Abstract
Objectives
To estimate the frequency and results of downstream testing following exercise treadmill tests (ETT).
Background
The utility of additional diagnostic testing following ETT is not well characterized.
Methods
We followed consecutive individuals without known CAD referred for clinical ETT at a large medical center. We measured the frequency and results of downstream imaging tests and invasive angiography within six months of ETT, and the combined endpoint of survival free from cardiovascular death, myocardial infarction, and coronary revascularization.
Results
Among 3,345 consecutive subjects who were followed for a mean of 2.5±1.1 years, 332 (9.0%) underwent noninvasive imaging while 84 (2.3%) were referred directly to invasive angiography after ETT. The combined endpoint occurred in 76 (2.2%) patients. The annual incidence of the combined endpoint following negative, inconclusive and positive ETT was 0.2%, 1.3% and 12.4% respectively (P<0.001). Rapid recovery of ECG changes during ETT was associated with negative downstream test results and excellent prognosis while typical angina despite negative ECG was associated with positive downstream tests and adverse prognosis (P<0.001). Younger age, female gender, higher METs achieved and rapid recovery of ECG changes were predictors of negative downstream tests.
Conclusions
Among patients referred for additional testing after ETT, the lowest yield was observed among individuals with rapid recovery of ECG changes or negative ETT while the highest yield was observed among those with typical angina despite negative ECG or a positive ETT. These findings may be used to identify patients who are most and least likely to benefit from additional testing.
Keywords: exercise testing, downstream testing, imaging
Coronary artery disease (CAD) remains the leading cause of death in both men and women. While advances in cardiovascular imaging have greatly improved our ability to diagnose and treat CAD, the rising costs of noninvasive testing have generated concern regarding the potential overutilization of such testing (1).
The current AHA and ACC guidelines recommend exercise treadmill tests (ETT) in the initial evaluation for ischemic heart disease in patients who are able to exercise and have a normal ECG at baseline (2,3). In addition to testing for ischemic ECG changes, this test provides other data that have important prognostic implications such as functional capacity, arrhythmias and exercise-induced symptoms (4).
Thus, a common and economically appealing strategy is to perform ETT as an initial test and then, among patients with positive or inconclusive results, selectively use noninvasive imaging or invasive angiography to guide further care (5). While recent studies assessed patterns of downstream testing after percutaneous coronary intervention (PCI) (6,7) and utilization of invasive angiography after stress testing (8), to our knowledge, no data exist on the incidence and results of downstream noninvasive testing after ETT in patients without known CAD.
Therefore, the aim of our study was to identify the frequency and results of downstream testing following ETT and identify predictors of patients who are most and least likely to benefit from additional testing.
Methods
Population
The study population consisted of all consecutive patients who underwent clinically indicated ETT at Brigham and Women’s Hospital between January 1, 2009 and December 31, 2010. We excluded patients with known CAD, prior coronary artery bypass grafting (CABG), PCI, or myocardial infarction (MI), and patients with non-clinical indications for testing such as participation in a research protocol or post-heart transplant evaluation. The Partners Institutional Review Board approved this study.
Clinical Information
Demographics, clinical history, medications and indications for testing were collected prospectively using a standardized patient interview. The electronic medical record, which includes all physicians’ notes, was used to identify the presence or absence of the following risk factors: hypertension, diabetes, hyperlipidemia and family history of CAD. We estimated pretest probability of CAD using the Morise score (9).
Exercise Treadmill Testing
Exercise treadmill tests were performed using a symptom-limiting Bruce protocol according to established guidelines (10) as part of routine clinical care. The target heart rate was determined as 85% of the maximum predicted heart rate (MPHR, which equals 220 – age). All ST segment measurements were performed 80 ms after the J point. The Duke Treadmill Score (DTS) was calculated for each patient who completed the Bruce protocol as: exercise time (minutes) − (5 x maximal ST-segment depression in millimeter) − (4 x angina index; 0, no angina; 1, angina; 2, angina as reason for stopping test) (11).
We categorized each test result as positive, negative, or inconclusive using conventional criteria (2). Positive tests were defined as upsloping ST depressions ≥ 1.5 mm, or downsloping or horizontal depressions ≥ 1.0 mm in at least two leads. Inconclusive tests were defined to include any result that may be interpreted as indeterminate, and comprised the following categories: (i) negative ECG with reduced sensitivity due to submaximal exercise (<85% MPHR and rate pressure product < 25,000); (ii) positive ECG with reduced specificity due to baseline ECG abnormalities; (iii) positive ECG with reduced specificity due to rapid recovery of ECG changes, defined as changes which resolve within 60 seconds; (iv) typical angina or (v) inappropriate dyspnea despite negative ECG findings, and (vi) clinically significant rhythm disturbances (any sustained arrhythmia or >3 beats of ventricular tachycardia). Typical angina was defined as exertional chest discomfort that was substernal and was relieved with rest or nitroglycerin.
Downstream testing
For each patient, we identified the use of all noninvasive imaging and invasive angiography tests performed within 6 months following ETT through review of the electronic medical record. The decision to undergo further testing was at the discretion of the referring physician. We chose the 6-month cutoff to capture any downstream testing that was likely triggered by the ETT results.
Noninvasive imaging
We included all possible subsequent noninvasive imaging tests available at our institution: nuclear stress tests, stress echocardiograms, coronary computed tomography angiography (CCTA), and stress magnetic resonance imaging (MRI). All tests were performed and reported according to institutional protocols.
We categorized all nuclear stress tests (PET and SPECT) results as follows: negative (summed stress score ≤ 2); inconclusive (equivocal scan results or negative perfusion imaging with submaximal heart rate response [<85% of MPHR]); and positive for ‘abnormal’ or ‘probably abnormal’ results (12).
We categorized CCTA results as negative for reports of no plaque or stenosis ≤50%; and positive for stenosis >70% (or >50% in the left main coronary). We defined as inconclusive for the evaluation of ischemia any studies that were uninterpretable or had moderate (51–70%) stenosis, given that such lesions may not be associated with ischemia and have uncertain hemodynamic significance (13).
We categorized cardiac MRI results as negative if no ischemia was detected; inconclusive if image quality precluded interpretation and positive if ischemia was identified.
We categorized results of echocardiograms as positive, negative, or inconclusive based on the presence or absence of stress-induced wall motion abnormalities. Inconclusive tests were defined as ones in which reduced image quality limited the evaluation, or if patients failed to achieve 85% of the MPHR.
