Abstract
We tested the independent utility of β amyloid precursor protein (β APP) immunohistochemical staining as evidence of brain trauma in the deaths of young children. Blinded reviewers retrospectively reviewed immunostained brain tissues from homicidal deaths, age matched control cases without evidence of trauma, as well as cases of sudden infant death syndrome (SIDS). The reviewers correctly identified five of the seven cases with documented inflicted head trauma. However, one of seven age matched control cases and one of 10 SIDS/sudden unexplained death in infancy (SUDI) cases demonstrated staining patterns similar to those seen in cases of inflicted trauma. We discuss these cases and the circumstances surrounding them with the intent to explain the difficulties associated with immunohistological interpretation of axonal injury. Although the utility of β APP is quite powerful if not confounded by global hypoxic ischemic injury, ultimately, β APP studies should be only one piece of information in the determination of cause and manner of death.
Keywords: forensic science, axonal injury, traumatic brain injury, amyloid precursor protein, pediatric, autopsy
Explanation of a young pediatric death can be a complicated task requiring different considerations than in determinations of cause and manner of death of adults. Information from a number of sources must be correlated by forensic pathologists and investigators to determine the cause and manner of death. Typical sources include birth history, metabolic screens if available, vaccination history, recent medical history from primary doctors and emergency personnel, information from the law enforcement investigation, information from the scene of death, and the results of the autopsy. Because infant deaths are usually unexpected and sometimes unexplained, there is inevitably heightened suspicion of foul play (1). Central nervous system (CNS) injury is not uncommonly a component of multiple inflicted injuries in infants and young children and may be demonstrated by the finding of intracranial hemorrhage, optic and/or retinal hemorrhage, gross parenchymal injury, and/or microscopic axonal injury (2,3). In some cases of postmortem examination of young pediatric death, investigation or inconsistent information from any of the above sources may raise suspicion of a non natural etiology without definitive gross evidence of injury or with equivocal gross and microscopic evidence of inflicted trauma. In such cases, it would be helpful to have a highly sensitive and specific test for occult, inflicted traumatic injuries. In our experience, it is not uncommon that additional special evaluation of the brain for evidence of microscopic axonal injury be entertained as an “afterthought” in hopes that such evaluation might demon strate additional evidence of inflicted injury.
Morphologic descriptions of axonal injury in human adult and various animal model populations sustaining trauma are well estab lished in medical and scientific literature (4,5). A number of reports have demonstrated β amyloid precursor protein (β APP) localization as a marker of axonal injury in both inflicted and accidental pediatric head trauma (3,6 8). Such reports have resulted in the frequent to the routine use of β APP immunostaining in the forensic evaluation of pediatric deaths. In our experience, the results of such studies are usually compiled with other data by the modern forensic pathologist when making his/her determination of the cause and manner of death. In cases with information from investigation and multiple gross injuries all pointing to a diagnosis of inflicted trauma, such additional “special studies,” if done routinely, may only be supportive of the overall diagnosis. However, is it possible that such additional studies might be used as preeminent evidence of occult trauma?
In 1999, the State of Maryland Office of the Chief Medical Examiner (OCME) investigated 153 deaths of subjects 3 years of age or younger. Of these, 97 deaths were natural (including 56 cases attributed to sudden infant death syndrome [SIDS]), 24 were accidental, 18 were homicides, and 14 were undetermined. Among the homicides, seven children sustained blunt force injuries to the head. We evaluated the staining pattern of β APP in multiple brain regions (frontal, temporal, and parietal cortices, cingulate gyrus/internal capsule / corpus callosum, and the cervicomedullary junction). We compared, in a blinded fashion, the β APP staining of the homicide cases to similar brain regions from 7 age matched control cases, in which death was because of a nontraumatic mechanism (other than SIDS), and 10 cases with similar ages, from the same calendar year in which death was attributed to SIDS.
Materials and Methods
Case Acquisition
Deaths in children under the age of 3, in the 1999 calendar year, were identified by electronic search of records of the Office of the Chief Medical Examiner (OCME) for the State of Maryland for cause of death, manner of death, and the presence of head injuries demonstrated at autopsy. Seven were cases in which the cause of death was some variation of multiple inflicted injuries, with evidence of head trauma, and the manner of death was ruled homicide. Seven nonconsecutive age matched cases were chosen as age matched controls, with manner of death deemed natural or accident (see Table 2). Ten additional deaths, also from the 1999 calendar year, were determined to be because of SIDS, according to the criteria for SIDS diagnosis used at that time. Upon the final review of the reports of these 10 cases, it was our opinion that three would not be classified as SIDS according to our current con sideration of cosleeping as a factor to be excluded for the diagnosis of SIDS. Thus, for the purposes of this review, these three cases are listed as sudden unexplained death in infancy (SUDI, Table 3). We chose to evaluate archival material from almost a decade ago to more effectively blind the reviewers and as a historical review of past determinations of SIDS at our institution.
TABLE 2.
Age matched control (nontraumatic) cases.
| Case | Age (days) |
Survival | Sex | Race | Cause /Manner of Death |
|---|---|---|---|---|---|
| 6 | 16 | No | F | White | Pneumonia/Natural |
| 7 | 60 | Resuscitation <1 h | F | White | Bronchopneumonia/Natural |
| 8 | 120 | Resuscitation <1 h | F | White | Pneumonia/Natural |
| 15 | 934 | 21 h | M | Black | Drowning/Accident |
| 19 | 139 | Resuscitation <1 h | F | White | Bronchiolitis/Natural |
| 22 | 396 | Resuscitation <1 h | M | Asian | Sepsis with early acute bronchopneumonia/Natural |
| 23 | 225 | No | F | White | Sudden infant death with tonsillitis/Natural |
TABLE 3.
