Abstract
Objective
Infusion reactions are common adverse reactions associated with antibody preparations. However, no studies have examined the time to onset of serious infusion reactions after administering cetuximab. We aimed to investigate the timing and severity of IRs affecting Japanese patients after administration of cetuximab.
Methods
Study subjects were identified from a nationwide prospective registry of 2126 metastatic colorectal cancer patients scheduled to receive cetuximab. Infusion reactions were examined in 2006 patients with adequate safety data.
Results
Infusion reactions of any grade occurred in 114 patients (5.7%), including Grade 3–4 infusion reactions in 22 patients (1.1%). Premedications were antihistamine plus corticosteroid (88.9% of patients with infusion reactions), antihistamine alone (9.2%) or corticosteroid alone (1.1%). In 95 patients (83.3%), infusion reactions occurred after the first dose. Twenty of the 22 Grade 3–4 infusion reactions occurred within 1 h of the first dose (the timing of the infusion reaction was unknown in one patient while another infusion reaction occurred after the fourth dose). Infusion reactions resolved in 111/114 patients (97.4%) while one patient recovered with sequelae, one patient died and one patient failed to recover within the follow-up period. Thirteen patients (15.7% of patients with infusion reactions) with Grade 1–2 infusion reactions showed recurrence after readministration of cetuximab; the recurrent infusion reactions were less severe than the initial reactions.
Conclusions
Grade 3–4 infusion reactions occurred in 1.1% of colorectal cancer patients, and most occurred within 1 h of receiving the first dose of cetuximab. Therefore, patients should be carefully observed following cetuximab infusion, especially during the first hour after the first infusion.
Keywords: cetuximab, chemotherapy, colorectal cancer, infusion reaction
INTRODUCTION
Infusion reactions (IRs) are occasionally associated with antibody preparations. In the field of anticancer therapy, IRs caused by several molecular-targeting drugs have been reported, including rituximab (1,2), trastuzumab (3,4), bevacizumab (5) and panitumumab (6). Mild-to-moderate IRs include chills, pyrexia and dizziness, which are often associated with hypersensitivity and allergic symptoms. Severe IRs include anaphylactoid symptoms, such as dyspnea, bronchospasm, urticaria, hypotension, loss of consciousness and shock, or even myocardial infarction or cardiac arrest in some patients. Therefore, IRs should be treated promptly to avoid exacerbation. It has been suggested that patients should be carefully observed after receiving the first dose of any antibody preparation (7). However, to our knowledge, no one has examined the time to onset of IRs after administering antibody preparations.
Cetuximab is an epidermal growth factor receptor (EGFR) inhibitor that is used to treat unresectable advanced or recurrent colorectal cancer. It is a human/mouse chimeric monoclonal immunoglobulin G1 antibody that inhibits the EGFR (8,9). Serious adverse reactions to cetuximab include IRs, cutaneous reactions and interstitial lung disease (11–13). An earlier clinical study identified Grade 3 and 4 IRs in 2% and 5% of 1373 patients, respectively, along with one death (10). The authors also reported that ∼90% of the IRs of any grade occurred after the first dose (10). To ameliorate and/or prevent IRs, premedication with an antihistamine before administering cetuximab is recommended. Indeed, the Monoclonal Antibody Cetuximab in a European Pre-License (MABEL) study showed that administration of a corticosteroid combined with an antihistamine reduced the incidence of IRs (14,15).
In Japan, the pharmaceutical regulatory authorities require patient registration studies to be conducted for all anticancer agents after approval is granted. The objective of such studies is to confirm, in the real-world clinical setting, the accuracy of the safety profile determined during the clinical studies that were conducted to support approval. These studies, also known as postmarketing surveillance studies, examine the safety and efficacy of anticancer agents in consecutively and prospectively registered patients. Registration commences immediately after the launch of the drug and is continued until the required number of patients has been enrolled. For cetuximab, a multicenter, prospective study was planned with a target sample size of 1800 patients. The following parameters were designated as priority items for assessment: IRs, cutaneous toxicity, interstitial pneumonia, hypomagnesemia and cardiotoxicity. A summary of the results of this study has already been reported (16). The present analysis focused on the incidence, timing of onset and severity of IRs affecting Japanese patients in the real-world clinical setting. We also attempted to identify risk factors for IRs occurring after the initial dose or after readministration of cetuximab.
