Abstract
Background
Women account for 23% of newly diagnosed HIV infections in the United States, but there are few recent, well-characterized cohorts of U.S. women in whom behavior characteristics and HIV acquisition have been well-described.
Objective
To evaluate HIV incidence and describe behaviors among U.S. women residing in areas of high HIV prevalence.
Design
Multisite, longitudinal cohort of women who had HIV rapid testing and audio computer-assisted self-interviews at baseline and every 6 months for up to 12 months. (ClinicalTrials.gov: NCT00995176)
Setting
10 urban and periurban communities with high HIV prevalence and poverty rates, located in the northeastern and southeastern United States.
Patients
Venue-based sampling was used to recruit women aged 18 to 44 years who recently had unprotected sex and had 1 or more additional personal or partner risk factors and no self-reported previous HIV diagnosis.
Measurements
HIV prevalence and incidence, frequency of HIV risk behaviors, and health status perceptions.
Results
Among 2099 high-risk women (85.9% black and 11.7% of Hispanic ethnicity), 32 (1.5%) were diagnosed with HIV infection at enrollment. Annual HIV incidence was 0.32% (95% CI, 0.14% to 0.74%). Older age, substance use, and knowing a partner had HIV were associated with HIV prevalence. Ten women died during the study (0.61% per year).
Limitations
Longitudinal assessment of risk behaviors was limited to a maximum of 12 months. There were few incident HIV infections, precluding identification of characteristics predictive of HIV acquisition.
Conclusion
This study enrolled a cohort of women with HIV incidence substantially higher than the Centers for Disease Control and Prevention national estimate in the general population of U.S. black women. Concerted efforts to improve preventive health care strategies for HIV and overall health status are needed for similar populations.
The HIV epidemic in the United States is concentrated in subpopulations as defined by geography, poverty indicators, race or ethnicity, and transmission method (1). Women constitute 23% of newly diagnosed HIV infections in the United States, most of which are acquired by heterosexual transmission (2). In 2010, surveillance data from 46 states found that 64% of newly diagnosed HIV infections among U.S. women occurred among black women, although black women constituted only 12% of the female population (3). Limited information about risk behaviors and HIV incidence in women, coupled with an inability to enroll U.S. women at high risk for HIV infection into research studies with HIV incidence as the end point, has impeded progress in HIV prevention for this population (4). To evaluate HIV incidence among U.S. women living in geographic areas with high rates of poverty and HIV prevalence and to assess factors that may increase risk for HIV and other health problems, the HIV Prevention Trials Network (HPTN) conducted study HPTN 064, the Women’s HIV SeroIncidence Study. The HPTN, funded by the National Institutes of Health, is a worldwide collaborative clinical trials network that develops and tests the safety and efficacy of primarily nonvaccine interventions for preventing HIV infection (5). The study was designed to increase understanding of the risk for HIV infection among certain populations of U.S. women and to provide information about risk behaviors.
Methods
Study Design
The HPTN 064 was a multisite, longitudinal cohort study. Eligible women were enrolled between May 2009 and July 2010 from 10 urban and periurban communities in 6 geographic areas of the United States (Atlanta, Georgia; Baltimore, Maryland; New York, New York; Newark, New Jersey; Raleigh/Durham, North Carolina; and Washington, DC). The study was approved by institutional review boards at each site and collaborating institutions, and a certificate of confidentiality was obtained.
Participants received HIV rapid testing and audio computer-assisted self-interviews at baseline and at 6-month intervals for up to 12 months. The audio computer-assisted self-interview included questions about socioeconomic factors, food insecurity, mental health (depression and posttraumatic stress disorder [PTSD]), sexual behavior, history of sexually transmitted infections, domestic violence, health perceptions, and social support. Many questions were derived from previous studies, such as the National HIV Behavioral Surveillance System surveys. The Center for Epidemiologic Studies Depression Scale used in HPTN 064 is a shortened version of the standard 32-item scale (6) and has been used by DiClemente and colleagues (7) in young black women. A score of 7 or higher (on an 8-item scale) was indicative of psychological distress or depressive symptoms. The Primary Care PTSD Screen, designed for use in general health care settings, indicated possible presence of PTSD if 3 or more questions had positive responses (8).
The protocol was designed to follow one half of the participants for 6 months and the other half for 12 months to provide the required person-months of observation, as assumed in the power calculations. However, during the study, based on a recommendation by the Scientific Monitoring Committee, we decided to increase total personyears of follow-up. Therefore, study participants in the 6-month group who had not completed follow-up at the time the decision was made and met extension criteria were eligible to continue participation for up to 12 months.
Inclusion Criteria and Definitions
Eligible persons were aged 18 to 44 years, identified themselves as women (transgender persons were eligible), reported at least 1 episode of unprotected vaginal or anal sex with a man in the 6 months before enrollment, and were willing to have HIV rapid testing and receive results. We used 2 additional inclusion criteria. The first was the reporting of 1 or more of the following in the past 6 months (except for incarceration, which could have occurred in the past 5 years): illicit injection or noninjection drug use (heroin, cocaine, crack cocaine, methamphetamine, or prescription drugs apart from those prescribed by a licensed provider); alcohol dependence (defined as CAGE [Cut Down, Annoyed, Guilty, and Eye Opener] score ≥2) (9); binge-drinking, defined as 4 or more drinks at a time; incarceration (jail or prison ≥24 hours); self-reported history of sexually transmitted infections, such as gonorrhea, chlamydia, or syphilis; exchange of sex for commodities, such as drugs, money, or shelter; or reported male sexual partner with reported history of either injection or noninjection drug use, sexually transmitted infections, HIV diagnosis, history of binge-drinking (≥5 drinks at a time), alcohol dependence (CAGE score ≥2) (9), or incarceration (jail or prison ≥24 hours within the past 5 years). The second additional requirement was that persons reside in census tracts (except the Bronx and Harlem in New York, where ZIP codes were used) that ranked in the top 30th percentile of HIV prevalence and with more than 25% of inhabitants living below the U.S. federal poverty threshold, as defined by the 2008 U.S. Census Bureau (10). These residential criteria are similar to those used by the Centers for Disease Control and Prevention–sponsored NHBS-HET (National HIV Behavioral Surveillance System of heterosexuals) study to define areas with high HIV burden (11, 12). Exclusion criteria included self-reported history of previous positive results on an HIV test, current HIV prevention trial enrollment, current or past participation in an HIV vaccine trial, or anticipated absence for more than 2 consecutive months during follow-up.
