Abstract
The increased development and use of nanoparticles in various fields may lead to increased exposure, directly affecting human health. Our current knowledge of the health effects of metal nanoparticles such as Cobalt and Titanium dioxide (Nano-Co and Nano-TiO2) is limited but suggests that some metal nanoparticles may cause genotoxic effects including cell cycle arrest, DNA damage and apoptosis. The growth arrest and DNA damage-inducible 45α protein (Gadd45α) has been characterized as one of the key players in the cellular responses to a variety of DNA damaging agents. The aim of this study was to investigate the alteration of Gadd45α expression in mouse embryo fibroblasts (PW) exposed to metal nanoparticles and the possible mechanisms. Non-toxic doses of Nano-Co and Nano-TiO2 were selected to treat cells. Our results showed that Nano-Co caused a dose- and time-dependent increase in Gadd45α expression, but Nano-TiO2 did not. To investigate the potential pathways involved in Nano-Co-induced Gadd45α up-regulation, we measured the expression of hypoxia inducible factor 1α (HIF-1α) in PW cells exposed to Nano-Co and Nano-TiO2. Our results showed that exposure to Nano-Co caused HIF-1α accumulation in the nucleus. In addition, hypoxia inducible factor 1α knock-out cells [HIF-1α (−/−)] and its wild-type cells [HIF-1α (+/+)] were used. Our results demonstrated that Nano-Co caused a dose- and time-dependent increase in Gadd45α expression in wild-type HIF-1α (+/+) cells, but only a slight increase in HIF-1α (−/−) cells. Pre-treatment of PW cells with heat shock protein 90 (Hsp90) inhibitor, 17-(Allylamino)-17-demethoxygeldanamycin (17-AAG), prior to exposure to Nano-Co significantly abolished the Nano-Co-induced Gadd45α expression. These results suggest that HIF-1α accumulation may be partially involved in the increased Gadd45α expression in cells exposed to Nano-Co. These findings may have important implications for understanding the potential health effects of metal nanoparticle exposure.
Keywords: metal nanoparticles, DNA damage, Gadd45α, HIF-1α, mouse embryo fibroblasts
Introduction
Nanotechnologies include the design, characterization, production, and application of structures, devices and systems by controlling shape and size at the nanometer scale (Kubik et al., 2005; Maynard et al., 2006). These technologies directly improve our lives in areas as diverse as engineering, information technology, and diagnostics. With the development of nanotechnology, a large number of metal nanoparticles will be developed and produced as new formulations with surface properties to meet novel demands. The importance of nanotechnology for sustaining economic growth is well recognized, but it is also clear that safeguarding the growth of this technology will require public understanding of the societal benefits as well as the health risks associated with its development and use (Baswas and Wu, 2005; Chow et al., 2005; Colvin, 2003; Kubik et al., 2005; Maynard et al., 2006; Oberdoster et al., 2005a, b; Owen and Depledge, 2005). The increased development and use of nanoparticles for various industries could lead to increased human exposure, affecting human health and the environment (Baswas and Wu, 2005; Chow et al., 2005; Colvin, 2003; Oberdoster et al., 2005a,b; Owen and Depledge, 2005). Our current knowledge of health effects of nanomaterials is limited, but suggests that they may exert effects at their portal of entry, such as lung, skin and gastrointestinal tract (Colvin, 2003; Oberdoster et al., 2005a,b).
Cobalt nanoparticles (Nano-Co), which have special characteristics such as high surface area, high magnetism and high active site, have been widely used not only in industry in the fields of electromagnetic-wave absorption, electromagnetic-wave radiation shielding for cellar phones, ferrofluids, high density magnetic storage, magnetic inks, and magnetic toner in xerograph, but also in biology and medicine in different forms from the simplest, such as cobalt oxide, to complex organic compounds or biopolymers (Wang et al., 2005; Yang et al., 2006). Magnetic metal nanoparticles, typically composed of iron, cobalt and nickel, have been increasingly used for MRI applications (Bouchard et al., 2009; Itoa et al., 2005; Pouponneau et al., 2009), and in an experimental cancer treatment called magnetic hyperthermia, which uses the heat that nanoparticles produced when they are placed in an alternative magnetic field to kill cancer cells (Itoa et al., 2005; Kale et al., 2012; Lu et al. 2007). In addition, some nanomaterials may cause genotoxic stress because of the metals used to make them.
The growth arrest and DNA damage-inducible (Gadd) gene 45 alpha (Gadd45α) is a member of a group of genes that are induced by DNA damaging agents and growth arrest signals (Liebermann et al., 2008; Siafakas et al., 2009; Zhan et al., 2005). Gadd45 gene was originally identified as the mRNA transcript that was rapidly induced in response to ultraviolet radiation (Carrier et al., 1999). Gadd45 protein is ubiquitously expressed 21kD acidic protein in response to genotoxic agents, and is involved in many biological processes related to the maintenance of genomic stability and apoptosis (Liebermann et al., 2008; Siafakas et al., 2009; Smith et al., 1994; Zhan et al., 2005). Therefore, Gadd45 genes have been implicated in the stress signaling in response to physiological or environmental stressors (Liebermann et al., 2008; Siafakas et al., 2009; Smith et al., 1994; Zhan et al., 2005), which results in cell cycle arrest, DNA repair, cell survival and senescence, or apoptosis. In addition, the tumor suppressor gene p53 plays an important role in the maintenance of genomic fidelity by controlling cell cycle checkpoints and apoptotic processes following cell exposure to genotoxic stress. The dependence of Gadd45 induction on normal cellular p53 function is well established (Carrier et al., 1999). In response to DNA damage, Gadd45 was found to contribute to the stability of p53 (Carrier et al., 1999; Liebermann et al., 2008; Siafakas et al., 2009; Smith et al., 1994; Zhan et al., 2005). Previous studies have shown that Nano-Co could cause cytotoxic and genotoxic effects (Wan et al., 2012), and Gadd45α may be involved in these Nano-Co-induced genotoxic effects. However, the underlying mechanisms are still unclear.