Invasive coronary angiography
We defined obstructive coronary artery disease as a stenosis greater than or equal to 50% in the left main coronary artery or greater than or equal to 70% in any other coronary vessel (14).
Patient follow-up for non-fatal MI and revascularization
We reviewed all patient charts to identify incident non-fatal myocardial infarction and coronary revascularization, which comprised all PCI and CABG procedures. MI was defined using universal criteria (15).
Patient follow-up for mortality and cause of death
We determined patients’ vital status using the Social Security Death Index, and the cause of death using chart review, autopsy findings, and hospice notes where available. If the chart lacked information to determine the cause of death, we used death certificates obtained from the Massachusetts Registry of Vital Records & Statistics. Two cardiologists blinded to ETT results adjudicated the cause of death for each patient. Deaths were considered to be of cardiovascular origin if the primary cause was acute MI, atherosclerotic coronary vascular disease, congestive heart failure, valvular heart disease, arrhythmic heart disease, stroke or sudden and unknown (16). Major adverse cardiovascular events were the combined endpoint of cardiovascular death, non-fatal MI and coronary revascularization.
Statistical analysis
Continuous variables are expressed as the mean ± standard deviation or median and interquartile range, as appropriate. Categorical variables are presented as frequencies. Differences between groups were tested using chi-square or Fisher’s exact tests for discrete variables and one-way analysis of variance (ANOVA) for continuous variables.
We constructed Kaplan-Meier curves to illustrate survival free from cardiovascular events, and tested for differences between sub-groups using the log-rank test. We built multivariable Cox proportional hazards models adjusted for age and gender. The assumption of non-proportional hazards was tested using Schoenfeld residuals and resulted in non-significant findings in all analyses. We performed a sensitivity analysis that excluded all patients without complete follow-up for all cardiovascular events.
To identify individuals in whom further testing may not be necessary, we built multivariable logistic regression models identifying predictors of negative downstream testing and event-free survival. Our model was based on the assumption that changes in patient management occur based on the identification of disease, and therefore, patients with negative test results who experienced event-free survival did not benefit from testing. Our models used all the clinical knowledge that physicians might have at hand when deciding whether or not to perform additional testing. Candidate predictor variables were selected among relevant clinical variables, as such an approach utilizing external clinical judgment has been shown to be superior to a p-value driven method (17). Model variables included age, gender, risk factors, symptoms, metabolic equivalents of task (METs) achieved and ETT results. The first model included age, gender, risk factors, and presenting symptoms. The second model added the ETT results to the first model. All tests were two-sided, and P <0.05 was considered statistically significant. Statistical analysis was performed using Stata version 12 (Statacorp, EUA).
Results
Study Population
Among 4,262 consecutive patients referred for ETT, we excluded 509 patients with prior diagnoses of CAD, 9 patients with age under 18, and 88 patients with indications other than CAD evaluation. The final study population included 3,656 patients, and 3,270 (90%) of these patients had complete follow-up for all clinical events.
Compared to those with complete follow-up, patients with incomplete follow-up achieved higher METs, had a higher Duke Treadmill Score, and were less likely to have typical angina symptoms (P<0.001 for all comparisons). Patients with incomplete follow-up for downstream clinical events (who still had complete information on downstream testing) also had a lower rate of downstream testing (6.5% vs. 11.9%, P=0.001). Additionally, 100% follow up for all-cause mortality was available. When evaluating for differences between those with complete versus incomplete follow up for cardiovascular outcomes, we found a similar annual incidence of all-cause mortality (0.53% vs. 0.49%, P=0.95).
A sensitivity analysis excluding all patients with incomplete follow-up had similar results (Appendix 1A).
Baseline Characteristics
The baseline characteristics of the patient population (age 54±13 years, 46.0% male) stratified by ETT exam results are presented in Table 1. As expected, patients with positive and inconclusive ETT results were of older age and had a higher frequency of risk factors.
Table 1.
Baseline characteristics stratified by ETT results.
| All (n=3,656) | Negative ETT (n=2,478) | Inconclusive ETT (n=1,043) | Positive ETT (n=135) | P-value | |
|---|---|---|---|---|---|
| Male | 1681 (46.0%) | 1141 (46.0%) | 471 (45.2%) | 69 (51.1%) | 0.423 |
| Age, years (standard deviation, SD) | 53 (13) | 52 (13) | 57 (12) | 61 (11) | <0.001 |
| Hypertension | 1681 (46.0%) | 1015 (41.0%) | 588 (56.4%) | 78 (57.8%) | <0.001 |
| Diabetes | 470 (12.9%) | 287 (11.6%) | 162 (15.5%) | 21 (15.6%) | 0.004 |
| Hyperlipidemia | 1537 (42.0%) | 975 (39.3%) | 494 (47.4%) | 68 (50.4%) | <0.001 |
| Current smoker | 468 (12.8%) | 267 (10.8%) | 181 (17.4%) | 20 (14.8%) | <0.001 |
| Family history of CAD | 1633 (44.7%) | 1096 (44.2%) | 470 (45.1%) | 67 (49.6%) | 0.449 |
| BMI (SD) | 28 (6) | 28 (6) | 29 (7) | 28 (6) | <0.001 |
| Morise score (SD) | 10.4 (4.5) | 9.8 (4.5) | 11.6 (4.3) | 12.6 (4.2) | 0.059 |
| Morise pre-test probability of CAD | 35% | 32% | 40% | 46% | <0.001 |
| Asymptomatic* | 214 (5.9%) | 143 (6%) | 66 (6%) | 5 (4%) | 0.202 |
| Non-anginal chest pain | 1676 (45.8%) | 1207 (48.7%) | 418 (40.1%) | 51 (37.8%) | <0.001 |
| Atypical chest pain | 297 (8.1%) | 200 (8.1%) | 80 (8%) | 17 (13%) | <0.001 |
| Typical chest pain | 241 (6.6%) | 113 (5%) | 111 (11%) | 17 (13%) | <0.001 |
| Dyspnea only | 345 (9.4%) | 206 (8.3%) | 126 (12%) | 13 (10%) | <0.001 |
| Arrhythmia or palpitations only | 440 (12.0%) | 315 (12.7%) | 116 (11.1%) | 9 (7%) | <0.001 |
| Other or unknown symptoms | 443 (12.1%) | 294 (11.9%) | 126 (12%) | 23 (17%) | <0.001 |
| Lipid-lowering therapy | 1045 (28.6%) | 627 (25.3%) | 366 (35.1%) | 52 (39%) | <0.001 |
| Aspirin | 1103 (30.2%) | 669 (27.0%) | 376 (36.0%) | 58 (43%) | <0.001 |
| β blocker | 746 (20.4%) | 300 (12.1%) | 405 (38.8%) | 41 (30%) | <0.001 |
| METs (SD) | 11 (4) | 12 (4) | 9 (3) | 10 (3) | <0.001 |
| Duke Treadmill Score (SD) | 9 (5) | 10 (4) | 6 (6) | −0.33 (5) | <0.001 |
| No symptoms during test | 2420 (66.2%) | 1831 (73.9%) | 524 (50.2%) | 65 (48%) | <0.001 |
| Typical angina during test | 147 (4%) | 0 | 113 (11%) | 34 (25%) | <0.001 |
| Atypical angina during test | 185 (5%) | 119 (5%) | 62 (6%) | 4 (3%) | 0.182 |
| Dyspnea during test | 459 (12.6%) | 193 (8%) | 238 (22.8%) | 28 (21%) | <0.001 |
| Other symptoms during test | 445 (12.2%) | 335 (13.5%) | 106 (10%) | 4 (3%) | <0.001 |
Asymptomatic individuals also included exams performed for pre-operative evaluation, pre-transplant evaluation and exercise prescription. Results are presented as mean (standard deviation) for continuous variables and frequency (percentage) for categorical variables.
