Abstract
Objective
To evaluate the presence of spinal inflammation with and without sacroiliac (SI) joint inflammation on magnetic resonance imaging (MRI) in patients with active nonradiographic axial spondyloarthritis (SpA), and to compare the disease characteristics of these subgroups.
Methods
ABILITY-1 is a multicenter, randomized, controlled trial of adalimumab versus placebo in patients with nonradiographic axial SpA classified using the Assessment of SpondyloArthritis international Society axial SpA criteria. Baseline MRIs were centrally scored independently by 2 readers using the Spondyloarthritis Research Consortium of Canada (SPARCC) method for the SI joints and the SPARCC 6–discovertebral unit method for the spine. Positive evidence of inflammation on MRI was defined as a SPARCC score of ≥2 for either the SI joints or the spine.
Results
Among patients with baseline SPARCC scores, 40% had an SI joint score of ≥2 and 52% had a spine score of ≥2. Forty-nine percent of patients with baseline SI joint scores of <2, and 58% of those with baseline SI joint scores of ≥2, had a spine score of ≥2. Comparison of baseline disease characteristics by baseline SI joint and spine scores showed that a greater proportion of patients in the subgroup with a baseline SPARCC score of ≥2 for both SI joints and spine were male, and patients with spine and SI joint scores of <2 were younger and had shorter symptom duration. SPARCC spine scores correlated with baseline symptom duration, and SI joint scores correlated negatively with the baseline Bath Ankylosing Spondylitis Disease Activity Index, but neither correlated with the baseline Ankylosing Spondylitis Disease Activity Score, total back pain, the patient's global assessment of disease activity, the Bath Ankylosing Spondylitis Functional Index, morning stiffness, nocturnal pain, or C-reactive protein level.
Conclusion
Assessment by experienced readers showed that spinal inflammation on MRI might be observed in half of patients with nonradiographic axial SpA without SI joint inflammation.
Magnetic resonance imaging (MRI) has become an important tool in the evaluation of patients with rheumatic diseases both in clinical trials and in daily clinical practice (1–6). The use of MRI in clinical practice has had a particularly notable impact on the investigation of patients with axial spondyloarthritis (SpA). Prior to MRI, there was reliance on radiographs as the most important imaging tool for identifying patients with sacroiliitis. With MRI, inflammation in the axial skeleton can now be visualized even in the absence of radiographic damage (7,8).
The Assessment of SpondyloArthritis international Society (ASAS) has established classification standards for patients with axial SpA, including patients with and without radiographic sacroiliitis (9,10). The imaging arm of the ASAS criteria requires the presence of sacroiliitis on MRI or radiography in addition to 1 SpA feature for patients with chronic low back pain with the onset of back pain at age ≤45 years. Positive MRI findings for sacroiliitis characteristic of SpA were further defined by the ASAS/Outcome Measures in Rheumatology MRI working group as the presence of bone marrow edema highly suggestive of SpA, with >1 bone marrow edema lesion on a single slice or 1 bone marrow edema lesion present on at least 2 consecutive slices (11).
The ASAS classification criteria for axial SpA were specifically developed to facilitate the conduct of clinical trials. Fulfillment of the imaging arm of the criteria takes into account the presence of MRI-documented inflammation only in the sacroiliac (SI) joints, but not in the spine (10). In daily clinical practice, MRI findings can be useful in confirming a clinical diagnosis of nonradiographic axial SpA and detecting objective evidence of active inflammation in patients with an established diagnosis of nonradiographic axial SpA (7,8). However, a sensitivity of MRI of only 38% for biopsy-proven sacroiliitis was reported (12).
Inflammation of the axial skeleton in nonradiographic axial SpA may involve the SI joints and/or spine. In the ASAS criteria validation study, eligibility criteria did not include a specified disease activity level. Of the 130 patients diagnosed as having axial SpA who underwent MRI of the SI joints and spine, only 5.4% had active inflammation in the spine but not in the SI joints (10). In the present study of patients with nonradiographic axial SpA who had active disease, we evaluated the presence of spinal inflammation on MRI at baseline among these patients with and without sacroiliitis on MRI, and we compared the disease characteristics of these patient subgroups.
