To the Editor:
We read with great interest the article by Pan et al,1 which appeared in the issue of August 2012 of the The American Journal of Surgical Pathology. The authors investigated the clinicopathologic features of 9 cases of Kaposi sarcoma–associated herpesvirus (KSHV)-associated large B-cell lymphomas without lymphomatous effusions in any body cavities. These lymphomas were originally termed “extracavitary KSHV-positive solid lymphoma.”2,3 Pan et al1 also reviewed additional 43 cases of extracavitary KSHV-positive solid lymphomas and compared them with 84 cases of classic primary effusion lymphoma (PEL)4 from the literature. The authors found different clinical presentations and some variations in immunophenotype between extracavitary KSHV-positive solid lymphomas and classic PEL and concluded that it is still uncertain whether it is justifiable to separate them as 2 distinct entities.1 Nevertheless, they recommended the diagnostic term “KSHV-associated large B-cell lymphoma (KSHV-LBL)” to replace many different names previously used.1
Previously, we found that the expression of a subset of genes, identified by gene expression profiling, distinguished PEL tumor cells from other HIV-related and unrelated large cell lymphomas.5 Importantly, the expression of this subset of genes was also found, by real time polymerase chain reaction and immunohistochemistry, in KSHV-positive solid lymphomas and was similar to that identified in PEL but distinct from other HIV-related and unrelated large cell lymphomas.3 Combined results suggested that KSHV-positive solid lymphoma may represent part of the spectrum of classic PEL.
In the present report, we would like to further contribute to the issue raised by Pan and colleagues by discussing new data derived from proteomic analysis of the secretome (cell conditioning media) of PEL (Gloghini et al, manuscript submitted). By applying proteomics techniques to analyze PEL secretome, we aimed at identifying putative new players in the interaction between PEL cells and microenvironmental cells and proteins that might be relevant for PEL pathogenesis. We identified secreted proteins that were shared by, or specifically found in, PEL secretomes. Among them we selected 11 proteins (Table 1) potentially related to PEL pathogenesis and cell adhesion. By immunohistochemistry we found that all these proteins were expressed in 4 cases of extracavitary KSHV-positive solid lymphomas and in several PEL cell lines and primary PEL samples tested (not shown). The profile shown in Table 1 and Figure 1 demonstrates that all the tested proteins were found to be expressed in the extracavitary KSHV-positive solid lymphomas. Almost all tumor cells were stained with a usually strong intensity. Consistent with these results, extracavitary KSHV-positive solid lymphomas show relatedness to the PEL profile in the protein expression as revealed by proteomic analysis of PEL secretome.
TABLE 1.
Extracavitary KSHV-positive Solid Lymphomas: Immunohistochemical Expression of 11 Secreted Proteins That Were Shared by, or Specifically Found in, PEL Secretomes
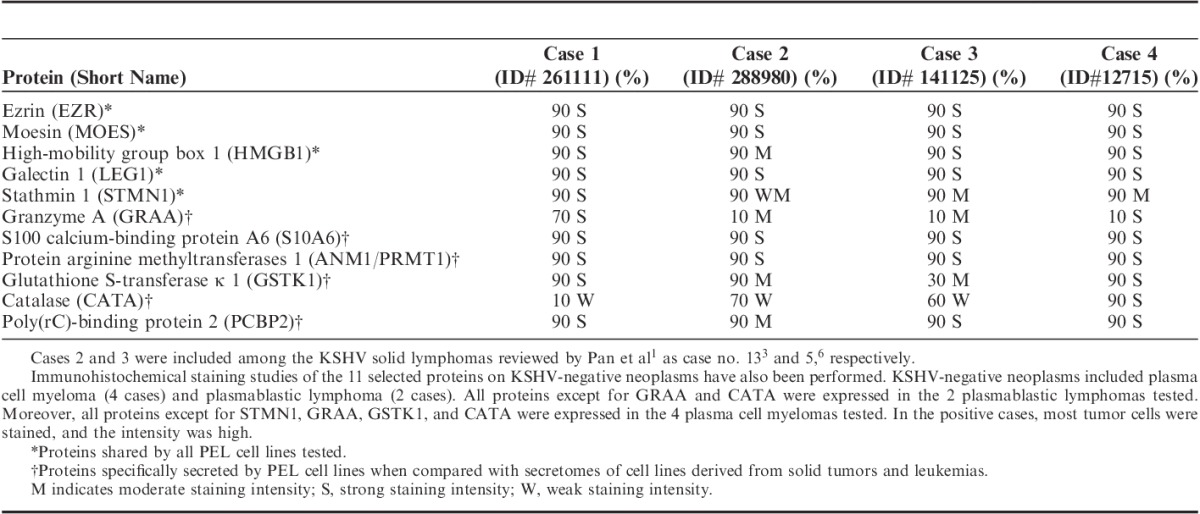
FIGURE 1.