Cobalt is well known to mimic hypoxia through activation of hypoxia inducible factor 1 α (HIF-1α). HIF-1 is a key transcription factor regulating cellular oxygen homeostasis. It is composed of HIF-1α and HIF-1β, both of which belong to the PAS family of basic helix-loop-helix transcription factors (Wang and Semenza, 1993a, b). HIF-1α is produced or activated in response to hypoxia (Wang and Semenza, 1993b; Yamashita et al., 2001), whereas HIF-1β is constitutively present regardless of oxygen tension (Liu et al., 2007). Under normoxia, the HIF-1α subunit is rapidly degraded by prolyl hydroxylase (PHD), the von Hippel-Lindau (VHL)/Elongin-C/Elongin-B E3 ubiquitin ligase complex, and the proteasome (Maxwell et al., 1999). However, under hypoxic conditions, HIF-1α translocates from the cytosol to the nucleus and heterodimerizes with HIF-1β to form the active HIF-1 protein, binding and activating hypoxia responsive genes, such as vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs) (Maxwell and Salnikow, Rey and Semenza, 2004). It has been reported that exposure to cobalt caused up- or down-regulation of many genes, and some up-regulated genes are HIF-1α-dependent. In addition, previous studies showed that Gadd45α mRNA was up-regulated during hypoxia (Price and Calderwood, 1992).
In the present study, we hypothesized that exposure to Nano-Co could up-regulate Gadd45α through activation of HIF-1α. We first investigated whether exposure of PW cells to Nano-Co or titanium dioxide nanoparticles (Nano-TiO2) would result in cell stress which was reflected by the up-regulation of Gadd45α. We then studied whether exposure to non-toxic doses of Nano-Co resulted in HIF-1α accumulation, and whether the up-regulation of Gadd45α induced by Nano-Co was due to the activation of HIF-1α pathway.
Materials and Methods
Metal nanoparticles
Nano-Co and Nano-TiO2 with a mean diameter of 20 nm and 28 nm, respectively, were provided by INABTA and Co., Ltd., Vacuum Metallurgical Co., Ltd., Japan. The nanostructure and composition of Nano-Co and Nano-TiO2 were characterized by transmission electron microscopy (TEM) (Hitach H-8000) and ancillary techniques including selected area electron diffraction (SAED) and energy-dispersive (X-ray) spectrometry (EDS). Nano-Co and Nano-TiO2 were dispersed in physiological saline and ultrasonicated for 30 min prior to each experiment. The characterization of these nanoparticles is summarized in Table 1, measured as previously reported (Wan et al., 2011).
Table 1.
Characterization of metal nanoparticles
| Metal | Particle size in powder (Diameter) (nm, average) | TEM (nm) Diameter | DLS (nm) Diameter | Specific surface area (m2/g) | Phase composition |
|---|---|---|---|---|---|
| Nano-Co | 20 | 10–40 | 260 | 47.9 | Co (85–90%) Co3O4 (10–15%) |
| Nano-TiO2 | 28 | 10–60 | 280 | 45.0 | Anatase (90%) Rutile (10%) |
Chemicals and reagents
The heat shock protein 90 (Hsp90) inhibitor, 17-allylamino-17-demethoxygeldanamycin (17-AAG), was purchased from Invitrogen (San Diego, CA). Polyclonal rabbit anti-Gadd45α antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA), monoclonal mouse anti-HIF-1α antibody from Abcam Inc. (Cambridge, MA), horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG from Santa Cruz Biotechnology (Santa Cruz, CA), and HRP-conjugated goat anti-rabbit IgG from CHEMICON (Temecula, CA). The ECL™ Western Blotting Detection Reagents was purchased from GE Healthcare, Amersham™ (Buckinghamshire, UK). Reagents for cell culture were purchased from Mediatech, Inc. (Herndon, VA) including Dulbecco’s Modification of Eagle’s Medium (DMEM), fetal bovine serum (FBS), 0.05% trypsin EDTA, nonessential amino acids, and penicillin-streptomycin solution. All other chemicals were purchased from Fisher Scientific (Pittsburgh, PA) unless otherwise stated.
Cell culture and treatment
Mouse embryo fibroblast PW cells, hypoxia inducible factor 1α knock-out cells [HIF-1α (−/−)] and its wild-type cells [HIF-1α (+/+)] were obtained from Dr. R. Johnson (University of California San Diego, San Diego, CA) and were cultured in DMEM supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin, and maintained in an incubator with a humidified atmosphere of 5 % CO2 at 37 °C.
In vitro cytotoxicity assay
The cytotoxicity of metal nanoparticles was analyzed by both an in vitro cytotoxicity assay kit (Sulforhodamine B Based, Sigma-Aldrich, St Louis, MO) (SRB assay) and the AlamarBlue™ assay (AbD Serotex, Oxford, UK) according to the manufacturers’ directions. Briefly, 5×103 PW, HIF-1α (−/−) and HIF-1α (+/+) cells were seeded into each well of 96-well plates and were allowed to attach to the growth surface by culturing overnight. Cells were then treated with different concentrations (0, 1.25, 2.5, 5, 7.5, 10, 20 μg/ml) of Nano-Co or Nano-TiO2 in a final volume of 200 μl per well for 6 h and 12 h. For the SRB assay, the adherent cells were fixed in situ with 50 % TCA at 4 °C, washed, and dyed with SRB. The incorporated dye was solubilized in 10 mM Tris base. The absorbance at 565 nm was recorded using a multidetection microplate reader (Synergy HT, BioTek, Vermont). The background absorbance at 690 nm was measured and subtracted from the measurement at 565 nm. The cell viability was expressed as the percentage of the control which was without treatment. Another method, AlamarBlue™ assay, is a colorimetric/fluorometric method for determining the number of metabolically active cells through oxidation-reduction indicator. This method was performed as described in a previous study (Wan et al., 2011).
