Abstract
The growing public awareness of nutrition and health care research substantiates the potential of phytochemicals such as polyphenols and dietary fiber on their health beneficial properties. Hence, there is in need to identify newer sources of neutraceuticals and other natural and nutritional materials with the desirable functional characteristics. Finger millet (Eleusine coracana), one of the minor cereals, is known for several health benefits and some of the health benefits are attributed to its polyphenol and dietary fiber contents. It is an important staple food in India for people of low income groups. Nutritionally, its importance is well recognised because of its high content of calcium (0.38%), dietary fiber (18%) and phenolic compounds (0.3–3%). They are also recognized for their health beneficial effects, such as anti-diabetic, anti-tumerogenic, atherosclerogenic effects, antioxidant and antimicrobial properties. This review deals with the nature of polyphenols and dietary fiber of finger millet and their role with respect to the health benefits associated with millet.
Keywords: Finger millet, Health benefits, Polyphenols, Non starch polysaccharides
Introduction
The incidence of diabetes and obesity are increasing in an exponential manner globally and to combat them, a raise in demand for food containing complex carbohydrates with higher levels of dietary fiber and health beneficial phytochemicals has been in demand (Shobana et al. 2007). Fortification of diets with food materials rich in phenolic acids was shown to impart antimutagenic, antiglycemic, and antioxidative properties, and this can be exploited in developing health foods (Friedman 1997). Utilization of Wholegrain cereals in food formulations is increasing worldwide, since they are rich sources of phytochemicals and dietary fiber which offer several health benefits (Jones and Engleson 2010). Millets are important crops in semiarid and tropical regions of the world due to their resistance to pests and diseases, short growing season, and productivity under hardy and drought conditions when major cereals cannot be relied upon to provide sustainable yields. Millets are underutilized in many developed countries. There is an immense potential to process millet grains into value-added foods and beverages in developing countries. Furthermore, millets, as they do not contain gluten and hence are advisable for celiac patients (Chandrasekara and Shahidi 2010).
Finger millet (Eleusine coracana L.) is important millet grown extensively in various regions of India and Africa, constitutes as a staple food for a large segment of the population in these countries. It ranks sixth in production after wheat, rice, maize, sorghum and bajra in India. It is a naked caryopsis with brick red-coloured seed coat and is generally used in the form of the whole meal for preparation of traditional foods, such as roti (unleavened breads or pancake), mudde (dumpling) and ambali (thin porridge). Epidemiological studies have demonstrated that regular consumption of whole grain cereals and their products can protect against the risk of cardiovascular diseases, type II diabetes, gastrointestinal cancers and a range of other disorders (McKeown 2002). Since the millets are normally prepared from the whole meal, the dietary fiber, minerals, phenolics and vitamins concentrated in the outer layer of the grain or the seed coat form the part of the food and offer their nutritional and health benefits (Antony et al. 1996). In case the millet is processed to separate out the seed coat matter as is normally done in millet malting and milling (Malleshi et al. 1995; Malleshi 2003), it could be used as an adjunct in special food applications.
Nutritional potential of millets in terms of protein, carbohydrate and energy values are comparable to the popular cereals like rice, wheat, barley or bajra (Tables 1 and 2). Finger millet contains about 5–8% protein, 1–2% ether extractives, 65–75% carbohydrates, 15–20% dietary fiber and 2.5–3.5% minerals (Chethan and Malleshi 2007a). It has the highest calcium content among all cereals (344 mg/100 g). However, the millet also contains phytates (0.48%), polyphenols, tannins (0.61%), trypsin inhibitory factors, and dietary fiber, which were once considered as “anti nutrients” due to their metal chelating and enzyme inhibition activities (Thompson 1993) but nowadays they are termed as neutraceuticals. The seed coat of the millet is an edible component of the kernel and is a rich source of phytochemicals, such as dietary fiber and polyphenols (0.2–3.0%) (Hadimani and Malleshi 1993; Ramachandra et al. 1977). It is now established that phytates, polyphenols and tannins can contribute to antioxidant activity of the millet foods, which is an important factor in health, aging and metabolic diseases (Bravo 1998). Although considerable literature is available on the nutritional and processing aspects of the millet, the information on the health benefits of its polyphenols and dietary fiber has not been reviewed.
Table 1.
Nutrient composition of cereal grains
| Cereals | Protein (%) | Fat (%) | Crude fiber (%) | Ash (%) | Starch (%) | Total dietary fiber (%) | Total phenol (mg/100 g) |
|---|---|---|---|---|---|---|---|
| Wheat | 14.4 | 2.3 | 2.9 | 1.9 | 64.0 | 12.1 | 20.5 |
| Rice | 7.5 | 2.4 | 10.2 | 4.7 | 77.2 | 3.7 | 2.51 |
| Maize | 12.1 | 4.6 | 2.3 | 1.8 | 62.3 | 12.8 | 2.91 |
| Sorghum | 11 | 3.2 | 2.7 | 1.8 | 73.8 | 11.8 | 43.1 |
| Barley | 11.5 | 2.2 | 5.6 | 2.9 | 58.5 | 15.4 | 16.4 |
| Oats | 17.1 | 6.4 | 11.3 | 3.2 | 52.8 | 12.5 | 1.2 |
| Rye | 13.4 | 1.8 | 2.1 | 2.0 | 68.3 | 16.1 | 13.2 |
| Finger millet | 7.3 | 1.3 | 3.6 | 3.0 | 59.0 | 19.1 | 102 |
| Pearl millet | 14.5 | 5.1 | 2.0 | 2.0 | 60.5 | 7.0 | 51.4 |
| Proso millet | 11 | 3.5 | 9.0 | 3.6 | 56.1 | 8.5 | – |
| Foxtail millet | 11.7 | 3.9 | 7.0 | 3.0 | 59.1 | 19.11 | 106 |
| Kodo millet | 8.3 | 1.4 | 9.0 | 3.6 | 72.0 | 37.8 | 368 |
Source: Saldivar (2003)
Table 2.
Mineral and vitamin composition of cereal grains
| Cereals | Ca (%) | P (%) | K (%) | Na (%) | Mg (%) | Fe (%) | Mn (%) | Zn (%) | Thiamin (mg/100gm) | Riboflavin (mg/100gm) | Nicotinic acid (mg/100gm) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wheat | 0.04 | 0.35 | 0.36 | 0.04 | 0.14 | 40.1 | 40.0 | 30.9 | 0.57 | 0.12 | 7.40 |
| Rice | 0.02 | 0.12 | 0.10 | 0.00 | 0.03 | 19.0 | 12.0 | 10.0 | 0.07 | 0.03 | 1.60 |
| Maize | 0.03 | 0.29 | 0.37 | 0.03 | 0.14 | 30.0 | 5.0 | 20.0 | 0.38 | 0.14 | 2.80 |
| Sorghum | 0.04 | 0.35 | 0.38 | 0.05 | 0.19 | 50.0 | 16.3 | 15.4 | 0.46 | 0.15 | 4.84 |
| Barley | 0.04 | 0.56 | 0.50 | 0.02 | 0.14 | 36.7 | 18.9 | 23.6 | 0.44 | 0.15 | 7.20 |
| Oats | 0.11 | 0.38 | 0.47 | 0.02 | 0.13 | 62.0 | 45.0 | 37.0 | 0.77 | 0.14 | 0.97 |
| Rye | 0.05 | 0.36 | 0.47 | 0.01 | 0.11 | 38.0 | 58.4 | 32.2 | 0.69 | 0.26 | 1.52 |
| Finger millet | 0.33 | 0.24 | 0.43 | 0.02 | 0.11 | 46.0 | 7.5 | 15.0 | 0.48 | 0.12 | 0.30 |
| Pearl millet | 0.01 | 0.35 | 0.44 | 0.01 | 0.13 | 74.9 | 18.0 | 29.5 | 0.38 | 0.22 | 2.70 |
| Proso millet | 0.01 | 0.15 | 0.21 | 0.01 | 0.12 | 33.1 | 18.1 | 18.1 | 0.63 | 0.22 | 1.32 |
| Foxtail millet | 0.01 | 0.31 | 0.27 | 0.01 | 0.13 | 32.6 | 21.9 | 21.9 | 0.48 | 0.12 | 3.70 |
| Kodo millet | 0.01 | 0.32 | 0.17 | 0.01 | 0.13 | 7.0 | – | – | 0.32 | 0.05 | 0.70 |
Source: Saldivar (2003)
Polyphenols
Nowadays, there has been a renewed interest in polyphenols as “life span essentials” due to their role in maintaining body functions and health throughout the adult and later phases of life (Chandrasekara and Shahidi 2010). Polyphenols are a large and diverse class of compounds, many of which occur naturally in a range of food plants. Phenolics (hydroxybenzenes) especially polyphenols (containing two or more phenolic groups) are ubiquitous in plant foods consumed by human and animals and one of the widest groups of a dietary supplements marketed worldwide (Ferguson 2001). The main polyphenols in cereals are phenolic acids and tannins, whilst flavonoids are present in small quantities (Rao and Muralikrishna 2002). Although, these compounds play no known direct role in nutrition (non-nutrients), many of them have properties, including antioxidant (Sripriya et al. 1996), anti-mutagenic, anti-oestrogenic, anti-carcinogenic and anti-inflammatory, antiviral effects and platelet aggregation inhibitory activity that might potentially be beneficial in preventing or minimising the incidence of diseases (Ferguson 2001). The tiny finger millet grain has a dark brown seed coat, rich in polyphenols compared to many other continental cereals such as barley, rice, maize and wheat (Viswanath et al. 2009).