PATIENTS AND METHODS
Patients
ABILITY-1 (NCT00939003) is an ongoing phase III, multicenter, randomized, controlled trial of adalimumab versus placebo in patients at least 18 years of age with nonradiographic axial SpA classified using the ASAS axial SpA criteria (10) who had an inadequate response to, intolerance to, or contraindication for nonsteroidal antiinflammatory drugs (13). As part of the assessment for fulfillment of the ASAS criteria, investigators reported whether or not patients had MRI evidence of active inflammatory lesions of the SI joints on either a prior or current MRI scan with definite bone marrow edema/osteitis highly suggestive of sacroiliitis. Active nonradiographic axial SpA was defined as a total back pain score of ≥4 cm on a 0–10-cm visual analog scale (VAS) and a Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) (14) score of ≥4 cm on a 0–10-cm VAS. Patients were excluded if they had a diagnosis of ankylosing spondylitis (AS) as defined by the modified New York criteria (15), past or present diagnosis of psoriasis or psoriatic arthritis, or prior exposure to biologic therapy.
MRI assessments
MRI of the SI joints and spine was performed at baseline. Images were scored, using the Spondyloarthritis Research Consortium of Canada (SPARCC) method for SI joints (16) and the SPARCC 6–discovertebral unit (6-DVU) method for the spine (17), independently by 2 central readers who were blinded with regard to time point and treatment. Scoring of the spine MRI was completed by the readers before seeing and scoring the corresponding MRI scans of the SI joints from the same time point. The mean scores from the 2 readers were used.
Six consecutive coronal slices were selected as representing the synovial compartment of the SI joints. Each SI joint, left and right, was divided into quadrants for a total of 8 per coronal slice. Each quadrant was assessed and scored for the presence (scored 1) or absence (scored 0) of bone marrow edema. Each coronal slice per SI joint was also given an additional score of 1 for the presence of an “intense” signal and an additional score of 1 for a “deep” lesion, defined as a homogeneous, unequivocal increase in signal >1 cm from the articular surface. The maximum possible score for all SI joints across 6 slices was 48 for the presence of bone marrow edema, 12 for intense edema, and 12 for deep edema, for a maximum possible total score of 72 (16).
For the spine, the 6 most severely affected DVUs were selected by an independent reviewer who did not perform the scoring. Each DVU was divided into 4 quadrants, with each quadrant assessed for the presence (scored 1) or absence (scored 0) of bone marrow edema. Each quadrant was scored on 3 consecutive sagittal slices per DVU, yielding a maximum possible score of 12 per DVU for bone marrow edema. Each sagittal slice per DVU was given an additional score of 1 for the presence of an “intense” signal and an additional score of 1 for a “deep” lesion, defined as a homogeneous, unequivocal increase in STIR signal >1 cm from the vertebral end plate. The maximum possible score for all 6 DVUs was 72 for the presence of bone marrow edema, 18 for intense edema, and 18 for deep edema, for a maximum possible total score of 108 (17).
Intra- and interreader reliability of baseline scores for MRI SPARCC scoring of both the SI joints and spine was assessed. Interreader correlations for the SI joints and spine were based on images from 177 and 176 patients, respectively. Intrareader correlations were based on a subset of 9 patients whose MRIs were reread by the same reader.
Statistical analysis
All patients were combined in these analyses, regardless of randomized treatment assignment. Analyses were performed only on baseline data obtained prior to randomization to study treatment. For the purpose of this analysis, positive evidence of inflammation on MRI was defined as an MRI SPARCC score of ≥2 for either the SI joints (11) or spine. Scoring of the baseline MR images was already completed when these cutoffs were determined. Descriptive statistics are presented for baseline demographic and clinical characteristics. Linear regression analysis was used to analyze the correlation of MRI SPARCC score with symptom duration and baseline disease activity measures. Analysis of variance was used to determine the intraclass correlation coefficient (ICC) for intra- and interreader reliability of MRI SPARCC scores. In addition, we evaluated agreement between readers' assessment of the spine or SI joint in terms of a SPARCC score of <2 versus ≥2.
RESULTS
Intra- and interreader reliability was strong for baseline SPARCC spine and SI joint MRI scores. The ICCs for intrareader reliability for SPARCC spine scores were 0.90 and 0.86 for readers 1 and 2, respectively. The ICCs for intrareader reliability for SPARCC SI joint scores were 0.70 and 0.92, respectively. The ICC for interreader reliability was 0.90 for SPARCC spine scores and 0.95 for SI joint scores. The 2 readers agreed on the assessment of the baseline MRI of the spine in terms of SPARCC score <2 versus ≥2 for 84% of the cases, and the 2 readers agreed on SPARCC SI joint scores for 79% of the cases.
At baseline, 89 of the 185 study patients (48%) were reported by the investigator to have past or present MRI evidence of sacroiliitis as required by the ASAS axial SpA criteria. A total of 181 patients had available baseline SPARCC MRI scores for both the SI joints and the spine (Table1), with 72 patients (40%) having a baseline SPARCC SI joint score of ≥2 and 95 patients (52%) having a baseline SPARCC spine score of ≥2. Of the 109 patients with a baseline SI joint score of <2 and the 72 patients with an SI joint score of ≥2, 53 patients (49%) and 42 patients (58%), respectively, had evidence of spinal inflammation with a baseline SPARCC spine score of ≥2.
