Abstract
The changes occurring in rice bran oil and its blend with sunflower oil during repeated frying cycles of dried and moist potato chips were monitored. The parameters assessed were: Colour, Refractive Index, SpecificGravity, Oryzanol Value, Free fatty acid, Iodine Value, Peroxide value, anisidine value, Saponification Value, trans fats and fatty acid composition. No significant changes (p≤0.05) were observed in the refractive index and specific gravity of rice bran oil, sunflower oil and their model blend. The colour of blended oil was lesser than RBO and the intensity of color increased after each frying cycle during the deep fat frying of moistened and dried potato chips. The oryzanol content and iodine value decreased with the frying cycles. The decrease in oryzanol value during the frying operation was more prominent in rice bran oil as compared to the blended oils. The increase in p-anisidine value was more in rice bran oil as compared to blended oil. No significant changes (P<0.05) in the myristic, palmitic and stearic acid composition was observed during the repeated deep fat frying cycles in both the rice bran oil and blended oils samples. The amount of unsaturated fatty acid decreased gradually during repeated deep fat frying cycles in both the oils. The trans fat increased with repeated deep fat frying cycles in both the rice bran and blended oils, when used to fry moistened and dried potato chips. Both the oil samples showed greater formation of trans fatty acids when the moistened potato chips were used during frying.
Keywords: Deep fat frying, Potato chips, Trans fats, Oryzanol content, P-anisidine value
Introduction
RBO contains unique component oryzanol which is linked with increase in good cholesterol and lowering down of bad cholesterol and triglycerides and also possesses high levels of phytosterols, gamma-oryzanol, tocotrienols as well as tocopherols (Taylor et al. 1996). These naturally occurring components impart a high resistance to thermal oxidation and deterioration of the oil. The high oxidative stability of RBO makes it preferred oil for frying and baking applications (Semwal and Arya 2001). Sunflower oil which consists of a combination of monounsaturated and polyunsaturated fats with low saturated fat levels is valued for its light taste, frying performance and health benefits. The relationship between oil temperature, viscosity and convective heat transfer coefficient is very much important in processed food industries and for process modelling. The physical properties of edible oils, such as viscosity and heat transfer, could be the primary factors that influence variation in fried food quality. However, there is a worldwide demand for the production of blended oil for culinary uses because of increasing consumer awareness, improved thermal stability, nutritional benefits, cheaper alternatives or substitutes to pure vegetable oils and ability to tailor the desired properties. According to Goburdhun and Jhurree (1995), frying of chips using soybean oil blended with palm kernel olein is stable in terms of hydrolysis of fats, oxidation of fats and rancidity as compared to pure soybean oil. Xu et al. (2000) reported that a blend of high oleic acid canola oil and palmolein showed higher oxidative stability, less free fatty acid and polar compound formation as well as higher heat stability than the palm olein alone. According to Su and White (2004), blending of soybean oil with high-oleate soybean oil showed a significant effect on color and oxidative stability during frying of bread cubes. However, extensive research has been carried out to describe the chemical changes in frying oils, but very few have attempted to relate the physical changes to the thermal properties of the different oils and oil blends. Blended frying oils provide desired low levels of viscosity and higher levels of heat transfer properties than the naturally occurring one and, therefore, are physico-thermally better suited for frying. Because blended oils were found to show lesser time to reach the frying temperature and consequently higher heat transfer coefficient, this may be a cost-effective proposition in the fried food industries. Therefore, by blending two oils in different proportions, a range of viscosities and heat transfer coefficients can be successfully achieved (Sukumar et al. 2008).
Deep fat frying is an important, ubiquitous and highly versatile process, which has been used since antiquity to cook a wide spectrum of products and is also considered a complex process involving a number of factors such as the actual process itself, the frying fat and the nature of the food to be fried (Varela et al. 1988). It involves a unique food processing operation, wherein the frying medium becomes a constituent of the fried food. Blended oils are gaining popularity worldwide due to advantages they offer such as improved thermal stability, oxidative stability, nutritional benefits (Sharma et al. 1996a) and an ability to tailor the desired properties. Also, they are considered cheaper alternatives or substitutes to pure vegetable oils. The blends of 50% palm kernel olein with 50% coconut oil were suitable, if crude oils with a coconut flavor were desired (Lee and Timms 1988). The pattern of oil uptake constituents during the frying of dehydrated potato chips has been reported (Sharma et al. 1996b). Premavalli et al. (1998) investigated the storage and thermal stabilities of refined cottonseed oil-mustard seed oil blends (80:20). Comparative studies on physical properties of vegetables oils and their blends after frying indicated a minimization in peroxide value using blended oils (Susheelamma et al. 2002). Repeated deep-fat frying was done by using dried potato chips in pure RBO (rice bran oil), SFO (safflower oil) and their model blends to study their thermal behavior. Pure RBO and SFO showed good thermal stability during the repeated deep-fat frying cycles. Although, all the blended oils, showed good thermal stability during repeated deep-fat frying cycles yet model blends constituting 20% RBO + 80% SFO showed better suitability during repeated deep-fat frying than other blended oil samples (Singh et al. 2007). The foods products containing varied amount of moisture are fried in the oil as a result alter the frying behavior. It is therefore very important to explore the changes in frying behavior of the blended oil, when used as a media to fry the foods consisting of varied amount of moisture. The present study was therefore aimed at exploring the frying behavior of blended oils, prepared from sunflower oil and rice bran oil, when used to fry the foods consisting of varied amount of moisture.
