Abstract
The present study investigated the effect of Dahi containing potential probiotic bacterial strains on macrophage functions in ageing mice. Probiotic Dahi was prepared by co-culturing Dahi bacteria (Lactococcus lactis ssp. cremoris and Lactococcus lactis ssp. lactis biovar diacetylactis) along with Lactobacillus acidophilus LaVK2 (La-Dahi) or combined L. acidophilus LaVK2 and Bifidobacterium bifidum BbVK3 (LaBb-Dahi) in buffalo milk. The effect of ageing on phagocytic function was evaluated on 4 mo, 12 mo and 16 mo old mice. The effect of probiotic Dahi was evaluated for macrophage functions in ageing mice (12 mo old) fed La-Dahi or LaBb-Dahi supplements for 4 months. The production of extracellular superoxide and H2O2 declined in peritoneal macrophages but enhanced in splenic macrophages, while intracellular superoxide declined in both peritoneal and splenic macrophages with ageing in mice. A decline in phagocytic activity of peritoneal macrophages was also observed in aged mice. Supplementation of diet with La-Dahi or LaBb-Dahi for 4 months improved production of reactive oxygen species and phagocytic and adherence indices of peritoneal macrophages in aged mice. These results suggest that oral administration of La-Dahi or LaBb-Dahi has potential to improve immune functions in ageing individuals.
Keywords: Ageing, Macrophages, Reactive oxygen intermediates, Phagocytosis, L. acidophilus, B. bifidum
Introduction
According to “The Immunologic Theory of Ageing” progressive loss of immune fidelity is responsible for pathologies associated with ageing and increased predisposition to illness and death (Walford 1969). In aged people, the decline in phagocytic activity is associated with increased susceptibility to infectious agents that contributes to infection-induced morbidity and mortality (Makinodan 1981). A reduction in number of phagocytes coupled with an intrinsic decline of their bactericidal activity has been observed in aged people (Strout and Suttles 2005). Also, there is a significant reduction in production of reactive oxygen intermediate (ROI) species in phagocytic cells (PMNs) in elderly. As a consequence of reduced respiratory burst the elimination of bacteria is hindered, and the elderly have infections of longer duration (Kumar et al. 2002).
Immunological recognition of antigen by macrophages is followed by a series of biochemical events producing plethora of microbicidal agents including reactive oxygen intermediates (ROI) and lytic enzymes. The ROI are generated in a complex process known as respiratory burst, wherein superoxide (O.-2) is initially formed during the reduction of molecular oxygen by single electron originating from NADPH dependent oxidase (also called phagocyte oxidase). This enzyme complex is dormant in resting cells (Babior 1984). Upon activation, the cytosolic proteins translocate to the membrane to form an electron-transfer system leading to O.-2 generation. The highly active O.-2 molecules get rapidly converted into other ROI moieties including hydrogen peroxide (H2O2), hydroxyl radical (OH) and hypochlorous acid (HOCl), through enzyme activities (superoxide dismutase, catalase, myeloperoxidase) (Casimir and Teahan 1994). These active radicals damage extracellular targets as well as destroy phagocytised pathogenic microorganism.
Lactic acid bacteria are well recognized to polarize (strain specifically) host immune status (Cross 2002; Jood et al. 2011). The metabolites produced by the action of probiotics on milk constituents also possess immuno-stimulating potentials (Cavallo et al. 1996). Recent studies showed that administration of Dahi prepared using these strains protects against enteric infection and gastrointestinal carcinogenesis (Rajpal and Kansal 2008, 2009), and ameliorates age related oxidative stress and molecular alterations in mice (Kaushal and Kansal 2011). The present study was conducted to examine the effect of probiotic Dahi prepared using these strains on age related decline in macrophage phagocytic functions in mice.