The exercise treadmill tests were negative for ischemia in 2,478 (67.7%), inconclusive in 1,043 (28.5%) and positive for ischemia in 135 (3.7%) patients. The most common types of inconclusive results were submaximal exercise (56.7%), followed by rapid recovery of ECG changes (12.9%) and typical angina despite no ECG changes (9.9%) (Figure 1A). Among 318 patients found to have ST depressions, 314 (98.7%) had horizontal or downsloping ST depressions ≥ 1.0 mm, while 4 (1.3%) had upsloping ST depressions ≥ 1.5 mm. For patients with rapid recovery of ECG changes, the median time to resolution of ST changes was 32 seconds (interquartile range: 27 – 60 seconds).
Figure 1.
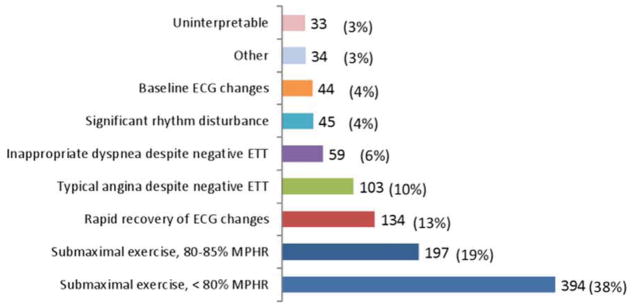
Frequency of inconclusive exercise treadmill test results (ETT) (1A). The flow diagram depicts patterns of downstream testing after ETT (1B). MPHR = maximum predicted heart rate.
Downstream Testing
Further testing was undertaken in 416 (11.4%) subjects within 6 months of ETT: 332 (9.1%) underwent noninvasive imaging while 84 (2.3%) were referred directly to invasive angiography (Figure 1B).
When evaluating the rate of all downstream testing by ETT results, 63 (3%) of individuals with negative ETT, 260 (24.9%) individuals with inconclusive ETT and 94 (70%) individuals with positive ETT underwent further testing. Notably, the 260 patients with inconclusive ETT accounted for the highest proportion (62.4%) of downstream tests. Within this group, 81 (60%) of 134 patients with rapid recovery of ECG changes underwent downstream testing compared to 41 (40%) of 103 patients with typical angina despite negative ECG.
Among patients referred for subsequent imaging, 270 (81.3%) underwent nuclear stress tests, 39 (12%) underwent stress echocardiograms, 17 (5%) underwent CCTA, and 6 (2%) underwent cardiac MRI. As only 12 patients (0.3%) received more than one noninvasive imaging test within 6 months of ETT (6 nuclear stress tests followed by CCTA, 1 nuclear stress tests followed by MRI, 5 nuclear stress tests followed by stress echo), only the first test following ETT was included in the present analysis. Overall, exercise stress testing was performed in 233 out of 315 (73.9%) imaging stress tests. However, among those who did not achieve adequate heart rate response on the initial ETT, the majority (46 out of 59, 78%) underwent pharmacological stress testing with SPECT or stress echocardiogram. Among the 12 patients who were referred for exercise testing with SPECT following a submaximal ETT, 8 (67%) received regadenoson at peak stress in order to induce maximal hyperemia and improve the diagnostic accuracy of the exam (18).
The results of all downstream tests are summarized in Table 2. Among the 26 patients with positive nuclear tests, 1 patient had a fixed defect, while all others had ischemia.
Table 2.
Yield of downstream testing after ETT.
| All (n=416, 11.4%) | Negative ETT (n=63, 3%) | Inconclusive ETT (n=260, 24.9%) | Positive ETT (n=94, 70%) | |
|---|---|---|---|---|
| Nuclear MPI | 270 (64.9%) | 40 (63%) | 184 (71%) | 47 (50%) |
| Normal | 233 (86%) | 37 (93%) | 159 (86%) | 37 (79%) |
| Inconclusive | 11 (4%) | 1 (3%) | 9 (5%) | 1 (2%) |
| Abnormal | 26 (10%) | 1 (3%) | 16 (9%) | 9 (19%) |
| Stress echocardiogram | 39 (9%) | 10 (16%) | 25 (10%) | 4 (4%) |
| Normal | 32 (82%) | 10 (100%) | 18 (72%) | 4 (100%) |
| Inconclusive* | 6 (15%) | 0 | 6 (24%) | 0 |
| Abnormal | 1 (3%) | 0 | 1 (4%) | 0 |
| Coronary CTA | 17 (4%) | 3 (5%) | 12 (5%) | 2 (2%) |
| No plaque, or stenosis < 50% | 13 (76%) | 2 (67%) | 11 (92%) | 0 |
| Inconclusive | ||||
| Uninterpretable | 1 (6%) | 0 | 0 | 1 (50%) |
| Stenosis 50–70% | 1 (6%) | 0 | 0 | 1 (50%) |
| Stenosis > 70% | 2 (12%) | 1 (33%) | 1 (8%) | 0 |
| Stress MRI (all negative) | 6 (1%) | 1 (2%) | 5 (2%) | 0 |
| Invasive Angiography | 84 (20%) | 9 (14%) | 34 (13%) | 41 (44%) |
| No obstructive CAD, no revascularization | 43 (51%) | 7 (78%) | 19 (56%) | 17 (41%) |
| Obstructive CAD, no revascularization | 4 (5%) | 0 | 3 (9%) | 1 (2%) |
| PCI | 24 (29%) | 1 (11%) | 12 (35%) | 11 (28%) |
| CABG | 13 (15%) | 1 (11%) | 0 | 12 (29%) |
all inconclusive stress echocardiogram results were due to submaximal exercise.
