Abstract
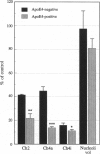
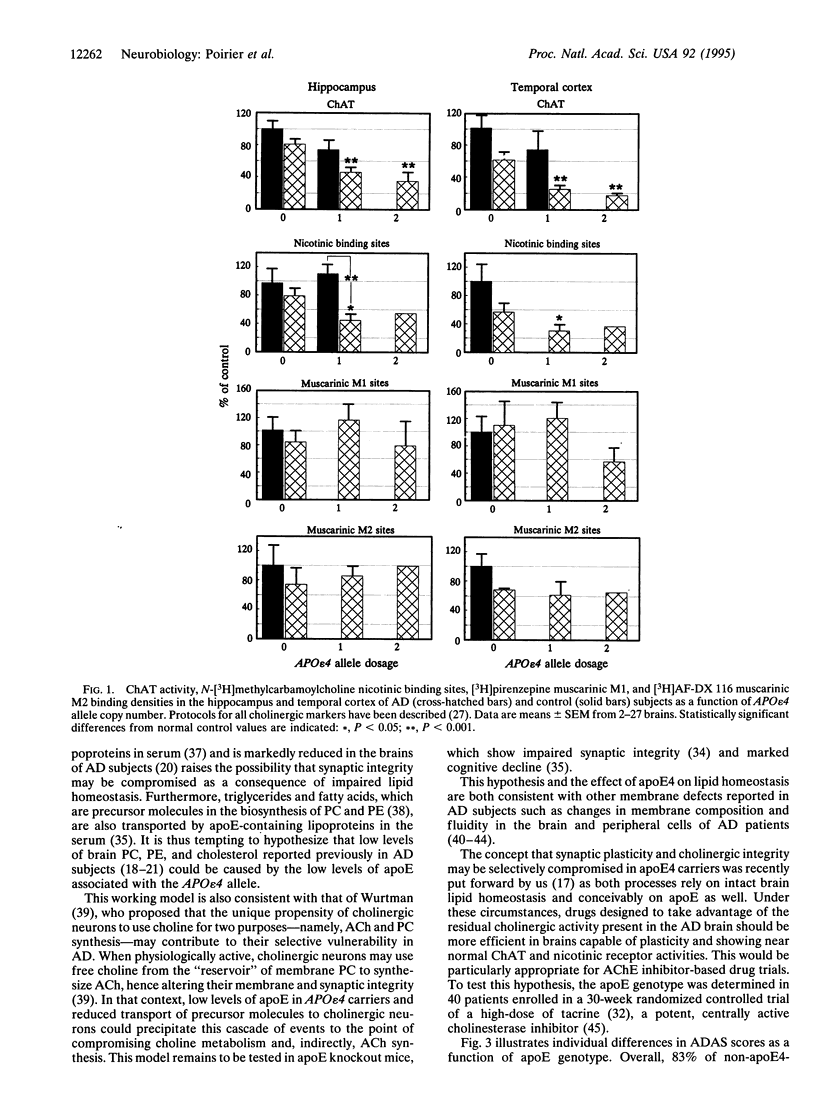
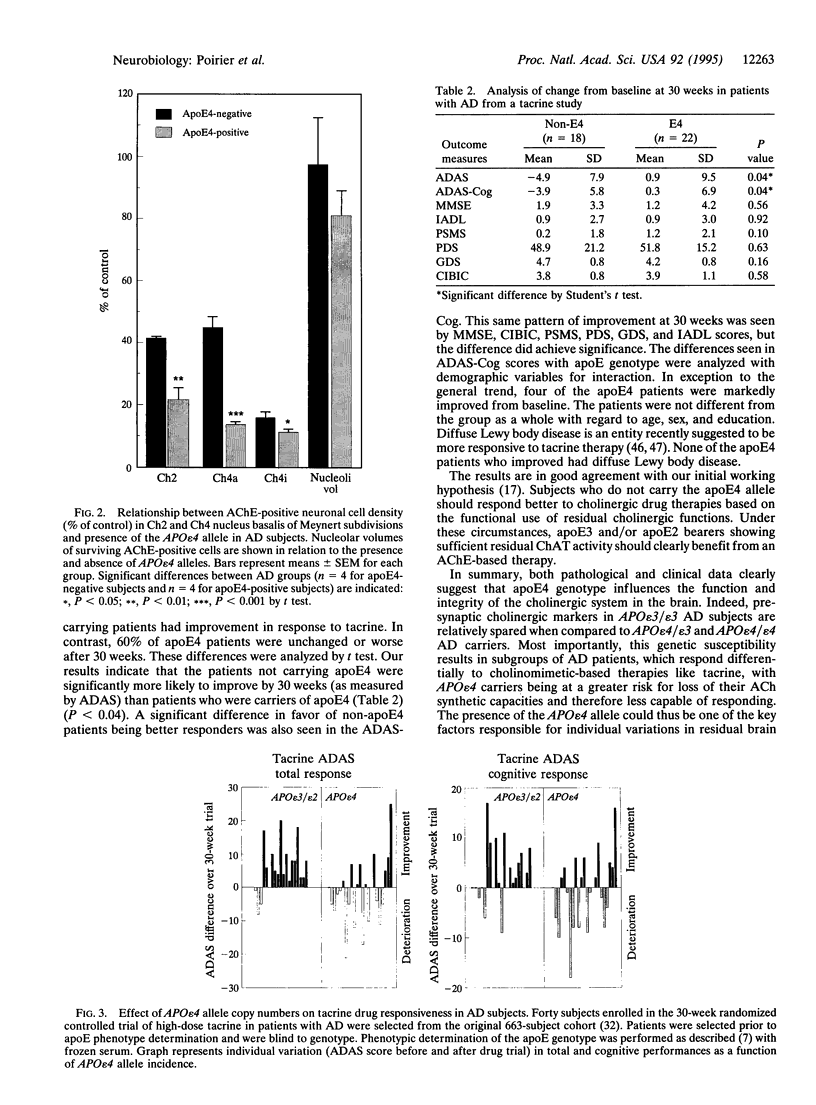
Apolipoprotein E (apoE) is critical in the modulation of cholesterol and phospholipid transport between cells of different types. Human apoE is a polymorphic protein with three common alleles, APO epsilon 2, APO epsilon 3, and APO epsilon 4. ApoE4 is associated with sporadic and late-onset familial Alzheimer disease (AD). Gene dose was shown to have an effect on risk of developing AD, age of onset, accumulation of senile plaques in the brain, and reduction of choline acetyltransferase (ChAT) activity in the hippocampus of AD subjects. To characterize the possible impact of the apoE4 allele on cholinergic markers in AD, we examined the effect of apoE4 allele copy number on pre- and postsynaptic markers of cholinergic activity. ApoE4 allele copy number showed an inverse relationship with residual brain ChAT activity and nicotinic receptor binding sites in both the hippocampal formation and the temporal cortex of AD subjects. AD cases lacking the apoE4 allele showed ChAT activities close or within age-matched normal control values. The effect of the apoE4 allele on cholinomimetic drug responsiveness was assessed next in a group (n = 40) of AD patients who completed a double-blind, 30-week clinical trial of the cholinesterase inhibitor tacrine. Results showed that > 80% of apoE4-negative AD patients showed marked improvement after 30 weeks as measured by the AD assessment scale (ADAS), whereas 60% of apoE4 carriers had ADAS scores that were worse compared to baseline. These results strongly support the concept that apoE4 plays a crucial role in the cholinergic dysfunction associated with AD and may be a prognostic indicator of poor response to therapy with acetylcholinesterase inhibitors in AD patients.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aubert I., Araujo D. M., Cécyre D., Robitaille Y., Gauthier S., Quirion R. Comparative alterations of nicotinic and muscarinic binding sites in Alzheimer's and Parkinson's diseases. J Neurochem. 1992 Feb;58(2):529–541. doi: 10.1111/j.1471-4159.1992.tb09752.x. [DOI] [PubMed] [Google Scholar]
- Bertrand P., Poirier J., Oda T., Finch C. E., Pasinetti G. M. Association of apolipoprotein E genotype with brain levels of apolipoprotein E and apolipoprotein J (clusterin) in Alzheimer disease. Brain Res Mol Brain Res. 1995 Oct;33(1):174–178. doi: 10.1016/0169-328x(95)00097-c. [DOI] [PubMed] [Google Scholar]