Materials and methods
Physically refined rice bran oil and sunflower oil samples were procured from the leading oil industry A. P. Organics Ltd located in Dhuri, Punjab, India. Rice bran oil and sunflower oil blend was prepared in the ratio of 60% rice bran oil (RBO) and 40% SnFO (sunflower oil) as the ratio had shown the better suitability during repeated deep fat frying than the remaining blended oils (Sharma et al. 2006). The oils in the specific proportions were blended using high shear mixer. The blending was carried out at 150 rpm and 60 °C temperature for 30 min.
Preparation of potato chips
The potato (variety-Kufri Jyoti). chips were prepared from the conventional method consisting of scrubbing, washing and then slicing using the slicer. The slices were immersed in water and washed repeatedly till the removal of starch. The washed slices were then dipped in the boiled water, consisting of salt, 1% for nearly 20 min. The slices obtained were wrapped in ordinary filter paper to remove the surface water. These potato chips, having the moisture of 64.77% were used for frying under the designed conditions.
To observe the frying behavior of the moistened chips and the dried chips, the drying of the potato chips was carried out under the sun (till the moisture of 1.09%) followed by drying in an oven at a temperature of 80° C till the constant weight. The finally dried chips obtained had the moisture of 0.5% (wb). Thickness of dried potato chips and moistened chips was 1.28 mm and 2.29 mm respectively.
Deep fat frying of potato chips
The deep fat frying process was carried out in the similar way as reported by Sharma et al. (2006). The frying of a known weight (50 g) of potato chips was carried out by drawing 500 ml of oil sample from control as well as blended oils separately in a frying domestic frier (diameter 28 cm, depth 6 cm) at a deep fat frying temperature of 210 °C for 8 s. Deep fat frying time and temperatures were decided based on preliminary experiments carried out in the laboratory. Another frying operation was carried out in 500 ml of oil under the same frying conditions. After frying, the oil samples from control as well as blended oils were cooled to room temperature and stored separately in PET bottles for 3 days for further frying. After 3 days, 80 ml of oil sample was taken from control and blended oils for analyzing physico-chemical parameters and lipid profile. After every frying cycle, the volume of all oil samples was again made up to 500 ml by adding oil from another frying operation carried out under similar conditions. The same frying processes were repeated six times with each and every oil sample after successive storage of oils for 3 days and withdrawing 80 ml oil sample. The total time for an experiment was 18 days.
Analytical procedures
After each frying cycle, the oil samples were analyzed for Free fatty acids (FFA) by titrating the free fatty acid with alkali in presence of ethyl alcohol as solvent [Ca 5a-40], Peroxide value (PV) was estimated by using sodium thiosulfate solution as titrating agent against the evolved iodine in the sample, after reacting the peroxides present in the sample with salt of iodine (KI) [Cd 8–53], Iodine value (IV) was determined by treating the sample with an excess of solutions of iodine monochloride (ICl) in glacial acetic acid. Unreacted iodine monochloride reacted with potassium iodide, converting it to iodine, whose concentration was determined by titration with sodium thiosulphate [Cd 1–25], Saponification value (SV) was determined by treating the sample with alkali and the unreacted parafins were then titrated against 0.5 N hydrochloric acid [Cd 3–25] and Refractive index (RI) was determined by using refractrometer with temperature adjusted to 37 °C [Cc 7–25]. The phosphorus content was determined spectrophotometricaly. The procedure was based on formation of ionic—associate of molybdophosphate with zinc oxide in an acidic medium and compared with the standard vanadomolybdate and molybdenum blue methods [Ca 12–55]. The p-anisidine value of the sample was estimated by using iso-octane as the solvent under 350 nm of wavelength [Cd 18–90] and specific gravity [Cc 10a-25] was determined by using standard methods (AOCS 2004). Color of the oil was measured by using Lovibond tintometer (Model F, Effem Technologies Pvt. Ltd., New Delhi, India). Oryzanol value of the sample was measured by using Spectrophotometer (UV-1700, Shimadzu Scientific Instruments, Columbia, North America) at 350 nm in presence of n-heptane solvent (Vijayalakshmi 2008). The presence of starch was indicated by the appearance of blue color from the addition of a drop of one percent iodine solution.
Determination of trans fats and fatty acid composition
Fatty acids of triglycerides were analyzed by preparing methyl esters according to a conventional procedure consisting of saponification followed by acidification and finally methylation using diazomethane as per the reported method (Sharma et al. 2006). The contents of trans-fatty acids were calculated by composition analysis using an integrator. Peaks of trans fats were identified by comparing their retention time with those of predetermined standards. Trans fatty acids mainly consisted of C18:1 t, C18:2 t, C18:3 t and C20:1 t and were expressed as percentages rounded to the second decimal place.
Methyl esterification
Methyl Esterification of samples used in the analyses was performed by BF3-MeOH method after alkaline hydrolysis. Mixture of 0.3 g of sample oils and 10 ml of NaOH-methanol solution was heated at 80–90 °C for 20 min. After cooling, 5 ml of BF3-MeOH reagent was added then the vessel was sealed and heated at 80–90 °C for 5 min. After cooling, 10 ml each of petroleum and saturated NaCl solution were added, followed by a thorough shaking. The resulting petroleum ether layer was used as a sample solution for GC.