Total RNA isolation, reverse transcription (RT) and real-time PCR
TRI Reagent (SIGMA, St. Louis, MO) was used to isolate total RNA according to the manufacturer’s instruction. RNA concentration was measured by absorbance at 260 nm with a DU 730 Spectrophotometer (Beckman Coulter, Fullerton, CA). 2 μg total RNA was reverse-transcribed at 42 °C for 60 min into cDNA using 1 μl M-MLV reverse transcriptase (Promega, Madison, WI) in a total volume of 25 μl which contains 2 μl of 0.5 μg/μl oligo(dT)18 primer, 1.25 μl of 10 mM dNTP, 0.75 μl RNasin Ribonuclease inhibitor, and 5 μl of 5 x M-MLV reaction buffer. Real-time PCR was performed by using a Bio-Rad iQ5 iCycler as previous described (Mo et al., 2009; 2012a,b)(43–45). Briefly, 1 μl cDNA from each sample was mixed with 1 μl of 5 μM of each primer, 10 μl of 2 x SYBR Green Supermix (Bio-Rad) in a total volume of 20 μl. PCR protocol consisted of four programs: (1) denaturation of the cDNA/RNA hybrid at 95 °C for 3 min; (2) amplification of cDNA for 50 cycles, each cycle using sequentially 95 °C for 10 s, 58 °C (β-actin and Gadd45α) for 30 s and 72 °C for 30 s; (3) analysis of the melting curve to confirm the single product amplification during the PCR assay; and (4) cooling the rotor and thermal chamber at 25 °C. The specific primers for mouse Gadd45α and β-actin (as the internal control) were as following: Gadd45α sense 5′-ATG ACT TTG GAG GAA TTC TCG-3′, antisense 5′-CAC TGA TCC ATG TAG CGA CTT-3′; β-actin sense 5′-GGC ATT GTT ACC AAC TGG GAC-3′, antisense 5′-ACC AGA GGC ATA CAG GGA CAG-3′. The relative expression level of each gene was calculated as fold dilution by using a standard curve for each gene. Standard curves were obtained by real-time PCR using 3 μl, 1 μl, and 1 μl of 10-fold dilution, 100-fold dilution and 1000-fold dilution respectively of cDNA obtained from PW, HIF-1α (−/−) or HIF-1α (+/+) cells. The expression level of Gadd45α was then normalized with the relative expression level of mouse β-actin in the same sample. Each sample was performed in triplicate.
Protein extraction and Western blot analysis
Cells treated with Nano-Co or Nano-TiO2 were collected. Nuclear proteins were prepared using NE-PERR Nuclear Extraction Reagent (Pierce, Rockford, IL) according to the manufacturer’s instruction. Proteins were quantified by the Bradford method. 100 μg of protein per sample was denatured at 100 °C for 5 min and separated on 7.5%~15% SDS-PAGE. After the protein was transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA), the nonspecific binding sites were blocked with 5 % skim milk in TBST (50 mM Tris-HCl, 140 mM NaCl, 0.05 % Tween 20, pH 7.2) for 2 h at room temperature. Then the membrane was incubated with the primary antibody at 4 °C overnight, followed by three washed with TBST, incubating with the second antibody for 1 h, three washed with TBST, and developing by using ECL™ Western Blotting Detection Reagents. Densitometric analyses were performed to quantify Western blotting signals by using NIH Image J software. Nuclear protein equal loading was verified by Coomassie Brilliant Blue staining.
Statistical analysis
Data are expressed as the mean ± SD. Differences among groups were evaluated with two-way analysis of variance; if the F-value was significant, groups were then compared at each dose by one-way analysis of variance (ANOVA) followed by Dunnett’s t-test. If a p value was less than 0.05, a difference was considered statistically significant. Statistical analyses were carried out using SigmaStat software (Jandel Scientific, San Raphael, CA).
Results
Cytotoxicity of Nano-Co or Nano-TiO2 in PW, HIF-1α (+/+) and HIF-1α (−/−) cells
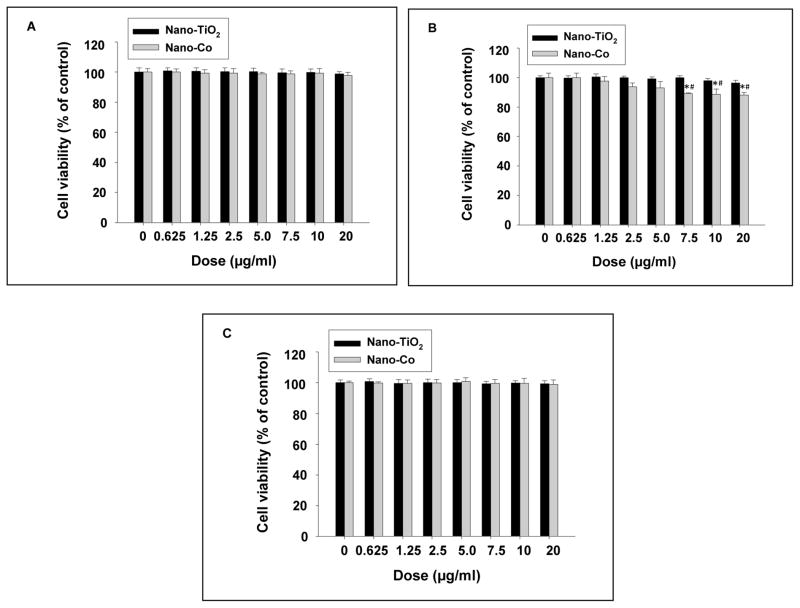
The effects of different concentrations (0–20 μg/ml) of Nano-Co or Nano-TiO2 on PW, HIF-1α (+/+) and HIF-1α (−/−) cells were assessed by SRB assay. No significant cytotoxicity was observed when cells were exposed to 20 μg/ml or less concentrations of Nano-Co for 6 h (Data not shown). However, exposure of HIF-1α (+/+) cells to Nano-Co at 7.5 μg/ml and beyond for 12 h caused significant cytotoxicity, while exposure of PW or HIF-1α (−/−) cells to Nano-Co at the same doses for 12 h did not cause any cytotoxic effects (Fig. 1 A–C). In contrast, exposure of cells to Nano-TiO2 did not cause any significant cytotoxic effects at all experimental dose and time points. The above results were further confirmed by the fluorescent AlamarBlue™ assay (data not shown). Non-toxic doses and time points were selected for the subsequent experiments.
Fig. 1 (A–C). Cytotoxicity of Nano-Co and Nano-TiO2 on PW (A), HIF-1α \(+/+) (B) and HIF-1α (−/−) (C) cells.
Cells were treated with different doses of Nano-Co or Nano-TiO2 for 12 h and cytotoxicity was determined by SRB assay. Cells without treatment were used as the control. Data are shown as mean ± SD of three experiments with six parallel culture in each experiment. * Significant difference as compared with the control, p<0.05; # Significant difference as compared with the same dose of Nano-TiO2-treated group, p<0.05.
