Abstract
Effects of microwave assisted hydrodistillation (MAHD) and conventional hydrodistillation (HD) methods on yield, composition, specific gravity, refractive index, and antioxidant and antimicrobial activities of essential oil of Rosmarinus officinalis L were studied. The main aroma compounds of rosemary essential oil were found as 1,8-cineole and camphor. Trolox equivalent antioxidant capacity (TEAC) values for essential oils extracted by MAHD and HD were 1.52 mM/ml oil and 1.95 mM/ml oil, respectively. DPPH radical scavenging activity of the oils obtained by MAHD and HD were found as 60.55% and 51.04% respectively. Inhibitory effects of essential oils obtained by two methods on linoleic acid peroxidation were almost the same. Essential oils obtained by two methods inhibited growth of Esherichia coli O157:H7, Salmonella typhimurium NRRLE 4463 and Listeria monocytogenes Scott A with the same degree. However, inhibitory activity of essential oil obtained by MAHD on Staphylococcus aureus 6538P was stronger than that of obtained by HD (p < 0.05).
Keywords: Antioxidant activity, DPPH, Microwave-assisted hydrodistillation (MAHD), Essential oils
Introduction
Rosmarinus officinalis L. (Lamiaceae), which is a small evergreen shrub (Atti-Santos et al. 2005), is used in cosmetics, in traditional medicine and for flavoring food (Ramírez et al. 2006). The highest quality essential oil of R. officinalis L. is obtained from the leaves (Lo Presti et al. 2005). The main producers are Turkey, Italy, Dalmatia, Spain, Greece, Egypt, France, Portugal and North Africa (Atti-Santos et al. 2005), while the United States, Japan, and some of the European Union countries are the principal importers (Flamini et al. 2002). Spices, herbs and their essential oils have varying degree of biological activity. There has been growing interest in the use of naturally occurring substances as antioxidants and antimicrobials in recent years (Pillai and Ramaswamy 2011). R. officinalis L. has been shown to exhibit antimicrobial and antioxidant activities (Baratta et al. 1998; Pintore et al. 2002; Angioni et al. 2004; Saccheti et al. 2005; Okoh et al. 2010; Karpiǹska-Tymoszezyk 2011).
Microwave has been applied in many areas in food processes such as pasteurization, cooking, reheating, drying, baking and thawing. Application of microwave heating for the extraction of essential oils is a new technique. Microwave-assisted hydrodistillation (MAHD) (Stashenko et al. 2004a, b), solvent-free microwave extraction (SFME) (Lucchesi et al. 2004a) and microwave hydrodiffusion and gravity (MHG) (Bousbia et al. 2009) methods are among the novel technologies developed for the extraction of thermo-sensitive compounds such as essential oils.
SFME has been used to obtain essential oils from ajowan, cumin, and star anise (Lucchesi et al. 2004a), basil, garden mint, and thyme (Lucchesi et al. 2004b), cardamom seed (Lucchesi et al. 2007), oregano (Bayramoglu et al. 2008), fresh orange peel (Ferhat et al. 2008), laurel (Bayramoglu et al. 2009; Uysal et al. 2010), melissa (Uysal et al. 2010) and rosemary (Okoh et al. 2010). In the literature, there are reported studies in which MAHD has been used for the extraction of essential oils from laurel (Kosar et al. 2005) and thyme (Golmakani and Rezaei 2008). Although MAHD of rosemary has also been studied (Lo Presti et al. 2005; Kosar et al. 2005; Tigrine-Kordjani et al. 2007), variation in the concentrations of the essential oil constituents during MAHD has not been investigated yet.
Aqueous, ethanolic and methanolic extracts of plants have been conducted in many studies regarding antioxidant activity of essential oils. However, a need for the assessment of antioxidant activity against different oxidants such as hydrophilic and lipophilic species is still continuing due to the wide range of antioxidant applications in the food industry. Therefore, antioxidant activity of essential oils was evaluated by using ABTS (2-2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) radical scavenging, DPPH (2,2-Diphenyl-1-picrylhydrazyl, D9132) radical scavenging and linoleic acid peroxidation assays in this study.
In the present study, the effects of extraction time on the yield of rosemary essential oil obtained by MAHD were investigated. Variation in the composition of the essential oils during MAHD and HD was also studied. Other quality parameters such as specific gravity and refractive index were also examined. In addition, antioxidant and antimicrobial activities of essential oils obtained with MAHD and HD were compared.
Materials and methods
Reagents and materials
The dried Rosmarinus officinalis L. having Sauter mean diameter of 1392.4 μm was obtained from Kutas (Kutas Tarim Urunleri Dis Tic. San. A.S., Manisa, Turkey).
The standard materials used for the qualitative and quantitative analysis of the essential oil constituents were α-pinene, β-myrcene, γ-terpinene, methyleugenol, p-cymene (Fluka, Ronkonkoma, NY, US), isopulegol, verbenone (Fluka, Tokyo, Japan), α-humulene, 4-terpineol, 3-carene, bornyl acetate, fenchol, terpinolene, (Fluka, Buchs, Switzerland), o-cimene, limonene (Fluka, Neu-Ulm, Germany), β-caryophyllene (Fluka, Madrid, Spain), borneol, thymol, carvacrol, α-terpineol, camphor, β-pinene, 1,8-cineole, methyl jasmonate, linalool (Sigma-Aldrich, Steinheim, Germany) and camphene (Supelco, Bellefonte, PA, USA). Nonane which was used as an internal standard was purchased from Fluka (Buchs, Switzerland). Sodium sulfate anhydrous was purchased from Riedel-de Haën (Seelze, Germany).
The chemicals used for the determination of antioxidant activity including DPPH (2,2-Diphenyl-1-picrylhydrazyl, D9132), linoleic acid (L1376), phosphate buffer tampon (P4417), hemoglobin (H2500), FeCl2 (372870), methanol were purchased from Sigma-Aldrich (Germany), ABTS [2-2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid] Diammonium salt (11557), was obtained from Fluka. Ammonium thiocyanate was purchased from Merck.