- Blennow K., Hesse C., Fredman P. Cerebrospinal fluid apolipoprotein E is reduced in Alzheimer's disease. Neuroreport. 1994 Dec 20;5(18):2534–2536. doi: 10.1097/00001756-199412000-00032. [DOI] [PubMed] [Google Scholar]
- Blusztajn J. K., Liscovitch M., Richardson U. I. Synthesis of acetylcholine from choline derived from phosphatidylcholine in a human neuronal cell line. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5474–5477. doi: 10.1073/pnas.84.15.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder E. H., Saunders A. M., Strittmatter W. J., Schmechel D. E., Gaskell P. C., Small G. W., Roses A. D., Haines J. L., Pericak-Vance M. A. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993 Aug 13;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Dawson R. M. Tacrine slows the rate of ageing of sarin-inhibited acetylcholinesterase. Neurosci Lett. 1989 May 22;100(1-3):227–230. doi: 10.1016/0304-3940(89)90689-7. [DOI] [PubMed] [Google Scholar]
- Diedrich J. F., Minnigan H., Carp R. I., Whitaker J. N., Race R., Frey W., 2nd, Haase A. T. Neuropathological changes in scrapie and Alzheimer's disease are associated with increased expression of apolipoprotein E and cathepsin D in astrocytes. J Virol. 1991 Sep;65(9):4759–4768. doi: 10.1128/jvi.65.9.4759-4768.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne P., Robitaille Y., Wood P., Gauthier S., Nair N. P., Quirion R. Nucleus basalis neuronal loss, neuritic plaques and choline acetyltransferase activity in advanced Alzheimer's disease. Neuroscience. 1986 Dec;19(4):1279–1291. doi: 10.1016/0306-4522(86)90142-9. [DOI] [PubMed] [Google Scholar]
- Farlow M., Gracon S. I., Hershey L. A., Lewis K. W., Sadowsky C. H., Dolan-Ureno J. A controlled trial of tacrine in Alzheimer's disease. The Tacrine Study Group. JAMA. 1992 Nov 11;268(18):2523–2529. [PubMed] [Google Scholar]
- Hicks N., Brammer M. J., Hymas N., Levy R. Platelet membrane properties in Alzheimer and multi-infarct dementias. Alzheimer Dis Assoc Disord. 1987;1(2):90–97. doi: 10.1097/00002093-198701020-00004. [DOI] [PubMed] [Google Scholar]
- Hyman B. T., Kromer L. J., Van Hoesen G. W. Reinnervation of the hippocampal perforant pathway zone in Alzheimer's disease. Ann Neurol. 1987 Mar;21(3):259–267. doi: 10.1002/ana.410210307. [DOI] [PubMed] [Google Scholar]