Materials and methods
Bacterial cultures and Dahi preparation
Lactobacillus acidophilus LaVK2 and Bifidobacterium bifidum BbVK3 are our laboratory isolates with probiotic attributes tested through in vitro tests as per FAO/WHO guidelines (FAO/WHO 2002). Lactococcus lactis ssp. cremoris and Lactococcus lactis ssp. lactis biovar diacetylactis were obtained from National Collection of Dairy Cultures, National Dairy Research Institute, Karnal, India. L. acidophilus LaVK2 and B. bifidum BbVK3 were propagated and maintained in MRS-broth and lactococci in M17 broth. B. bifidum BbVK3 was cultured and propagated in anaerobic conditions. Bacterial cultures were revitalized three times in reconstituted and autoclaved skim milk prior to use for preparation of fermented milk. Buffalo milk (pooled milk from several buffalos) obtained from cattle yard of National Dairy Research Institute, Karnal, adjusted to 3.0% fat (using skim milk), was heated to 90 °C for 15 min and then cooled to 37 °C. Dahi was prepared culturing fat adjusted buffalo milk with Dahi starter (Lc. lactis ssp. cremoris and Lc. lactis ssp. lactis biovar diacetylactis, 1% each) at 30 °C for 8 h. Probiotic La-Dahi was prepared culturing standardized buffalo milk with L. acidophilus LaVK2 and Dahi starter. For preparation of LaBb-Dahi, a mixture of L. acidophilus LaVK2, B. bifidum BbVK3 and Dahi starter was employed. The final bacterial counts in fermented milk product was determined by the method described elsewhere (Terzaghi and Sandine 1975; Dave and Shah 1996). The final product contained lactococci, 1–2 × 109 cfu/g, L. acidophilus, 2–20 × 107 cfu/g and B. bifidus 2–20 × 107 cfu/g.
Animals and diet
Male Swiss albino mice obtained from Small Animal House of National Dairy Research Institute, Karnal, India were grown on animal stock diet upto the age of 4 months (young group) and 12 months (ageing group). Guidelines for the care and use of animals were followed and approved by Ethical Committee of National Dairy Research Institute, Karnal, India. The effect of ageing on phagocytic function was evaluated on 4 mo, 12 mo and 16 mo old mice (each group consisted of 6 animals). For evaluation of the effect of probiotic Dahi on phagocytic function, ageing mice (12 mo old) grouped according to their body weights were divided into the following five groups (n = 6) with mean initial body weight 40 ± 2 g : 1) control group, fed basal diet; 2) milk group, fed buffalo milk supplements along with basal diet; 3) Dahi group, fed Dahi supplements along with basal diet; 4) La-Dahi group, fed La-Dahi supplements along with basal diet and 5) LaBb-Dahi group, fed LaBb-Dahi supplements along with basal diet. The animals housed in polypropylene cages in an air conditioned room (24 ± 1 °C) were provided basal diet and water ad libitum for 4 months. Each mouse was fed 5 g test supplement prior to basal diet for a period of 4 months. The basal diet comprised of starch (63%), casein (20%), soybean oil (5.5%), vitamin mixture (1%), mineral mixture (5%), choline chloride (0.2%), cellulose (5%) and methionine (0.2%). Vitamin and mineral mixtures were prepared and mixed according to AOAC (2005).
Macrophages harvest
Peritoneal fluid was harvested from the animals killed by cervical dislocation. The abdominal skin was swabbed with alcohol (70%) and removed to expose peritoneal wall. DMEM Hams F-12 medium was injected into peritoneal cavity, and following a gentle massage of abdomen, the abdominal fluid was collected by aspiration.
Splenic macrophages were isolated by teasing spleen and RBCs were lysed using lysis buffer (one part of 0.17 M Tris HCl plus 9 parts of 0.6 M NH4Cl and pH adjusted to 7.2). The cells plated in 35 mm cell culture plate were incubated at 37 °C for 1 h in an atmosphere of 5% CO2 in air. The adherent cells were released by jetting chilled DMEM on to the cells and counted using Neubauer’s chamber.
Respiratory burst
For assay of respiratory burst, the aliquots of peritoneal or splenic cell suspensions (100 μl/well) were dispensed in 96-well flat bottom tissue culture microplates and macrophages were allowed to adhere, incubating the plates for 2 h at 37 °C in CO2 incubator (SHEL LAB model no. IR2424, Cornelius, USA) perfused with 5% CO2 in air. Non-adherent cells (erythrocytes and lymphocytes) were removed, and the adhered cells (established macrophages) were assayed for hydrogen peroxide and superoxide production in vitro.
The intracellular superoxide ion production was estimated using opsonized zymosan (1 mg/ml, from Saccharomyces cerevisiae) as stimulant and following NBT test (Choi et al. 2006). Briefly, aliquots (100 μl/well) of NBT solution (1 mg/ml) were added to established macrophages in the presence or absence of opsonized zymosan. The reference wells contained SOD (superoxide dismutase) added into NBT solution (300 U/ml). The NBT deposited inside the cells were dissolved (120 μl of 2 M KOH and 140 μl of DMSO) by gentle shaking for 10 min at room temperature and read against blank using microplate reader (Microscan, EC Co., India Pvt Ltd., Hyderabad) at 620 nm and results expressed as A620nm per mg macrophage protein.