The analysis of fatty acid methyl esters was carried out using Gas chromatography (GC) (Agilent Technologies J&W, 7820A) with full electronic pneumatics control (EPC) for inlets and detectors, ensuring excellent reproducibility, as well as reliable accuracy and precision. A DB-23 capillary column of high polarity phase (Agilent Technologies J&W) with 60 m length, 0.250 μm internal diameter, and 0.25 μm film thicknesses was used. This column is specially designed for trans fat estimation and was made from fused silica (stationary phase) and were coated with a thin uniform liquid phase (50% Cyanopropyl- methylpolysiloxane). The injector and detector temperatures were maintained at 250 °C. The column temperature was set at 140 °C for 5 min and then ramped at a rate of 6 °C/min to 180 °C and then kept for 7 min, then again ramped at a rate of 2 °C/min to 230 °C and kept for 17 min. The total time for analysis was 60 min. Fatty acids were tentatively identified by comparison with retention times of authentic reference samples.
Estimation of oil uptake by the fried food products
The extraction of fat in presence of hexane 100 ml/5g sample, (solvent) was carried out by rapid Soxtec extractor (Socs Plus, Pelican Equipment, Model No. SCS-6, Chennai) and the percent oil uptake was determined on the basis of the sample taken and residue left out after extraction.
Statistical analysis
The ANOVA and Tukey’s test was applied to the experimental data. The Microsoft office excel software was used for analysis. Three replicates were conduct for each analysis.
Results and discussion
The initial quality of rice bran, sunflower and blended (60%RBO and 40% SnFO) oil sample is shown in Table 1. Rice bran oil sample had trans fat and p-anisidine value in the proportion of 1.27% and 46.02 whereas sunflower oil had 0.50% and 9.05 respectively. The oryzanol content of rice bran oil was 15,196 ppm whereas it was found absent in sunflower oil as expected. Blended oil sample had trans fat, oryzanol value and p-anisidine value in the proportion of 1.15%, 9812 ppm and 39.11 respectively while the free fatty acid (ffa), peroxide value (PV) iodine value (IV), specific gravity, color, saponification value (SV), p-content and refractive index (RI) were found as per the regulatory standards (Codex Alimentarius Commission 2007). The major fatty acids composition, C14:0, C16:0, C18:0, C18:1, C18:2, C18:3 were found in the reported range (Sharma et al. 2006).
Table 1.
Behavior of rice bran and blended oil (RBO: SUNF – 60:40) after frying the potato chips having moisture 0.5% (wb)
| Parameter | Sample | Control | After 1st frying | After 2nd frying | After 3rd frying | After 4th frying | After 5th frying | After 6th frying |
|---|---|---|---|---|---|---|---|---|
| FFA | RBO | 0.08a | 0.09acf | 0.09acfh | 0.10acfhk | 0.11acfhkl | 0.12bcfhklm | 0.13bdgjklm |
| Blend | 0.07d | 0.09dg | 0.09dgi | 0.10dgik | 0.10dgikl | 0.11fgiklm | 0.13fhjklm | |
| Colour | RBO | 10a | 10.50ac | 10.50ace | 11.50aceg | 12acegh | 13bdfghj | 13.50bdfghj |
| Blend | 9.00a | 9.00af | 9.50afh | 10.00afhj | 10.50afhjk | 10.50afhjkl | 11.50dgijkl | |
| PV | RBO | 0.59a | 1.02af | 1.58afh | 2.04afhk | 2.57bfhkl | 2.99bghklp | 3.61bgjklp |
| Blend | 1.49a | 1.89ae | 2.43aeg | 2.85aegk | 3.07aegkl | 3.66dfgklt | 4.01dfgklt | |
| IV | RBO | 99.88a | 98.19ad | 97.12adf | 95.31adfh | 93.11cdfhk | 92.79cdfhkt | 90.21ceghkt |
| Blend | 105.60a | 103.06ac | 100.65acf | 99.15acfg | 98.09acfgh | 96.57befghj | 94.91befghj | |
| p-anisidine | RBO | 46.02a | 46.21ac | 46.67acf | 48.33acfg | 51.04bcfgh | 53.94befghk | 56.45befghk |
| Blend | 39.11a | 39.85ac | 41.03acf | 43.47acfh | 46.43acfhk | 50.01bcfhkl | 53.02beghkl | |
| OC | RBO | 15,196a | 15101ac | 14978ace | 14645aceg | 14307acegh | 14088bdeghk | 13914bdfghk |
| Blend | 9812a | 9685ac | 9578acf | 9391acfh | 9261acfhj | 9031befhjk | 8875beghjk | |
| Oil uptake | RBO | 11.05a | 11.79ac | 13.95ace | 14.75acef | 16.28bcefg | 17.24bdefgk | 18.77bdefgk |
| Blend | 8.97a | 10.08af | 11.56afh | 13.32afhk | 13.99afhkl | 15.18eghklp | 17.01egjklp |
*Means within the same rows sharing a common small letter are not significantly different at P < 0.05. FFA free fatty acid (%), colour (unit), PV peroxide value(meq/kg), IV iodine value (Wijs method), p-anisidine value, OC Oryzanol content (ppm), oil uptake (%). Results are average of three individual experiments
Tables 2 and 3 depict the behavior of rice bran oil and its blended oil, based on the various physico-chemical parameters during the repeated frying cycles under the controlled conditions, when used as a medium to fry dried and moistened potato chips. No significant changes (p ≤ 0.05) were observed in the refractive index and specific gravity of rice bran oil, sunflower oil and their model blend during repeated deep fat frying cycles of dried and moistened potato chips.
