Abstract
This study aimed to evaluate the influence of the methanol extracts of mushrooms Lentinus edodes and Agaricus blazei on the retention of tocopherols in soybean oil, when subjected to an accelerated storage test. The following treatments were subjected to an accelerated storage test in an oven at 60 °C for 15 days: Control (soybean oil without antioxidants), TBHQ (soybean oil + 100 mg/kg of TBHQ), BHT (soybean oil + 100 mg/kg of BHT), L. edodes (soybean oil + 3,500 mg/kg of L. edodes extract) and A. blazei (soybean oil + 3,500 mg/kg of A. blazei extract). The samples were analyzed for tocopherols naturally present in soybean oil and mass gain. The results showed, the time required to reach a 0.5% increase in mass was 13 days for TBHQ and 15 days for A. blazei. The content of tocopherols for TBHQ was 457.50 mg/kg and the A. blazei, 477.20 mg/kg.
Keywords: Mushrooms, Antioxidants, Mass gain, Tocopherols
Introduction
The lipid oxidation is the main cause of quality loss of fatty foods during processing and storage. This degradation can cause changes of taste, odor and color, destroying fat soluble vitamins and polyunsaturated fatty acids. The food industry tries to minimize such damage with the use of synthetic antioxidants, namely BHA, BHT and TBHQ. However, the use of additives is strictly controlled because of the human health damage that it can cause (González-Montelongo et al. 2010).
In Canada and the European Community the use of TBHQ is not allowed (Reische et al. 2002). The Codex Alimentarium limited to 200 mg/kg the use of BHT in vegetable oils (FAO 2010). The concern regarding food safety among consumers leads the food industry to seek safer alternatives that can replace synthetic antioxidants. Many researchers have been devoted to the search for natural compounds from by-products such as grape marc (Lafka et al. 2007; Pinelo et al. 2005), orange peel (Xu et al. 2008), pomegranate (Li et al. 2006; Devatkal et al. 2011) and star fruit (Shui and Leong 2006).
Mushrooms have also been the target of research because they have significant amounts of phenolic compounds and some varieties, like L. edodes and A. blazei, provide the strengthening of the immune system when consumed frequently (Choi et al. 2006; Huang and Mau 2006). The in vivo action of mushrooms was also evaluated in the treatment of diabetes, hypertension and hepatitis (Aida et al. 2009). However, few studies focus on the antioxidant activity of mushroom extracts against lipid oxidation.
Tocopherols are monophenol compounds existing in plants, mainly in oil seeds and leaves, which have an antioxidant activity and vitamin E. The nomenclature of these compounds gets the prefix of α, β, γ e δ, depending on the number and position of the methyl group attached to aromatic ring. The α-tocopherol is the compound that has greater activity of vitamin E (Mau et al. 2004; Yang et al. 2002).
Thus, the aim of this work was to evaluate the influence of the methanol extracts of L. edodes and A. blazei on the retention of tocopherols in soybean oil when subjected to an accelerated storage test.
Materials and methods
Mushrooms and soybean oil
Approximately 4.0 kg of raw mushroom (Lentinus edodes), produced and marketed in the region of Salto, in the state of São Paulo, on April/2009, was used. It was packaged and cooled and sent directly to the Laboratory of Oils and Fats, at the Department of Engineering and Food Technology, at UNESP. The Agaricus blazei was acquired in the region of São José do Rio Preto, in the State of São Paulo, during the months of April, May and September/2009, totaling about 3.0 kg. Immediately after harvest, raw A. blazei was washed and sent to the Laboratory of Oils and Fats, Department of Engineering and Food Technology, at UNESP.
The samples were frozen at −30 °C for 24 h and lyophilized in lyophilizer brand Liotop model L101 and crushed in a grinding mill, brand Erbele, model 2508.001-2. The powder obtained was stored in dark plastic containers, sealed with screw caps and labeled for later analysis. The samples of A. blazei were homogenized in order to minimize possible variations between batches.