Phenolic compounds
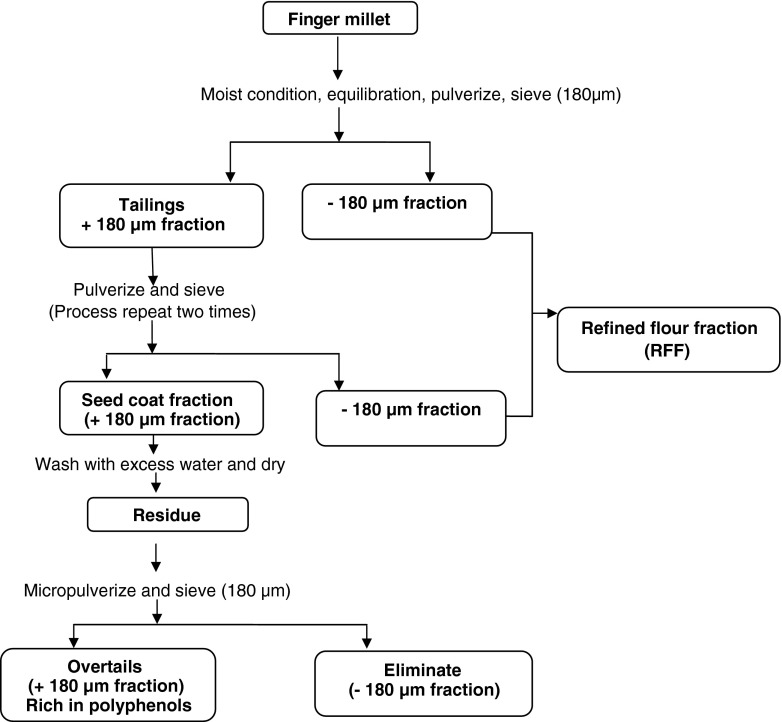
Efforts have been made towards identification of the polyphenols in different anatopical parts of the millet seed using histochemical as well as chemical analysis of milling fractions. Phenolics are not equally distributed in the grain, and are mainly concentrated in the outer layers, namely, the aleurone layer, testa, and pericarp, which form the main components of the bran fraction. Histochemical examination of the millet kernel indicates that nearly 60% of the polyphenols of the millet are concentrated in the seed coat tissue which accounts for about 12% of the seed mass. The method for preparation of polyphenol rich seed coat fractions of the millet (Fig. 1) has been worked out (Chethan and Malleshi 2007a). Phenolic compounds in grains exist as free, soluble conjugates and insoluble bound forms. According to Hilu et al. (1978), majority of the phenolic compounds present in the millet exist in the form of glycosides, whereas Rao and Muralikrishna (2002) reported ferulic acid as the major bound phenolic acid (18.60 mg/100 g) and protocatechuic acid as the major free phenolic acid (45.0 mg/100 g) of the millet. The major bound phenolics present in finger millets are ferulic acid and p-coumaric acid, and the bound phenolic fraction account for 64–96 and 50–99% of total ferulic acid and p-coumaric acid contents of millet grains, respectively.
Fig. 1.
Protocol for preparation of polyphenol rich seed coat fractions of finger millet (Chethan and Malleshi 2007a)
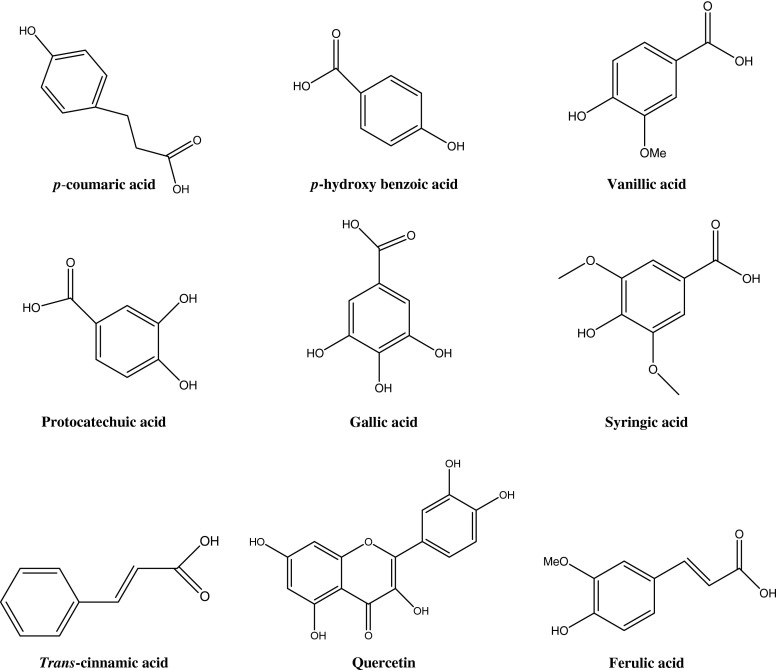
Acidic methanol (1% HCl in methanol) has been shown to be very effective solvent for extraction of the millet polyphenols (Ramachandra et al. 1977; Sripriya et al. 1996; Chethan and Malleshi 2007a). The millet phenolics are heat stable but pH sensitive and are largely unstable under alkaline conditions (Chethan and Malleshi 2007a). Fractionation of the polyphenols extracted by high performance liquid chromatography (HPLC) showed that the analytics were derivatives of benzoic acid (gallic acid, proto-catechuic acid and p-hydroxy benzoic acid) and cinnamic acid (p-coumaric acid, syringic acid, ferulic acid and trans-cinnamic acid) and a flavanoid compound (quercetin) (Table 3). Benzoic acid derivatives accounted for about 85% of the total phenolic compounds (Chethan et al. 2008b) (Fig. 2). In addition to these phenolic compounds, direct infusion electrospray ionisation mass spectrometry of the seed coat extract showed the presence of naringenin, kaempferol, luteolin glycoside, phloroglucinol, apigenin, (+)-catechin/(-)-epicatechin, trans-feruloyl- malic acid, dimer of prodelphinidin (epi/gallocatechins; 2GC), diadzein, catechin gallates, trimers and tetramers of catechin (Shobana et al. 2009).
Table 3.
Phenolic compounds identified in finger millet
| Class | Basic skeleton | Compounds | References |
|---|---|---|---|
| Phenolic acids Hydroxybenzoic acid derivatives | C6–C1 | Gallic acid, protocatechuic acid, p-hydroxybenzoic acid, vanillic acid, syringic acid | McDonough et al. (1986), Rao and Muralikrishna (2002), Chethan and Malleshi (2007a), Chethan et al. (2008a, b), Shobana et al. (2009) |
| Hydroxycinnamic acid derivatives | C6–C3 | Ferulic acid, trans—cinnamic acid, p- coumaric acid, caffeic acid, sinapic acid, | |
| Flavonoids | C6-C3–C6 | Quercetin | Chethan et al. (2008a, b), Dykes and Rooney (2006), Chandrasekara and Shahidi (2010) |
| Proanthocyanidins (Condensed tannins) |
Fig. 2.
Structure of major phenolic compounds present in finger millets
Finger millets varieties are also reported to contain proanthocyanidins, also known as condensed tannins (Dykes and Rooney 2006). Procyanidins, are high-molecular weight polyphenols that consist of polymerized flavan-3-ol and/or flavan-3,4-diol units. They are biologically active and when present in sufficient quantities, may lower the nutritional value and biological availability of proteins and minerals (Chavan et al. 2001). Several in vivo assays have demonstrated their anti-inflammatory, antiviral, antibacterial and antioxidant properties. Condensed tannins are generally more potent antioxidants than their corresponding monomers. Among the millet varieties studied, finger (local) millet had the highest content (311.28 ± 3.0 μmol of catechin equivalent/g of defatted meal) followed by finger (Ravi), foxtail, little, pearl, and proso millets. The values reported for millets were higher than those for barley (Chandrasekara and Shahidi 2010).
Varietal variations in polyphenol contents
Total phenolics and tannin contents varied across finger millet grain genotypes (Table 4). Light-coloured grain types contain much lower total phenolics and tannins compared to brick red pigmented types. The pigmented testa in the red coloured varieties is known to contain much tannin content and the tannins were located in the said tissue of the grain (Siwela et al. 2007). Studies conducted with respect to the contents of phenolic acids and tannins in different varieties of the millet indicate considerable differences, with respect to the polyphenol contents of the seven popular high yielding Indian cultivars. They observed that brown varieties contained (1.2–2.3%) higher proportions of polyphenols than white (0.3–0.5%) varieties (Ramachandra et al. 1977). Likewise, among 85 Indian finger millet varieties, considerable differences (0.19–3.37%) in the total polyphenol contents (as catechin equivalents) has been reported (Shankara 1991). Tannin content was also estimated in hilly region varieties and found to be less compared to base region varieties (Wadikar et al. 2006). The noticeable difference between polyphenols content in white and brown varieties could be due to the presence of the red pigments, such as anthocyanins, which are generally polymerized phenolics present in brown cultivars.
Table 4.
Total polyphenol content in few brown and white finger millet varieties
| Number of Varieties | Polyphenols (%) | Tannins (%) | References |
|---|---|---|---|
| Brown | |||
| 26 | 0.08–2.44 | 0.12–3.47 | Ramachandra et al. (1977) |
| 1 | – | 0.36 | Rao and Prabhavati (1982) |
| 3 | 0.55–0.59 | 0.17–0.32 | McDonough et al. (1986) |
| 12 | – | 0.35–2.39 | Rao and Deosthale (1988) |
| 1 | 0.1 | – | Sripriya et al. (1997) |
| 5 | 1.3–2.3 | – | Chethan and Malleshi (2007a) |
| 18 | 0.34–1.84 | 0.02–2.08 | Siwela et al. (2010) |
| White | |||
| 6 | 0.06–0.09 | 0.04–0.06 | Ramachandra et al. (1977) |
| 1 | 0.003 | – | Sripriya et al. (1997) |
| 2 | 0.3–0.5 | – | Chethan and Malleshi (2007a) |
| 4 | ND-0.09 | ND | Siwela et al. (2010) |
| Hilly region | |||
| 3 | – | 0.34 | Wadikar et al. (2006) |
| Base region | |||
| 7 | – | 0.53 | Wadikar et al. (2006) |
ND—not detected
Influence of processing on polyphenols contents and their characteristics
Processing technologies such as decortication, soaking, germination, fermentation, puffing and cooking of the millet are known to reduce the levels of tannins and phenols and thereby increase the bioavailability of proteins, starch and minerals. Nutrient composition and polyphenols content changes during processing are listed in Table 5. Decortication of the millet reduces the total polyphenol content of millet by 74.7% and also causes significant reduction in tannin content (Ramachandra et al. 1977; Shobana and Malleshi 2007). Studies on the changes in free and bound phenolic acids during malting of finger millet revealed that malting for 96 h decreased bound caffeic, coumaric, and ferulic acid levels by 45%, 41%, and 48%, respectively (Rao and Muralikrishna 2001). On the other hand, the level of gallic, vanillic, coumaric, and ferulic acids, the free phenolics increased considerably after 96 h of malting (Rao and Muralikrishna 2002). Rao and Deosthale (1988) reported 0.91% tannin contents in ungerminated millet, which decreased by 72% on 72 h germination, whereas Sripriya et al. (1996) reported 35% decrease in the total polyphenols on germination and 34% increase on fermentation. Increase in polyphenols during fermentation of millets may be due to microbial activity, which may hydrolyse the condensed tannins to lower molecular weight phenolics (Khetarpaul and Chauhan 1991). In contrast, Antony and Chandra (1998) reported that phenolics decrease by 26–29%, while tannins show a more marked reduction of 44–52% by 48 h of fermentation and they attributed that the reduction may be due to the release of fiber bound tannins and polyphenol oxidase activity by fermenting microbes. Chethan et al. (2008a) reported nearly 44% of loss of polyphenols of the millet during the first 24 h and about 80% after 120 h of germination. Tannin content was reduced significantly after 24 and 48 h germination respectively. The reason for the decrease in the bound phenolics might be due to the action of esterase developed during germination, which decreases the various phenolic acid esters linked either to arabinoxylans or other non-starch polysaccharides (Maillard et al. 1996).
Table 5.