Assay of extra cellular superoxide ion production follows the reduction of ferricytochrome c, the specificity of which being controlled by its inhibition by superoxide dismutase (Pick 1986). Briefly, established macrophages were incubated with ferricytochrome c (100 μl of 160 μM) with the reference wells containing SOD solution (30 units/well) to preserve specificity of reaction. After 2 h of incubation at 37 °C in an atmosphere of 5% CO2 in air, the plates were read at 550 nm, and O.-2 (superoxide) production was calculated using molar extinction coefficient of cytochrome c 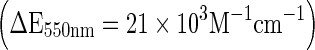 .
.
Assay of hydrogen peroxide production follows horseradish peroxidase-dependent oxidation of phenol red (Pick 1986). Briefly, the established macrophages in microplates were incubated with phenol red solution (100 μl of 0.56 mM phenol red and 20 U/ml horseradish peroxidase) in the presence or absence of opsonized zymosan. After 2 h of incubation at 37 °C in atmosphere of 5% CO2 in air, the reaction was interrupted with 1 M NaOH (10 μl/well) and the color was read at 600 nm against the reference prepared adding 1 M NaOH (10 μl) prior to phenol red solution to established macrophages. The concentration of H2O2 was calculated using molar extinction coefficient of oxidized minus native phenol red at 600 nm 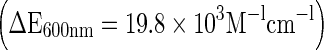 .
.
Protein content in macrophages
The macrophages in microplate wells were dissolved in 1 M NaOH (0.05 ml) incubating overnight at 37 °C and the protein concentration determined (Lowry et al. 1951) using bovine serum albumin as a standard.
Adherence activity of macrophages
Aliquots (500 μl) of a peritoneal cell suspension were incubated in centrifuge tubes (1.5 ml) at 37 °C in a shaking water bath for 60 min. Non-adherent macrophages were counted in the supernatant and adherence index calculated (Forner et al. 1994).
In-vitro phagocytosis assay
The phagocytic activity of peritoneal macrophages was determined using sonicated yeast cells (Saccharomyces cerevisiae) as foreign particles as described earlier (Rajpal and Kansal 2009). The phagocytosis index calculated as percentage of macrophages containing one or more yeast cells times the mean number of yeast cells.
Statistical analysis
Results are expressed as mean ± SE (n = 6). Analysis of variance was performed using GraphPad PRISM version 5.0 statistical software package and the difference between groups was tested using Tukey–Kramer post-hoc test. The critical level of statistical significance for all tests was p < 0.05.
Results and discussion
Reactive oxygen intermediates (ROI) production by peritoneal and splenic macrophages
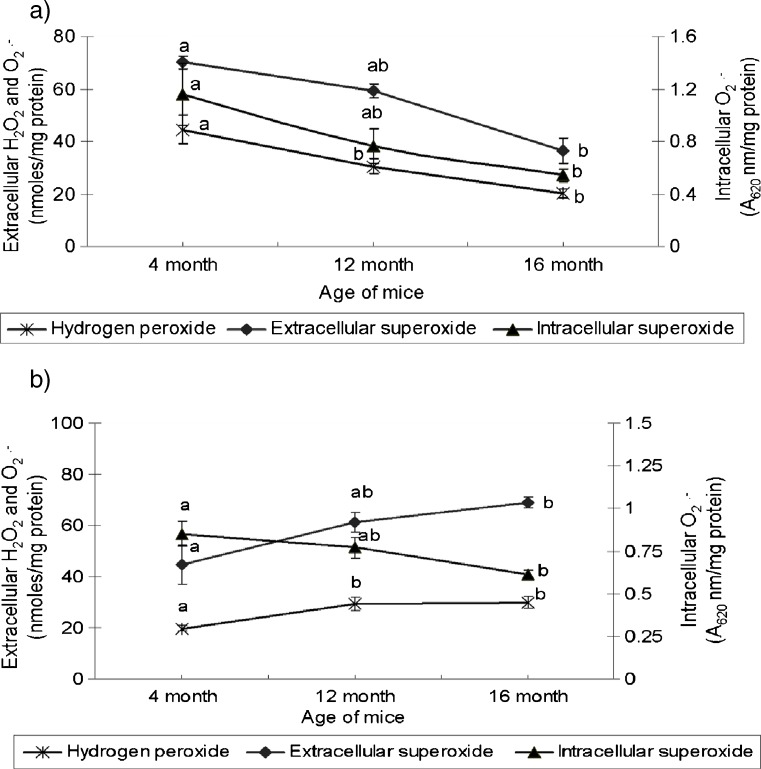
A decline in ability of peritoneal macrophage to produce reactive oxygen intermediates was observed in ageing mice (Fig. 1a). The production of hydrogen peroxide by peritoneal macrophages declined progressively in 12 mo (31.5%) and 16 mo (57.5%) old mice, opposed to 4 mo old ones. A similar decline in extracellular and intracellular O.-2 production was also observed in aged mice, relative to their young counterparts. These observations are concurrent with previous report (Lavie et al. 1992).
