Abstract
Background
Although increased levels of S-adenosylmethionine (SAM) and S-adenosylhomocysteine (SAH) have been implicated as markers for renal and vascular dysfunction, until now there have been no studies investigating their association with clinical post-transplant events such as organ rejection and immunosuppressant nephrotoxicity.
Methods
A newly developed and validated liquid chromatography–tandem mass spectrometry (LC–MS/MS) assay for the quantification of SAM and SAH in human EDTA plasma was used for a clinical proof-of-concept pilot study. Retrospective analysis was performed using samples from a longitudinal clinical study following de novo kidney transplant patients for the first year (n = 16).
Results
The ranges of reliable response were 8 to 1024 nmol/l for SAM and 16 to 1024 nmol/l for SAH. The inter-day accuracies were 96.7–103.9% and 97.9–99.3% for SAM and SAH, respectively. Inter-day imprecisions were 8.1–9.1% and 8.4–9.8%. The total assay run time was 5 min. SAM and SAH concentrations were significantly elevated in renal transplant patients preceding documented acute rejection and nephrotoxicity events when compared to healthy controls (n = 8) as well as transplant patients void of allograft dysfunction (n = 8).
Conclusion
The LC-MS/MS assay will provide the basis for further large-scale clinical studies to explore these thiol metabolites as molecular markers for the management of renal transplant patients.
Keywords: S-adenosylmethionine, S-adenosylhomocysteine, Acute rejection, Nephrotoxicity, HPLC-MS-MS
1. Introduction
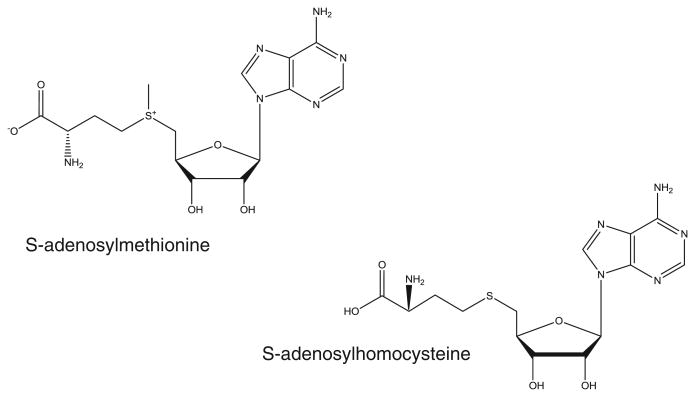
Kidney transplantation is the preeminent treatment for patients with terminal kidney failure, both in terms of survival advantage and quality of life [1,2]. Although short-term allograft survival has significantly improved over the years, long-term graft survival past 5 y has remained largely unchanged [3,4]. Considerable advances have been made in the discovery and qualification of novel molecular markers that have shown promise as predictive markers of renal dysfunction and nephropathy, however, allograft biopsy in addition to monitoring changes in serum creatinine have remained the ‘gold standard’ for assessing renal function in clinical settings [2,5,6]. Nevertheless, the lack of sensitivity and specificity of serum creatinine and the risk of damaging the allograft during a biopsy have underlined the need for more sensitive and specific noninvasive diagnostic tools capable of monitoring and predicting kidney dysfunction in a clinical setting. While chronic allograft dysfunction is the primary cause of renal allograft loss after the first year, cardiovascular complications remain the foremost cause of death [1]. The increased prevalence of cardiovascular disease in transplant recipients is the result of multiple factors, and homocysteinemia was found in approximately 60% of renal transplant patients [7]. Unfortunately, due to large variation in endogenous homocysteine levels, its application as a molecular marker has often been unsuccessful [8,9]. As a result, primary focus has shifted towards methylation cycle intermediates, in particular transmethylation pathway intermediates S-adenosylmethionine (SAM) and S-adenosylhomocysteine (SAH) Fig. 1 [8–11]. Methionine sulfur metabolism is thought to occur primarily via the trans-sulfuration pathway which results in the transfer of the sulfur from methionine, an essential amino acid that is primarily metabolized in the liver, to serine resulting in the formation of cysteine [12,13]. The first step in methionine metabolism is the formation of S-adenosylmethionine (SAM) in a reaction catalyzed by methionine adenyltransferase (MAT) [12]. Under normal conditions, most of the SAM generated in this process is used in trans-methylation reactions, whereby SAM acts as a universal methyl donor for a large variety of acceptor compounds by conversion into S-adenosylhomocysteine through the trans-methylation pathway [13]. This reaction is catalyzed by S-adenosylhomocysteine hydrolase and is reversible with the equilibrium favoring formation of SAH [12]. Several studies have shown evidence that elevated concentrations of S-adenosylmethionine (SAM) and S-adenosylhomocysteine (SAH) can be used as sensitive indicators of vascular disease [8]. Moreover, decreases in the SAM/SAH ratio, which is also commonly referred to as “methylation potential” have been observed in patients with end-stage renal failure suggesting a link between disturbed trans-methylation reactions, vascular dysfunction and impaired renal function [10,11,14]. Although previous studies have identified both SAM and SAH as potential markers for cardiovascular and renal dysfunction, to date, no studies have been performed evaluating SAM and SAH concentrations and/or ratios in plasma from renal transplant patients as an early marker for acute rejection and nephrotoxicity. One of the major limitations has been the availability of reliable high-throughput assays that can be used for larger clinical trials and, later, potentially for clinical monitoring. While several quantitative assays have been developed to measure both metabolites in human plasma, utilizing coulometric electrochemical detection, liquid chromatography with ultraviolet detection (LC–UV), as well as liquid chromatography mass spectrometry (LC–MS), these assays involve tedious sample preparation, extended analysis times, and/or lack of proper bioanalytical method validation following current guidelines [14–18].
