Abstract
OBJECTIVE
Airborne endotoxin exposure has both adverse and protective health effects. Studies show males have augmented acute inflammatory responses to endotoxin. In this longitudinal cohort study we investigated the effect of long-term exposure to endotoxin in cotton dust on health, and determined whether these effects differ by gender.
METHODS
In the Shanghai Textile Worker Study, 447 cotton and 472 control silk textile workers were followed from 1981 to 2011 with repeated measures of occupational endotoxin exposure, spirometry, and health questionnaires. Impaired lung function was defined as a decline in forced expiratory volume in one second to less than the 5th percentile of population predicted. Death was ascertained by death registries. We used Cox proportional hazards models to assess the effect of endotoxin exposure on the time to development of impaired lung function and death.
RESULTS
128 deaths and 164 diagnoses of impaired lung function were ascertained between 1981 and 2011. Hazard ratios (HRs) for the composite endpoint of impaired lung function or death was 1.47 (95% CI 1.09-1.97) for cotton vs. silk workers and 1.04 (95% CI 1.01-1.07) per 10,000 endotoxin units (EU)/m3-years increase in exposure. HRs for all-cause mortality was 1.36 (95% CI 0.93-1.99) for cotton vs. silk workers and 1.04 (95% CI 0.99-1.08) per 10,000 EU/m3-years. The risk associated with occupational endotoxin exposure was elevated only in men.
CONCLUSIONS
Occupational endotoxin exposure is associated with an increase in the risk of impaired lung function and all-cause mortality in men.
Keywords: endotoxin, organic dust, gender, lung function, mortality
INTRODUCTION
Endotoxin, a component of the cell wall in Gram-negative bacteria, causes profound inflammatory responses in humans. Endotoxin is ubiquitous in the environment and is present at high concentrations in urban schools,1 homes burning biomass fuel,2 tobacco smoke,3 and a number of occupational settings, particularly cotton textile work.4 Endotoxin is an unusual exposure in that it is protective against childhood development of asthma5 and adult development of lung cancer,6 but harmful in terms of asthma development and morbidity with later life exposures,7 malignancies,8 cardiovascular disease,9 heart failure,10 and hepatic disease.11
There remains debate about whether chronic exposure to the endotoxin component of organic dust ultimately leads to abnormal lung function12 in a healthy working population with often better than predicted lung function. The effects of cotton dust have been best studied in the context of respiratory effects, where an asthma-like syndrome called byssinosis was first described,13 with later studies demonstrating an accelerated decline in lung function.14 While early longitudinal studies in cotton textile workers suggested that men had a greater loss of lung function compared to women,15,16 many of these studies have been limited by the healthy worker survivor effect,17,18 incomplete exposure assessment, loss to follow-up exceeding 50% of the original cohort, relatively short duration of follow-up, and most importantly, inadequate control of smoking as a confounder. Thus it remains unclear, for example, whether any observed adverse pulmonary effects or differential gender effects were due to residual confounding from smoking. Additionally, there did not seem to be a clear biologic basis to suspect that gender differences would exist in the context of cotton dust exposure. It must be noted that many of these longitudinal studies focused on dust and not endotoxin as the exposure of interest. As cotton from various regions or growing conditions can contain different amounts of endotoxin, gravimetric measurements of dust exposure cannot always serve as a proxy for endotoxin exposure.19
In recent years, however, studies performed in both animal models and experimental human studies show an augmented inflammatory response to endotoxin in males compared to females.20-22 Most studies of endotoxin exposure are performed in the acute setting, and it remains unknown whether these gender differences persist in the chronic setting where repeated high level exposure occurs.
The Shanghai Textile Worker Study provides a unique opportunity to study the associations between gender, occupational endotoxin exposure, and time to development of impaired lung function or death. In this cohort study, cotton and silk textile workers have been prospectively followed with repeated measurement of occupational endotoxin, smoking habits, and lung function every five years, with a 74% follow-up rate at 30 years. Cotton textile work is associated with extremely high levels of airborne endotoxin because as cotton plants grow and the boll opens, they become increasingly colonized by gram negative bacteria, leading to one of the highest endotoxin levels measured in any setting.4 In contrast, silkworm cocoons are boiled to prevent the larvae from emerging and destroying the silk fibers as well as to facilitate unraveling, and so the boiled cocoons have minimal bacterial contamination and minimal endotoxin (endotoxin is water soluble). The raw material provided to silk textile mills in this study was raw spooled silk, while the raw material provided to the cotton textile mills were ginned cotton bales heavily contaminated with endotoxin. Thus this natural experiment allowed us to assess the time to development of impaired lung function and death in relation to occupational endotoxin exposure in the Shanghai Textile Worker Study, and furthermore to determine whether the effects of exposure differ by gender.
METHODS
Study population
Between October and December 1981, workers in the yarn preparation areas of two cotton textile mills and a silk thread processing mill in the same industrial sector in Shanghai, China were enrolled in the Shanghai Textile Worker Study. Workers were included if they had at least two years of work in the mill (so subjects were prevalent hires), and excluded if they had a history of respiratory disease diagnosed prior to entry into the textile workforce. Surveys were performed at baseline and in 1986, 1992, 1996, 2001, 2006, and 2011, with eligibility for retesting defined as membership in the original cohort. There was no crossover between cotton and silk textile workers. At each survey, trained interviewers administered a modified version of the American Thoracic Society standardized respiratory symptom questionnaire,23 height and weight was recorded, and pulmonary function tests were performed. Pulmonary function tests were performed before subjects entered the work area on the first day of work after a two-day rest in active workers, or at study follow-up in retired workers. Forced expiratory maneuvers with up to 7 trials to produce three acceptable curves were performed under the direction of a trained technician on a calibrated 8L water-sealed field spirometer (W.E. Collins Co., Braintree, MA) according to American Thoracic Society guidelines.24 Spirometric curves were read manually by the same trained expert with all values corrected to conditions of body temperature and pressure saturated with water vapor; spirometry that did not meet ATS criteria despite 7 trials were excluded from the analysis. The highest values for forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) were used given that they were technically acceptable tests. All spirometry was performed without the administration of a bronchodilator.
