, point to the need to understand and address sources of variability in placebo response in order to improve AED trial design.
Extended-release formulations that reduce AED dosing frequency can be more convenient for patients, with the potential for better patient adherence and greater effectiveness. In the interval since seminal studies with AEDs by Cramer et al. 15,16 were published, a large body of evidence has demonstrated that adherence rates with multiple-daily dosing are significantly lower than with once-daily dosing 17,18. Several studies have documented associations between adherence/non-adherence and outcomes in patients with epilepsy such as mortality, 19 emergency department/inpatient visits due to serious seizures 20,21, and injuries due to motor vehicle accidents 19,22 – all of which support an association between adherence and AED effectiveness in controlling seizures.
However, few blinded randomized studies have directly compared ER AEDs to their IR counterparts, and no blinded randomized study has documented greater effectiveness of a once-daily ER formulation vs its IR formulation solely on the basis of once-daily vs b.i.d administration. Nonetheless, an ER AED formulation has been shown, albeit indirectly, to be more effective than the IR formulation due to improved tolerability associated with ‘flattening’ the plasma concentration curve from the ER formulation 23. Two separate but similarly designed blinded randomized trials compared lamotrigine with b.i.d. CBZ-IR 24 or b.i.d. CBZ-ER 25. Despite identical dosing frequency (b.i.d. administration) in the two studies, therapeutic success measured as patient retention was much greater with CBZ-ER (67% retention) 25 than with CBZ-IR (42% retention) 24 because CBZ-ER was better tolerated and therefore allowed more patients to derive the benefits of AED therapy.
The observations with CBZ-ER vs CBZ-IR are especially relevant to SPN-804 and OXC-IR, given the similarities of CBZ and OXC. For both AEDs, which act directly on voltage-gated sodium channels, pharmacodynamic activity mirrors plasma concentration fluctuations, as demonstrated by the appearance of transient side effects when plasma concentrations are at their peak. SPN-804 slows OXC absorption and reduces plasma MHD exposure ∼16% (Cmin) to ∼19% (AUC, Cmax) relative to OXC-IR 600 mg b.i.d 7.
A crossover PK study of 1200 mg SPN-804 administered once daily vs OXC-IR (Trileptal) 600 mg b.i.d. in healthy volunteers (n = 30) initially signaled the potential for improved tolerability with SPN-804. Dizziness was not reported during the SPN-804 period, whereas 11/30 (37%) reported dizziness with b.i.d. OXC-IR exposure (unpublished data). Indirect comparisons across similarly designed studies in patients with refractory epilepsy are also suggestive of the potential for improved tolerability with SPN-804. Namely, in the OXC-IR dose-ranging study 4, 36.2% of patients assigned to OXC-IR 600 mg b.i.d. discontinued due to adverse events; the rate of discontinuations due to adverse events was 14.8% with 1200 mg SPN-804 in the PROSPER study. The most common adverse events with OXC-IR 600 mg b.i.d. – dizziness, 32%; diplopia, 30%; somnolence, 28%; vomiting, 25%, nausea, 25%, ataxia, 17% – were reported less frequently in patients receiving 1200 mg SPN-804 once daily. Similar differences were observed for OXC-IR and SPN-804 at 2400 mg. It is notable that in the Barcs et al. study, 26% of patients randomized to 2400 mg OXC-IR completed the study and only 19% completed on the assigned dose 4. Despite ∼19% reduction in MHD exposure with SPN-804 vs OXC-IR at the same mg total daily dose, SPN-804 was associated with ∼50% reductions in the frequency of treatment-limiting adverse events. Indirect comparisons across similarly designed studies are fraught with confounding factors that may account for between-study differences. In the case of the PROSPER study and the OXC-IR dose-ranging study, OXC-IR was titrated in half the time as SPN-804, for example, 6 and 14 days to 1200 mg/day, respectively, which would impact tolerability. Study durations were different for the two studies (Barcs et al., 26 weeks; PROSPER, 16 weeks), which could also influence the results. However, the seizure-free rates in the two studies hint at the potential therapeutic benefit if tolerability and retention can be improved. In the pragmatic ITT analysis of seizure-free rates in the PROSPER study, 11.4% of patients assigned to SPN-804 2400 mg were seizure free for the 16-week double-blind treatment phase; with the higher discontinuation rates in the OXC-IR study (treatment-limiting adverse events: 600 mg b.i.d., 36%; 1200 mg b.i.d., 67%), the pragmatic ITT seizure-free rate was 1.3% 11. These observations of potential improvements in tolerability and effectiveness associated with the use of SPN-804 as adjunctive therapy in patients with epilepsy must be confirmed, however, in blinded comparative studies.