Fig. 1.
Age related changes in extra cellular hydrogen peroxide (H2O2) and superoxide radical (O.-2) and intracellular O.-2 production by mouse (a) peritoneal and (b) splenic macrophages. Values (mean ± SE for n = 6), expressed per mg macrophage protein, among age groups with different superscript letters are significantly different (p < 0.05)
Contrary to peritoneal macrophages, the production of ROI by splenic macrophages increased in aged mice. The production of hydrogen peroxide by splenic macrophages from 12 to 16 mo old mice increased by 48–57%, when compared with 4 mo old animals. The increase in extra cellular O.-2 production by splenic macrophages from 12 to 16 mo aged mice was 37.4–54.5%. The production of intracellular O.-2 decreased with age, however, the difference among three age groups was statistically non significant (Fig. 1b). Similar dysregulation of peritoneal and splenic macrophage functions has been reported in tumor bearing animals suggesting this might be responsible for predisposing aged animals to spontaneous tumor development (Gardner et al. 1995).
The difference in ROI production potential of peritoneal and splenic macrophages could be due to microenvironment factors that contribute to differential modulation of macrophage functions in different tissues including initiating or inhibitory signals such as cytokines, antioxidant status and hormones (Hayakawa et al. 1995; Kohut et al. 2004). Enhanced H2O2 production in spleen suppresses lymphocyte proliferative function and could be one of the factors responsible for loss of splenic lymphocyte proliferative functions in aged (Metzger et al. 1980).
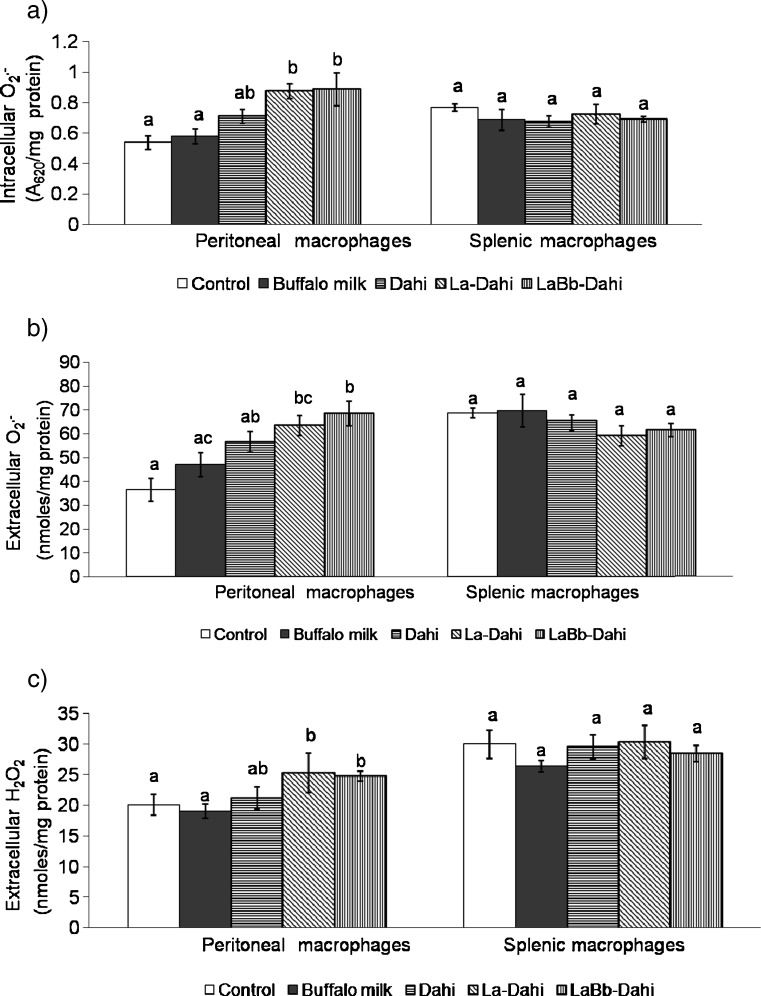
Feeding ageing mice with Dahi containing potential probiotic bacterial strains significantly enhanced the ability of peritoneal macrophages to produce ROI. The production by peritoneal macrophages of intracellular superoxide increased by over 60% in probiotic Dahi fed groups (Fig. 2a), and that of extracellular O.-2 by 73.7% and 87.6% in La-Dahi and LaBb-Dahi fed groups, respectively, compared to the age matched control group (Fig. 2b). Also, an increasing trend in production of extracellular hydrogen peroxide was seen in groups fed with La-Dahi or LaBb-Dahi, compared to age matched control group (Fig. 2c). The changes in gut flora by feeding probiotic strains