For analysis of oxidative stability, the refined soybean oil was used, without the addition of synthetic antioxidants (TBHQ and citric acid), Cargil Agrícola S/A brand, purchased in packs of 900 mL in the local market.
Extracts and antioxidants
The methanol extracts of mushrooms were obtained by slow extraction (120 rpm, 3 h on shaker). The extract was obtained stirring 8 g of mushroom with 80 mL of methanol, in the dark and at room temperature. Then, the mixture was centrifuged at 3,000 rpm for 5 min and the supernatant was filtered with vacuum pump and subjected to rotary evaporator under reduced pressure at 40 °C. The dry extract was weighed and resuspended in methanol, yielding a stock solution containing 1.0 g of extract and 10 mL of methanol, used for direct application in soybean oil.
The synthetic antioxidants terc butyl hydroquinone (TBHQ) (100 mg/kg) and butylated hydroxytoluene (BHT) (100 mg/kg) were used in their commercial form, supplied by Danisco A/S.
Experimental testing
The following treatments were subjected to an accelerated storage test in an oven at 60 °C for 15 days: Control (soybean oil without antioxidants), TBHQ (soybean oil + 100 mg/kg of TBHQ), BHT (soybean oil + 100 mg/kg of BHT), L. edodes (soybean oil + 3,500 mg/kg of L. edodes extract) and A. blazei (soybean oil + 3,500 mg/kg of A. blazei extract), with 50 mL beakers containing 30 mL of sample with surface/volume ratio 0.4 cm-1. All samples, at different time intervals (0, 3, 6, 9, 12 and 15 days), were collected and flushed with nitrogen gas and stored at approximately −18 °C until analysis of tocopherols naturally present in soybean oil added to extracts of mushrooms. Simultaneously the test was conducted to mass gain, in which glass Petri dishes containing the oil samples were taken daily from the oven for weighing. For application of the mushroom extract in soybean oil, several extract concentrations were tested (0 to 3,500 mg/kg). By linear regression, it was observed that the highest antioxidant activity, measured by the oxidative stability, agreed with the highest concentration of shiitake and almond mushroom extracts, which is of 3.500 mg/kg.
Mass gain
For mass gain analysis, 2.0 g of each sample were placed in glass Petri dishes, which were kept in an oven at 60 °C. The rate of oxidation, in terms of mass increase, was recorded at 24 h intervals up to 16 days. The time required for a 0.5% mass increase for oil was taken as the index of stability and the mass gain was calculated as percentage of the original weight (Iqbal and Bhanger 2007). The weighing machine used was Ohaus, model AS200 (number of digit 0,0001 g and precision 0,0025 g).
Tocopherols
For the chromatographic analysis of tocopherols, performed using the method AOCS Ce 8–89 (1997), the high performance liquid chromatography was used, consisting of a Varian pro Star 210, with a fluorescence detector. Under the conditions of analysis, it was used silica column of 250 × 4.6 mm with pores of 5 μm, flow 1.2 mL/min, wavelength of excitation at 290 nm and emission at 330 nm and as a mobile phase the mixture of 99.5% of n-hexane and 0.5% of isopropanol, all with purity for HPLC. The identification of tocopherols was made by comparison with the retention time of standards Sigma 95% purity. These were quantified by external standard and the levels of tocopherols expressed as mg/kg.
Statistical analysis
The results obtained for tocopherols and mass gain, in duplicate, were submitted to analysis of variance to determine the influence of the factors (treatments and storage period) on the modification of oils subjected to the accelerated storage test. The 5 × 6 factorial experiment was performed in a completely randomized delineation (Gacula and Singh 1984). Both the analysis of variance and the Tukey test for the 5% average were obtained using the ESTAT (System for Statistical analysis, version 2.0).