Hydrodistillation (HD)
In conventional hydrodistillation, Clevenger apparatus was used. Heating was achieved using a hemisphere heater (Termal Laboratory Equipments, Istanbul, Turkey) with 200 W of power. For the experiments, 350 g of distilled water and 50 g of dried R. officinalis L. were placed in a flat bottom flask. The process was continued until no more essential oil was obtained. The essential oil samples were collected at different extraction times and stored in amber colored vials, dehydrated with anhydrous sodium sulfate, capped under nitrogen and kept at 4°C until being analyzed. Experiments were conducted twice for each condition.
Microwave-assisted hydrodistillation (MAHD)
MAHD was conducted in a domestic microwave oven operating at 2450 MHz and 622 W (White-Westinghouse, Pittsburg, USA). The delivered power level of the microwave oven was measured using IMPI-2 L test (Buffler 1993).
The interior cavity of the microwave oven was 29 × 37 × 40 cm. Flat bottom flask having a capacity of 1000 mL was placed inside the cavity for the MAHD experiments. The flask was connected to Clevenger apparatus through the hole at the top of the oven which was previously drilled. As in the case of conventional HD, 350 g of distilled water and 50 g of dried R. officinalis L. were placed in flat-bottom flask combined to a Clevenger apparatus. Then, MAHD was performed until no more essential oil was obtained. Experiments were performed starting from the beginning each time to collect the essential oil samples at different extraction times since taking continuous data was not possible. For each condition, experiments were replicated twice. The essential oil samples were collected in amber colored vials, dehydrated with anhydrous sodium sulfate, capped under nitrogen and kept at 4°C until being analyzed.
Analysis of essential oil
Yield
Essential oil yield was expressed in terms of the volume of the oil collected in mL per gram of dry plant material.
Composition
The essential oils obtained at different conditions were analyzed by gas chromatography (Agilent Technologies 6890N Network GC System, Palo Alto, CA, US) and gas chromatography coupled to mass spectrometry (Agilent Technologies 6890N Network GC System coupled to Agilent Technologies 5973 Network Mass Selective Detector, Palo Alto, CA, US). In order to perform quantitative analysis with FID at the same time with the component characterization of MSD, a two holes ferrule was used in which two columns were placed. By this way, injection of the sample from one injection block was distributed equally into two columns. The capillary columns used for both of the analysis were HP-5MS (30 m × 0.25 mm × 0.25 μm) with a 5% phenyl methyl siloxane stationary phase. GC-MS conditions were as follows: carrier gas, He; flow rate, 0.8 mL/min; splitless; injection volume 1 μL; injection temperature 250°C; oven temperature program, holding at 50°C for 2 min, rising to 225°C with 3°C/min; MSD transfer line temperature, 250°C; MSD quadrupole temperature, 150°C; ionization temperature, 230°C; ionization mode, electronic impact at 70 eV. Solvent delay was for 4.5 min. The GC analysis was performed with the following conditions: flow rate, 0.4 mL/min; FID temperature, 275°C; make-up gas type, He with a make-up flow rate of 45 mL/min.
For the quantitative analysis of R. officinalis L. essential oil, γ-terpinene, methyl jasmonate, verbenone, 1,8-cineole, β-pinene, p-cymene, β-caryophyllene, bornyl acetate, methyl eugenol, carvacrol, 4-terpineol, camphor and fenchol were used as external standards (7 data points) and nonane was used as an internal standard. For the quantitation of the other constituents of rosemary essential oil, the approach of Schoenmakers et al. (2000) was used. Following this approach, monoterpene hydrocarbons, phenols, alcohols, ketones, esters, ethers and sesquiterpenes in the essential oil of R. officinalis L. were quantified using the relative response factors of β-pinene (~0.943), carvacrol (~1.073), 4-terpineol (~1.092), camphor (~1.059), bornyl acetate (~1.245), 1,8-cineole (~1.068) and β-caryophyllene (~0.935), respectively. For the quantification of aldehydes, the relative response factor of camphor was used since Zhu et al. (2005) claimed that the relative response factors of aldehydes were close to those of ketones. In the mentioned reference, two dimensional GC×GC was compared with GC-MS. In GC, FID detector was used.
The components of the essential oils were identified by comparison of their retention times with those of available standards and with library matching of their mass spectra (NIST98, Wiley7n, Flavor2). The data were analyzed by a software program, MSD ChemStation (G1701 DA D.02.00.275).
Specific gravity and Refractive index measurements
Specific gravity and refractive index of the essential oil samples obtained using conventional HD and MAHD at the end of the processes were measured.
Specific gravities were calculated by dividing the weight of 10 μL essential oil to that of 10 μL distilled water. Weight measurements were made in triplicate using the balance having an accuracy of ±0.00001 g (Denver Instrument, Gottingen, Germany) at 22 ± 2 °C.
Refractive index measurements were made in triplicate using the Bellingham Stanley Ltd. RFM 330 refractometer (Kent, England). Measurement temperatures were 25 ± 2 °C.
Antioxidant activity
Antioxidant activities of essential oil samples obtained from HD and MAHD were measured by using ABTS free radical scavenging, DPPH free radical scavenging and linoleic acid inhibition methods. All analyses were performed as two parallels and triplicates.
ABTS free radical–scavenging assay
ABTS radical scavenging activities of the samples were determined by the method of Re et al. (1999). Briefly, ABTS radical solution was diluted with ethanol to an absorbance of 0.70 (±0.02) at 734 nm. After addition of 1.0 ml diluted ABTS radical solution to 10 μl of the sample, absorbance reading (Cary 50 Scan UV-Visible Spectrophotometer) was taken 5 min after initial mixing. Percent inhibition was calculated by using the equation below.
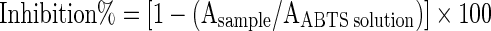 |
Where;
A sample: Absorbance reading obtained for the mixture (sample + ABTS radical solution)
AABTS solution: Absorbance reading obtained for ABTS radical solution
Trolox equivalent antioxidant capacity (TEAC)
The Trolox equivalent antioxidant capacity assay based on the reaction of ABTS radical with Trolox was performed in order to compare radical scavenging activity of a sample with antioxidant activity of Trolox. The antioxidant activities of the samples were estimated within the range of the dose-response curve of Trolox and expressed as the Trolox equivalent antioxidant capacity. The latter is defined as the concentration of Trolox having the antioxidant capacity equivalent to 1.0 mmol/l solution of the substance under investigation. In this study, the TEAC values were expressed as mM TEAC per ml sample (Miller et al. 1993).