SIDS and SUDI cases.
| Case | Age (days) | Survival | Sex | Race | Cause of Death |
|---|---|---|---|---|---|
| 1 | 65 | <1 h resuscitation | M | White | SIDS |
| 2 | 149 | No | M | White | SIDS |
| 3 | 190 | No | M | Black | SIDS |
| 4 | 109 | No | M | Black | SIDS |
| 10 | 129 | No | M | Black | SUDI |
| 12 | 134 | 9 h | F | Black | SUDI |
| 16 | 90 | No | M | Black | SIDS |
| 18 | 162 | No | M | White | SIDS |
| 21 | 94 | No | M | White | SIDS |
| 24 | 79 | No | M | Black | SUDI |
SIDS, sudden infant death syndrome; SUDI, sudden unexplained death in infancy.
In all 24 cases, as is routine at our institution for patients of this age, the brains were formalin fixed and sectioned by a neuropathologist 2 4 weeks after the initial autopsy. A separate neuropathology report was issued and attached to the final autopsy report. Archival formalin fixed, paraffin embedded brain tissues from these 24 autopsy cases were pulled. Histologic sections from five general locations frontal lobe, parietal lobe, mesiotemporal and inferior temporal lobe, peristriatal internal capsule and the body of the corpus callosum, and cervicomedullary junction were processed for β APP immunohistochemical staining, for each of the cases.
Immunohistochemistry
All tissue was processed for microtomy according to established methods. Blocks were cut at 5 µm, and sections were mounted on charged glass slides. Sections were de paraffinized and re hydrated by xylene and graded dilute ethanol rinses. Antigen retrieval was performed by boiling the sections in pH 6.8 citrate buffer. The slides were hand stained with diluted antisera (MAB349; Millipore) to amyloid beta (A4) precursor protein at room temperature. Optimal conditions were determined using appropriate control human autopsy tissues from cases of known cerebral trauma. After extensive washing in phosphate buffered solutions, the slides were incubated with diluted horseradish peroxidase conjugated secondary antisera at room temperature. Signal amplification was performed using avidin/biotin complex reagents (ABC, DAKO). After final rinsing, the staining was visualized with diaminobenzadine chromogen.
Interpretation
These immunohistochemical preparations were reviewed separately by three of the authors (MWJ, LL, AR) who viewed the immunohistochemical stains only and did not have any other information about the cases, at the time of review. One of the reviewers, who was responsible for accruing the cases for study, was aware of the numbers of cases in each category (homicide, natural/accidental control, SIDS/SUDI). A semiquantitative methodology was used to quantify the presence, focality, and density of immunohistochemical labeling within white matter only. Gray matter staining, if present, was not tabulated.
Each of the authors recorded their opinion of the labeling pat tern on each slide as negative, focally mild, focally moderate, focally severe, multi focally mild, multi focally moderate, or multi focally severe. Focal was defined as staining within a single area of the slide, whereas multi focal involved multiple areas of the same microscopic slide separated by more than a 10× power field. Mild was defined as 1 3 axonal profiles per high powered field (40×, hpf). Moderate was 3 10 per hpf, and severe was greater than 10 profiles per hpf. After scoring was accomplished, each of the reviewing pathologists reported, with a yes or no answer, whether the immunohistochemical changes were consistent with traumatic injury. Prior reports have described differentiating patterns induced by hypoxic ischemic injury versus traumatic acceleration deceleration type injury under the terminologies of vascular axonal injury and various other traumatic axonal injury descriptors, respectively (5,7,8). Additional note was taken of the general appearance and pattern of axonal injury in each histologic section. The results from each of the reviewers were then compared, and the cases were un blinded for correlation and interpretation.
Results and Case Reviews
The general demographic information, including postinjury resuscitation and survival time as well as autopsy findings of the seven homicide victims, is summarized in Table 1. The demographic information, causes/manners of death, and resuscitation/survival times of the seven age matched control cases and the 10 SIDS/SUDI cases are shown in Tables 2 and 3, respectively.
TABLE 1.
General demographic information and autopsy findings of homicide cases.
| Case | Age (days) |
Resuscitation/ Survival Postinjury |
Sex | Race | Head Impact Injury* |
Skull Fracture |
EDH† | SDH† | SAH† | Gross Cerebral Parenchymal Injury |
Eyes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 54 | 2 days | Female | White | Yes | Yes | Yes | Yes | Yes | Hypoxic ischemic encephalopathy | Retinal, intra, and subdural hemorrhages |
| 9 | 861 | Deceased at scene | Female | Black | Yes | No | No | Yes | Yes | Hypoxic ischemic encephalopathy | Intra and subdural hemorrhages |
| 11 | 28 | Resuscitation <1 h | Female | Black | Yes | Yes | No | No | No | Acute and remote contusions (frontal, occipital) | Normal |
| 13 | 123 | 5 days | Female | Asian | Yes | Yes | Yes | Yes | Yes | Bilateral cortical infarcts | Retinal, intra, and subdural hemorrhages |
| 14 | 120 | Resuscitation <1 h | Male | Black | Yes | Yes | Yes | No | Yes | Remote contusions (right parietal) | Subdural hemorrhages |
| 17 | 571 | 5 h | Male | Black | Yes | No | Yes (spinal cord) | No | No | None | Normal |
| 20 | 66 | Resuscitation <1 h | Female | Black | Yes | No (vertebral fracture) | Yes (spinal cord) | Yes | No | Acute spinal contusion | Normal |
Evidence of head impact injury scalp subcutaneous and/or subgaleal hemorrhage with or without external evidence of injury or intracranial injuries.
EDH, epidural hemorrhage; SDH, subdural hemorrhage; SAH, subarachnoid hemorrhage; unless otherwise stated, hemorrhage was documented within the cranium.