Table 1.
Baseline demographic and clinical characteristics of the patients by baseline MRI evidence of SI joint and/or spine inflammation*
| SI joint score <2 | SI joint score ≥2 | |||
|---|---|---|---|---|
| Spine score <2 (n = 56) | Spine score ≥2 (n = 53) | Spine score <2 (n = 30) | Spine score ≥2 (n = 42) | |
| Female, % | 60.7 | 62.3 | 56.7 | 35.7 |
| White, % | 98.2 | 98.1 | 100.0 | 97.6 |
| Age, years | 35.3 ± 9.6 (19.0–63.0) | 40.5 ± 11.8 (19.0–72.0) | 35.9 ± 9.2 (21.0–59.0) | 39.6 ± 11.4 (19.0–65.0) |
| Symptom duration, years† | 7.9 ± 6.4 (0.2–24.2) | 11.6 ± 10.3 (0.6–42.4) | 11.0 ± 9.0 (0.8–38.2) | 10.6 ± 9.4 (0.4–38.0) |
| Duration since diagnosis, years | 3.0 ± 4.4 (0.1–18.1) | 2.9 ± 4.1 (0.1–21.2) | 2.4 ± 3.1 (0.1–11.3) | 3.0 ± 4.2 (0.1–17.0) |
| Sacroiliitis on MRI, past or present, reported by the investigator, % | 37.5 | 32.1 | 70.0 | 69.0 |
| HLA–B27 positive, % | 76.8 | 84.9 | 80.0 | 71.4 |
Except where indicated otherwise, values are the mean ± SD (range). MRI = magnetic resonance imaging.
n = 52 for sacroiliac (SI) joint score <2 and spine score <2, and n = 28 for SI joint score ≥2 and spine score <2.
Compared to the other subgroups, a greater proportion of patients in the subgroup with a baseline SPARCC score of ≥2 for both SI joints and spine were male (Table1). The mean age was lower in the subgroup with a SPARCC spine score of <2.
In the overall study population, the mean duration of SpA symptoms was ∼10 years. Mean symptom duration was lower in the subgroup with an MRI SPARCC score of <2 for both SI joints and spine. Based on linear regression analysis, SPARCC spine scores were significantly associated with symptom duration (P = 0.015), while SPARCC SI joint scores were not. Nonetheless, approximately one-third of patients with evidence of spinal inflammation (i.e., SPARCC spine scores of ≥2) regardless of the presence of SI joint inflammation had a symptom duration of <5 years (Figure 1).
Figure 1.
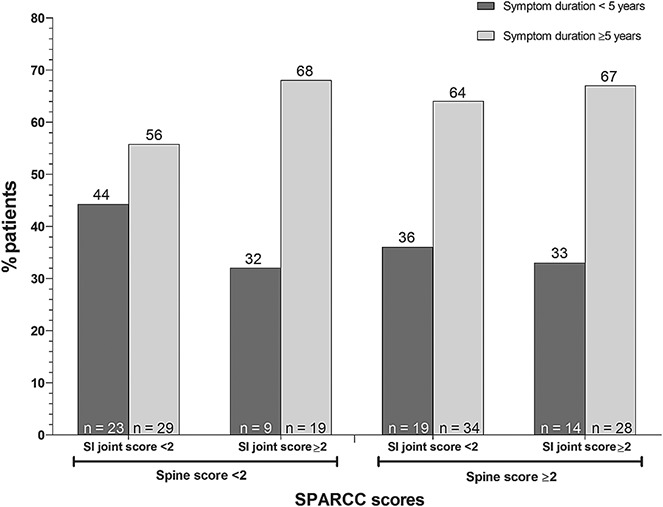
Spondyloarthritis Research Consortium of Canada (SPARCC) sacroiliac (SI) joint and spine magnetic resonance imaging scores by duration of spondyloarthritis symptoms.
Fewer patients in the subgroup with a SPARCC score of <2 for both SI joints and spine had an elevated C-reactive protein (CRP) level at baseline compared to the other subgroups (Table2). Baseline disease activity measures such as the BASDAI and AS Disease Activity Score (ASDAS) (18) were similar regardless of the presence or absence of inflammation in the SI joints and/or spine.
Table 2.