Nutrient composition and polyphenol content changes during processing of finger millet (g/100 g)
| Parameter | Whole flour | Husk | 3% flour | 5% flour | 7% flour | Hyrdrothermally processed | Decorticated |
|---|---|---|---|---|---|---|---|
| Moisture | 7.7 | 8.7 | 8.7 | 8.9 | 9.70 | 11.10 | 10.46 |
| Protein | 7.4 | 15.4 | 5.7 | 4.9 | 3.50 | 6.90 | 4.43 |
| Fat | 1.2 | 3.0 | 1.1 | 1.0 | 0.90 | 1.40 | 0.9 |
| Acid insoluble ash | 0.3 | 0.5 | 0.1 | 0.1 | 0.10 | 0.08 | 0.07 |
| Dietary fiber | 22.5 | 53.3 | 9.9 | 6.0 | 4.20 | 21.10 | 14.7 |
| Starch | 71.1 | 30.1 | 77.8 | 82.3 | 89.10 | 65.00 | 74.0 |
| Phenolic content | 7.3 | 12.6 | 4.3 | 3.6 | 3.30 | 1.19 | 0.52 |
| Calcium | 0.34 | 0.64 | 0.28 | 0.24 | 0.14 | 0.31 | 0.18 |
| Phosphorus | 0.25 | 0.49 | 0.19 | 0.15 | 0.08 | 0.157 | 0.109 |
| Iron | 0.003 | 0.003 | 0.002 | 0.002 | 0.002 | 0.006 | 0.003 |
Total phenolics reduced during cooking of millet flours probably due to thermal degradation and also due to the changes in chemical reactivity or formation of insoluble complexes with food components such as proteins. Enzymatic treatments of untreated, cooked, soaked, germinated millet with phytase and tyrosinase for 24 h resulted in 20, 40, 26, 32% decrease of total polyphenols content and may be due to condensation of phenolic compounds (Matuschek et al. 2001). Extrusion cooking is one of the most efficient and versatile food processing technologies that can be used to produce pre-cooked and dehydrated foods. During preparation of uji, a thin porridge prepared from maize-finger millet blend, tannin contents reduced by 40% after extrusion of the unfermented blend and further to 10% after fermentation and extrusion. Extrusion of the blends with lactic or citric acids also counteracted thermal degradation of tannins and results in decrease of tannin content (Onyango et al. 2005). Puffing of millets is a well known traditional method of processing. It is generally carried out by conditioning of grains to higher moisture content and roasting in hot sand. Puffing of finger millet varieties (3 varieties from hilly region and 7 varieties from base region) leads to 3–18% decrease in tannin content. Decreases in tannin content during puffing were less in hill than base region varieties and nutritional quality of finger millet were improved (Wadikar et al. 2006).
Effect of phenolics on the millet grain and malt quality
Chethan et al. (2008a) suggested that phenolics in finger millet grain are detrimental to its malt quality, as they inhibited malt amylases. Siwela et al. (2010) determined type of phenolics type, fungal load, germinative energy (GE) and the malt quality of finger millet grains differing in colour and phenolic contents and reported that phenolics influenced malt quality positively by contributing to attenuation of the fungal load on the germinating grain. Finger millet types with higher level of phenolics had superior malt quality than the low-phenol varieties, with respect to diastatic power (DP), and α- and β-amylase activities. According to them, GE, DP and α-amylase activity positively correlated with total phenolics and the phenolics content (p < 0.05) and negatively correlated with total fungal count (p < 0.01).
Functional role of polyphenols
Polyphenols offer several health beneficial and antifungal activities and the beneficial properties of phenols present in finger millets are outlined in Table 6.
Table 6.
Beneficial properties of finger millet polyphenols
| Properties | Functional Role | References |
|---|---|---|
| Antimicrobial properties | (i) Seed coat phenolic extract—active against Bacillus cereus, Aspergillus niger | Viswanath et al. (2009) |
| (ii) Fermented finger millet extract—suppress growth of Salmonella sp., Escherichia coli | Antony et al. (1998) | |
| (iii) Germinated and ungerminated millet phenol extract—against Bacillus cereus, Staphylococcus aureus, Yersinia enterocolitica, Escherichia coli, Listeria monocytogenes, Streptococcus pyogenes, Pseudomonas aeruginosa, Serrtia marcescens, Klebsiella pneumonia | Chethan and Malleshi (2007b) | |
| Antioxidant properties | (i) Whole flour methanol extract—Antioxidant activity through β-carotene—linoleic acid assay, DPPH radical, hydroxyl quenching action—27%, 94%, 77% respectively | Viswanath et al. (2009), Sripriya et al. (1996) |
| (ii) Seed coat methanol extract—Antioxidant activity (β-carotene—linoleic acid assay)—86% | Viswanath et al. (2009) | |
| (iii) DPPH scavenging effect IC50 (μg/ml)—Crude phenolic extract—90.12; Gallic acid—26.9; Protocatechuic acid—77.63; p—Hydroxy benzoic acid—183.7; p-coumaric acid—112.01; Vanillic acid—176.5; Syringic acid—155.6; Ferulic acid—189.1; Trans-cinnamic acid 96.7; Quercetin—56.8 | Chethan et al. (2008a) | |
| Anti diabetic properties | in vitro studies | |
| (i) millet phenolics inhibits—Malt amylase, α—glucosidase, pancreatic amylase—reduce postprandial hyperglycemia by partially inhibiting the enzymatic hydrolysis of complex carbohydrates | Shobana et al. (2009), Chethan et al. (2008b) | |
| (ii) Inhibits—Aldose reductase—prevents the accumulation of sorbitol—reduce the risk of diabetes induced cataract diseases | Chethan et al. (2008a) | |
| (iii) Methanolic extract—prevents glycation and crosslinking of collagen—reduce complication of diabetes and aging due to presence of free radical scavangers | Hegde et al. (2002) | |
| in vivo studies | ||
| (i) Whole grain millet meal flour protect against hyperglycemic and oxidative stress | Hegde et al. (2005) | |
| (ii) Finger millet feeding controls blood glucose level, improve antioxidant status and hastens the dermal wound healing process in diabetic rats | Rajasekaran et al. (2004) | |
Antimicrobial properties
Plant phenolics have been implicated for minimising the intensity of several diseases and also to inhibit the in vitro growth of an assortment of fungal genera (Baranowski et al. 1980; Bravo 1998). Seetharam and Ravikumar (1994) indicated that finger millet grain phenolics including tannins may be involved in resistance of the grain to fungal attack. Phenolic compounds, particularly tannins in the outer layers of the grain serve as a physical barrier to the fungal invasion. The acidic methanol extracts from the seed coat showed high antibacterial and antifungal activity compared to whole flour extract due to high polyphenols content in seed coat (Viswanath et al. 2009). Siwela et al. (2010) reported that the fungal load (total fungal load and infection levels) of the unmalted millet grain and its malt, were negatively correlated (p < 0.05) with total phenolics and phenolic type (condensed tannins, anthocyanins and flavan-4-ols). Oxidation of microbial membranes and cell components by the free radicals formed, irreversible complexation with nucleophilic amino acids leading to inactivation of enzymes are major biochemical benefits of polyphenols towards the antifungal activity. Besides, loss of their functionality and also the interaction of phenolic compounds, especially tannins with biopolymers such as proteins and polysaccharides and complexing with metal ions making them unavailable to micro-organisms are some of the mechanisms involved in the inhibitory effect of phenolic compounds on microorganisms (Cowan 1999; Scalbert 1991). The extremely good storage property of finger millet and its processed foods could be attributed to its polyphenol content.
Antioxidant properties
Antioxidant compounds are gaining importance due to their main roles as lipid stabilizers and as suppressors of excessive oxidation that causes cancer and ageing (Namikii 1990). Their stable radical intermediates prevent the oxidation of various food ingredients, particularly fatty acids and oils (Cuvelier et al. 1992; Maillard et al. 1996). Phenolic acids and their derivatives, flavonoids and tannins present in millet seed coat are of multifunctional and can act as reducing agents (free radical terminators), metal chelators, and singlet oxygen quenchers (Shahidi et al. 1992; Sripriya et al. 1996). The potency of phenolic compounds to act as antioxidants arise from their ability to donate hydrogen atoms via hydroxyl groups on benzene rings to electron-deficient free radicals and in turn form a resonance-stabilized and less reactive phenoxyl radical. Studies were carried out on the natural antioxidants in edible flours of small millets. Total antioxidant capacity of finger, little, foxtail and proso millets were found to be higher and their total carotenoids content varied from 78–366 mg/100 g in the millet varieties. Total tocopherol content in finger and proso millet varieties were higher (3.6–4.0 mg/100 g) than in foxtail and little millet varieties (~1.3 mg/100 g). HPLC analysis of carotenoids for the presence of β-carotene showed its absence in the millets, and vitamin E indicated a higher proportion of γ-and α-tocopherols; however, it showed lower levels of tocotrienols in the millets. Edible flours of small millets are good source of endogenous antioxidants (Asharani et al. 2010).
Free radical quenching potential of six different millets kodo millet, finger millet, little millet, foxtail millet, barnyard millet (kudiraivali), great millet (jowar) and their white varieties by electron spin resonance (ESR) spectroscopic studies revealed that kodo millet extract quenched 70% of 1, 1, Diphenyl -2- picrylhydrazyl (DPPH), followed by great millet, finger millet and other extracts which showed 15–53%. Processing methods such as cooking by roasting and boiling, germination and/or fermentation decreased the free radical quenching activity which might be due to hydrolysis of tannins and the white varieties of millets showed lower activity than their coloured counterparts, indicating that phenolics in the seed coat could be responsible for the antioxidant activities (Hegde and Chandra 2005; Sripriya et al. 1996). The reducing power of the seed coat extracts was significantly higher than that of whole flour extract. The antioxidant capacity of phenolic acids changes during malting of finger millet. Rao and Muralikrishna (2002) reported that the antioxidant activity of a free phenolic acid mixture was found to be higher compared to that of a bound phenolic acid mixture. An increase in an antioxidant activity coefficient was observed in the case of free phenolic acids whereas the same decreased in bound phenolic acids upon 96 h of malting. Soluble and insoluble-bound phenolic extracts of several varieties of whole grain millets (kodo, finger, foxtail, proso, pearl, and little millets) evaluated for their phenolic contents and antioxidative efficacy using trolox equivalent antioxidant capacity (TEAC), reducing power (RP), and β-carotene-linoleate model system as well as ferrous chelating activity showed high antioxidant activities, although the order of their efficacy was assay dependent. The potential of whole millets as natural sources of antioxidants could be due to varietal differences existed in the contents of phenolics as well as antioxidant capacities between soluble and insoluble bound phenolic fractions (Chandrasekara and Shahidi 2010). The extent of antioxidant activity of phenolics depends on the position and extent of hydroxylation of the phenolic rings (Miyake and Shibamoto 1997). Many other structural features play a significant role in determining the extent of antioxidant activity (Bravo 1998). Ferulic acid exhibits very strong antioxidant, free radical scavenging and anti-inflammatory activity (Castelluccio et al. 1995; Shahidi et al. 1992).