Participant Recruitment and Retention
Venue-based recruitment using time–space sampling, a method used successfully in previous studies to obtain large, diverse samples from hard-to-reach populations (13–15), was conducted in an effort to sample women who may not be reached using standard recruitment methods. Specific venues (or locations) in which young women from the target census tracts (or ZIP codes) could reasonably be expected to congregate were identified by several methods, including focus groups of similarly aged community women, interviews of knowledgeable community experts (for example, personnel from previous survey studies, such as the NHBS-HET) who provided a list of potential locations frequented by young women, and individual discussions with community members. A list of potential venues (such as laundromats, street corners, or liquor stores) was created by each study site. Venues were then evaluated by study personnel, examining whether the target population (that is, women likely to meet eligibility criteria) frequented the location and when. Those frequented by few women between ages 18 and 44 years were eliminated. In addition, whether the venues were feasible recruitment locations was assessed; those considered unsafe or physically inadequate or for which permission (if required) could not be procured were also eliminated from the final venue list. A sampling frame of venues for specified periods during the day and evening was constructed. Venues for specified periods were randomly selected each month from the sampling frame to construct a sampling event calendar. To minimize selection bias, women present at a designated venue were systematically approached for prescreening when they entered a predetermined “recruitment area” (for example, study staff designated the exact space that a potential participant must enter before she was approached and asked about possible study participation). Women giving verbal approval for prescreening were asked a limited number of eligibility questions in a more private area of the recruitment venue (for example, outside the direct line of foot traffic). Women who met eligibility criteria and provided written consent to participate in the HPTN 064 study were subsequently enrolled in an area that provided additional privacy (that is, the clinical research site, mobile van, or private room at the venue). To encourage participants to return for scheduled follow-up appointments, study staff contacted participants monthly (usually by phone) to update contact information, after which a nominal amount of money was remunerated. At each in-person follow-up visit, participants were compensated for their time and transportation and provided with free condoms and counseling on HIV prevention measures.
Retention, calculated at 6 and 12 months, was defined as the number of persons who completed the required study visit divided by the total eligible number. Participants enrolled for 12 months who missed the 6-month visit were permitted to complete the 12-month visit. Participants who tested HIV-positive at enrollment or any time during the study were retained in the cohort and referred to HIV care.
Outcomes
The primary outcome of the study was a composite measure of HIV incidence that included infection acquired shortly before enrollment (recent infections), acute infection detected at study entry, and seroconversion that occurred during study follow-up. Acute HIV infection was detected using a 4th-generation antigen/antibody assay or an HIV RNA test (16). Recent HIV infection was assessed at study entry using a multiassay algorithm that included the HIV-1 subtypes B, E, and D (BED) capture enzyme immunoassay (which measures the proportion of IgG that is HIV-specific [17]), an assay that measures the avidity of anti-HIV antibodies for target antigens (18), CD4 cell count, and HIV RNA viral load (19). Women with HIV were characterized as recently infected if they had all of the following test results: BED capture enzyme immunoassay less than 1.0 normalized optical density units, avidity index less than 80%, CD4 count greater than 0.200 × 109 cells/L, and HIV RNA viral load greater than 400 copies/ mL (16). During follow-up, HIV seroconversion was assessed using HIV rapid test screening with Western blot confirmation.
Information about participants’ deaths was ascertained by research staff. For persons lost to follow-up, the Social Security index, local obituaries, and other relevant sources were searched. Mortality rate was calculated by dividing the total number of deaths by the sum of the follow-up time for all participants.
Sample Size Assessment
The goal was enrollment of 210 women per participating site for a sample size of 2100 women. Assuming 5% HIV prevalence at enrollment and 10% loss to follow-up, approximately 1350 person-years of follow-up were needed to estimate the 95% CI for the annual HIV incidence with a width of 1.5% (assuming that the incidence would be approximately 2%). We assumed that analysis of infections acquired shortly before enrollment would yield an additional 830 person-years of data and, when pooled with the longitudinal data, would yield a CI with a width of 1.32% or less.
Statistical Analysis
The decision to pool HIV incidence findings from all communities was decided a priori. Overall, incidence was determined by combining results from all 3 methods (recent infections at study entry, acute infections at study entry, and seroconversion during follow-up) using the total number of these events as the numerator and the 3 non-overlapping periods of risk (recent, acute, and longitudinal) as the denominator. A CI for overall incidence was computed on the log scale using an SE obtained by a weighted combination of SEs for the incidence from the 3 periods and included adjustment for window period uncertainty where appropriate (20).
Because high-risk behaviors were entry criteria for this study, sensitivity analyses (Appendix, available at www.annals.org) were done to determine whether changes in risk behaviors between baseline and 6 and 12 months could be explained by regression to the mean.
Generalized linear regression with a log link and binomial errors was used to estimate the relative risk between prevalent HIV infection and individual covariates. Each regression used all available nonmissing data for the covariate in the current regression. All analyses were done using SAS, version 9.2 (SAS Institute, Cary, North Carolina).
Role of the Funding Source
The study was funded by the National Institutes of Health (specifically, the National Institute of Allergy and Infectious Diseases, National Institute on Drug Abuse, and National Institute of Mental Health). The funding source reviewed the study design but had no role in the conduct or analysis of the study or in the decision to submit the manuscript for publication.