- Jones O. T., McNamee M. G. Annular and nonannular binding sites for cholesterol associated with the nicotinic acetylcholine receptor. Biochemistry. 1988 Apr 5;27(7):2364–2374. doi: 10.1021/bi00407a018. [DOI] [PubMed] [Google Scholar]
- KARNOVSKY M. J., ROOTS L. A "DIRECT-COLORING" THIOCHOLINE METHOD FOR CHOLINESTERASES. J Histochem Cytochem. 1964 Mar;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- Knapp M. J., Knopman D. S., Solomon P. R., Pendlebury W. W., Davis C. S., Gracon S. I. A 30-week randomized controlled trial of high-dose tacrine in patients with Alzheimer's disease. The Tacrine Study Group. JAMA. 1994 Apr 6;271(13):985–991. [PubMed] [Google Scholar]
- Levy R., Eagger S., Griffiths M., Perry E., Honavar M., Dean A., Lantos P. Lewy bodies and response to tacrine in Alzheimer's disease. Lancet. 1994 Jan 15;343(8890):176–176. doi: 10.1016/s0140-6736(94)90966-0. [DOI] [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L. Lipoprotein receptors and cholesterol homeostasis. Biochim Biophys Acta. 1983 May 24;737(2):197–222. doi: 10.1016/0304-4157(83)90001-1. [DOI] [PubMed] [Google Scholar]
- Mayeux R., Stern Y., Ottman R., Tatemichi T. K., Tang M. X., Maestre G., Ngai C., Tycko B., Ginsberg H. The apolipoprotein epsilon 4 allele in patients with Alzheimer's disease. Ann Neurol. 1993 Nov;34(5):752–754. doi: 10.1002/ana.410340527. [DOI] [PubMed] [Google Scholar]
- Mesulam M. M., Mufson E. J., Levey A. I., Wainer B. H. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol. 1983 Feb 20;214(2):170–197. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- Nalbantoglu J., Gilfix B. M., Bertrand P., Robitaille Y., Gauthier S., Rosenblatt D. S., Poirier J. Predictive value of apolipoprotein E genotyping in Alzheimer's disease: results of an autopsy series and an analysis of several combined studies. Ann Neurol. 1994 Dec;36(6):889–895. doi: 10.1002/ana.410360614. [DOI] [PubMed] [Google Scholar]
- Nitsch R. M., Blusztajn J. K., Pittas A. G., Slack B. E., Growdon J. H., Wurtman R. J. Evidence for a membrane defect in Alzheimer disease brain. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1671–1675. doi: 10.1073/pnas.89.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payami H., Kaye J., Heston L. L., Bird T. D., Schellenberg G. D. Apolipoprotein E genotype and Alzheimer's disease. Lancet. 1993 Sep 18;342(8873):738–738. [PubMed] [Google Scholar]