might have played a role in stimulating immunocyte (macrophage) functions. A decline in superoxide anion (O-2) producing ability of phorbol myristate acetate stimulated peritoneal macrophages was reported in germ free mice opposed to age-matched conventional mice (Mitsuyama et al. 1986). Supplementation of lactic acid bacterial cultures, L. reuteri, L. johnsonii and L. animalis are reported to enhance ROI production by mouse peritoneal macrophages (Marcinkiewicz et al. 2007) and by fish head kidney leucocytes (Panigrahi et al. 2005). Contrary to peritoneal macrophages, splenic macrophages from mice fed La-Dahi, LaBb-Dahi, regular Dahi or buffalo milk exhibited no increase in ROI production (Fig. 2).
Fig. 2.
Effect of feeding cultured milk preparations to aged mice on production of (a) intracellular superoxide radical (O.-2), (b) extra cellular O.-2 and (c) extra cellular hydrogen peroxide (H2O2) by mouse peritoneal and splenic macrophages. Values (mean ± SE for n = 6), expressed per mg macrophage protein, with different superscript letters are significantly different (p < 0.05)
Phagocytic activity and adherence index of peritoneal macrophages
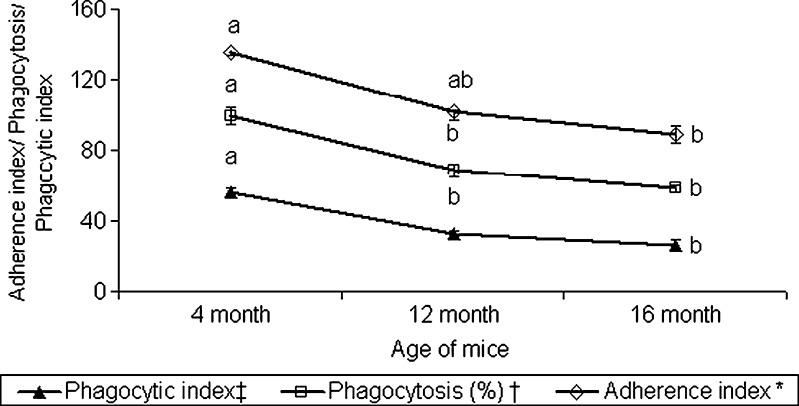
Adherence of phagocytes is the first event to the inflammatory response, and their adherence to plastic surface is comparable to tissue adherence capacity (Noga et al. 1984). The percentage of active phagocytic population (percent phagocytosis) in peritoneal exudate and their phagocytic index declined significantly in 12 and 16 mo old mice, compared to their 4 mo old counterparts (Fig. 3). A significant decline in the adherence index was also observed in 16 mo old mice. Concurrent with our observation, Ortega et al. (2000) observed a significant decline in adherence index during 22–48 weeks of age in female balb/c mice. The decline in adherence index of peritoneal macrophages with ageing could be because of decline in the ability of macrophages to form pseudopods (Johnson et al. 1978), and decline in expression of various cell adhesion factors (ICAM-1 or VCAM-1) as reported in case neutrophils and monocytes of elderly (De Martinis et al. 2004). The decline in adherence index of peritoneal macrophages with ageing correlated with a decline in phagocytic efficiency and diminished production of ROI as reported earlier (Nagel et al. 1986).
Fig. 3.