Results
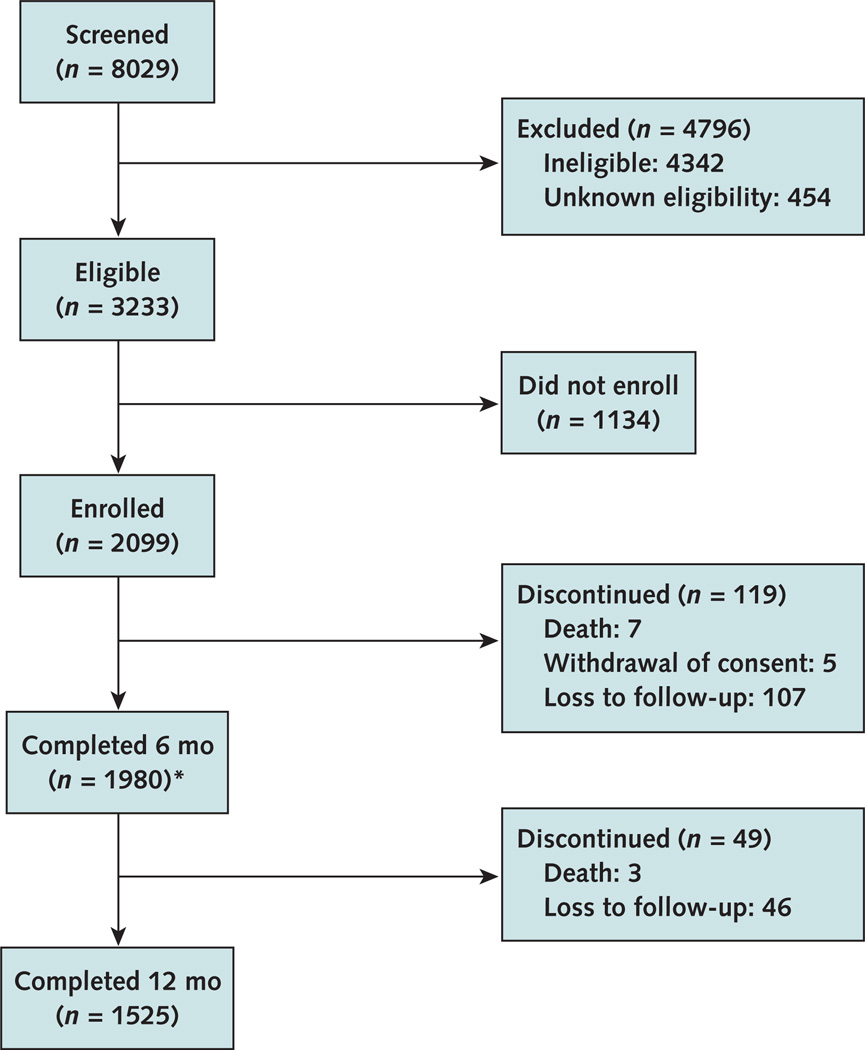
The Figure shows screening, recruitment, and retention data, as well as reasons for study discontinuation. Of the 8029 women screened, 3233 (40%) were eligible for enrollment and 2099 (26%) enrolled between May 2009 and August 2010. Most women who were eligible but did not enroll either did not return to the clinical research site for enrollment or could not complete enrollment procedures (for example, unsuccessful phlebotomy). As individual identifiers could not be attached to eligibility screening data, differences among the eligible population that enrolled versus that which did not are unknown. Most participants (73%) reported both individual and partner risk factors. Eighty-six percent of participants reported 1 or more individual risk factors, 34% reported 3 or more individual risk factors, and 87% reported male partner risk factors.
Figure.
Study flow diagram
1953 attended the visit and 27 missed the visit but returned at 12 mo. A total of 406 women completed the study at the 6-mo visit.
Four hundred six women completed the study at the 6-month visit before the protocol amendment extending follow-up was approved. Of the 627 women eligible for extending follow-up, 87% were successfully contacted and reconsented for 12-month follow-up. Participant retention was 93% at 6 months and 94% at 12 months. Twenty-seven women missed the 6-month visit but completed the 12-month visit. Ten participants died during follow-up (0.61% per year).
Cohort Characteristics
Approximately 85.9% of participants were black, 6.8% white, 2.6% mixed, and 4.8% “other” race. Hispanic ethnicity was reported by 11.7% of participants, and the median age was 29 years (Table 1). Nearly all participants (97%) were born in the United States. Thirty-seven percent had not completed high school, 36.8% had a high school diploma, and the remainder had education beyond high school. Most participants (53.8%) were single, and 44.4% reported an annual household income of less than $10 000. At baseline, 46.3% of participants reported being concerned about having sufficient food for themselves and their families over the past 6 months. Only 6% of participants considered themselves to be commercial sex workers; however, 31% of the 1885 participants who did not reported exchanging sex for goods (such as food, money, or shelter).
Table 1.
Baseline Characteristics of Participants*
| Characteristic | Participants (n = 2099) |
|---|---|
| Median age (interquartile range), y | 29 (23–38) |
| Race/ethnicity | |
| Black | 1802 (85.9) |
| White | 143 (6.8) |
| Mixed | 54 (2.6) |
| Other | 100 (4.8) |
| Hispanic | 245 (11.7) |
| Education | |
| Less than high school graduation | 777 (37.0) |
| High school graduation | 772 (36.8) |
| Education beyond high school | 550 (26.2) |
| Marital status | |
| Single, separated, divorced, or widowed | 1258 (59.9) |
| Married or not married but living with partner | 638 (30.4) |
| Other | 152 (7.2) |
| Unknown | 51 (2.4) |
| Annual household income | |
| ≤$10 000 | 933 (44.4) |
| $10 001–$20 000 | 225 (10.7) |
| >$20 000 | 197 (9.4) |
| Unknown | 744 (35.5) |
| Food insecurity | |
| Concerned about having sufficient food for self and family | 971 (46.3) |
| Unknown | 27 (1.3) |
Values are numbers (percentages) unless otherwise indicated. Percentages may not sum to 100 due to rounding.
Illicit drug use (excluding cannabis) was reported by 22% of participants in the 6 months preceding study entry, and 24.1% reported at least weekly binge-drinking (Table 2).
Table 2.