Exposure assessment
Throughout the study, personal samplers were not available for cotton dust measurements and thus full-shift measurements of airborne cotton dust were performed at each survey with stationary vertical elutriators (General Metalworks Corp., Mequon, WI, USA) according to guidelines set by the National Institute for Occupational Safety and Health. Endotoxin from collected dust was measured in a single laboratory using the chromogenic Limulus amoebocyte lysate assay (Kinetic-QCL, BioWhittaker, Walkersville, MD, USA) as previously described.25 Exposure measurements collected in the first survey were used to estimate pre-1981 levels. A limited number of full-shift samples were also taken in the silk mill; endotoxin levels were below the limit of detection and thus silk workers were considered unexposed to endotoxin. A detailed work history was also obtained from each worker at each survey which included records dating back to the beginning of employment. Endotoxin exposure for each subject was calculated using geometric mean levels of endotoxin exposure multiplied by years of work in each work area, resulting in a cumulative index of exposure (in endotoxin units/meters3-years or EU/m3-yrs), with an interpretation analogous to that of pack-years for smoking.
Assessment of impaired lung function and mortality
Impaired lung function was defined as a decline in forced expiratory volume at 1 second (FEV1) to the fifth percentile of the population predicted based on age and gender adjusted prediction equations derived from a healthy Chinese population.26 Death was determined through 2011 based on records of the Shanghai Textile Industry Bureau and the death registry in Shanghai.
Analysis
Impaired lung function and death are semi-competing risks; a worker diagnosed with impaired lung function can subsequently die, whereas a worker who dies cannot subsequently be diagnosed with impaired lung function.27 To address the issue of semi-competing risks, we used a composite outcome as our primary outcome,28,29 where an event was defined as the earlier of either low lung function or death. The biological motivation for the use of a composite outcome is as follows. Large population based studies have demonstrated that low FEV1 is a predictor of death from ischemic heart disease, stroke, and cancer and not just of death from respiratory causes.30-34 Furthermore, endotoxin exposure has been associated with pleiotropic effects on health. 6-11 Thus from a biological perspective, both low lung function and death are important clinical events and it is biologically plausible that the effect of endotoxin on both low lung function and death are in the same direction. From a statistical standpoint, the use of a composite endpoint is also appropriate as it has been demonstrated that when the exposure is associated with both outcomes in the same direction, using a composite endpoint increases statistical power.29
We used Cox proportional hazards models to estimate hazard ratios (HRs) and their 95% confidence intervals (CIs) for the association between endotoxin exposure and a composite outcome of the earlier of impaired lung function or death. In addition to the composite outcome, we also estimated the hazard ratios associated with exposure for each component of the composite outcome as recommended by reporting guidelines.28 Specifically, we report the cause-specific hazard ratio associated with impaired lung function and the hazard ratio associated with death from any cause. This also allows us to verify that the exposure is associated with both outcomes in the same direction.
Endotoxin exposure was modeled as either a binary variable based on cotton vs. silk worker status, or as a continuous variable based on measured occupational endotoxin exposure as a time-varying covariate. Covariates were chosen based on a priori biological knowledge, with covariates derived from baseline and follow-up surveys. Cumulative numbers of years worked, pack-years smoked, and height were obtained at each survey. Age and gender were obtained at the baseline survey. Age, years worked from start of employment, and cumulative pack-years smoked were modeled as continuous time-varying covariates while height and gender were modeled as non-time varying covariates. For any given survey, if the subject participated, all covariate information was obtained.
Event times for the composite outcome and all-cause mortality were subject to left truncation since subjects who died between the time of employment and the baseline survey in 1981 would not have been part of the study. In addition, a total of 47 subjects were diagnosed with impaired lung function at the baseline survey in 1981, and hence their event times for the composite outcome and impaired lung function were also subject to left censoring. To account for both left truncation and left censoring when evaluating the composite outcome and the impaired lung function outcome, risk sets were calculated incorporating the left truncation time, and consequently subjects are not at risk until the baseline survey. Thus when evaluating the composite outcome and the impaired lung function outcome, the 47 subjects with impaired lung function in 1981 effectively do not contribute to the partial likelihood.35
As gender and smoking habits were highly correlated, a sensitivity analysis was performed by restricting the cohort to only lifetime nonsmokers (n=583) and repeating the analysis. The proportional hazards assumption was verified using Schoenfeld residuals. All statistical analyses were performed with SAS (version 9.3). Kaplan Meier plots were generated with the KMPLOT9 SAS macro.