Age related changes in adherence and phagocytic indices of mouse peritoneal macrophages. * Adherence index: Percentage of macrophages attached to the plastic surface. † Phagocytosis: Number of macrophages with yeast cells internalized per 100 macrophages. ‡ Phagocytic index: Percentage of macrophages containing one or more yeast cells times the mean number of yeast cells per positive macrophage. Values (mean ± SE for n = 6) among age groups with different superscript letters are significantly different (p < 0.05)
The ability of peritoneal macrophages to adhere to substrate (adherence index) increased significantly (15–20%) in mice by feeding probiotic Dahi preparations. Though feeding La-Dahi, LaBb-Dahi, regular Dahi or buffalo milk to ageing mice did not alter percent phagocytosis, the phagocytic index increased significantly by feeding former two preparations containing potential probiotic bacterial strains (Table 1). The effect well correlated with their improved ability to produce ROI. Free radicals have been reported to increase the expression of various adhesion factors on macrophage surface (Breviario et al. 1988; Cheng et al. 1988). The effect of lactic acid bacteria on phagocytosis is strain specific. The mice fed with S. thermophilus were observed to have lower ability to clear colloidal carbon than the mice fed with L. casei, L. bulgaricus, L. acidophilus or milk fermented with a mixture of L. casei and L. acidophilus (Perdigon et al. 1995). Structural differences in cell wall composition of lactobacilli strains are suggested to be responsible for the observed difference in their efficacy to stimulate phagocytic activity of macrophages (Erickson and Hubbard 2000).
Table 1.
Effect of fermented milk preparations on phagocytic activity of mouse peritoneal macrophages
| Phagocytosisd (%) | Phagocytic indexe | Adherence indexc | |
|---|---|---|---|
| Control | 32.1a ± 1.99 | 26.1a ± 1.88 | 30.6a ± 0.98 |
| Buffalo milk | 34.1a ± 0.89 | 28.0ab ± 0.99 | 34.3ab ± 0.99 |
| Dahi | 32.1a ± 1.10 | 30.0ab ± 1.01 | 34.8ab ± 1.38 |
| La-Dahi | 32.8a ± 0.64 | 31.5b ± 1.38 | 35.8b ± 0.95 |
| LaBb-Dahi | 35.1a ± 2.02 | 38.3b ± 4.42 | 35.1b ± 0.68 |
a,bValues (mean ± SE for n = 6) with different superscript letters are significantly different (p < 0.05)
cAdherence index: Percentage of macrophages attached to the plastic surface
dPhagocytosis: Number of macrophages with yeast cells internalized per 100 macrophages
ePhagocytic index: Percentage of macrophages containing one or more yeast cells times the mean number of yeast cells per positive macrophage
Conclusion
The present study shows that the Dahi preparation containing selected probiotic strains of Lactobacillus acidophilus LaVK2 or combined L. acidophilus LaVK2 and Bifidobacterium bifidum BbVK3 can potentially ameliorate age induced deficits in phagocytic activity of peritoneal macrophages with corresponding increase in production of reactive oxygen species. Exploring traditional dairy products for delivery of probiotic bacteria is an interesting perspective for stimulation of immune functions in aged population. Further studies are needed to explore the possible mechanism of action of probiotics in immune stimulation and delaying the process of immunosenescence associated with ageing.
Acknowledgement
The authors thankfully acknowledge the Council of Scientific and Industrial Research (CSIR), New Delhi, for awarding fellowship grants to one of the authors (Deepti Kaushal).