Glycemic response
Diabetes mellitus is a chronic metabolic disorder characterised by hyperglycemia, resulting from insufficient or inefficient insulin secretion, with alterations in carbohydrate, protein and lipid metabolism. Recent reports indicate that hyperglycemia could induce non-enzymatic glycosylation of various proteins, resulting in the development of chronic complications in diabetes (Lebovitz 2001). Therefore, control of postprandial blood glucose surge is critical for treatment of diabetes and for reducing chronic vascular complications (Baron 1998; Lebovitz 2001) which can be controlled by intake of high complex carbohydrate and high fiber diet. The millet diet is known for high sustaining power and is usually recommended for diabetics. Research has shown that the carbohydrates present in finger millet are slowly digested and assimilated than those present in other cereals (Kavitha and Prema 1995). Regular consumption of finger millet is known to reduce the risk of diabetes mellitus (Gopalan 1981) and gastrointestinal tract disorders (Tovey 1994) and these properties were attributed to its high polyphenols and dietary fiber contents (Chethan et al. 2008b). The beneficial effect of phenolics is due to partial inhibition of amylase and α-glucosidase during enzymatic hydrolysis of complex carbohydrates and delay the absorption of glucose, which ultimately controls the postprandial blood glucose levels (Shobana et al. 2009). Beneficial effect of dietary fiber is usually attributed either to slower gastric emptying or formation of un-absorbable complexes with available carbohydrates in the gut lumen and these two properties might result in delayed absorption of carbohydrates and in the reduction of absolute quantity absorbed (Kawai et al. 1987; Rasmussen et al. 1991).
Ramananthan and Gopalan (1957) studied glucose levels in the blood of six normal male subjects and two diabetics (one male and one female) after consumption of meals made up of cooked rice, parboiled rice, wheat, ragi, rice starch, or ragi starch. Ragi flour and ragi starch gave the lowest glycemic response. Ragi starch released less glucose into the blood than did rice starch while after in vitro enzymic digestion, the differences between the two starches disappeared. Feeding of finger millets for 28 days at 55% level in rat diet supplemented with casein, oil, minerals, vitamins, and corn starch to Alloxan induced rats increased body weight by 43% in the control group, by 6% in the diabetic rats fed corn starch and casein, and by 28% in the group of diabetic rats fed finger millet. Glycemic index of the finger millet based diet in diabetic animals was lower significantly compared to normal diet in the diabetic animal groups. Glucose level was controlled by whole grain flour diet rich in phenolic antioxidants, suggesting that millets can provide valuable health protective properties against diet-related chronic disease (Hegde et al. 2005). Shobana et al. (2007) formulated four different foods from whole wheat, decorticated ragi, popped, and flaked (expanded) rice, and a mixture of bengal gram, green gram, and black gram flours. Spices and condiments including cumin, pepper, cinnamon, asafoetida, turmeric powder and tamarind powder, fenugreek, guar gum, amla, and gurmar (Gymnema sylvestre) were added to a total of 11%. Oil, skimmed milk powder, and vitamins and minerals were then added in the extent of 9, 6 and 1% respectively. A 50 g equivalent carbohydrate portion of the foods in the form of thick porridge was provided to eight healthy adult subjects and the postprandial blood glucose response was determined. The Glycemic Index (GI) values were less for wheat and ragi-based foods. After the decortication process also, the glycemic index of ragi was lower than of the two rice products. Wheat based and finger millet based formulations are suitable as a food supplement or meal replacer for non insulin dependent diabetes mellitus (NIDDM) subjects. Lakshmi Kumari and Sumathy (2002) studied the effect of consumption of finger millet on hyperglycemia in six NIDDM men and reported that glycemic responses were lower in whole finger millet based roti and dosa and germinated finger millet roti. Geetha and Parvathi (1990) reported that supplementation of diets with ragi for a month showed a higher reduction of fasting and post prandial glucose levels than did supplementation with other millets. In contrast, Patel et al. (1968) found no reduction in blood glucose levels when the diets of eight diabetic males (40–80 years of age) were changed from rice to one with ragi as the staple grain. Another independent study on the glycemic response on feeding rice, finger millet, tapioca and wheat diet on normal humans for 15 days did not alter the GI values. However, the plasma cholesterol profile was benefited significantly by finger millet and tapioca (Kurup and Krishnamurthy 1993).
Shobana et al. (2010) provided evidence for hypoglycaemic, hypocholesterolaemic, nephroprotective and anti-cataractogenic properties of finger millet, the ‘health-grain’. Feeding a diet containing 20% millet seed coat matter (SCM) to streptozotocin induced diabetic rats for 6 weeks exhibited lesser degree of fasting hyperglycemia and partial reversal of abnormalities in serum albumin, urea and creatinine compared to diabetic control. Hypercholesterolaemia, hypertriacylglycerolaemia, nephropathy and neuropathy associated with diabetes were notably reversed in diabetic group fed with the diet containing millet seed coat matter.
Oxidative stress and glycemic status
ESR spectroscopic studies suggest that patients with diabetes mellitus (DM) are susceptible to increased levels of oxidative stress (Davison et al. 2002). Oxidative stress and hyperglycemia in diabetes produce reactive oxygen species, which causes peroxidation of membrane lipids, protein glycation, and health complications such as retinopathy, neuropathy, nephropathy, and vasculopathy (Monnier 1990). Antioxidants inhibit glycation by scavenging reactive oxygen species and superoxide dismutase (SOD) and metal chelators protect against alloxan-induced diabetes in animals (Chattopadhyay et al. 1997). Effects of the antioxidant properties of millet species on oxidative stress and glycemic status in alloxan-induced rats was investigated by Hegde et al. (2005). The alloxan induced rats fed with finger millet enriched diet (55% by weight) showed a greater reduction in blood glucose (36%) and cholesterol level (13%). Glycation of tail tendon collagen was 40% in the finger millet–fed rats. The levels of enzymatic (glutathione, vitamins E and C) and non-enzymatic antioxidants (superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase) and lipid peroxides were significantly reduced in diabetic animals and restored to normal levels in the millet-fed groups. This could be due to the presence of phenolics, tannins, and phytates in finger millets.
Inhibition of collagen glycation and crosslinking
The chemical reaction between the aldehyde group of reducing sugars and the amino group of proteins termed non enzymatic glycosylation is a major factor responsible for the complications of diabetes and aging. Increased oxidative stress and hyperglycemia contribute significantly to the accelerated accumulation of advanced glycation end products and the cross-linking of collagen in diabetes mellitus (Monnier 1990). Free radicals play major role in non-enzymatic glycosylation of collagen and crosslinking whereas antioxidative conditions and free radical scavengers inhibit these reactions (Fu et al. 1992). Hegde et al. (2002) studied the effects of methanolic extracts of finger millet and kodo millet on glycation and crosslinking of collagen. The collagen incubated with glucose (50 mM) and 3 mg methanolic extratcs of finger millet inhibited glycation. This may be due to natural antioxidants primarily of polyphenolic nature and other phytochemicals extracted from the seed coats of the millet grains. Finger millets could have a potent therapeutic role as dietary supplements for the prevention of glycation induced complications, as in diabetes or aging.
Wound healing process
The process of wound healing is determined by inflammation (Khodr and Khalil 2001), a vital and protective response offered by the injured cells at the wound site that actually starts the process of tissue repair (Adam et al. 1999). The perfect wound healing process is interrupted in diseased conditions like diabetes and age associated biochemical phenomenon due to increased level of reactive oxygen species (ROS). The diabetic conditions had a deleterious influence on the wound healing process through abnormal physiological response. Free oxygen radicals damage the cells in the zone of stasis, which lead to necrosis and conversion of superficial wound into a deeper wound (King 2001). Antioxidants significantly prevent tissue damage and stimulate the wound healing process.
Antioxidant effects of finger millet on the dermal wound healing process in diabetes induced rats with oxidative stress-mediated modulation of inflammation were studied by Rajasekaran et al. (2004). They reported that the role of finger millet feeding on skin antioxidant status, nerve growth factor (NGF) production and wound healing parameters in healing the impaired early diabetic rats. Hyperglycemic rats received food containing 50 g/100 g finger millet (FM) and the non-diabetic controls and diabetic controls received balanced nutritive diet. Full-thickness excision skin wounds were made after 2 weeks prior to feeding of finger millet diet. They studied the intensity of wound, levels of collagen, hexosamine and uronic acid in the granulation tissue, skin antioxidant status and lipid peroxide concentration. The healing process was hastened with an increased rate of wound contraction in hyperglycemic rats fed with finger millet diet and skin antioxidant levels of glutathione (GSH), ascorbic acid and α-tocopherol in alloxan-induced diabetic rat was lower as compared to non-diabetics. Altered activities of superoxide dismutase (SOD) and catalase (CAT) were also recorded in diabetic rats. The thiobarbituric acid reactive substances (TBARS) levels of both normal and wounded skin tissues were significantly elevated (P < 0.001) when compared with control (nondiabetic) and diabetics fed with FM. Increased expression of NGF determined by ELISA and immunocytochemical evaluation were observed in hyperglycemic rats supplemented with FM diet. Histological and electron microscopic examinations revealed the epithelialization, increased synthesis of collagen, activation of fibroblasts and mast cells in FM-fed animals. Increased levels of oxidative stress markers accompanied by decreased levels of antioxidants, causes delaying in wound healing of diabetic rats. Feeding the diabetic animals with finger millet for 4 weeks, regulated the glucose levels and improved the antioxidant status, this hastened the dermal wound healing process and could be due to the structure, anti-oxidative mechanism and the synergistic effects of different phenolic compounds. It is attributed that the phenolic antioxidants present in FM partially protected the insulin-producing cells from alloxan-mediated cell damage, and hence promoted the healing process (Rajasekaran et al. 2004).
Millet phenolics and the enzyme inhibition
Inhibition of malt amylases, pancreatic amylase, intestinal α-glucosidase
Polyphenols are known to inhibit the activity of digestive enzymes such as amylase, glucosidase, pepsin, trypsin and lipases and the subject has been studied extensively (Rohn et al. 2002). They may act as inhibitors of amylase and glucosidase (similar to acarbose, miglitol and voglibose) leading to a reduction in post-prandial hyperglycemia (Bailey 2001). Synergy between phenolics and dietary fiber may play a role in mediating amylase inhibition and therefore, have the potential to contribute to the management of type II Diabetes mellitus (Saito et al. 1998; Toeller 1994). Chethan et al. (2008a) studied the mode of inhibition of finger millet malt amylases by the millet phenolics and reported that the crude polyphenolic extract exerts mixed non-competitive type inhibition, whereas the individual phenolic compounds isolated from the extract exhibit uncompetitive inhibition. Trans-cinnamic acid exhibited a higher degree of inhibition (79.2%) as compared to other phenolic compounds and syringic acid was found to be a weaker inhibitor (~56% inhibition). Depending on the structure, the phenolics react with proteins/enzymes and alter various properties of biopolymers such as the molecular weight, solubility and in vitro digestibility. It has also been shown that the decrease in enzyme activity depends on the concentration as well as the number and position of hydroxyl groups of the phenolics (Rohn et al. 2002).