- Piletz J. E., Sarasua M., Whitehouse P., Chotani M. Intracellular membranes are more fluid in platelets of Alzheimer's disease patients. Neurobiol Aging. 1991 Sep-Oct;12(5):401–406. doi: 10.1016/0197-4580(91)90064-q. [DOI] [PubMed] [Google Scholar]
- Pitas R. E., Boyles J. K., Lee S. H., Foss D., Mahley R. W. Astrocytes synthesize apolipoprotein E and metabolize apolipoprotein E-containing lipoproteins. Biochim Biophys Acta. 1987 Jan 13;917(1):148–161. doi: 10.1016/0005-2760(87)90295-5. [DOI] [PubMed] [Google Scholar]
- Poirier J. Apolipoprotein E in animal models of CNS injury and in Alzheimer's disease. Trends Neurosci. 1994 Dec;17(12):525–530. doi: 10.1016/0166-2236(94)90156-2. [DOI] [PubMed] [Google Scholar]
- Poirier J., Baccichet A., Dea D., Gauthier S. Cholesterol synthesis and lipoprotein reuptake during synaptic remodelling in hippocampus in adult rats. Neuroscience. 1993 Jul;55(1):81–90. doi: 10.1016/0306-4522(93)90456-p. [DOI] [PubMed] [Google Scholar]
- Poirier J., Davignon J., Bouthillier D., Kogan S., Bertrand P., Gauthier S. Apolipoprotein E polymorphism and Alzheimer's disease. Lancet. 1993 Sep 18;342(8873):697–699. doi: 10.1016/0140-6736(93)91705-q. [DOI] [PubMed] [Google Scholar]
- Poirier J., Hess M., May P. C., Finch C. E. Astrocytic apolipoprotein E mRNA and GFAP mRNA in hippocampus after entorhinal cortex lesioning. Brain Res Mol Brain Res. 1991 Sep;11(2):97–106. doi: 10.1016/0169-328x(91)90111-a. [DOI] [PubMed] [Google Scholar]
- Poirier J., Hess M., May P. C., Finch C. E. Cloning of hippocampal poly(A) RNA sequences that increase after entorhinal cortex lesion in adult rat. Brain Res Mol Brain Res. 1991 Feb;9(3):191–195. doi: 10.1016/0169-328x(91)90002-f. [DOI] [PubMed] [Google Scholar]
- Roheim P. S., Carey M., Forte T., Vega G. L. Apolipoproteins in human cerebrospinal fluid. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4646–4649. doi: 10.1073/pnas.76.9.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders A. M., Strittmatter W. J., Schmechel D., George-Hyslop P. H., Pericak-Vance M. A., Joo S. H., Rosi B. L., Gusella J. F., Crapper-MacLachlan D. R., Alberts M. J. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology. 1993 Aug;43(8):1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- Seta K., Nakamura H., Okuyama T. Determination of alpha-tocopherol, free cholesterol, esterified cholesterols and triacylglycerols in human lipoproteins by high-performance liquid chromatography. J Chromatogr. 1990 Aug 31;515:585–595. doi: 10.1016/s0021-9673(01)89358-4. [DOI] [PubMed] [Google Scholar]