PATIENTS AND METHODS
Patients
A more detailed description of this registry study is provided elsewhere (16). In brief, 2126 patients scheduled to receive cetuximab at 637 institutions in Japan were prospectively enrolled in a central registry between 19 September 2008 and 5 January 2009 (16). Only patients receiving cetuximab in accordance with the approved indications were enrolled. All of the patients had EGFR-positive colorectal cancer, no history of hypersensitivity to any ingredients of the drug, a performance status (PS) of 0–1, no interstitial lung disease and a resistant/refractory tumor or intolerance of prior chemotherapy. Cetuximab was administered by intravenous infusion once weekly, with no limit on the number of doses or duration of treatment. The first dose (400 mg/m2) was infused over 2 h, and subsequent doses (250 mg/m2) were given over 1 h (10). When the study was planned, there were no Japanese data on the efficacy or safety of cetuximab combined with oxaliplatin-based regimens, so irinotecan or FOLFIRI regimens (folinic acid, fluorouracil and irinotecan) could be used instead. The product information for cetuximab states that an antihistamine should be given as premedication before administration and concomitant use of a corticosteroid may reduce the risk of IRs. Therefore, premedication with an antihistamine and/or corticosteroid was recommended.
Evaluation and Analysis
The observation period was defined as the time between the first and last doses of cetuximab. The attending physicians were instructed to submit case report forms documenting safety and efficacy in Weeks 4 and 16 after the first dose, and after the final dose. The safety and efficacy results were described in an earlier report (16). Adverse event data were compiled by the Central Data Centre, and the severity of adverse events was assessed according to Common Terminology Criteria for Adverse Events (CTCAE) Version 3.0. Adverse events are described using CTCAE terminology. Priority items included the presence or absence and severity of IRs, cutaneous disorders, interstitial lung disease, electrolyte abnormalities (e.g. hypomagnesemia), cardiotoxicity, gastrointestinal disorders, thrombosis/embolism, delayed wound healing and ocular disorders (e.g. keratitis). Detailed information on IRs was obtained from the case report forms, and included seriousness, date of onset, time from starting treatment to onset, outcome and premedication(s), and whether or not readministration was attempted.
Statistical Analysis
Data were compiled and analyzed using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA). The incidence of IRs was evaluated in relation to patient characteristics (sex, age, stage, PS, complications, previous illnesses, history of allergy, premedication and concomitant drugs) in univariate analyses using the χ2 test, Fisher's exact test, or Wilcoxon two-sample test as was appropriate. In all analyses, P<0.05 was considered to indicate statistical significance.
RESULTS
Patient Characteristics
A total of 154 patients who were enrolled did not receive cetuximab and were excluded from analysis. The remaining 2006 patients were included in the safety analysis set. Their median age was 64 years (range: 18–87 years), and the male/female ratio was 1 : 0.6. The PS was 0 or 1 in 2000 patients (99.7%) and 3 or 4 in six patients (0.3%). Concurrent diseases were present in 974 patients (48.6%), including hypertension in 447 patients (22.3%), diabetes mellitus in 284 patients (14.2%), liver dysfunction in 93 patients (4.6%), hyperlipidemia in 86 patients (4.3%) and heart diseases in 79 patients (3.9%). Overall, 405 patients (20.2%) had a history of clinically relevant diseases, including allergy in 306 patients (15.4%), heart disease in 27 patients (1.3%) and interstitial lung disease in four patients (0.2%).
The median duration of cetuximab treatment was 15.3 weeks (1–73.9 weeks), and 1869 patients (93.2%) received cetuximab as third-line or later treatment. The number of doses of cetuximab was ≤3 in 12.6%, 4–15 in 44.9%, 32–47 in 12.7% and ≥48 in 3.7% of patients. Four hundred and sixty patients (22.9%) received cetuximab alone while 1546 patients (77.1%) received cetuximab in combination with chemotherapy. The chemotherapy regimens included irinotecan in 1255 patients (62.6%) and FOLFIRI in 256 patients (12.8%). Premedication was given to 1991 patients (99.3%).