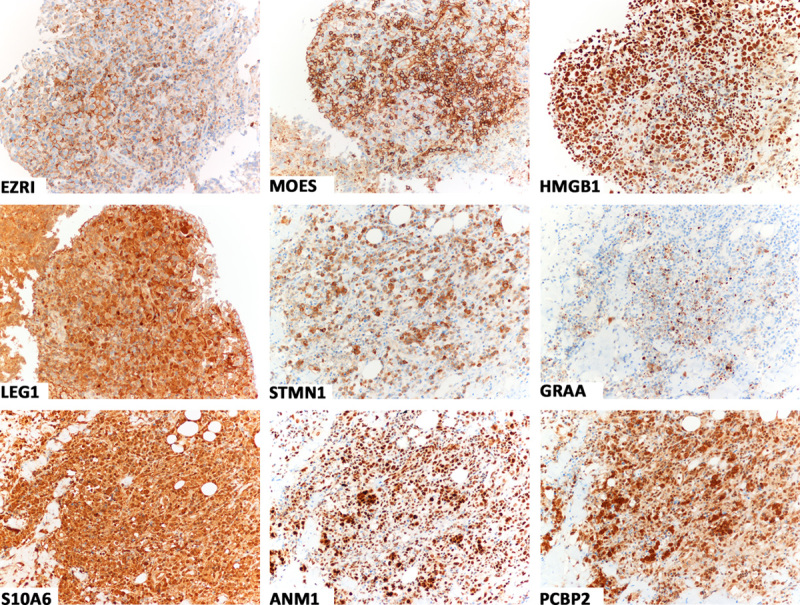
Immunostains showing tumor cell positivity for ezrin (EZRI), moesin (MOES), high-mobility group box 1 (HMGB1), galectin 1 (LEG1), and stathmin 1 (STMN1) in case 3, and granzyme A (GRAA), S100 calcium-binding protein A6 (S10A6), protein arginine methyltransferases 1 (ANM1), and poly(rC)-binding protein 2 (PCBP2) in case 2. Almost all tumor cells were stained; the intensity of staining was usually strong (immunoperoxidase, hematoxylin counterstain).
On the basis of previous gene expression profiling–derived observations3,5 and the present findings, extracavitary KSHV-positive solid lymphomas can be considered as part of a continuous spectrum of classic PEL, and the diagnostic term of “extracavitary KSHV-positive solid lymphoma” may be recommended to define this tissue-based variant of PEL.
Antonino Carbone*
Chiara C. Volpi†
Dario Caccia‡
Ambra V. Gualeni†
Anna M. Cilia*
Italia Bongarzone‡
Annunziata Gloghini†
*Department of Pathology, Centro di Riferimento Oncologico Aviano, Istituto Nazionale Tumori, IRCCS, Aviano
†Department of Diagnostic Pathology and Laboratory Medicine
‡Proteomics Laboratory, Department of Experimental Oncology and Molecular Medicine, Fondazione IRCCS Istituto Nazionale dei Tumori, Milano, Italy
Footnotes
Conflicts of Interest and Source of Funding: The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
REFERENCES
- 1.Pan ZG, Zhang QY, Lu ZB, et al. Extracavitary KSHV-associated large B-Cell lymphoma: a distinct entity or a subtype of primary effusion lymphoma? Study of 9 cases and review of an additional 43 cases.Am J Surg Pathol. 2012;36:1129–1140 [DOI] [PubMed] [Google Scholar]
- 2.Chadburn A, Hyjek E, Mathew S, et al. KSHV-positive solid lymphomas represent an extra-cavitary variant of primary effusion lymphoma.Am J Surg Pathol. 2004;28:1401–1416 [DOI] [PubMed] [Google Scholar]
- 3.Carbone A, Gloghini A, Vaccher E, et al. Kaposi’s sarcoma-associated herpesvirus/human herpesvirus type 8-positive solid lymphomas: a tissue-based variant of primary effusion lymphoma.J Mol Diagn. 2005;7:17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cesarman E, Chang Y, Moore PS, et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas.N Engl J Med. 1995;332:1186–1191 [DOI] [PubMed] [Google Scholar]
- 5.Klein U, Gloghini A, Gaidano G, et al. Gene expression profile analysis of AIDS-related primary effusion lymphoma (PEL) suggests a plasmablastic derivation and identifies PEL-specific transcripts.Blood. 2003;101:4115–4121 [DOI] [PubMed] [Google Scholar]
- 6.Carbone A, Gloghini A, Vaccher E, et al. KSHV/HHV-8 associated lymph node based lymphomas in HIV seronegative subjects. Report of two cases with anaplastic large cell morphology and plasmablastic immunophenotype.J Clin Pathol. 2005;58:1039–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]