As an extended-release formulation that allows once-daily dosing and reduces peak plasma MHD concentrations, SPN-804 (Oxtellar XR) may positively impact treatment effectiveness via its effects on the complex interactions of dosing frequency, tolerability, and patient adherence as well as the ability to achieve higher, potentially more effective dosages. As an extended-release OXC formulation, SPN-804 represents a valuable alternative for patients in whom effectiveness of OXC-IR therapy is compromised by poor tolerability and/or non-adherence. Dosage adjustments may be required in patients who are switched from b.i.d. OXC-IR to once-daily SPN-804 to accommodate differences in plasma MHD concentrations.
Acknowledgments
The authors thank Karen Malley, Malley Research Programming Inc, for statistical programming support and also Verna Ilacqua, ID&A, for editorial assistance. Funding for this study was provided by Supernus Pharmaceuticals Inc.
PROSPER Investigators
United States: Bassel Abou-Khalil, Nashville, TN; Richard Brower, El Paso, TX; David Burdette, Detroit, MI; Julio Cantero, Sarasota, FL; Melissa Carran, Camden, NJ; Warren Chumley, Lexington, KY; Steve Chung, Phoenix, AZ; John DeCerce, Jacksonville, FL; Vithalbhai Dhaduk, Dunmore, PA; Stephen Evans, Springfield, IL; Jessica Feldman, Hatfield, PA; Gerald Ferencz, Toms River, NJ; James Fessler, Rochester, NY; Mark Fisher, Oklahoma City, OK; Stephen Flitman, Phoenix, AZ; Robert Gerner, Los Angeles, CA; Gordon Gibson, Little Rock, AR; Michael Harris, Orem, UT; Richard Hull, Huntsville, AL; Waseem Ibrahim, Riverside, CA; Alan F. Jacobson, Miami, FL; Batool Kirmani, Temple, TX; Pavel Klein, Bethesda, MD; Michael M. Mahdad, Fountain Valley, CA; Gregory Marsella, Boca Raton, FL; Laszlo Mate, West Palm Beach, FL; Selwyn-Lloyd McPherson, Kent, OH; George Morris, Milwaukee, WI; Piotr Olenjiczak, New Orleans, LA; Trudy Pang, Boston, MA; Ricardo Pardo, Baytown, TX; John Pollard, Philadelphia, PA; Jan Savit, Johnstown, PA; Bashir Shihabuddin, Little Rock, AR; Laura Strom, Aurora, CO; Thomas Swanson, Missoula, MT; David Teeple, Tucson, AZ; Alexandre Todorov, Northport, AL; Sala Uddin, Birmingham, AL; Blanca Vazquez, New York, NY; Reinaldo Verson, Columbus, GA; Thomas Vidic, Elkhart, IN; Roi Ann Wallis, Los Angeles, CA; Elizabeth Waterhouse, Richmond, VA; Robert Yapundich, Hickory, NC. Canada: S. Nizam Ahmed, Edmonton; Jean-Francois Clement, Greenfield Park; Neelan Pillay, Calgary; Martin Veilleux, Montreal. Mexico: Jose Alemán-Pedroza, Zapopan; Freddy Castro-Farfan, Mexico City; Eduardo Díaz-Juarez, Durango; Juan Escamilla-Garza, Monterrey; Juan Pérez-Garcia, Puebla; Ricardo Rángel-Guerra, Monterrey; Mariela Renteria-Bilbao, Ciudad Juarez; Sarug Reyes-Morales, Aguascalientes; Ildefonso Rodriguez-Leyva, San Luis Postosi; Jose Ruiz-Sandoval, Guadalajara; Gustavo Sanchez-Arrioja, Toluca; Sandra Silva-Sanchez, Chihuahua; Felipe Vega-Boada, Mexico City. Bulgaria: Nadezhda Deleva, Varna; Rosen Kalpachki, Sofia; Sasho Kastrev, Blogoevgrad; Dimitar Maslarov, Sofia; Neli Petrova, Ruse; Penko Shotekov, Sofia; Ivan Staikov, Sofia; Paraskeva Stamenova, Sofia; Plamen Tsvetanov, Pleven; Zahari Zahariev, Plovdiv. Croatia: Silvio Basic, Zagreb; Josip Glavic, Dubrovnik; Hrvoje Hecimovic, Zagreb; Ante Jurjevic, Rijeka; Anamarija Mrden, Zadar; Zdravka Poljakovic, Zagreb; Renata Susak, Osijek. Poland: Waldemar Brola, Konskie; Piotr Czapinski, Krakow; Anna Czlonkowska, Warszawa; Wieslaw