Table 2.
Behavior of rice bran and blended oil (RBO: SUNF – 60:40) after frying the potato chips having moisture 64.77% (wb)
| Parameter | Sample | Control | After 1st frying | After 2nd frying | After 3rd frying | After 4th frying | After 5th frying | After 6th frying |
|---|---|---|---|---|---|---|---|---|
| FFA | RBO | 0.08a | 0.09ac | 0.10ace | 0.10acef | 0.12bcefg | 0.13bcefgh | 0.14bdefgh |
| Blend | 0.07a | 0.09af | 0.10afh | 0.13afhk | 0.14bfhkl | 0.16bghklt | 0.17bgjklt | |
| Colour | RBO | 10a | 10.50ac | 12acf | 12.50acfg | 13bcfgk | 13bcfgkl | 14.50bdfgkl |
| Blend | 9a | 10ae | 10.50aeg | 11aegh | 11.50aeghj | 12.50ceghjk | 13cfghjk | |
| PV | RBO | 0.59a | 1.86ac | 3.39acf | 4.51acfh | 6.07bcfhk | 7.61beghkl | 8.56begjkl |
| Blend | 1.49a | 2.32af | 3.17afk | 3.92afkl | 4.68afklm | 5.26dfklmt | 6.59dgklmt | |
| IV | RBO | 99.88a | 98.47aq | 96.66aqs | 95.91aqsy | 92.52pqsyl | 91.81pqsylt | 89.02prtylt |
| Blend | 105.60b | 103.43bd | 101.09bdf | 98.21bdfk | 96.53bdfkl | 93.03cefklq | 90.79cegklq | |
| p-anisidine | RBO | 46.02a | 53.21af | 59.43afh | 63.96efhj | 68.51efhjk | 73.69efhjkl | 78.64eghjkl |
| Blend | 39.11a | 44.44af | 48.58afi | 51.47afik | 59.88dfikl | 65.36dfiklm | 70.71dgjklm | |
| OC | RBO | 15,196a | 14,851ac | 14,603ace | 14,296bcef | 14,043bcefg | 13,856bdefgh | 13,622bdefgh |
| Blend | 9812a | 9671ac | 9432acf | 9163acfg | 8874bcfgh | 8624befghj | 8566befghj | |
| Oil uptake | RBO | 12.65a | 13.77ac | 14.81ace | 17.56aceg | 18.60bcegh | 19.82bdeghl | 21.79bdfghl |
| Blend | 11.27a | 11.98af | 13.70afh | 15.32afhl | 16.56afhlm | 18.03eghlmt | 20.26egklmt |
*Means within the same rows sharing a common small letter are not significantly different at P < 0.05. FFA free fatty acid (%), colour (unit), PV peroxide value (meq/kg), IV iodine value (Wijs method), p-anisidine value, OC Oryzanol content (ppm), oil uptake (%). Results are average of three individual experiments
Table 3.
Fatty acid composition and trans fats of pure rice bran and blended oil (RBO: SNFO – 60:40) after frying the dried potato chips (moisture 0.5% wb)
| % Fatty acid composition | Oil sample | Control | After 1st frying | After 2nd frying | After 3rd frying | After 4th frying | After 5th frying | After 6th frying |
|---|---|---|---|---|---|---|---|---|
| C14:0 | RBO | 0.27NA | 0.27NA | 0.28NA | 0.28NA | 0.29NA | 0.29NA | 0.30NA |
| Blend | 0.20NA | 0.20NA | 0.20NA | 0.19NA | 0.19NA | 0.20NA | 0.20NA | |
| C16:0 | RBO | 19.43a | 19.43ac | 19.44ace | 19.43aceg | 19.45acegk | 19.53acegkl | 19.51acegkl |
| Blend | 13.40a | 13.48ab | 13.48abe | 13.37acdf | 13.41abefg | 13.47abefgh | 13.49acdfgi | |
| C18:0 | RBO | 2.20a | 2.22ab | 2.23abd | 2.24abdf | 2.27abdgh | 2.29abdfkj | 2.31aceghj |
| Blend | 2.58a | 2.61ac | 2.63ace | 2.66aceg | 2.67acfhk | 2.69acegkm | 2.73adeglm | |
| C18:1 | RBO | 42.59a | 42.25ac | 42.30ace | 42.05bcef | 41.82bcefg | 41.90bcefgh | 41.65bdefgh |
| Blend | 43.37a | 43.31ac | 43.25ace | 43.33acef | 43.09bcefg | 43.19bcefgh | 43.02bdefgh | |
| C18:2 | RBO | 32.64a | 32.56ac | 32.56ace | 32.40aceg | 32.25bcegh | 32.30bceghk | 32.01bdfghk |
| Blend | 37.87a | 37.72ac | 37.69acg | 37.57acgk | 37.29bcgkl | 37.35bcgklp | 37.11behklp | |
| C18:3 | RBO | 0.99a | 0.96ac | 0.95ace | 0.86aceg | 0.81acegh | 0.75bdeghj | 0.68bdfghj |
| Blend | 0.65a | 0.65ac | 0.62acf | 0.59acfh | 0.59acfhj | 0.55befhjk | 0.57beghjk | |
| Trans Fat (%) | RBO | 1.27a | 1.51ac | 1.69acf | 1.80acfg | 1.99bcfgh | 2.18bcfghj | 2.37befghj |
| Blend | 1.15a | 1.25ad | 1.32adf | 1.40adfh | 1.53adfhk | 1.66befhkl | 1.80begjkl |
*C14:0 -Myristic acid, C16:0 -Palmitic acid, C18:0 -Stearic acid, C18:1 -Oleic acid, C18:2 -Linoleic acid, C18:3 -Linolenic acid. Results are average of three individual experiments. NA Not analysed statistically
Rice bran oil is known for the oryzanol content present in it and the derived potential benefits. However, it is absent in the sunflower oil therefore the resultant blended oils from the rice bran oil and sunflower oils contained lesser oryzanol content. The oryzanol content decreased with the frying cycles in both the oils, when used as frying media for moistened and dried potato chips. However, the decrease during the frying operation was more prominent in rice bran oil as compared to the blended oils. The oryzanol content reduction was more in rice bran oil, when used to fry the moistened potato chips as compared to the dried potato chips. Significant changes in the oryzanol content of rice bran oil and blended oil were observed after 3rd and 4th frying cycles respectively, when used to fry dried and moistened potato chips. The higher moisture content in the potato chips sharply brought changes much earlier in the oryzanol content of rice bran oil, which may be due to the hydrolytic degradation of rice bran oil when exposed to higher temperature. Shin et al. (1997) also found that increased moisture content and extrusion temperature reduces the oryzanol content. Oryzanol (Gamma) acts as an antioxidant in the oil but is lost during the thermal oxidation.
