Abstract
Background
In rodents, hypothalamic brain-derived neurotrophic factor (BDNF) expression appears to be regulated by melanocortin-4 receptor (MC4R) activity. The impact of MC4R genetic variation on circulating BDNF in humans is unknown.
Objective
To compare BDNF concentrations of subjects with loss-of-function (LOF) and gain-of-function (GOF) MC4R variants to those of controls with common sequence MC4R.
Methods
Circulating BDNF was measured in two cohorts with known MC4R sequence: 148 subjects of Pima Indian heritage ([mean±SD]: age 15.7±6.5y, BMI-Z 1.63±1.03), and 69 subjects of Hispanic heritage (10.8±3.6y, BMI-Z 1.57±1.07). MC4R variants were characterized in vitro by cell surface expression, receptor binding, and cAMP response after agonist administration. BDNF single nucleotide polymorphisms (SNPs) rs12291186, rs6265, and rs7124442 were also genotyped.
Results
In the Pima cohort, no significant differences in serum BDNF was observed for 43 LOF-subjects versus 65 LOF-matched controls [age-, sex-, and BMI-matched] (P=0.29), or 20 GOF-subjects versus 20 GOF-matched controls (P=0.40). Serum BDNF was significantly associated with genotype for BDNF rs12291186 (P=0.006) and rs6265 (P=0.009), but not rs7124442 (P=0.99); BDNF SNPs did not interact with MC4R status to predict serum BDNF. In the Hispanic cohort, plasma BDNF was not significantly different among 21 LOF-subjects, 20 GOF-subjects, and 28 controls (P=0.79); plasma BDNF was not predicted by BDNF genotype or BDNF-x-MC4R genotype interaction.
Conclusions
Circulating BDNF concentrations were not significantly associated with MC4R functional status, suggesting that peripheral BDNF does not directly reflect hypothalamic BDNF secretion and/or that MC4R signaling is not a significant regulator of the bulk of BDNF expression in humans.
Keywords: BDNF, MC4R, ELISA, obesity, leptin
Introduction
Brain-derived neurotrophic factor (BDNF) is believed to function downstream of the leptin-proopiomelanocortin (POMC) signaling pathway to play a key role in the regulation of energy balance.1–4 Bdnf heterozygous knockout mice display hyperphagia and obesity,5, 6 as do mice where depletion of hypothalamic BDNF has been produced.7 Human BDNF haploinsufficiency, either due to heterozygous deletion in patients with WAGR/11p deletion syndrome8 or disruption of BDNF expression in a child with interstitial 11p inversion,9 is associated with decreased serum BDNF concentrations, hyperphagia, and obesity. Patients with Prader-Willi syndrome, a disorder caused by lack of expression of paternally-derived genes on chromosome 15q11-13 and characterized by hyperphagia and obesity, have decreased serum and plasma BDNF concentrations compared to BMI-matched controls.10 These findings suggest the possibility that alterations in BDNF may be a mechanism through which other disorders of energy homeostasis affect food intake and body weight.
The melanocortin-4 receptor (MC4R) is a G protein-coupled receptor that is highly expressed in the hypothalamic paraventricular nucleus and dorsal motor nucleus of the vagus.11 In animal studies, MC4R appears to serve an intermediary role within the leptin pathway, acting downstream of the leptin receptor and upstream of BDNF signaling.1–4 Leptin-receptor deficient db/db mice have decreased hypothalamic BDNF expression;12 their obesity and impaired glucose metabolism are ameliorated by intracerebroventricular administration of BDNF.13 In rodents, both POMC- and AgRP-expressing neurons in the arcuate nucleus project to the ventromedial hypothalamus, where BDNF is highly expressed.1, 14 MC4R activation induces BDNF expression in cultured rat astrocytes,4 and Mc4r homozygous knockout mice are hyperphagic, obese, and have decreased hypothalamic Bdnf expression.1 The anorexic effects of MC4R activation can be blocked by administering an anti-BDNF antibody in the third ventricle,2 and the orexigenic effects of MC4R antagonism are abrogated by BDNF co-administration in the fourth ventricle.3 These data suggest an important role for BDNF downstream of MC4R within the central nervous system.
In humans, inactivation of MC4R is the most common monogenic cause of severe, early-onset obesity,15 with more pronounced weight-gain in childhood compared to adulthood.16 Additionally, two MC4R polymorphisms, V103I and 1251L, which cause decreased sensitivity to AgRP inhibition and increased MC4R activity, respectively,17 appear to confer protection against obesity.18 Despite the strong animal evidence for MC4R’s role as a regulator of BDNF, there have been no previous studies examining BDNF concentrations in patients with loss-of-function (LOF) or gain-of-function (GOF) MC4R variants. The brain is believed to be the primary source of BDNF, and circulating BDNF concentrations are thought to reflect cerebral output of BDNF.19 Therefore, examination of peripheral BDNF in subjects with MC4R variants could yield insights for the role of human MC4R signaling as a regulator of BDNF secretion. We hypothesized that BDNF would be higher in subjects with GOF MC4R variants and lower in subjects with LOF MC4R variants compared to BDNF in subjects with common sequence or nonfunction-altering MC4R variants. We secondarily hypothesized that the effects of MC4R variants on BDNF concentrations within individuals would be more pronounced in childhood than in adulthood. Finally, because BDNF single nucleotide polymorphisms (SNPs) have been associated with obesity in genome-wide association studies,20 we hypothesized that BDNF SNPs would be associated with altered BDNF concentrations and would modify the effects of MC4R functional variants.
Materials and Methods
Subjects
Two cohorts that had previously undergone MC4R genotyping were studied:
Pima cohort
Children and adults, age ≥ 5 years, of Pima Indian heritage were participants in an NIH longitudinal health study of members of the Gila River Indian Community in Arizona, as previously described.21 Sequencing of the MC4R coding region was performed in 6 760 subjects, as previously described.16 Non-diabetic subjects were included in the current study if baseline height, weight, and stored fasting serum (collected during 1970–2006) were available. All adults provided written informed consent; a parent or guardian provided informed consent for children. This study was approved by the institutional review board of NIDDK. Forty subjects with heterozygous LOF MC4R variants and 3 subjects with homozygous LOF MC4R variants were individually matched with common sequence MC4R control subjects (LOF-C) by sample storage time (±2 years), sex, age (±1 year), and body mass index [BMI] (±1 kg/m2). Attempts were made to match 2 LOF-C for each LOF, but due to limitations in serum availability from children with high BMI z-scores (BMI-Z), some LOF subjects had only 1 LOF-C match, for a total of 65 LOF-C. Of these individuals, 23 LOF and 43 LOF-C had measurements both in childhood and adulthood. Twenty subjects with GOF MC4R variants were individually matched 1:1 with 20 common sequence MC4R control subjects (GOF-C).
