Abstract
Altered expression of miR-29b is implicated in the pathogenesis and progression of liver fibrosis. We and others previously demonstrated that miR-29b down-regulates the expression of several extracellular-matrix (ECM) genes including Col 1A1, Col 3A1 and Elastin via directly targeting their 3′-UTRs. However, whether or not miR-29b plays a role in the post-translational regulation of ECM biosynthesis has not been reported. Heat shock protein 47 (HSP47) and lysyl oxidase (LOX) are known to be essential for ECM maturation. In this study we have demonstrated that expression of HSP47 and LOX was significantly up-regulated in culture-activated primary rat hepatic stellate cells (HSCs), TGF-β stimulated LX-2 cells and liver tissue of CCl4-treated mice, which was accompanied by a decrease of miR-29b level. In addition, over-expression of miR-29b in LX-2 cells resulted in significant inhibition on HSP47 and LOX expression. Mechanistically, miR-29b inhibited the expression of a reporter gene that contains the respective full-length 3′-UTR from HSP47 and LOX gene, and this inhibitory effect was abolished by the deletion of a putative miR-29b targeting sequence from the 3′-UTRs. Transfection of LX-2 cells with miR-29b led to abnormal collagen structure as shown by electron-microscopy, presumably through down-regulation of the expression of molecules involved in ECM maturation including HSP47 and LOX. These results demonstrated that miR-29b is involved in regulating the post-translational processing of ECM and fibril formation.
Keywords: Heat shock protein 47, lysyl oxidase, miR-29b, fibrosis, extracellular matrix
INTRODUCTION
Liver fibrosis is a wound-healing response characterized by an increased and altered deposition of extracellular matrix (ECM) components, particularly collagen typeIand III [1]. It has been demonstrated that liver fibrosis can be inhibited by suppressing the synthesis of ECM such as collagen.
The biosynthesis of collagen is a multi-step process which involves many intracellular and extracellular factors such as HSP47 and LOX. HSP47 is an endoplasmic reticulum (ER)-resident molecular chaperone specific for collagen synthesis, and it plays an essential role in procollagen processing. After the synthesis of -polypeptide chains, HSP47 assists the correct folding and stabilization of triple-helical procollagen molecules [2]. This process is crucial for subsequent secretion, cleavage and fibril formation of collagen [3]. The role of HSP47 on collagen maturation has been demonstrated both in vitro and in vivo [3,4,5,6].
Lysyl oxidase (LOX) is an extracellular enzyme that catalyzes the oxidative deamination of hydroxylysine and lysine residues in collagen and elastin. The resulting peptidyl aldehyde products spontaneously form covalent cross-links with unmodified lysine residues or with other peptidyl aldehyde residues. These cross-links are necessary for the formation of insoluble collagen and elastic fibers as well as mature functional extracellular matrix [7]. Abnormally increased lysyl oxidase enzyme activity can lead to excessive accumulation of insoluble collagen fibers and is directly associated with fibrotic diseases [8,9].
MiRNAs are small non-coding RNAs that repress the expression of target genes by interacting with their 3′-UTRs, causing mRNA degradation or translational repression [10]. Accumulating data have demonstrated the critical role of miRNAs in fibrotic diseases [11,12,13]. Among the reported miRNA species that were involved in liver fibrosis, miR-29 is emerging as a key suppressor of fibrotic changes [14,15]. MiR-29 is significantly down-regulated during liver fibrosis in mice and humans [14], and extensive interest has been focused on the mechanism by which miR-29b inhibits fibrosis. For example, Kwiecinski et al. have identified the profibrogenic growth factors PDGF-B, PDGF-C, IGF-I and VEGF-A as target genes of miR-29 [16]. We and others recently showed that members of miR-29 family can down-regulate multiple genes coding for ECM proteins including collagens, fibrillins, and elastin [17,18,19]. This regulation is achieved at post-transcriptional or translational level by targeting to their 3′-UTR.
In addition to altering the ECM expression at mRNA or translational level, microRNAs can also regulate the post-translational maturation of ECM by targeting relevant genes. For example, we have recently shown that over-expression of miR-122 markedly attenuated the expression of prolyl 4-hydroxylase subunit alpha-1 (P4HA1), leading to decreased collagen maturation and ECM production [20]. However, a possible role of miR-29b in post-translational modifications of ECM remains unexplored. In the present work, we investigated the role of miR-29b in ECM maturation through a post-translational mechanism.