FFA content increased after the deep fat frying cycles but no significant difference (P < 0.05) was observed in the FFA content of RBO and blended oil samples between consecutive frying cycles, 1st to 3rd for the moistened potato chips and 1st to 4th for the dried potato chips. Rice bran oil and blended oil samples obtained after 5th and 6th frying cycles had significantly different FFA composition when used as a media to fry moistened and dried potato chips respectively. The significant changes in the FFA composition after 5th frying cycles in rice bran and blended oils when used to fry moistened potato chips indicate greater formation of free fatty acids as compare to the samples used to fry dried potato chips. However, no sample can be declared inferior in terms of thermal stability on the basis of this parameter even after the sixth frying cycle because all the oil samples had FFA content lower than 0.5 (PFA 1954).
Color value index of blended oil was lesser than rice bran oil and the intensity of color increased after each frying cycle for both dried and moistened products (Tables 2 and 3). The color value of rice bran oil and blended oil increased to 14.5 and 13 (when used to fry moistened product) and 13.5 and 11.5, when used to fry dried product, after the sixth frying cycle compared to the control value of 10 and 9 (Table 1). This may be attributed to the fact that food when fried at a high temperature can introduce various components into the oil such as carbohydrates, phosphates, sulphur compounds, trace metals etc. The formation of nonvolatile decomposition products is due primarily to thermal oxidation and polymerization of the unsaturated fatty acids in fat. Many of these compounds contribute to color formation along with other changes (Whitfield 1992).
Initial peroxide value was higher for blended oil as compared to rice bran oil but the changes in the peroxide value during frying were more prominent in the rice bran oil as compared to the blended oils, when used a medium for the dried and moistened potato chips (Tables 2 and 3). For both rice bran oil and blended oil samples, PV increased with frying cycles. The peroxide value was increased to 8.56 & 6.59 and 3.61 & 4.01 in rice bran oil and blended oil when used to fry moistened and dried potato chips respectively. The peroxide value was significantly different (P < 0.05) after 4th and 5th frying cycles in case of rice bran oil and blended oil respectively during the frying of both types of potato chips under the study. This indicates the better frying stability of blended oils as compare to the pure rice bran oil.
Pure rice bran oil and sunflower oil had iodine values of 99.88 and 111.58 respectively (Table 1). Their model blend had iodine value, 105.60 before frying. The iodine values of rice bran oil and blended oil were gradually decreased during repeated deep fat frying cycles. Pure rice bran oil & blended oil, when used to fry moistened and dried potato chips, iodine values decreased to 89.02 & 90.79 and 90.21 and 94.91 respectively after the sixth repeated frying cycle. The decrease in iodine value may be attributed to the oxidative and thermal degradation reactions during the deep fat frying process (Sharma et al. 2006). The decrease in iodine number was more prominent in case of blended oil as compare to pure rice bran oil. The rice bran oil and blended oil, showed significant changes (P < 0.05) in the iodine values after 4th and 5th repeated deep fat frying cycles, when used to fry dried and moistened potato chips respectively. The data indicated that the quality deterioration started earlier when the product, containing high moisture content was fried.
The p-anisidine value was 46.02 and 9.05 for rice bran oil and sunflower oil respectively (Table 1) whereas the blended oil had 39.11 before frying. Thermal and oxidative degradation of oil leads to the formation of conjugated dienes and p-anisidine (Khan et al. 2011). The p-anisidine values of rice bran oil and blended oil were gradually increased during repeated deep fat frying cycles. Pure rice bran oil & blended oil, when used a to fry moistened and dried potato chips, p-anisidine value increased to 78.64 & 70.71 and 56.45 & 53.02 respectively after the sixth repeated frying cycle. The increase in p-anisidine value may be due to the formation of secondary oxidative compounds during the deep fat frying process. The increase in p-anisidine value was more prominent in the oils there in moistened potato chips were fried. The p-anisidine value was much higher for the oils, used to fry moistened potato chips; which may probably be due to the rapid formation of secondary oxidative compounds in the oils.