The millet polyphenols may affect the amylases in several ways, for instance, by competing with the substrate to bind to the active site of the enzyme or by disrupting irreversibly the catalytic process. The heterogeneity of phenolics having different structural features in crude extract may be the reason for the mode of inhibition of amylases. The mode of inhibition also depends on the substrate specificity of the enzymes. The inhibitory constant (Ki) for the crude polyphenol extract was 66.7 μg, but the dissociation constants (Ki′) of phenolic compounds were in the range of 4.6 × 10−7 M–7.3 × 10−7 M. Kinetics of amylase inhibition by phenolic compounds indicated the presence of secondary binding sites in malted finger millet amylases similar to other cereal amylases. Finger millet phenolics also showed strong inhibition towards α-glucosidase and pancreatic amylase and the IC50 values were 16.9 and 23.5 μg of phenolics, respectively. Kinetic studies of these enzymes in the presence of millet phenolics revealed a non-competitive type of inhibition. The inhibitory constant (Ki) for α-glucosidase and pancreatic amylase were 5.0 and 10 μg of finger millet phenolics respectively, whereas the dissociation constant (K′i) for α-glucosidase and pancreatic amylase were 2.5 and 7 μg of phenolic compounds respectively. The phenolic compounds present in the millet seed coat may regulate the glucose uptake from the intestinal lumen by inhibiting carbohydrate digestion and absorption, leading to glucose homeostasis and reduce postprandial hyperglycemia. Hence finger millet phenolics can be used as amylase and α-glucosidase inhibitors for modulation of carbohydrate breakdown and regulation of glycemic index of foods thus reducing the chronic pathologies such as diabetes mellitus.
Inhibition of aldose reductase
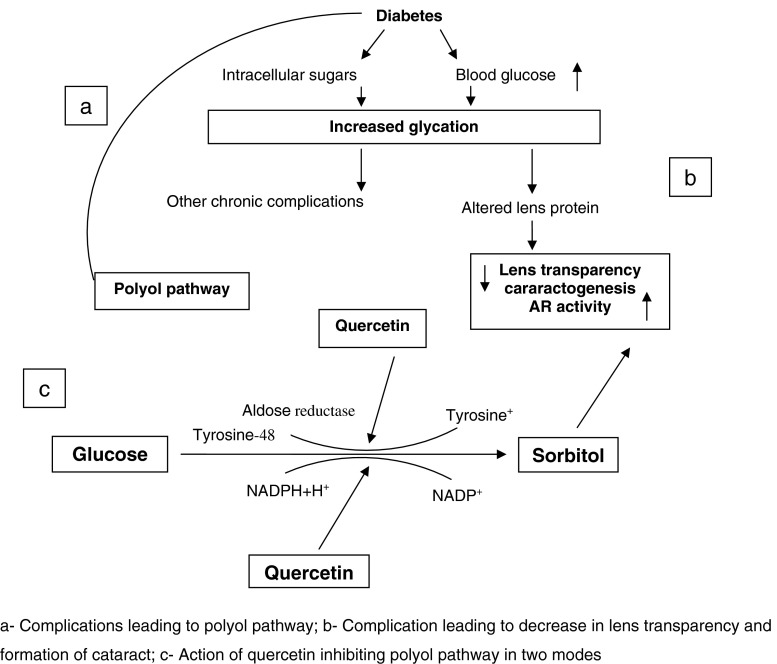
Mode of inhibition of aldose reductase from cataracted eye lenses by finger millet polyphenols was studied by Chethan et al. (2008b). Diabetes induced cataract is characterized by an accumulation of sorbitol, which is mediated by the action of a key enzyme aldose reductase (AR). The non-enzymatic glycation (binding of glucose to protein molecule) induced during diabetes appear to be the key factor for AR mediated sugar-induced cataract. AR enzyme is crucial in cataractogenesis via a polyol pathway (Fig. 3). Crude phenolic extracts from finger millet exhibited the strong inhibitory effects on AR activity and showed an IC50 of 60.12 μg/ml. Mode of inhibition of polyphenols on aldose reductase could be by preventing either the enzymatic conversion of glyceraldehyde to glycerol and glucose to sorbitol, thereby replenishing the depletion of NADPH levels. Phenolic constituents in finger millet phenolics such as gallic, protocatechuic, p-hydroxy benzoic, p-coumaric, vanillic, syringic, ferulic, trans-cinnamic acids and the quercetin was found to inhibit cataract effectively. Structure and function analysis revealed that phenolics with hydroxy group at 4th position is important for aldose reductase inhibitory property. Furthermore, the presence of neighboring O-methyl group in phenolics denatured the AR activity. Quercetin is the most potent AR inhibitory component among the finger millet polyphenolic constituents with IC50 at 25.23 ± 2.2 μg/ml. The activity was correlated with antioxidant potency with the correlation coefficient (r = 0.99, p ≤ 0.1) between antioxidant and AR inhibitory effect of phenolic constituents suggesting that the proton abstracting ability is responsible for AR inhibitory effect. Quercetin exhibits non competitive inhibition on AR enzyme and it may render reversible inhibition by successfully blocking the polyol pathway leading to cataractogenesis. The strong hydrogen abstracting ability of quercetin may replace the proton donation from AR-Histidine-110/Tyrosine-48, which is a key step in the NADPH regenerating potential substantiating the effective AR blockade activity. AR inhibitions potentially resulted in no or only trace accumulation of sorbitol, which is beneficial to overcome the osmotic pressure that may also affect eye lens.
Fig. 3.
Etiology of complications of diabetes (Chethan et al. 2008a)
Dietary fiber (DF)
Finger millet like any other cereal is a source of dietary carbohydrates but the proportion of dietary fiber in finger millet is relatively higher than many other cereals. Finger millet carbohydrates (72%) comprises of starch as the main constituent and the non starchy polysaccharides (NSP) which amounts to 15–20% of the seed matter as an unavailable carbohydrate. DF, principally the NSP and lignin of the plant origin, is not digested by endogenous enzymes within the human intestinal tract, but is an important component of our diet (DeVries et al. 1999). DF can be divided into two categories according to their water solubility. Each category provides different therapeutic effects. Water-soluble fibre (SDF) consists of NSP, mainly β-glucan and arabinoxylan. Water-insoluble fibre (IDF) contains lignin, cellulose, hemicelluloses (Bingham 1987; Marlett 1990), and NSP such as water-unextractable arabinoxylan. In millets, NSP form the quantitatively most important source of both soluble and insoluble dietary fibers (Bunzel et al. 2001). In cereal botanical components, the majority of dietary fibres generally occur in decreasing amounts from the outer pericarp to the endosperm, except arabinoxylan, which is also a major component of endosperm cell wall materials.
The health benefits associated with high fibre foods are delayed nutrient absorption, increased faecal bulk, lowering of blood lipids, prevention of colon cancer, barrier to digestion, mobility of intestinal contents, increased faecal transit time and fermentability characteristics (Tharanathan and Mahadevamma 2003). SDF fractions are important in foods because they trap fatty substances in the gastro-intestinal tract and therefore, reduce cholesterol level in the blood and lower the risk of heart disease. SDF in general has a wide range of functionality due to its ability to absorb water and form gel like structure, and is almost fully fermented in the large intestine microflora, bringing about many desired metabolic effects of fiber (Lopez et al. 1999). The ability of SDF to retard absorption of glucose in the small intestine is also a desirable characteristic in the development of foods for diabetic individuals (Onyango et al. 2004). The increase in the soluble fibre content of the product has special nutritional significance because of its physiological advantages in terms of hypoglycemic and hypocholesterolemic characteristics (Shobana and Malleshi 2007). Soluble fibre also decreases serum cholesterol, postprandial blood glucose, and insulin contents in the human body. Insoluble fiber has a major impact on gastrointestinal transit times, binds water, speeds up intestinal transit, faecal bulk and binds some carcinogens. It reduces contact time for faecal mutagens to interact with the intestinal epithelium and also modifies the activity of digestive microflora and leads to modification or reduction in the production of mutagens. Some fibers can adsorb mutagenic agents and are eliminated in the faeces (Thebaudin et al. 1997).
Formation of the resistant starch (RS) also contributes towards dietary fibre content and complements the health benefits of the millet (Shobana and Malleshi 2007). This residual starch can be quantified in the soluble dietary fiber residue and is highly susceptible to fermentation in the large intestine. RS, a functional fiber fraction is also present in ragi, which escapes the enzymatic digestion imparts beneficial effects by preventing several intestinal disorders (Annison and Topping 1994; Gee et al. 1992). Similar to oligosaccharides, especially fructooligosaccharides, it escapes digestion and provides fermentable carbohydrates for colonic bacteria. It has also been shown to provide benefits such as the production of desirable metabolites, including short-chain fatty acids in the colon, especially butyrate, which seems to stabilize colonic cell proliferation as a preventive mechanism for colon cancer (Englyst et al. 1992). In addition to its therapeutic effects, resistant starch provides better appearance, texture, and mouth feel than conventional fibres (Martinez-Flores et al. 1999).
Composition of the millet DF and changes in contents during processing
Dietary fiber content of finger millet varieties (hilly and base region) has been extensively studied (Premavalli et al. 2004). Dietary fiber content of ten varieties (3 from hilly region and 7 from base region) ranged from 7–21.2% and base region varieties showed higher dietary fiber than hilly region varieties. The total NSP content in native millet is 17.11 g/100 g in which IDF and SDF contributes 15.70, 1.40 g/100 g respectively (Dharmaraj and Malleshi 2010). DF profile of ragi such as total dietary fiber (TDF), IDF, SDF, neutral detergent fiber (NDF), acid detergent fiber, crude fiber, hemicellulose, lignin content is 17.6, 15.7, 1.8, 15.6, 5.2, 4.0, 10.4, 1.3% respectively (Navita and Sumathi 1992). The compositions of neutral detergent fiber in millet viz. hemicellulose, lignin, cellulose and cutin & silica are 34.41 ± 0.99, 29.98 ± 0.99, 27.58 ± 0.85, 9.02 ± 0.28 g/100 g respectively (Thomas et al. 1990).
During processing, the starch molecule undergoes several physical changes depending on its type and the processing methods employed (Goni et al. 1996). Changes in types of dietary fiber viz., TDF, SDF and IDF during different types of processing methods in millets are listed in Table 7. Extrusion processing influences the amount of dietary fiber and resistant starch in foods. Unlu and Faller (1998) have reported that adding certain forms of starch or citric acid to corn meal prior to extrusion modifies RS and dietary fibre. Fermentation of sorghum-based foods before extrusion has also been reported to counteract the formation of RS, whereas direct acidification does not (Knudsen and Munck 1985). Extrusion cooking of Uji reduced total dietary fibre by 39–68%, redistributed soluble to insoluble fibre ratios and had a negligible effect on the formation of resistant starch (less than 1 g/100 g). In extrusion cooking the combined effects of shear along with heat and pressure are mainly responsible for the modification of starch properties. During the process, maximum gelatinization of starch occurs at a temperature of 100˚C and a highest feed moisture content of 23%. As the extrusion temperature increases, the puffing expansion also increases to a maximum of 170˚C discharge temperature and the products will have improved carbohydrate digestibility. This could probably be the reason for the decreased formation of RS during extrusion cooking (Mangala et al. 1999).