Hispanic cohort
Children and adolescents, ages 4–19 years, of Hispanic heritage were participants in the USDA/ARS Children’s Nutrition Research Center/Baylor College of Medicine sponsored Viva La Familia longitudinal study in Texas, as previously described.22 Sequencing of the entire MC4R gene plus 3′ and 5′ flanking regions was performed in 376 parents, and the 25 identified SNPs, including 7 non-synonymous SNPs in the coding region of MC4R, were genotyped in 1 016 children and 613 additional parents, as previously described.23 Subjects were included in the current cross-sectional study if baseline height, weight, and stored fasting plasma (collected during 2000–2004) were available. Subjects with diabetes at baseline visit, and subjects with incomplete genotyping results for the 7 non-synonymous coding region SNPs or a mixture of LOF and GOF MC4R variants were excluded from the current study. All children and their parents gave written informed consent or assent. The protocol was approved by the Institutional Review Board for Human Subject Research for Baylor College of Medicine and Affiliated Hospitals and Texas Biomedical Research Institute (formerly, Southwest Foundation for Biomedical Research). Subjects with the common MC4R sequence (n=25) or non-function-altering MC4R variants (n=3) were categorized together as controls for comparison with subjects who had LOF (n=21) and GOF (n=20) variants. Control subjects were selected to be similar in age, sex, and BMI-Z with LOF and GOF subjects but were not individually matched. Body composition was determined by dual-energy X-ray absorptiometry with a Delphi-A whole-body scanner (Hologic Inc, Waltham, MA). Total body and regional estimates of fat mass and fat-free mass were obtained by using the manufacturer’s software (version 11.2).
Measurement of BDNF and leptin concentrations
In the Pima cohort, a morning fasting venous blood sample was obtained in a serum separator tube, permitted to clot at room temperature for 30 minutes, and then centrifuged at 1 500g to separate serum, which was stored at −80°C. In the Hispanic cohort, a morning fasting venous blood sample was obtained in a potassium ethylenediaminetetraacetic acid (EDTA) tube and centrifuged at 1 000g to separate plasma, which was stored at −80°C. BDNF concentrations were measured using a commercial enzyme-linked immunosorbent assay (ELISA) with intra- and inter-assay variabilities of 3.8% and 7.6%, respectively, and a minimum detection limit of 20 pg/mL (Human BDNF Quantikine ELISA, R&D Systems, Minneapolis, MN). Samples were diluted 1:20 for serum and 1:5 for plasma prior to BDNF assay, and the average value of duplicate measurements was used for data analyses. In the Pima cohort, leptin concentrations were measured using a commercial ELISA kit with intra- and inter-assay variabilities of 3.0% and 4.2%, respectively, and a minimum detection limit of 7.8 pg/mL (Human Leptin Quantikine ELISA, R&D Systems, Minneapolis, MN). Serum samples were diluted 1:100 prior to leptin assay, and the average of duplicate measurements was used for data analyses. In the Hispanic cohort, leptin concentrations were measured by a commercial radioimmunoassay kit, as previously described.23
Functional characterization of MC4R variants
Several previously-identified variants found in subjects of the present study had been described as LOF in prior studies24: D37Stop,16 G55V,25, 26 R165G,16 R165Q,16, 17 I269N,16, 26 and A303P.16 Two variants were described as GOF in a prior study: V103I17 and I251L.17 One variant, F202L,27, 28 was described as non-function-altering in prior studies. Independent confirmatory functional characterization was performed for G55V, R165G, R165Q, I269N, F202L, and A303P, as well as for two previously-undescribed MC4R variants, C172R and M208V. In vitro mutagenesis of MC4R, stable transfections, binding studies of α-MSH and (Nle4, D-Phe7)-α-MSH [NDP-MSH, a super-potent agonist for the MC4R29] in intact cells, stimulation of intracellular 3′-5′-cyclic adenosine monophosphate (cAMP) production by α-MSH and NDP-MSH, and imaging of cells stably expressing MC4R by confocal microscopy were performed using procedures previously described.30
Genotyping of BDNF SNPs
SNPs in BDNF (rs12291186, rs6265, and rs7124442, located within an intron, within the protein coding exon, and in the 3′UTR, respectively, and spanning the major LD block of BDNF with high D′ 0.93 and low R2 0.2) were genotyped to examine their potential modification of MC4R functional effect on BDNF expression. In the Pima cohort, these SNPs were genotyped using SNPlex Genotyping System 48-plex (Applied Biosystems, Foster City, CA) on an automated DNA capillary sequencer (model 3730; Applied Biosystems). In the Hispanic cohort, rs6265 and rs7124442 were genotyped using the Illumina HumanOmni1-Quad v1.0 BeadChips, as previously described,31 while rs12291186 was genotyped by TaqMan SNP Genotyping Assay (Applied Biosystems).