Incidence and Severity of IRs
IRs were reported in 114/2006 patients (5.7%) and were classified as Grade 3 in 13 patients and Grade 4 in nine patients (Grades 3–4: 1.1%). The most common IRs were classified (using CTCAE terminology) as general disorders and administration site conditions (e.g. infusion-related reactions and pyrexia), which occurred in 114 (5.7%, any grade) of patients, including 1.1% classified as Grades 3–4. Other IRs (which were observed in individuals who were also classified as having general disorders and administration site reactions) included respiratory, thoracic and mediastinal disorders (e.g. dyspnea) in 26 patients (1.3%; Grades 3–4: 0.4%), skin and subcutaneous tissue disorders in 23 patients (1.1%; Grades 3–4: 0.1%), vascular disorders (e.g. flushing) in 15 patients (0.7%), immune system disorders (including anaphylactic shock and hypersensitivity) in 14 patients (0.7%; Grades 3–4: 0.4%), nervous system disorders (≤0.1%), cardiac disorders (≤0.1%), gastrointestinal disorders (0.1%) and investigations (0.3%).
Timing of IRs
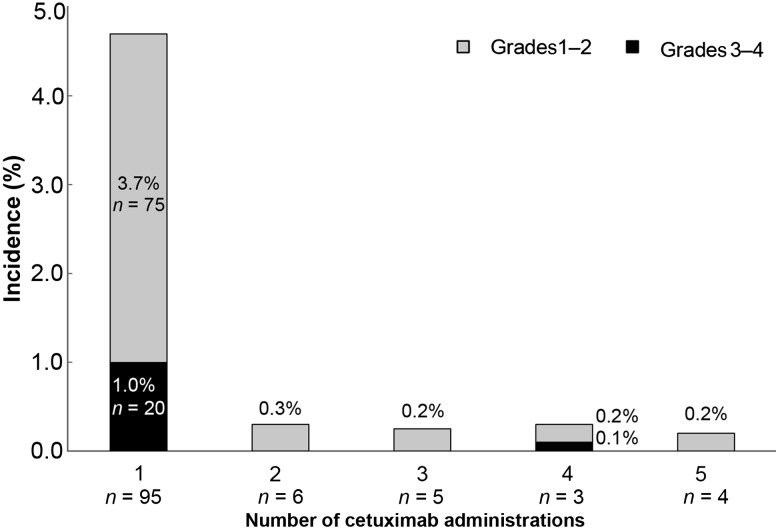
Of the 114 patients, 95 (83.3%), 6 (5.3%), 5 (4.4%), 3 (2.6%) and 4 (3.5%) experienced IRs after the first, second, third, fourth and fifth or subsequent doses of cetuximab, respectively (Fig. 1). Grade 3–4 IRs occurred in 22 patients (Grade 3 in 13 patients and Grade 4 in nine patients). Among them, 20 patients (90.9%) experienced IRs after the first dose and one patient (4.5%) did so after the fourth dose, while the timing was unknown for one patient (4.5%).
Figure 1.
Timing of infusion reactions (IRs) in relation to the number of cetuximab doses. One patient who had a Grade 3 event with unknown timing was excluded from the analysis.
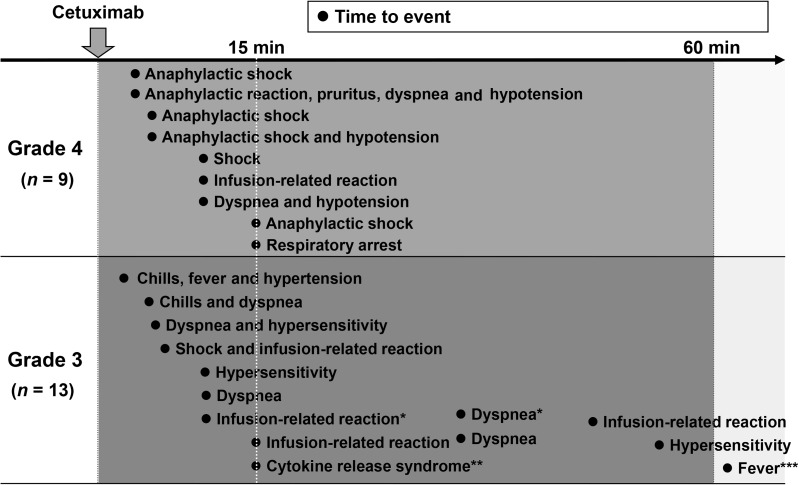
The timing of each symptom after the start of cetuximab infusion in the 22 patients with Grade 3 or 4 IRs is shown in Fig. 2. The median time to the onset of reactions in these 22 patients was 10 min (range: 2 min to 8 h). In 18 patients, IRs occurred within 15 min after the start of infusion. Except for fever at 8 h after the start of cetuximab infusion in one patient (which was considered by the investigator to be at least partly related to a biliary tract infection in this patient), all of the other IRs occurred within 1 h of starting infusion. Interestingly, all of the Grade 4 IRs occurred within 15 min of starting infusion.