Exposure of PW cells to Nano-Co resulted in increased Gadd45α expression
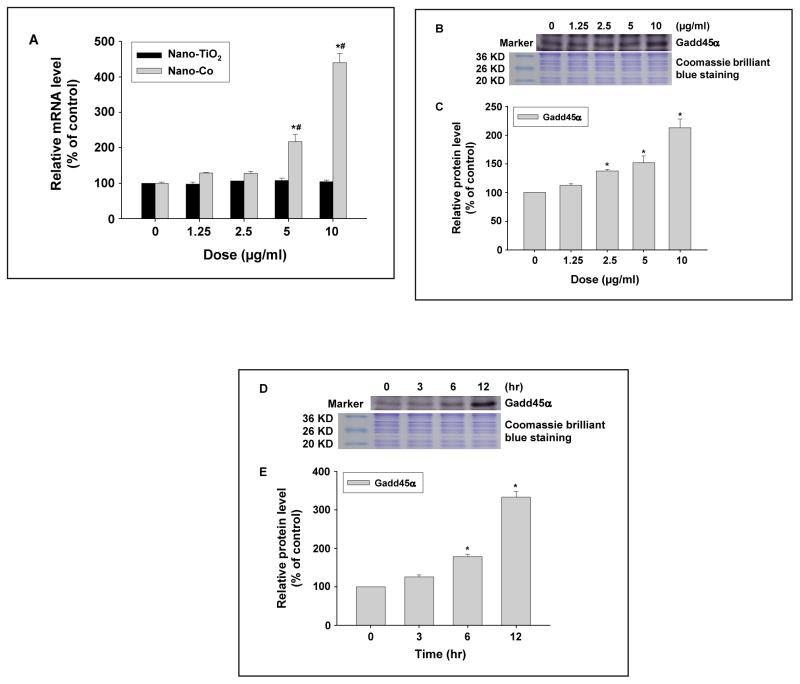
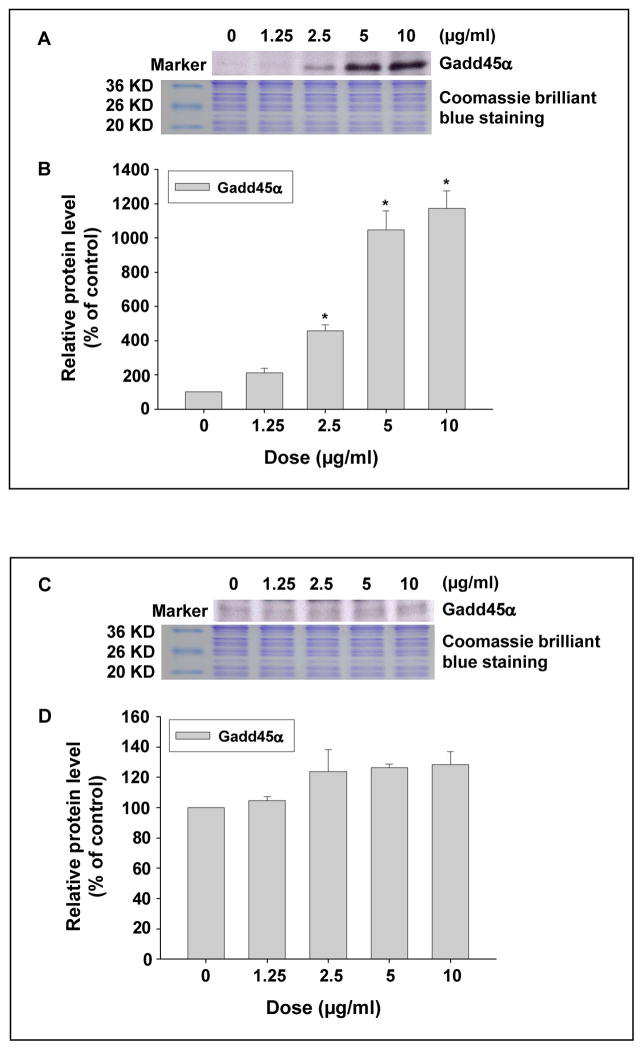
The effects of metal nanoparticles on Gadd45α expression in PW cells were determined by both real-time PCR and Western blot. Our results showed that there was a dose-response increase in Gadd45α at both mRNA and protein levels after exposure of PW cells to 0, 1.25, 2.5, 5, and 10 μg/ml of Nano-Co for 6h (Fig. 2 A–C), whereas Nano-TiO2 did not induce any significant Gadd45α up-regulation (Fig. 2 A). Our results also showed a time-response increase in Gadd45α expression in PW cells exposed to 5 μg/ml of Nano-Co for 3, 6 and 12 h (Fig. 2 D–E).
Fig. 2 (A–E). Dose- and time- response increase in Gadd45α expression in PW cells exposed to Nano-Co.
PW cells were treated with 0, 1.25, 2.5, 5, and 10 μg/ml of Nano-Co or Nano-TiO2 for 6 h (A–C), or with 5 μg/ml of Nano-Co for 3, 6, and 12 h (D–E). Cells without treatment were used as the control. Gadd45α mRNA level was determined by real-time PCR (A) while protein level was determined by Western blot (B–E). (A) shows the real-time PCR results as mean ± SD of three experiments with triplicate in each experiment. (B and D) show the results of a single Western blot experiment. (C and E) represent normalized band densitometry readings averaged from three independent experiments ± SD of Western results. * Significant difference as compared with the control, p<0.05; # Significant difference as compared with the same dose of Nano-TiO2-treated group, p<0.05.
Exposure of PW cells to Nano-Co caused HIF-1α accumulation
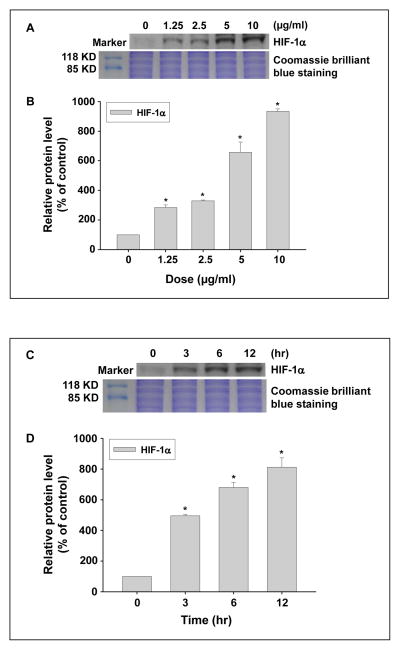
To examine whether exposure to Nano-Co caused HIF-1α accumulation, PW cells were treated with Nano-Co and the accumulation of HIF-1α was determined by Western blot. The results showed that exposure to 1.25, 2.5, 5, and 10 μg/ml of Nano-Co for 6 h caused significant HIF-1α accumulation in PW cells (Fig. 3 A–B). Our results also showed a time-response increase in HIF-1α accumulation in PW cells exposed to 5 μg/ml of Nano-Co for 3, 6 and 12 h (Fig. 3 C–D).