MATERIALS AND METHODS
Animals and cell culture
Primary rat HSCs were isolated from retired male Sprague-Dawley rats (Charles River Laboratories. Wilmington, MA) [21]. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. The immortalized human hepatic stellate cell line LX-2 was kindly provided by Dr. Scott L. Friedman (Mount Sinai School of Medicine, New York, NY, USA) [22]. All of the cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 4.5 mg/mL glucose, supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 g/mL streptomycin at 37°C in 5% CO2. All products for cell culture were purchased from Invitrogen (Carlsbad, CA). To establish an animal model of liver fibrosis, carbon tetrachloride (CCl4; Merck; 0.6 mL/kg of body weight) was injected to mice via intraperitoneal route twice a week for six weeks [20].
RNA isolation and qRT-PCR
Total RNA was extracted from cells and tissues with TRIzol reagent, and the first-strand cDNA was synthesized using SuperScript III reverse transcriptase according to manufacturer’s instructions (Invitrogen, San Diego, CA). The qRT-PCR was performed using SYBR Green-based assays with the ABI Prism 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA) [17]. The analysis of miR-29b expression was performed using stem-loop real-time RT-PCR (SLqRT-PCR) according to our previous protocol [20]. The primers for qRT-PCR were obtained from MWG Biotech, and their sequences are listed in Table S1. The relative transcript abundance was analyzed as previously reported [17] and calculated from three independent experiments.
Western Blot analysis
Forty-eight h after transfection with 50 nM of control miRNA or miR-29b, the whole-cell lysates of LX-2 cells were prepared as described previously[17]. Equal amounts of protein (30 μg) were separated by 10% SDS-polyacrylamide gel and transferred to PVDF membrane (Millipore, Billerica, MA). The membranes were blocked in phosphate-buffered saline with 0.05% Tween 20 (TPBS) containing 5% skim milk and incubated with specific primary antibody (rabbit anti-HSP47 IgG, rabbit anti- β-actin IgG or goat-anti-LOX IgG, Santa Cruz Biotechnology) overnight, followed by incubation with secondary antibody (horseradish peroxidase-conjugated goat anti-rabbit IgG or horseradish peroxidase-conjugated donkey anti-goat IgG, Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 h. ECL chemiluminescence kit (Amersham Biosciences, Piscataway, NJ) was used to detect the signals from HSP47 and β-actin, and the LOX signal was detected with the SuperSignal West Femto Chemiluminescent Substrate (Thermo Fisher Scientific).
Plasmid Constructs
The wild-type 3′-UTR of HSP47 and LOX genes were PCR amplified from human genomic DNA using the primers listed in Table S1. The mutant 3′-UTR of HSP47 without putative miR-29b-binding sequence was also amplified. To clone the mutant 3′-UTR of LOX without the putative miR-29b binding sites, two fragments of LOX 3′-UTR were amplified separately and joined by overlap extension PCR according to a published method [23]. The PCR products of wild-type or mutant 3′-UTR for HSP47 were then cloned into the MluI-HindIII site downstream of the stop codon in the pMIR-REPORT Firefly Luciferase reporter vector (Ambion). The segments of wild-type or mutant 3′-UTR for LOX were cloned into the same vector at SacI-MluI site. The sequences of the generated constructs were confirmed by restriction digestion and sequencing.
Transfection and luciferase assays
LX-2 cells were grown in 96-well plates. Reporter plasmids and miRNAs (ABI, Applied Biosystems, Foster City, CA) were co-transfected into LX-2 cells using Lipofectamine2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s instruction. To normalize the value of luciferase activity for transfection efficiency and cell viability after transfection, the pCMV-βgal plasmid was co-transfected [24]. Cells were washed and lysed 24h following transfection as described [25], and the luciferase activity and β-galactosidase activity were measured. Transfection experiments were repeated three times independently and in each case were done in triplicate. Data were presented as relative luciferase activity of control miRNA.
LOX Activity Assays
To measure the effect of miR-29b on extracellular LOX activity, LX-2 cells were transfected with control miRNA or miR-29b and cultured in complete medium until confluent at which time it was replaced by serum free, phenol red free DMEM. At 3 days post-confluence, the conditioned media were collected and concentrated using 10,000 molecular weight cut-off Amicon Ultra centrifugal filter units (Millipore) [26]. The LOX enzyme activity of concentrated media was measured using the Amplex Red fluorescence assay as previously described [27]. The difference of fluorescent intensity value between the samples with or without BAPN was used to calculate the specific LOX activity. Data was normalized by total protein amount and expressed as the fold induction over control samples. All samples were assayed in triplicate.