Statistical analyses
The primary outcome measure was BDNF concentration. We calculated that a sample size of 20 subjects per MC4R functional status group would have >80% power to detect a difference in BDNF concentration of 7 ng/mL (~1 SD10) at significance of p<0.05. In vitro MC4R characterization data were analyzed using Prism software (GraphPad Software, Inc., San Diego, CA) to determine half-maximal inhibitory concentration (IC50) for the binding studies, and half-maximal effective concentration (EC50) and maximal response (Rmax) for the stimulation studies. Maximal binding (Bmax), expressed as a percentage of the maximal amount of binding to common sequence MC4R, was calculated based on binding of 125I-NDP-MSH to variant MC4R in the absence of unlabeled competitor. Human subject data were analyzed using SPSS Statistics software, v17.0 (IBM, Armonk, NY). BMI standard deviation scores (BMI-Z) were calculated based on normative standards for age and sex32 using the modified LMS method.33 Normative data for age 20 years were used to calculate BMI-Z from the measured height and weight of subjects older than 20 years. Subject characteristics were compared using independent samples t tests, Mann-Whitney U tests, analyses of variance with post-hoc LSD, or Kruskal-Wallis tests with post-hoc U tests. Longitudinal changes were assessed by paired t tests or Wilcoxon Signed Ranks, and repeated-measures ANCOVA adjusting for change in BMI-Z. Percentages were compared by Fisher’s exact and chi-square tests. Spearman and partial Pearson correlations assessed association between sample storage time and analyte concentrations. For analyses of covariance (ANCOVAs) and linear regressions, BDNF and leptin were normalized by log-transformation, and percent Indian heritage and percent body fat transformed by arcsine (square-root). Covariates included age, sex, BMI-Z, sample storage time, BDNF genotypes, and in the Pima cohort, degree of Indian heritage based on self-reported ethnicities to the nearest eighth. Means ± standard deviations (SDs) or medians [25–75th percentile] are shown for subject characteristics. Adjusted means ± standard error of the mean (SEMs) or back-transformed adjusted means ± 95% confidence intervals (CIs) are shown for ANCOVAs. Genetic linkage disequilibrium analyses were performed using CubeX.34
Results
MC4R Variant Functional Characterization
The cell surface expression, ligand binding, and signaling properties of common and variant MC4Rs in response to agonist stimulation are shown in Table 1 and Supplemental Figures 1–4. The two MC4R variants with unknown function, C172R and M208V, were categorized as LOF because they had decreased ligand binding and lower basal and stimulated cAMP generation compared to common sequence MC4R (Tables 1 and Supplemental Figures 3–4). The finding from the other 6 variants we examined (G55V, R165G, R165Q, I269N, F202L, and A303P) confirmed all prior reported categorizations (non-function-altering for F202L, and LOF for the other 5 variants). Categorization of all subjects within the Pima and Hispanic cohorts are shown in Table 2.
Table 1.
The Ligand Binding and Signaling Properties of Common and Variant MC4Rs in Response to NDP-MSH or α-MSH Stimulation
| MC4R Genotype | Bmax (%C)a | NDP-MSH Binding | NDP-MSH-stimulated cAMP | α-MSH Binding | α-MSH-stimulated cAMP | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| IC50 (nM) | EC50 (nM) | Rmax (%C)b | IC50 (nM) | EC50 (nM) | Rmax (%C)c | ||
|
|
|
|
|||||
| Common | 100 | 33.93 ± 4.19 | 1.60 ± 0.27 | 100 | 606.28 ± 139.34 | 10.81 ± 5.04 | 100 |
| G55V | 55.40 ± 9.63e | 47.63 ± 16.26 | 5.41 ± 1.17d | 97.08 ± 6.46 | N/D | 612.34 ± 210.05d | 84.87 ± 4.82d |
| R165G | 37.18 ± 3.07f | 3.24 ± 0.40e | 1.34 ± 0.22 | 135.46 ± 13.02 | 271.20 ± 65.53 | 5.18 ± 1.32 | 112.50 ± 4.51 |
| R165Q | 34.75 ± 4.44f | 2.78 ± 0.35e | 0.91 ± 0.08d | 142.68 ± 6.64d | 365.83 ± 86.87 | 4.46 ± 0.50 | 134.40 ± 5.28d |
| C172R | N/D | N/D | 9.60 ± 2.22d | 32.86 ± 6.69e | N/D | 1627.96 ± 430.15e | 39.21 ± 7.91e |
| F202L | 99.24 ± 9.32 | 26.74 ± 5.92 | 2.15 ± 0.50 | 125.99 ± 8.55 | 492.63 ± 83.57 | 2.79 ± 0.58 | 92.67 ± 12.58 |
| M208V | 74.16 ± 6.18e | 70.38 ± 15.20 | 17.77 ± 5.14d | 113.88 ± 13.65 | 913.47 ± 224.19 | 270.75 ± 122.34d | 139.40 ± 22.24 |
| I269N | 42.86 ± 3.08f | 10.73 ± 1.39d | 0.63 ± 0.23 | 131.12 ± 9.65 | 561.20 ± 42.92 | 40.42 ± 23.73 | 121.30 ± 6.83d |
| A303P | 22.42 ± 4.55f | 2.29 ± 0.34e | 0.57 ± 0.13e | 98.49 ± 9.23 | 54.98 ± 14.23d | 1.00 ± 0.06 | 108.30 ± 2.97 |
Abbreviations: MC4R, melanocortin-4 receptor; NDP-MSH, 4-Norleucine, 7-D-phenylalanine-alpha-melanocyte-stimulating hormone (ultra-potent MC4R agonist); α-MSH, alpha-melanocyte-stimulating hormone; Bmax, maximal binding of 125I-NDP-MSH to cells transiently transfected with common or variant MC4R; %C, percentage of value for common sequence of MC4R; IC50, half-maximal inhibitory concentration; EC50, half-maximal effective concentration; cAMP, 3′-5′-cyclic adenosine monophosphate; Rmax, maximal response; N/D, could not be detected or calculated; SEM, standard error of the mean.
Bmax are mean ± SEM of at least six independent experiments, and other parameters are mean ± SEM of at least three independent experiments. Independent samples t tests compared variant MC4Rs with common MC4R.
Rmax of common MC4R was 3544.33 ± 471.12 pmol/106 cells with NDP-MSH stimulation.
Rmax of common MC4R was 3117.01 ± 472.62 pmol/106 cells with α-MSH stimulation.
Significantly different from common MC4R, P <0.05.
Significantly different from common MC4R, P <0.01.
Significantly different from common MC4R, P <0.001.
Table 2.
Summary of Functional Properties of Variant MC4Rs and Their Distribution in the Cohorts Studied
| MC4R Construct | Surface Expression | Binding | Signaling cAMP
|
Functional Category | Pima Cohort | Hispanic Cohort | |
|---|---|---|---|---|---|---|---|
| Basal | Stimulated | ||||||
| G55V | + | ↓ | ↓ | ↓ | LOF | 0 | 5a |
| R165G | ↓ | ↓ | + | + | LOF | 9 | 0 |
| R165Q | ↓ | ↓ | + | ↑ | LOF | 19 | 0 |
| C172R | ↓ | ↓ | ↓ | ↓ | LOF | 0 | 5a |
| F202L | ND | + | + | + | NFA | 0 | 3 |
| M208V | + | ↓ | ↓ | ↓ | LOF | 0 | 1 |
| I269N | ↓ | ↓ | + | + | LOF | 0 | 11 |
| A303P | ↓ | ↓ | ↑ | + | LOF | 3 | 0 |
|
| |||||||
| D37Stop | ↓ | LOFb | 12 | 0 | |||
| V103I | + | + | + | + | GOFc | 11 | 8 |
| I251L | + | + | ↑ | + | GOFc | 9 | 12 |
Abbreviations: MC4R, melanocortin-4 receptor; cAMP, 3′-5′-cyclic adenosine monophosphate; LOF, loss-of-function; NFA, non-function-altering; GOF, gain-of-function; +, no significant difference compared with common MC4R, ND, not done.