Fig. 1.
Structures of SAM and SAH.
2. Materials and methods
2.1. Reagents and reference materials
Solvents and reagents (HPLC grade acetonitrile, water, formic acid, and ammonium formate) used for sample preparation and as mobile phases were from Fisher Scientific (Fair Lawn, NJ) and used without further purification. SAM, SAH and DL-homocysteine reference materials as well as dithiothreitol were from Sigma Aldrich (St. Louis, MO). SAH hydrolase was purchased from Abcam (Cambridge, MA). d5-Adenosine was from Cambridge Isotopes (Andover, MA), deuterated internal standard (d3-SAM) was purchased from C/D/N Isotopes (Pointe-Claire, QC, Canada), and deuterated internal standard (d5-SAH) was synthesized as previously described by Stabler et al. [18]. Six separate lots of human plasma were supplied by Bonfils Blood Center (Denver, CO). Six separate lots of human plasma with elevated triglyceride and bilirubin levels for matrix interference testing were supplied by Bioreclamation (Westbury, NY). The use of de-identified blood bank plasma for assay validation, calibration and quality control was considered exempt by the Colorado multi-institutional Review Board (COMIRB, Aurora, CO).
2.2. Sample extraction
Internal standard solution with d3-S-adenosylmethionine and d5-S-adenosylhomocysteine was prepared at a concentration of 5 μmol/l in 0.1% formic acid. Two hundred microliters of plasma sample was transferred into a microfuge tube. Each sample was spiked with 50 μl internal standard solution, vortexed for 5 min and incubated at 4 °C for 10 min. Five hundred and fifty microliters acetone stored at −20 °C was then added to each sample, vortexed for 10 min and incubated at 4 °C for an additional 10 min. Samples were then centrifuged for 10 min at 13400 ×g at 4 °C and 500 μl of the clear supernatant was transferred into an HPLC auto sampler vial.
2.3. LC-MS/MS assay for the quantification of SAM and SAH
Samples were analyzed using an Agilent 1200 series HPLC system consisting of a G1312 binary pump, a G1322A vacuum degasser, and a G1316A thermostated column compartment (Agilent Technologies, Palo Alto, CA) in combination with a Leap CTC PAL auto sampler (Carrboro, NC). The HPLC system was interfaced with an ABSciex 5000 triple quadrupole mass spectrometer (Foster City, CA) operating with an electrospray ionization source (ESI) using nitrogen (purity: 99.99%). Twenty microliters of the extracted sample were injected onto a 3.0 × 150 mm, 3.5 μm RP-Amide column, Supelco (St Louis, MO). The starting mobile phase consisted of 5% acetonitrile and 95% 10 mmol/l ammonium formate buffer (pH 3.4) with a flow of 0.6 ml/min for the first minute. After 1 min, the flow rate was increased to 0.8 ml/min and a gradient from 5% to 95% acetonitrile within 2.5 min was run. Acetonitrile was then held at 95% for 0.5 min. The column was re-equilibrated for 1 min to starting conditions. Themass spectrometer was run in the multiple reaction monitoring (MRM) mode with the interface heated to 500 °C. Nitrogen of >99.999% purity was used as collision activated dissociation (CAD) and curtain gas. The first quadrupole (Q1) was set to selectthe protonated molecular ion [M + H]+ of each compound, SAM (m/z = 399.0), SAM-d3 (m/z = 402.0), SAH (m/z = 385.1) and SAH-d5 (m/z = 390.0). The declustering potential (DP) was 90 V and the entrance potential (EP) 10 V. Collision energy (CE) settings were 28 eV for SAH and SAH-d5 and 32 eV for SAM and SAM-d3. The second quadrupole (Q2) was used as collision chamber, and the third quadrupole (Q3) to select the characteristic product ions of SAM (m/z = 250.1 and m/z = 136.2), SAM-d3 (m/z = 250.1 and m/z = 136.2), SAH (m/z = 136.2), and SAH-d5 (m/z = 137.2).