Table 7.
Effect of processing on dietary fiber fractions of finger millet (%)
| Processing | TDF | IDF | SDF |
|---|---|---|---|
| Native flourb | 19.8 ± 0.5 | 18.1 ± 00.4 | 0.7 ± 0.9 |
| Cookingb | 14.9 ± 0.2 | 13.0 ± 0.2 | 1.9 ± 0.2 |
| Pressure cookingb | 15.7 ± 0.5 | 14.0 ± 0.2 | 1.6 ± 0.1 |
| Autoclavingb | 15.4 ± 0.1 | 13.9 ± 0.2 | 1.5 ± 0.1 |
| Re-autoclavingb | 14.7 ± 0.2 | 12.8 ± 0.1 | 1.9 ± 0.1 |
| Puffingb | 20.3 ± 0.2 | 19.6 ± 0.2 | 0.8 ± 0.2 |
| Roastingb | 14.7 ± 0.1 | 13.1 ± 0.4 | 1.6 ± 0.2 |
| Bakingb | 9.7 ± 0.4 | 8.6 ± 0.2 | 1.2 ± 0.3 |
| Fryingb | 12.2 ± 1.0 | 11.1 ± 0.9 | 1.1 ± 0.3 |
| Germinationb | 10.7 ± 0.4 | 8.9 ± 0.3 | 1.8 ± 0.1 |
| Maltingb | 12.0 ± 0.4 | 8.8 ± 0.1 | 3.3 ± 0.1 |
| Toasting (Roti)b | 13.6 ± 0.3 | 12.6 ± 0.2 | 1.1 ± 0.3 |
| Toasting (Dosa)b | 11.1 ± 0.2 | 9.8 ± 0.1 | 1.3 ± 0.1 |
| Hydrothermally treateda | 21.1 ± 1.6 | 19.1 ± 1.2 | 2.0 ± 0.6 |
| Decorticateda | 14.7 ± 1.8 | 12.3 ± 1.0 | 2.4 ± 0.5 |
According to Onyango et al. (2004), extrusion of the unfermented maize-finger millet increased SDF and reduced IDF, whereas extrusion of the fermented or acidified blends reduced SDF and increased IDF fractions. Total NSP decreased 50% in the unfermented-extruded blend and further to a range of 10% and 12% in blends that were fermented or treated with different molarities of lactic or citric acids before extrusion. The decrease in total NSP is attributable to the high extrusion temperature and intense mechanical shear that disrupts glycosidic linkages and weak bonds between polysaccharide chains of dietary fibre polysaccharides. The decrease in total NSP after extrusion was accompanied by a redistribution of SDF to IDF fractions in all the blends. The proportion of SDF in the raw blend was 39% and increased to 52% in the unfermented-extruded blend. Increased SDF fraction after extrusion or canning is associated with solubilization of some IDF fractions, disruption of ligno-cellulose links in the cell walls and disintegration of larger molecules of fibre resulting in the formation of low molecular weight soluble fragments such as arabinose, xylose, galactose and glucose (Björk et al. 1984; Fornal et al. 1987; Periago et al. 1996, 1997). Solubilization of fibre increases its availability to bacterial flora in the colon making it easier to ferment than insoluble fibre.
SDF fractions in the fermented, lactic or citric acid treated maize-finger millet blends decreased on extrusion. The proportion of SDF in total NSP decreased from 39% in the raw blend to 19% when the blend was fermented before extrusion. The ratio of SDF in total NSP decreased from 30% to 19% and 45% to 30% with increasing molarities of citric and lactic acids, respectively. Extrusion at acidic conditions facilitates conversion of SDF to IDF by polymerization of the short chain fibre fragments to form large insoluble complexes or Maillard compounds that are consequently analysed as lignin (Camire 2001). It is also possible that the diverse bacterial and yeast flora in the backslop fermented blend may have utilized some SDF for their metabolic processes. The decrease in SDF and increase in IDF fractions indicates the increased fibre availability for faecal bulking and water binding in the colon resulting in more frequent and softer bowel motions, reduced risk of constipation and increased volume of waste material (Onyango et al. 2004). In contrast, extrusion processing increases approximately 10% soluble dietary fiber than the unprocessed cereal based weaning foods (Malleshi et al. 1996).
Hydrothermal processing of millets results in 39% decrease in soluble fiber however there is no change in total dietary fiber. Decortication reduced the dietary fibre content of the millet by about 33.2% but at the same time, the proportion of soluble fibre content increased considerably by 170% (Shobana and Malleshi 2007; Dharmaraj and Malleshi 2010). The total NSP content of native, hydro thermally processed and decorticated millet was 20.27, 20.11 and 17.11 g/100 g, respectively. The non-starchy polysaccharide constituents also underwent considerable changes during hydrothermal processing in their composition but their content remained almost unchanged. However, decortication caused qualitative as well as quantitative changes in all the NSP fractions, mainly because of separation of the cellulose rich seed coat matter from the HM. The cold-water and the hot water solubles as well as the hemicellulose-B fractions of NSP decreased after hydrothermal processing, whereas, the pectic polysaccharides, the hemicellulose-A and also the cellulose fractions increased. Decortication results in slight increase in cold-water solubles, significant increase in hot water solubles and decrease in hemicellulose A and B fractions, pectic polysaccharides and cellulosic fraction (Dharmaraj and Malleshi 2010).
Processing of cereals such as wheat, sorghum, maize, ragi, bajra into chapathi, rice processed by pressure cooking had no effect on their TDF, IDF contents with the exception of ragi, whereas the significant increase in TDF and IDF could be due to resistant of tannin bound proteins to enzymatic hydrolysis of proteins (Ramulu and Udayasekhara Rao 1997). Among the cereal based Indian food preparations viz., chapathi, idli, pongal, poori, ragi roti, rice roti, rice flakes upma, semolina idli and upma and the accompaniments such as cooked dhal, chutney and potato palya, the TDF and IDF were higher in ragi roti. The TDF, IDF, RS of ragi roti increased with accompaniment chutney (Sharavathy et al. 2001). Effects of primary processing on dietary fiber profile of sorghum, bajra, ragi and wheat showed the highest and lowest total dietary fiber in ragi and bajra respectively. TDF and IDF contents of unprocessed and milled processed finger millet flour were found too high among all millets (Navita and Sumathi 1992).
The starch fractions based on digestibility are nutritionally recognized as important because of their impact on physiological functions. The starch fractions of 3 hilly and 7 base varieties of finger millets were extensively studied. Rapidly digestible starch (RDS) in hilly varieties ranged from 8.4–11.2% with an average of 10.0 ± 1.4% while in base varieties it ranged from 8.6–9.9% with an average of 9.2 ± 0.6%. Slowly digestible starch (SDS) varied from 32–35% and 26–30% in hilly and base varieties respectively, while total starch (TS) varied from 44–53% and 39–41% in hilly and base varieties respectively. Resistant starch was 0.9% in hilly varieties and ranged from 0.8–1% in base varieties. Relatively, hilly varieties had higher RDS, SDS, TS while RS was less as compared to base varieties. In puffed ragi flour, the results indicated that RDS increased by 5–18.9% and total available starch decreased drastically which indicated its hydrolysis during puffing. Relatively, the increase in RDS and decrease in SDS was higher in hilly varieties than the base ones, while TS was slightly lower in hilly varieties. RS, decreased in the range of 19–26% in hilly varieties and 14–31% in the base ones (Roopa and Premavalli 2008).
RS formation was influenced by different processing methods. Increase in RS was observed during pressure cooking and roasting whereas it was decreased in other process. Repeated autoclaving further decreased the RS (Roopa and Premavalli 2008). However, Mangala et al. (1999) showed RS formation was more pronounced in autoclaving and a drastic increase (five fold) in its content during repeated autoclaving (heating and cooling) because the net crystallinity of RS is increased by cooling in between successive autoclaving cycles. In addition to that, all the processes-popping, roller drying, extrusion, flaking, parboiling, malting increased the RS formation both in rice and ragi flours. Mangala et al. (1999) reported an increase of RS by 9–10% in popped ragi. In contrast Prachure and Kulkarni (1997) reported a decrease in RS during roasting, pressure cooking, frying and cooking methods. In rice, Sagum and Arcot (2000) reported a reduction in RS during boiling. During roller drying of cereal flour, starch becomes fully gelatinized, resulting in a decrease in the molecular entanglement, hence less formation of resistant starch. During preparation of Uji, RS was not detected in the raw blend and only a minimal amount (0.6 g/100 g) was formed when the unfermented blend was extruded. Formation of RS was counteracted when the pH of the blends was lowered either by fermentation or increasing molarities of lactic or citric acids. Formation of RS is associated with retrogradation of amylose (Englyst et al. 1992; Shamai et al. 2003) during which enzyme resistant amylose–amylose linkages are formed. This implies that starchy foods with high amylose content are expected to have high RS content after extrusion.