Although immunohistochemical staining on all the cases was carried out at the same time, with the same reagents, and with the same staining methodology, the resulting labeling was somewhat variable from case to case. In some cases, there was considerable background neuropil staining. In some, robust neuronal cytoplasmic staining within the gray matter was present (Fig. 1A), whereas in others, significant neuronal staining was not present. Occasional perivascular staining was present in some traumatic, nontraumatic controls, and SIDS/SUDI cases (Fig. 1B). Superficial staining of ependymal surfaces was variable. All of these findings were noted, but the reviewers limited their concentration and interpretation to punctate or solidly stained profiles that had the morphology of axons. In particular, these structures were usually oblong or linearly elongate and often present in bundles within the white matter. Some were bizarrely swollen such that they were spherical and many times the caliber of adjacent “normal” axons. Because of the neuronal staining in some cases, we did not attempt to quantify staining patterns within superficial cortical or deep gray matter.
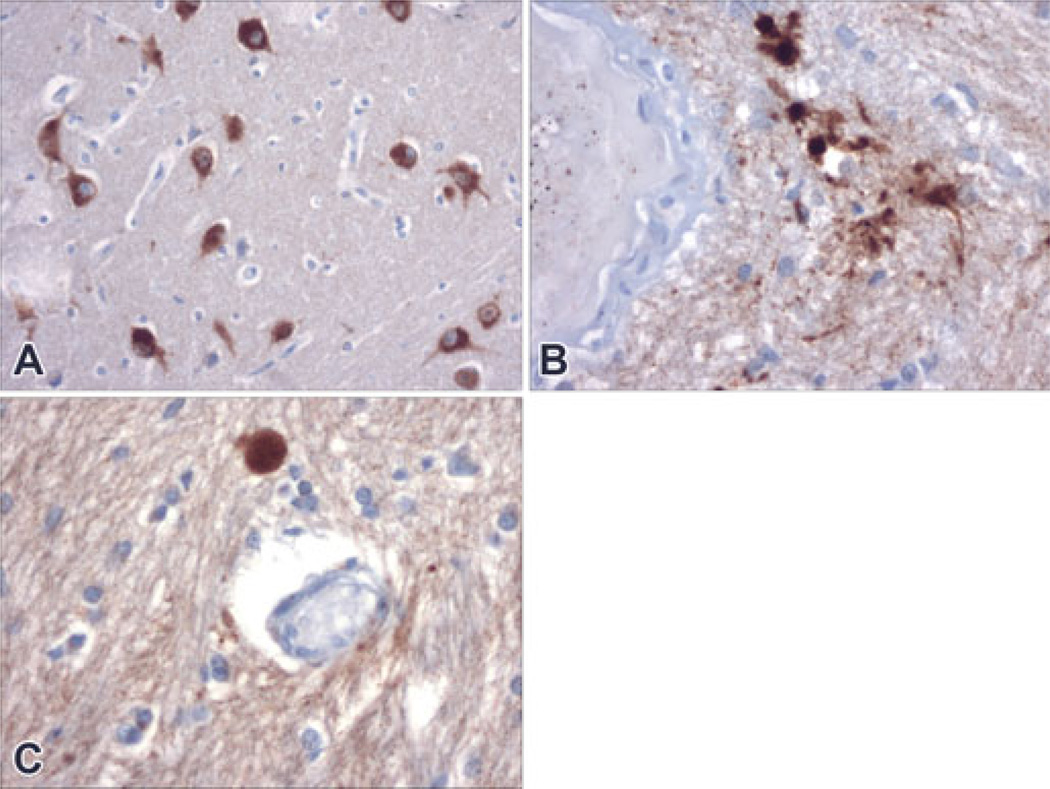
FIG. 1.
Antisera to Beta amyloid (β APP) precursor protein strongly labeled neurons in some cases (Panel A, Case #12, 200× magnification). In the majority of the cases, β APP staining was entirely absent or showed a single focus of staining. Case #16 showed a focal cluster of perivascular staining (Panel B, 400× magnification) in one brain region and an individual swelling in another (Panel C, also Case #16, 400× magnification). Although definite staining, these were not considered significant with regard to evidence of inflicted injury (see Results and Case Reviews).
As described in the Materials and Methods section, three reviewers recorded the distribution and semiquantitative density of stained axons and axonal swellings in the white matter within five general locations: frontal lobe, parietal lobe, temporal lobe, the body of the corpus callosum and internal capsule adjacent to the striate cortex, and the cervicomedullary junction. Each of the three reviewers also independently recorded their overall interpretation of the meaning of the staining patterns (consistent with trauma or no evidence of trauma). It should be noted that interpretation of the staining pattern was not discussed prior to independent evaluation, so there were no specific agreed upon criteria used by the reviewers in deciding whether the pattern of β APP staining was indicative of inflicted trauma. Subsequent to independent review, the three reviewers compared their findings. There was complete agreement regarding the overall impressions of the cases, despite the absence of an agreed upon criteria. Differences in semiquantitative assessments varied only slightly and were limited to difference in opinion regarding adjacent assessment categories (i.e., mild vs. moderate or moderate vs. severe staining). The reviewers had complete agreement about the focality of the staining (focal vs. multifocal). Consensus results, as determined by unanimous or majority determination of each of the parameters by the three reviewers, are listed in Table 4.
TABLE 4.