Electron Microscopy
For the TEM study, LX-2 cells were cultured in 10% fetal bovine serum /DMEM in the presence of ascorbic acid phosphate (136 μg/mL). The EM study was performed 3-5 days after the cell culture became confluent. Cultured cells were rinsed in PBS and fixed with cold 2.5% glutaraldehyde in 0.1 M PBS. The specimens were rinsed in PBS, post-fixed in 1% Osmium Tetroxide with 0.1% potassium ferricyanide, rinsed in PBS, dehydrated through a graded series of ethanol and embedded in Epon. Semi-thin (300 nm) sections were cut on a Reichart Ultracut, stained with 0.5% Toluidine Blue and examined under the light microscope. Ultrathin sections (65 nm) were stained with uranyl acetate and Reynold’s lead citrate and examined on Jeol 1011 transmission electron microscope.
Statistical Analysis
Unpaired Student’s t-test was performed on data of qRT-PCR and luciferase assays. All data are reported as means ± SEM unless otherwise stated. P < 0.05 was considered statistically significant.
RESULTS and DISCUSSION
We hypothesized that miR-29b is involved in the post-translational modification of ECM proteins in addition to its role in regulating ECM expression at mRNA and translational levels. Computational prediction by the algorithms Target scan and miRanda has identified HSP47 and LOX as potential target genes of miR-29. Therefore, we further hypothesized that miR-29b inhibits ECM maturation through targeting HSP47 and LOX.
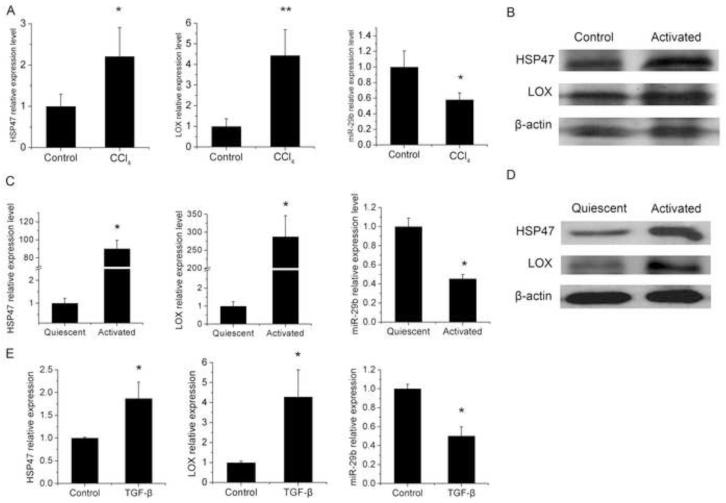
To test this hypothesis, we first examined the expression level of HSP47, LOX and miR-29b in a CCl4-induced model of liver fibrosis. I.P. injection of CCl4 to mice for 6 weeks resulted in significant upregulation of HSP47 and LOX mRNA in liver tissue, with significantly decreased miR-29b expression (Fig.1A). Similarly, the protein expression of HSP47 and LOX in the liver of CCl4 treated mice was significantly increased compared with vehicle-treated group (Fig. 1B). This is consistent with the reported data [28] [29]. Since HSCs are the major source of ECM and become activated during liver fibrosis, we then examined the gene expression in primary-cultured rat HSCs. It is apparent that the expression of HSP47 and LOX in culture-activated HSCs was dramatically increased at both mRNA (Fig. 1C) and protein levels (Fig. 1D) compared with that in quiescent cells, accompanied by the reduced expression of miR-29b (Fig. 1C). This is consistent with a recent report which shows inverse changes in the expression of miR-29 and LOX in HSCs treated with a HDAC inhibitor, MC1568 [30].
Fig. 1. Expression of HSP47, LOX and miR-29b in mouse liver with CCl4-induced fibrosis, culture-activated rat HSCs and TGF-β treated LX-2 cells.
CD-1 mice were treated with corn oil or CCl4 for 6 weeks (A-B). Quiescent and activated HSCs of rat were isolated and harvested as described in the Materials and methods (C-D). LX-2 cells were treated with TGF-β (5ng/ml) and harvested at 24h after treatment (E). Quantitative PCR was conducted to detect the expression levels of HSP47 and LOX mRNA and miR-29b. Gene expression level was normalized against the control groups, and data represent quantification of four independent experiments, *P < 0.05 (A, C and E). Western blots were conducted to detect the protein expression levels of HSP47 and LOX (B and D).