Results and discussion
Mass gain
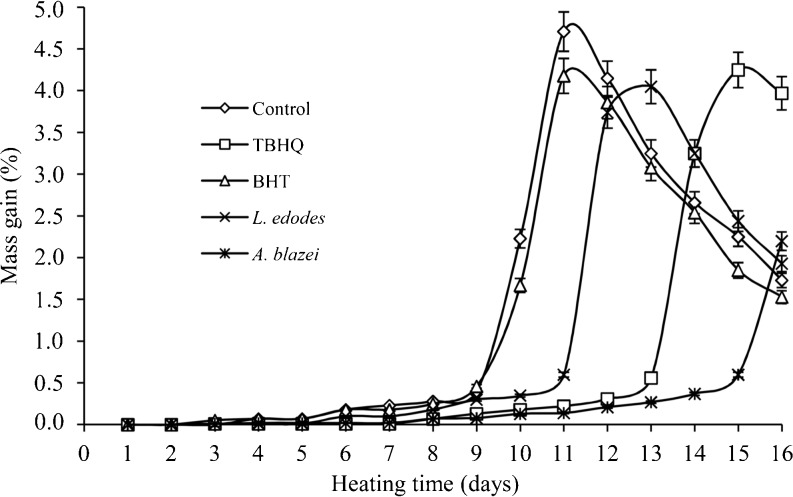
Mass gain is usually employed to estimate the amount of oxygen added to the unsaturated content of lipid molecules and formation of hydroperoxides during oxidation. The mass gain was measured for all the samples at 24 h intervals for 16 days and results, in percentage, are shown in Fig. 1.
Fig. 1.
Mass gain of treatments subjected to accelerated storage. Data are mean ± SD (n = 2)
Figure 1 shows that the time required to reach a 0.5% increase in mass were 10, 13, 10, 11 and 15 days for the Control, TBHQ, BHT, L. edodes and A. blazei, respectively. According to Evans et al. (1973), each day of storage in an oven at 60 °C is equivalent to 1 month storage at room temperature. Initially, the mass gain was not significant, but a sudden increase was observed in all samples followed by a decline for Control, TBHQ, BHT and L. edodes along the storage.
The decline of mass gain is due to the fact that peroxides, primary products of oxidation formed during accelerated storage, can be degraded into low molecular weight compounds including volatile substances. The Control remained stable until the 9th day, followed by a sudden increase until the 11th day, assuming the maximum 4.71% increase in mass gain followed by a sharp decrease until the end of the storage period.
The treatment BHT had similar behavior to Control, assuming greater mass gain in 11 days of storage, with 4.18%. The L. edodes was efficient against oxidation during the first 11 days of storage, and the maximum amount reported was 4.05% after 13 days of storage. The TBHQ showed no significant increase until the 13th day since A. blazei maintained this protection until the 15th day, followed by a sharp increase, becoming more efficient in protecting the oil against oxidation compared to other treatments.
Iqbal and Bhanger (2007) investigated the mass gain of sunflower oil with the addition of garlic extract (250, 500 and 1,000 mg/kg) subjected to an accelerated storage test in an oven at 65 °C for 14 days. The BHT (200 mg/kg) was used as a comparison. The authors found that the garlic extract at 1,000 mg/kg was more effective in protecting the oil, followed by BHT. The sunflower oil without antioxidants, unlike soybean oil, showed mass gain in the first days of storage. It shows that the sunflower oil is more susceptible to oxidation because its unsaturation (89.0%) is higher than that in soybean oil (83.4%).
Tocopherols
The retention of tocopherols in soybean oil is one way to evaluate the efficiency of mushroom extracts added to oil during the accelerated storage and compare them with synthetic antioxidants.
It is observed that the levels of α-tocopherol remained constant in treatments TBHQ and A. blazei, while in the other treatments the levels of this isomer decreased with storage period. At the end of 15 days it was found that there was no significant difference either in the content of α-tocopherol for TBHQ and A. blazei treatments or for Control and BHT.
The only treatment that effectively protected the contents of β-tocopherol was the A. blazei. For the other treatments, there was a slight decrease in the quantity of the isomer, especially at the end of the process of storage, the TBHQ and A. blazei were statistically equal to 15 days, the same as the Control, BHT and L. edodes.