Fig. 3 (A–D). Dose- and time- response increase in HIF-1α expression in PW cells exposed to Nano-Co.
PW cells were treated with 0, 1.25, 2.5, 5, and 10 μg/ml of Nano-Co for 6 h (A and B) or treated with 5 μg/ml of Nano-Co for 3, 6, and 12 h (C and D). Cells without particle treatment were used as the control. (A and C) show the results of a single Western blot experiment. (B and D) represent normalized band densitometry readings averaged from three independent experiments ± SD of Western results. * Significant difference as compared with the control, p<0.05.
Effects of Nano-Co on Gadd45α expression in HIF-1α (+/+) and HIF-1α (−/−) cells
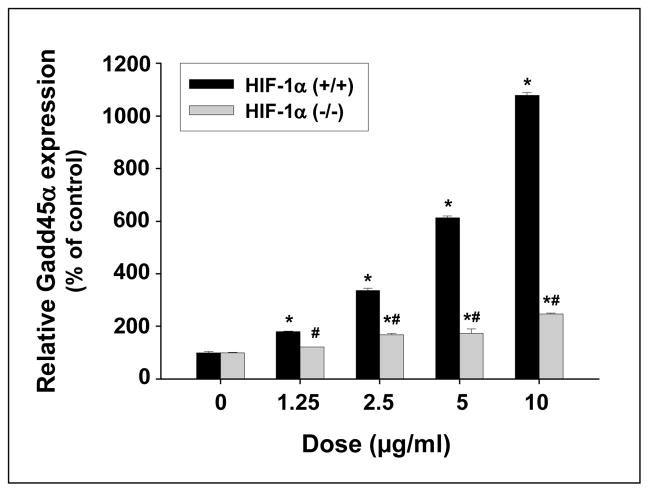
To investigate the potential pathways involved in Nano-Co-induced Gadd45α upregulation, HIF-1α (+/+) and HIF-1α (−/−) cells were treated with various doses of Nano-Co for various times. Although Nano-Co induced a dose-response increase in Gadd45a mRNA level in both HIF-1α (+/+) and HIF-1α (−/−) cells, the increase in HIF-1α (+/+) cells was significantly higher than that in HIF-1α (−/−) cells (Fig. 4). 5 and 10 μg/ml of Nano-Co caused 6- and 11- fold increase in Gadd45α mRNA level in HIF-1α (+/+) cells, but only a slightly increase in HIF-1α (−/−) cells. The Gadd45α protein level was also measured in both HIF-1α (+/+) and HIF-1α (−/−) cells after exposure to various doses of Nano-Co for 6 h. Our results demonstrated that Nano-Co up-regulated Gadd45α protein levels in HIF-1α (+/+) cells, but not in HIF-1α (−/−) cells (Fig. 5 A–D). These results suggest that HIF-1α pathway may be involved in the Nano-Co-induced increased Gadd45α expression.
Fig. 4. Dose-response induction of Gadd45α mRNA expression in HIF-1α (+/+) and HIF-1α (−/−) cells exposed to Nano-Co.
Cells were treated with 1.25, 2.5, 5, and 10 μg/ml of Nano-Co for 6 h. Cells without particle treatment were used as the control. Gadd45a mRNA level was measured by real-time PCR. The results showed mean ± SD of three experiments with triplicate in each experiment. * Significant difference as compared with the control, p<0.05; # Significant difference as compared with the same dose of Nano-Co-treated HIF-1α (+/+) cells, p<0.05.
Fig. 5 (A–D). Dose-response induction of Gadd45α protein expression in HIF-1α (+/+) cells (A and B) exposed to Nano-Co, but not in HIF-1α (−/−) cells (C and D).
Cells were treated with 1.25, 2.5, 5, and 10 μg/ml of Nano-Co for 6 h. Cells without particle treatment were used as the control. Gadd45α protein level was measured by Western blot. (A and C) show the results of a single Western blot experiment. (B and D) represent normalized band densitometry readings averaged from three independent experiments ± SD of Western results. * Significant difference as compared with the control, p<0.05.
Effects of heat-shock protein 90 (Hsp90) inhibitor, 17-AAG, on Nano-Co-induced Gadd45α up-regulation in PW cells
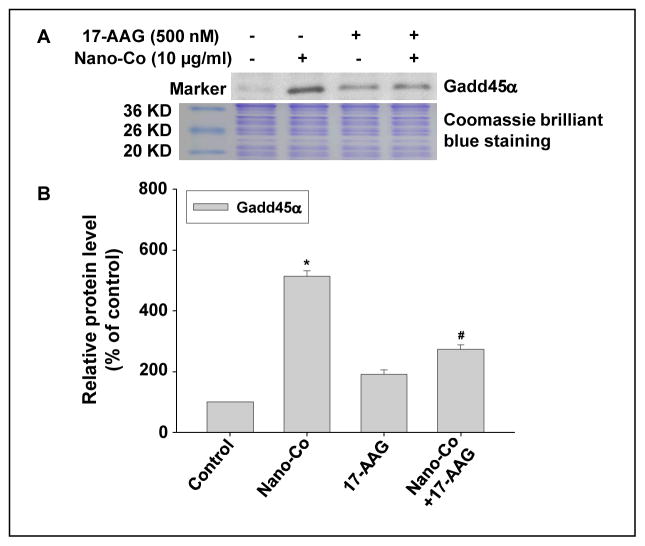
To further examine whether HIF-1α was involved in Nano-Co-induced Gadd45α upregulation, PW cells were pre-treated with 17-AAG, an Hsp90 inhibitor, for 4 h prior to 10 μg/ml of Nano-Co treatment for another 6 h. The results showed that Nano-Co-induced Gadd45α up-regulation was attenuated significantly when PW cells were pre-treated with 17-AAG (Fig. 6 A–B). These results indicated that Nano-Co-induced HIF-1α accumulation was involved in Nano-Co-induced Gadd45α up-regulation.
Fig. 6 (A–B). Hsp90 inhibitor (17-AAG) inhibited Nano-Co-induced Gadd45α accumulation in PW cells.
PW cells were pretreated with 500 nM 17-AAG for 4 h prior to exposure to 10 μg/ml of Nano-Co for another 6 h. Cells without particle treatment were used as the control. (A) shows the results of a single Western blot experiment. (B) represents normalized band densitometry readings averaged from three independent experiments ± SD of Western results. * Significant difference as compared with the control, p<0.05; # Significant difference as compared with Nano-Co-treated group, p<0.05.