Figure 2.
Time to onset of Grade 3–4 IRs. The median time was 10 min (range: 2 min to 8 h). Dots indicate individual patients. Asterisks indicate multiple events in the same patient. *Identical patients. **This patient had dyspnea, bronchospasm, tachycardia and nausea. ***Considered related to biliary tract infection and liver metastasis.
Outcome
The IR resolved/improved in 111/114 patients (97.4%). One patient experienced flushing (a vascular symptom and IR) and other symptoms associated with the infusion, although the nature of these symptoms was not reported. This patient did not recover from these symptoms during the follow-up period of this survey. One patient who had pruritus recovered with sequelae. One patient with Grade 4 IRs died. In this patient, the Grade 4 IRs were dyspnea and low blood pressure, which were detected 10 min after the start of infusion. The median time to recovery was 1 day (range: 0–366 days) in patients with Grade 1–2 IRs and 1 day (range: 1–15 days) in patients with Grade 3–4 IRs.
Incidence of IRs According to Premedication
Premedication consisted of an antihistamine plus a corticosteroid in 1783 patients (88.9% of the patients in the safety analysis set), an antihistamine alone in 185 patients (9.2%) and a corticosteroid alone in 23 patients (1.1%). The incidence of IRs was similar between patients pretreated with an antihistamine plus corticosteroid and those pretreated with an antihistamine alone [5.9% (105/1783) versus 4.3% (8/185)]. The incidence of Grade 3–4 IRs was 1.1% (20/1783 patients) in patients pretreated with an antihistamine plus corticosteroid and 0.5% in those pretreated with an antihistamine alone (1/185 patients).
Recurrence of IRs after Cetuximab Readministration
Overall, 85 of 114 patients who experienced IRs after the first infusion underwent cetuximab readministration (83 patients with Grade 1–2 IRs and two patients with Grade 3–4 IRs). Of these 85 patients, 14 (16.3%) developed an IR following readministration. The product information for cetuximab in Japan and other countries states that it should be immediately discontinued in patients with Grade 3–4 IRs and that such patients should not receive readministration. However, it was readministered in two patients with Grade 3–4 IRs, but no adverse events were observed in either patient.
Risk Factors for IRs
Univariate analysis was conducted to identify possible risk factors for IRs. It was found that the incidence of IRs was significantly higher in patients with a history of heart disease or interstitial lung disease than in patients without a history of such diseases (8.6 versus 5.0%, P = 0.0077). The incidence of IRs was also significantly higher in patients treated with cetuximab alone than in those treated with cetuximab plus chemotherapy (8.0 versus 5.0%, P = 0.0158). Furthermore, the incidence of IRs was slightly, but not significantly, higher in patients with a history of allergy than in patients without a history of allergy (8.4 versus 5.3%, P = 0.0822). No association was found with other characteristics, such as sex, age, chemotherapy stage and PS.
DISCUSSION
The present survey showed that IRs occurred in 114/2006 patients (5.7%) included in the safety analysis set while Grade 3–4 IRs occurred in 22 patients (1.1%). The majority of Grade 3–4 IRs were detected within 1 h of starting infusion, which suggests that careful observation is necessary for ≥1 h after first administering cetuximab in routine clinical practice.
Most of the IRs occurred after the first dose of cetuximab, including 90% of Grade 3–4 IRs, although one patient developed a severe IR after the fourth dose. Therefore, careful observation is particularly important after the first dose but physicians should continue to be vigilant for possible IRs after subsequent doses of cetuximab.