2.4. Assay validation
The assay was validated following the FDA Guidelines on Bioanalytical Method Validation [19] as considered fit-for-purpose. Calibrators were prepared by spiking known concentrations of SAM and SAH into human plasma 1/5 diluted with PBS at 8 levels (8, 16, 32, 64, 128, 256, 512, and 1024 nmol/l). Quality Control samples (QC) were prepared at 5 different levels (37.5, 75, 150, 300 and 900 nmol/l). The high QC samples (150, 300 and 900 nmol/l) were prepared by spiking known concentrations into undiluted human EDTA plasma. The low QC samples (37.5 and 75 nmol/l) were prepared by enriching 1/5 diluted human plasma to minimize the effect of endogenous SAM and SAH levels. To account for endogenous SAM and SAH levels, the ratios of endogenous peak areas divided by the IS peak areas of non-enriched matrix were subtracted from the area ratios of enriched samples (corrected analyte area/IS area ratio). Calibration curves were constructed by plotting the peak area ratios of the corresponding analyte and internal standard against nominal analyte concentrations of the aforementioned calibrators. For validation purposes 6 calibration curves were run for the first day and two additional curves each day for a total of 20 days. The linearity of the method was investigated by calculation of the regression line using the least squares method. Quality control samples were prepared (n = 6) for day 1 and (n = 3) for the remaining days at the previously mentioned concentrations. The lower limit of quantitation was the lowest calibrator that consistently showed ±20% or less deviation from the nominal concentration as well as a precision of ≤20%. The upper limit of quantitation was set as the highest calibrator that consistently showed ±15% or less deviation from the nominal concentration as well as a precision of ≤15%. Accuracy and precision were verified over 20 days. Intra-day and inter-day accuracies and precisions were calculated using the equations as set forth in Clinical Laboratory and Standards Institute guideline EP5A2 [20].
2.5. Matrix interferences, ion suppression/enhancement, absolute extraction recoveries, dilution integrity and exclusion of carry-over
Interferences caused by matrix signals were excluded by extraction and analysis of plasma samples collected from 6 different individuals following the strategy as described by Matuszewski et al. [21]. In brief, to detect changes in ionization efficiency by co-eluting matrix substances, human EDTA plasma samples from 6 different healthy individuals were extracted and then enriched with appropriate amounts of the analytes to result in all previously described quality control concentrations for SAM and SAH. Signal areas were compared to those of neat solutions containing corresponding amounts of the analytes. In addition, using a “post-column infusion” approach to assess potential matrix effects, SAM-d3 and SAH-d5 (20 μM dissolved in 0.1% formic acid in H2O/methanol, 8:2, v/v) was infused post-column via a tee at a rate of 50 μl/min using a syringe pump (Harvard Scientific, Holliston, MA). The extent of ion suppression was established by monitoring the signal intensities of the ion currents in MRM mode (m/z = 402.0 → m/z = 250.1) and (m/z = 390.0 → m/z = 136.2) at the retention times of analyte and IS after injection of extracted non-enriched and undiluted plasma samples collected from six different individuals.
Interferences caused by lipemia, icterus and hemolysis were excluded by extraction and analysis of EDTA plasma samples collected from six different individuals with increased triglyceride and bilirubin concentrations as well as 6 lots of hemolytic plasma. Samples were enriched with appropriate amounts of the analytes to result in all previously described quality control concentrations for SAM and SAH and then extracted and analyzed. To account for endogenous SAM and SAH levels, the ratios of endogenous peak areas divided by the IS peak areas of 1/5 diluted and undiluted non-enriched matrix were subtracted from area ratios of enriched samples for low (37.5, 75 and 150 nmol/l) and high (300 and 900 nmol/l) SAM and SAH concentrations. Within-batch accuracy and precisions were determined.
To estimate absolute extraction recoveries, extracted plasma samples were enriched with all previously described quality control concentrations for SAM and SAH (n = 3) and compared to a freshly extracted set of quality control samples.
Dilution integrity was established by preparing samples containing 20 μmol/l SAM and SAH. These samples were then diluted 1:25, 1:50, and 1:100 with 1/5 diluted plasma (n = 3). Potential carry-over was assessed by alternately analyzing plasma samples spiked with concentrations of SAM and SAH at the upper limit of quantitation (n = 6) followed by blank acetone/PBS (75/25, v/v) samples.
2.6. Stability testing
Bench top stabilities of the analytes were tested for plasma and stock solutions (24 h) at room temperature. To assess stability in plasma, quality control samples were prepared (n = 15/concentration) at low (37.5 nmol/l), medium (150 nmol/l) and high (900 nmol/l) concentrations for SAM and SAH. One set of samples as well as undiluted and diluted plasma containing endogenous concentrations were measured immediately for both compounds (baseline) using a newly prepared calibration curve while the other 4 sets remaining on the bench top over the test period (1, 2, 6, and 24 h). The remaining samples were analyzed and accuracies were compared to the baseline samples. To test bench top stock solution stability, a SAM and SAH (40 μmol/l) stock solution was freshly prepared. One set of QC samples was prepared immediately from the newly made stock at matching concentrations as described above. These samples were then analyzed using a newly made calibration curve while the original stocks were left on the bench over the test period (6 and 24 h). After the corresponding time had passed, the stocks were used to prepare another corresponding set of QC samples. These samples were then analyzed and accuracies were compared to those of the baseline samples.
Autosampler stability was determined by leaving extracted quality control samples of all 3 levels in the autosampler at 4 °C for 24 h and 48 h. Samples were analyzed and the results were compared with the results of the analyses immediately after extraction (baseline).
Freeze-thaw stability was determined by preparing QC samples with previously described concentrations (n = 3/concentration). Samples were then exposed to up to three freeze-thaw cycles. Results were compared to those of freshly prepared baseline QC samples at the corresponding concentrations. As a result of previously published reports recommending that plasma acidification is necessary to avoid degradation during long term storage [16,18], long-term stability was assessed by preparing QC samples at previously described concentrations both acidified and non-acidified. Acidification was performed by adding 2 μl of 10% formic acid. One set of QC samples both acidified and non-acidified was extracted and analyzed immediately (time zero) while the remaining set was stored at −80 °C for 1 and 3 months (n = 3). These samples were then analyzed with freshly prepared calibration curves and the results were compared to those measured at baseline. In all cases stability was assumed if the results fell within 85– 115% of the measured concentrations at baseline.