The percent oil uptake was evaluated in the products during the deep fat frying process. The results indicated that the oil uptake was higher in RBO as compared to the blended oil, when used as the frying medium for the dried and moistened potato chips (Tables 2 and 3). The oil uptake was increased with the repeated deep fat frying cycles for both RBO and blended oils. The oil uptake was significantly higher in the moistened potato chips as compare to the dried potato chips as expected. Significant difference (P < 0.05) was observed in the oil uptake of the samples after 4th and 5th repeated deep fat frying cycles in case of rice bran oil and blended oil respectively during the frying of both type of potato chips. The percent oil uptake was 21.79% & 20.26% and 18.77% & 17.01% after 6th frying cycles in case of rice bran oil and blended oil, when used as a medium to fry moistened and dried potato chips respectively.
Tables 3 and 4 illustrate the behavior of rice bran oil and its blended oil, based on the different fatty acids composition during the repeated deep fat frying cycles under the controlled conditions, when used as a medium to fry dried and moistened potato chips. Pure sunflower oil and rice bran oil contained myristic, palmitic and stearic acid in the proportions of 0.07% and 0.27%, 6.03% and 19.43% and 3.06% and 2.20% respectively. Compositions of myristic, palmitic and stearic acid in pure rice bran and sunflower oil have been reported to be 1.0% and 0.5%, 18–20% and 3–10%, and 2.5–3.5% and 1–10% respectively (Orthoefer and Smith 1996). Blended oil had myristic, palmitic and stearic acid composition, 0.20%, 13.40% and 2.58% respectively. The chromatograms for the pure rice bran, sunflower and blended oil are given in Figs. 1A, B, C. No significant changes (P < 0.05) in the myristic, palmitic and stearic acid composition was observed during the repeated fat frying cycles in both the rice bran oil and blended oils samples when used as the medium to fry the dried and moistened potato chips (Tables 3 and 4).
Table 4.
Fatty acid composition and trans fats of pure rice bran and blended oil (RBO: SNFO – 60:40) after frying the moistened potato chips (moisture 64.77% wb)
| % Fatty acid composition | Oil sample | Control | After 1st frying | After 2nd frying | After 3rd frying | After 4th frying | After 5th frying | After 6th frying |
|---|---|---|---|---|---|---|---|---|
| C14:0 | RBO | 0.27 NA | 0.27aNA | 0.29NA | 0.29 NA | 0.30 NA | 0.30aNA | 0.30bNA |
| Blend | 0.20NA | 0.19NA | 0.19NA | 0.19NA | 0.20NA | 0.20NA | 0.21NA | |
| C16:0 | RBO | 19.43a | 19.49ac | 19.58ack | 19.55ackq | 19.64ackqt | 19.62ackqtv | 19.68bfmqtv |
| Blend | 13.40a | 13.42ac | 13.46acf | 13.45acfg | 13.48acfgh | 13.48acfghj | 13.52acfghj | |
| C18:0 | RBO | 2.20a | 2.21ac | 2.23acf | 2.25acfg | 2.26acfgh | 2.27acfghi | 2.27acfghi |
| Blend | 2.58a | 2.59ad | 2.62adg | 2.65adgh | 2.67adghj | 2.70afghkl | 2.72adghjl | |
| C18:1 | RBO | 42.59a | 42.18ac | 42.45acf | 41.91acfg | 41.55bcfgk | 41.64bdfgkl | 40.81bdfgkl |
| Blend | 43.37a | 43.38ac | 43.09acf | 42.87acfg | 42.68bcfgh | 42.74bcfghj | 42.55befghj | |
| C18:2 | RBO | 32.64a | 32.46ac | 32.61ace | 32.35acef | 31.91bcefg | 31.94bcefhk | 31.57bdefhk |
| Blend | 37.87a | 37.65ac | 37.42acf | 37.30acfg | 37.12bcfgh | 36.97befghl | 36.78befghl | |
| C18:3 | RBO | 0.99a | 0.95ac | 0.90acg | 0.83acgl | 0.78bcglm | 0.76bfhlmp | 0.65bfhlmp |
| Blend | 0.65a | 0.63ac | 0.59acg | 0.60acgl | 0.55acglm | 0.48bfglmp | 0.45bfglmp | |
| Trans Fat (%) | RBO | 1.27a | 1.49ac | 1.69acg | 1.96acgj | 2.21acgjk | 2.64bfgjkl | 2.91bfhjkl |
| Blend | 1.15a | 1.36ac | 1.58acg | 1.65acgl | 1.86acglm | 2.23bfglmp | 2.48bfhlmp |
*C14:0 -Myristic acid, C16:0 -Palmitic acid, C18:0 -Stearic acid, C18:1 -Oleic acid, C18:2 -Linoleic acid, C18:3 -Linolenic acid. Results are average of three individual experiments. NA Not analysed statistically
Fig. 1.