DPPH free radical–scavenging assay
The free radical scavenging activity using DPPH radical was determined according to the method described by Braca et al. (2001). In this experiment 50 μL of each extract was added to 950 μL of 0.030 mg/mL methanol solution of DPPH. Then, the mixture was shaken vigorously and left in darkness for 5 min. Finally, the absorbance of the mixture was measured against methanol (blank) at 515 nm by using a spectrophotometer (Cary 50 Scan UV-Visible Spectrophotometer) The DPPH scavenging activity was expressed as the percent inhibition of free radical DPPH (Braca et al. 2001).
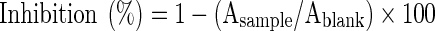 |
Where;
A sample : Absorbance of the mixture (the sample + DPPH radical solution).
A blank : Absorbance of the DPPH radical solution
Inhibition of the linoleic acid peroxidation
Inhibitory effect of the samples on the linoleic acid peroxidation was determined by the method of Kuo et al. (1999). 10 μl of sample, 0.37 ml of 0.05 M phosphate buffer (pH 7.0) containing 0.05% Tween 20 and 4 mM of linoleic acid were mixed in a test tube. This mixture was equilibrated at 37 °C for 3 min. The peroxidation of linoleic acid in the mixture was initiated by adding 20 μl of 0.035% hemoglobin prepared in water. Then mixture incubated at 37 °C in a shaking water bath under 100 rpm for 10 min. Reaction was stopped by the addition of 5 ml of 0.6% HCl prepared in ethanol. The hydroperoxide formed was determined according to ferric thiocyanate method. Absorbance readings were taken at 480 nm with a spectrophotometer (Cary 50 Scan UV-Visible Spectrophotometer). Antioxidant activity of the sample was calculated according to the equation below.
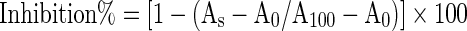 |
Where;
A0 = Absorbance obtained for reaction mixture that does not contain hemoglobin.
A100 = Absorbance obtained for reaction mixture that does not contain sample
As = Absorbance obtained for reaction mixture
Antimicrobial activity
Gram-positive and gram- negative bacterial species used in this study were kindly obtained from the culture collection of Microbiology laboratory in Food Engineering Department of Ege University, Turkey. The bacteria species include: Listeria monocytogenes Scott A, Staphyloccous aureus 6538P, Esherichia coli O157:H7 and Salmonella typhimurium NRRLE 4463.
Trypton Soya Broth (TSB, Oxoid CM 129) was used as the media for the development of the strains of pathogen cultures, whereas Plate Count Agar (Oxoid CM 325) was used for the enumeration.
Inocula used in the antimicrobial assay were obtained from cultures grown on TSB at 35 °C for 24 h. Essential oils were sterilized by filtration through 0.45 μm Millipore filters. Antimicrobial tests were then carried out by the disc diffusion method using 100 μL of suspension containing 108 CFU/ml of pathogen bacteria spread on Nutrient Agar (NA Oxoid CM0003). The sterilized paper-discs (6 mm in diameter) were impregnated aseptically with 10 μL of essential oil placed on the inoculated agar. Three discs were placed on each petri plate. Sterilized water was used as a control. Plates were kept at ambient temperature for 1 h and then incubated at 37 °C for 24 h. Antimicrobial activity was evaluated by measuring the inhibition zone (Güllüce et al. 2007).
Statistical analysis
Each assay was performed in parallels and triplicates. Statistical analysis was performed using SPSS for Windows (Version 10.0). Two-way analysis of variance (ANOVA) was performed to determine significant differences in essential oil yields. Paired sample t-test was used to determine effect of extraction methods (MAHD and HD) on antioxidant and antimicrobial activities of the essential oils. To evaluate differences in the antimicrobial activity against pathogen cultures, one way ANOVA was conducted, and Tukey HSD multiple range test were used to determine significant differences at p < 0.05.
Results and discussion
Essential oil yield
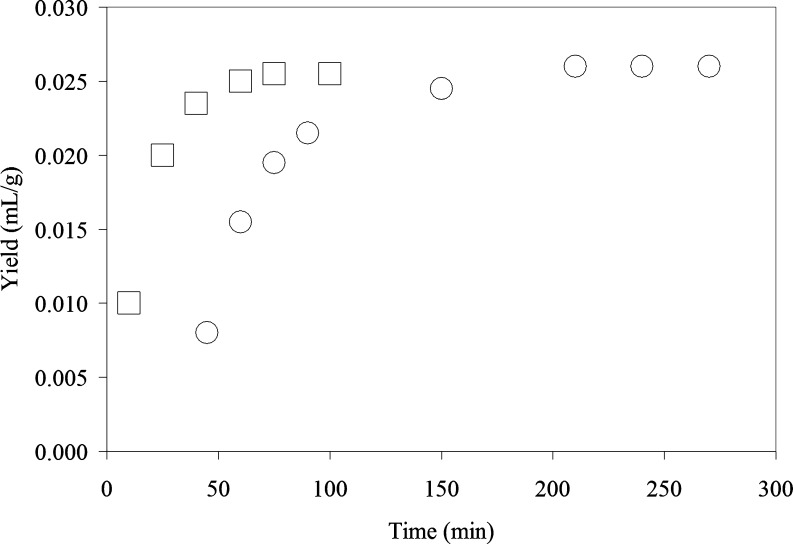
Figure 1 shows the variation of essential oil yield with processing time during conventional HD and MAHD methods. The maximum essential oil yields obtained in MAHD and HD were found to be the same as 0.026 mL/g. The time needed for the complete extraction of rosemary essential oil was found as 75 min for MAHD and 210 min for HD. That is, the extraction time was reduced by about 65% by using microwave. The reason for the reduction in the processing time is the heat generated by microwave heating which results in high pressure gradient inside the product. Microwaves interact selectively with the free water molecules present in the gland and vascular systems which leads to localized heating. As a result of internal superheating, a dramatic expansion and consequently rupture of cell walls occurs allowing the extraction of essential oil.
Fig. 1.