Consensus* interpretation of immunohistochemical stains.
| Case # | None | Mild Focal | Mod Focal |
Freq Focal |
Mild Multifocal |
Mod Multifocal |
Freq Multifocal | Reviewer Impressions |
Cause or Manner of Death |
|---|---|---|---|---|---|---|---|---|---|
| 1 | A,B,C,D,E | Not Trauma | SIDS | ||||||
| 2 | A,C,E | B | D | Not Trauma | SIDS | ||||
| 3 | A,B,E | C,D | Not Trauma | SIDS | |||||
| 4 | A,E | B,C,D | Not Trauma | SIDS | |||||
| 5 | C | A | B | D,E | Trauma | Homicide | |||
| 6 | A,B,C,D,E | Not Trauma | Pneumonia | ||||||
| 7 | A,B,C,D,E | Not Trauma | Bronchopneumonia | ||||||
| 8 | B | A,C,D,E | Not Trauma | Pneumonia | |||||
| 9 | E | A,B,C,D | Trauma | Homicide | |||||
| 10 | E | A,B,C,D | Not Trauma | SUDI | |||||
| 11 | C | B | D | A,E | Trauma | Homicide | |||
| 12 | A,B,C,E | D | Trauma | SUDI | |||||
| 13 | B | A,C,D,E | Trauma | Homicide | |||||
| 14 | A,B | D,E | C | Trauma | Homicide | ||||
| 15 | A | B | D | C,E | Trauma | Drowning | |||
| 16 | A,B,C,E | D | Not Trauma | SIDS | |||||
| 17 | A,B,E | C,D | Not Trauma | Homicide | |||||
| 18 | A,B,C,E | D | Not Trauma | SIDS | |||||
| 19 | A,C,D,E | B | Not Trauma | Bronchiolitis | |||||
| 20 | A,B,E | C,D | Not Trauma | Homicide | |||||
| 21 | A,C,E | B,C | Not Trauma | SIDS | |||||
| 22 | A,B,C,E | D | Not Trauma | Sepsis | |||||
| 23 | A,E | B,C,D | Not Trauma | Tonsillitis | |||||
| 24 | A,B | C,D,E | Not Trauma | SUDI |
Consensus: These results are the unanimous or majority opinion of the three reviewers. All the reviewers agreed upon the focality/multifocality of the staining. Reviewer opinion differed only amongst categories of staining density, and difference of opinion was limited to adjacent categories (i.e., mild vs. moderate or moderate vs. frequent).
A Frontal lobe; B Parietal lobe; C Temporal lobe; D Internal capsule/corpus callosum; E cervicomedullary junction. SIDS, sudden infant death syndrome; SUDI, sudden unexplained death in infancy.
Upon unblinded review of the autopsy information, it was revealed that the reviewers identified five of the seven cases of inflicted blunt force trauma by evaluation of β APP staining alone. As can be seen by cursory comparison of the semiquantitative data and overall interpretation, the focality or multifocality of the staining correlated completely with reviewer interpretation (Table 4). Cases with multifocal axonal staining were interpreted as being consistent with the effects of inflicted trauma by all three reviewers.
In the identified homicide cases (#5, #9, #11, #13, and #14, see Table 4), four of the cases demonstrated frequent β APP stained profiles (Fig. 2) within multiple foci on multiple slides. One of the cases (#5) demonstrated only mild to moderate numbers of β APP stained profiles, but injured axons in this case were also demonstrated to be within multiple foci on multiple slides. Two cases in which the death was not because of traumatic inflicted injury also demonstrated multiple foci of frequent β APP axonal profiles (Fig. 3). In one case (#12), the cause of death was ruled SUDI. In the other (#15), the death resulted from near drowning. Detailed information about each of the cases flagged by the reviewers as “consistent with inflicted trauma,” as well as “false negative” cases, is below.
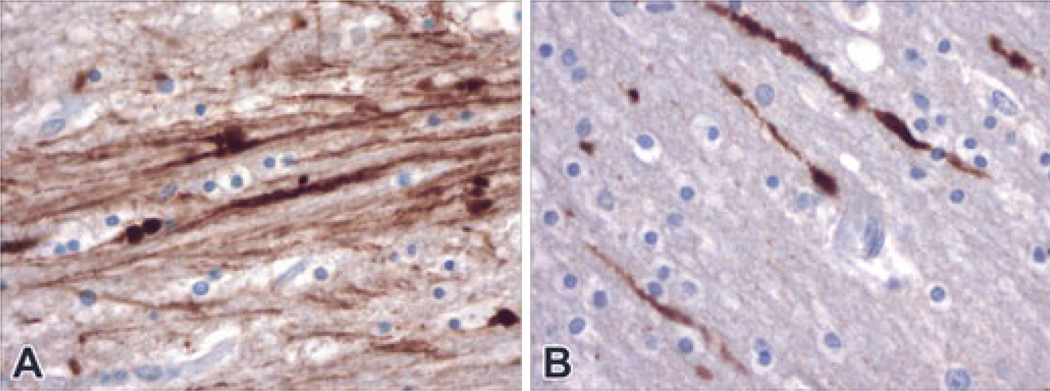
FIG. 2.
Numerous labeled clustered and individual stained axons were demonstrated within multiple foci of several brain regions in cases of inflicted trauma (homicides, both at 400× magnification). Panel A is a photomicrograph from Case #13. Panel B is a photomicrograph from Case #9.
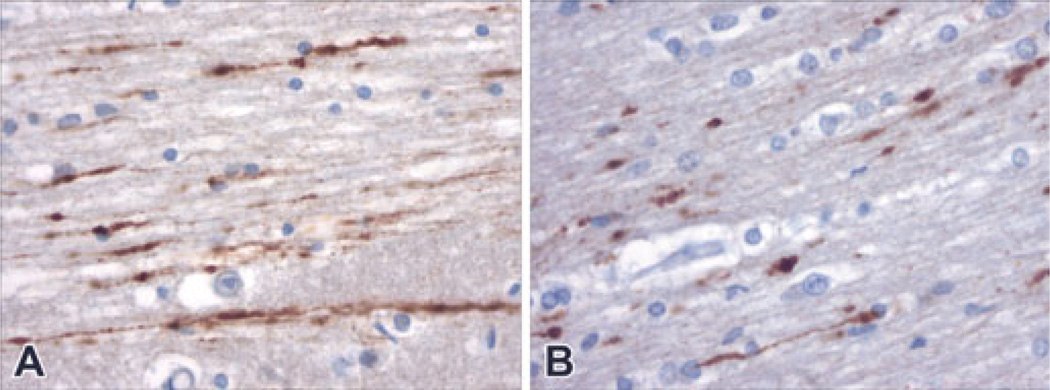
FIG. 3.