Baseline disease activity by baseline evidence of SI joint and/or spine inflammation*
| SI joint score <2 | SI joint score ≥2 | |||
|---|---|---|---|---|
| Spine score <2 (n = 56) | Spine score ≥2 (n = 53) | Spine score <2 (n = 30) | Spine score ≥2 (n = 42) | |
| SPARCC SI joint score, 0–72 | 0.4 ± 0.5 (0.0–1.5) | 0.5 ± 0.6 (0.0–1.5) | 8.5 ± 6.7 (2.0–25.0) | 14.0 ± 15.4 (2.0–60.5) |
| SPARCC spine score, 0–108 | 0.5 ± 0.5 (0.0–1.5) | 7.4 ± 5.5 (2.0–27.5) | 0.4 ± 0.5 (0.0–1.5) | 8.4 ± 6.9 (2.0–29.5) |
| BASDAI, 0–10-cm VAS | 6.6 ± 1.4 (1.9–9.9) | 6.6 ± 1.7 (1.6–10.0) | 6.0 ± 1.4 (4.0–8.6) | 6.4 ± 1.5 (2.9–9.3) |
| ASDAS† | 3.2 ± 0.8 (1.4–5.1) | 3.3 ± 0.8 (2.0–4.8) | 3.3 ± 0.8 (2.0–5.1) | 3.4 ± 0.7 (2.1–5.3) |
| Total back pain, 0–10-cm VAS | 7.1 ± 1.6 (3.4–10.0) | 7.1 ± 1.7 (2.4–10.0) | 6.6 ± 1.8 (1.4–9.8) | 6.8 ± 1.8 (1.9–9.9) |
| Patient's global assessment of disease activity, 0–10-cm VAS | 6.8 ± 1.7 (3.4–9.6) | 6.8 ± 2.0 (1.6–10.0) | 6.7 ± 1.9 (1.9–9.5) | 6.8 ± 1.6 (3.4–10.0) |
| BASFI, 0–10-cm VAS‡ | 4.8 ± 2.2 (0.4–9.4) | 4.8 ± 2.3 (0.1–9.3) | 4.2 ± 2.1 (0.3–8.0) | 4.7 ± 2.0 (1.7–8.3) |
| Inflammation (morning stiffness), 0–10-cm VAS§ | 6.5 ± 1.9 (1.7–9.9) | 6.6 ± 2.1 (1.7–10.0) | 5.9 ± 2.2 (0.1–8.9) | 7.0 ± 1.9 (2.7–9.9) |
| Nocturnal back pain, 0–10-cm VAS | 6.6 ± 2.2 (0.50–9.9) | 6.6 ± 2.4 (0.0–10.0) | 6.1 ± 2.3 (0.0–9.3) | 6.5 ± 2.1 (2.1–10.0) |
| C-reactive protein (pooled), mg/liter¶ | 5.4 ± 8.6 (0.2–43) | 6.3 ± 9.7 (0.2–50.5) | 8.2 ± 10.1 (0.4–34.3) | 9.4 ± 14.6 (0.3–77.2) |
| C-reactive protein (pooled), abnormal, % | 25.0 | 37.7 | 43.3 | 38.1 |
Except where indicated otherwise, values are the mean ± SD (range). SPARCC = Spondyloarthritis Research Consortium of Canada; VAS = visual analog scale; ASDAS = Ankylosing Spondylitis Disease Activity Score; BASFI = Bath Ankylosing Spondylitis Functional Index.
n = 53 for sacroiliac (SI) joint score <2 and spine score <2, n = 52 for SI joint score <2 and spine score ≥2, n = 29 for SI joint score ≥2 and spine score <2, and n = 40 for SI joint score ≥2 and spine score ≥2.
n = 52 for SI joint score <2 and spine sore ≥2.
Mean of items 5 and 6 of the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI).
Includes both standard and high-sensitivity C-reactive protein values.
A small though significant negative correlation was noted between the SPARCC SI joint score and the BASDAI at baseline (r = −0.187, P = 0.01) (Table3). No significant correlations were found between the baseline SPARCC SI joint or spine score and baseline ASDAS, total back pain, patient's global assessment of disease activity, Bath Ankylosing Spondylitis Functional Index (19), inflammation (morning stiffness), nocturnal back pain, or CRP level.
Table 3.
Pearson correlation coefficients for mean baseline SPARCC MRI scores and baseline disease activity variables*
| Variable | SI joint score (n = 182) | Spine score (n = 181) |
|---|---|---|
| BASDAI | −0.187† | −0.030 |
| ASDAS‡ | 0.022 | 0.123 |
| Total back pain | −0.105 | −0.069 |
| Patient's global assessment of disease activity | −0.096 | 0.017 |
| BASFI§ | −0.105 | 0.043 |
| Inflammation (morning stiffness)¶ | −0.028 | <0.001 |
| Nocturnal back pain | −0.082 | 0.063 |
| C-reactive protein (pooled)# | 0.094 | 0.142 |
MRI = magnetic resonance imaging (see Table2 for other definitions).