2.7. Clinical sample analysis
Control EDTA plasma samples were obtained from 8 healthy volunteers and stored at −80 °C until analysis. Patient samples were obtained from a retrospective longitudinal, one-arm, single-center clinical trial including 47 de-novo single kidney transplant patients. All patients were scheduled to undergo or undergoing de novo renal transplantation at the Charité Campus Virchow (Berlin, Germany). The study was approved by the Charité's internal review board and all patients gave their written informed consent. The study was conducted in accordance with the rules of good clinical practice and in compliance with all other applicable national and international guidances and regulations. Exclusion criteria were the presence of HIV-positive patients, and re-transplantation or concomitant transplantation of another organ such as liver or pancreas. After informed consent, 14 ml of blood (4 ml heparinized, 10 ml EDTA) was taken from each patient prior to transplantation and on days 1, 3, 7 and months 1, 3, 6, 9 and 12 after transplantation. These samples were all obtained during routine blood draws either during the hospital phase immediately after transplantation or during routine follow-up visits. Samples were centrifuged at 3000g for 10 min (+4 °C), aliquoted, kept on ice and snap frozen at − 80 °C within 1 h of collection. Histopathology revealed a diagnosis of acute rejection in sixteen of 47 patients at one or more time-points; 18 patients had histological changes consistent with immunosuppressant nephrotoxicity; and 7 patients were diagnosed with both at different biopsy time-points. A total of 24 samples collected from 16 patients were analyzed as well as samples from the aforementioned 8 healthy individuals. Patients were selected based on the presence of histopathological changes consistent with acute rejection (n = 4), immunosuppressant nephrotoxicity (n = 4) as well as patients void of any allograft dysfunction or injury (n = 8). Selection of samples from patients with allograft injury included two samples: one sample taken at a time point preceding changes in graft function and the other one collected at a time point thereafter when kidney graft function had recovered and/or stabilized.
2.8. Statistical analysis
Non-parametric one-way analysis of variance (ANOVA) was used to test for differences among groups. Dunn's test was used for pairwise, post-hoc comparisons. Statistical significance was set at the p ≤ 0.05 level. If not mentioned otherwise, results are presented as mean (coefficient of variance). All statistical analyses were performed using SPSS, ver. 21.0 (IBM/SPSS, Chicago, IL).
3. Results
3.1. Assay development and validation
MS and MS/MS spectra were recorded after direct infusion of S-adenosylmethionine, S-adenosylhomocysteine and their deuterated internal standards S-adenosylmethionine-d3 and S-adenosylhomocysteine-d5 into the electrospray source via syringe pump (Harvard Scientific, Holiston, MA). Compounds were dissolved at a concentration of 50 nmol/l and infused at a rate of 70 μl/min. The following ion transitions were the predominant transitions and thus were selected for quantification: m/z = 399.0 [M + H]+ → 250.1 for S-adenosylmethionine, m/z = 385.1 [M + H]+ → 136.2 for S-adenosylhomocysteine, m/z = 402.0 [M + H]+ → 250.1 for the deuterated S-adenosylmethionine-d3 internal standard and m/z = 390.0 [M + H]+ → 137.2 for deuterated S-adenosylhomocysteine-d5 internal standard. The upper limit of quantitation was 1024 nmol/l for both SAM and SAH. The lower limits of quantitation were 8 and 16 nmol/l for SAM and SAH, respectively. The limits of detection for SAM and SAH obtained using the empirical approach previously described by Armbruster et al. and defined as the analyte concentration corresponding to a signal-to-noise ratio of 3, were 1 nmol/l and 8 nmol/l respectively [22]. Calibration curves for both analytes were generated using linear curve fit with 1/× weighting which proved to give the most reliable results as seen in Fig. 2. The correlation coefficients (r) for the calibration curves were r2 ≥ 0.99 encompassing a concentration range of 8–1024 nmol/l for SAM and 16-1024 nM for SAH. Intra- and inter-day accuracies and precisions were within predefined acceptance criteria as seen in Table 1.
Fig. 2.
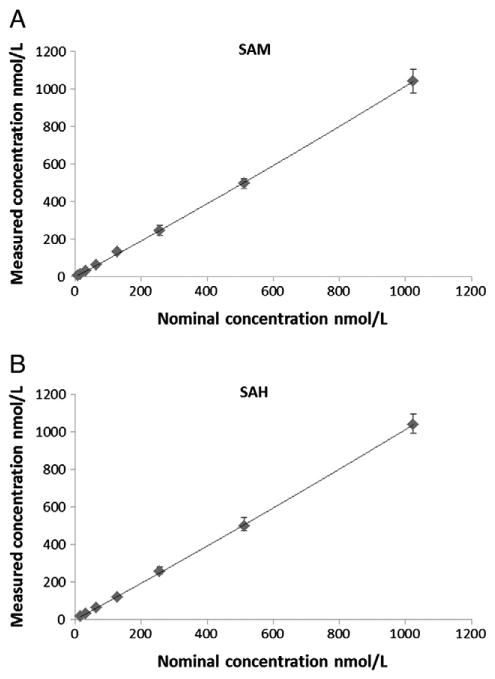
Calibration curves for SAM and SAH(n = 6). SAM:y = −0.008857x + 0.004292, r2 = 0.9942; SAH: y = 0.01156x + 0.012, r2 = 0.9925.