In two cases in which there was not inflicted cerebral trauma, multiple foci of labeled axons were demonstrated. Panel A demonstrates multiple longitudinal profiles of labeled axons within the temporal lobe of a child who was resuscitated and survived for almost a day after a near drowning (Case #15, 400× magnification). Panel B shows similar profiles of labeled axons within the white matter of sudden unexplained death in an infant who was resuscitated and survived for about 12 h (Case #12, 400× magnification).
Case #5
This 7 week old female Caucasian child survived for 2 days after she was brought to an emergency unit by a family member who reported that the child “had fallen down the stairs.” The autopsy demonstrated multiple bilateral cutaneous contusions of the head associated with subgaleal hemorrhage, bilateral parietal skull fractures, and frontal bone fracture with associated acute subdural hemorrhages and acute subarachnoid hemorrhage. There were epidural and subdural hemorrhages of the cervical cord as well as optic subdural and retinal hemorrhages. Additionally, there were gross hypoxic ischemic changes of the cerebrum and cerebellum, and hemosiderin laden macrophages within microscopic sections of the cervical epidural hemorrhage. The death was ruled due to blunt force injuries of the head. The manner was homicide. At a later date, a family member pled guilty to child abuse resulting in death.
β APP labeling highlighted multiple foci of axonal profiles in the corpus callosum and internal capsule. Less dense, but multiple foci of similar labeled axons were noted in the parietal white matter. At the cervicomedullary junction, several foci of moderately dense axonal staining were present within the lateral white matter. Neuronal cytoplasms of the inferior olives were prominently stained.
Case #9
This 2 year old African American female infant was found dead in an adult bed upon arrival of medical and law enforcement per sonnel. The autopsy demonstrated subgaleal hemorrhage beneath the frontal and parietal scalp, a subdural hematoma and subarachnoid hemorrhage over the left cerebrum, lower back paraspinal hemorrhage and subdural hemorrhage of the lumbar spinal cord, intradural and subdural optic nerve hemorrhages, and acute hypoxic ischemic brain injury. The death was determined to be due to blunt force injuries of the head, malnutrition, and dehydration; the manner of death was ruled homicide.
Slides demonstrated frequent multifocal β APP stained axonal swellings of the frontal, temporal, and parietal lobes, periventricular white matter, and corpus callosum. No definitive axonal swellings were demonstrated within the cervicomedullary sections. The staining distribution was robust and clustered in areas, but individual axonal swellings were also noted scattered throughout the white matter of all the affected areas. Rare microscopic fields of β APP staining in a geographic / vascular type pattern were noted within cingulate white matter at the angle of the lateral ventricle.
Case #11
This 28 day old African American female was transported by emergency services to a hospital after her mother called 911 and reported her “unresponsive.” Despite approximately 30 min of unsuccessful resuscitative attempts, she was declared deceased at the hospital. Autopsy demonstrated contusions of the face and right side of the forehead, old fractures of multiple ribs with callous formation, remote contusions of the bilateral frontal and right occipital white matter associated with some fresh hemorrhage, and axonal degeneration of the cortico spinal tracts. Her death was determined to be because of child abuse with brain injuries and skeletal fractures. The manner of death was homicide.
β APP stained axonal profiles were noted in a thin rim like pat tern just below the attenuated surface of remote cortical contusions of the frontal and parietal lobe. There was surrounding gliosis. Nongeographic, diffusely distributed axonal swellings were also present in the internal capsule and cervicomedullary bilateral pyramidal tracts.
Case #13
This 4 month old Vietnamese female child was brought into an emergency department by her parents. At presentation, the child was noted to have head swelling and orbital swelling, and she was lethargic. Bilateral craniotomies were performed, but she died after a 5 day protracted course. The autopsy demonstrated contusions of the extremities, scalp hemorrhage, left occipital skull fracture with epidural, subdural, and subarachnoid hemorrhage, cerebral edema with tonsilar herniation, multiple cerebral and cerebellar infarcts and hypoxic ischemic encephalopathy (HIE), retinal and vitreous hemorrhages, and optic subdural and subarachnoid hemorrhage. The death was ruled as a result of blunt force trauma to the head. The manner of death was homicide.
Histology demonstrated frequent and multifocal β APP stained axonal profiles in the frontal and temporal lobes, internal capsule, cingulate cortex white matter, and corpus callosum. Most prominent were sweeping longitudinal profiles of axons arching around the periventricular white matter. Scattered stained axons were also noted within the caudate nucleus. In the white matter of the inferior temporal lobe, there was geographic staining with a faint “zig zag” distribution. At the cervicomedullary junction, there were multifocal clusters of extensive axonal staining of the bilateral dorsal and lateral white matter.
Case #14
This 4 month old African American male child was pronounced dead on arrival after he was reported to be face down and unresponsive in bed, by his mother. The autopsy demonstrated subgaleal hemorrhage beneath the left parietal scalp, nondisplaced fracture of the parietal skull, epidural hemorrhage, and remote cortical contusions and white matter injury of the parietal lobe. The cause of death was determined to be child abuse with skull fracture, epidural hemorrhage, and brain injuries. The manner of death was homicide. A family member subsequently admitted to striking the child in the head on multiple occasions over weeks to months prior to his death.