The levels of γ-tocopherol were maintained throughout the storage for all treatments, except the L. edodes that showed a decrease at the end of storage. The same trend was observed for the δ-tocopherol isomer.
For contents of total tocopherols, it was found that the protective effect of Control and BHT were reduced during storage, showing 351.95 and 378.10 mg/kg of total tocopherols, respectively, at the end of 15 days of storage.
L. edodes was effective in protecting the total tocopherols from soybean oil until the 9th day of storage, with 363.05 mg/kg of tocopherols on the 15th day, statistically equal to Control and BHT. For treatments TBHQ and A. blazei, the levels of total tocopherols did not reduce during the storage time featuring an average of 446.07 and 476.73 mg/kg of total tocopherols, respectively.
Overall, it was observed that the effect of treatments on the content of tocopherols and their counterparts was more significant compared to the effect of storage period. This is because the test was conducted in mild temperature, that is, 60 °C. Studies on thermoxidation of oil, simulating frying processes, show that the most severe temperatures are applied to the oil reaching 180 °C. In these cases, the loss of the protective effect of tocopherols by antioxidants can be seen more explicitly (Angelo and Jorge 2008; Ramalho and Jorge 2008).
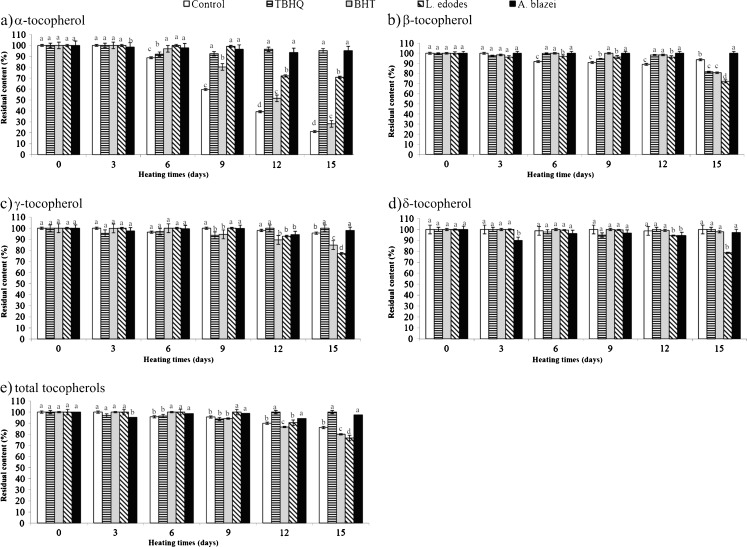
To better assess the protection of tocopherols naturally present in soybean oil, the residual content (%) of tocopherols during the storage period was calculated. These values are represented in Fig. 2. It was observed that the degradation of tocopherols was just significant, that is, below 50%, for treatments Control and BHT in α-tocopherol. With the exception of Control, all treatments protected the α-tocopherol of soybean oil up to the 6th day of storage. Starting on the 9th day it was observed a loss of α-tocopherol for BHT. At the end of the test, the antioxidant which was most effective in retaining α-tocopherol was TBHQ (95.18%), followed by A. blazei extract (95.17%), L. edodes extract (70.84%), BHT (28.18%) and Control (21.17%).
Fig. 2.
Residual content (%) of total tocopherols and their isomers in soybean oil with the addition of antioxidants. Data are mean ± SD (n = 2)
The L. edodes extract was the less effective treatment in the retention of β, γ and δ isomers in soybean oil at the end of storage. On average, it has 76.23% of residual tocopherols.
For total tocopherols, the most outstanding treatments were TBHQ and A. blazei, with 100 and 97.50% of residual content tocopherols, followed by Control (86.11%), BHT (80.02%) and L. edodes (76.64%).