RESULTS
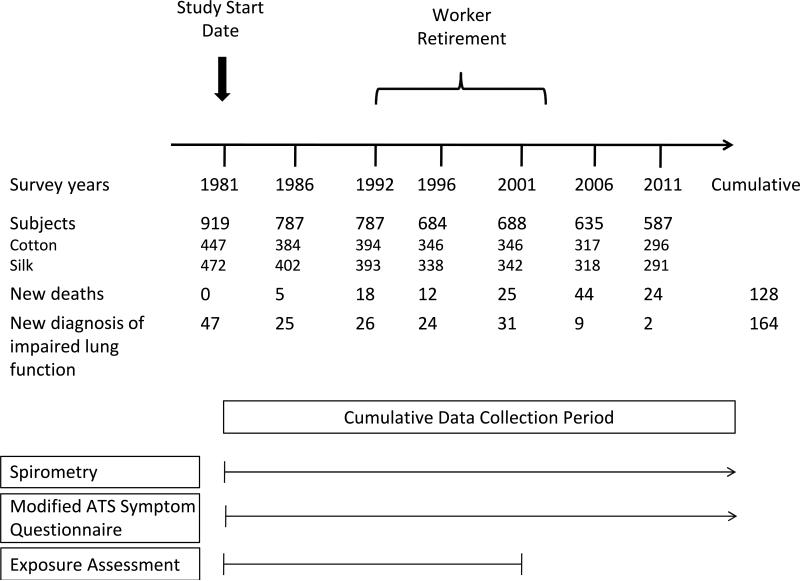
Of the 1021 textile workers who met our eligibility criteria in the 3 mills, 919 (90%) participated in the 1981 baseline survey. There were 472 silk and 447 cotton textile workers. The median follow-up time was 30 years with no significant differences in average follow-up time between cotton and silk workers (see Figure 1 for schema detailing study design, events, and follow-up). Table 1 shows the baseline demographic and lung function characteristics of the study population in 1981. Cotton textile workers were more likely to be smokers and to have been employed for over 5 years. Males were more likely to be older, smoke, and among cotton textile workers, to have been exposed to higher cumulative levels of endotoxin.
Figure 1. Study schema.
Study design, event, and follow-up rates for the Shanghai Textile Worker Study.
Table 1.
Characteristics of study population in 1981a
| Characteristic | Silk (n=472) | Cotton (n=447) |
|---|---|---|
| Male | 200 (42.4%) | 214 (47.9%) |
| Age, years | 33 [26-47] | 34 [27-47] |
| Male | 43 [29-50] | 41 [29-49] |
| Female | 31 [26-45] | 33 [26-45] |
| Follow-up time, yearsb | 30 [29-30] | 30 [29-30] |
| Height, cm | 162.5 (±7.3) | 163.9 (±7.5) |
| Current smoking | 118 (25.0%) | 159 (35.6%) |
| Male | 117 (58.5% of males) | 149 (69.6% of males) |
| Female | 1 (0.9% of females) | 10 (4.3% of females) |
| Pack-yearsc | 2.4 (±6.5) | 3.1(±7.2) |
| Nonsmoker | 348 (73.5%) | 284 (65.5%) |
| Male | 77 (38.5% of males) | 61 (28.5% of males) |
| Female | 271 (99.1% of females) | 223 (95.7% of females) |
| Years employed | 15 [6-25] | 15 [6-25] |
| Employed ≤5 years | 96 (20.3%) | 72 (16.1%) |
| Male | 47 (23.5% of males) | 27 (12.6% of males) |
| Female | 85 (31.3% of females) | 46 (19.7% of females) |
| Occupational endotoxin exposure, EU/m3-yrsb | - | 37,688 [15,517-65,554] |
| Male | - | 47,992 [16,339-86,532] |
| Female | - | 29.860 [15,517-65,554] |
| FEV1, liters | 2.84 (±0.67) | 2.90 (±0.72) |
| FVC, liters | 3.46 (±0.75) | 3·53 (±0.78) |
| FEV1/FVC ratio | 0.84 (±0.09) | 0·83 (±0.09) |
| % predicted FEV1 | 99.2 (±14.7) | 98·4 (±15.2) |
Values reported are means (±standard deviation) or median [interquartile range]
Total follow-up time and cumulative endotoxin exposure calculated based on all available data up until 30-year survey
For smokers
128 deaths and 164 diagnoses of impaired lung function were ascertained between October 1981 and August 2011. Causes of death were related to neoplasms (n=60, with 11 due to lung cancer), diseases of the central nervous system (n=35), circulatory system (n=19, of whom 4 had chronic pulmonary heart disease or cor pulmonale), respiratory system (n=6), digestive system (n=2), genitourinary system (n=3), injury, poisoning, and certain other consequences of external causes (n=1), other external causes (n=1), and abnormal clinical and laboratory findings not otherwise classified(n=1). The diagnosis of impaired lung function was made more frequently in men vs. women (106 vs. 58, men vs. women), and deaths occurred more frequently in men (89 vs. 39, men vs. women).