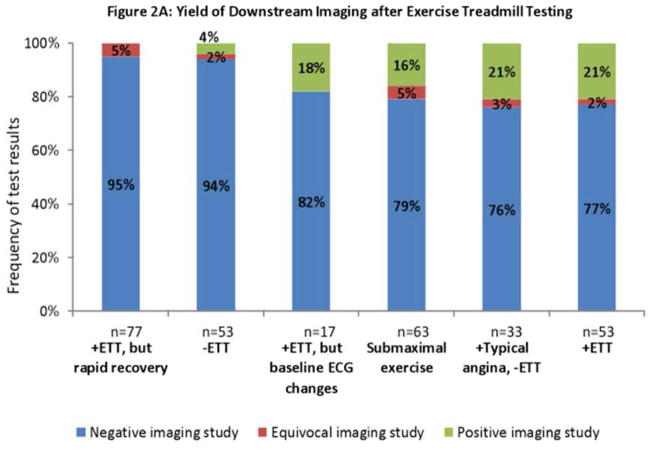
Among the different indications, the yield of downstream noninvasive imaging tests varied from 0% to 21% (Figure 2A). The lowest yield was observed among tests that demonstrated rapid recovery of ECG changes, as 0 out of 77 imaging tests were found to be positive. Notably, this was similar to the yield observed after negative ETT (2 out of 53, 4%). On the other hand, the highest yield (21%) was observed among tests that demonstrated typical angina despite no ECG changes, as 7 of 33 were found to be positive. This was similar to the positive imaging rate after positive ETT (11 of 53, 21%). Similar results were obtained when the analysis of yield of downstream testing was restricted to patients undergoing nuclear stress testing (Appendix 1B).
Figure 2.
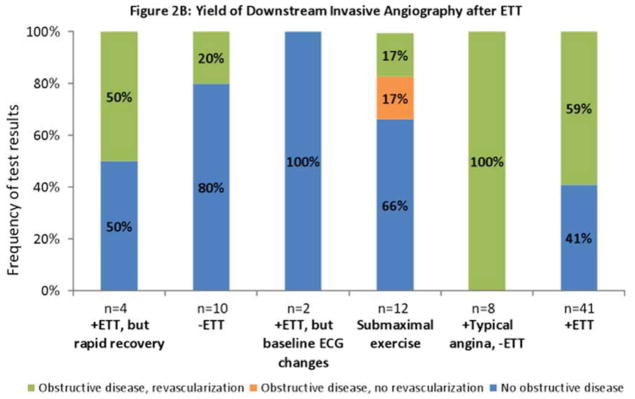
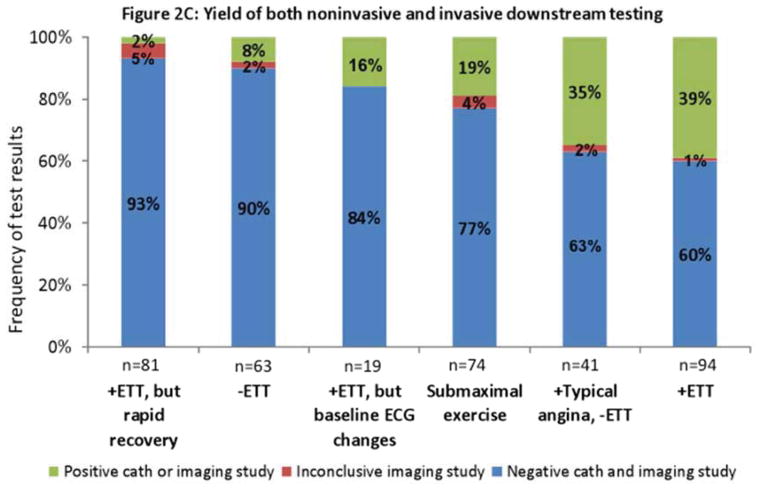
Results of downstream testing. Results of noninvasive imaging (2A), invasive angiography (2B), and all imaging (2C) obtained within six months after exercise treadmill testing (ETT) stratified by test results. The rate of positive results (yield) ranges from 0% to 21%, being lowest after rapid recovery of ECG changes; and highest after typical angina despite negative ECG or a positive ETT. 2C combines results from 2A and 2B. Obstructive disease denotes stenosis ≥ 50% in the left main coronary or ≥70% in all other coronary vessels. Positive angiography results denote either obstructive disease or coronary revascularization within six months after the most recent test. Negative angiography denotes absence of obstructive disease.
Among patients with typical angina but no ECG changes who were referred directly to invasive angiography after ETT, all 8 (100%) underwent coronary revascularization. Among those referred with rapid recovery of ECG changes, 2 of 4 (50%) patients underwent coronary revascularization (Figure 2B).
A yield similar to that of noninvasive imaging was observed when the results of both noninvasive and invasive imaging were combined (Figure 2C). The rates of positive downstream testing following negative, inconclusive and positive ETT were 8%, 14% and 39% respectively. While the overall number of patients with high risk DTS (−11 or lower) was low, when examining the yield of downstream testing in those with a low risk (5 or higher) versus intermediate risk DTS (between −10 and 4), there was no significant difference (P= 0.268). Comparing patients with positive versus negative downstream tests, a higher proportion of patients with positive downstream tests were male, older, and had a lower Duke Treadmill Score and typical angina during ETT (Appendix 1C).
Among patients with typical angina and no ECG changes, patients with positive downstream tests were more likely to be male (79% vs. 37%, P=0.012) and older (62 vs. 53 years, P=0.049) than those with negative or equivocal downstream test; however METs achieved and Duke Treadmill Score were not significantly different between these groups. Among the 7 patients who had positive imaging tests after ETTs with typical angina but no ST depressions, 1 was found to have severe stenosis (>70%) on CCTA. Among the 6 patients who had positive nuclear imaging, 3 patients had mild ischemia (summed difference score < 4), 1 patient had moderate ischemia (summed difference score 4–7), and 2 patients had severe ischemia (summed difference scores 8 or larger). One patient who had severe ischemia also had transient ischemic dilatation of the left ventricle as well as a decrease in ejection fraction from rest to stress on PET imaging. Among patients with negative downstream testing in this group (n=25) there were no high risk features such as transient ischemic dilatation of the left ventricle or a decrease in ejection fraction from rest to peak-stress.