Reported Participant and Partner Characteristics at Baseline*
| Characteristic | Participants† | Missing Responses, % |
|---|---|---|
| Participant | ||
| Median number of partners in previous 6 mo (IQR) | 2 (1–3) | <1 |
| Exchange sex for commodities | 776 (37.3) | <1 |
| HIV status of man with whom had last vaginal sex unknown | 865 (41.4) | <1 |
| Condom used at last vaginal sex | 376 (18.0) | <1 |
| Anal sex | 796 (38.0) | <1 |
| Condom used at last anal sex | 143 (18.0) | 2 |
| Concurrency‡ | 776 (37.1) | <1 |
| Self-reported STI (gonorrhea, syphilis, or chlamydia) | 232 (11.1) | 1.6 |
| At least weekly substance use (including drug use or binge-drinking [≥4 drinks on 1 occasion]) | 782 (37.8) | 1.5 |
| At least weekly binge-drinking (≥4 drinks on 1 occasion) | 498 (24.1) | 1.5 |
| At least weekly drug use (excluding cannabis) | 459 (22.0) | <1 |
| Depressive symptoms (CES-D score ≥7) | 692 (35.6) | 7.5 |
| PTSD (positive responses to ≥3 items on the Primary Care PTSD Screen) | 600 (29.3) | 2.5 |
| Any history of abuse | 768 (37.0) | <1 |
| Any childhood abuse | 934 (45.1) | <1 |
| Partner | ||
| HIV seropositive diagnosis | 28 (1.3) | <1 |
| Reported STI (gonorrhea, syphilis, or chlamydia) | 215 (10.2) | <1 |
| Illicit drug use (injection or noninjection, excluding cannabis) | 752 (35.8) | <1 |
| Binge-drinking (≥5 drinks on 1 occasion) | 1179 (56.2) | <1 |
| Alcohol dependence (CAGE score ≥2) | 869 (41.4) | <1 |
| Incarceration within previous 5 y | 1233 (58.7) | <1 |
CAGE = Cut Down, Annoyed, Guilty, and Eye Opener; CES-D = Center for Epidemiologic Studies Depression scale; IQR = interquartile range; PTSD = posttraumatic stress disorder; STI = sexually transmitted infection.
All characteristics described were within 6 mo of the study start, unless otherwise indicated. Data are based on 2099 participants.
Values are presented as numbers (percentages) unless otherwise indicated.
Self-report of sex with a man while involved in a sexual relationship with another man during the same period.
Various types of abuse were reported by participants: 45.1% reported a history of childhood abuse, and 37% reported some type of abuse (physical, emotional, or sexual) during the 6 months before study entry. At baseline, 35.6% of participants reported symptoms indicative of psychological distress or depressive symptoms and 29.3% screened positive for PTSD.
At baseline, the median number of sex partners reported by participants in the previous 6 months was 2 (mean, 4.2), and 38% reported anal sex in the 6 months before study entry (Table 2). Approximately 41.4% and 44% of participants at baseline reported not knowing the HIV status of their last partner with whom they had vaginal sex and anal sex, respectively. One percent reported that the partner with whom they last had vaginal or anal sex had HIV. Condom use was infrequent; 18% reported use at last vaginal or anal sex.
Risky behaviors decreased during follow-up: Condom use at last vaginal sex increased from 18% at baseline to 35% at 6 months and 37% at 12 months. Sensitivity analyses suggest that 40% to 70% of the increase in condom use was a study effect.
HIV Infections
Thirty-eight women were newly identified as having HIV during the study. Thirty-two women (1.5%) had new diagnoses of HIV at study entry (confirmed by a Western blot test). Two of the 32 women were identified as having recently acquired HIV. Two additional women who had nonreactive HIV rapid test results had acute infection at study entry. Four women who were not infected at study entry became infected during follow-up (documented by HIV seroconversion). The overall annual HIV incidence for this cohort was 0.32% (95% CI, 0.14% to 0.74%).
There were no associations of either participant or partner characteristics reported at baseline with incident HIV infection, probably because of the limited number of incident infections. However, increasing participant age and substance use (at least weekly use of illicit drugs or binge-drinking over the past 6 months) were associated with prevalent HIV infection (Table 3). The only partner risk factor associated with prevalent HIV infection in participants was known HIV infection of the partner (relative risk, 8.19 [CI, 2.64 to 25.42]).
Table 3.
Univariate Analysis of Potential Factors Associated With Prevalent HIV Infection*
| Characteristic | Prevalence in Women With HIV at Study Entry, % |
Association With HIV Infection at Study Entry RR (95% CI) |
P Value |
|---|---|---|---|
| Participant | |||
| At least weekly substance use (including drug use or binge-drinking) | 60 | 2.52 (1.22–5.21) | 0.013 |
| Concurrency† | 40 | 1.13 (0.55–2.33) | 0.85 |
| Education beyond high school (reference: high school graduation or less education) | 13.3 | 0.43 (0.15–1.24) | 0.142 |
| Food insecurity | 33.3 | 0.57 (0.27–1.21) | 0.148 |
| Anal sex | 26.7 | 0.59 (0.27–1.33) | 0.26 |
| At least weekly binge-drinking (≥4 drinks on 1 occasion) | 33.3 | 1.57 (0.74–3.33) | 0.28 |
| At least weekly drug use (excluding cannabis) | 43.3 | 2.71 (1.33–5.53) | 0.006 |
| Age 27–33 y (reference: 18–26 y) | 23.3 | 5.83 (1.22–27.96) | 0.028 |
| Age ≥34 y (reference: 18–26 y) | 70 | 11.54 (2.71–49.05) | 0.001 |
| Depressive symptoms (CES-D score ≥7) | 44.4 | 1.45 (0.68–3.07) | 0.42 |
| Positive PTSD responses (reference: negative PTSD responses) | 21 | 0.63 (0.26–1.54) | 0.41 |
| Any history of emotional, physical, or sexual abuse | 20 | 0.43 (0.18–1.04) | 0.061 |
| History of childhood abuse | 43.3 | 0.93 (0.46–1.91) | 0.85 |
| Partner, as reported by female participant | |||
| Illicit drug use (injection or noninjection) | 46.7 | 1.57 (0.77–3.19) | 0.25 |
| Incarceration within previous 5 y | 46.7 | 0.61 (0.30–1.25) | 0.189 |
| Reported STI (gonorrhea, syphilis, or chlamydia) | 10 | 0.97 (0.30–3.17) | 0.96 |
| HIV seropositive diagnosis | 10 | 8.19 (2.64–25.42) | 0.007 |
| Binge-drinking (≥5 drinks on 1 occasion) | 70 | 1.82 (0.84–3.96) | 0.140 |
| Alcohol dependence | 50 | 1.42 (0.70–2.88) | 0.35 |
CES-D = Center for Epidemiologic Studies Depression scale; PTSD = posttraumatic stress disorder; RR = relative risk; STI = sexually transmitted infection.