P = 0.01.
n = 175 for SI joint score, and n = 174 for spine score.
n = 181 for SI joint score, and n = 180 for spine score.
Mean of items 5 and 6 of the BASDAI.
Includes both standard and high-sensitivity C-reactive protein values.
The most frequently involved DVUs with bone marrow edema on MRI were found in the lower thoracic and lumbar spine regardless of the presence or absence of concurrent MRI sacroiliitis (Figure 2).
Figure 2.
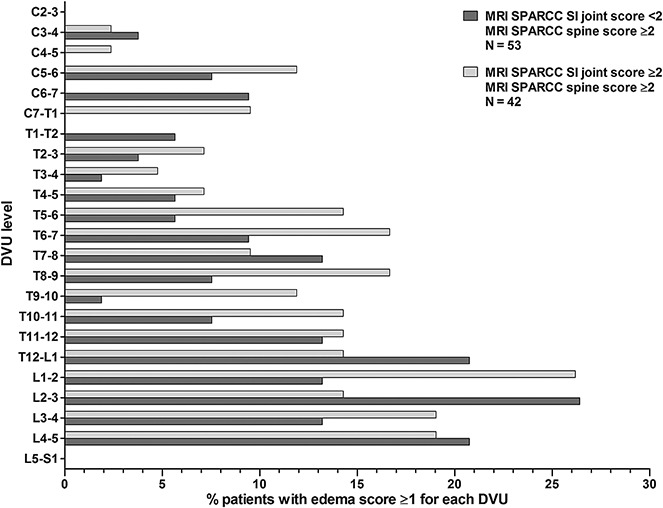
Proportion of patients with a bone marrow edema score of at least 1 for each discovertebral unit (DVU) by baseline SPARCC SI joint magnetic resonance imaging (MRI) score, among those with inflammation evidenced on MRI of the spine (SPARCC score ≥2). C = cervical; T = thoracic; L = lumbar; S = sacral (see Figure 1 for other definitions).
The distribution of SPARCC spine scores observed was similar regardless of the presence or absence of SI joint inflammation on MRI (SI joint score of ≥2 or <2) (Figure 3A). However, greater SI joint inflammation was observed among patients with evidence of inflammation in the spine (SPARCC spine score of ≥2) (Figure 3B). The distribution of SPARCC spine scores was similar in males and females. Greater SI joint inflammation was observed in males than in females (data not shown).
Figure 3.
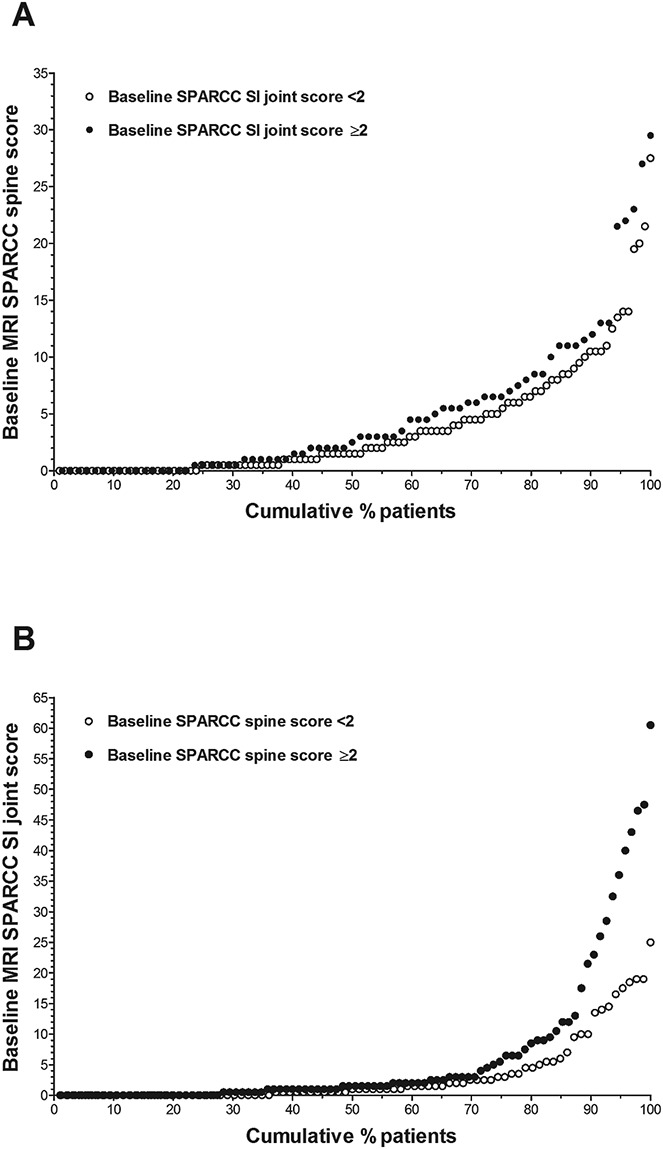
Distribution of baseline SPARCC spine and SI joint magnetic resonance imaging (MRI) scores. A, Cumulative probability of various baseline SPARCC spine scores by SPARCC SI joint score. B, Cumulative probability of various baseline SPARCC SI joint scores by SPARCC spine score. See Figure 1 for other definitions.