Discussion
Although cobalt plays a physiological role as a cofactor of vitamin B12, cobalt cannot be regarded only as an essential element. It is well known that exposure to cobalt or its compound particles can cause pulmonary fibrosis, interstitial pneumonitis and asthma (De Boeck et al., 2003; IARC, 1992). Cobalt has been classified as “possibly carcinogenic to humans” (group 2B) by the International Agency for Research on Cancer (IARC) (IARC, 1992). Cobalt nanoparticles have been widely used in industrial and biological applications as organometal compound or as a biopolymer (Wang et al., 2005; Yang et al., 2006). Due to its small size and high surface area, Nano-Co may have unpredictable toxic or genotoxic properties. Previous inhalation studies showed that exposure to Nano-Co induced lung inflammation in rats, and damage to type I and type II pneumocytes (Kyono et al., 1992). Previous studies also showed that Nano-Co induced more severe lung inflammation than standard-sized (5 μm) cobalt particles (Zhang et al. 2000). While studies have demonstrated the potential genotoxic effects of some nanomaterials, the mechanisms are still unclear. In the present study, we selected Nano-Co as a ‘model’ of metal nanoparticles, because of its industrial interest, to examine its genotoxic effects and the underlying mechanisms. We studied whether exposure to metal nanoparticles such as Nano-Co or Nano-TiO2 resulted DNA damage and repair. In order to explore the potential mechanisms involved in these effects, three kinds of cells were used, mouse embryo fibroblast PW, HIF-1α knock out cell HIF-1α (−/−) and its control HIF-1α (+/+) cells which contains wild-type HIF-1α.
Our results showed that exposure of HIF-1α (+/+) cells to 7.5 μg/ml or more of Nano-Co for 12 h caused significant toxicity. However, this effect was not observed in PW and HIF-1α (−/−) cells. Exposure of these three kinds of cells to Nano-Co for 6 h did not produce significant cytotoxicity. In addition, there were no toxic effects on these cells with exposure to Nano-TiO2 at all experimental doses and time points. Several factors may affect cytotoxic effects of nanoparticles, including their small diameter, high surface area, and chemical composition (Colvin, 2003; Oberdorster et al., 2005b). Although it is difficult to estimate the degree of human health effects at such concentrations or quantities of Nano-Co that might be absorbed from dose-response studies, Nano-Co may accumulate in the body. Using doses that are lower than the cytotoxicity dose can help us to identify the potential health effects of Nano-Co other than those due to cytotoxicity itself.
The Gadd45α gene is a member of a group of genes that are induced by DNA damaging agents and growth arrest signals. Gadd45α gene encodes a conserved 165 amino acid protein with nuclear localization and is widely expressed in normal tissues, particularly in quiescent cellular populations. It is well known that regulation of cell cycle and growth is imperative for cell survival, and the intricate mechanisms that control proliferation and cell cycle are numerous (Liebermann and Hoffman, 2008; Siafakas and Richardson, 2009; Zhan, 2005). An interesting result of the current study was that exposure to some metal nanoparticles, such as Nano-Co, resulted in the up-regulation of Gadd45α both at mRNA and protein levels. These results suggest that exposure to Nano-Co may cause DNA damage, because Gadd45α plays an important role in the response to DNA damage in normal cells. Recent studies suggested that exposure of human A549 cells to Nano-Co caused DNA damage (Wan et al., 2012). The results from this study are also consistent with previous research reports which demonstrated that exposure to infrared and ultraviolet irradiation, and metals such as cadmium and arsenic, caused increase in Gadd45α expression (Bower et al., 2006; Liebermann and Hoffman, 2008; Siafakas and Richardson, 2009; Smith et al., 1994; Son et al., 2010; Zhan, 2005). Although this study did not determine Nano-Co–induced apoptosis, other previous studies have shown that increased Gadd45α expression could promote cell apoptosis.
Oxidative stress, pro-inflammatory cytokines, and MAK kinases have been shown to play important roles in the regulation of Gadd45α expression in several types of cells, including human type II cells and fibroblasts (Liebermann and Hoffman, 2008; Siafakas and Richardson, 2009; Zhan, 2005). Other transcription factors, such as HIF-1 may also be involved in the regulation of Gadd45α expression or activity (Piece and Calderwood, 1992). HIF-1 is a key transcription factor regulating cellular oxygen homeostasis. Our results showed that in HIF-1α (−/−) cells, the expression of Gadd45α induced by Nano-Co exposure was very low, almost the same as that in control cells. The data, therefore, pointed to a possible role of HIF-1α in the regulation of Gadd45a expression. It is also possible, however, that an upstream event (e.g. stabilization of p53) is HIF-1α-dependent.
Inhibition of Hsp90 also causes O2/PHD/VHL-independent degradation of HIF-1α. Hsp90 competes with the receptor of activated protein kinase C (RACK1) for binding to the PAS A-domain for HIF-1α. Hsp90 inhibition leads to increased RACK1 binding and recruitment of the Elongin C ubiquitin-ligase complex that mediates ubiquitination and proteasomal degradation of HIF-1α (Liu et al., 2007). Previous in vitro and in vivo studies have clearly shown that exposure to cobalt caused HIF-1α accumulation (Busch et al., 2010; Saini et al., 2010a, b). Our results demonstrated a dose- and a time-related increase in HIF-1α accumulation after exposure to non-toxic doses of Nano-Co. It has been reported that hypoxia can induce Gadd45α expression in cells (Saletta et al., 2011; Xiong et al., 2009). This raises an interesting question, whether the HIF-1α pathway is involved in Nano-Co-induced Gadd45α expression. To investigate the role of HIF-1α in Nano-Co-induced Gadd45α expression imbalance, the Hsp90 inhibitor 17-AAG was used to pre-treat PW cells. Our results clearly showed that pre-treatment with 17-AAG significantly inhibited Nano-Co-induced up-regulation of Gadd45α expression. The results suggest that HIF-1α may be involved in the regulation of Gadd45α.
In conclusion, our studies showed that exposure to Nano-Co caused an increase in Gadd45α expression in cells which could invoke various cellular responses such as cell cycle arrest, apoptosis and importantly, DNA repair. For DNA repair, Gadd45α is the critical component that is required for establishing a delicate and efficient cellular defense network in response to genotoxic stress and maintaining genomic stability. The HIF-1α signaling pathway may be involved in this process via Nano-Co-induced hypoxia, although the detailed mechanisms need to be further studied. These results provide evidence for the potential health effects of metal nanoparticle exposure. However, these health effects need to be further investigated, since the consequences of long-term and low-dose exposure to Nano-Co are still unclear. Further studies are needed to explore the precise molecular mechanism(s) by which this stress protein plays roles in Nano-Co-induced DNA damage-activated biological events, including the cell cycle checkpoint, DNA repair, and apoptosis.