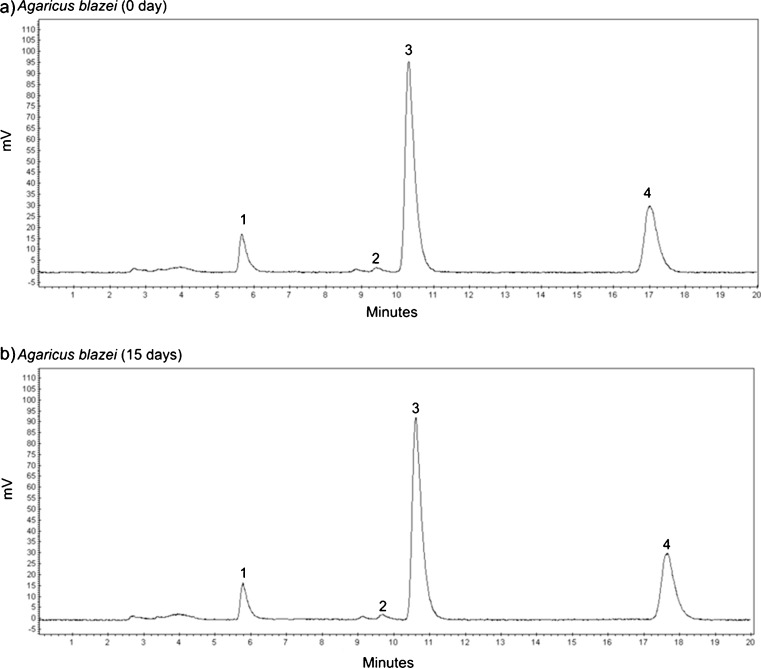
The Fig. 3 represents the chromatograms of A. blazei at the 0 day (a) and 15 days (b) of storage, respectively.
Fig. 3.
HPLC chromatograms of A. blazei at the 0 day a and 15 days b of storage, respectively. (1) α-tocopherol, (2) β-tocopherol, (3) γ-tocopherol, (4) δ-tocopherol
Ramalho and Jorge (2008) found a retention factor of 95% for soybean oil added to rosemary extract (1,000 mg/kg) subjected to 10 h of termoxidation and 70% for soybean oil naturally, showing good efficiency of the rosemary to protect the soybean oil. For the same period of thermoxidation, Angelo and Jorge (2008) determined 26.70% of retention factor of α-tocopherol for the sunflower oil, and at the end of 30 h, this figure dropped to 4.0%.
Conclusion
The extracts of A. blazei and the THBQ were the most effective treatments on the retention of α-tocopherol naturally present in soybean oil. In all treatments, the isomers β, γ and δ-tocopherol were more resistant to storage period when compared to α-tocopherol, with high residual levels after 15 days of storage. Regarding the retention of total tocopherols content, the TBHQ and A. blazei extract were the most efficient, followed by BHT, Control and L. edodes. Therefore, natural extracts of mushrooms can be applied to vegetable oils as a way to reduce the degradation caused by lipid oxidation.
Acknowledgments
The authors wish to thank Regina H. C. Garcia and Edalcy Ferrano (Estância Vilamar) who generously provide the A. blazei samples of and Denise Abackerli for the L. edodes.