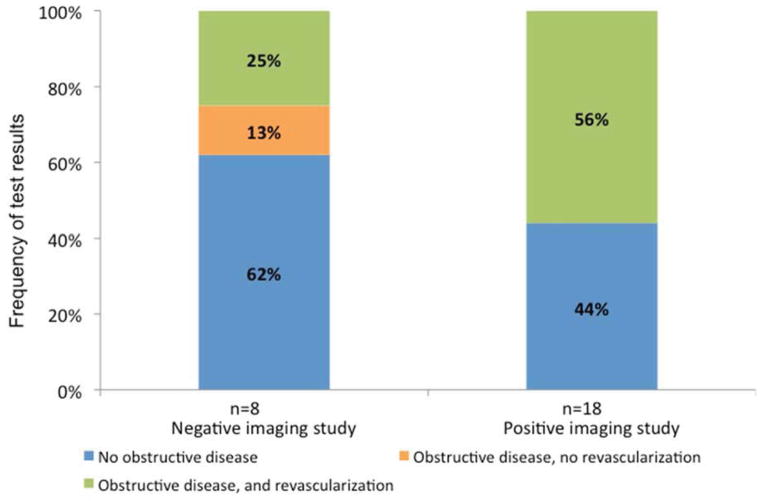
Figure 3 depicts the yield of invasive angiography following imaging tests that followed ETT. Subsequent revascularization was performed in 10 of 17 (59%) of patients with positive imaging, and 2 of 8 (25%) of patients with negative imaging.
Figure 3.
The results of invasive angiography in patients who underwent imaging after exercise treadmill testing. The figure includes all invasive angiography studies that occurred within 6 months after imaging. The yield of invasive angiography was higher after positive imaging (56%) than after negative imaging (25%). Obstructive disease denotes stenosis ≥ 50% in the left main coronary or ≥70% in all other coronary vessels.
Outcomes
During the 8,282 person-years of follow-up (median 2.7 years, interquartile range 2.1 to 3.3 years), 47 (1.3%) patients died; 9 (0.25%) due to cardiovascular causes, 11 (0.3%) patients had MI, and 69 (2%) patients required coronary revascularization. Overall, 76 (2.2%) patients experienced the combined endpoint of major adverse cardiovascular events. The annual incidence of the combined endpoint following negative, inconclusive and positive ETT was 0.2%, 1.3% and 12.4% respectively (P<0.001). Among patients with rapid recovery of ECG changes, the annual incidence was 0.7% while among those with typical angina but negative ECG the incidence was 7.4%.
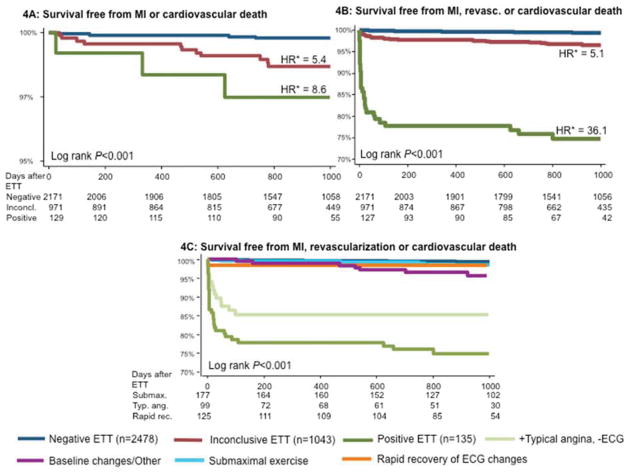
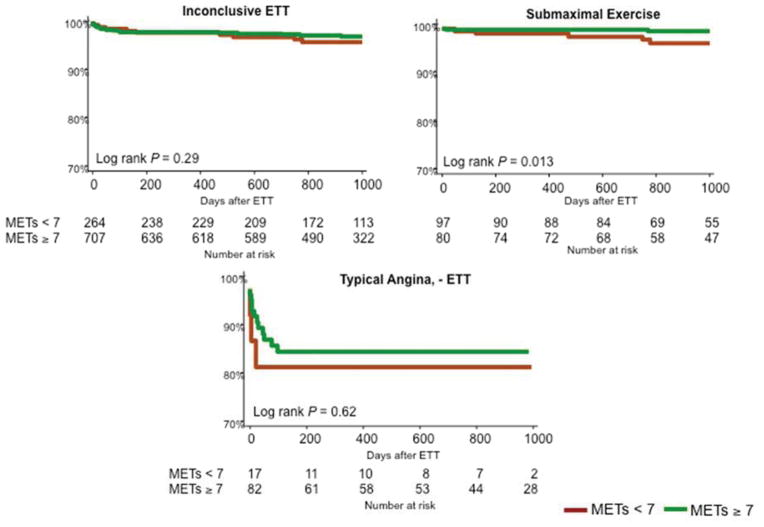
Figure 4 shows unadjusted Kaplan-Meier cumulative event curves for non-fatal MI or cardiovascular death (4A), and non-fatal MI, revascularization or cardiovascular death (4B, 4C) according to ETT results. Patients with inconclusive ETT had a lower survival free from cardiovascular death or MI than those with a negative ETT (P<0.001, Figure 4A). When specifically examining patients with inconclusive ETT, rapid recovery of ECG changes was associated with favorable prognosis, while typical angina despite negative ECG findings was associated with adverse prognosis (Figure 4C). When further stratifying the most common inconclusive groups by functional capacity, a higher exercise capacity was associated with a better prognosis only for the group with submaximal exercise (P=0.013, Figure 5).
Figure 4.
Kaplan-Meier Curves examining outcomes according to exercise treadmill testing (ETT) results. Panel A shows survival free from myocardial infarction (MI) or cardiovascular death (CVD). The rates of MI or CVD were higher among patients with inconclusive ETT (hazard ratio, (HR) 5.4, P=0.004) and positive ETT (HR 8.6, P=0.006) than among those with negative ETT. Panels C and D show survival free from the combined endpoint of MI, CVD, or coronary revascularization. The rates of the combined endpoint were higher among patients with inconclusive ETT (HR 5.1, P<0.001) and positive ETT (HR 36.1, P<0.001). Patients with typical angina despite negative ECG had a higher incidence of the combined endpoint than other types of inconclusive ETT. *Hazard ratios were derived from Cox proportional hazards models adjusted for age and gender. HR = hazard ratio.