Table 1.
Intra-day and inter-day accuracies and precisions as well as extraction recoveries for S-adenosylmethionine and S-adenosylhomocysteine.
| Analyte | Quality control concentration | Intra-day accuracy ± coefficient of variation | Inter-day accuracy ± coefficient of variation | Recovery ± coefficient of variation |
|---|---|---|---|---|
|
|
|
|
|
|
| nmol/L | [%] | [%] | [%] | |
| SAM | 37.5 | 93.4 ± 6.6 | 96.5 ± 9.2 | 99.7 ± 7.2 |
| 75 | 96.6 ± 5.3 | 99.3 ± 8.6 | 99.8 ± 2.4 | |
| 150 | 91.9 ± 4.4 | 97.4 ± 8.9 | 99.2 ± 2.1 | |
| 300 | 105.5 ± 2.4 | 101.5 ± 8.7 | 104.6 ± 3.0 | |
| 900 | 98.9 ± 7.1 | 103.9 ± 8.1 | 103.3 ± 2.2 | |
| SAH | 37.5 | 95.0 ± 8.1 | 98.9 ± 8.7 | 96.7 ± 5.8 |
| 75 | 90.3 ± 4.3 | 99.3 ± 9.6 | 95.4 ± 2.3 | |
| 150 | 89.7 ± 3.0 | 97.9 ± 9.8 | 92.7 ± 3.4 | |
| 300 | 98.5 ± 5.3 | 99.0 ± 9.7 | 97.1 ± 1.1 | |
| 900 | 90.3 ± 2.5 | 98.9 ± 8.4 | 103.5 ± 4.4 |
Analysis of plasma from six different individuals in addition to a post-column infusion matrix effect experiment confirmed the absence of ion suppression/enhancement caused by the matrix for both analytes (for further details please see the Supplementary Materials, Table S1 and Fig. S1). Extraction recoveries for SAM and SAH ranged from 99.2 to 104.6% and 92.7 to 103.5% (Table 1). Using water/acetone (7/3, v/v) as autosampler wash solvents with additional needle wash steps incorporated into the injection cycle resulted in lack of detectable carry-over. The concentrations of SAM and SAH in the three sets of diluted samples (1/25, 1/50, and 1/100) were found to be within predefined acceptance criteria (85–115%) with mean accuracies of 106.6, 108.6, and 113.3% for SAM and 94.0, 99.5, and 107.8% for SAH.
Increased triglyceride and bilirubin concentrations as well as hemolysis did not interfere with the quantification of both SAM and SAH with within-batch accuracies and precisions meeting predefined acceptance criteria (accuracy: 85–115% of nominal concentrations, precision ≤ 15%, for details please see Table S2).
None of the extracted samples were affected by 48-h storage in the autosampler at 4 °C with both analytes measuring within ±15% of the nominal concentration (Table S3). Both SAM and SAH showed no degradation through three freeze-thaw cycles (Table S4). Short-term storage of stock solutions under the conditions tested through 24 h did not have an effect on the integrity of the data. However, short term storage of non-extracted quality control samples displayed minimal degradation through 6 h but significant degradation after 24 h storage at room temperature as seen in Table S5. Long term storage of both acidified and non-acidified quality control samples showed negligible degradation after three months of storage at −80 °C (Table S6).
The representative ion chromatograms for an endogenous 1/5 diluted human plasma sample, 1/5 diluted human plasma spiked with 8 nmol/l SAM and SAH, and a representative patient sample are shown in Fig. 3A, B, and C.
Fig. 3.
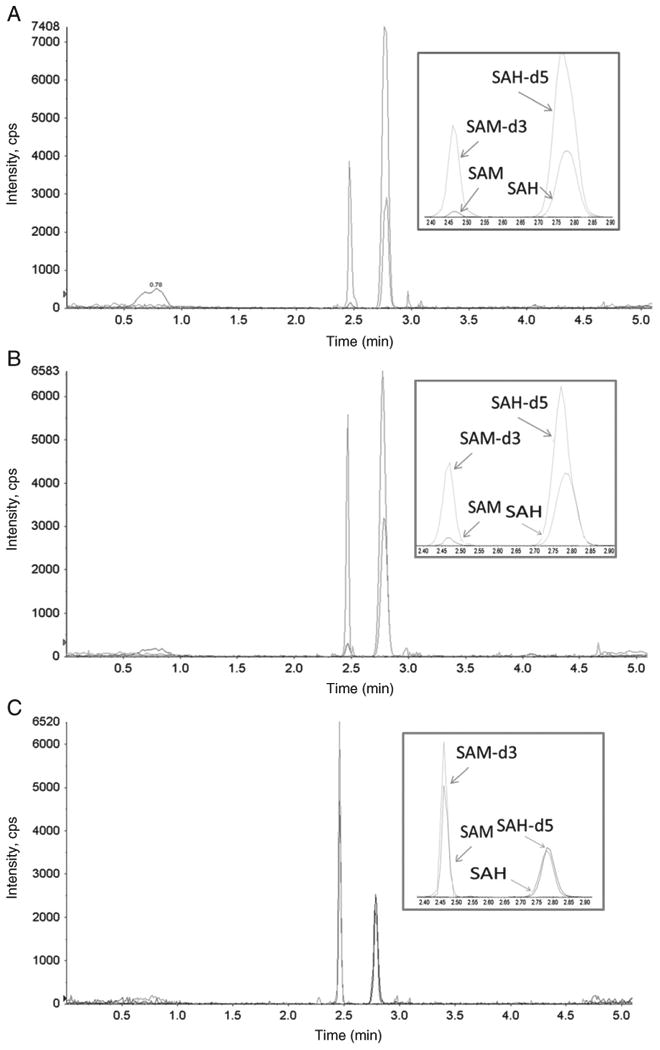
Representative ion chromatogram from 1/5 diluted neat plasma (A), 1/5 diluted neat plasma spiked with 8 nmol/l SAM and SAH (B), as well as a renal allograft recipient (C). The following concentrations were found: SAM: 115.1 nmol/l and SAH: 87.5 nmol/l.