References
- Aida FMNA, Shuhaimi M, Yazid M, Maaruf AG. Mushroom as a potential source of prebiotics: a review. Trends in Food Sci Tech. 2009;20:567–575. doi: 10.1016/j.tifs.2009.07.007. [DOI] [Google Scholar]
- Angelo PM, Jorge N. Antioxidant evaluation of extract and ascorbyl palmitate in sunflower oil under thermoxidation. J American Oil Chem Soc. 2008;85:1045–1049. doi: 10.1007/s11746-008-1296-9. [DOI] [Google Scholar]
- AOCS American Oil Chemists Society (1997) Official methods and recommended practices of the American Oil Chemists’ Society
- Gacula MC, Singh J (1984) Statistical methods in food and consumer research. Academic Press
- Choi Y, Lee SM, Chun J, Lee HB, Lee J. Influence of heat treatment on the antioxidant activities and polyphenolic compounds of shiitake (Lentinus edodes) mushroom. Food Chem. 2006;99:381–387. doi: 10.1016/j.foodchem.2005.08.004. [DOI] [Google Scholar]
- Devatkal SK, Narsaiah K, Borah A. The effect of salt, extract of kinnow and pomegranate fruit by-products on colours and oxidative stability of raw chicken patties during refrigerated storage. J Food Sci Technol. 2011;48:472–477. doi: 10.1007/s13197-011-0256-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CD, List GR, Moser HA, Cowan JC. Long-term storage of soybean and cotton salad oil. Journal American Oil Chem Soc. 1973;50:218–222. doi: 10.1007/BF02640494. [DOI] [PubMed] [Google Scholar]
- FAO—Food and Agriculture Organization (2010) Available at: http://www.codexalimentarius.net/gsfaonline/additives/details.html?id=189. Accessed on 02 May 2010
- González-Montelongo R, Lobo MG, González M. The effect of extraction temperature, time and number of steps on the antioxidant capacity of methanolic banana peel extracts. Sep Purif Technol. 2010;71:347–355. doi: 10.1016/j.seppur.2009.12.022. [DOI] [Google Scholar]
- Huang SJ, Mau JL. Antioxidant properties of methanolic extracts from Agaricus blazei with various doses of γ-irradiation. Lebensm-Wiss Technol. 2006;39:707–716. doi: 10.1016/j.lwt.2005.06.001. [DOI] [Google Scholar]
- Iqbal S, Bhanger MI. Stabilization of sunflower oil by garlic extract during accelerated storage. Food Chem. 2007;100:246–254. doi: 10.1016/j.foodchem.2005.09.049. [DOI] [Google Scholar]
- Lafka TI, Sinanoglou V, Lazos ES. On the extraction and antioxidant activity of phenolic compounds from winery wastes. Food Chem. 2007;104:1206–1214. doi: 10.1016/j.foodchem.2007.01.068. [DOI] [Google Scholar]
- Li Y, Guo C, Yang J, Wei J, Xu J, Cheng S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006;96:254–260. doi: 10.1016/j.foodchem.2005.02.033. [DOI] [Google Scholar]
- Mau JL, Chang CN, Huang SJ, Chen CC. Antioxidant properties of methanolic extracts from Grifola frondosa, Morchella esculenta and termitomyces albunosus mycelia. Food Chem. 2004;87:111–118. doi: 10.1016/j.foodchem.2003.10.026. [DOI] [Google Scholar]
- Pinelo M, Rubilar M, Jerez M, Sineiro J, Núñez MJ. Effect of solvent, temperature and solvent-to-solid ratio on the total phenolic content and antiradical activity of extracts from different components of grape pomace. J Agr Food Chem. 2005;53:2111–2117. doi: 10.1021/jf0488110. [DOI] [PubMed] [Google Scholar]
- Ramalho VC, Jorge N. Antioxidant action of Rosemary extract in soybean oil submitted to the thermoxidation. Grasas y Aceites. 2008;59:128–131. doi: 10.3989/gya.2008.v59.i2.500. [DOI] [Google Scholar]
- Reische DW, Lillard DA, Eitenmiller RR. Antioxidants. In: Akoh CC, Min DB, editors. Food lipids: chemistry, nutrition and biotechnology. 2. New York: Marcel Dekker; 2002. pp. 489–515. [Google Scholar]
- Shui G, Leong LP. Residue from star as valuable source for functional food ingredients and antioxidant nutraceuticals. Food Chem. 2006;97:277–284. doi: 10.1016/j.foodchem.2005.03.048. [DOI] [Google Scholar]
- Xu GH, Chen JC, Liu DH, Zhang YH, Jiang P, Ye XQ. Minerals, phenolic compounds, and antioxidant capacity of citrus peel extract by hot water. J Food Sci. 2008;73:11–18. doi: 10.1111/j.1750-3841.2007.00546.x. [DOI] [PubMed] [Google Scholar]
- Yang JH, Lin HC, Mau JL. Antioxidant properties of several commercial mushrooms. Food Chem. 2002;77:229–235. doi: 10.1016/S0308-8146(01)00342-9. [DOI] [Google Scholar]