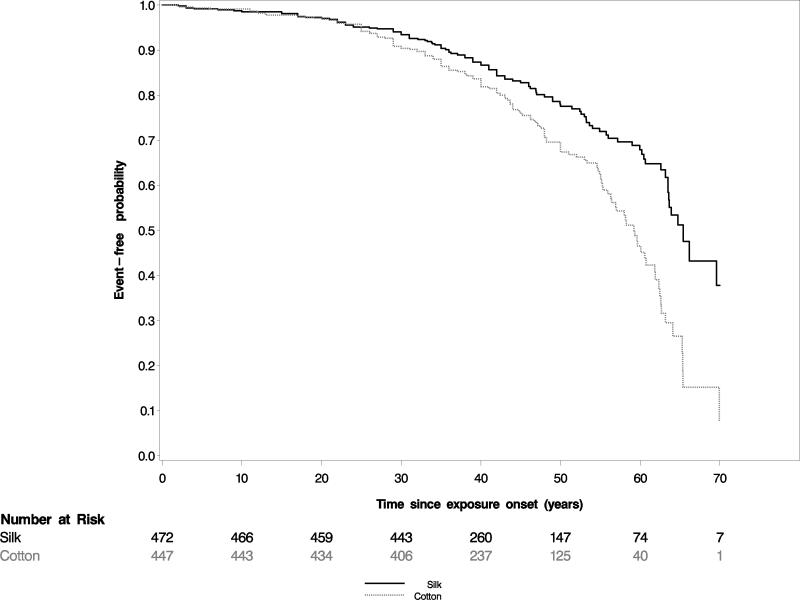
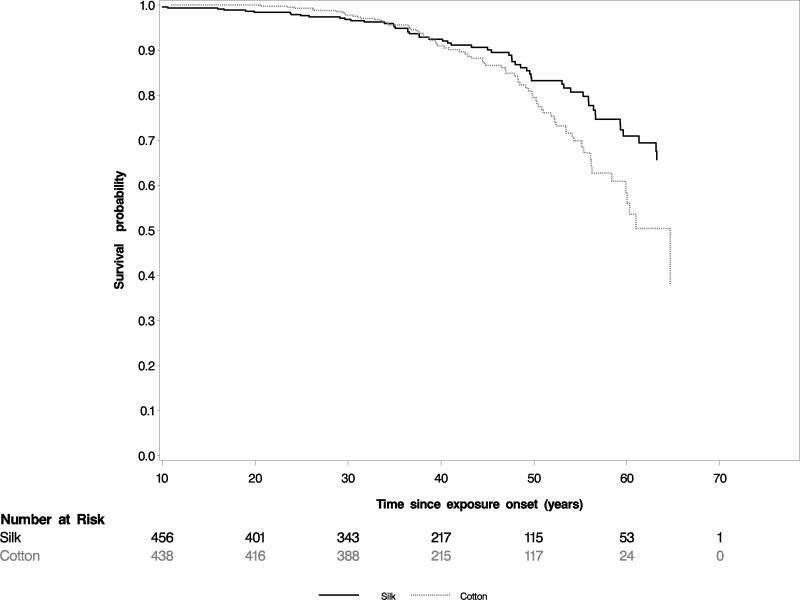
Kaplan-Meier curves for the unadjusted risk in cotton vs. silk workers of the composite endpoint of impaired lung function and death (Figure 2), and for the unadjusted risk of all-cause mortality (Figure 3) showed significantly worse outcomes for cotton vs. silk workers.
Figure 2. Kaplan-Meier plots of cotton vs. silk workers for the composite endpoint of low lung function and death.
Event-free survival was plotted against time from onset of employment (and therefore onset of occupational exposure to endotoxin in cotton workers). Cotton workers had higher event probabilities than silk workers in this unadjusted analysis.
Figure 3. Kaplan-Meier plots of cotton vs. silk workers for all-cause mortality.
Survival was plotted against time from onset of employment (and therefore onset of occupational exposure to endotoxin in cotton workers). Cotton workers had lower survival than silk workers in this unadjusted analysis. Note left truncation.
In multivariate models, cotton vs. silk worker status was associated with a 47% increase in the risk of a composite outcome of impaired lung function or death (HR 1.47, 95% CI 1.09 – 1.97), Table 2. The association differed by gender (cotton by male interaction p-value=0.04), and was elevated only in male textile workers (HR 1.84, 95% CI 1.27 – 2.67 in males vs. HR 1.00, 95% CI 0.62 – 1.61 in females). When exposure was modeled as cumulative occupational endotoxin exposure, it was likewise associated with an increased risk of impaired lung function and death, with each 10,000 EU/m3-yrs increase in cumulative endotoxin exposure associated with a 4% increase in risk (HR 1.04 95% CI 1.01 – 1.07). Risk was elevated in males (HR 1.05, 95% CI 1.01 – 1.08) but not females (HR 1.00, 95% CI 0.91 – 1.10).
Table 2. Hazard ratios (HR) for the composite endpoint, all-cause mortality, and cause specific hazard (CSH) ratio for impaired lung function in relation to occupational endotoxin exposure in the entire cohort (n=919, 414 men, 505 women).
Hazards calculated using the Cox proportional hazards model accounting for left censoring and left truncation. Endotoxin exposure was modeled as either cotton vs. silk textile work, or lifetime occupational endotoxin exposure as a time varying covariate. Hazard ratios were adjusted for gender and height as non-time varying covariates, and for years worked in the textile industry, age, and lifetime pack-years smoked as time varying covariates.
| FEV1 at fifth percentile1 | All-cause mortality | Composite endpoint1,2 | ||||
|---|---|---|---|---|---|---|
| Events | CSH (95% CI) | Events | HR (95% CI) | Events | HR (95% CI) | |
| Cotton vs. silk | ||||||
| All | 164/919 | 1.36 (0.93-2.00) | 128/919 | 1.36 (0.93–1.99) | 253/919 | 1.47 (1.09–1.97) |
| Males | 106/414 | 1.42 (0.89-2.28) | 89/414 | 1.63 (1.04–2.57) | 163/414 | 1.84 (1.27–2.67) |
| Females | 58/505 | 1.26 (0.67-2.38) | 39/505 | 0.92 (0.48–1.77) | 90/505 | 1.00 (0.62–1.61) |
| Occupational endotoxin (per 10,000 EU/m3-yrs) | ||||||
| All | - | 1.04 (0.99-1.08) | - | 1.04 (0.99–1.08) | - | 1.04 (1.01–1.07) |
| Males | - | 1.03 (0.99-1.08) | - | 1.04 (1.00–1.08) | - | 1.05 (1.01–1.08) |
| Females | - | 1.05 (0.94-1.18) | - | 1.00 (0.89–1.14) | - | 1.00 (0.91–1.10) |
47 (28 men, 19 women) subjects were diagnosed with impaired lung function at the start of the study in 1981 and were effectively excluded from the analysis to address the issue of left censoring.
An event was defined as the earlier of either impaired lung function or death. 39 subjects (32 men, 7 women) were first diagnosed with impaired lung function and subsequently died during the follow-up period.