TGF-β signaling is known to play an important role in stimulating stellate cell activation and ECM synthesis [31,32]. To define a role of TGF-β signaling in regulating the expression of HSP47, LOX, and miR-29b during HSC transactivation, the mRNA expression levels of these genes were examined in LX-2 cells with or without TGF-β1 treatment. LX-2 is an immortalized human hepatic stellate cell line that exhibits typical features of primary HSCs such as over-expression of α-SMA and responsiveness to transforming growth factor-β (TGF-β) [22]. Fig. 1E showed that the expression of HSP47 and LOX was significantly up-regulated following TGF-β treatment. TGF-β-treatment also led to significant increases in the mRNA expression levels of HSP47 and LOX in LX-2 cells (Fig. 1E). Again, these changes were associated with a decrease in the expression level of miR-29b (Fig. 1E).These results strongly suggest a role of TGF-β signaling in regulating the expression of HSP47, LOX, and miR-29b during HSC transactivation.
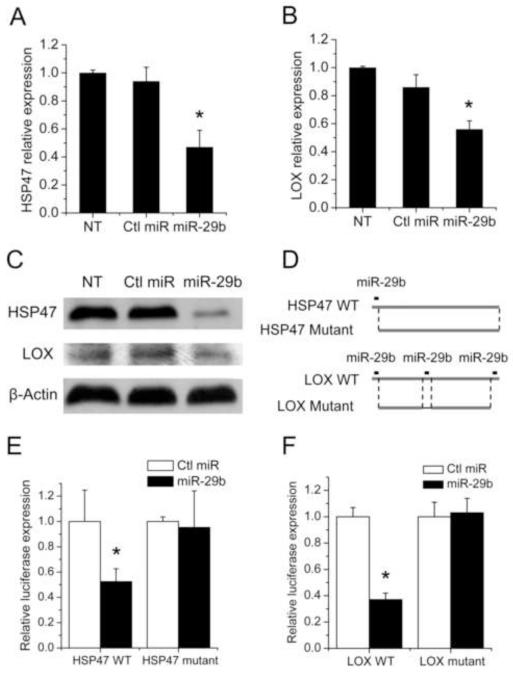
The above studies clearly show an inverse correlation between miR-29b and HSP47/LOX gene expression in both fibrotic liver and transactivated HSCs. To evaluate the contribution of decreased miR-29b expression to the increased expression of HSP47 and LOX during fibrotic changes, we investigated the effect of forced expression of miR-29b on the HSP47 and LOX expression level in LX-2 cells. LX-2 cells were transfected with miR-29b or a scramble-miR control sequence, and the mRNA expression of HSP47 and LOX was examined 24 h later by real-time RT-PCR. Fig. 2 showed that transfection of LX-2 cells with miR-29b led to a significant inhibition of HSP47 and LOX expression at both mRNA (Fig. 2A-B) and protein (Fig. 2C) levels. To confirm whether HSP47 and LOX are direct targets of miR-29b, the 3′-UTR of HSP47 or LOX gene with or without the putative target sites (Fig. 2D) was subcloned into a luciferase reporter vector. Each plasmid construct was co-transfected with miR-29b or control miRNA into LX-2 cells, followed by measurement of luciferase activity. As shown in Fig. 2E, miR-29b significantly inhibited the luciferase activity of the wild-type HSP47-3′-UTR construct. In contrast, such an inhibitory effect was essentially abolished when the putative miR-29b target sequence is deleted from the reporter construct (Fig. 2E). This is consistent with a recent report that miR-29a targets HSP47 in cervical squamous cell carcinoma [33]. Similar results were observed with the plasmid construct containing the 3′-UTR of LOX gene (Fig. 2F). These data suggest that miR-29b down-regulated the expression of HSP47 and LOX via targeting the putative binding sequence on the respective 3′-UTR of HSP47 and LOX mRNA. Taken together, all of the data above suggest a causal effect of decreased miR-29b expression on the upregulation of HSP47 and LOX during fibrotic changes.
Fig. 2. miR-29b decreased the expression of HSP47 and LOX by directly interacting with the 3-‘UTR of their mRNAs.