Drozdowski, Bialystok; Urszula Fiszer, Warszawa; Magdelena Kapelusiak-Pielok, Poznan; Maria Mazurkiewicz-Beldinska, Gdansk; Wojciech Moskal, Wilkowice; Ewa Motta, Katowice; Marcin Nastaj, Lublin; Grzegorz Opala, Katowice; Krystyna Pierzchala, Zabrze; Artur Radman, Gizycko; Tomascz Rosochowicz, Lipno; Jacek Rozniecki, Lodz; Andrzej Tutaj, Olsztyn; Agata Wlodek, Siedlce; Maria Zubiel, Lodz. Romania: Corneliu Angelo Bulboaca, Cluj-Napoca; Mirela Chiru, Bucharest; Silviu Manescu, Campulung Muscel; Ioan Marginean, Cluj-Napoca; Cornelia Zaharia, Craiova. Russia: Alina Agafina, St. Petersburg; Gagik Avakyan, Moscow; Anna Belova, Nizhny Novgorod; Boris Beyn, Kirov; Enver Bogdanov, Kazan; Alexander Gofman, Moscow; Alexander Gustov, Nizhny Novgorod; Nadezhda Koroleva, St. Petersburg; Vitaliy Laskov, Kursk; Natalia Maslova, Smolensk; Gennadiy Mishin, Pyatigorsk; Eugeny Pankratov, Novosibirsk; Natalia Pizova, Yaroslavl; Irina Poverenova, Samara; Alexander Skoromets, St. Petersburg; Elena Vostrikova, Novosibirsk; Eduard Yakupov, Kazan; Leonid Zaslavskiy, St. Petersburg.
Conflict of interests and source of funding
J. French serves as the President of the Epilepsy Study Consortium, a non-profit organization. Dr. French receives 25% salary support from the Epilepsy Study Consortium. The consortium receives funding from Supernus Pharmaceuticals Inc., as well as many other companies with AEDs in development or marketed AEDs, for work performed (principal investigator, FDA submission, consulting) by Dr. French on behalf of the Epilepsy Study Consortium. P. Baroldi, S.T. Brittain, and J.K. Johnson are current (S.T. Brittain, J.K. Johnson) or past (P. Baroldi) employees of Supernus Pharmaceuticals Inc. The PROSPER study was sponsored by Supernus Pharmaceuticals Inc.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
PROSPER Study Key Inclusion/Exclusion Criteria.
References
- Koch MW, Polman SK. Oxcarbazepine versus carbamazepine monotherapy for partial onset seizures. Cochrane Database Syst Rev. 2009:CD006453. doi: 10.1002/14651858.CD006453.pub2. [DOI] [PubMed] [Google Scholar]
- Flesch G, Czendlik C, Renard D, et al. Pharmacokinetics of the monohydroxy derivative of oxcarbazepine and its enantiomers after a single intravenous dose given as racemate compared with a single oral dose of oxcarbazepine. Drug Metab Dispos. 2011;39:1103–10. doi: 10.1124/dmd.109.030593. [DOI] [PubMed] [Google Scholar]
- Flesch G, Czendlik C, Renard D, et al. Trileptal prescribing information. Novartis Pharmaceuticals. Revised March, 2011.
- Barcs G, Walker E, Elger C, et al. Oxcarbazepine placebo-controlled, dose-ranging trial in refractory partial epilepsy. Epilepsia. 2000;41:1597–607. doi: 10.1111/j.1499-1654.2000.001597.x. [DOI] [PubMed] [Google Scholar]
- Edelbroek PM, Augustijn PB, de Haan GJ, et al. Change in oxcarbazepine (Trileptal) formulation is associated with more side effects and higher blood concentrations. J Neurol Neurosurg Psychiatry. 2001;71:708–9. doi: 10.1136/jnnp.71.5.708a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striano S, Striano P, di Nocera P, et al. Relationship between serum mono-hydroxy-carbazepine concentrations and adverse effects in patients with epilepsy on high-dose oxcarbazepine therapy. Epilepsy Res. 2006;69:170–6. doi: 10.1016/j.eplepsyres.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Striano S, Striano P, Di Nocera P, et al. Oxtellar prescribing information. Supernus Pharmaceuticals, Inc. Revised October 2012.