Cotton vs. silk worker status had a similar magnitude of risk for all-cause mortality as for the composite outcome, although this association was not statistically significant in the overall cohort (HR 1.36 95% CI 0.93 – 1.99). There was a higher risk of death in male cotton compared to male silk workers (HR 1.63, 95% CI 1.04 – 2.57); this was not observed in female cotton vs. female silk workers (HR 0.92, 95% CI 0.47– 1.77). There was no statistically significant increase in death associated with cumulative endotoxin exposure in the overall cohort (HR 1.04, 95% CI 0.99 – 1.09) although the magnitude of risk again was again elevated in males (HR 1.04, 95% CI 1.00 – 1.08) but not in females (HR 1.00, 95% CI 0.89-1.14).
We also report the cause-specific hazard associated with exposure for the development of impaired lung function in order to verify that exposure is associated with both components of the composite outcome in the same direction. This analysis has to be interpreted carefully in light of the issues of semi-competing risks and the known association between low FEV1 and death from both pulmonary and non-pulmonary causes. We observed a similar magnitude of risk for development of impaired lung function associated with exposure. Given the low event rates (note the 47 subjects who already had impaired lung function in 1981 were effectively excluded from this analysis), the point estimates for the gender specific analyses are less precise and therefore more difficult to interpret.
While we adjusted for the confounding effects of smoking, given that 94.8% of the female but only 22.9% of the male cotton textile workers remained nonsmokers throughout the duration of the study, we performed a sensitivity analysis to verify that the gender differential in the effects of occupational endotoxin exposure on health was not due to residual confounding from smoking. In the 583 textile workers (313 silk, 270 cotton) who remained nonsmokers throughout the duration of the study, we observed the same patterns of risk as observed in the analysis on the entire cohort (Supplemental Table 1). As expected, confidence intervals were wider due to lower event rates in this non-smoking subgroup.
DISCUSSION
In the largest observational study of textile workers to date with the longest duration of sequential follow-up, we found that occupational endotoxin exposure is associated with an increased risk of either impaired lung function or death; a similar increase in risk is seen for all-cause mortality alone. The observed risk was elevated only in male and not in female cotton textile workers.
We identified a striking gender effect on the impact of long term occupational endotoxin exposure on impaired lung function and death; this is the first study to clearly demonstrate that risk associated with occupational endotoxin exposure has significant gender differences. While other longitudinal studies of cotton workers have found a trend towards a greater annual decline in lung function in men as compared to women, they have been limited by small study size, lack of control for confounders, no exposure assessment for endotoxin, and high loss to follow-up. 16,15,36
This is also the first study to evaluate the effects of occupational endotoxin exposure beyond pulmonary health with the use of a composite outcome. This was motivated primarily from the biological standpoint to address the known pleiotropic health effects of endotoxin although it also addressed the issue of semi-competing risks with increased statistical power.29 While the respiratory health effects of endotoxin are the best studied, the health effects of endotoxin clearly extend beyond the respiratory system.5-11 When evaluating the components of the composite outcome in a sensitivity analysis, we find the same consistent relationship; cotton dust exposure is associated with an increased risk of both low lung function and death. If the relationship between exposure and each outcome was not consistent, then a loss of power would result when combining these two outcomes, and not a gain of power as we have observed.
Gender effects on the risk associated with occupational endotoxin exposure could be explained by a number of different factors. Occupational endotoxin exposure was higher in male compared to female cotton textile workers; men were more likely than women to work in highly exposed areas such as opening, carding, and blowing, which exposed them to higher levels of endotoxin (although no additional occupational exposures besides cotton dust was present in these work areas). Thus a threshold effect of occupational endotoxin on health could be postulated. In any observational study there is the potential for uncontrolled confounding, and of greatest concern in this study was that almost none of the female workers were smokers and thus gender and smoking was highly correlated. When smokers were excluded from the analysis, however, we observed the same differential effect of occupational endotoxin exposure on both endpoints, suggesting that the interaction between gender and endotoxin exposure is not due to residual confounding from smoking. Alternatively, a true gender effect could be present. Human studies have demonstrated that women have an attenuated physiologic response to low dose endotoxin injection21 and decreased production of inflammatory cytokines such as TNF-α37 when compared to men. Estradiol appears to play a role in modulating the response to endotoxin in in vitro studies,22 and animal studies have demonstrated that intratracheal endotoxin administration leads to exaggerated inflammatory responses in male compared to female mice; this effect is attenuated with gonadectomy in male mice and similarly accentuated in females with the administration of testosterone.20 Thus a biologic explanation for the differential response to occupational endotoxin exposure by gender is clearly present, and would identify men as a vulnerable subgroup in these highly endotoxin-exposed settings.
Only one other study38 has compared mortality rates in cotton vs. silk textile workers although this study focused on COPD-related mortality and reported a higher risk in silk compared to cotton textile workers. This registry study based on the Shanghai Textile Industry Bureau involved 267,400 female textile workers with mortality data obtained from 1989 to 2000. Chronic obstructive pulmonary disease (COPD)-related mortality as reported on death certificates was higher in cotton workers compared to the general textile population but unexpectedly highest in silk workers. Curiously, COPD deaths were observed only among silk workers who did not smoke; no COPD deaths were recorded in smoking silk workers. One explanation could be that sicker silk workers would be less likely to smoke than healthy silk workers, i.e. a “healthy smoker effect” that has been previously described.39 Given the lack of exposure assessment and detailed medical histories in these subjects as well as low event rates,18,40it is difficult to determine whether silk dust actually represents a toxic exposure or whether these results are related to a differential healthy worker survivor effect that depends on the toxicity of the exposure where cotton dust represents the more toxic exposure.40,41 It is likely that the general cotton textile population is healthier than with general silk textile population. This is consistent with prior correspondence between one of the authors (DC) and physicians working for the Shanghai Textile Bureau indicating that by policy, candidate workers with any prior respiratory disease are automatically excluded from cotton textile work. Our study population was drawn from a subset of this registry based study – we explicitly excluded silk workers with a prior history of active tuberculosis or asthma, thus selecting for healthy silk workers as a comparison group. We observed decreased, not increased overall mortality when comparing previously healthy silk with previously healthy cotton textile workers, although our sample size did not allow us to specifically address COPD-specific mortality.