Cells were transfected with either non-specific control miRNA or miR-29b at a concentration of 50 nM. The mRNA expression levels of HSP47 (A) and LOX (B) were analyzed by qRT-PCR at 24 h post-transfection. Western blots were conducted to detect the HSP47 and LOX expression at 48 h post-transfection (C). (D) Scheme of wild-type and mutant 3′-UTRs of human HSP47 and LOX. Wild type 3′-UTRs include the putative binding sites highlighted. Deletion mutant eliminates the putative binding sites. (E) LX-2 cells were transfected with a luciferase construct with wildtype or mutant HSP47-3′-UTR in the presence of 50 nM miR-29b or non-specific control miRNA. Luciferase assay was performed 24 h post-transfection. (F) The same experiment with LOX 3′-UTR luciferase vectors as in (E), miR concentration=10nM. Luciferase activity was normalized against the control groups. Data shown in the panels represent means± (SD) of the fold increase over the control. N = 3. *P < 0.05 (vs. control miRNA).
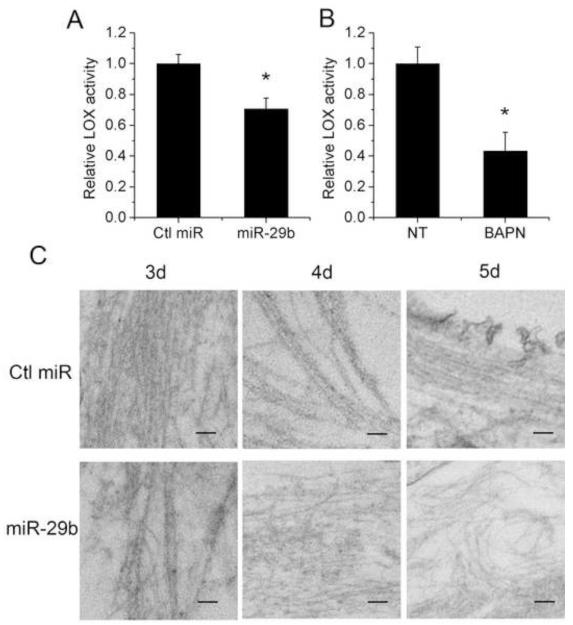
To investigate the physiological significance of the inhibitory effect of miR-29b on LOX expression, we transfected LX-2 cells with miR-29b and examined its effect on LOX enzymatic activity in the conditioned medium. Fig. 3 showed that LOX activity was significantly decreased by miR-29b (Fig. 3A) and LOX inhibitor BAPN (Fig. 3B). Then we further investigated the role of miR-29b in the post-translational maturation of ECM. LX-2 cells were transfected with miR-29b and the morphology of extracellular fibrils was examined by electron microscopy. The extracellular fibrils of miR-29b transfected cells were relatively thin and sparse with more branches compared with control miRNA treated cells at day 5 post-confluence (Fig. 3C). These observations are consistent with the reported data generated from HSP47−/− embryonic fibroblasts [3] or BAPN-treated osteoblasts [34]. These results suggest that miR-29b over-expression can lead to defects of extracellular fibrils, with the potential contribution from HSP47 and LOX inhibition.
Fig. 3. Transfection of LX-2 cells with miR-29b affects their extracellular LOX activity and morphology of extracellular fibrils.
(A) Extracellular LOX enzyme activity was significantly reduced in the supernatant of miR-29b transfected LX-2 cells 72 h post-transfection compared with that of control miRNA transfected cells. (B) LOX activity in the conditioned media was significantly inhibited by 100 μM BAPN. Data represent mean ± SD, n = 3. (*P < 0.05). (C) LX-2 cells were transfected with control miRNA or miR-29b. The extracellular fibrils were observed by Transmission Electron Microscopy at 3 d, 4 d and 5 d after the cells became confluent. Bar, 100 nm.
The lysyl oxidase family consists of five members: lysyl oxidase (LOX) and lysyl oxidase-like 1–4 (LOXL1-LOXL4). All members share conserved C-terminal catalytic domains that contribute to the enzyme activity in catalyzing the cross-linking of collagen and elastin molecules [35]. Recently Chou et al. [36] reported that miR-29b can inhibit tumor metastasis by targeting LOX, LOXL2 and LOXL4 in breast cancer cells, which indicates the implication of miR-29b and LOX in the regulation of tumor microenvironment besides ECM synthesis.