Histology demonstrated multifocal clusters of longitudinally punctate labeled axons, similar in caliber to their unstained neighbors, within the internal capsule and scattered swollen axons within the internal capsule and corpus callosum. Less prominently, but multifocally, were scattered swollen axons within the temporal lobe and within the pyramids and dorsal medulla. The remote contusion and white matter injury seen grossly were not sampled for histology. Only rare labeled axonal swellings were noted in the parietal cortex.
Case #17
This 19 month old African American male child died 5 h after he was brought to an emergency department by his mother and the mother’s friend, with complaints of diarrhea and vomiting. The autopsy demonstrated abrasions and contusions of the face and scalp with multiple areas of subgaleal hemorrhage, contusions of the chest, hemorrhage of the superficial muscles of the back associated with cervical and thoracic epidural hemorrhage, lacerations of the liver, laceration of the vena cava with 400 cc of hemoperitoneum, laceration of the pancreas, perigastric hemorrhage, and perites ticular hemorrhage. There was no injury to the skull or gross intracranial injury. The cause of death was determined to be multiple blunt force injuries. The manner of death was homicide. Subsequent to the autopsy, the mother’s friend admitted to “stomping on the child’s stomach after he urinated on himself.”
Immunohistochemistry demonstrated no evidence of axonal injury in any of the regions surveyed.
Case #20
This 2 month old African American female was pronounced deceased on arrival, after being transported to a hospital less than an hour after her father reported her to be unresponsive. The autopsy demonstrated five separate cutaneous contusions of the submental area and left side of the face, <10 mL of combined subdural hemorrhage over the left and right cerebral convexities and beneath the left occipital lobe, evidence of asphyxia in the form of soft tissue hemorrhage and scleral hemorrhage, hemothoraces, fracture of the thoracic spine, rib fractures of varying age, liver laceration, spinal epidural hemorrhage, contusions and lacerations of the thoracic cord, and thymus hemorrhage. The cause of death was determined to be because of multiple blunt force injuries. The manner of death was homicide.
Immunohistochemical labeling demonstrated only a single swollen axonal profile within the corpus callosum.
Case #12
This 4 1/2 month old African American female was cosleeping in a queen size bed with two adult parents. Upon waking, the parents found the infant in a supine position, unresponsive. Emergency services were summoned, and they were able to resuscitate the child, who survived for 12 h. At autopsy, there was no evidence of external or internal injury. Special examination of the brain demon strated remote focal intradural hemorrhage, attributed to birth, but no other gross findings. Although the cause of death in this case was listed as SIDS, such a case with cosleeping would be considered SUDI according to our current practice (see above).
Immunohistology demonstrated multiple foci of labeled axons, in cross section and longitudinal profiles, both in groups and individually, within the corpus callosum. Rare stained axonal profiles were also noted within multiple areas of the internal capsule and the temporal and parietal white matter.
Case #15
This 2 year old male child was found, by a passerby, floating face down in a lake used by locals for swimming. Per report, the child had been in the vicinity of a pier by which a number of children were swimming. The child had been reported missing by his mother to lifeguard staff, about 20 min prior to his discovery. The person who found the child started cardiopulmonary resuscitation, and resuscitation attempts were continued by lifeguard staff and emergency staff. A pulse was restored, and although he never regained consciousness, the child survived for 21 h at a local hospital. The autopsy demonstrated multiple superficial abrasions to the skin around the eyes and upon the left side of the face, water in the sphenoid sinus, and cerebral edema with gross dusky discoloration consistent with HIE. The cause of death was drowning, and the manner was accident.
Immunohistochemistry demonstrated multifocal clustered groups and diffusely scattered labeled axonal swellings within the white matter of the temporal cortex, the internal capsule adjacent to the striatum, and within the corpus callosum. In none of these areas was the staining pattern particularly geographic or in a visible vascular pattern. Frequent labeled axonal swellings were also noted in the dorsal and lateral white matter of the cervicomedullary junction.
Discussion
The purpose of this study was to “test” some of the concepts regarding evidence of microscopic axonal injury available from multiple reports in the literature. Specifically, we wanted to test the utility of β APP labeling in the determination of the cause and manner of young pediatric deaths. Prior reports have demonstrated that β APP labeled axonal swellings are common findings in inflicted and accidental pediatric brain injury (2,3,5 8). But in the hypothetical situation that the determination of a case is equivocal with regard to the manner of death, should microscopic β APP distribution be invoked as a determinant? Most forensic pathologists may remember those one or several cases in which, although there were suspicions or focal physical findings suggestive of inflicted trauma, data from the investigation and/or the cumulative findings at autopsy were not definitive. It has been our anecdotal experience that in such cases, there is a tendency to more aggressively utilize specialist consultants, such as forensic neuropathologists, in the hope of finding that “one piece of additional information” that might clarify the case investigation.
Complicating the use of β APP stains is the well established fact that similar, but perhaps not identical, histologic evidence of axonal injury can be induced in the white matter by hypoxia ischemia (3). As we started this study, we envisioned potential “pitfalls” associated with the interpretation of β APP staining in cases in which there might be agonal apnea or HIE in a lingering injured or sick child. We speculated that microscopic findings in children who died as a result of SIDS or as a result of an unexplained “overlay,” might prove confusing, but we hoped that the pattern of staining would be useful in discerning traumatic from HIE related axonal injury. Thus, we queried the independent utility of immunohisto chemical evidence of axonal injury in discriminating traumatic injury from nontrauma.