Variation of essential oil yield of Rosmarinus officinalis L. during hydrodistillation (HD) and microwave-assisted hydrodistillation (MAHD) during the process (□, MAHDa; ○, HDa) (*means extraction conditions with different letters are significantly different, p ≤ 0.05)
Composition
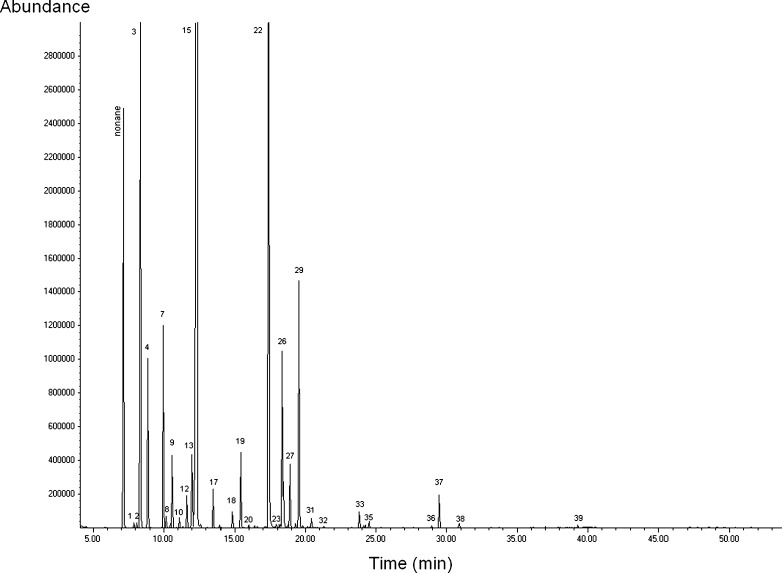
The total ion chromatogram of the rosemary essential oil is given in Fig. 2. The composition of the essential oil of R. officinalis L. obtained by MAHD and conventional HD methods is given in Table 1. The compositions of the essential oils obtained by both of the methods were found to be almost the same qualitatively, whereas some quantitative differences were observed. The main components of the essential oil of R. officinalis L. were determined as 1,8-cineole (436–450 mg/mL) followed by camphor (153–167 mg/mL), α-pinene (~85 mg/mL), α-terpineol (35–41 mg/mL), borneol (35–40 mg/mL), β-pinene (~33 mg/mL), and camphene (~25 mg/mL). It was also found that the essential oil mainly composed of oxygenated compounds (77–79%) while monoterpene hydrocarbons and sesquiterpenes constituted 21–22% and 0.5–0.6% of it, respectively.
Fig. 2.
Total ion chromatogram (obtained by GC-MS analysis) of the Rosmarinus officinalis L. essential oil extracted by MAHD (1: tricyclene, 2: á-thujene, 3: á-pinene, 4: camphene, 7: â-pinene, 8: 1-octen-3-ol, 9: â-myrcene, 10: á-phellandrene, 12: á-terpinene, 13: p-cymene, 15: 1,8-cineole, 17: ãterpinene, 18: terpinolene, 19: linalool, 20: fenchol, 22: camphor, 23: isopulegol, 26: borneol, 27: 4- terpineol, 29: á-terpineol, 31: verbenone, 32: citronellol(?), 33: bornyl acetate, 35: carvacrol, 36: methyl eugenol, 37: â-caryophyllene, 38: á-humulene, 39: methyl jasmonate)
Table 1.
Concentrations of the compounds present in the essential oil of Rosmarinus officinalis L. obtained by different methods
| No | Compounds | RT 1a (min) | RT 2b (min) | Concentration (mg mL−1) | |||
|---|---|---|---|---|---|---|---|
| 45 min | 210 min | 10 min | 75 min | ||||
| Hydrodistillation | MAHD | ||||||
| 1 | Tricyclene | 7.910 | 13.401 | 0.903 | 0.837 | 1.177 | 1.523 |
| 2 | α-thujene | 8.095 | 13.549 | 0.841 | 0.718 | 0.991 | 0.868 |
| 3 | α -pinene | 8.350 | 13.889 | 79.625 | 85.710 | 120.695 | 84.323 |
| 4 | Camphene | 8.874 | 14.534 | 20.404 | 25.464 | 30.442 | 24.211 |
| 5 | Verbenenec | 9.101 | 14.810 | – | 0.281 | – | – |
| 6 | Sabinenec | 9.869 | 15.629 | – | – | 0.248 | 0.224 |
| 7 | β-pinene | 9.970 | 15.760 | 27.696 | 32.847 | 41.549 | 33.161 |
| 8 | 1-octen-3-ol | 10.176 | 16.096 | 2.118 | 1.423 | 1.787 | 1.332 |
| 9 | β -myrcene | 10.605 | 16.291 | 9.108 | 11.579 | 14.622 | 11.234 |
| 10 | α -phellandrene | 11.119 | 16.949 | 1.300 | 1.799 | 1.817 | 1.643 |
| 11 | 3-carene | 11.378 | 17.213 | 0.000 | 0.742 | 0.661 | 0.000 |
| 12 | α -terpinene | 11.648 | 17.502 | 3.059 | 4.846 | 4.324 | 4.279 |
| 13 | P-cymene | 12.014 | 17.875 | 7.556 | 11.010 | 9.536 | 10.046 |
| 14 | Limonene | – | 18.080 | 4.138 | 15.151 | 16.454 | 14.826 |
| 15 | 1,8-cineole | 12.363 | 18.234 | 538.797 | 436.875 | 409.754 | 450.188 |
| 16 | Cis-Ocimene | 12.644 | 18.909 | – | – | – | – |
| 17 | γ-terpinene | 13.502 | 19.407 | 3.521 | 6.219 | 4.764 | 5.582 |
| 18 | Terpinolene | 14.826 | 20.771 | 1.763 | 5.332 | 2.447 | 3.081 |
| 19 | Linalool | 15.413 | 21.214 | 13.520 | 14.352 | 12.248 | 13.583 |
| 20 | Fenchol | 15.975 | 21.955 | 1.532 | 2.342 | 1.049 | 1.509 |
| 21 | Campholaldehydec | 16.589 | 22.528 | 0.591 | 0.696 | 0.297 | 0.586 |
| 22 | Camphor | 17.399 | 23.420 | 139.592 | 153.297 | 149.583 | 167.174 |
| 23 | Isopulegol | 17.939 | 23.903 | 1.074 | 0.887 | 1.059 | 0.843 |
| 24 | Pinocamphone/isopinocamphone | 18.146 | 24.140 | 1.030 | 0.727 | 0.843 | 0.764 |
| 25 | Pinocarvone/trans-pinocarvone | 18.236 | 24.252 | 0.299 | – | 0.344 | – |
| 26 | Borneol | 18.352 | 24.330 | 21.488 | 35.582 | 28.329 | 39.672 |
| 27 | 4-terpineol | 18.892 | 24.837 | 7.568 | 10.497 | 8.153 | 11.294 |
| 28 | p-cymene-8-olc | 19.295 | 25.143 | 0.223 | 0.893 | 0.857 | 1.145 |
| 29 | α-terpineol | 19.539 | 25.401 | 18.104 | 36.869 | 26.356 | 41.003 |
| 30 | Myrtenolc | 19.814 | 25.937 | – | 0.401 | 0.199 | 0.886 |
| 31 | Verbenone | 20.418 | 26.315 | 1.389 | 2.939 | 2.133 | 3.273 |
| 32 | Citronellolc | 21.312 | 26.916 | – | 0.824 | – | 0.846 |
| 33 | Bornyl acetate | 23.812 | 29.552 | 2.241 | 3.212 | 2.991 | 3.261 |
| 34 | Thymol | 24.108 | 29.794 | – | – | – | 0.666 |
| 35 | Carvacrol | 24.500 | 30.068 | 1.005 | 1.112 | 1.197 | 1.617 |
| 36 | Methyl eugenol | 28.964 | 34.339 | – | 1.040 | – | 1.169 |
| 37 | β -caryophyllene | 29.478 | 35.195 | 2.669 | 4.035 | 4.796 | 4.895 |
| 38 | α -humulene | 30.886 | 36.534 | 0.169 | 0.481 | 0.652 | 0.850 |
| 39 | Methyl jasmonate | 38.496 | 43.609 | – | 1.079 | – | 2.643 |
| % of total | 99.49 | 96.69 | 99.05 | 97.89 | |||
| Monoterpene hydrocarbons | 159.91 | 202.53 | 249.73 | 195.00 | |||