Figure 5.
Kaplan-Meier curves describing survival free from the combined endpoint of myocardial infarction, revascularization or cardiovascular death for inconclusive exercise treadmill tests (ETT) stratified by functional capacity as measured by metabolic equivalents of task (METs) achieved during ETT (4). While there are no differences in event-free survival by functional capacity in the inconclusive ETT and typical angina groups, among patients with submaximal exercise, patients who achieved ≥ 7 METs had a lower event rate (P=0.013 by the log-rank test).
Individuals less likely to benefit from additional testing
Table 3 shows the results of multivariable models predicting negative downstream tests and no downstream events after ETT. Younger age (P<0.001), female gender (P<0.001), higher METs (P=0.037) and rapid recovery of ECG changes (P=0.013) were found to be independent predictors of negative downstream tests and event-free survival.
Table 3.
Predictors of negative downstream tests and no events
| Univariate predictors (CI) | P-value | Multivariable model 1 | Multivariable model 2 | |||
|---|---|---|---|---|---|---|
| Fit statistic | P-value | Fit statistic | P-value | |||
| Likelihood ratio χ2 | – | 61.49 | <0.001 | 80.67 | 0.002* | |
| ROC | – | 0.7149 | – | 0.7451 | 0.018* | |
| AIC | – | 560.26 | – | 551.07 | – | |
| Odds Ratio (CI) | Odds Ratio (CI) | |||||
| Age (per 10 years) | 0.67 (0.56 – 0.78) | <0.001 | 0.65 (0.54 – 0.78) | <0.001 | 0.72 (0.60 – 0.88) | 0.001 |
| Female gender | 2.69 (1.81 – 4.01) | <0.001 | 2.89 (1.89 – 4.44) | <0.001 | 3.17 (2.00 – 5.01) | <0.001 |
| ≤ 1 risk factors† | 1.00 | reference | 1.00 | reference | 1.00 | reference |
| ≥ 2 risk factors† | 0.66 (0.42 – 1.01) | 0.0564 | 0.59 (0.37 – 0.95) | 0.030 | 0.65 (0.40 – 1.06) | 0.087 |
| Presenting symptoms | ||||||
| None | 1.00 | reference | 1.00 | reference | 1.00 | reference |
| Non-anginal chest pain | 1.16 (0.74 – 1.81) | 0.527 | 0.83 (0.51 – 1.35) | 0.442 | 0.89 (0.54 – 1.48) | 0.656 |
| Atypical angina | 0.65 (0.33 – 1.28) | 0.216 | 0.52 (0.25 – 1.07) | 0.079 | 0.62 (0.29 – 1.31) | 0.209 |
| Typical angina | 0.53 (0.29 – 0.94) | 0.029 | 0.40 (0.21 – 0.76) | 0.005 | 0.50 (0.25 – 1.01) | 0.052 |
| ETT results | ||||||
| METs achieved (per METs) | 1.08 (1.03 – 1.15) | 0.005 | – | – | 1.09 (1.01–1.17) | 0.037 |
| Negative ETT | 1.00 | reference | – | – | 1.00 | reference |
| Typical Angina, -ECG | 0.73 (0.35 – 1.52) | 0.401 | – | – | 0.95 (0.40 – 2.26) | 0.911 |
| Rapid Recovery of ECG changes | 3.64 (1.62 – 8.19) | 0.002 | – | – | 2.67 (1.15 – 6.22) | 0.013 |
| Inconclusive for other reasons | 1.06 (0.62 – 1.81) | 0.832 | – | – | 1.32 (0.71 – 2.45) | 0.380 |
| Positive ETT | 0.59 (0.33 – 1.06) | 0.076 | – | – | 0.64 (0.34 – 1.21) | 0.173 |
Comparing models 1 and 2;
Risk factors included hypertension, diabetes, dyslipidemia, current smoking, family history of premature CAD.
CI = confidence intervals
Discussion
In this study, we examined patterns of downstream testing and outcomes in a cohort of 3,656 patients without prior CAD referred for exercise treadmill testing. Although we found an overall low rate of referral for downstream testing (11%) – consistent with the high prevalence of negative ETT results – there was a substantially higher rate of referral for additional testing among patients with positive (70%) or inconclusive (25%) test results. Moreover, we found that 28% of the patients in our study had inconclusive ETT results, a group which despite a favorable prognosis accounted for 63% of all the downstream tests. In our analysis of inconclusive results, we identified that patients who had rapid recovery of ECG changes had an excellent prognosis and a low yield of additional testing while patients who had typical angina despite negative ECG results had a worse prognosis and higher yield of downstream testing.
An important finding of our study is that patients with typical angina but negative ETT represent a relatively higher risk cohort, and one in which the yield of additional testing is relatively higher and comparable to the yield after positive ETT. These results are particularly important in light of a recent study by Cheng et al (19), which suggested that the prevalence of obstructive CAD by CCTA is vastly overestimated in patients with typical angina. However, the study by Cheng et al utilized patient-reported symptoms, while, in our study, physicians and exercise physiologists identified symptoms at the time of testing. Our results suggest that patients who develop typical angina during ETT, even in the presence of a good exercise capacity, warrant further evaluation. Similar to our findings, Bairey et al (20) showed that among 190 patients with typical angina but negative ETT, an abnormal thallium myocardial perfusion study was found in 24% and that such testing provided significant prognostic value.
Another noteworthy finding from our study is that patients with rapid recovery of ECG changes had a low yield on subsequent imaging and few adverse outcomes. While it is recognized that ST depressions that resolve rapidly have a lower specificity for detecting obstructive CAD (21), to our knowledge, no prior studies have investigated the yield of imaging and prognosis of such patients. In our study, 81 (60%) of the 134 patients who had positive ECG changes with rapid recovery underwent downstream testing. Among the 77 patients referred for noninvasive imaging, there were no positive results and no subsequent deaths or myocardial infarctions. Two out of 4 patients, who, per treating physician discretion, were referred directly to coronary angiography, underwent coronary revascularization procedures. One of these patients achieved 10.1 METs and had typical angina with 2.0 mm horizontal ST depressions in leads V3–V6 which resolved within 1 minute into recovery, while the second patient achieved 7.8 METs and had dyspnea on exertion with 1.0 mm horizontal ST depressions in leads V5–V6 which resolved 30 seconds into recovery.