- Soininen H., Kosunen O., Helisalmi S., Mannermaa A., Paljärvi L., Talasniemi S., Ryynänen M., Riekkinen P., Sr A severe loss of choline acetyltransferase in the frontal cortex of Alzheimer patients carrying apolipoprotein epsilon 4 allele. Neurosci Lett. 1995 Mar 3;187(2):79–82. doi: 10.1016/0304-3940(95)11343-6. [DOI] [PubMed] [Google Scholar]
- Svennerholm L., Gottfries C. G. Membrane lipids, selectively diminished in Alzheimer brains, suggest synapse loss as a primary event in early-onset form (type I) and demyelination in late-onset form (type II). J Neurochem. 1994 Mar;62(3):1039–1047. doi: 10.1046/j.1471-4159.1994.62031039.x. [DOI] [PubMed] [Google Scholar]
- Van Broeckhoven C., Backhovens H., Cruts M., Martin J. J., Crook R., Houlden H., Hardy J. APOE genotype does not modulate age of onset in families with chromosome 14 encoded Alzheimer's disease. Neurosci Lett. 1994 Mar 14;169(1-2):179–180. doi: 10.1016/0304-3940(94)90385-9. [DOI] [PubMed] [Google Scholar]
- Wallin A., Gottfries C. G., Karlsson I., Svennerholm L. Decreased myelin lipids in Alzheimer's disease and vascular dementia. Acta Neurol Scand. 1989 Oct;80(4):319–323. doi: 10.1111/j.1600-0404.1989.tb03886.x. [DOI] [PubMed] [Google Scholar]
- Whitehouse P. J., Price D. L., Struble R. G., Clark A. W., Coyle J. T., Delon M. R. Alzheimer's disease and senile dementia: loss of neurons in the basal forebrain. Science. 1982 Mar 5;215(4537):1237–1239. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]
- Wilcock G. K., Scott M. I. Tacrine for senile dementia of Alzheimer's or Lewy body type. Lancet. 1994 Aug 20;344(8921):544–544. doi: 10.1016/s0140-6736(94)91933-x. [DOI] [PubMed] [Google Scholar]
- Wurtman R. J. Choline metabolism as a basis for the selective vulnerability of cholinergic neurons. Trends Neurosci. 1992 Apr;15(4):117–122. doi: 10.1016/0166-2236(92)90351-8. [DOI] [PubMed] [Google Scholar]
- Zubenko G. S. Hippocampal membrane alteration in Alzheimer's disease. Brain Res. 1986 Oct 15;385(1):115–121. doi: 10.1016/0006-8993(86)91552-0. [DOI] [PubMed] [Google Scholar]
- Zubenko G. S., Wusylko M., Cohen B. M., Boller F., Teply I. Family study of platelet membrane fluidity in Alzheimer's disease. Science. 1987 Oct 23;238(4826):539–542. doi: 10.1126/science.3659926. [DOI] [PubMed] [Google Scholar]