One subject was compound heterozygous for G55V and C172R.
Nine subjects heterozygous, 3 subjects homozygous for this frameshift mutation that results in premature termination prior to the ligand binding domain. Decreased stimulated cAMP generation previously reported.16 Surface expression, ligand binding, and basal activity have not been reported.
Categorized as GOF because V103I previously reported to have decreased agouti-related peptide potency, while I251L has increased basal activity.17
Pima Cohort
Subject Characteristics
A total of 148 subjects of Pima Indian heritage [age (mean ± SD) 15.7 ± 6.5y, 50% female, BMI-Z 1.63 ± 1.03] were included in baseline cross-sectional analyses. Sixty-six of these subjects (23 LOF and 43 LOF-C) were also included in longitudinal analyses (baseline age: 13.8 ± 1.7y; follow-up age: 23.3 ± 3.4y; interval: 9.5 ± 4.3y; characteristics shown in Supplemental Table 1). Characteristics of Pima subjects grouped by MC4R functional status are shown in Table 3. LOF and GOF subjects were well matched with the control groups for age, sex, BMI, and length of serum sample storage time (all P’s > 0.3, Table 3). Higher BMI-Z was observed for subjects with LOF variants compared to those with GOF variants (P = 0.004, Table 3), even after accounting for the higher individual mean percentage Indian heritage among LOF compared to GOF subjects (Figure 1A). Using percent Indian versus percent Pima heritage as covariates yielded similar results for all analyses.
Table 3.
Subject Characteristics in the Pima Cohort
| LOF (n=43) | LOF-C (n=65) | LOF vs. LOF-C P value | GOF (n=20) | GOF-C (n=20) | GOF vs. GOF-C P value | LOF vs.GOF P value | |
|---|---|---|---|---|---|---|---|
| Age (y)a | 15.6 ± 7.1 | 15.2 ± 5.7 | 0.73 | 16.5 ± 7.1 | 16.7 ± 7.3 | 0.92 | 0.65 |
| Sex (% female)b | 46.5 | 46.2 | 0.97 | 60.0 | 60.0 | 1.00 | 0.42 |
| Indian heritage (%)a,c | 95 ± 13 | 92 ± 18 | 0.31 | 66 ± 20 | 83 ± 23 | 0.02 | <0.001 |
| BMI (kg/m2)a | 31.4 ± 7.1 | 30.7 ± 6.6 | 0.61 | 26.1 ± 7.8 | 26.2 ± 7.9 | 0.96 | 0.009 |
| BMI-Za | 1.91 ± 0.83 | 1.85 ± 0.89 | 0.69 | 0.97 ± 1.16 | 0.96 ± 1.19 | 0.97 | 0.003 |
| Sample storage (y)a | 25.3 ± 8.9 | 24.1 ± 7.9 | 0.48 | 16.2 ± 9.0 | 16.4 ± 8.8 | 0.95 | <0.001 |
| Serum leptin (ng/mL)d | 22.7 [6.4–39.8] | 16.4 [7.7–32.5] | 0.51 | 10.3 [1.2–20.6] | 10.8 [3.6–28.6] | 0.48 | 0.02 |
| Serum BDNF (ng/mL)d | 23.3 [19.1–28.1] | 21.9 [17.9–25.0] | 0.12 | 25.0 [19.1–26.0] | 19.0 [15.3–23.2] | 0.06 | 0.99 |
Abbreviations: BMI, body mass index; BMI-Z, standard deviation score for BMI by age and sex,32 BDNF, brain-derived neurotrophic factor; LOF, subjects with loss-of-function MC4R variant; GOF, subjects with gain-of-function MC4R; LOF-C and GOF-C, control subjects with common MC4R matched with LOF and GOF subjects, respectively, by age, sex, BMI, and storage time of serum sample; SD, standard deviation.
Mean ± SD shown. Independent samples t tests compared groups.
Fisher’s exact tests compared percentages between groups.
Percent Indian heritage calculated based on self-reported ethnicities of great-grandparents.
Median [interquartile range: 25th to 75th percentile] shown because non-normal distribution. Nonparametric U tests compared groups.
Figure 1.
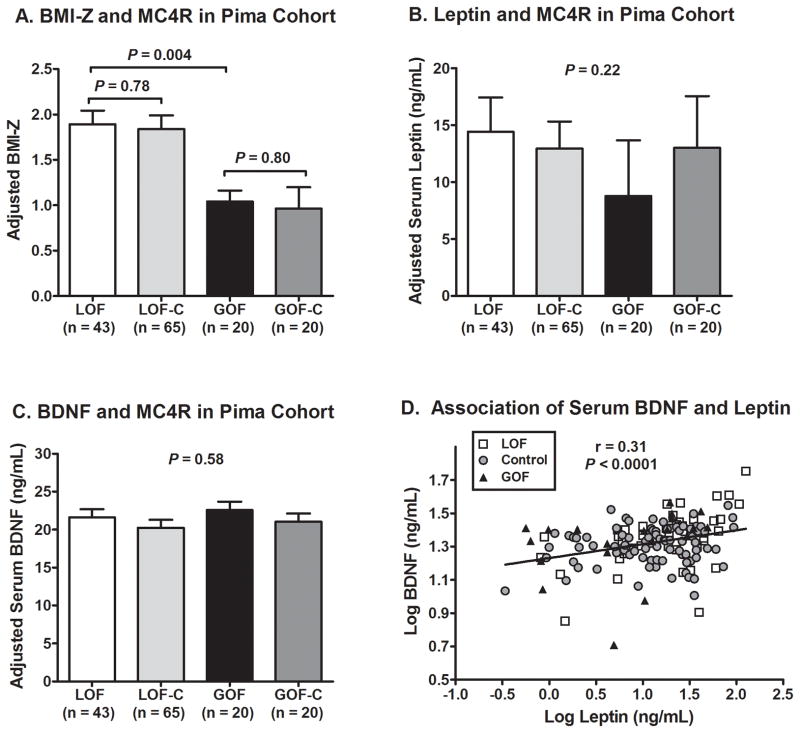
Comparison of subjects in the Pima cohort by MC4R functional status. (A) BMI-Z (adjusted for age, sex, and percent Indian heritage) was similar for LOF vs. LOF-C and for GOF vs. GOF-C. The LOF and LOF-C groups had higher adjusted BMI-Z than the GOF and GOF-C groups. Adjusted means ± SEMs and P values from post-hoc least significant difference (LSD) pairwise comparisons are shown. (B) Serum leptin concentration (adjusted for age, sex, BMI-Z, percent Indian heritage, and sample storage time) was not significantly different between groups. (C) Serum BDNF concentration (adjusted for age, sex, BMI-Z, percent Indian heritage, and sample storage time) was not significantly different between groups. (D) Serum BDNF and leptin concentrations were positively correlated, but there was no significant difference in this relationship by MC4R functional status (P = 0.08). For (B)–(D), log values were used for calculations. For (B)–(C), back-transformed adjusted means ± 95% CIs and overall P value from ANCOVAs are shown.