The major strengths of this study are its prospective design with working matched controls, duration and frequency of follow-up, serial assessment of endotoxin exposure, smoking habits, spirometry, and high participation rate even at 30 years after the baseline survey. Few studies have repeated spirometry performed in subjects without overt respiratory disease. Additionally, we have carefully accounted for the presence of left truncation, left censoring, and semi-competing risks present in this study. We have also performed sensitivity analyses to demonstrate the consistency of our findings.
We acknowledge that our study has several limitations. First, we estimated occupational endotoxin exposure from job histories and area samplers for dust and endotoxin rather than from personal samplers. Additionally, exposure assessment was not performed prior to 1981 and the assumption was that endotoxin and dust levels did not change significantly in the cotton textile mills. No significant changes to the infrastructure and ventilation system of the mills were made since the 1950s although if the origin of the baled cotton used in the mills changed significantly in the decades prior to 1981,42 this assumption may be less valid. These above factors may contribute to exposure misclassification. Personal samplers are still not available for measurement of cotton dust exposure, and by study design we could not have obtained measurements of endotoxin and dust levels prior to 1981. However, such exposure misclassification would be expected to be non-differential and bias results towards the null; in our study we still observed significant results despite these limitations. Second, our cohort was not a newly-hired cohort but rather was composed of prevalent hires raising the concern for a healthy worker survivor effect.18,40 We adjusted for the number of years worked as well as left truncation in order to address this issue; An analysis using Cox proportional hazards models stratified by the number of years worked at study inception to account for the healthy worker survivor effect also showed no significant differences in our results. If present, the healthy worker survivor effect would again be expected to bias our results towards the null. Third, it is possible that other exposures correlated with endotoxin exposure may play a causative role in disease development. Animal studies have supported the role of endotoxin in causing respiratory pathology43 and in particular the etiologic role of endotoxin in cotton dust.44 Other agents such as β-glucan, a fungal cell wall component, may also play a role,45 but evidence to date suggests that it is the endotoxin component of cotton dust that is the major contributor to respiratory disease. Fourth, while a 74% follow-up rate in a 30 year longitudinal study is unusually high, one may argue that those lost to follow-up may not be representative of those that continued to participate in the study. Since the primary research question relates to the effect of cotton vs. silk dust on the outcomes, if the loss to follow-up was different between cotton vs. silk workers, and if this was not adjusted for in our analysis, then there is the potential for biased estimates of the effect of cotton dust on low lung function and death. Survival analysis is particularly suited to dealing with incomplete (censored) time to event data and one reason why we chose this method of analysis. The censoring is allowed to be dependent on the exposures. In our case, since we always include the exposure in our models as it is precisely our primary covariate of interest (whether as cotton vs. silk worker status or as measured endotoxin exposure), we still get valid estimates of the effect of cotton dust or endotoxin exposure on the time to development of low lung function or death even if follow-up rates were to differ between cotton vs. silk workers.
The exact nature of the observed impaired lung function associated with occupational endotoxin exposure is unclear from this study. Prior studies have shown an association between endotoxin containing organic dust and the development of obstructive lung disease,14,46 although it is unclear whether the etiology of the observed airflow obstruction is due to small airways disease or emphysema. Post-bronchodilator spirometry and lung volumes were not obtained in this study, and thus we are currently unable to specifically address this issue. A study using high resolution computed tomography to answer this question is ongoing in this cohort.
In summary, occupational endotoxin exposure is associated with an increased risk of impaired lung function and all-cause mortality in Chinese textile workers. The observed risk was seen only in male and not female cotton textile workers, even after careful adjustment for the effects of smoking. High levels of endotoxin have been detected in cigarette smoke, homes burning biomass fuel, and various occupational settings such as agricultural work sewage treatment plants,2-4 suggesting that our results have broader implications beyond the textile industry. If men are more vulnerable to the health effects of endotoxin than women, then they may represent a subgroup that will benefit from targeted interventions such as pre-emptive screening for sub-clinical disease, workplace measures to lower endotoxin exposure, and lifestyle modifications such as smoking cessation to reduce the risk of adverse health effects.
Supplementary Material
What this paper adds
Endotoxin exposure has been associated with both harmful and beneficial effects on health in humans. The best studied health effects relate to lung function and mortality.
Animal studies and short term experimental human studies have demonstrated that men have an augmented inflammatory response to endotoxin compared to women. Whether these gender effects are seen with chronic exposure in humans is unclear.
In the present analysis we examined the effect of occupational endotoxin exposure in cotton dust on the time to development of impaired lung function and death in male and female cotton textile workers with silk workers as a control group. Occupational endotoxin exposure was associated with an increased risk of developing impaired lung function and death in male but not female cotton textile workers
Our results demonstrate that occupational endotoxin exposure is associated with overall harmful effects on health in men. The strong gender effect suggests that men in high endotoxin exposure occupations would benefit from pre-emptive screening for sub-clinical disease, workplace measures to lower endotoxin exposure, and lifestyle modifications such as smoking cessation to reduce the harmful effects of long-term endotoxin exposure.
ACKNOWLEDGMENTS
None.
FUNDING
This study was supported by National Institutes of Health (NIH) grants ES00002, F32 ES020082, K23ES023700, and National Institute for Occupational Safety and Health (NIOSH) grant OH002421.