Data based on 30 participants. All characteristics described were within 6 mo of the study start, unless otherwise indicated.
Self-report of sex with a man while involved in a sexual relationship with another man during the same period.
Perceived Health Status and Death
At baseline, general health status was reported as “excellent” or “very good” by 46% of participants (Table 4). Four hundred seventeen women (20%) reported that they could not obtain needed health care in the 6 months preceding enrollment; 61% of these women indicated inability to afford care as the reason it was not obtainable (Table 4). Ten women died during the study, for an annual mortality rate of 0.61% per year. Causes of death were available for 5 of the 10 deceased participants and spanned a range of preventable causes (Table 5). The only individual risk behavior statistically significantly associated with death was drug use (at least weekly, excluding cannabis) (P < 0.005).
Table 4.
Perceived Health Status and Medical Care Access*
| Characteristic | Baseline (n = 2099) |
Month 6 (n = 1953) |
Month 12 (n = 1525) |
|---|---|---|---|
| General perception of health, n (%) | |||
| Excellent | 323 (15) | 368 (19) | 324 (21) |
| Very good | 646 (31) | 610 (31) | 478 (31) |
| Good | 782 (37) | 655 (34) | 492 (32) |
| Fair | 318 (15) | 283 (14) | 208 (14) |
| Poor | 26 (1) | 29 (1) | 21 (1) |
| Missing | 4(<1) | 8 (<1) | 1 (<1) |
| Needed medical care but could not get it during previous 6 mo, n (%) | |||
| Yes | 417 (20) | 295 (15) | 219 (14) |
| No | 1679 (80) | 1650 (84) | 1304 (86) |
| Missing | 3(<1) | 8 (<1) | 2 (<1) |
| Main reason needed medical care was not received, n (%) | |||
| Could not afford care | 257 (61) | 172 (58) | 134 (61) |
| Did not know where to find care | 26 (6) | 16 (5) | 18 (8) |
| Could not get an appointment anywhere | 34 (8) | 30 (10) | 23 (11) |
| None available | 15 (4) | 10 (3) | 6 (3) |
| Did not think it was necessary | 19 (5) | 12 (4) | 5 (2) |
| Thought it was necessary, but never tried to get care | 38 (9) | 21 (7) | 9 (4) |
| Did not know where to find a physician speaking the same language | 5 (1) | 4 (1) | 4 (2) |
| Other | 23 (6) | 28 (9) | 20 (9) |
| Missing | 0 (0) | 2 (1) | 0 (0) |
Percentages may not sum to 100 due to rounding.
Table 5.
Recorded Participant Deaths
| Participant | Cause of Death | Time Between Enrollment and Death, d |
|---|---|---|
| 1 | Unknown | 104 |
| 2 | Cardiac arrest | 50 |
| 3 | Unknown | 187 |
| 4 | Unknown | 84 |
| 5 | Diabetic coma | 60 |
| 6 | Unknown | 181 |
| 7 | End-stage AIDS, cryptococcal meningitis (CD4 count at baseline, 0.011 × 109 cells/L) | 304 |
| 8 | Combined toxic effects of cocaine, opiates, and doxepin | 294 |
| 9 | Homicide | 183 |
| 10 | Unknown | 146 |
Discussion
To our knowledge, this is the first study to estimate HIV incidence using a comprehensive approach that includes a longitudinal incidence assessment based on HIV seroconversion, detection of recently acquired HIV at study entry, and detection of acute HIV infection at study entry. The overall (composite) annual incidence estimate is 0.32%, which is substantially higher than the 2009 national estimate from the Centers for Disease Control and Prevention on HIV incidence in the general population of U.S. black women of similar age (0.05%) (21), suggesting that the recruitment methods successfully identified women at risk for HIV. The HIV incidence reported in this study is similar to that estimated for the general adult population in some sub-Saharan African countries (Congo, 0.28% [CI, 0.23% to 0.35%]; Nigeria, 0.38% [CI, 0.33% to 0.44%]; and Kenya, 0.53% [CI, 0.34% to 0.70%]) (22), underscoring the substantial ongoing HIV transmission within specific U.S. populations, including women at risk, as defined in this study.
Venue-based recruitment with time–space sampling was successful in recruiting women with elevated risk for HIV infection. Recruitment venues located in high HIV prevalence areas and selected for the frequency with which young women were present offered easy access to study entry for the target population. The systematic procedures for recruitment may be used in future studies involving similar populations. It is noteworthy that this study’s methods were not designed to recruit a representative community sample, but rather to systematically sample specific community members (in this case, young women living in areas of high HIV prevalence).
The overall annual incidence found in this study may underestimate the actual incidence in this population because the behavior of women in the study cohort may have changed in response to participation (because risk reduction counseling was done at each study visit and free condoms were available). Findings from behavioral assessments during the study suggest the likelihood of this effect on the basis of the demonstrated decrease in sexual risk behavior, with reported condom use at last vaginal sex increasing from 18% at baseline to 35% at the 6-month visit to 37% at the 12-month visit. Because unprotected sex was an enrollment criterion, some of this change may have been due to regression to the mean; however, sensitivity analyses suggest that 40% to 70% of the increase in condom use was a study effect. Nonetheless, approximately two thirds of participants still reported unprotected vaginal sex at the 6- and 12-month study visits, clearly demonstrating that further research is needed to minimize HIV risk among subpopulations similar to this cohort. Financial insecurity was common and may have motivated the exchange of sex for basic commodities, which was reported by nearly one third of participants.