3.2. Clinical sample analysis
Analysis of patient samples collected from stable patients without any biopsy proven clinical events affecting the transplant kidney (SAR), samples collected from patients with biopsy-proven allo-immune reaction against the transplant kidney or immunosuppressive drug nephrotoxicity at a time point after kidney graft function had stabilized again (CAR), as well as samples collected from healthy individuals (HV) had SAM (Fig. 4A) and SAH concentrations (Fig. 4B) in the same concentration range. This was confirmed by non-parametric analysis of variance in combination with Dunn's post-hoc test that did not indicate any statistically significant differences between these sample sets. However, SAM (Fig. 4A) and SAH concentrations (Fig. 4B) were significantly higher in samples collected prior to the diagnosis of biopsy-proven allo-immune reaction against the transplant kidney or immunosuppressive drug nephrotoxicity (EAR) than in the aforementioned sample sets (Dunn's post-hoc test: SAM: p < 0.001 and SAH p < 0.002 when compared to samples collected from the same patients after kidney transplant function had stabilized again) (also see Supplemental Data Table S7).
Fig. 4.
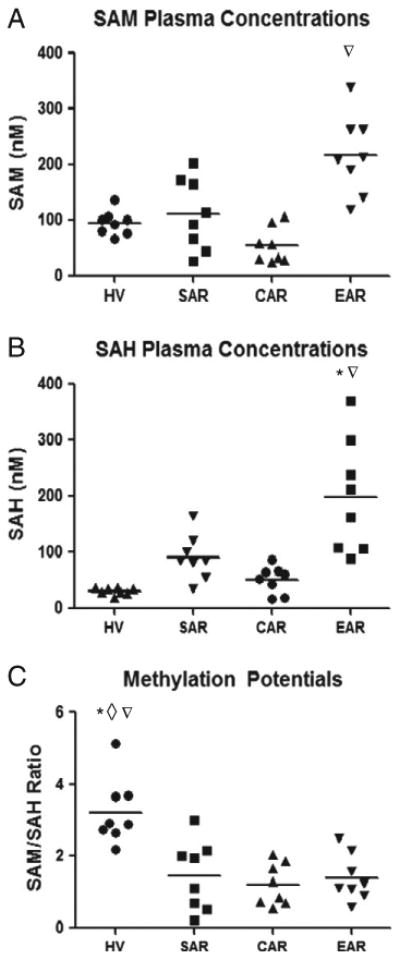
Scatter plot showing comparison of (A) S-adenosylmethionine (SAM), (B) S-adenosylhomocysteine (SAH) and (C) methylation potentials between healthy volunteers (HV) (n = 8), stable allograft recipients (SAR) without any documented allograft dysfunction (n = 8), control allograft recipients (CAR) with documented acute rejection (n = 4) and nephrotoxicity events (n = 4) but samples collected during time points void of any graft dysfunction, and samples collected prior to the diagnosis of biopsy-proven clinical events such as allo-immune rejection or immunosuppressant toxicity (EAR) A) ANOVA: 0.0004, post-hoc Dunn's test: EAR versus CAR p < 0.001∇. B) ANOVA: 0.0001, post-hoc Dunn's test: EAR versus HV, and CAR p < 0.001*, 0.05∇. (C) ANOVA: 0.001, post-hoc Dunn's test: HV versus SAR, CAR, and EAR p = 0.05*, 0.01◊, 0.05∇.
Although individual concentrations of both SAM and SAH increased markedly prior to biopsy-proven allo-immune rejection against the transplant kidney and prior to immunosuppressive drug nephrotoxicity, methylation potentials (SAM/SAH ratio), which have previously been shown to possess diagnostic potential [10,11,14], remained largely unchanged in the samples collected from transplant patients. As seen in Fig. 4C, methylation potentials were significantly lower in samples from kidney transplant patients with averages of 1.48 ± 0.34, 1.21 ± 0.94 and 1.38 ± 0.23 (means ± SD) for SAR, CAR, and EAR respectively, when compared to healthy volunteers which showed an average of 3.23 ± 0.33 (mean ± SD).