References
- AOAC (2005) In W. Horowitz (Ed.), Official methods of analysis (18th ed.). Gaithersburg, MD: Association of Official Analytical Chemists 45:75–76
- Babior BM. Oxidants from phagocytes: agents of defense and destruction. Blood. 1984;64:959–966. [PubMed] [Google Scholar]
- Breviario F, Bertocchi F, Dejana E, Bussolino F. IL-1 induced adhesion of polymorphonuclear leukocytes to cultured human endothelial cells. Role of platelet-activating factor. J Immunol. 1988;141:3391–3397. [PubMed] [Google Scholar]
- Casimir CM, Teahan CG. The respiratory burst of neutrophils and its deficiency. In: Hellewell PG, Williams TJ, editors. Immunopharmacology of neutrophils. London: Academic; 1994. pp. 27–54. [Google Scholar]
- Cavallo MG, Fava D, Monetini L, Barone F, Pozzilli P. Cell-mediated immune response to beta-casein in recent onset insulin-dependent diabetes: implications for disease pathogenesis. Lancet. 1996;348:926–928. doi: 10.1016/S0140-6736(95)12065-3. [DOI] [PubMed] [Google Scholar]
- Cheng JJ, Wung BS, Chao YJ, Wang DL. Cyclic strain-induced reactive oxygen species involved in ICAM-1 gene induction in endothelial cells. Hypertension. 1988;31:125–130. doi: 10.1161/01.HYP.31.1.125. [DOI] [PubMed] [Google Scholar]
- Choi HS, Kim JW, Cha YN, Kim C. A quantitative nitroblue tetrazolium assay for determining intracellular superoxide anion production in phagocytic cells. J Immunoassay Immunochem. 2006;27:31–44. doi: 10.1080/15321810500403722. [DOI] [PubMed] [Google Scholar]
- Cross ML. Microbes versus microbes: immune signals generated by probiotic lactobacilli and their role in protection against microbial pathogens. FEMS Immunol Med Microbiol. 2002;34:245–253. doi: 10.1111/j.1574-695X.2002.tb00632.x. [DOI] [PubMed] [Google Scholar]
- Dave RI, Shah NP. Evaluation of media for selective enumeration of Streptococcus thermophilus, Lactobacillus delbrueckii subsp. bulgaricus, Lactobacillus acidophilus and Bifidobacterium sp. J Dairy Sci. 1996;79:1529–1536. doi: 10.3168/jds.S0022-0302(96)76513-X. [DOI] [PubMed] [Google Scholar]
- De Martinis M, Modesti M, Ginaldi L. Phenotypic and functional changes of circulating monocytes and polymorphonuclear leucocytes from elderly persons. Immunol Cell Biol. 2004;82:415–420. doi: 10.1111/j.0818-9641.2004.01242.x. [DOI] [PubMed] [Google Scholar]
- Erickson KL, Hubbard NE. Probiotic immunomodulation in health and disease. J Nutrition. 2000;130:403S–409S. doi: 10.1093/jn/130.2.403S. [DOI] [PubMed] [Google Scholar]
- FAO/WHO (2002) Guidelines for evaluation of probiotics in food. Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food. London, Ontario, Canada, April 30 and May 1, 2002
- Forner MA, Collazos ME, Barriga C, De la Fuente M, Rodríguez AB, Ortega E. Effect of age on adherence and chemotaxis capacities of peritoneal macrophages. Influence of physical activity stress. Mech Ageing Dev. 1994;75:179–189. doi: 10.1016/0047-6374(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Gardner TE, Naama H, Daly JM. Peritoneal and splenic macrophage functions in the tumor-bearing host. J Surg Res. 1995;59:305–310. doi: 10.1006/jsre.1995.1169. [DOI] [PubMed] [Google Scholar]
- Hayakawa H, Sato A, Yagi T, Uchiyama H, Ide K, Nakano M. Superoxide generation by alveolar macrophages from aged rats: improvement by in vitro treatment with IFN-γ. Mech Ageing Dev. 1995;80:199–211. doi: 10.1016/0047-6374(95)01573-I. [DOI] [PubMed] [Google Scholar]
- Johnson DR, Fernandes G, Douglas SD. Age related decline in cytoplasmic spreading of mouse peritoneal macrophages. Dev Comp Immunol. 1978;2:347–354. doi: 10.1016/S0145-305X(78)80077-9. [DOI] [PubMed] [Google Scholar]
- Jood S, Khetarpaul N, Goyal R (2011) Efficacy of barley based probiotic food mixture in treatment of pathogenic E.coli induced diarrhoea in mice. J Food Sci Technol. doi:10.1007/s13197-011-0270-y [DOI] [PMC free article] [PubMed]
- Kaushal D, Kansal VK (2011) Probiotic Dahi containing Lactobacillus acidophilus and Bifidobacterium bifidum alleviates age-inflicted oxidative stress and improves expression of biomarkers of ageing in mice. Mol Biol Rep. doi:10.1007/s11033-011-0920-1 [DOI] [PubMed]