| Oxygenated compounds | 750.57 | 705.04 | 647.18 | 743.45 | |||
| Sesquiterpenes | 2.84 | 4.52 | 5.45 | 5.74 | |||
a RT 1: Retention time in min on HP-5MS column obtained by MSD;
b RT 2: Retention time in min on HP-5MS column obtained by FID
c Compounds determined using mass spectrum consistent with spectra found in the literature, but not using the retention time of standard compounds
(n = 6)
It was observed that concentrations of sesquiterpenes increased with increase in time in both HD and MAHD (Table 1). This is probably due to their higher molecular weights, lower volatilities and lower solubilities in water than the other constituents. They need more time to reach their maximum levels in the essential oil. Concentration of monoterpene hydrocarbons decreased with time in MAHD, while they increased with time in HD. On the other hand, concentration of oxygenated compounds was slightly decreased with time in HD, while they slightly increased with time in MAHD.
At the end of the process, the concentration of monoterpene hydrocarbons, oxygenated compounds and sesquiterpenes were almost the same in both methods (Table 1). The oxygenated compounds detected in rosemary essential oil were found to be mainly composed of ethers (~60%), ketones (22–24%), alcohols (15–17%). Ethers were found to be constituted almost entirely of 1,8-cineole. The other oxygenated constituents of the oil were determined as aldehydes, esters, and phenols.
Specific gravity and refractive index of essential oils
The mean values for the specific gravities of the rosemary essential oil extracted by HD and MAHD were found as 0.875 ± 0.0020 and 0.879 ± 0.0020, respectively. The mean values for the refractive index of the rosemary oil extracted by HD and MAHD were found to be the same as 1.465 ± 0.0005. Both specific gravity and refractive index values were found to be in good agreement with the ones for the essential oil of R. officinalis L. in literature (Atti-Santos et al. 2005).
Antioxidant activity
Many test systems are available to measure antioxidant activity. Some assays are based on the screening antioxidant activity of hydrophilic compounds and the others are able to determine antioxidant activity of hydrophobic compounds. In addition, the chemical complexity of essential oils, often a mixture of dozens of compounds with different functional groups, polarity and chemical behaviour, could lead to scattered results, depending on the test employed (Saccheti et al. 2005). For this reason, antioxidant activity of essential oils was evaluated by using ABTS radical scavenging, DPPH radical scavenging and linoleic acid peroxidation assays.
Antioxidant activity against ABTS and DPPH radicals
ABTS radical scavenging activities of rosemary essential oils obtained by MAHD and HD were found as 69.75% and 81.99% respectively. TEAC values of these oils were 1.52 mM/ml oil (1.54 mmol/L oil) for MAHD extract and 1.95 mM/ml oil (1.97 mmol/L oil) for HD extract. No significant differences were obtained for radical scavenging abilities and TEAC values of the essential oils obtained by two methods (Table 2). This may be due to the similar composition of the essential oils obtained by HD and MAHD (Table 1). Bousbia et al. (2009) reported that antioxidant activities of rosemary essential oils, obtained by microwave hydrodiffusion and gravity (MHG) and hydrodistillation were 4.53 mmol of Trolox per liter of sample and 3.68 mmol of Trolox per liter of sample, respectively. These differences explained by higher amounts of the oxygenated compounds that MHG essential oils contained. Although oxygenated compounds we found in the essential oil were higher (77–79%) than oxygenated compounds Bousibia et al., determined (26.16–29.54%), TEAC values we determined were lower than those of Bousbia et al. (2009) reported. Erkan et al. (2008) reported that ABTS radical scavenging activity of rosemary extract obtained by Soxhlet extraction, was found in between 15.5 and 15.7 (TEAC, mM) according to reaction time (1–6 minutes). Similarly Dorman et al. (2003) found that ABTS radical scavenging activity of water fraction of rosemary obtained by hydrodistillation was changed from 10.3 to 14.1 (TEAC, mM) according to reaction time (1–10 minutes). Their results cannot be comparable with our results. This may be due to the differences in growth stages and ecological conditions of plant. Essential oils of R. officinalis obtained by MAHD and HD inhibited oxidation generated by DPPH radical by the percentage of 60.55 and 51.04 respectively (Table 2). Mata et al. (2007) reported that water and ethanol extracts of rosemary had the same antioxidant activity against DPPH radical. Dorman et al. (2003) reported that aqueous extracts of rosemary and sage showed the highest activity against DPPH radical. IC50 values for rosemary and sage were 236.5 μg/ml and 265.8 μg/ml respectively.