With regard to the timing of IRs, all of the Grade 3–4 reactions occurred within 1 h of starting infusion, except in one patient who developed an IR at 8 h. However, that patient's symptom was fever, which is not a typical IR symptom and was considered to be at least partly related to biliary tract infection and liver metastasis.
The IRs resolved/improved in 97.4% of the patients, with a median recovery time of 1 day. IRs caused by cetuximab appear to resolve promptly by appropriate treatment. However, persistence of the IR, recovery with sequelae and death were reported in one patient each. Clinicians should be fully aware of these risks of IRs and inform patients of them before starting cetuximab therapy.
IRs associated with cetuximab were extensively described in the MABEL study (14,15), a multinational, Phase 2 study that examined the efficacy and safety of cetuximab combined with irinotecan in 1147 patients with metastatic colorectal cancer refractory to irinotecan monotherapy. A similar number of patients received this combination in the present study (1546). Premedication was administered to 1991/2006 patients (99.3%) in our study and to 1122/1147 patients (97.8%) in the MABEL study. In that study, IRs occurred in 175/1122 patients (15.6%) and Grade 3–4 IRs occurred in 27 patients (2.4%), compared with 5.7 and 1.1%, respectively, in our study. Although we found no difference in the incidence or IRs between patients receiving premedication with an antihistamine alone and those treated with an antihistamine plus a corticosteroid, it is notable that 88.9% of our patients received antihistamine plus corticosteroid premedication compared with 61.0% in the MABEL study. Therefore, it seems likely that concomitant corticosteroid use was protective against IRs. Indeed, the lower rate of Grade 3–4 IRs in our study (1.1%) compared with that in the MABEL study (2.4%) may be attributable to a higher proportion of patients premedicated with antihistamines combined with corticosteroids. The lack of a difference in the incidence of IRs between patients receiving premedication with an antihistamine alone and those treated with an antihistamine plus a corticosteroid in the present study is probably due to the small number of events and the small number of patients treated with an antihistamine alone.
The incidence of IRs varies greatly among antibody preparations. For example, the incidence of IRs occurring within 24 h after the start of administration is ∼90% for rituximab (chimeric), ∼40% for trastuzumab (humanized) and<3% for bevacizumab (humanized) (1,3,5). The incidence of IRs caused by cetuximab in the present survey (5.7%) was higher than that caused by panitumumab (3%: 43/1336 patients; Grade 3–4 IRs: 1%, six patients) (6), which is a fully human anti-EGFR antibody.
Regarding readministration of cetuximab after the occurrence of mild-to-moderate IRs (Grades 1–2), cetuximab was readministered to 83 of 92 patients with Grade 1–2 IRs, and 13 (15.7%) developed recurrent IRs. However, none of those IRs was more severe than the initial reactions. Based on these results, it may be necessary to change the premedication or reduce the infusion rate in patients with Grade 1–2 IRs who undergo readministration of cetuximab.
Some limitations of this study should be discussed. First, this was a nonrandomized and uncontrolled study. Therefore, it is possible that there is a bias in the data obtained; however, given the sample size of 2006 patients, we believe such a bias is unlikely. Second, the case report forms did not allow the clinicians to record the cause of death or sequelae in patients who did not recover from IRs; therefore, it is possible that there were serious events related to cetuximab infusion that we were unable to identify. Likewise, because the symptoms were reported by the investigators, those symptoms that did not recover by the end of the follow-up period were classified as unrecovered. Third, although the quality of the data is different from that of controlled prospective clinical trials, this prospective registry provided us with valuable information on the safety and effectiveness of cetuximab in real-world usage.
In summary, Grade 3–4 IRs caused by cetuximab occurred in 1.1% of patients in this prospective registry. Most Grade 3–4 IRs occurred within 60 min after the initial administration. Therefore, we think that patients should be very carefully observed following infusion of cetuximab, especially during the first hour after initial administration. Notably, there were no severe IRs after readministration of cetuximab after Grade 1–2 IRs. Therefore, clinicians should consider the potential risks/benefits when readministering cetuximab after IRs and ensure the patients are fully informed of the possible risks of cetuximab therapy.
Funding
This work was supported by Bristol-Myers K.K and Merck Serono Co., Ltd., Japan. Funding to pay the Open Access publication charges for this article was provided by Bristol-Myers K.K and Merck and Serono Co., Ltd., Japan.