Acknowledgments
This work was partly supported by American Lung Association (RG-872-N), American Heart Association (086576D), KSEF-1686-RED-11, CTSPGP 20018 and IRIG 50753 from University of Louisville, T32-ES011564 and ES01443, and grants 11-ZC02 and 2013RCB002 from Department of Health, Zhejiang, R. R. of China.
Abbreviations
- 17-AAG
17-(Allylamino)-17-demethoxygeldanamycin
- Gadd45α
growth arrest and DNA damage-inducible 45α protein
- HIF-1α
hypoxia inducible factor 1α
- Nano-Co
nano-sized cobalt
- Nano-TiO2
nano-sized titanium dioxide
Footnotes
The authors declare that there are no conflicts of interest.
References
- Baswas P, Wu CY. Critical review: nanoparticles and the environmental. J Air and Waste Manage Associ. 2005;55:708–746. doi: 10.1080/10473289.2005.10464656. [DOI] [PubMed] [Google Scholar]
- Bouchard LS, Anwar MS, Liu GL, Hann B, Xie ZH, Gray JW, Wang X, Pines A, Chen FF. Picomolar sensitivity MRI and photoacoustic imaging of cobalt nanoparticles. Proc Natl Acad Sci U S A. 2009;106(11):4085–4089. doi: 10.1073/pnas.0813019106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JJ, Leonard SS, Chen F, Shi X. As(III) transcriptionally activates the gadd45α gene via the formation of H2O2. Free Radic Biol Med. 2006;41(2):285–294. doi: 10.1016/j.freeradbiomed.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Busch W, Kühnel D, Schirmer K, Scholz S. Tungsten carbide cobalt nanoparticles exert hypoxia-like effects on the gene expression level in human keratinocytes. BMC Genomics. 2010;11:65. doi: 10.1186/1471-2164-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier F, Georgel PT, Pourquier P, Blake M, Kontny HU, Antinore MJ, Gariboldi M, Myers TG, Weinstein JN, Pommier Y, Fornace AJ., Jr Gadd45, a p53-responsive stress protein, modifies DNA accessibility on damaged chromatin. Mol Cell Biol. 1999;19:1673–1685. doi: 10.1128/mcb.19.3.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow JC, Watson JG, Savage N, Solomon CJ, Cheng YS, McMurry PH, Corey LM, Bruce GM, Pleus RC, Biswas P, Wu CY. Nanoparticles and the Environment. J Air and Waste Manage Associ. 2005;55:1411–1417. doi: 10.1080/10473289.2005.10464743. [DOI] [PubMed] [Google Scholar]
- Colvin VL. The potential environmental impact of engineered nanomateroials. Nat Biotechnol. 2003;21(10):1166–1170. doi: 10.1038/nbt875. [DOI] [PubMed] [Google Scholar]
- De Boeck M, Krisch-Volders M, Lison D. Cobalt and antimony: genotoxicity and carcinogenicity. Mut Res. 2003a;533:135–152. doi: 10.1016/j.mrfmmm.2003.07.012. [DOI] [PubMed] [Google Scholar]
- IARC working group. IARC monographs on the evaluation of the carcinogenic risk of chemicals to man. Vol. 52. Lyon: IARC; 1992. Cobalt and cobalt compounds; pp. 263–473. [Google Scholar]
- Itoa A, Shinkaib M, Hondaa H, Kobayashic T. Medical application of functionalized magnetic nanoparticles. 2005;100 (1):1–11. doi: 10.1263/jbb.100.1. [DOI] [PubMed] [Google Scholar]
- Kale SN, Jadhav AD, Verma S, Koppikar SJ, Kaul-Ghanekar R, Dhole SD, Ogale SB. Characterization of biocompatible NiCo2O4 nanoparticles for applications in hyperthermia and drug delivery. Nanomedicine. 2012;8(4):452–459. doi: 10.1016/j.nano.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Kubik T, Bogunia-Kubik K, Sugusaka M. Nanotechnology on duty in medical application. Curr Pharm Biotechnol. 2005;6:17–33. doi: 10.2174/1389201053167248. [DOI] [PubMed] [Google Scholar]
- Kyono H, Kusaka Y, Homma K, Kubota H, Endo-ichikawa Y. Reversible lung lesions in rats due to short-term exposure to ultrafine cobalt particles. Ind Health. 1992;30:103–118. doi: 10.2486/indhealth.30.103. [DOI] [PubMed] [Google Scholar]
- Liebermann AD, Hoffman B. Gadd45 in stress signaling. J Mol Signal. 2008;3:15. doi: 10.1186/1750-2187-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YV, Baek JH, Zhang H, Diez R, Cole RN, Semenza GL. RACK1 competes with HSP90 for binding to HIF-1alpha and is required for O(2)-independent and HSP90 inhibitor- induced degradation of HIF-1alpha. Mol Cell. 2007;25:207–217. doi: 10.1016/j.molcel.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu AH, Salabas EL, Schuth F. Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew Chem Int Ed. 2007;46:1222–1244. doi: 10.1002/anie.200602866. [DOI] [PubMed] [Google Scholar]
- Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumor suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- Maxwell P, Salnikow K. HIF-1: an oxygen and metal responsive transcription factor. Cancer Biol Ther. 2004;3:29–35. doi: 10.4161/cbt.3.1.547. [DOI] [PubMed] [Google Scholar]
- Maynard AD, Aitken RJ, Butz T, Colvin V, Donaldson K, Oberdorster G, Philbert MA, Ryan J, Seaton A, Stone V, Tinkle SS, Tran L, Walker NJ, Warheit DB. Safe handing of nanotechnology. Nature. 2006;444:267–269. doi: 10.1038/444267a. [DOI] [PubMed] [Google Scholar]
- Mo Y, Wan R, Wang J, Chien S, Tollerud DJ, Zhang Q. Diabetes is associated with increased sensitivity of alveolar macrophages to urban particulate matter exposure. Toxicology. 2009;262(2):130–7. doi: 10.1016/j.tox.2009.05.019. [DOI] [PubMed] [Google Scholar]