4. Discussion
Unlike previously published assays which require complex multi-step sample preparation involving either solid phase extractions [15,16,18] or multi-step acidification and protein precipitation extractions [16,18], in addition to extended chromatographic run times to achieve proper separation [14,17,18], our assay requires a simple one-step cold acetone precipitation with a total assay time of only 5 min between injections. Moreover, the assay presented here is validated following current FDA guidelines for bioanalytical method validation and thus can be considered fit for clinical sample analysis. A common issue with developing assays for the quantification of endogenous compounds is the selection of a proper blank matrix for validation purposes and for the preparation of calibrators and quality control samples, as, in contrast to drug analysis, blank plasma that does not contain the analyte (the endogenous compound at physiological concentrations) is often not available. An adequate blank surrogate matrix should mimic the characteristics of the clinical sample matrix as closely as possible [19,20]. In particular, the matrix effects exerted by surrogate matrices should be analogous to the matrix effects exerted by patient samples [23]. While most previously published methods have used inorganic surrogate matrices such as an aqueous buffer or the mobile phase, our assay utilizes a surrogate matrix consisting of 1/5 diluted human plasma (for the lower concentrations) and undiluted human EDTA plasma (for higher concentrations) for calibrators and quality control samples. Subsequently, as alluded earlier, when using complex matrices such as plasma, ion suppression becomes a major obstacle and extensive sample cleanup is often required [24]. This constitutes the inherent risk that simplified extraction methods, as developed during the present study, may result in extracted samples that are more prone to matrix effects. Consequently, ion suppression effects were thoroughly investigated performing both post-column infusion experiments as well as extraction and analysis of blank plasma samples collected from six different individuals following the strategy as described by Matuszewski et al. [21]. With these experiments we were able to exclude any meaningful matrix effects as seen in Table S1 and Fig. S1 confirming that our rapid one-step extraction procedure was a valid approach. It also needs to be considered that one of the major strengths of the present assay is the use of appropriate isotope-labeled internal standards that add another layer of protection against potential matrix effects.
While, as aforementioned, chronic allograft dysfunction is the primary cause of renal allograft loss after the first year, cardiovascular complications remain the foremost cause of death of kidney transplant patients [1]. Although the heightened risk of cardiovascular disease in transplant recipients is the result of multiple factors, immunosuppressant side effects on endothelial function are principal contributors [25]. As mentioned earlier, recent studies have shown evidence that elevated concentrations of S-adenosylmethionine (SAM) and S-adenosylhomocysteine (SAH) can be used as sensitive indicators of vascular disease [8]. Moreover, decreases in the SAM/SAH ratio, which is also commonly referred to as “methylation potential” have been observed in patients with end stage renal failure and pediatric renal transplant patients suggesting a link between disturbed transmethylation reactions, endothelial dysfunction and impaired renal function [7,10,11,14]. After successful validation, we tested our assay in a retrospective, clinical pilot study. The results suggested that (A) samples collected during or prior to clinical events affecting the kidney graft such as biopsy-proven acute allo-immune reaction and immunosuppressive drug nephrotoxicity showed significantly higher concentrations of SAM and SAH than samples from transplant patients during time periods of stable kidney graft function or from health individuals; and (B) that SAM/SAH ratios in kidney transplant patients were consistently lower than in healthy individuals independent of the clinical status of the transplant patients. There was no difference in SAM/SAH ratios when samples were collected prior to biopsy-proven allo-immune rejection or immunosuppressant toxicity or during times of stable kidney graft function, but all samples collected from transplant patients had significantly lower SAM/SAH ratios than those found in healthy individuals.
5. Conclusion
These results provide the rationale for further adequately powered, prospective clinical trials systematically assessing the potential value of monitoring SAM and SAH concentrations and their longitudinal changes using the present assay in individual patients as diagnostic markers for clinical risk evaluation and mitigation strategies (REMS) for the management of kidney transplant patients. But most importantly for the present study, the results provided first proof-of-concept that our LC–MS/MS assay for the quantification of SAM and SAH in human EDTA plasma is suitable for the analysis of clinical trial samples.
Supplementary Material
Acknowledgments
This work was supported by the United States National Institutes of Health, grant R01HD070511.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.cca.2013.03.003.