Without any other information, axonal injury highlighted by β APP immunohistochemical labeling was sufficient to allow three independent reviewers to unanimously identify five of seven cases of inflicted trauma with associated head injuries. Two of these cases had acute and chronic injuries (Cases 11 and 14). Although most were dead upon arrival of emergency services, three of the homicide cases, one of which demonstrated no microscopic evidence of axonal injury, were alive several days after the initial injury (see Table 1). Although a relatively small group of cases for meaningful calculations of sensitivity and specificity, approximately 71.4% of the cases were correctly identified. In contrast, immunolabeling in two of 17 of the nonhomicide cases could not be differentiated from the β APP staining patterns of the homicide cases (specificity = 88.2%). The qualitative and semiquantitative patterns of axonal labeling in these 24 cases did not allow the reviewers to differentiate traumatic injury from what we can only presume to be purely hypoxic ischemic axonal injury in the two nonhomicide cases.
The two homicide cases that were not identified by immunohistochemical evidence of axonal injury alone are illustrative. The first was a child (Case #17) who had demonstrated evidence of head injury in the form of scalp and subgaleal contusions at autopsy, but no evidence of intracranial hemorrhage or gross brain injury. Rather the visible CNS injuries in this child were restricted to the spine, as a result of blunt force injuries centered on the torso. The second case was similar in that the child also had head injuries, the cause of death was because of multiple injuries, and the bulk of injuries were centered primarily in the torso. However, in contrast, this second child (Case #20) did demonstrate scant subdural hemorrhages, without other evidence of gross brain injury, at the time of autopsy. Given that subdural hemorrhage, be it on the brain or around the optic nerve, is often touted as a concerning indicator for inflicted pediatric brain injury (9), the lack of apparent axonal injury was surprising. There are several possible explanations for the discrepancy. The survival time in this infant may not have been long enough to allow for the development of axonal swellings and axonal transport dysfunction sufficient to lead to accumulation of β APP. These axonal changes take time to develop, and although they have been reported to occur as early as 35 min in adults (10), they are generally thought to occur several hours after cerebral injuries (11,12). Another possibility is that, although there was superficial head injury, it was not severe enough to cause white matter injury at least in the areas that were surveyed.
With regard to the two nontraumatic cases that could not be differentiated from those with inflicted trauma, there are also a number of useful concepts. One of these children (Case #15) experienced a near drowning and was resuscitated to survive, with ventilatory assistance, for 21 h. Information from his hospitalization and the appearance of his brain at autopsy were consistent with global HIE. In the other (Case #12), the child was cosleeping in an adult bed with his adult parents and was found unresponsive in that setting. This child was also resuscitated and survived for about 12 h, with ventilatory assistance. Several reports have detailed histologic patterns of axonal injury and attributed them to different mechanisms such as vascular compromise, metabolic disease, and trauma because of global acceleration/deceleration which are purported to account for the findings of diffuse axonal injury (5,7,8). With their descriptive categories in mind, we reviewed the cases to determine whether we could spot patterns in the homicides and atraumatic cases. Although there were focal areas of vascular injury type geographic staining (“zig zag” pattern), for the most part in all of the cases described above, β APP highlighted bundles and individual fibers as they arched through the white matter, in a pattern more typical, according to the above authors, of nonvascular axonal injury. Furthermore, when there was significant staining, the patterns were similar in traumatic and nontraumatic cases.
Six cases of pediatric death because of various natural causes did not demonstrate significant β APP staining within axonal profiles. Most brain regions in these cases were completely negative for staining, and some areas showed a single labeled axonal swelling. When present, these focal stainings were not confused with evidence of traumatic cerebral injury.
In our study, we also reviewed 10 cases that were originally determined to be because of SIDS. SIDS is, to paraphrase a general working definition, sudden natural death of an infant in which specific natural causes or environmental factors were not identified to account for the death. At least one theory regarding SIDS is that these infants may experience repeated or cataclysmic episodes of apnea which result in “sudden” death (13,14). Since 1999, from which all of these archived cases were pulled, a number of opinions have changed. SIDS has always been a “diagnosis of exclusion,” as generally understood in the paraphrased statement above, but only relatively recently (the last decade) have forensic pathologists made much consideration of the sleeping environment of these children. Cosleeping with adults and/or other large children in a small bed can result in asphyxia from “overlay,” which although sometimes reported by family members, may only be suspected by investigators. Thus currently, in the State of Maryland, cases in which there is concern for the sleeping environment which might include occupants of the bed, bedding materials, etc. the diagnosis of SUDI is usually used to differentiate such a death from what is often assumed to be an occult natural cause in cases of SIDS (15). In the 10 cases pulled from our archives for this study by searching for the SIDS diagnosis, three, upon review of the records for this study, were more consistent with our current diagnosis of SUDI. In the seven cases attributed to SIDS, β APP staining was negative or only focally positive. In one case (Case #16), there was a cluster of perivascular staining that, although noteworthy upon review, was not sufficient to cause the reviewers to consider this to be evidence of trauma. Two of the three SUDI cases also did not demonstrate significant β APP staining. As described above, one of the SUDI cases was confused with inflicted trauma, and we speculate that the robust β APP staining was because of the long postresuscitation survival time with poor brain perfusion.
A number of studies have attempted to evaluate SIDS cases for hypoxic ischemic axonal injury using β APP staining. In a 2003 reported study of nonhomicidal pediatric autopsies, Reichard et al. (7) demonstrated β APP labeling of axonal profiles, using a three tiered semiquantitative methodology, in eight cases of death that were attributed to SIDS. They reported “mild” staining in all of the cases but moderate patchy to extensive staining in three of the eight cases. On the other hand, Sawaguchi et al. (16) in 2003 reported no evidence of β APP axonal staining in their review of 26 cases of SIDS. Other reports, in which SIDS cases were reviewed as “nontraumatic controls,” demonstrated no significant β APP staining (3,17). The lack of β APP staining in our SIDS/SUDI cases, without resuscitation and significant survival time, supports the latter publications. The discrepancies amongst our findings and these various reports are likely due to differences in the immunohistochemical material and staining methods or in the reviewer interpretation of staining patterns. It is our opinion that significant β APP staining is inconsistent with the diagnosis of SIDS.