Table 2.
Antioxidant activity of rosemary essential oil against ABTS and DPPH radicals and linoleic acid peroxidation*
| Extraction methods | Inhibition of ABTS radical oxidation (%) | TEAC (mM/ml oil) | Inhibition of DPPH radical oxidation (%) | Inhibition of linoleic acid peroxidation (%) |
|---|---|---|---|---|
| MAHD | 69.7 ± 5.73a | 1.5 ± 0.10a | 60.5 ± 10.68a | 92.5 ± 4.90a |
| HD | 82.0 ± 14.63a | 1.9 ± 0.45a | 51.0 ± 18.39a | 91.5 ± 3.58a |
Results were given as mean ± SD, (n = 6)
*Different letters means that there are significant differences between different extraction methods in the same column (p < 0.05)
TEAC: Trolox equivalent antioxidant capacity
Inhibition of linoleic acid peroxidation
Apart from ABTS and DPPH radical scavenging effect, inhibition of linoleic acid peroxidation is an indicator that the sample is an effective inhibitor of lipid peroxidation. Antioxidant effect against linoleic acid peroxidation of rosemary oils obtained by MAHD and HD (Table 2) were found to be similar (p > 0.05). However, essential oils inhibited linoleic acid peroxidation by greater proportion (92.49% for MAHD and 91.53% for HD) than oxidation generated by ABTS and DPPH radicals. Frutos and Hernández-Herrero (2005), studied the effect of rosemary extract on the promoted oxidation in a dressing consisting of sunflower oil, garlic and parsley and found that rosemary extract caused an antioxidant effect with the acceptable sensory properties except for the formulation in which 6 g rosemary/L was used. There are many studies regarding antioxidant activities of rosemary, rosemary extracts and rosemary essential oils especially in meat and meat products (Balentine et al. 2006; O’Grady et al. 2006; Hernández-Hernández et al. 2009). Estévez and Cava (2006) reported that the addition of rosemary essential oil to different types of frankfurters inhibited the development of lipid and protein oxidation depending on the level of added essential oil and characteristic of the frankfurter. Our study revealed that rosemary essential oil can be used to retard linoleic acid peroxidation. Therefore, essential oil of rosemary can be a good alternative to protect foods from being oxidized.
Antimicrobial activity
R. officinalis essential oil showed antimicrobial activity against two gram positive and two gram negative bacteria with different degree. The antimicrobial activity of the essential oils of R. officinalis has been attributed to the presence of α-pinene, 1,8-cineole, camphor, verbenone and borneol Borneol was found to be the most potent compound and it was followed by camphor and verbinone (Santoya et al. 2005). The composition of the essential oil we studied was 1,8-cineole (436–450 mg/mL), camphor (153–167 mg/mL), α-pinene (~85 mg/mL), α-terpineol (35–41 mg/mL), borneol (35–40 mg/mL), β-pinene (~33 mg/mL), and camphene (~25 mg/mL) (Table 1). Extraction methods did not affect antimicrobial activity of the essential oil against E. coli (inhibition zone = 3.5 mm), S. typhimurium NRRLE (inhibition zone = 1.5 mm) 4463 and L. monocytogenes Scott-A (inhibition zone = 2.5 mm). However, essential oil obtained by MAHD displayed greater antimicrobial activity against S. aureus 6538 P (inhibition zone = 3.5 mm) than the essential oil obtained by HD (inhibition zone = 2.5 mm) (p < 0.05). Among the bacteria tested S. typhimurium NRRLE 4463 was the most resistant bacteria to the essential oil (p < 0.05). Okoh et al. (2010) reported that essential oil from R. officinalis obtained through hydrodistillation and solvent free microwave extraction inhibited growth of Staphylococucus aureus, Bacillus subtilis, Escherichia coli and Klebsiella pneumonia. Minimum inhibitory concentration values were found between 0.23 mg/ml and 7.5 mg/ml. It was reported that essential oil has greater antimicrobial activity on gram positive bacteria than gram negative bacteria (Kokoska et al. 2002; Okoh et al. 2010). This result explained with the differences in the cell membranes of these bacterial groups. External membrane of gram negative bacteria renders their surfaces highly hydrophobic whereas the lipophilic ends of the lipoteichoic acids of the cell membrane of gram positive bacteria may facilitate penetration by hydrophobic compounds (Okoh et al. 2010).
Conclusion
No significant differences were obtained in the essential oil yields obtained by MAHD and conventional HD although the process time was found to be reduced substantially in the case of MAHD. Composition, specific gravity and refractive index of the oils obtained with different methods were also the same. In addition, antioxidant activity of the essential oils obtained by MAHD and HD were not statistically different. Similarly, antimicrobial activity of the essential oils obtained with two methods was also the same except for S. aureus 6538P. Essential oil obtained by MAHD displayed greater antimicrobial activity against S. aureus 6538P. Therefore, it can be concluded that MAHD is a good alternative for the extraction of essential oils from R officinalis L. since it provides essential oils of similar quality, antioxidant and antimicrobial activity compared to conventional HD while reducing the time of the process drastically.