In view of the critical role of HSP47 and LOX in stabilizing collagen fibrils, both have been investigated as molecular targets in developing new therapies for the treatment of fibrotic diseases including liver fibrosis. An unique feature of miRNA-mediated gene regulation is that individual miRNA can regulate hundreds of genes at the same time [37]. For example, SPARC [38], BMP-1 [19] and fibronectin [39] have been reported as target genes of miR-29b, and those molecules may also contribute to the altered fibril formation besides HSP47 and LOX. This property of miRNAs allows them to coordinate complex programs of gene expression to achieve similar consequences. More studies are needed to better understand the regulation of each factor by miR-29 and their respective contribution to the altered ECM biosynthesis under various pathological conditions. Nevertheless, our observations strongly support a role of miR-29b in the regulation of ECM maturation during liver fibrosis.
In conclusion, we have shown that miR-29b plays a role in collagen maturation. These data, together with other published works, suggest complex mechanisms by which miR-29 regulates ECM biosynthesis at multiple steps. Our data further support the notion that miR-29 may become a promising therapeutics for the treatment of liver fibrosis.
Supplementary Material
Highlights.
▶Enhanced HSP47 and LOX expression is associated with decreased miR-29b level in liver fibrosis
▶miR-29b down-regulates HSP47 and LOX expression
▶The suppression of HSP47 and LOX by miR-29b is mediated by putative sites at their 3′-UTRs
▶miR-29b inhibits extracellular LOX activity and collagen maturation
ACKNOWLEDGEMENTS
This work was supported by a NIH grant (HL091828), a grant from University of Pittsburgh Central Research Development Fund, and National Natural Science Foundation of China (81228005 and 81273226).
Abbreviations
- ECM
extracellular matrix
- HSP47
heat shock protein 47
- LOX
lysyl oxidase
- HSCs
hepatic stellate cells
Footnotes
DISCLOSURES No conflicts of interest are declared by the author(s).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Olaso E, Friedman SL. Molecular regulation of hepatic fibrogenesis. J Hepatol. 1998;29:836–847. doi: 10.1016/s0168-8278(98)80269-9. [DOI] [PubMed] [Google Scholar]
- [2].Nagata K. HSP47: A collagen-specific molecular chaperone. Trends Biochem Sci. 1996;21:23–26. doi: 10.1016/0968-0004(96)80881-4. [DOI] [PubMed] [Google Scholar]
- [3].Ishida Y, Kubota H, Yamamoto A, Kitamura A, Bächinger HP, Nagata K. Type I collagen in Hsp47-null cells is aggregated in endoplasmic reticulum and deficient in N-propeptide processing and fibrillogenesis. Mol Biol Cell. 2006;17:2346–2355. doi: 10.1091/mbc.E05-11-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hosokawa N, Hohenadl C, Satoh M, Kuhn K, Nagata K. HSP47, a collagen-specific molecular chaperone, delays the secretion of type III procollagen transfected in human embryonic kidney cell line 293: A possible role for HSP47 in collagen modification. J Biochem. 1998;124:654–662. doi: 10.1093/oxfordjournals.jbchem.a022162. [DOI] [PubMed] [Google Scholar]
- [5].Nagai N, Hosokawa M, Itohara S, Adachi E, Matsushita T, Hokawa N, Nagata K. Embryonic lethality of molecular chaperone HSP47 knockout mice is associated with defects in collagen biosynthesis. J Cell Biol. 2000;150:1499–1505. doi: 10.1083/jcb.150.6.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rocnik EF, van der Veer E, Cao H, Hegele RA, Pickering JG. Functional linkage between the endoplasmic reticulum protein HSP47 and procollagen expression in human vascular smooth muscle cells. J Biol Chem. 2002;277:38571–38578. doi: 10.1074/jbc.M206689200. [DOI] [PubMed] [Google Scholar]
- [7].Kagan HM, Trackman PC. Properties and function of lysyl oxidase. Am J Respir Cell Mol Biol. 1991;5:206–210. doi: 10.1165/ajrcmb/5.3.206. [DOI] [PubMed] [Google Scholar]