- Mo Y, Wan R, Feng L, Chien S, Tollerud DJ, Zhang Q. Combination effects of cigarette smoke extract and ambient ultrafine particles on endothelial cells. Toxicol In Vitro. 2012a;26(2):295–303. doi: 10.1016/j.tiv.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo Y, Wan R, Zhang Q. Application of Reverse Transcription-PCR and Real-Time PCR in Nanotoxicity Research. Methods Mol Biol. 2012b;926:99–112. doi: 10.1007/978-1-62703-002-1_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdoster G, Maynard A, Donaldson K, Castranova V, Fitzpatrick Ausman K, Carter J, Jarn B, Kreyling W, Lai D, Olin S, Monteiro-Riviere N, Warheit D, Yang H. A report from the ILSI Research Foundation/risk science Institute Nanomaterial Toxicity Screening Working. Principle for characterizing the potential human health effects from exposure to nanomaterilas: elements of a screening strategy. Particles and Fiber Toxicology. 2005a;2:8. doi: 10.1186/1743-8977-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdorster G, Oberdoster E, Oberdorster L. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005b;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R, Depledge M. Nanotechnology and the environment: risk and rewards. Marine Pollution Bulletin. 2005;50:609–612. doi: 10.1016/j.marpolbul.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Pouponneau P, Leroux JC, Martel S. Magnetic nanoparticles encapsulated into biodegradable microparticles steered with an upgraded magnetic resonance imaging system for tumor chemoembolization. Biomaterials. 2009;30(31):6327–6332. doi: 10.1016/j.biomaterials.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Price BD, Calderwood SK. Gadd45 and Gadd153 messenger RNA levels are increased during hypoxia and after exposure of cells to agents which elevate the levels of the glucose-regulated proteins. Cancer Res. 1992;52(13):3814–3817. [PubMed] [Google Scholar]
- Rey S, Semenza GL. Hypoxia-inducible factor-1-dependent mechanisms of vascularization and vascular remodelling. Cardiovasc Res. 2010;86:236–242. doi: 10.1093/cvr/cvq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini Y, Kim KY, Lewandowski R, Bramble LA, Harkema JR, Lapres JJ. Role of hypoxiainducible factor 1α in modulating cobalt-induced lung inflammation. Am J Physiol Lung Cell Mol Physiol. 2010a;298(2):L139–L147. doi: 10.1152/ajplung.00252.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini Y, Greenwood KK, Merrill C, Kim KY, Patial S, Parameswaran N, Harkema JR, LaPres JJ. Acute cobalt-induced lung injury and the role of hypoxia-inducible factor 1alpha in modulating inflammation. Toxicol Sci. 2010b;116(2):673–681. doi: 10.1093/toxsci/kfq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saletta F, Suryo Rahmanto Y, Siafakas AR, Richardson DR. Cellular iron depletion and the mechanisms involved in the iron-dependent regulation of the growth arrest and DNA damage family of genes. J Biol Chem. 2011;286(41):35396–35406. doi: 10.1074/jbc.M111.273060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siafakas AR, Richardson DR. Growth arrest and DNA damage-45 alpha (Gadd45a) Intern J of Biochem & Cell Bio. 2009;41:986–989. doi: 10.1016/j.biocel.2008.06.018. [DOI] [PubMed] [Google Scholar]
- Smith ML, Chen IT, Zhan Q, Bae I, Chen CY, Gilmer TM, Kastan MB, O’Connor PM, Fornace AJ., Jr Interaction of the p53-regulated protein Gadd45 with proliferating cell nuclear antigen. Science. 1994;266:1376–1380. doi: 10.1126/science.7973727. [DOI] [PubMed] [Google Scholar]
- Son YO, Lee JC, Hitron JA, Pan J, Zhang Z, Shi X. Cadmium induces intracellular Ca2+- and H2O2-dependent apoptosis through JNK- and p53-mediated pathways in skin epidermal cell line. Toxicol Sci. 2010;113(1):127–137. doi: 10.1093/toxsci/kfp259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan R, Mo Y, Chien S, Li Y, Li Y, Tollerud DJ, Zhang Q. The role of hypoxia inducible factor-1α in the increased MMP-2 and MMP-9 production by human monocytes exposed to nickel nanoparticles. Nanotoxicology. 2011;5(4):568–582. doi: 10.3109/17435390.2010.537791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan R, Mo Y, Feng L, Chien S, Tollerud DJ, Zhang Q. DNA damage caused by metal nanoparticles: involvement of oxidative stress and activation of ATM. Chem Res Toxicol. 2012;25(7):1402–1411. doi: 10.1021/tx200513t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GL, Semenza GL. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci USA. 1993a;90:4304–4308. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GL, Semenza GL. Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J Biol Chem. 1993b;268:21513–21518. [PubMed] [Google Scholar]
- Wang K, Xu JJ, Chen HY. A novel glucose biosensor based on the nanoscaled cobalt phthalocyanine glucose oxidase biocomposite. Biosens Bioelectron. 2005;20:1388–1396. doi: 10.1016/j.bios.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Liebermann DA, Tront JS, Holtzman EJ, Huang Y, Hoffman B, Geifman-Holtzman O. Gadd45α stress signaling regulates sFlt-1 expression in preeclampsia. J Cell Physiol. 2009;220(3):632–639. doi: 10.1002/jcp.21800. [DOI] [PubMed] [Google Scholar]
- Yang MH, Jiang JH, Yang YH, Chen XH, Shen GL, Yu RQ. Carbon nanotube/cobalt hexacyanoferrate nanoparticle-biopolymer system for the fabrication of biosensors. Biosens Bioelectron. 2006;21:1791–1797. doi: 10.1016/j.bios.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Yamashita K, Discher DJ, Hu J, Bishopric NH, Webster KA. Molecular regulation of the endothelin-1gene by hypoxia. Contributions of hypoxia-inducible factor-1, activator protein-1, GATA-2, and p300/CBP. J Biol Chem. 2001;276:12645–12653. doi: 10.1074/jbc.M011344200. [DOI] [PubMed] [Google Scholar]
- Zhan Q. Gadd45a, a p53 and BRCA1-reguated stress protein, in cellular response to DNA damage. Mutat Rea. 2005;569:133–143. doi: 10.1016/j.mrfmmm.2004.06.055. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Kusaka Y, Donaldson K. Comparative rat pulmonary responses caused by exposure to standard cobalt and ultrafine cobalt. J Occup Health. 2000;42:179–184. [Google Scholar]