Structural and functional features of NSP
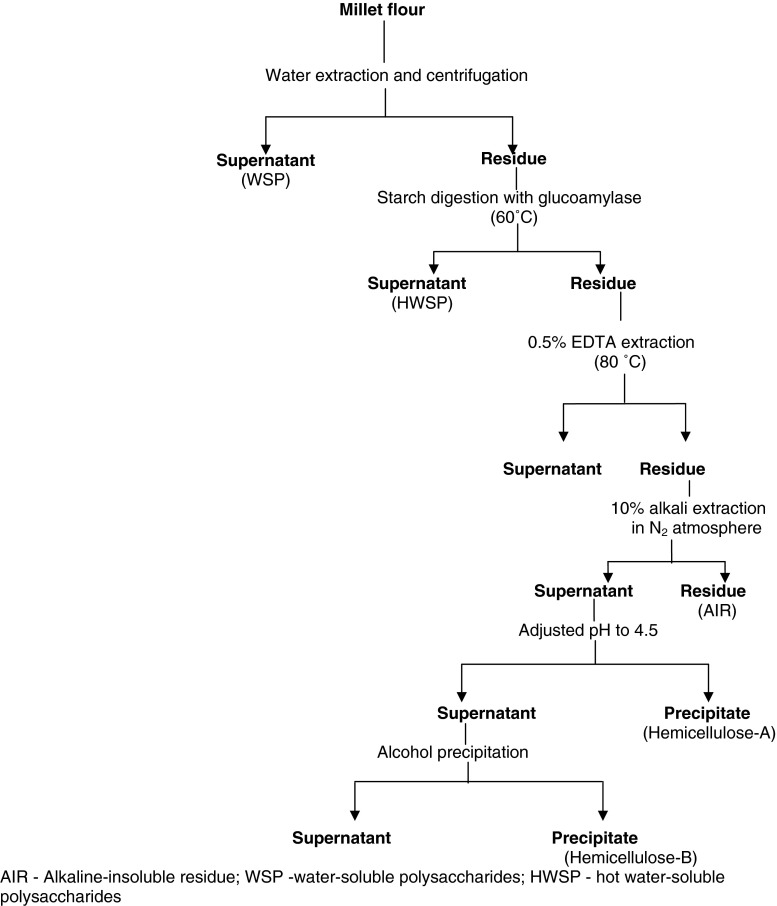
The NSP includes arabinoxylans, 1-3/1-4 β-D-glucans, pectins and arabinogalactans (Izydorczyk and Biliaderis 1995). The schematic procedure for isolation of non starch polysaccharides from the millet is depicted in Fig. 4. Yield of water-soluble NSP, hemicellulose-B and cellulose polysaccharides increase upon malting of the millet causing a substantial decrease in the yield of hemicellulose-A (Rao and Muralikrishna 2001). Arabinoxylans, along with some amount of β-D-glucans, are the major components of soluble dietary fiber. The main water soluble NSP exhibit a wide range of functional properties and health benefits. They are known to have many beneficial roles in human nutrition and health such as lowering cholesterol and fat contents, reducing the disease symptoms of constipation and the risk of diabetes, atherosclerosis and colorectal cancer (Morris et al. 1977; Plaami 1997; Willett 1994). Arabinoxylans are also proposed to have wound dressing potential. Structural elucidation of purified arabinoxylans isolated from finger millet and its malt by methylation followed by fractionation in GLC–MS, periodate oxidation, Smith degradation, NMR, IR, optical rotation, and oligosaccharide analysis indicated that the backbone of the molecule was a 1,4-β-D-xylan, with the majority of the residues substituted at C-3. The structural analysis of oligosaccharide generated by endo xylanase treatment showed that it contained eight xylose and six arabinose residues, substituted at C-3 (monosubstituted) and at both C-2 and C-3 carbons (disubstituted) (Rao and Muralikrishna 2004).
Fig. 4.
Procedure for isolation of non starch polysaccharides from millets (Rao and Muralikrishna 2001)
Feraxans are shown to be low molecular weight polysaccharides with high amounts of arabinose, galactose, uronic acid and ferulic acid. Ferulic acid, a major bound phenolic acid, is known to exist as ester linked mainly to arabinoxylans and influence their physicochemical properties (Ishii 1997). Ferulic acid is supposed to have a number of health benefits. It is known to decrease total cholesterol and increase bioavailability of vitamin-E, vitality of sperms and offers a good protective action against UV radiation–induced skin damage. It is known to have anti-tumor and anti-cancer effects (Mori et al. 1999). Apart from dietary fiber, several reports have proven that ferulic acid is a potential chemo-preventive agent for colorectal cancer (Kawabata et al. 2000; Mori et al. 1999). Feruloyl arabinoxylans were shown to be highly antioxidant, and this property is correlated with their molecular architecture (Rao and Muralikrishna 2006).
Structural characterization of the purified water-soluble feruloyl arabinoxylans (feraxans) from native and malted millet was studied in order to correlate the structure-functional relationship, with specific reference to the bound ferulic acid. Characterization of feraxans by methylation, followed by GLC–MS, and also by 1H NMR and 13C NMR spectroscopy, indicated very high branching and presence of high amounts of O2 substituted xylans. Malting brought dynamic changes in the physicochemical/structural features of feraxans and led to decrease molecular weight (140 kDa to 38.9 kDa) but increased ferulic acid content (161 μg/g to 950 μg/g) of feraxans, due to the action of xylanase. The amount of O2, 3 disubstituted xylopyranosyl residues and the arabinose: xylose ratio was higher in malt feraxans (Rao and Muralikrishna 2007). Water soluble feraxans from the millet exhibited very strong antioxidant activity, which could be 5000 times higher than the activity exerted by sulphated polysaccharides. Apart from phenolic acids, presence of sugars with > C = O (uronyl/acetyl) groups and degree/nature of polymerization impart strong antioxidant activity to the polysaccharides. The ferulic acid present in cereals exhibited strong antioxidant activity in its bound form and thus it need not get digested and be released in the colon through the action of microflora to exert its activity (Ohta et al. 1994, 1997; Rao and Muralikrishna 2006).
Functional characteristics of NSP obtained from native and malted finger millet indicated that the millet can be incorporated as a source of dietary fiber both in the native and malted forms, in the preparation of various health foods, bakery products without affecting the quality of the end-product. Addition of water-soluble NSP also imparted positive effect on the properties of wheat dough. It resulted in increased water absorption, decreased dough development time, increase in dough extensibility, improvement in starch pasting characteristics and good foam stabilization activity, besides a significant increase in loaf volume and softness of the bread (Rao et al. 2007).
Potential contribution of dietary fiber to the health effects of finger millets
DF has gained importance during the last two decades due to its role in decreasing the risk diseases such as diabetes, cardiovascular diseases, colon cancer, constipation and diverticulosis (Ramulu and Udayasekhara Rao 1997). Physical attributes of the fiber causes change in morphology of the intestine and these changes could be associated with functional changes in the gastrointestinal tract through different mechanisms. Consumption of dietary fiber that are viscous lowers blood glucose levels and helps to maintain it and also helps to treat cardiovascular and type II diabetes. Fibers are incompletely or slowly fermented by microflora in the colon promotes normal laxation which prevents constipation, diverticulosis and diverticulitis. Daily intake of fiber is 20–35 g/day for healthy individuals and age plus 5 g/day for children is recommended.
Dietary fiber has major effects on the rate of gastrointestinal absorption; sterol metabolism; ceacal fermentation and stool weight. Rate of intestinal absorption in the upper gastrointestinal tract dietary fiber prolongs gastric emptying time and retards the absorption of nutrients. Both processes are dependent on the physical form of the fiber, and particularly on viscosity. The physiological effects of dietary fiber in relation to functions of intestines are given in Table 8. An important function of insoluble fibers is to increase luminal viscosity in the intestine. The inclusion of viscous polysaccharides in carbohydrate meals reduces the postprandial blood glucose level concentrations in humans. The direct effect of fiber on sterol metabolism may be through one of several mechanisms: altered lipid absorption; altered bile acid metabolism in the cecum; reduced bile acid absorption in the cecum; indirectly via short chain fatty acids, especially propionic acid, resulting from fiber fermentation. Fermentation in colon involves nutrient salvage so that dietary fiber, resistant starch, fat, and protein are utilized by bacteria and the end products are absorbed and used by the body. The functions of dietary fiber in the colon are susceptible to bacterial fermentation, ability to increase bacterial mass and saccharolytic enzyme activity and water holding capacity of the fiber residue after fermentation. The most important mechanism whereby dietary fiber increases stool weight is through the water-holding capacity of unfermented fiber (Eastwood 1992). Potential negative effects of dietary fiber are reduced absorption of vitamins, minerals and proteins. Fermentation of dietary fiber by anaerobic bacteria in the large intestine produces gas such as hydrogen, methane and carbon dioxide, which causes flatulence problems.
Table 8.
Physiological effects of DF in relation to intestinal functions
| Characteristics | Effects | Physiological implications |
|---|---|---|
| DF and small intestinal functions | ||
| Dispersibility in water | Increases volume, dilution of metabolites formed | Slower digestion, promotes nutrient absorption with reduction of plasma cholesterol |
| Bulk | Increases bulk, alters mixing of contents | Alters transit time |
| Viscosity | Slows gastric emptying | Alters mixing and diffusion |
| Adsorption-binding | Increases bile acid excretion | Reduction in plasma cholesterol |
| DF and large intestinal functions | ||
| Dispersibility in water | Provides an aqueous phase for penetration of microbes | Increased polysaccharide break down by microflora |
| Bulk | Increases bulk/volume | Aids laxation |
| Adsorption-binding | Increases bile acid concentration | Bile acid excretion increased |
| Fermentability | Growth of microflora, microbial adaptation to polysaccharide structures | Increased microbial mass and products of metabolism |
Source: Barbara (1999)
Ragi husk, a natural fiber composed of many types of indigestible fractions incorporated to 9% protein diet at a level of 10% promoted better growth in albino rats (Kanchana and Shurpalekar 1988b). Addition of finger millet husk at 8% level to 9% protein diet increased the small bowel length; the villous height in the duodenum and ileum and elevated the activity of the chymotrypsin in both pancreas and intestine and no marked difference was seen in pH profile and activity of trypsin and these results indicated no deleterious effect on the gastrointestinal tract of the albino rats (Kanchana and Shurpalekar 1988a). Tovey (1974) and Jayaraj et al. (1976) found that unrefined wheat, rice bran and certain unrefined grains (finger millet) had a significant buffering effect in vitro digestibility and protect rats against experimental ulceration. NDF has cholesterol lowering action and high hemicellulose content of the dietary fiber content is positively correlated with the effect on cholesterol metabolism. Hypocholesterolemic action of ragi NDF fed in rats showed the lower concentration of cholesterol, triglycerides in serum and tissues and concentration of hepatic bile acids, faecal bile acids, faecal sterols are higher compared to isocaloric fiber free diet fed rats. In vitro binding of NDF with bile acids is found to be low (Thomas et al. 1990). Whole grains of foxtail millet and proso millet fed as diet for a period of 5 weeks to hyperlipidemic rats reduced the concentrations of serum triglycerides and concentrations of serum total, high density lipoprotein (HDL), and low-density lipoprotein (LDL)-cholesterol was found to be lower. Levels of C-reactive protein were significantly lower in the foxtail millet group than the white rice, sorghum, and proso millet groups and these millets may prevent cardiovascular disease by reducing plasma triglycerides (Lee et al. 2010).
Conclusion
Increased nutritional awareness challenges the food industries in developing new food products with special health-enhancing characteristics. The dietary fiber and polyphenols in finger millet are known to offer several health benefits such as antidiabetic, antioxidant, hypocholesterolaemic, antimicrobial effects and protection from diet related chronic diseases to its regular consumers. The millet polyphenols is a complex mixture of benzoic acid and cinnamic acid derivatives and exhibit enzyme inhibitory and anti-cataractogenic activities also. The non starchy polysaccharides of the millet form bulk of its dietary fiber constituents and offer several health benefits including delayed nutrient absorption, increased faecal bulk and lowering of blood lipids. Regular consumption of finger millet as a food or even as snacks helps in managing diabetes and its complications by regulation of glucose homeostasis and prevention of dyslipideamia. This review provides a scientific rationale for the use of finger millet as a therapeutic and health building food.