Effect of Sample Storage Time
Because the collection of samples in the Pima cohort spanned several decades, we examined the effect of storage time. Serum leptin concentration was inversely correlated with storage time within the GOF (rho = -0.70, P = 0.001) and GOF-C (rho = -0.64, P = 0.002) groups, but not within the LOF (P = 0.79) and LOF-C (P = 0.25) groups, suggesting instability of leptin during long-term storage at lower concentrations since the GOF and GOF-C groups had lower leptin than the LOF and LOF-C groups attributable to their lower BMIs (Table 3). Serum leptin remained significantly associated with storage time after adjusting for age, sex, BMI-Z, percent Indian heritage, and MC4R functional status (r = -0.17, P = 0.048). Serum BDNF concentrations were not significantly associated with storage time (P = 0.24).
Leptin and BDNF Associations
Leptin, which is expected to be proportional to adiposity, was positively associated with BMI-Z (r2 = 0.51, P < 0.001, Supplemental Figure 5A), but there was no significant difference by MC4R functional status for unadjusted values (P = 0.52) as well as after adjustment for age, sex, BMI-Z, percent Indian heritage, and sample storage time (P = 0.22, Figure 1B). Serum BDNF concentration was significantly but weakly associated with BMI-Z (r2 = 0.07, P = 0.001, Supplemental Figure 5B). There was no difference by MC4R functional status for unadjusted BDNF values (P = 0.54) as well as after adjustment for age, sex, BMI-Z, percent Indian heritage, and sample storage time (P = 0.58, Figure 1C). Of note, the 3 subjects who were homozygous LOF MC4R variants had serum BDNF concentrations that were all above the median value for LOF-C, GOF-C, GOF, and the entire cohort combined. Serum BDNF and leptin concentrations were positively correlated in unadjusted analysis (r = 0.31, P <0.0001, Figure 1D) and after adjustment for age, sex, BMI-Z, percent Indian heritage, and sample storage time (r = 0.24, P = 0.003), but there was no significant difference in this relationship by MC4R functional status (p=0.08). Sex did not significantly interact with MC4R functional status to predict BDNF concentrations (p=0.07). BDNF concentrations were higher in childhood compared to adulthood (median [25–75th percentile]: 22.8 [18.5–26.2] vs.18.7 [14.9–24.1] ng/mL, p=0.002), including after adjustment for change in BMI-Z (p<0.001), but this difference was not significantly associated with MC4R status (p=0.09).
BDNF Genotypes
Distribution of SNPs and linkage data are shown in Supplemental Table 1. Serum BDNF concentrations were significantly associated with BDNF rs12291186 (Supplemental Figure 6A P = 0.006) and rs6265 (Supplemental Figure 6B P = 0.009), but not rs7124442 (P = 0.99) genotype. When both rs12291196 and rs6265 were included in the model, each SNP genotype remained independently associated with BDNF (P = 0.01 and P = 0.04, respectively). None of the BDNF SNPs, however, were associated with MC4R functional status, and none interacted with MC4R in the linear models predicting serum BDNF concentrations (all P’s > 0.3).
Hispanic Cohort
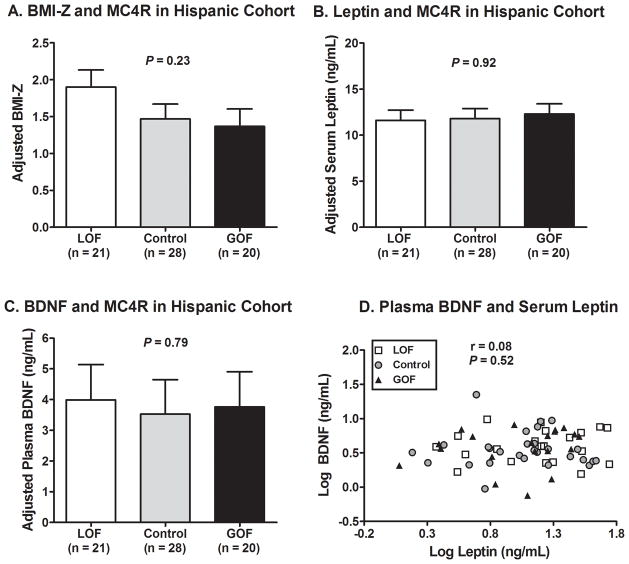
A total of 69 subjects of Hispanic heritage [age (mean ± SD) 10.8 ± 3.6y, 45% female, BMI-Z 1.57 ± 1.07) were included in this study. Subject characteristics grouped by MC4R functional status are shown in Table 4. We observed no differences between LOF, GOF, and controls for age (P = 0.77), sex (P = 0.95), BMI-Z (P = 0.25), serum leptin concentration (P = 0.50), or plasma BDNF concentration (P = 0.45). As shown in Figure 2, after adjustment for age, sex, and BMI-Z, no differences were observed for serum leptin (P = 0.92) or plasma BDNF (P = 0.79). Plasma BDNF and serum leptin concentrations were not correlated (r = 0.08, P = 0.52, Figure 2D) and were not associated with MC4R functional status (P = 0.65). Sex did not significantly interact with MC4R functional status to predict BDNF concentrations (p=0.60). Distribution of SNPs and linkage data are shown in Supplemental Table 2. BDNF SNPs rs12291186, rs6265, and rs7124442 were not associated with plasma BDNF (P’s > 0.3) and there were no significant interactions between BDNF SNPs with MC4R functional status in predicting plasma BDNF (all P’s > 0.23). Removal of the 3 subjects with MC4R variants that were non-function-altering from the control cohort (leaving only subjects homozygous for the common genomic sequence for MC4R) did not significantly alter any findings. Examination of each variant separately did not reveal any significant differences in BDNF compared to the common sequence. Use of percent body fat or fat mass and fat free mass instead of BMI-Z as covariates did not significantly alter any findings.