Given concerns regarding overutilization of imaging tests, a key objective of our study was to identify individuals who can safely avoid further testing after ETT. As is consistent with established risk factors for obstructive CAD, a younger age, female gender and higher exercise capacity during ETT were all independently associated with negative downstream testing and event-free survival. In addition, rapid recovery of ECG changes was independently associated with negative downstream testing and event-free survival.
Patel et al (22) have demonstrated a low yield of identification of obstructive CAD in noninvasive testing compared to invasive angiography, however, their study included several types of tests (including rest-only echocardiogram and ECG). In our study, among patients referred for invasive angiography after a positive imaging study, the yield was 59% (Figure 3), higher than the 41% observed by Patel et al. However, it should be recognized that discordant findings are not unexpected when tests that evaluate ischemia are compared to an anatomical reference standard (13).
Our findings are similar to Mudrick et al (8) who followed 80,676 patients who underwent stress testing (54% nuclear; 25% ETT; 21% stress echocardiography): within 60 days after testing, the incidence of invasive angiography among ETT patients was 6.9% while the incidence of coronary revascularization was 2.3%. Interestingly, these rates were higher than those observed in our study. This could be explained by increased use of noninvasive imaging in our population, although Mudrick et al, who utilized an administrative database, did not report data on the use of noninvasive testing.
Consistent with prior studies (8), the rates of positive ETT and adverse events observed in our study were low, in keeping with the fact that patients referred for ETT alone are generally lower risk that those who are referred for noninvasive imaging. Furthermore, we excluded patients with CAD. These results highlight the challenges of using diagnostic testing to mitigate the risk of events in an already low-risk population. For instance, approximately 40% of patients with a positive ETT had a positive downstream test, reflecting the low specificity of ECG changes for predicting abnormal imaging or angiography. Nevertheless, despite the overall low event rate of our cohort, we were able to identify subgroups of patients (e.g. typical angina) in which additional testing provided clinically important data as well as sub-groups (e.g. rapid recovery of ECG changes) in which excessive testing was done despite of an event rate that was lower than that observed among individuals who had negative ETT testing.
When selecting an imaging test following equivocal ETT results, the reason for the inconclusive ETT results should be considered. For instance, among patients who are unable to achieve their target heart rate with exercise, future testing should employ pharmacological stress testing with either vasodilators or dobutamine (23). In our study, some patients were referred for exercise SPECT despite a suboptimal heart rate response on ETT and were administered regadenoson at peak exercise in order to induce maximal hyperemic response, and thus improve the diagnostic accuracy while reducing the likelihood of inconclusive results (18).
Limitations
Given the observational single-center design of our study, our results may be less applicable to other institutions. For instance, centers likely vary by how they interpret and report ETT findings as well as which testing options are available or used when further testing is obtained. However, our primary aim was to identify the frequency of downstream testing following ETT and identify predictors of patients who are most and least likely to benefit from additional testing. While the pattern of downstream utilization of imaging modalities differs across centers, we would not expect our findings to change even if different modalities of downstream testing were obtained. Nevertheless, our center is well suited for this study given that: (a) ETT is commonly used as the first test to evaluate patients with no CAD, and (b) we have an integrated multi-modality noninvasive lab with a detailed database which includes all patient characteristics as well as all noninvasive and invasive testing performed. While we assumed that any testing within 6 months of the ETT exam was attributable to the test results, it is possible that some downstream testing occurred because of changes in clinical status.
Certain assumptions are implied by our analysis. We defined a ‘benefit’ (e.g. yield) of a downstream test as a positive result, as we assumed that positive test results are more likely to impact patient management. However, our definition of yield might discount the value of reassurance provided by negative test results. Moreover, since not all patients in our cohort underwent secondary testing, referral bias may influence the frequency of positive downstream test results across some of the sub-groups of patients referred for invasive angiography or non-invasive testing. However, our aim was to observe the yield in actual clinical practice, as such results may be more applicable to patients for whom physicians are contemplating performing additional testing on.
We defined inconclusive ETT results broadly in order to compare different sub-groups. As a result, the rate of inconclusive studies may be higher than would be expected if a more conservative definition were used. However, our rate of inconclusive studies (28%) was similar to that reported by others (3,24). Moreover, our findings regarding the yield and prognosis associated with various ETT findings would not be expected to change even if some of the inconclusive sub-groups listed in Figure 1 would be reclassified as positive or negative. Nevertheless, several of the subgroups of patients with inconclusive ETT results were small, thus limiting our power to detect differences in outcomes. Finally, our findings at this time represent only medium-term follow-up; and it is thus possible that longer term follow-up would identify more events.
Clinical Implications
To our knowledge, this is the first study assessing downstream non-invasive testing following ETT among patients without CAD. In recognition of the increased concerns regarding overutilization of cardiovascular imaging, it is essential to develop effective patient-centered algorithms to identify the best initial testing option for a given patient. Given the overall good prognosis as well as low need for downstream testing, our results suggest that ETT represents a reasonable initial testing option for many patients without prior CAD who are able to exercise and have a normal baseline ECG. Furthermore, we identify that in the absence of any high-risk features, patients with rapid recovery of ECG changes do not benefit from additional diagnostic testing. On the other hand, we found that those who develop typical angina have an absolutely low but relatively higher yield of downstream testing, suggesting some of these patients might warrant further evaluation and/or treatment. Our findings could be used to reduce the number of inconclusive results and downstream testing by more definitely reclassifying ETT results as positive or negative. In establishing the optimal threshold for any diagnostic test, there is a trade-off between sensitivity and specificity. Our findings imply that when it comes to patients with rapid recovery of ECG changes, the potential gain in sensitivity is extremely small, and is likely unjustified.
Supplementary Material
Acknowledgments
This study was supported in part by the National Heart Lung and Blood Institute of the National Institutes of Health, Bethesda, Maryland under award number K23HL092299.
We acknowledge the outstanding dedication of the exercise physiologists and imaging technologists at Brigham and Women’s Hospital.