DISCUSSION
The findings described in this report confirm previously published data and add new insights to the growing body of literature on MRI in axial SpA. We showed that among patients with active nonradiographic axial SpA as defined by BASDAI and total back pain scores, spinal inflammation on MRI may be observed in half of those without MRI evidence of SI joint inflammation. Sex differences were noted in MRI findings. More males were in the subgroup with MRI evidence of inflammation of both the spine and SI joints at study entry. An observation that has important clinical relevance is the finding that SPARCC spine MRI scores correlated with symptom duration. Only approximately one-third of patients in this study with bone marrow edema in the spine regardless of the presence or absence of sacroiliitis seen on MRI had symptom duration of <5 years, but approximately two-thirds of patients had symptom duration of ≥5 years. Previous studies in patients with duration of axial SpA symptoms of <5 years and MRI evidence of either SI joint or spine inflammation have shown lower percentages of patients with MRI evidence of spine inflammation than in our study (20,21). Although the patient populations in those studies were somewhat different from our patient population, the data suggest that MRI evidence of spine inflammation in the absence of MRI evidence of SI joint inflammation may not be a lesser concern in patients with shorter symptom duration.
The small but significant negative correlation noted between the SPARCC SI joint score and the BASDAI is most likely a chance finding; this is underscored by a lack of correlation of baseline SPARCC SI joint and spine scores with other baseline clinical disease activity measures in nonradiographic axial SpA. This is generally consistent with previous reports in patients with axial SpA (22–25). However, the lack of correlation with CRP level is surprising (22–25) and indicates that both CRP level and MRI findings should be considered in the evaluation for active disease in patients with nonradiographic axial SpA. The distribution of DVU involvement along the spine in our study population confirms previous findings that the thoracic and lumbar regions of the spine are the areas most frequently affected by inflammation in axial SpA (26–29).
This study has several limitations. The study population had longstanding nonradiographic axial SpA. The scope of the analyses did not include evaluation of correlation of MRI findings with treatment response or change in disease activity. We used a definition of positive MRI evidence of active inflammation in the spine—a SPARCC spine score of ≥2—that has not been previously validated. The analyses were focused on evaluating MRI evidence of active inflammation in the spine and SI joints in patients with established nonradiographic axial SpA that is clinically active. Therefore, the findings and conclusions do not necessarily address the approach to imaging used in clinical practice when making a diagnosis of axial SpA. Typically, the SI joints are imaged first in the diagnostic evaluation of a patient in whom axial SpA is suspected. An important question for clinicians is whether imaging of the spine should be performed if the SI joint scan does not reveal axial SpA but the disease is strongly suspected. This study was not designed to address that important question. There was also no group of patients without axial SpA who served as a control for this exercise.
In summary, spinal inflammation without evidence of SI joint inflammation on MRI was observed in patients with longstanding nonradiographic axial SpA. Further studies are needed to confirm these findings in patients with nonradiographic axial SpA with shorter symptom duration and to investigate the optimal use of SI joint and spine MRI in clinical practice for monitoring disease activity and response to therapy.
Acknowledgments
We thank Kathleen V. Kastenholz, PharmD, MS of AbbVie for medical writing support.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Pangan had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. van der Heijde, Sieper, Maksymowych, Brown, Rathmann, Pangan.
Acquisition of data. Brown, Lambert, Pangan.
Analysis and interpretation of data. van der Heijde, Sieper, Maksymowych, Brown, Lambert, Rathmann, Pangan.
ROLE OF THE STUDY SPONSOR
AbbVie Inc. funded the study, participated in the design of the study, and contributed to the collection, analysis, and interpretation of the data and to the writing, review, and approval of the manuscript. AbbVie Inc. paid for the services of a medical writer. Publication of the manuscript was contingent upon approval by AbbVie Inc.