Table 4.
Subject Characteristics in the Hispanic Cohort
| LOF (n=21) | Control (n=28) | GOF (n=20) | P value | |
|---|---|---|---|---|
| Age (y)a | 10.5 ± 3.5 | 11.2 ± 3.9 | 10.5 ± 3.3 | 0.77 |
| Sex (% female)b | 47.6 | 42.9 | 45.0 | 0.95 |
| BMIa | 27.4 ± 10.4 | 24.4 ± 6.0 | 24.1 ± 6.9 | 0.31 |
| BMI-Za | 1.89 ± 1.11 | 1.48 ± 1.04 | 1.36 ± 1.04 | 0.25 |
| Total Body Fat (%)a | 34.9 ± 9.1 | 32.3 ± 7.9 | 33.1 ± 9.3 | 0.61 |
| Serum leptin (ng/mL)c | 16.8 [6.5–33.1] | 13.2 [6.1–19.1] | 13.8 [5.1–20.6] | 0.50 |
| Plasma BDNF (ng/mL)c | 4.0 [2.3–6.4] | 3.3 [2.4–4.2] | 4.4 [2.9–6.4] | 0.45 |
Abbreviations: BMI, body mass index; BMI-Z, standard deviation score for BMI by age and sex,32 BDNF, brain-derived neurotrophic factor; LOF, subjects with loss-of-function MC4R variant; GOF, subjects with gain-of-function MC4R; SD, standard deviation; ANOVA, analysis of variance.
Mean ± SD shown. ANOVAs compared groups.
Chi-square test compared percentages among groups.
Median [interquartile range: 25th to 75th percentile] shown because non-normal distribution. Nonparametric Kruskal-Wallis tests compared groups.
Figure 2.
Comparison of subjects in the Hispanic cohort by MC4R functional status. (A) BMI-Z (adjusted for age and sex) was not significantly different between LOF, controls, and GOF groups. (B) Serum leptin concentration (adjusted for age, sex, and BMI-Z) was not significantly different between groups. (C) Plasma BDNF concentration (adjusted for age, sex, and BMI-Z) was not significantly different between groups. (D) Plasma BDNF and serum leptin concentrations were not correlated within the Hispanic cohort, and there was no significant difference in this relationship by MC4R functional status (P = 0.65). For (B)–(D), log values were used for calculations. For (B)–(C), back-transformed adjusted means ± 95% CIs and overall P value from ANCOVAs are shown.
Discussion
Contrary to our primary hypothesis, we did not observe any significant differences in circulating BDNF concentrations in subjects with function-altering MC4R variants studied from two separate cohorts. The association of leptin with BMI-Z was similar among MC4R genotypes, suggesting that variant MC4R functioning does not alter leptin production or sensitivity independent of adiposity. Serum and plasma BDNF concentrations were not associated with MC4R functional status, suggesting that peripheral BDNF may not directly reflect hypothalamic BDNF expression or that MC4R signaling is not a significant regulator of the bulk of BDNF expression in humans. In fact, even within the hypothalamus, the subpopulation of BDNF-expressing neurons that are under the regulation of MC4R may be very few and region specific. Xu et al. reported that the caudal portion of the ventral medial hypothalamus is the region with the most severe reduction of BDNF expression in Ay mice that overexpress Agouti, an MC4R antagonist.1 Other regions of the hypothalamus, including the paraventricular, lateral and dorsomedial nuclei, as well as other regions of the brain, including the hippocampus and cerebral cortex, are not affected in the Ay mouse. Therefore, overall output of central BDNF may not be significantly affected by function-altering MC4R variants, which may exert their effects on energy balance via a small subpopulation of hypothalamic neurons through direct neuronal projections or local paracrine effects. In contrast, conditions that result in global decreases in BDNF expression due to BDNF haploinsufficiency are associated with lower peripheral BDNF concentrations as well as with hyperphagia and obesity.8, 9
We observed a positive correlation between serum BDNF and serum leptin, significant even after adjustment for BMI-Z; we also observed lack of interaction between MC4R genotype and this association. Together, these findings suggest that there may be associations between leptin and BDNF that are body mass- and MC4R- independent. In our longitudinal analyses, we observed higher serum BDNF concentrations in adolescents compared to young adults, consistent with age-related differences in BDNF,35, 36 but these differences were not associated with MC4R functional status.
Several differences were observed when comparing findings in the Pima and Hispanic cohorts. In the Pima cohort, GOF subjects had significantly lower BMI-Z compared to LOF. However, in the Hispanic cohort, although GOF had mean BMI-Z that was ~0.5 SD units lower than LOF (Table 4), this difference was not significant. Our sample sizes were powered based on the primary outcome measure, BDNF, rather than for BMI-Z, which would have required 60 subjects in each of the 3 MC4R groups within the Hispanic cohort to have 80% power to detect a significant difference; thus, the available sample size of individuals with GOF and LOF MC4R variants is a limitation of the study. The observation of relatively high BMI values among subjects with GOF MC4R variants (mean of 83rd percentile and 91st percentile in the Pima and Hispanic cohorts, respectively) is attributable to the very high prevalence of obesity in individuals of Pima descent (such that children with somewhat protective alleles have a high BMI compared to United States norms but below average BMI within their own population)16 and the selection criteria for the Hispanic cohort, which enrolled probands and their family members based on probands having BMI ≥ 95th percentile and fat mass ≥ 85th percentile.22
In the Pima group, we observed a positive correlation between serum BDNF and leptin, as well as associations of BDNF rs12291186 and rs6265 with serum BDNF concentration. Rs12291186 is located within an intron of BDNF, and the sequence change alters a putative binding site for the YY1 transcription factor (TRANSFAC 7.0 Public 2005). Rs6265 causes a Val66Met substitution in the amino-terminal portion of the BDNF prohormone, which is thought to impair intracellular trafficking of BDNF, leading to decreased activity-dependent secretion of BDNF.37 However, plasma concentration in the Hispanic cohort was associated with neither serum leptin nor any of the BDNF SNPs, which may be attributable to the 2-fold higher coefficient of variability in plasma BDNF concentration. Because BDNF in peripheral circulation is stored in and released from platelets,38 plasma BDNF concentrations are expected to be several fold lower than serum BDNF concentrations, and trace hemolysis can result in falsely elevated BDNF concentrations in plasma. Conversely, insufficient clotting of blood prior to separation of serum can lead to falsely diminished BDNF concentrations. A limitation of this study is the lack of platelet count values which are often included as a covariate for analyses.39 Another limitation is the lack of body composition data for the Pima cohort. A further limitation of our study is the lack of samples from individuals during infancy and toddlerhood, ages at which genetic influences on energy homeostasis may be especially pronounced; however, comparison of longitudinal data during adolescence and adulthood in a subset of our Pima subjects showed no interaction between MC4R functional status and age in contributing to differences in BDNF.