- [8].Kagan HM. Intra- and extracellular enzymes of collagen biosynthesis as biological and chemical targets in the control of fibrosis. Acta Trop. 2000;77:147–152. doi: 10.1016/s0001-706x(00)00128-5. [DOI] [PubMed] [Google Scholar]
- [9].Sommer P, Gleyzal C, Raccurt M, Delbourg M, Serrar M, Joazeiro P, Peyrol S, Kagan H, Trackman PC, Grimaud JA. Transient expression of lysyl oxidase by liver myofibroblasts in murine schistosomiasis. Lab Invest. 1993;69:460–470. [PubMed] [Google Scholar]
- [10].He L, Hannon GJ. MicroRNAs: Small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- [11].Ji J, Zhang J, Huang G, Qian J, Wang X, Mei S. Over-expressed microRNA-27a and 27b influence fat accumulation and cell proliferation during rat hepatic stellate cell activation. FEBS Lett. 2009;583:759–766. doi: 10.1016/j.febslet.2009.01.034. [DOI] [PubMed] [Google Scholar]
- [12].Guo CJ, Pan Q, Li DG, Sun H, Liu BW. miR-15b and miR-16 are implicated in activation of the rat hepatic stellate cell: An essential role for apoptosis. J Hepatol. 2009;50:766–778. doi: 10.1016/j.jhep.2008.11.025. [DOI] [PubMed] [Google Scholar]
- [13].Lakner AM, Steuerwald NM, Walling TL, Ghosh S, Li T, McKillop IH, Russo MW, Bonkovsky HL, Schrum LW. Inhibitory effects of microRNA 19b in hepatic stellate cell-mediated fibrogenesis. Hepatology. 2012;56:300–310. doi: 10.1002/hep.25613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Roderburg C, Urban GW, Bettermann K, Vucur M, Zimmermann H, Schmidt S, Janssen J, Koppe C, Knolle P, Castoldi M, Tacke F, Trautwein C, Luedde T. Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology. 2011;53:209–218. doi: 10.1002/hep.23922. [DOI] [PubMed] [Google Scholar]
- [15].van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A. 2008;105:13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kwiecinski M, Noetel A, Schievenbusch S, Strack I, Elfimova N, Drebber U, Töx U, Dienes HP, Odenthal M. microRNA-29 regulates the expression of profibrogenic mediators in hepatic stellate cells. Z Gastroenterol. 2010;48:P1_22. [Google Scholar]
- [17].Li J, Zhang Y, Kuruba R, Gao X, Gandhi CR, Xie W, Li S. Roles of miR-29a in the antifibrotic effect of FXR in hepatic stellate cells. Mol Pharmacol. 2011 doi: 10.1124/mol.110.068247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sekiya Y, Ogawa T, Yoshizato K, Ikeda K, Kawada N. Suppression of hepatic stellate cell activation by microRNA-29b. Biochem Biophys Res Commun. 2011;412:74–79. doi: 10.1016/j.bbrc.2011.07.041. [DOI] [PubMed] [Google Scholar]
- [19].Luna C, Li G, Qiu J, Epstein DL, Gonzalez P. Role of miR-29b on the regulation of the extracellular matrix in human trabecular meshwork cells under chronic oxidative stress. Mol Vis. 2009;15:2488–2497. [PMC free article] [PubMed] [Google Scholar]
- [20].Li J, Ghazwani M, Zhang Y, Lu J, Fan J, Gandhi CR, Li S. miR-122 regulates collagen production via targeting hepatic stellate cells and suppressing P4HA1 expression. J Hepatol. 2012;58:522–528. doi: 10.1016/j.jhep.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Thirunavukkarasu C, Uemura T, Wang LF, Watkins SC, Gandhi CR. Normal rat hepatic stellate cells respond to endotoxin in LBP-independent manner to produce inhibitor(s) of DNA synthesis in hepatocytes. J Cell Physiol. 2005;204:654–665. doi: 10.1002/jcp.20366. [DOI] [PubMed] [Google Scholar]
- [22].Xu L, Hui AY, Albanis E, Arthur MJ, O’Byrne SM, Blaner WS, Mukherjee P, Friedman SL, Eng FJ. Human hepatic stellate cell lines, LX-1 and LX-2: new tools for analysis of hepatic fibrosis. Gut. 2005;54:142–151. doi: 10.1136/gut.2004.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- [24].Umesono K, Murakami KK, Thompson CC, Evans RM. Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell. 1991;65:1255–1266. doi: 10.1016/0092-8674(91)90020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].He F, Li J, Mu Y, Kuruba R, Ma Z, Wilson A, Alber S, Jiang Y, Stevens T, Watkins S, Pitt B, Xie W, Li S. Downregulation of endothelin-1 by farnesoid X receptor in vascular endothelial cells. Circ Res. 2006;98:192–199. doi: 10.1161/01.RES.0000200400.55539.85. [DOI] [PubMed] [Google Scholar]