Abbreviations
- COPD
Chronic obstructive pulmonary disease
- EU/m3-yrs
endotoxin units/meters3-years
- FEV1
Forced expiratory volume in one second
- FVC
Forced vital capacity
Footnotes
CONTRIBUTORS
PL, XL, DC, TC participated in the data analysis and interpretation of the results. PL participated in the writing of the report with input from all authors. JH, FZ, BZ, HD, LS, and DC participated in the study implementation. PL and DC contributed to the conception of this study. DC is the Principal Investigator of the Shanghai Textile Workers Study.
COMPETING INTERESTS
The authors have no competing financial interests to declare.
Additional details of the statistical methods are provided in the Supplemental Methods.
REFERENCES
- 1.Jacobs JH, Krop EJ, Wind SD, et al. Endotoxin levels in homes and classrooms of Dutch school children and respiratory health. Eur Respir J. 2012 doi: 10.1183/09031936.00084612. [DOI] [PubMed] [Google Scholar]
- 2.Semple S, Devakumar D, Fullerton DG, et al. Airborne endotoxin concentrations in homes burning biomass fuel. Environ Health Perspect. 2010;118:988–91. doi: 10.1289/ehp.0901605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasday JD, Bascom R, Costa JJ, et al. Bacterial endotoxin is an active component of cigarette smoke. Chest. 1999;115:829–35. doi: 10.1378/chest.115.3.829. [DOI] [PubMed] [Google Scholar]
- 4.Liebers V, Raulf-Heimsoth M, Brüning T. Health effects due to endotoxin inhalation (review). Arch Toxicol. 2008;82:203–10. doi: 10.1007/s00204-008-0290-1. [DOI] [PubMed] [Google Scholar]
- 5.Braun-Fahrlander C, Riedler J, Herz U, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002;347:869–77. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- 6.Lenters V, Basinas I, Beane-Freeman L, et al. Endotoxin exposure and lung cancer risk: a systematic review and meta-analysis of the published literature on agriculture and cotton textile workers. Cancer Causes Control. 2010;21:523–55. doi: 10.1007/s10552-009-9483-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu AH. Endotoxin exposure in allergy and asthma: reconciling a paradox. J Allergy Clin Immunol. 2002;109:379–92. doi: 10.1067/mai.2002.122157. [DOI] [PubMed] [Google Scholar]
- 8.Applebaum KM, Ray RM, Astrakianakis G, et al. Evidence of a paradoxical relationship between endotoxin and lung cancer after accounting for left truncation in a study of Chinese female textile workers. Occup Environ Med;Published Online First. 2013 Jun 12; doi: 10.1136/oemed-2012-101240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiedermann CJ, Kiechl S, Dunzendorfer S, et al. Association of endotoxemia with carotid atherosclerosis and cardiovascular disease: prospective results from the Bruneck Study. J Am Coll Cardiol. 1999;34:1975–81. doi: 10.1016/s0735-1097(99)00448-9. [DOI] [PubMed] [Google Scholar]
- 10.Charalambous BM, Stephens RC, Feavers IM, et al. Role of bacterial endotoxin in chronic heart failure: the gut of the matter. Shock. 2007;28:15–23. doi: 10.1097/shk.0b013e318033ebc5. [DOI] [PubMed] [Google Scholar]
- 11.Thuy S, Ladurner R, Volynets V, et al. Nonalcoholic fatty liver disease in humans is associated with increased plasma endotoxin and plasminogen activator inhibitor 1 concentrations and with fructose intake. J Nutr. 2008;138:1452–5. doi: 10.1093/jn/138.8.1452. [DOI] [PubMed] [Google Scholar]
- 12.Buck M, Wall J, Schachter E. Airway constrictor response to cotton bract extracts in the absence of endotoxin. Br J Ind Med. 1986;43:220–226. doi: 10.1136/oem.43.4.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schilling RS. Byssinosis in the British cotton textile industry. Br Med Bull. 1950;7:52–6. doi: 10.1093/oxfordjournals.bmb.a073798. [DOI] [PubMed] [Google Scholar]
- 14.Wang X-R, Zhang H-X, Sun B-X, et al. A 20-year follow-up study on chronic respiratory effects of exposure to cotton dust. Eur Respir J. 2005;26:881–6. doi: 10.1183/09031936.05.00125604. [DOI] [PubMed] [Google Scholar]
- 15.Glindmeyer HW, Lefante JJ, Jones RN, et al. Exposure-related declines in the lung function of cotton textile workers. Relationship to current workplace standards. Am Rev Respir Dis. 1991;144:675–83. doi: 10.1164/ajrccm/144.3_Pt_1.675. [DOI] [PubMed] [Google Scholar]
- 16.Zuskin EE, Valić FF. Change in the respiratory response to coarse cotton dust over a ten-year period. Am Rev Respir Dis. 1975;112:417–421. doi: 10.1164/arrd.1975.112.3.417. [DOI] [PubMed] [Google Scholar]
- 17.Arrighi HM, Hertz-Picciotto I. The evolving concept of the healthy worker survivor effect. Epidemiology. 1994;5:189–96. doi: 10.1097/00001648-199403000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Su WL, Chen YH, Liou SH, et al. Meta-analysis of standard mortality ratio in cotton textile workers. Eur J Epidemiol. 2004;19:989–97. doi: 10.1007/s10654-004-0917-3. [DOI] [PubMed] [Google Scholar]
- 19.Castellan R, Olenchock S, Kinsley K, et al. Inhaled endotoxin and decreased spirometric values. New England Journal of Medicine. 1987;317:605–610. doi: 10.1056/NEJM198709033171005. [DOI] [PubMed] [Google Scholar]
- 20.Card JW, Carey MA, Bradbury JA, et al. Gender differences in murine airway responsiveness and lipopolysaccharide-induced inflammation. J Immunol. 2006;177:621–30. doi: 10.4049/jimmunol.177.1.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coyle SM, Calvano SE, Lowry SF. Gender influences in vivo human responses to endotoxin. Shock. 2006;26:538–43. doi: 10.1097/01.shk.0000232589.39001.4d. [DOI] [PubMed] [Google Scholar]