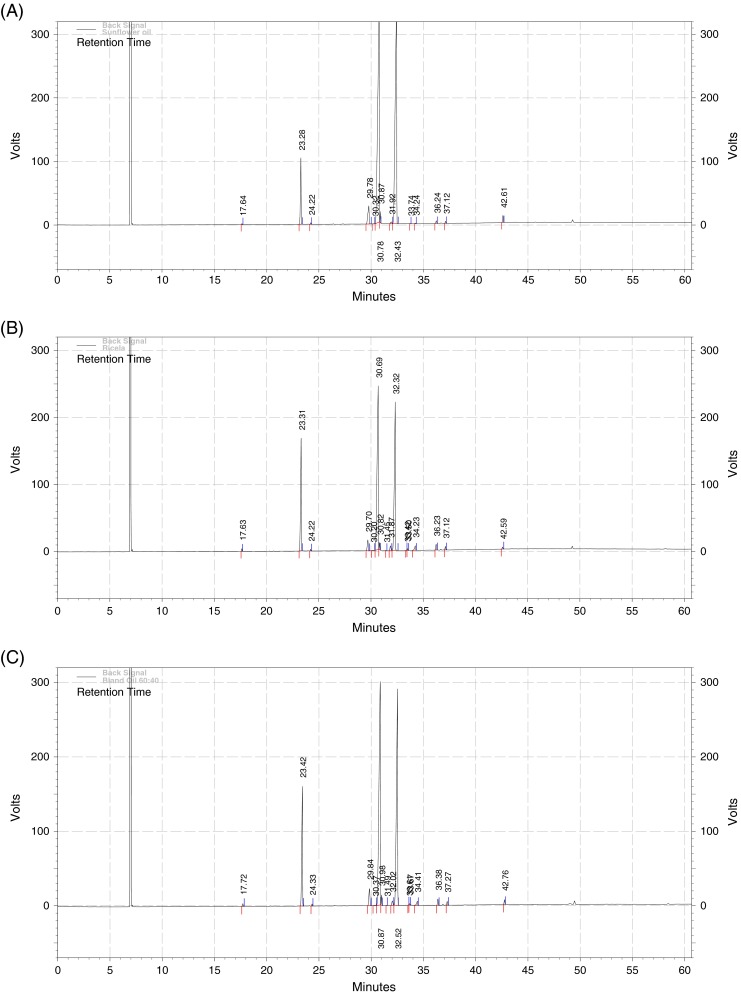
Fatty acid profile for a Pure Sunflower Oil, b Pure Rice Bran Oil and c Blended oil (RBO:SUNFO-60:40). Peak Names - 1) Myristic acid (RT – 17.64), 2) Palmitic acid (RT - 23.28), 3) Stearic acid (RT – 29.78), 4) Elaidic acid (C18:1 trans – RT- 30.32), 5) Oleic acid (RT – 30.78), 6) Linoleic (C18:2 trans – RT- 31.92), 7) Linoleic acid (RT – 32.43), 8) Linolenic (C18:3 trans – RT – 33.74), 9) Linolenic acid (RT – 36.24), 10) C20:1 trans (RT – 34.24)
Pure sunflower oil and rice bran oil had oleic, linoleic and linolenic acid in the proportions of 44.97% and 42.59%, 43.60% and 32.64%, and 0.26% and 0.99% respectively. Compositions of oleic, linoleic and linolenic acid in pure rice bran and sunflower oil have been reported to be 40–42% and 14–65%, 32–35% and 20–75% and 1–1.5% and 0.7% respectively (Orthoefer and Smith 1996). The blended oil had oleic, linoleic and linolenic acid as 43.37%, 37.87% and 0.65% respectively. The data showed that the amount of unsaturated fatty acid decreased gradually during repeated deep fat frying cycles in both the oils. The fatty acids; C18:1, C18:2 and C18:3 decreased to 41.65% & 43.02%, 32.01% & 37.11% and 0.68% & 0.57% after sixth repeated deep fat frying cycles in rice bran oil and blended oil respectively, when used to fry dried potato chips. This may be attributed to oxidative and thermal degradation reactions during repeated deep fat frying cycles in the unsaturated fatty acid constituents of triacylglycerols (Garrido-Polonio et al. 1994). Significant differences (P < 0.05) in C18:1 and C18:2 were observed after 4th repeated deep fat frying cycles for rice bran and blended oil respectively, when used to fry moistened potato chips. However, significant changes in C18:3 were observed after 4th and 5th repeated deep fat frying cycles in the rice bran oil and blended oil respectively, when used to fry moistened potato chips and after 5th repeated deep fat frying cycle in the oils, when used to fry dried potato chips. The comparable changes were observed in the blended oil also as compared to the rice bran oil. The results are in agreement with the findings of Sharma et al. (2006).
The total trans fat was 0.50% in sunflower oil as compared to 1.27% in RBO whereas the blended oil had 1.15%. The trans fat increased with repeated deep fat frying cycles in both the rice bran and blended oils, when used to fry dried and moistened potato chips (Tables 3 and 4). The percent trans fat was found 2.37% & 1.80% and 2.91% & 2.48% after 6th frying cycle for rice bran oil and blended oils when used to fry dried potato chips and moistened potato chips respectively. Both the oil samples showed greater formation of trans fatty acids when the moistened potato chips were used frying. These data clearly revealed the rapid degradation of the quality of oils, when used to fry the products consisting of higher moisture. The blended oil samples were found better as the samples were acceptable after even sixth repeated deep fat frying, when used to fry dried potato chips however the significant differences (P < 0.05) in the total trans fats was observed after 5th repeated deep fat frying cycles.