Although self-reported history of HIV infection was an exclusion criterion, 32 women (1.5%) were newly diagnosed with HIV infection at study entry, 2 of whom were likely to have been recently infected. Finding this many newly identified infections at study entry was remarkable because testing guidelines in the United States have recommended opt-out HIV testing in medical settings since 2006, and several study communities (such as Washington, DC, and the Bronx) had ongoing HIV testing campaigns. Despite these participants living in high HIV prevalence areas of the United States, testing programs or the test results did not effectively reach them. Although known HIV infection in a sexual partner was strongly associated with infection in this study, 41.4% and 44% of the women were unaware of the status of their last vaginal and anal sex partners, respectively. These findings emphasize the importance of testing strategies that provide HIV diagnosis opportunities and prevention education to populations at greatest risk, consistent with recommendations of the National HIV/AIDS Strategy (23). The importance of partner characteristics in HIV acquisition has received recent attention (24). Most HPTN 064 participants (87%) reported male sex partners with HIV risk factors, suggesting a need to enhance HIV prevention initiatives among men who have sex with women. Clearly, clinicians, researchers, public health practitioners, and preventive health programs should be aware of the importance of HIV testing as well as the heightened risk of female patients whose demographic characteristics and risk factors are similar to those of the women in this study.
A prominent finding was the high mortality rate. Ten participants died during follow-up, resulting in an annual mortality rate of 0.61% per year, which is considerably higher than the expected age-adjusted mortality rate of 0.11% per year (25). Causes of death included diabetic coma, drug overdose, homicide, and HIV or AIDS, all of which are preventable. However, necessary medical care was often unavailable as reported by a substantial proportion of the participants (Table 4). One in 5 women in the study who needed medical care in the previous 6 months reported that they were not able to obtain care. Most women (61%) cited financial reasons for lack of access, highlighting the critical need for reforms that improve access for persons living in poverty. However, structural barriers other than financial need also precluded care, as 18% of women reported not accessing needed care because they did not know where to find care, could not get an appointment, or concluded that care was unavailable. Depressive symptoms and PTSD were very common in this cohort, suggesting that mental health services are needed. In this cohort of relatively young women, only 15% characterized their health as excellent (Table 4). Although reasons underlying perceptions of health are unknown, one is left to wonder whether those perceptions would improve in a setting of better access to quality care.
The study has several strengths. It included a careful assessment of participant characteristics and behaviors and used a novel combination of laboratory methods to assess HIV incidence. The HPTN 064 used a rigorous approach to recruit and retain participants representative of U.S. women at high risk for HIV; study results apply to U.S. women with risk profiles similar to those enrolled in this study.
In terms of limitations, all studies based on self-reported sensitive behavioral data, including this one, are limited by an inherent social desirability bias (26). The use of audio computer-assisted self-interviews, however, probably decreased this bias because they have been found to increase reporting of sensitive behaviors (27, 28). Study follow-up was limited to 12 months; it is unknown whether the reported decreases in risk behaviors in this study are durable beyond that time. Few incident infections were seen, precluding our ability to identify specific risk factors associated with incident HIV infection.
In summary, this study successfully used novel recruitment methods to identify U.S. women at increased risk for HIV. Indeed, 1 in 300 women in this cohort acquired HIV infection annually, suggesting that concerted efforts are needed to define effective prevention strategies for similar populations. Populations analogous to that constituted in HPTN 064 should be considered appropriate for future HIV prevention research and programming. Finally, this study, conducted in the United States, shows that enhanced risk for HIV infection occurs in the setting of increased mortality rates and inability to obtain needed medical care, suggesting that prevention strategies addressing a broader agenda than just HIV are needed.
Context.
There have been few longitudinal studies of HIV incidence and prevalence among young women living in U.S. urban and periurban areas with high poverty indicators.
Contribution
In a venue-based longitudinal study, such women had an estimated HIV incidence rate of 0.32%, similar to that of some sub-Saharan countries, and an annual mortality rate of 0.61%. Deaths were largely due to preventable causes, including HIV infection. Women reported difficulty obtaining medical care.
Caution
The HIV incidence rate found may actually be an underestimate due to behavior change secondary to study participation.
Implication
Improved programs for HIV testing, disease prevention, and linkage to general medical care should be offered to young women in areas with high HIV prevalence.
—The Editors
Acknowledgment
The authors thank the study participants, community stakeholders, and staff from each study site. In particular, they acknowledge Lynda Emel, Jonathan Lucas, Nirupama Sista, Kathy Hinson, Elizabeth DiNenno, Ann O’Leary, Lisa Diane White, Waheedah Shabaaz-El, Quarraisha Abdool-Karim, and Sten Vermund.
Grant Support: By the National Institute of Allergy and Infectious Diseases, National Institute on Drug Abuse, and National Institute of Mental Health (cooperative agreement UM1 AI068619, U01-AI068613, and UM1-AI068613); Centers for Innovative Research to Control AIDS, Mailman School of Public Health, Columbia University (5UM1A1069466); University of North Carolina Clinical Trials Unit (AI069423); University of North Carolina Clinical Trials Research Center of the Clinical and Translational Science Award (RR 025747); University of North Carolina Center for AIDS Research (AI050410); Emory University HIV/AIDS Clinical Trials Unit (5UO1AI069418), Center for AIDS Research (P30 AI050409), and Clinical and Translational Science Award (UL1 RR025008); Johns Hopkins Adult AIDS Clinical Trials Unit (AI069465) and Clinical and Translational Science Award (ULI RR025008); Johns Hopkins Clinical and Translational Science Award (ULI RR025005); and The Terry Beirn Community Programs for Clinical Research on AIDS Clinical Trials Unit.
Primary Funding Source: National Institutes of Health.
Appendix
Sensitivity Analysis Method
Because high-risk behaviors were entry criteria for this study, sensitivity analyses were done to determine whether changes in risk behaviors between baseline, 6 months, and 12 months could be explained by regression to the mean. For example, 18% of women reported condom use at last sex at enrollment and this increased to 35% at 6 months. It can be shown that the proportion of this change that would be predicted by regression to the mean is approximately
| (1) |
where s is the population mean of the selection criteria (“any unprotected sex in the last 6 months”; s was varied between 0.65 and 0.80), r is the correlation between “condom use at last sex” at baseline and 6 months (measured at 0.2), and ρ is the correlation between the selection criteria and “condom use at last sex” (estimated as −0.35 based on the correlation between “any unprotected sex in last 6 months with last 3 partners” and “condom use at last sex”).