- 22.Pioli PA, Jensen AL, Weaver LK, et al. Estradiol attenuates lipopolysaccharide-induced CXC chemokine ligand 8 production by human peripheral blood monocytes. J Immunol. 2007;179:6284–90. doi: 10.4049/jimmunol.179.9.6284. [DOI] [PubMed] [Google Scholar]
- 23.Ferris BG. Epidemiology Standardization Project (American Thoracic Society). Am Rev Respir Dis. 1978;118:1–120. [PubMed] [Google Scholar]
- 24.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 25.Olenchock S, Christiani D, Mull J, et al. Airborne endotoxin concentrations in various work areas within two cotton textile mills in the People's Republic of China. Biomed Environ Sci. 1990;3:443–51. [PubMed] [Google Scholar]
- 26.Ip MS, Ko FW, Lau AC, et al. Updated spirometric reference values for adult Chinese in Hong Kong and implications on clinical utilization. Chest. 2006;129:384–92. doi: 10.1378/chest.129.2.384. [DOI] [PubMed] [Google Scholar]
- 27.Fine J, Jiang H, Chappell R. On semi-competing risks data. Biometrika. 2001;88:907–919. [Google Scholar]
- 28.Cordoba G, Schwartz L, Woloshin S, et al. Definition, reporting, and interpretation of composite outcomes in clinical trials: systematic review. BMJ. 2010;341:c3920. doi: 10.1136/bmj.c3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varadhan R, Weiss CO, Segal JB, et al. Evaluating health outcomes in the presence of competing risks: a review of statistical methods and clinical applications. Med Care. 2010;48:S96–105. doi: 10.1097/MLR.0b013e3181d99107. [DOI] [PubMed] [Google Scholar]
- 30.Young RP, Hopkins R, Eaton TE. Forced expiratory volume in one second: not just a lung function test but a marker of premature death from all causes. Eur Respir J. 2007;30:616–22. doi: 10.1183/09031936.00021707. [DOI] [PubMed] [Google Scholar]
- 31.Lange P, Nyboe J, Appleyard M, et al. Spirometric findings and mortality in never-smokers. J Clin Epidemiol. 1990;43:867–73. doi: 10.1016/0895-4356(90)90070-6. [DOI] [PubMed] [Google Scholar]
- 32.Hole DJ, Watt GC, Davey-Smith G, et al. Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. BMJ. 1996;313:711–5. doi: 10.1136/bmj.313.7059.711. discussion 715-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest. 2005;127:1952–9. doi: 10.1378/chest.127.6.1952. [DOI] [PubMed] [Google Scholar]
- 34.Stavem K, Aaser E, Sandvik L, et al. Lung function, smoking and mortality in a 26-year follow-up of healthy middle-aged males. Eur Respir J. 2005;25:618–25. doi: 10.1183/09031936.05.00008504. [DOI] [PubMed] [Google Scholar]
- 35.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. Springer-Verlag; New York: 2010. [Google Scholar]
- 36.Berry G, McKerrow C, Molyneux M, et al. A study of the acute and chronic changes in ventilatory capacity of workers in Lancashire cotton mills. Br J Ind Med. 1973;30:25–36. doi: 10.1136/oem.30.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Imahara SD, Jelacic S, Junker CE, et al. The influence of gender on human innate immunity. Surgery. 2005;138:275–82. doi: 10.1016/j.surg.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 38.Cui L, Gallagher L, Ray R, et al. Unexpected excessive chronic obstructive pulmonary disease mortality among female silk textile workers in Shanghai, China. Occupational and Environmental Medicine. 2011;68:883–887. doi: 10.1136/oem.2010.062034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Becklake MR, Lalloo U. The ‘healthy smoker’: a phenomenon of health selection? Respiration. 1990;57:137–44. doi: 10.1159/000195837. [DOI] [PubMed] [Google Scholar]
- 40.Bakirci N, Kalaca S, Fletcher AM, et al. Predictors of early leaving from the cotton spinning mill environment in newly hired workers. Occup Environ Med. 2006;63:126–30. doi: 10.1136/oem.2005.021352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castellan R, Olenchock S, Hankinson J, et al. Acute bronchoconstriction induced by cotton dust: dose-related responses to endotoxin and other dust factors. Ann Intern Med. 1984;101:157–163. doi: 10.7326/0003-4819-101-2-157. [DOI] [PubMed] [Google Scholar]
- 42.Lane SR, Sewell RD. The bacterial profile of cotton lint from worldwide origins, and links with occupational lung disease. Am J Ind Med. 2007;50:42–7. doi: 10.1002/ajim.20412. [DOI] [PubMed] [Google Scholar]
- 43.Brass DM, Hollingsworth JW, Cinque M, et al. Chronic LPS inhalation causes emphysema-like changes in mouse lung that are associated with apoptosis. Am J Respir Cell Mol Biol. 2008;39:584–90. doi: 10.1165/rcmb.2007-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Castranova V, Robinson V, Frazer D. Pulmonary reactions to organic dust exposures: development of an animal model. Environ Health Perspect. 1996;104:41–53. doi: 10.1289/ehp.96104s141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rylander R, Holt PG. (1-->3)-beta-D-glucan and endotoxin modulate immune response to inhaled allergen. Mediators Inflamm. 1998;7:105–10. doi: 10.1080/09629359891252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lai PS, Christiani DC. Long-term respiratory health effects in textile workers. Curr Opin Pulm Med. 2013;19:152–7. doi: 10.1097/MCP.0b013e32835cee9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.