Conflict of interest statement
Kensei Yamaguchi has provided consultancy services to, and received honoraria from Merck Serono Co. Ltd. and Bristol-Myers K.K.; Toshiaki Watanabe has received honoraria and research funding from Merck Serono Co. Ltd. and Bristol-Myers K.K.; Taroh Satoh has received honoraria from Merck Serono Co. Ltd. and Bristol-Myers K.K.; Makiko Izawa is an employee of Bristol-Myers K.K.; Shogo Inoshiri is an employee of Merck Serono Co. Ltd.; Kenichi Sugihara has received honoraria and research funding from Merck Serono Co. Ltd. and Bristol-Myers K.K.; Yuh Sakata has received honoraria from Merck Serono Co. Ltd. and Bristol-Myers K.K. Megumi Ishiguro has no conflict of interest to declare.
Acknowledgements
We are grateful to the physicians, patients and their families for their cooperation. We also thank Nicholas D. Smith, PhD and Daniel McGowan, PhD, for their editorial support.
References
- 1.Rituxan® (rituximab) Package Insert. South San Francisco, CA: Genentech, Inc.; (Revised: 04/2011). [Google Scholar]
- 2.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large B-cell lymphoma. N Engl J Med. 2002;346:235–42. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 3.Herceptin® (trastuzumab) Package Insert. South San Francisco, CA: Genentech, Inc; (Revised: 10/2010). [Google Scholar]
- 4.Cook-Bruns N. Retrospective analysis of the safety of Herceptin immunotherapy in metastatic breast cancer. Oncology. 2001;61(Suppl 2):58–66. doi: 10.1159/000055403. [DOI] [PubMed] [Google Scholar]
- 5.Avastin® (bevacizumab) Package Insert. South San Francisco, CA: Genentech, Inc; (Revised: 12/2011). [Google Scholar]
- 6.Vectibix® (panitumumab) Package Insert. Thousand Oaks, CA: Amgen Inc; (Revised: 12/2011). [Google Scholar]
- 7.Lenz HJ. Management and preparedness for infusion and hypersensitivity reactions. Oncologist. 2007;12:601–9. doi: 10.1634/theoncologist.12-5-601. [DOI] [PubMed] [Google Scholar]
- 8.Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358:1160–74. doi: 10.1056/NEJMra0707704. [DOI] [PubMed] [Google Scholar]
- 9.Ciardiello F, Tortora G. A novel approach in the treatment of cancer: targeting the epidermal growth factor receptor. Clin Cancer Res. 2001;7:2958–70. [PubMed] [Google Scholar]
- 10.Erbitux® (cetuximab) Package Insert. New York, NY: ImClone Systems Incorporated; Princeton, NJ: Bristol-Myers Squibb Company. (Revised: 01/2012) [Google Scholar]
- 11.Jonker DJ, O'Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040–8. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 12.Sobrero AF, Maurel J, Fehrenbacher L, et al. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:2311–9. doi: 10.1200/JCO.2007.13.1193. [DOI] [PubMed] [Google Scholar]
- 13.Van Cutsem E, Kohne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–17. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 14.Siena S, Glynne-Jones R, Adenis A, et al. Reduced incidence of infusion-related reactions in metastatic colorectal cancer during treatment with cetuximab plus irinotecan with combined corticosteroid and antihistamine premedication. Cancer. 2010;116:1827–37. doi: 10.1002/cncr.24945. [DOI] [PubMed] [Google Scholar]
- 15.Wilke H, Glynne-Jones R, Thaler J, et al. Cetuximab plus irinotecan in heavily pretreated metastatic colorectal cancer progressing on irinotecan: MABEL Study. J Clin Oncol. 2008;26:5335–43. doi: 10.1200/JCO.2008.16.3758. [DOI] [PubMed] [Google Scholar]
- 16.Ishiguro M, Watanabe T, Yamaguchi K, et al. A Japanese post-marketing surveillance of cetuximab (Erbitux®) in patients with metastatic colorectal cancer. Jpn J Clin Oncol. 2012;42:287–94. doi: 10.1093/jjco/hys005. [DOI] [PMC free article] [PubMed] [Google Scholar]