- [26].Fogelgren B, Polgár N, Szauter KM, Újfaludi Z, Laczkó R, Fong KSK, Csiszar K. Cellular fibronectin binds to lysyl oxidase with high affinity and is critical for its proteolytic activation. J Biol Chem. 2005;280:24690–24697. doi: 10.1074/jbc.M412979200. [DOI] [PubMed] [Google Scholar]
- [27].Palamakumbura AH, Trackman PC. A fluorometric assay for detection of lysyl oxidase enzyme activity in biological samples. Anal Biochem. 2002;300:245–251. doi: 10.1006/abio.2001.5464. [DOI] [PubMed] [Google Scholar]
- [28].Masuda H, Fukumoto M, Hirayoshi K, Nagata K. Coexpression of the collagen-binding stress protein HSP47 gene and the alpha 1(I) and alpha 1(III) collagen genes in carbon tetrachloride-induced rat liver fibrosis. J Clin Invest. 1994;94:2481–2488. doi: 10.1172/JCI117617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Furui K, Takahara T, Ito H, Nakayama Y, Miyabayashi C, Higuchi K, Watanabe A, Ooshima A. Gene expression of lysyl oxidase in CCl4-induced liver injury. Int Hepatol Commun. 1995;3:3–3. [Google Scholar]
- [30].Mannaerts I, Eysackers N, Onyema OO, Van Beneden K, Valente S, Mai A, Odenthal M, van Grunsven LA. Class II HDAC inhibition hampers hepatic stellate cell activation by induction of microRNA-29. PLoS ONE. 2013;8:e55786. doi: 10.1371/journal.pone.0055786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gressner AM. Cytokines and cellular crosstalk involved in the activation of fat-storing cells. J Hepatol. 1995;22:28–36. [PubMed] [Google Scholar]
- [32].Gressner AM, Lotfi S, Gressner G, Haltner E, Kropf J. Synergism between hepatocytes and Kupffer cells in the activation of fat storing cells (perisinusoidal lipocytes) J Hepatol. 1993;19:117–132. doi: 10.1016/s0168-8278(05)80185-0. [DOI] [PubMed] [Google Scholar]
- [33].Yamamoto N, Kinoshita T, Nohata N, Yoshino H, Itesako T, Fujimura L, Mitsuhashi A, Usui H, Enokida H, Nakagawa M, Shozu M, Seki N. Tumor-suppressive microRNA-29a inhibits cancer cell migration and invasion via targeting HSP47 in cervical squamous cell carcinoma. Int J Oncol. 2013;43:1855–1863. doi: 10.3892/ijo.2013.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hong HH, Pischon N, Santana RB, Palamakumbura AH, Chase HB, Gantz D, Guo Y, Uzel MI, Ma D, Trackman PC. A role for lysyl oxidase regulation in the control of normal collagen deposition in differentiating osteoblast cultures. J Cell Physiol. 2004;200:53–62. doi: 10.1002/jcp.10476. [DOI] [PubMed] [Google Scholar]
- [35].Kagan HM, Ryvkin F. Lysyl Oxidase and Lysyl Oxidase-Like Enzymes. In: Mecham RP, editor. The Extracellular Matrix: an Overview. Springer; Berlin Heidelber: 2011. [Google Scholar]
- [36].Chou J, Lin JH, Brenot A, Kim J.-w., Provot S, Werb Z. GATA3 suppresses metastasis and modulates the tumour microenvironment by regulating microRNA-29b expression. Nat Cell Biol. 2013;15:201–213. doi: 10.1038/ncb2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Satoh J, Tabunoki H. Comprehensive analysis of human microRNA target networks. BioData Min. 2011;4:17. doi: 10.1186/1756-0381-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhu XC, Dong QZ, Zhang XF, Deng B, Jia HL, Ye QH, Qin LX, Wu XZ. microRNA-29a suppresses cell proliferation by targeting SPARC in hepatocellular carcinoma. Int J Mol Med. 2012;30:1321–1326. doi: 10.3892/ijmm.2012.1140. [DOI] [PubMed] [Google Scholar]
- [39].Kadler KE, Hill A, Canty-Laird EG. Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators. Curr Opin Cell Biol. 2008;20:495–501. doi: 10.1016/j.ceb.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.