Derivation of the equation for predicting what proportion of observed change in risk behavior can be attributed to regression to the mean.
A key risk factor is the behavior “condom use at last sex.” In the Women’s HIV SeroIncidence Study, this increased from 18% at baseline to 35% at 6 months to 37% at 12 months. However, a key entry criterion for the study was “Unprotected sex within the last 6 months” (100% at baseline; not measured at 6 and 12 months). Because unprotected sex was an entry criterion, one would expect condom use at last sex to be low at baseline and naturally increase at later time points, even in the absence of any intervention or study participation (a phenomenon known as “regression to the mean”).
The question of interest is, “What proportion of the observed change is due to regression to the mean?”
Define:
m = long-term mean of risk behavior among participants in absence of an intervention effect
s = long-term mean of selection criteria among participants in absence of an intervention effect
X = observed mean of risk behavior at baseline among participants
Z = observed mean of selection criteria at baseline among participants
Yo = observed mean of risk behavior at 6 months among participants
Yp = predicted mean of risk behavior at 6 months among participants in absence of an intervention effect and let X, Z, Yo, and Yp ~ N(σ2)
Then:
| (2) |
where r is the correlation between observations on the risk behavior 6 months apart. That is, r captures the regression to the mean effect. Thus, the proportion of the observed change from baseline to 6 months that can be explained by regression to the mean is:
| (3) |
The difference between the observed mean risk behavior at baseline and the long-term mean for that risk behavior is unknown but can be related to the selection criteria by the following relationship:
| (4) |
where ρ is the correlation between the selection criteria and the risk behavior of interest. Thus:
| (5) |
In the current application, Yo = 0.35, X = 0.18, Z = 1, r = 0.2 is the measured correlation between “condom use at last sex” at baseline and 6 months, ρ is estimated as −0.35 based on the correlation between “any unprotectnd sex in last 6 months with last 3 partners” and “condom use at last sex,” and s was varied between 0.65 and 0.80 in sensitivity analyses.
Footnotes
Disclaimer: The views expressed herein are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases, the National Institute of Mental Health, the National Institutes of Health, the HPTN, or its funders.
Potential Conflicts of Interest: Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M12-1057.
Reproducible Research Statement: Study protocol: Available at www.HPTN.org. Statistical code and data set: Not available.
Author Contributions: Conception and design: S.L. Hodder, J. Justman, J.P. Hughes, D.F. Haley, C.E. Golin, L. Soto-Torres, S.H. Eshleman, W.M. El-Sadr.
Analysis and interpretation of the data: S.L. Hodder, J. Justman, J.P.
Hughes, J. Wang, A.A. Adimora, C. Del Rio, C.E. Golin, S.B.
Mannheimer, L. Johnson-Lewis, S.H. Eshleman, W.M. El-Sadr.
Drafting of the article: S.L. Hodder, J. Justman, J.P. Hughes, D.F.
Haley, A.A. Adimora, C. Del Rio, L. Soto-Torres, S.B. Mannheimer, S.H. Eshleman, W.M. El-Sadr.
Critical revision of the article for important intellectual content: S.L.
Hodder, J. Justman, J.P. Hughes, D.F. Haley, A.A. Adimora, C.E. Golin, I. Kuo, A. Rompalo, S.B. Mannheimer, L. Johnson-Lewis, S.H. Eshleman, W.M. El-Sadr.
Final approval of the article: S.L. Hodder, J. Justman, J.P. Hughes, D.F.
Haley, A.A. Adimora, C. Del Rio, C.E. Golin, I. Kuo, A. Rompalo, L.
Soto-Torres, S.B. Mannheimer, S.H. Eshleman, W.M. El-Sadr.
Provision of study materials or patients: S.L. Hodder, J. Justman, D.F.
Haley, C. Del Rio, C.E. Golin, I. Kuo, A. Rompalo, S.B. Mannheimer.
Statistical expertise: J.P. Hughes, J. Wang.
Obtaining of funding: S.L. Hodder, J. Justman, C.E. Golin, W.M. El-Sadr.
Administrative, technical, or logistic support: D.F. Haley, W.M. El-Sadr.
Collection and assembly of data: S.L. Hodder, C. Del Rio, C.E. Golin, I. Kuo, A. Rompalo, S.H. Eshleman.
References
- 1.El-Sadr WM, Mayer KH, Hodder SL. AIDS in America—forgotten but not gone. N Engl J Med. 2010;362:967–970. doi: 10.1056/NEJMp1000069. [PMID: 20147707] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. HIV Among Women. 2011 Aug; Accessed at www.cdc.gov/hiv/topics/women/pdf/women.pdf on 6 September 2012.
- 3.Centers for Disease Control and Prevention. HIV Surveillance in Women. [updated 6 April 2012]; Accessed at www.cdc.gov/hiv/topics/surveillance/resources/slides/women/index.htm on 6 September 2012.