Conclusion
The study concluded that the moisture content in the product has a dominating effect in more deterioration of oils during deep fat frying process. The higher moisture content in the potato chips sharply brought changes much earlier in the gamma-oryzanol content of rice bran oil. Blended oil showed better stability as compared to pure rice bran oil when used to fry both dried and moistened potato chips. This is evident from the lower values of different physico-parameters i.e. peroxide value, free fatty acid, iodine values, colour, p-anisidine values and gamma-oryzanol values during the repeated deep fat frying cycles. The oil uptake was increased with the repeated deep fat frying cycles for both RBO and blended oils. The oil uptake was significantly higher in the moistened potato chips as compare to the dried potato chips. Trans fatty acids increased with repeated deep fat frying cycles in both the rice bran and blended oils, when used to fry moistened and dried potato chips. Both the oil samples showed greater formation of trans fatty acids when the moistened potato chips were used during frying. However, the blended oil samples were found better as the samples were acceptable even after sixth cycle of deep fat frying, when used to fry dried potato chips
References
- AOCS (2004) Official methods of analysis. 5th ed. American oil chemist’s society chamaign, Illinois, Washington DC
- Codex Alimentarius Commission (2007) Report of the twentieth session of the Codex Committee on fats and oils. Joint FAO/WHO Food Standards Programme, 30th session, London, UK
- Garrido-Polonio MC, Sanchez-Muniz FJ, Arroyo R, Cuesta C. Small scale frying of potatoes in sunflower oil thermooxidative alteration of the fat content in the frying products. Z Ernahrungswissensch. 1994;33:267–276. doi: 10.1007/BF01614432. [DOI] [PubMed] [Google Scholar]
- Goburdhun D, Jhurree B. Effect of deep-fat frying on fat oxidation in soybean oil. Int J Food Sci Nutr. 1995;46(4):363–371. doi: 10.3109/09637489509012568. [DOI] [PubMed] [Google Scholar]
- Khan HN, Khan JS, Ali S, Hussain K, Alam MS, Habib A. Vegetable oil blends after frying and sample stored in different storage packs. Current Bot. 2011;2(5):20–25. [Google Scholar]
- Lee LS, Timms RE. Use of palm kernel ole in as frying oil. Asian Food J. 1988;3:11–16. [Google Scholar]
- Orthoefer FT, Smith J. Rice bran oil and sunflower oil. In: Hui YH, editor. Bailey’s Industrial oil and fat products vol. 2. 5. NewYork: Wiley; 1996. pp. 393–410. [Google Scholar]
- Prevention of food adulteration act along with rules and notification and commodity index. 16. Lucknow: EBC Publishing Company; 1954. [Google Scholar]
- Premavalli KS, Madhura CV, Arya SS. Storage and thermal stability of refined cottonseed oil mustard oil blend. J food Sci Tech. 1998;35(6):530–532. [Google Scholar]
- Semwal AD, Arya SS. Studies on the stability of some edible oils and their blends during storage. J Food Sci Technol, India. 2001;38(5):515–518. [Google Scholar]
- Sharma HK, Singhal RS, Kulkarni PR. Blended oils - New entrants in India. J Sci Ind Res. 1996;4:95–98. [Google Scholar]
- Sharma HK, Singhal RS, Kulkarni PR. Studies on deep fat frying using blended oils and potato chips. J Food Lipids. 1996;3:155–159. doi: 10.1111/j.1745-4522.1996.tb00063.x. [DOI] [Google Scholar]
- Sharma HK, Kaur B, Sarkar B, Singh C. Thermal behaviour of pure rice bran oil, sunflower oil and their model blends during deep fat frying. GRASAS Y ACEITES. 2006;57(4):376–381. [Google Scholar]
- Shin TS, Godber JS, Martin DE, Wells JH. Hydrolytic stability and changes in e vitamers and oryzanol of extruded rice bran during storage. J Food Sci. 1997;62(4):704–728. doi: 10.1111/j.1365-2621.1997.tb15440.x. [DOI] [Google Scholar]
- Singh A, Sharma HK, Sarkar BC, Singh C, Shitandi AA. Thermal behaviour of pure rice bran oil, safflower oil and their model blends during deep fat frying. J Food Sci Technol. 2007;44:52–55. [Google Scholar]
- Su C, White P. Frying stability of high-oleate and regular soybean oil blends. J Am Oil Chem Soc. 2004;81(8):783–788. doi: 10.1007/s11746-004-0978-4. [DOI] [Google Scholar]
- Sukumar D, Vidyarty SK, Singh RP. Impact of blending of frying oils on viscosity and heat transfer coefficient at elevated temperatures. J Food Process Eng. 2008;33:144–161. [Google Scholar]
- Susheelamma NS, Asha MR, Ravi R, Kumar AKV. Comparative studies on physical properties of vegetable oils and their blends after frying. J Food Lipids. 2002;9(4):259–276. doi: 10.1111/j.1745-4522.2002.tb00225.x. [DOI] [Google Scholar]
- Taylor JB, Richar TM, Wilhelm CL, Chrysam MM, Otterburn M, Leveille GA (1996) Rice bran oil antioxidant. U.S. Patent No. 5,552,167
- Varela G, Bender AE, Morton ID. Frying of foods, principles, changes, new approaches. England: Ellis Horwood Ltd Chichester; 1988. [Google Scholar]
- Vijayalakshmi P (2008) Processing and analytical methodologies of oils and fats. Centre for Lipid Research, Indian Institute of Chemical Technology, Hyderabad, pp 10–11
- Whitfield FB. Volatiles from interactions of Millard reactions and lipids. Crit Rev Food Sci Nutr. 1992;31:1–58. doi: 10.1080/10408399209527560. [DOI] [PubMed] [Google Scholar]
- Xu XQ, Tran VH, Palmer MV, White K, Salisbury P. Chemical, physical and sensory properties of canola oil, palm olein and their blends in deep frying trials. Food Aust. 2000;52(3):77–82. [Google Scholar]