References
- Angioni A, Barra A, Cereti E, Barile D, Coisson JD, Arlorio M, Dessi S, Coroneo V, Cabras P. Chemical composition, plant genetic differences, antimicrobial and antifungal activity investigation of the essential oil of Rosmarinus officinalis L. J Agric Food Chem. 2004;52:3530–3535. doi: 10.1021/jf049913t. [DOI] [PubMed] [Google Scholar]
- Atti-Santos AC, Rossato M, Pauletti GF, Rota LD, Rech JC, Pansera MR, Agostini F, Serafini LA, Moyne P. Physico chemical evaluation of Rosmarinus officinalis L. essential oils. Braz Arc Biol Techn. 2005;48:1035–1039. doi: 10.1590/S1516-89132005000800020. [DOI] [Google Scholar]
- Balentine CW, Crandall PG, O’Bryan CA, Duong DQ, Pohlman FW. The pre- and post-grinding application of rosemary and its effects on lipid oxidation and color during storage of ground beef. Meat Sci. 2006;73:413–421. doi: 10.1016/j.meatsci.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Baratta MT, Dorman HJD, Deans SG, Figueiredo AC, Barroso JG, Ruberto G. Antimicrobial and antioxidant properties of some commercial essential oils. Flavour Frag J. 1998;13:235–244. doi: 10.1002/(SICI)1099-1026(1998070)13:4<235::AID-FFJ733>3.0.CO;2-T. [DOI] [Google Scholar]
- Bayramoglu B, Sahin S, Sumnu G. Solvent-free microwave extraction of essential oil from oregano. J Food Eng. 2008;88:535–540. doi: 10.1016/j.jfoodeng.2008.03.015. [DOI] [Google Scholar]
- Bayramoglu B, Sahin S, Sumnu G. Extraction of essential oil from laurel leaves by using microwaves. Sep Sci Technol. 2009;44:722–733. doi: 10.1080/01496390802437271. [DOI] [Google Scholar]
- Bousbia N, Vian MA, Ferhat MA, Petitcolas E, Meklati BY, Chemat F. Comparison of two isolation methods for essential oil from rosemary leaves: hydrodistillation and microwave hydrodiffusion and gravity. Food Chem. 2009;114:355–362. doi: 10.1016/j.foodchem.2008.09.106. [DOI] [Google Scholar]
- Braca A, Tommasi ND, Bari LD, Pizza C, Politi M, Morelli I. Antioxidant principles from Bauhinia terapotensis. J Nat Prod. 2001;64:892–895. doi: 10.1021/np0100845. [DOI] [PubMed] [Google Scholar]
- Buffler C. Microwave cooking and processing: engineering fundamentals for the food scientist. New York: Avi Book; 1993. [Google Scholar]
- Dorman HJD, Peltoketo A, Hiltunen R, Tikkanen MJ. Characterisation of the antioxidant properties of de-odourised aqueous extracts from selected Lamiaceae herbs. Food Chem. 2003;83:255–262. doi: 10.1016/S0308-8146(03)00088-8. [DOI] [Google Scholar]
- Erkan N, Ayrancı G, Ayrancı E. Antioxidant activities of rosemary (Rosmarinus officinalis L.) extract, blackseed (Nigella sativa L.) essential oil carnosic acid rosmarinic acid and sesamol. Food Chem. 2008;110:76–82. doi: 10.1016/j.foodchem.2008.01.058. [DOI] [PubMed] [Google Scholar]
- Estévez M, Cava R. Effectiveness of rosemary essential oil as an inhibitor of lipid and protein oxidation: contradictory effects in different types of frankfurters. Meat Sci. 2006;72:348–355. doi: 10.1016/j.meatsci.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Ferhat MA, Meklati BY, Visinoni F, Vian MA, Chemat F. Solvent free microwave extraction of essential oils – Green chemistry in the teaching laboratory. Chim Oggi. 2008;26:48–50. [Google Scholar]
- Flamini G, Cioni PL, Morelli I, Macchia M, Ceccarini L. Main agronomic-productive characteristics of two ecotypes of Rosmarinus officinalis L. and chemical composition of their essential oils. J Agric Food Chem. 2002;50:3512–3517. doi: 10.1021/jf011138j. [DOI] [PubMed] [Google Scholar]
- Frutos MJ, Hernández-Herrero JA. Effects of rosemary extract (Rosmarinus officinalis) on the stability of bread with an oil garlic and parsley dressing. LWT-Food Sci Technol. 2005;38:651–655. doi: 10.1016/j.lwt.2004.08.013. [DOI] [Google Scholar]
- Golmakani MT, Rezaei K. Comparison of microwave-assisted hydro distillation with the traditional hydrodistillation method in the extraction of essential oils from Thymus vulgaris L. Food Chem. 2008;109:925–930. doi: 10.1016/j.foodchem.2007.12.084. [DOI] [PubMed] [Google Scholar]
- Güllüce M, Şahin F, Sökmen M, Özer H, Daferera D, Sökmen A, Poissiou M, Adıgüzel A, Özkan H. Antimicrobial and antioxidant properties of the essential oils and methanol extract from Mentha longifolia L.ssp longifolia. Food Chem. 2007;103:1449–1456. doi: 10.1016/j.foodchem.2006.10.061. [DOI] [Google Scholar]
- Hernández-Hernández E, Ponce-Alquicira E, Jaramillo-Flores ME, Legarreta IG. Antioxidant effect of rosemary (Rosmarinus officinalis L.) and oregano (Origanum vulgare L.) extracts on TBARS and color of model raw pork batters. Meat Sci. 2009;81:410–417. doi: 10.1016/j.meatsci.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Karpiǹska-Tymoszezyk M (2011) The effects of oil soluble rosemary extract, sodium erythorbate and a mixture of oil-soluble rosemary extract and sodium erythorbate on the quality of cooked meatballs. J Food Sci Technol. doi:10.1007/s13197-011-0359-3 [DOI] [PMC free article] [PubMed]
- Kokoska L, Polesny Z, Rada V, Nepovim A, Vanek T. Screening of some Siberian medicinal plants for antimicrobial activity. J Ethnopharmacol. 2002;82:51–53. doi: 10.1016/S0378-8741(02)00143-5. [DOI] [PubMed] [Google Scholar]
- Kosar M, Tunalier Z, Ozek T, Kurkcuoglu M, Baser KHC. A simple method to obtain essential oils from Salvia Triloba L. and Laurus nobilis L. by using microwave-assisted hydrodistillation. Z Naturforsch C. 2005;60:501–504. doi: 10.1515/znc-2005-5-620. [DOI] [PubMed] [Google Scholar]