We conclude that even though peripheral BDNF concentrations are, on average, lower in patients with disorders that cause global central nervous system BDNF insufficiency,8,9 circulating BDNF may not be an adequate proxy measure for specific changes in MC4R-regulated hypothalamic BDNF secretion. Post-mortem cadaveric studies of human hypothalamic tissue from individuals with MC4R functional variants would be needed to address these questions more fully.
Supplementary Material
Acknowledgments
Funding for this study was provided by the International Hyperphagia Conference Best Idea Grant from the Prader-Willi Syndrome Association (USA) (J.C.H., J.A.Y.), by the NIH Intramural Research Programs of NICHD and NIDDK, American Diabetes Association grant 1-12-BS212 (Y-X T), NIH (R01 DK59264 (N.F.B.), R01 DK080457 (N.F.B.), C06 RR013556, and C06 RR017515) and USDA/ARS under Cooperative Agreement 58-6250-51000-037. J.K. and J.A.Y. are Commissioned Officers of the United States Public Health Service.
Footnotes
Disclosures: The authors have no conflicts of interest to disclose.
Clinical Trials Registry: Subjects in the Pima cohort were enrolled in observational, noninterventional studies, registered as NCT00339482. Subjects in the Hispanic cohort were enrolled in observational, noninterventional studies.
Supplementary information is available at the International Journal of Obesity’s website
References
- 1.Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, Jones KR, et al. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci. 2003;6(7):736–42. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicholson JR, Peter JC, Lecourt AC, Barde YA, Hofbauer KG. Melanocortin-4 receptor activation stimulates hypothalamic brain-derived neurotrophic factor release to regulate food intake, body temperature and cardiovascular function. J Neuroendocrinol. 2007;19(12):974–82. doi: 10.1111/j.1365-2826.2007.01610.x. [DOI] [PubMed] [Google Scholar]
- 3.Bariohay B, Roux J, Tardivel C, Trouslard J, Jean A, Lebrun B. Brain-derived neurotrophic factor/tropomyosin-related kinase receptor type B signaling is a downstream effector of the brainstem melanocortin system in food intake control. Endocrinology. 2009;150(6):2646–53. doi: 10.1210/en.2008-1184. [DOI] [PubMed] [Google Scholar]
- 4.Caruso C, Carniglia L, Durand D, Gonzalez PV, Scimonelli TN, Lasaga M. Melanocortin 4 receptor activation induces brain-derived neurotrophic factor expression in rat astrocytes through cyclic AMP-protein kinase A pathway. Mol Cell Endocrinol. 2013;348(1):47–54. doi: 10.1016/j.mce.2011.07.036. [DOI] [PubMed] [Google Scholar]
- 5.Kernie SG, Liebl DJ, Parada LF. BDNF regulates eating behavior and locomotor activity in mice. Embo J. 2000;19(6):1290–300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyons WE, Mamounas LA, Ricaurte GA, Coppola V, Reid SW, Bora SH, et al. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci U S A. 1999;96(26):15239–44. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Unger TJ, Calderon GA, Bradley LC, Sena-Esteves M, Rios M. Selective deletion of Bdnf in the ventromedial and dorsomedial hypothalamus of adult mice results in hyperphagic behavior and obesity. J Neurosci. 2007;27(52):14265–74. doi: 10.1523/JNEUROSCI.3308-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han JC, Liu QR, Jones M, Levinn RL, Menzie CM, Jefferson-George KS, et al. Brain-derived neurotrophic factor and obesity in the WAGR syndrome. N Engl J Med. 2008;359(9):918–27. doi: 10.1056/NEJMoa0801119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gray J, Yeo GS, Cox JJ, Morton J, Adlam AL, Keogh JM, et al. Hyperphagia, severe obesity, impaired cognitive function, and hyperactivity associated with functional loss of one copy of the brain-derived neurotrophic factor (BDNF) gene. Diabetes. 2006;55(12):3366–71. doi: 10.2337/db06-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han JC, Muehlbauer MJ, Cui HN, Newgard CB, Haqq AM. Lower brain-derived neurotrophic factor in patients with prader-willi syndrome compared to obese and lean control subjects. J Clin Endocrinol Metab. 2010;95(7):3532–6. doi: 10.1210/jc.2010-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu H, Kishi T, Roseberry AG, Cai X, Lee CE, Montez JM, et al. Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. J Neurosci. 2003;23(18):7143–54. doi: 10.1523/JNEUROSCI.23-18-07143.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stranahan AM, Arumugam TV, Mattson MP. Lowering corticosterone levels reinstates hippocampal brain-derived neurotropic factor and Trkb expression without influencing deficits in hypothalamic brain-derived neurotropic factor expression in leptin receptor-deficient mice. Neuroendocrinology. 2011;93(1):58–64. doi: 10.1159/000322808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakagawa T, Ono-Kishino M, Sugaru E, Yamanaka M, Taiji M, Noguchi H. Brain-derived neurotrophic factor (BDNF) regulates glucose and energy metabolism in diabetic mice. Diabetes Metab Res Rev. 2002;18(3):185–91. doi: 10.1002/dmrr.290. [DOI] [PubMed] [Google Scholar]
- 14.Bagnol D, Lu XY, Kaelin CB, Day HE, Ollmann M, Gantz I, et al. Anatomy of an endogenous antagonist: relationship between Agouti-related protein and proopiomelanocortin in brain. J Neurosci. 1999;9(18):RC26. doi: 10.1523/JNEUROSCI.19-18-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farooqi IS, Yeo GS, Keogh JM, Aminian S, Jebb SA, Butler G, et al. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J Clin Invest. 2000;106(2):271–9. doi: 10.1172/JCI9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thearle MS, Muller YL, Hanson RL, Mullins M, Abdussamad M, Tran J, et al. Greater impact of melanocortin-4 receptor deficiency on rates of growth and risk of type 2 diabetes during childhood compared with adulthood in Pima Indians. Diabetes. 2012;61(1):250–7. doi: 10.2337/db11-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiang Z, Litherland SA, Sorensen NB, Proneth B, Wood MS, Shaw AM, et al. Pharmacological characterization of 40 human melanocortin-4 receptor polymorphisms with the endogenous proopiomelanocortin-derived agonists and the agouti-related protein (AGRP) antagonist. Biochemistry. 2006;45(23):7277–88. doi: 10.1021/bi0600300. [DOI] [PubMed] [Google Scholar]