REFERENCES
- Bird P, Conaghan P, Ejbjerg B, McQueen F, Lassere M, Peterfy C, et al. The development of the EULAR-OMERACT rheumatoid arthritis MRI reference image atlas. Ann Rheum Dis. 2005;64(Suppl I):i8–10. doi: 10.1136/ard.2004.031807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergaard M, Peterfy C, Conaghan P, McQueen F, Bird P, Ejbjerg B, et al. OMERACT rheumatoid arthritis magnetic resonance imaging studies: core set of MRI acquisitions, joint pathology definitions, and the OMERACT RA-MRI scoring system. J Rheumatol. 2003;30:1385–6. [PubMed] [Google Scholar]
- Menashe L, Hirko K, Losina E, Kloppenburg M, Zhang W, Li L, et al. The diagnostic performance of MRI in osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2012;20:13–21. doi: 10.1016/j.joca.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun J, Baraliakos X, Golder W, Hermann KG, Listing J, Brandt J, et al. Analysing chronic spinal changes in ankylosing spondylitis: a systematic comparison of conventional x rays with magnetic resonance imaging using established and new scoring systems. Ann Rheum Dis. 2004;63:1046–55. doi: 10.1136/ard.2003.019968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landewe RB, Hermann KG, van der Heijde DM, Baraliakos X, Jurik AG, Lambert RG, et al. Scoring sacroiliac joints by magnetic resonance imaging: a multiple-reader reliability experiment. J Rheumatol. 2005;32:2050–5. [PubMed] [Google Scholar]
- Hermann KG, Baraliakos X, van der Heijde DM, Jurik AG, Landewe R, Marzo-Ortega H, et al. Assessment in SpondyloArthritis international Society (ASAS) Descriptions of spinal MRI lesions and definition of a positive MRI of the spine in axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI study group. Ann Rheum Dis. 2012;71:1278–88. doi: 10.1136/ard.2011.150680. on behalf of the. [DOI] [PubMed] [Google Scholar]
- Weber U, Lambert RG, Ostergaard M, Hodler J, Pedersen SJ, Maksymowych WP. The diagnostic utility of magnetic resonance imaging in spondylarthritis: an international multicenter evaluation of one hundred eighty-seven subjects. Arthritis Rheum. 2010;62:3048–58. doi: 10.1002/art.27571. [DOI] [PubMed] [Google Scholar]
- Rudwaleit M, van der Heijde D, Khan MA, Braun J, Sieper J. How to diagnose axial spondyloarthritis early. Ann Rheum Dis. 2004;63:535–43. doi: 10.1136/ard.2003.011247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudwaleit M, Landewe R, van der Heijde D, Listing J, Brandt J, Braun J, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis. 2009;68:770–6. doi: 10.1136/ard.2009.108217. [published erratum appears in Ann Rheum Dis 2011;70:1519]. [DOI] [PubMed] [Google Scholar]
- Rudwaleit M, van der Heijde D, Landewe R, Listing J, Akkoc N, Brandt J, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68:777–83. doi: 10.1136/ard.2009.108233. [DOI] [PubMed] [Google Scholar]
- Rudwaleit M, Jurik AG, Hermann KG, Landewe R, van der Heijde D, Baraliakos X, et al. Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI group. Ann Rheum Dis. 2009;68:1520–7. doi: 10.1136/ard.2009.110767. [DOI] [PubMed] [Google Scholar]
- Gong Y, Zheng N, Chen SB, Xiao ZY, Wu MY, Liu Y, et al. Ten years' experience with needle biopsy in the early diagnosis of sacroiliitis. Arthritis Rheum. 2012;64:1399–406. doi: 10.1002/art.33453. [DOI] [PubMed] [Google Scholar]
- Sieper J, van der Heijde D, Dougados M, Mease PJ, Maksymowych WP, Brown MA, et al. Efficacy and safety of adalimumab in patients with non-radiographic axial spondyloarthritis: results of a randomised placebo-controlled trial (ABILITY-1) Ann Rheum Dis. 2013;72:815–22. doi: 10.1136/annrheumdis-2012-201766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol. 1994;21:2286–91. [PubMed] [Google Scholar]
- Van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis: a proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361–8. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]
- Maksymowych WP, Inman RD, Salonen D, Dhillon SS, Williams M, Stone M, et al. Spondyloarthritis Research Consortium of Canada magnetic resonance imaging index for assessment of sacroiliac joint inflammation in ankylosing spondylitis. Arthritis Rheum. 2005;53:703–9. doi: 10.1002/art.21445. [DOI] [PubMed] [Google Scholar]