- Kohut ML, Senchina DS, Madden KS, Martin AE, Felten DL, Moynihan JA. Age effects on macrophage function vary by tissue site, nature of stimulant, and exercise behavior. Exp Gerontol. 2004;39:1347–1360. doi: 10.1016/j.exger.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Kumar P, Pai K, Pandey H, Sundar S. NADH-oxidase, NADPH-oxidase and myeloperoxidase activity of visceral leishmaniasis patients. J Med Microbiol. 2002;51:832–836. doi: 10.1099/0022-1317-51-10-832. [DOI] [PubMed] [Google Scholar]
- Lavie L, Weinreb O, Cershon D. Age-related alterations in superoxide anion generation in mouse peritoneal macrophages studied by repeated stimulations and heat shock treatment. J Cell Physiol. 1992;152:382–388. doi: 10.1002/jcp.1041520220. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin Phenol Reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Makinodan T (1981) Cellular basis of immunologic aging. In: Schimke RT (ed) Biologic Mechanisms in Aging, USDA, NIH, pp 488–500
- Marcinkiewicz J, Ciszek M, Bobek M, Strus M, Heczko PB, Kurnyta M, Biedroń R, Chmielarczyk A. Differential inflammatory mediator response in vitro from murine macrophages to lactobacilli and pathogenic intestinal bacteria. Int J Exp Pathol. 2007;88:155–164. doi: 10.1111/j.1365-2613.2007.00530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger Z, Hoffeld JT, Oppenheim JJ. Macrophage-mediated suppresion I. Evidence for participation of both hydrogen peroxide and prostaglandin of supperssion in murine lymphocyte proliferation. J Immunol. 1980;124:938–988. [PubMed] [Google Scholar]
- Mitsuyama M, Ohara R, Amako K, Nomoto K, Yokokura T. Ontogeny of macrophage function to release superoxide anion in conventional and germfree mice. Infect Immun. 1986;52:236–239. doi: 10.1128/iai.52.1.236-239.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel JE, Han K, Coon PJ, Adler WH, Bender BS. Age differences in phagocytosis by polymorphonuclear leukocytes measured by flow cytometry. J Leukoc Biol. 1986;39:399–407. doi: 10.1002/jlb.39.4.399. [DOI] [PubMed] [Google Scholar]
- Noga SJ, Normann SJ, Weiner RS. Methods in laboratory investigation. Isolation of guinea pig monocytes and Kurloff cells: characterization of monocyte subsets by morphology, cytochemistry, and adherence. Lab Invest. 1984;51:244–252. [PubMed] [Google Scholar]
- Ortega E, Garcia JJ, De la Fuente M. Ageing modulates some aspects of the non-specific immune response of murine macrophages and lymphocytes. Exp Physiol. 2000;85:519–525. doi: 10.1017/S0958067000020509. [DOI] [PubMed] [Google Scholar]
- Panigrahi A, Kiron V, Puangkaew J, Kobayashi T, Satoh S, Sugita H. The viability of probiotic bacteria as a factor influencing the immune response in rainbow trout Oncorhynchus mykiss. Aquaculture. 2005;243:241–254. doi: 10.1016/j.aquaculture.2004.09.032. [DOI] [Google Scholar]
- Perdigon G, Alvarez S, Rachid M, Aguro G, Gobbato N. Immune system stimulation by probiotics. J Dairy Sci. 1995;78:1597–1606. doi: 10.3168/jds.S0022-0302(95)76784-4. [DOI] [PubMed] [Google Scholar]
- Pick E. Microassays for superoxide and hydrogen peroxide production and nitroblue tetrazolium reduction using an enzyme immunoassay microplate reader. Methods Enzymol. 1986;132:407–421. doi: 10.1016/S0076-6879(86)32026-3. [DOI] [PubMed] [Google Scholar]
- Rajpal S, Kansal VK. Buffalo milk probiotic Dahi containing Lactobacillus acidophilus, Bifidobacterium bifidum and Lactococcus lactis reduces gastrointestinal cancer induced by dimethylhydrazine dihydrochloride in rats. Milchwissenschaft. 2008;63:122–125. [Google Scholar]
- Rajpal S, Kansal VK. Probiotic Dahi containing Lactobacillus acidophilus and Bifidobacterium bifidum stimulates immune system in mice. Milchwissenschaft. 2009;64:147–150. [Google Scholar]
- Strout RD, Suttles J. Immunosenescence and macrophage functional plasticity: dysregulation of macrophage function by age-associated microenvironmental changes. Immunol Rev. 2005;205:60–71. doi: 10.1111/j.0105-2896.2005.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzaghi BE, Sandine W. Improved medium for lactic acid streptococci and their bacteriophages. Appl Microbiol. 1975;29:807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walford RL. The Immunologic theory of aging. Copenhagen: Munksgaard; 1969. [Google Scholar]