In conclusion, β APP immunohistochemical staining of brain material is a useful tool for the confirmation of brain injury. However, its application is not a panacea. Children who did not sustain head trauma but who survive resuscitation to linger on ventilator support may have extensive axonal staining that may be interpreted as false positive evidence of traumatic injury. Therefore, β APP should be used carefully as a marker of brain injury in determination of cause of death. However, having said that, the absence of staining is a useful test in ruling out traumatic injury in children without long survival times, such as in SIDS/SUDI and most sudden natural deaths.
Footnotes
Presented at the 62nd Annual Meeting of the American Academy of Forensic Sciences, February 22 27, 2010, in Seattle, WA.
References
- 1.Li L, Fowler D, Liu L, Ripple MG, Lambros Zoe, Smialek JE. Investigation of sudden infant deaths in the State of Maryland (1990 2000) Forensic Sci Int. 2005;148:85–92. doi: 10.1016/j.forsciint.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 2.Geddes JF, Hackshaw AK, Vowles GH, Nickols CD, Whitwell HL. Neuropathology of inflicted head injury in children I. Patterns of brain damage. Brain. 2001;124:1290–1298. doi: 10.1093/brain/124.7.1290. [DOI] [PubMed] [Google Scholar]
- 3.Geddes JF, Vowles GH, Hackshaw AK, Nickols CD, Scott IS, Whitwell HL. Neuropathology of inflicted head injury in children II. Microscopic brain injury in infants. Brain. 2001;124:1299–1306. doi: 10.1093/brain/124.7.1299. [DOI] [PubMed] [Google Scholar]
- 4.Adams JH, Doyle D, Ford I, Gennarelli TA, Graham DI, Mclellan DR. Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathology. 1989;15:49–59. doi: 10.1111/j.1365-2559.1989.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 5.Geddes JF, Whitwell HL, Graham DI. Traumatic axonal injury: practical issues for diagnosis in medicolegal cases. Neuropathol Appl Neurobiol. 2000;26:105–116. doi: 10.1046/j.1365-2990.2000.026002105.x. [DOI] [PubMed] [Google Scholar]
- 6.Shannon P, Smit CR, Deck J, Ang LC, Ho M, Becker L. Axonal injury and the neuropathology of shaken baby syndrome. Acta Neuropathol. 1998;95:625–631. doi: 10.1007/s004010050849. [DOI] [PubMed] [Google Scholar]
- 7.Reichard RR, White CL, III, Hladik CL, Dolinak D. Beta amyloid precursor protein staining in nonhomicidal pediatric medicolegal autopsies. J Neuropathol Exp Neurol. 2003;62:237–247. doi: 10.1093/jnen/62.3.237. [DOI] [PubMed] [Google Scholar]
- 8.Reichard RR, White CL, III, Hladik CL, Dolinak D. Beta amyloid precursor protein staining of nonaccidental central nervous system injury in pediatric autopsies. J Neurotrauma. 2003;4:347–355. doi: 10.1089/089771503765172309. [DOI] [PubMed] [Google Scholar]
- 9.Jayawant S, Rawlinson A, Gibbon F, Price J, Schulte J, Sharples P, et al. Subdural haemorrhages in infants: population based study. BMJ. 1998;317:1558–1561. doi: 10.1136/bmj.317.7172.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hortobagyi T, Wise S, Hunt N, Cary N, Djurovic V, Fegan Earl A, et al. Traumatic axonal damage in the brain can be detected using beta APP immunohistochemistry within 35 min after head injury to human adults. Neuropathol Appl Neurobiol. 2007;33:226–237. doi: 10.1111/j.1365-2990.2006.00794.x. [DOI] [PubMed] [Google Scholar]
- 11.Sherriff FE, Bridges LR, Sivaloganathan S. Early detection of axonal injury after human head trauma using immunocytochemistry for B amyloid precursor protein. Acta Neuropathol. 1994;87:55–62. doi: 10.1007/BF00386254. [DOI] [PubMed] [Google Scholar]
- 12.McKenzie KJ, McLellan DR, Gentleman SM, Mawell WL, Gennarelli TA, Graham DI. Is beta APP a marker of axonal damage in short surviving head injury? Acta Neuropathol. 1996;92:608–613. doi: 10.1007/s004010050568. [DOI] [PubMed] [Google Scholar]
- 13.Leiter JC, Bohm I. Mechanisms of pathogenesis in the sudden infant death syndrome. Respir Physiol Neurobiol. 2007;159:127–138. doi: 10.1016/j.resp.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 14.Kinney HC, Thach BT. The sudden infant death syndrome. N Engl J Med. 2009;361:795–805. doi: 10.1056/NEJMra0803836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L, Zhang Y, Zielke RH, Ping Y, Fowler D. Observations on increased accidental asphyxia deaths in infancy while cosleeping in the state of Maryland. Am J Forensic Med Pathol. 2009;30:318–321. doi: 10.1097/PAF.0b013e31819df760. [DOI] [PubMed] [Google Scholar]
- 16.Sawaguchi T, Patricia F, Kadhim H, Groswasser J, Sottiaux M, Nishida H, et al. Investigation into the correlation in SIDS victims between Alzheimer precursor protein A4 in the brainstem and sleep apnea. Early Hum Dev. 2003;75:S21–S30. doi: 10.1016/j.earlhumdev.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Gleckman AM, Bell MD, Evans RJ, Smit TW. Diffuse axonal injury in infants with nonaccidental craniocerebral trauma. Arch Pathol Lab Med. 1999;123:146–151. doi: 10.5858/1999-123-0146-DAIIIW. [DOI] [PubMed] [Google Scholar]