- Maksymowych WP, Inman RD, Salonen D, Dhillon SS, Krishnananthan R, Stone M, et al. Spondyloarthritis Research Consortium of Canada magnetic resonance imaging index for assessment of spinal inflammation in ankylosing spondylitis. Arthritis Rheum. 2005;53:502–9. doi: 10.1002/art.21337. [DOI] [PubMed] [Google Scholar]
- Machado P, Landewe R, Lie E, Kvien TK, Braun J, Baker D, et al. Assessment of SpondyloArthritis International Society. Ankylosing Spondylitis Disease Activity Score (ASDAS): defining cut-off values for disease activity states and improvement scores. Ann Rheum Dis. 2011;70:47–53. doi: 10.1136/ard.2010.138594. [DOI] [PubMed] [Google Scholar]
- Calin A, Garrett S, Whitelock H, Kennedy LG, O'Hea J, Mallorie P, et al. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol. 1994;21:2281–5. [PubMed] [Google Scholar]
- Barkham N, Keen HI, Coates LC, O'Connor P, Hensor E, Fraser AD, et al. Clinical and imaging efficacy of infliximab in HLA–B27–positive patients with magnetic resonance imaging– determined early sacroiliitis. Arthritis Rheum. 2009;60:946–54. doi: 10.1002/art.24408. [published erratum appears in Arthritis Rheum 2010;62:3005]. [DOI] [PubMed] [Google Scholar]
- Song IH, Hermann KG, Haibel H, Althoff CE, Listing J, Burmester GR, et al. Effects of etanercept versus sulfasalazine in early axial spondyloarthritis on active inflammatory lesions as detected by whole-body MRI (ESTHER): a 48-week randomised controlled trial. Ann Rheum Dis. 2011;70:590–6. doi: 10.1136/ard.2010.139667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksymowych WP, Dhillon SS, Park R, Salonen D, Inman RD, Lambert RG. Validation of the Spondyloarthritis Research Consortium of Canada magnetic resonance imaging spinal inflammation index: is it necessary to score the entire spine? Arthritis Rheum. 2007;57:501–7. doi: 10.1002/art.22627. [DOI] [PubMed] [Google Scholar]
- Kiltz U, Baraliakos X, Karakostas P, Igelmann M, Kalthoff L, Klink C, et al. The degree of spinal inflammation is similar in patients with axial spondyloarthritis who report high or low levels of disease activity: a cohort study. Ann Rheum Dis. 2012;71:1207–11. doi: 10.1136/annrheumdis-2011-200508. [DOI] [PubMed] [Google Scholar]
- Machado P, Landewe RB, Braun J, Baraliakos X, Hermann KG, Hsu B, et al. MRI inflammation and its relation with measures of clinical disease activity and different treatment responses in patients with ankylosing spondylitis treated with a tumour necrosis factor inhibitor. Ann Rheum Dis. 2012;71:2002–5. doi: 10.1136/annrheumdis-2012-201999. [DOI] [PubMed] [Google Scholar]
- Lambert RG, Salonen D, Rahman P, Inman RD, Wong RL, Einstein SG, et al. M03-606 Study Group. Adalimumab significantly reduces both spinal and sacroiliac joint inflammation in patients with ankylosing spondylitis: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2007;56:4005–14. doi: 10.1002/art.23044. [DOI] [PubMed] [Google Scholar]
- Baraliakos X, Landewe R, Hermann KG, Listing J, Golder W, Brandt J, et al. Inflammation in ankylosing spondylitis: a systematic description of the extent and frequency of acute spinal changes using magnetic resonance imaging. Ann Rheum Dis. 2005;64:730–4. doi: 10.1136/ard.2004.029298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett AN, Rehman A, Hensor EM, Marzo-Ortega H, Emery P, McGonagle D. Evaluation of the diagnostic utility of spinal magnetic resonance imaging in axial spondylarthritis. Arthritis Rheum. 2009;60:1331–41. doi: 10.1002/art.24493. [DOI] [PubMed] [Google Scholar]
- Weber U, Hodler J, Jurik AG, Pfirrmann CW, Rufibach K, Kissling RO, et al. Assessment of active spinal inflammatory changes in patients with axial spondyloarthritis: validation of whole body MRI against conventional MRI. Ann Rheum Dis. 2010;69:648–53. doi: 10.1136/ard.2009.108274. [DOI] [PubMed] [Google Scholar]
- Althoff CE, Sieper J, Song IH, Haibel H, Weiss A, Diekhoff T, et al. Active inflammation and structural change in early active axial spondyloarthritis as detected by whole-body MRI. Ann Rheum Dis. 2013;72:967–73. doi: 10.1136/annrheumdis-2012-201545. [DOI] [PubMed] [Google Scholar]