- 18.Stutzmann F, Vatin V, Cauchi S, Morandi A, Jouret B, Landt O, et al. Non-synonymous polymorphisms in melanocortin-4 receptor protect against obesity: the two facets of a Janus obesity gene. Hum Mol Genet. 2007;16(15):1837–44. doi: 10.1093/hmg/ddm132. [DOI] [PubMed] [Google Scholar]
- 19.Krabbe KS, Nielsen AR, Krogh-Madsen R, Plomgaard P, Rasmussen P, Erikstrup C, et al. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia. 2007;50(2):431–8. doi: 10.1007/s00125-006-0537-4. [DOI] [PubMed] [Google Scholar]
- 20.Thorleifsson G, Walters GB, Gudbjartsson DF, Steinthorsdottir V, Sulem P, Helgadottir A, et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet. 2009;41(1):18–24. doi: 10.1038/ng.274. [DOI] [PubMed] [Google Scholar]
- 21.Knowler WC, Pettitt DJ, Saad MF, Charles MA, Nelson RG, Howard BV, et al. Obesity in the Pima Indians: its magnitude and relationship with diabetes. Am J Clin Nutr. 1991;53(6 Suppl):1543S–1551S. doi: 10.1093/ajcn/53.6.1543S. [DOI] [PubMed] [Google Scholar]
- 22.Butte NF, Cai G, Cole SA, Comuzzie AG. Viva la Familia Study: genetic and environmental contributions to childhood obesity and its comorbidities in the Hispanic population. Am J Clin Nutr. 2006;84(3):646–54. doi: 10.1093/ajcn/84.3.646. [DOI] [PubMed] [Google Scholar]
- 23.Cole SA, Butte NF, Voruganti VS, Cai G, Haack K, Kent JW, Jr, et al. Evidence that multiple genetic variants of MC4R play a functional role in the regulation of energy expenditure and appetite in Hispanic children. Am J Clin Nutr. 2010;91(1):191–9. doi: 10.3945/ajcn.2009.28514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tao YX. The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocr Rev. 2010;31(4):506–43. doi: 10.1210/er.2009-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cai G, Cole SA, Butte N, Bacino C, Diego V, Tan K, et al. A quantitative trait locus on chromosome 18q for physical activity and dietary intake in Hispanic children. Obesity (Silver Spring) 2006;14(9):1596–604. doi: 10.1038/oby.2006.184. [DOI] [PubMed] [Google Scholar]
- 26.Tan K, Pogozheva ID, Yeo GS, Hadaschik D, Keogh JM, Haskell-Leuvano C, et al. Functional characterization and structural modeling of obesity associated mutations in the melanocortin 4 receptor. Endocrinology. 2009;150(1):114–25. doi: 10.1210/en.2008-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tao YX, Segaloff DL. Functional analyses of melanocortin-4 receptor mutations identified from patients with binge eating disorder and nonobese or obese subjects. J Clin Endocrinol Metab. 2005;90(10):5632–8. doi: 10.1210/jc.2005-0519. [DOI] [PubMed] [Google Scholar]
- 28.Xiang Z, Proneth B, Dirain ML, Litherland SA, Haskell-Luevano C. Pharmacological characterization of 30 human melanocortin-4 receptor polymorphisms with the endogenous proopiomelanocortin-derived agonists, synthetic agonists, and the endogenous agouti-related protein antagonist. Biochemistry. 2010;49(22):4583–600. doi: 10.1021/bi100068u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sawyer TK, Sanfilippo PJ, Hruby VJ, Engel MH, Heward CB, Burnett JB, et al. 4-Norleucine, 7-D-phenylalanine-alpha-melanocyte-stimulating hormone: a highly potent alpha-melanotropin with ultralong biological activity. Proc Natl Acad Sci U S A. 1980;77(10):5754–8. doi: 10.1073/pnas.77.10.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tao YX, Segaloff DL. Functional characterization of melanocortin-4 receptor mutations associated with childhood obesity. Endocrinology. 2003;144(10):4544–51. doi: 10.1210/en.2003-0524. [DOI] [PubMed] [Google Scholar]
- 31.Comuzzie AG, Cole SA, Laston SL, Voruganti VS, Haack K, Gibbs RA, et al. Novel genetic loci identified for the pathophysiology of childhood obesity in the Hispanic population. PLoS One. 2012;7(12):e51954. doi: 10.1371/journal.pone.0051954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002;11(246):1–190. 2000. [PubMed] [Google Scholar]
- 33.Cole TJ. The LMS method for constructing normalized growth standards. Eur J Clin Nutr. 1990;44 (1):45–60. [PubMed] [Google Scholar]
- 34.Gaunt TR, Rodriguez S, Day IN. Cubic exact solutions for the estimation of pairwise haplotype frequencies: implications for linkage disequilibrium analyses and a web tool ‘CubeX’. BMC Bioinformatics. 2007;8:428. doi: 10.1186/1471-2105-8-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katoh-Semba R, Wakako R, Komori T, Shigemi H, Miyazaki N, Ito H, et al. Age-related changes in BDNF protein levels in human serum: differences between autism cases and normal controls. Int J Dev Neurosci. 2007;25(6):367–72. doi: 10.1016/j.ijdevneu.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Webster MJ, Herman MM, Kleinman JE, Shannon Weickert C. BDNF and trkB mRNA expression in the hippocampus and temporal cortex during the human lifespan. Gene Expr Patterns. 2006;6(8):941–51. doi: 10.1016/j.modgep.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 37.Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–69. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 38.Fujimura H, Altar CA, Chen R, Nakamura T, Nakahashi T, Kambayashi J, et al. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb Haemost. 2002;87(4):728–34. [PubMed] [Google Scholar]
- 39.El-Gharbawy AH, Adler-Wailes DC, Mirch MC, Theim KR, Ranzenhofer L, Tanofsky-Kraff M, et al. Serum brain-derived neurotrophic factor concentrations in lean and overweight children and adolescents. J Clin Endocrinol Metab. 2006;91(9):3548–52. doi: 10.1210/jc.2006-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.