References
- 1.Christians U, Klawitter J, Brunner N, Schmitz V. Biomarkers of immunosuppressant organ toxicity after transplantation: status, concepts and misconceptions. Expert Opin Drug Metab Toxicol. 2011;7:175–200. doi: 10.1517/17425255.2011.544249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merville P. Combating chronic renal allograft dysfunction: optimal immunosuppressive regimens. Drugs. 2005;65:615–31. doi: 10.2165/00003495-200565050-00004. [DOI] [PubMed] [Google Scholar]
- 3.Chapman JR. Clinical renal transplantation: where are we now, what are our key challenges? Transplant Proc. 2010;42:S3–6. doi: 10.1016/j.transproceed.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 4.Nankivell BJ, Alexander SI. Rejection of the kidney allograft. N Engl J Med. 2010;363:1451–62. doi: 10.1056/NEJMra0902927. [DOI] [PubMed] [Google Scholar]
- 5.Alachkar N, Rabb H, Jaar BG. Urinary biomarkers in acute kidney transplant dysfunction. Nephron Clin Pract. 118:c173–c181. doi: 10.1159/000321381. [DOI] [PubMed] [Google Scholar]
- 6.Korbely R, Wilflingseder J, Perco P, et al. Molecular biomarker candidates of acute kidney injury in zero-hour renal transplant needle biopsies. Transpl Int. 2011;24:143–9. doi: 10.1111/j.1432-2277.2010.01162.x. [DOI] [PubMed] [Google Scholar]
- 7.Andrade F, Rodriguez-Soriano J, Prieto JA, et al. Methylation cycle, arginine-creatine pathway and asymmetric dimethylarginine in paediatric renal transplant. Nephrol Dial Transplant. 2011;26:328–36. doi: 10.1093/ndt/gfq404. [DOI] [PubMed] [Google Scholar]
- 8.Wagner C, Koury MJ. S-adenosylhomocysteine: a better indicator of vascular disease than homocysteine? Am J Clin Nutr. 2007;86:1581–5. doi: 10.1093/ajcn/86.5.1581. [DOI] [PubMed] [Google Scholar]
- 9.Kerins DM, Koury MJ, Capdevila A, Rana S, Wagner C. Plasma S-adenosylhomocysteine is a more sensitive indicator of cardiovascular disease than plasma homocysteine. Am J Clin Nutr. 2001;74:723–9. doi: 10.1093/ajcn/74.6.723. [DOI] [PubMed] [Google Scholar]
- 10.Jabs K, Koury MJ, Dupont WD, Wagner C. Relationship between plasma S-adenosylhomocysteine concentration and glomerular filtration rate in children. Metabolism. 2006;55:252–7. doi: 10.1016/j.metabol.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 11.Valli A, Carrero JJ, Qureshi AR, et al. Elevated serum levels of S-adenosylhomocysteine, but not homocysteine, are associated with cardiovascular disease in stage 5 chronic kidney disease patients. Clin Chim Acta. 2008;395:106–10. doi: 10.1016/j.cca.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 12.Brosnan JT, Brosnan ME. The sulfur-containing amino acids: an overview. J Nutr. 2006;136:1636S–40S. doi: 10.1093/jn/136.6.1636S. [DOI] [PubMed] [Google Scholar]
- 13.Chiang PK, Gordon RK, Tal J, et al. S-adenosylmethionine and methylation. FASEB J. 1996;10:471–80. [PubMed] [Google Scholar]
- 14.Jiang Z, Liang Q, Luo G, Hu P, Li P, Wang Y. HPLC-electrospray tandem mass spectrometry for simultaneous quantitation of eight plasma aminothiols: application to studies of diabetic nephropathy. Talanta. 2009;77:1279–84. doi: 10.1016/j.talanta.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 15.Kirsch SH, Knapp JP, Geisel J, Herrmann W, Obeid R. Simultaneous quantification of S-adenosyl methionine and S-adenosyl homocysteine in human plasma by stable-isotope dilution ultra performance liquid chromatography tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:3865–70. doi: 10.1016/j.jchromb.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 16.Struys EA, Jansen EE, de Meer K, Jakobs C. Determination of S-adenosylmethionine and S-adenosylhomocysteine in plasma and cerebrospinal fluid by stable-isotope dilution tandem mass spectrometry. Clin Chem. 2000;46:1650–6. [PubMed] [Google Scholar]
- 17.Melnyk S, Pogribna M, Pogribny IP, Yi P, James SJ. Measurement of plasma and intracellular S-adenosylmethionine and S-adenosylhomocysteine utilizing coulometric electrochemical detection: alterations with plasma homocysteine and pyridoxal 5′-phosphate concentrations. Clin Chem. 2000;46:265–72. [PubMed] [Google Scholar]
- 18.Stabler SP, Allen RH. Quantification of serum and urinary S-adenosylmethionine and S-adenosylhomocysteine by stable-isotope-dilution liquid chromatography-mass spectrometry. Clin Chem. 2004;50:365–72. doi: 10.1373/clinchem.2003.026252. [DOI] [PubMed] [Google Scholar]
- 19.CDER; U.S. Department of Health and Human Services, Food and Drug Administration, (CVM) CfDEaRaCfVM. Guidance for the industry Bioanalytical method validation. 2001 2001:< http://www.fda.gov/cder/guidance>.
- 20.Tholen D. CLSI evaluation protocols. MLO Med Lab Obs. 2006;38(38):40–1. [PubMed] [Google Scholar]
- 21.Matuszewski BK, Constanzer ML, Chavez-Eng CM. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC–MS/MS. Anal Chem. 2003;75:3019–30. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- 22.Armbruster DA, Pry T. Limit of blank, limit of detection and limit of quantitation. Clin Biochem Rev. 2008;29(Suppl. 1):S49–52. [PMC free article] [PubMed] [Google Scholar]
- 23.Vogeser M, Seger C. Pitfalls associated with the use of liquid chromatography–tandem mass spectrometry in the clinical laboratory. Clin Chem. 2010;56:1234–44. doi: 10.1373/clinchem.2009.138602. [DOI] [PubMed] [Google Scholar]
- 24.Annesley TM. Ion suppression in mass spectrometry. Clin Chem. 2003;49:1041–4. doi: 10.1373/49.7.1041. [DOI] [PubMed] [Google Scholar]
- 25.Nickel T, Schlichting CL, Weis M. Drugs modulating endothelial function after transplantation. Transplantation. 2006;82:S41–6. doi: 10.1097/01.tp.0000231505.91988.26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.