- 4.Hodder SL, Justman J, Haley DF, Adimora AA, Fogel CI, Golin CE HIV Prevention Trials Network Domestic Prevention in Women Working Group. Challenges of a hidden epidemic: HIV prevention among women in the United States. J Acquir Immune Defic Syndr. 2010;55(Suppl 2):S69–S73. doi: 10.1097/QAI.0b013e3181fbbdf9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.HIV Prevention Trials Network. Accessed at www.hptn.org/index.htm on 6 September 2012. [Google Scholar]
- 6.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 7.DiClemente RJ, Wingood GM, Crosby RA, Sionean C, Brown LK, Rothbaum B, et al. A prospective study of psychological distress and sexual risk behavior among black adolescent females. Pediatrics. 2001;108:E85. doi: 10.1542/peds.108.5.e85. [PMID: 11694669] [DOI] [PubMed] [Google Scholar]
- 8.Prins A, Ouimette P, Kimerling RP, Hugelshofer DS, Shaw-Hegwer J, Thrailkill A, et al. The primary care PTSD screen (PC-PTSD): development and operating characteristics. Primary Care Psychiatry. 2004;9:9–14. [Google Scholar]
- 9.Ewing JA. Detecting alcoholism. The CAGE questionnaire. JAMA. 1984;252:1905–1907. doi: 10.1001/jama.252.14.1905. [PMID: 6471323] [DOI] [PubMed] [Google Scholar]
- 10.DeNavas-Walt C, Proctor BD, Smith JC. U.S. Census Bureau. Current Population Reports: Consumer Income, P60–236. Washington, DC: U.S. Government Printing Office; 2009. Income, poverty, and health insurance coverage in the United States: 2008. Accessed at www.census.gov/prod/2009pubs/p60-236.pdf on 3 November 2012. [Google Scholar]
- 11.Gallagher KM, Sullivan PS, Lansky A, Onorato IM. Behavioral surveillance among people at risk for HIV infection in the U.S.: the National HIV Behavioral Surveillance System. Public Health Rep. 2007;122(Suppl 1):32–38. doi: 10.1177/00333549071220S106. [PMID: 17354525] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen DR, Finlayson T, Abdul-Quader A, Lansky A. The role of formative research in the National HIV Behavioral Surveillance System. Public Health Rep. 2009;124:26–33. doi: 10.1177/003335490912400106. [PMID: 19413025] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacKellar D, Valleroy L, Karon J, Lemp G, Janssen R. The Young Men’s Survey: methods for estimating HIV seroprevalence and risk factors among young men who have sex with men. Public Health Rep. 1996;111(Suppl 1):138–144. [PMID: 8862170] [PMC free article] [PubMed] [Google Scholar]
- 14.Muhib FB, Lin LS, Stueve A, Miller RL, Ford WL, Johnson WD, et al. Community Intervention Trial for Youth Study Team. A venue-based method for sampling hard-to-reach populations. Public Health Rep. 2001;116(Suppl 1):216–222. doi: 10.1093/phr/116.S1.216. [PMID: 11889287] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stueve A, O’Donnell LN, Duran R, San Doval A, Blome J. Time-space sampling in minority communities: results with young Latino men who have sex with men. Am J Public Health. 2001;91:922–926. doi: 10.2105/ajph.91.6.922. [PMID: 11392935] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eshleman SH, Hughes JP, Laeyendecker O, Wang J, Brookmeyer R, Johnson-Lewis L, et al. Use of a multi-faceted approach to assess HIV incidence in a cohort study of women in the United States. J Infect Dis. 2013 doi: 10.1093/infdis/jis658. [Forthcoming]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall HI, Song R, Rhodes P, Prejean J, An Q, Lee LM, et al. HIV Incidence Surveillance Group. Estimation of HIV incidence in the United States. JAMA. 2008;300:520–529. doi: 10.1001/jama.300.5.520. [PMID: 18677024] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masciotra S, Dobbs T, Candal D, Hanson D, Delaney K, Rudolph D, et al., editors. Antibody avidity-based assay for identifying recent HIV-1 infections based on genetic systems TM 1/2 plus O EIA; Presented at 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, California. 16–19 February 2010; Paper 937. Accessed at http://retroconference.org/2010/PDFs/937.pdf on 3 November 2012. [Google Scholar]
- 19.Laeyendecker O, Brookmeyer R, Cousins MM, Mullis CE, Donnell, Celum C, et al. HIV incidence determination in the United States: a multiassay approach. J Infect Dis. 2013 doi: 10.1093/infdis/jis659. [Forthcoming]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brookmeyer R. Accounting for follow-up bias in estimation of human immunodeficiency virus incidence rates. J R Stat Soc Ser A. 1997;160:127–140. [Google Scholar]
- 21.Prejean J, Song R, Hernandez A, Ziebell R, Green T, Walker F, et al. HIV Incidence Surveillance Group. Estimated HIV incidence in the United States, 2006–2009. PLoS One. 2011;6:e17502. doi: 10.1371/journal.pone.0017502. [PMID: 21826193] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joint United Nations Programme on HIV/AIDS (UNAIDS) Global Report: UNAIDS Report on the Global AIDS Epidemic. 2010 Accessed at www.unaids.org/documents/20101123_globalreport_em.pdf on 3 November 2012. [Google Scholar]
- 23.The White House Office of National AIDS Policy. National HIV/AIDS Strategy for the United States. 2010 Jul; Accessed at http://aids.gov/federal-resources/national-hiv-aids-strategy/nhas.pdf on 3 November 2011.
- 24.Millett GA, Flores SA, Peterson JL, Bakeman R. Explaining disparities in HIV infection among black and white men who have sex with men: a meta-analysis of HIV risk behaviors. AIDS. 2007;21:2083–2091. doi: 10.1097/QAD.0b013e3282e9a64b. [PMID: 17885299] [DOI] [PubMed] [Google Scholar]
- 25.Murphy SL, Xu J, Kochanek KD. Deaths: preliminary data for 2010. Centers for Disease Control and Prevention. National Vital Statistics Reports. 2012;60 Accessed at www.cdc.gov/nchs/data/nvsr/nvsr60/nvsr60_04.pdf on 3 November 2012. [Google Scholar]
- 26.Fisher R. Social desirability bias and the validity of indirect questioning. J Consum Res. 1993;20:303–315. [Google Scholar]
- 27.Turner CF, Ku L, Rogers SM, Lindberg LD, Pleck JH, Sonenstein FL. Adolescent sexual behavior, drug use, violence: increased reporting with computer survey technology. Science. 1998;280:867–873. doi: 10.1126/science.280.5365.867. [PMID: 9572724] [DOI] [PubMed] [Google Scholar]
- 28.Locke SE, Kowaloff HB, Hoff RG, Safran C, Popovsky MA, Cotton DJ, et al. Computer-based interview for screening blood donors for risk of HIV transmission. JAMA. 1992;268:1301–1305. [PMID: 1507376] [PubMed] [Google Scholar]