- Bancaud J, Henriksen O, Rubio-Donnadieu F, et al. Proposal for revised clinical and electroencephalographic classification of epileptic seizures. From the Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia. 1981;22:489–501. doi: 10.1111/j.1528-1157.1981.tb06159.x. [DOI] [PubMed] [Google Scholar]
- Noether GE. Sample size determination for some common nonparametric tests. J Am Stat Ass. 1987;82:645–7. [Google Scholar]
- Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75:800–2. [Google Scholar]
- Gazzola DM, Balcer LJ, French JA. Seizure-free outcome in randomized add-on trials of the new antiepileptic drugs. Epilepsia. 2007;48:1303–7. doi: 10.1111/j.1528-1167.2007.01136.x. [DOI] [PubMed] [Google Scholar]
- Rheims S, Perucca E, Cucherat M, et al. Factors determining response to antiepileptic drugs in randomized controlled trials. A systematic review and meta-analysis. Epilepsia. 2011;52:219–33. doi: 10.1111/j.1528-1167.2010.02915.x. [DOI] [PubMed] [Google Scholar]
- French JA, Krauss GL, Biton V, et al. Adjunctive perampanel for refractory partial-onset seizures: randomized phase III study 304. Neurology. 2012;79:589–96. doi: 10.1212/WNL.0b013e3182635735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt D, Beyenburg S, D'souza J, et al. Clinical features associated with placebo response in refractory focal epilepsy. Epilepsy Behav. 2013;27:393–8. doi: 10.1016/j.yebeh.2013.01.015. [DOI] [PubMed] [Google Scholar]
- Cramer J, Vachon L, Desforges C, et al. Dose frequency and dose interval compliance with multiple antiepileptic medications during a controlled clinical trial. Epilepsia. 1995;36:1111–7. doi: 10.1111/j.1528-1157.1995.tb00469.x. [DOI] [PubMed] [Google Scholar]
- Cramer JA, Mattson RH, Prevey ML, et al. How often is medication taken as prescribed? A novel assessment technique. JAMA. 1989;261:3273–7. [PubMed] [Google Scholar]
- Coleman CI, Limone B, Sobieraj DM, et al. Dosing frequency and medication adherence in chronic disease. J Manag Care Pharm. 2012;18:527–39. doi: 10.18553/jmcp.2012.18.7.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava K, Arora A, Kataria A, et al. Impact of reducing dosing frequency on adherence to oral therapies: a literature review and meta-analysis. Patient Prefer Adherence. 2013;7:419–34. doi: 10.2147/PPA.S44646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faught E, Duh MS, Weiner JR, et al. Nonadherence to antiepileptic drugs and increased mortality: findings from the RANSOM study. Neurology. 2008;71:1572–8. doi: 10.1212/01.wnl.0000319693.10338.b9. [DOI] [PubMed] [Google Scholar]
- Ettinger AB, Manjunath R, Candrilli SD, et al. Prevalence and cost of nonadherence to antiepileptic drugs in elderly patients with epilepsy. Epilepsy Behav. 2009;14:324–9. doi: 10.1016/j.yebeh.2008.10.021. [DOI] [PubMed] [Google Scholar]
- Manjunath R, Davis KL, Candrilli SD, et al. Association of antiepileptic drug nonadherence with risk of seizures in adults with epilepsy. Epilepsy Behav. 2009;14:372–8. doi: 10.1016/j.yebeh.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Davis KL, Candrilli SD, Edin HM. Prevalence and cost of nonadherence with antiepileptic drugs in an adult managed care population. Epilepsia. 2008;49:446–54. doi: 10.1111/j.1528-1167.2007.01414.x. [DOI] [PubMed] [Google Scholar]
- Perucca E. Extended-release formulations of antiepileptic drugs: rationale and comparative value. Epilepsy Curr. 2009;9:153–7. doi: 10.1111/j.1535-7511.2009.01326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie M, Overstall P, Giorgi L. Multicentre, double-blind, randomised comparison between lamotrigine and carbamazepine in elderly patients with newly diagnosed epilepsy. Epilepsy Res. 1999;37:81–7. doi: 10.1016/s0920-1211(99)00039-x. [DOI] [PubMed] [Google Scholar]
- Saetre E, Perucca E, Isojarvi J, et al. An international multicenter randomized double-blind controlled trial of lamotrigine and sustained-release carbamazepine in the treatment of newly diagnosed epilepsy in the elderly. Epilepsia. 2007;48:1292–302. doi: 10.1111/j.1528-1167.2007.01128.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PROSPER Study Key Inclusion/Exclusion Criteria.