Abbreviations
- ETT
exercise treadmill test
- MI
myocardial infarction
- METs
metabolic equivalents of task
- CAD
coronary artery disease
- PCI
percutaneous coronary intervention
- CABG
coronary artery bypass grafting
- MPHR
maximum predicted heart rate
- DTS
Duke treadmill score
- CCTA
coronary computed tomography angiography
- MRI
magnetic resonance imaging
- PET
positron emission tomography
- SPECT
single-photon emission computed tomography
Footnotes
No conflicts of interest or relationships with industry exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Iglehart JK. The new era of medical imaging--progress and pitfalls. The New England journal of medicine. 2006;354:2822–8. doi: 10.1056/NEJMhpr061219. [DOI] [PubMed] [Google Scholar]
- 2.Gibbons RJ, Balady GJ, Bricker JT, et al. ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines) Circulation. 2002;106:1883–92. doi: 10.1161/01.cir.0000034670.06526.15. [DOI] [PubMed] [Google Scholar]
- 3.Amsterdam EA, Kirk JD, Bluemke DA, et al. Testing of low-risk patients presenting to the emergency department with chest pain: a scientific statement from the American Heart Association. Circulation. 2010;122:1756–76. doi: 10.1161/CIR.0b013e3181ec61df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourque JM, Holland BH, Watson DD, Beller GA. Achieving an exercise workload of > or = 10 metabolic equivalents predicts a very low risk of inducible ischemia: does myocardial perfusion imaging have a role? J Am Coll Cardiol. 2009;54:538–45. doi: 10.1016/j.jacc.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hendel RC, Berman DS, Di Carli MF, et al. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 appropriate use criteria for cardiac radionuclide imaging: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine. Circulation. 2009;119:e561–87. doi: 10.1161/CIRCULATIONAHA.109.192519. [DOI] [PubMed] [Google Scholar]
- 6.Mudrick DW, Shah BR, McCoy LA, et al. Patterns of stress testing and diagnostic catheterization after coronary stenting in 250 350 medicare beneficiaries. Circulation Cardiovascular imaging. 2013;6:11–9. doi: 10.1161/CIRCIMAGING.112.974121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah BR, Cowper PA, O’Brien SM, et al. Patterns of cardiac stress testing after revascularization in community practice. J Am Coll Cardiol. 2010;56:1328–34. doi: 10.1016/j.jacc.2010.03.093. [DOI] [PubMed] [Google Scholar]
- 8.Mudrick DW, Cowper PA, Shah BR, et al. Downstream procedures and outcomes after stress testing for chest pain without known coronary artery disease in the United States. American heart journal. 2012;163:454–61. doi: 10.1016/j.ahj.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morise A, Evans M, Jalisi F, Shetty R, Stauffer M. A pretest prognostic score to assess patients undergoing exercise or pharmacological stress testing. Heart. 2007;93:200–4. doi: 10.1136/hrt.2006.093799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibbons RJ, Balady GJ, Bricker JT, et al. ACC/AHA 2002 guideline update for exercise testing: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines) J Am Coll Cardiol. 2002;40:1531–40. doi: 10.1016/s0735-1097(02)02164-2. [DOI] [PubMed] [Google Scholar]
- 11.Shaw LJ, Peterson ED, Shaw LK, et al. Use of a prognostic treadmill score in identifying diagnostic coronary disease subgroups. Circulation. 1998;98:1622–30. doi: 10.1161/01.cir.98.16.1622. [DOI] [PubMed] [Google Scholar]
- 12.Abidov A, Hachamovitch R, Hayes SW, et al. Are shades of gray prognostically useful in reporting myocardial perfusion single-photon emission computed tomography? Circulation Cardiovascular imaging. 2009;2:290–8. doi: 10.1161/CIRCIMAGING.108.815811. [DOI] [PubMed] [Google Scholar]
- 13.Blankstein R, Di Carli MF. Integration of coronary anatomy and myocardial perfusion imaging. Nature reviews Cardiology. 2010;7:226–36. doi: 10.1038/nrcardio.2010.15. [DOI] [PubMed] [Google Scholar]
- 14.Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for ercutaneous Coronary Intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:2574–609. doi: 10.1161/CIR.0b013e31823a5596. [DOI] [PubMed] [Google Scholar]
- 15.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–35. doi: 10.1161/CIR.0b013e31826e1058. [DOI] [PubMed] [Google Scholar]
- 16.Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–51. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 17.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. American journal of epidemiology. 1989;129:125–37. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 18.Partington SL, Lanka V, Hainer J, et al. Safety and feasibility of regadenoson use for suboptimal heart rate response during symptom-limited standard Bruce exercise stress test. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2012;19:970–8. doi: 10.1007/s12350-012-9562-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng VY, Berman DS, Rozanski A, et al. Performance of the traditional age, sex, and angina typicality-based approach for estimating pretest probability of angiographically significant coronary artery disease in patients undergoing coronary computed tomographic angiography: results from the multinational coronary CT angiography evaluation for clinical outcomes: an international multicenter registry (CONFIRM) Circulation. 2011;124:2423–32. 1–8. doi: 10.1161/CIRCULATIONAHA.111.039255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bairey CN, Rozanski A, Maddahi J, Resser KJ, Berman DS. EXERCISE TL-201 SCINTIGRAPHY AND PROGNOSIS IN TYPICAL ANGINA-PECTORIS AND NEGATIVE EXERCISE ELECTROCARDIOGRAPHY. American Journal of Cardiology. 1989;64:282–287. doi: 10.1016/0002-9149(89)90520-1. [DOI] [PubMed] [Google Scholar]
- 21.Sakuragi S, Takaki H, Taguchi A, et al. Diagnostic value of the recovery time-course of ST slope on exercise ECG in discriminating false-from true-positive ST-Segment depressions. Circ J. 2004;68:915–922. doi: 10.1253/circj.68.915. [DOI] [PubMed] [Google Scholar]
- 22.Patel MR, Peterson ED, Dai D, et al. Low diagnostic yield of elective coronary angiography. The New England journal of medicine. 2010;362:886–95. doi: 10.1056/NEJMoa0907272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blankstein R, Devore AD. Selecting a noninvasive imaging study after an inconclusive exercise test. Circulation. 2010;122:1514–8. doi: 10.1161/CIRCULATIONAHA.109.903351. [DOI] [PubMed] [Google Scholar]
- 24.Diercks DB, Gibler WB, Liu T, Sayre MR, Storrow AB. Identification of patients at risk by graded exercise testing in an emergency department chest pain center. The American journal of cardiology. 2000;86:289–92. doi: 10.1016/s0002-9149(00)00916-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.