- Kuo JM, Yeh DB, Pan BS. Rapid photometric assay evaluating antioxidative activity in edible plant material. J Agric Food Chem. 1999;47:3206–3209. doi: 10.1021/jf981351o. [DOI] [PubMed] [Google Scholar]
- Lo Presti M, Ragusa S, Trozzi A, Dugo P, Visinoni F, Fazio A, Dugo G, Mondello L. A comparison between different techniques for the isolation of rosemary essential oil. J Sep Sci. 2005;28:273–280. doi: 10.1002/jssc.200400037. [DOI] [PubMed] [Google Scholar]
- Lucchesi ME, Chemat F, Smadja J. An original solvent free microwave extraction of essential oils from spices. Flavour Frag J. 2004;19:134–138. doi: 10.1002/ffj.1274. [DOI] [Google Scholar]
- Lucchesi ME, Chemat F, Smadja J. Solvent-free microwave extraction of essential oil from aromatic herbs: comparison with conventional hydro-distillation. J Chromatogr A. 2004;1043:323–327. doi: 10.1016/j.chroma.2004.05.083. [DOI] [PubMed] [Google Scholar]
- Lucchesi M, Smadja J, Bradshaw S. Solvent free microwave extraction of Elletaria cardamomum L.: a multivariate study of a new technique for the extraction of essential oil. J Food Eng. 2007;79:1079–1086. doi: 10.1016/j.jfoodeng.2006.03.029. [DOI] [Google Scholar]
- Mata AT, Poença C, Ferreira AR, Serralheiro MLM, Nogueira JMF, Araujo MEM. Antioxidant and antiacetylcholinesterase activities of five plants used as Portoguese food spices. Food Chem. 2007;103:778–786. doi: 10.1016/j.foodchem.2006.09.017. [DOI] [Google Scholar]
- Miller NJ, Rice-Evans CA, Davies MJ, Gopinathan V, Milner A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clinical Sci. 1993;84:407–412. doi: 10.1042/cs0840407. [DOI] [PubMed] [Google Scholar]
- O’Grady MN, Maher M, Troy DJ, Moloney AP, Kerry JP. An assessment of dietary supplementation with tea catechins and rosemary extract on the quality of fresh beef. Meat Sci. 2006;73:132–143. doi: 10.1016/j.meatsci.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Okoh OO, Sadimenko AP, Afolayan AJ. Comparative evaluation of the antibacterial activities of the essential oils of Rosmarinus officinalis L. obtained by hydrodistillation and solvent free microwave extraction methods. Food Chem. 2010;120:308–312. doi: 10.1016/j.foodchem.2009.09.084. [DOI] [Google Scholar]
- Pintore G, Usai M, Bradesi P, Juliano C, Boatto G, Tomi F, Chessa M, Cerri R, Casanova J. Chemical composition and antimicrobial activity of Rosmarinus officinalis L. oils from Sardinia and Corsica. Flavour Frag J. 2002;17:15–19. doi: 10.1002/ffj.1022. [DOI] [Google Scholar]
- Ramírez P, Garcia-Risco MR, Santoyo S, Senorans FJ, Ibanez E, Reglero G. Isolation of functional ingredients from rosemary by preparative-supercritical fluid chromatography (Prep-SFC) J Pharmaceut Biomed. 2006;41:1606–1613. doi: 10.1016/j.jpba.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Re R, Pelegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad Biol and Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Pillai P, Ramaswamy K (2011) Effect of naturally occuring antimicrobials and chemical preservatives on the growth of Aspercillus parasiticus. J Food Sci Technol. doi:10.1007/s13197-011-0275-6 [DOI] [PMC free article] [PubMed]
- Saccheti G, Maietti S, Muzzoli M, Scaglianti M, Manfredini S, Radice M, Bruni R. Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem. 2005;91:621–632. doi: 10.1016/j.foodchem.2004.06.031. [DOI] [Google Scholar]
- Santoya S, Cavero S, Jaime L, Ibanez E, Senorans FJ, Reglero G. Chemical composition and antimicrobial activity of Rosmarinus officinalis L. essential oil obtained via supercritical fluid extraction. J Food Protect. 2005;68:790–795. doi: 10.4315/0362-028x-68.4.790. [DOI] [PubMed] [Google Scholar]
- Schoenmakers PJ, Oomen JLMM, Blomberg J, Genuit W, Van Velzen G. Comparison of comprehensive two-dimensional gas chromatography and gas chromatography – mass spectrometry for the characterization of complex hydrocarbon mixtures. J Chromatogr A. 2000;892:29–46. doi: 10.1016/S0021-9673(00)00744-5. [DOI] [PubMed] [Google Scholar]
- Stashenko EE, Jaramillo BE, Martinez JR. Comparison of different extraction methods for the analysis of volatile secondary metabolites of Lippia alba (Mill.) NE brown, grown in Colombia, and evaluation of its in vitro antioxidant activity. J Chromatogr A. 2004;1025:93–103. doi: 10.1016/j.chroma.2003.10.058. [DOI] [PubMed] [Google Scholar]
- Stashenko EE, Jaramillo BE, Martinez JR. Analysis of volatile secondary metabolites from Columbian Xylopia aromatica (Lamarck) by different extraction and headspace methods and gas chromatograhpy. J Chromatogr A. 2004;1025:105–113. doi: 10.1016/j.chroma.2003.10.059. [DOI] [PubMed] [Google Scholar]
- Tigrine-Kordjani N, Chemat F, Meklati BY, Tuduri L, Giraudel JL, Montury M. Relative characterization of rosemary samples according to their geographical origins using microwave-accelerated distillation, solid-phase microextraction and Kohonen self-organizing maps. Anal Bioanal Chem. 2007;389:631–641. doi: 10.1007/s00216-007-1441-6. [DOI] [PubMed] [Google Scholar]
- Uysal B, Sozmen F, Buyuktas BS. Solvent-free microwave extraction of essential oils from Laurus nobilis and Melissa officinalis: comparison with conventional hydrodistillation and ultrasound extraction. Nat Prod Commun. 2010;5:111–114. [PubMed] [Google Scholar]
- Zhu SK, Lu X, Dong L, Xing J, Su XL, Kong HW, Xu GW, Wu CY. Quantitative determination of compounds in tobacco essential oils by comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry. J Chromatogr A. 2005;1086:107–114. doi: 10.1016/j.chroma.2005.04.007. [DOI] [PubMed] [Google Scholar]