References
- Adam J, Singer MD, Richard AF, Clark MD. Cutaneous wound healing. N Engl J Med. 1999;341(10):738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- Annison G, Topping DP. Nutritional role of resistant starch: chemical structure and physiological function. Annu Rev Nutr. 1994;14:297–320. doi: 10.1146/annurev.nu.14.070194.001501. [DOI] [PubMed] [Google Scholar]
- Antony U, Chandra TS. Antinutrient reduction and enhancement in protein, starch and mineral availability in fermented flour of finger millet (Eleusine coracana) J Agric Food Chem. 1998;46:2578–2582. [Google Scholar]
- Antony U, Sripriya G, Chandra TS. Effect of fermentation on the primary nutrients in finger millet (Eleusine coracana) J Agric Food Chem. 1996;44:2616–2618. [Google Scholar]
- Antony U, Moses LG, Chandra TS. Inhibition of Salmonella typhimurium and Escherichia coli by fermented flour of finger millet (Eleusine coracana) World J Microbiol Biotechnol. 1998;14:883–886. [Google Scholar]
- Asharani VT, Jayadeep A, Malleshi NG. Natural antioxidants in edible flours of selected small millets. Int J Food Prop. 2010;13(1):41–50. [Google Scholar]
- Bailey CJ. New approaches to the pharmacotherapy of diabetes. In: Pickup JC, William G, editors. Text book of diabetes. 3. UK: Blackwell Science Ltd; 2001. [Google Scholar]
- Baranowski JD, Davidson PM, Nagel CW, Brannen RL. Inhibition of Saccharomyces cerevisiae by naturally occurring hydroxyl cinnamates. J Food Sci. 1980;45:592–594. [Google Scholar]
- Barbara OS. Fiber, inulin and oligofructose: similarities and differences. J of Nutr. 1999;129:1424S–1430S. doi: 10.1093/jn/129.7.1424S. [DOI] [PubMed] [Google Scholar]
- Baron AD. Postprandial hyperglycemia and alpha-glucosidase inhibitors. Diabetes Res Clin Pract. 1998;40:S51–S55. doi: 10.1016/s0168-8227(98)00043-6. [DOI] [PubMed] [Google Scholar]
- Bingham S. Definitions and intakes of dietary fiber. Am J Clin Nutr. 1987;45:1226–1231. doi: 10.1093/ajcn/45.5.1226. [DOI] [PubMed] [Google Scholar]
- Björk I, Nyman M, Asp NG. Extrusion cooking and dietary fibre: effects on dietary fibre content and on degradation in the rat intestinal tract. Cereal Chem. 1984;61(2):174–179. [Google Scholar]
- Bravo L. Polyphenols: chemistry, dietary sources, metabolism and nutritional significance. Nutr Rev. 1998;56:317–333. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- Bunzel M, Ralph J, Marita JM, Hatfield RD, Steinhart H. Diferulates as structural components in soluble and insoluble cereal dietary fiber. J Sci Food Agric. 2001;81:653–660. [Google Scholar]
- Camire ME (2001) Extrusion cooking: technologies and applications. In: Guy R (ed) Woodhead Publishing Co, Cambridge, pp 109–129
- Castelluccio C, Paganga G, Melikian N, Bolwett GP, Pridham J, Sampson J, Rice-Evans C. Antioxidant potential of intermediates in phenylpropanoid metabolism in higher plants. FEBS Lett. 1995;368:188–192. doi: 10.1016/0014-5793(95)00639-q. [DOI] [PubMed] [Google Scholar]
- Chandrasekara A, Shahidi F. Content of insoluble bound phenolics in millets and their contribution to antioxidant capacity. J Agric Food Chem. 2010;58:6706–6714. doi: 10.1021/jf100868b. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S, Ramanathan M, Das J, Bhattacharya SK. Animal models in experimental diabetes mellitus. Indian J Exp Biol. 1997;35:141–145. [PubMed] [Google Scholar]
- Chavan UD, Shahidi F, Naczk M. Extraction of condensed tannins from beach pea (Lathyrus maritmus L.) as affected by different solvents. Food Chem. 2001;75:509–512. [Google Scholar]
- Chethan S, Malleshi NG. Finger millet polyphenols: optimization of extraction and the effect of pH on their stability. Food Chem. 2007;105:862–870. [Google Scholar]
- Chethan S, Malleshi NG. Finger millet polyphenols: characterization and their nutraceutical potential. Am J Food Technol. 2007;2:582–592. [Google Scholar]
- Chethan S, Dharmesh SM, Malleshi NG. Inhibition of aldose reductase from cataracted eye lenses by finger millet (Eleusine coracana) polyphenols. Bioorg Med Chem. 2008;16:10085–10090. doi: 10.1016/j.bmc.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Chethan S, Sreerama YN, Malleshi NG. Mode of inhibition of finger millet malt amylases by the millet phenolics. Food Chem. 2008;111:187–191. [Google Scholar]
- Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuvelier ME, Richard H, Berset C. Comparison of the antioxidative activity of some acid–phenols: structure activity relationship. Biosci Biotechnol Biochem. 1992;56(2):325. [Google Scholar]
- Davison GW, George L, Jackson SK, Young IS, Davies B, Bailey DM, Peters JR, Ashton T. Exercise, free radicals and lipid peroxidation in type 1 diabetes mellitus. Free Radical Biol Med. 2002;33:1543–1551. doi: 10.1016/s0891-5849(02)01090-0. [DOI] [PubMed] [Google Scholar]
- DeVries JW, Prosky L, Li B, Cho S. A historic perspective on defining dietary fiber. Cereal Foods World. 1999;44:367–369. [Google Scholar]
- Dharmaraj U, Malleshi NG (2010) Changes in carbohydrates, proteins and lipids of finger millet after hydrothermal processing. LWT Food Sci Technol. doi:10.1016/j.lwt.2010.08.014
- Dykes L, Rooney LM. Sorghum and millet phenols and antioxidants. J Cereal Sci. 2006;44:236–251. [Google Scholar]
- Eastwood MA. The physiological effect of dietary fiber. Annu Rev Nutr. 1992;12:19–35. doi: 10.1146/annurev.nu.12.070192.000315. [DOI] [PubMed] [Google Scholar]
- Englyst HN, Kingman SM, Cummings JH. Classification and measurement of nutritionally important starch fractions. Eur J Clin Nutr. 1992;46:S33–S50. [PubMed] [Google Scholar]
- Ferguson LR. Role of plant polyphenols in genomic stability. Mutat Res. 2001;475:89–111. doi: 10.1016/s0027-5107(01)00073-2. [DOI] [PubMed] [Google Scholar]
- Fornal L, Soral-Smietana M, Smietana Z, Szpendowski J. Chemical characteristics and physicochemical properties of the extruded mixtures of cereal starches. Starch/Stärke. 1987;39(3):75–79. [Google Scholar]
- Friedman M. Chemistry, biochemistry and dietary role of potato polyphenols—a review. J Agric Food Chem. 1997;45:1523–1540. [Google Scholar]
- Fu MX, Knecht KJW, Thorpe SR, Baynes JW. Role of oxygen in cross linking and chemical modification of collagen by glucose. Diabetes. 1992;41:42–48. doi: 10.2337/diab.41.2.s42. [DOI] [PubMed] [Google Scholar]
- Gee JM, Johnson IT, Lind L. Physiological properties of resistant starch. Eur J Clin Nutr. 1992;46:S125–S131. [PubMed] [Google Scholar]
- Geetha C, Parvathi EP. Hypoglycaemic effect of millet incorporated breakfast on selected non-insulin dependent diabetic patients. Indian J Nutr Diet. 1990;27:316–320. [Google Scholar]
- Goni L, Garcia-Diz Manas E, Galixto FS. Analysis of resistant starch: a method for foods and food products. Food Chem. 1996;56(4):445–449. [Google Scholar]
- Gopalan C (1981) Carbohydrates in diabetic diet. India: Bulletin of Nutrition Foundation p3
- Hadimani NA, Malleshi NG. Studies on milling, physicochemical properties, nutrient composition and dietary fiber content of millets. J Food Sci Technol. 1993;30:17–20. [Google Scholar]
- Hegde PS, Chandra TS. ESR spectroscopic study reveals higher free radical quenching potential in kodo millet (Paspalum scrobiculatum) compared to other millets. Food Chem. 2005;92:177–182. [Google Scholar]
- Hegde PS, Chandrakasan G, Chandra TS. Inhibition of collagen glycation and cross linking in vitro by methanolic extracts of Finger millet (Eleusine coracana) and Kodo millet (Paspalum scrobiculatum) J Nutr Biochem. 2002;13:517–521. doi: 10.1016/s0955-2863(02)00171-7. [DOI] [PubMed] [Google Scholar]
- Hegde PS, Rajasekaran NS, Chandra TS. Effects of the antioxidant properties of millet species on oxidative stress and glycemic status in alloxan-induced rats. Nutr Res. 2005;25:1109–1120. [Google Scholar]
- Hilu KW, De Wet JMJ, Seigler D. Flavonoid patterns and systematics in Eleusine. Biochem Syst Ecol. 1978;6:247–249. [Google Scholar]
- Ishii T. Structure and functions of feruloylated polysaccharides. Plant Sci. 1997;127:111–127. [Google Scholar]
- Izydorczyk MS, Biliaderis CG. Cereal arabinoxylans: advances in structure and physicochemical properties. Carbohydr Polym. 1995;28:33–48. [Google Scholar]
- Jayaraj AP, Tovey FI, Clark CG. The possibility of dietary protective factors in duodenal ulcer. II. An investigation into the effect of pre-feeding with different diets and of instillation of foodstuffs into the stomach on the incidence of ulcers in pylorus-ligated rats. Postgrad Med J. 1976;52:640–644. doi: 10.1136/pgmj.52.612.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JM, Engleson J. Whole grains: benefits and challenges. Annu Rev Food Sci Technol. 2010;1:19–40. doi: 10.1146/annurev.food.112408.132746. [DOI] [PubMed] [Google Scholar]
- Kanchana S, Shurpalekar KS. Effect of ragi (Eleusine coracana) husk on the gastrointestinal tract of albino rats. Nutr Rep Int. 1988;38(4):711–717. [Google Scholar]
- Kanchana S, Shurpalekar KS. Effect of different levels of ragi (Eleusine coracana) husk in semisynthetic diet on the growth of albino rats. Nutr Rep Int. 1988;38(5):1067–1071. [Google Scholar]
- Kavitha MS, Prema L. Post prandial blood glucose response to meals containing different CHO in diabetics. Indian J Nutr Diet. 1995;32:123–126. [Google Scholar]
- Kawabata K, Yamamoto T, Hara A, Shimizu M, Yamada Y, Matsunaga K, Tanaka T, Mori H. Modifying effects of ferulic acid on azoxymethane-induced colon carcinogenesis in F344 rats. Cancer Lett. 2000;157:15–21. doi: 10.1016/s0304-3835(00)00461-4. [DOI] [PubMed] [Google Scholar]
- Kawai K, Murayama Y, Okuda Y, Yamashita K. Post prandial glucose, insulin and glucagon responses to meals with different nutrient compositions in NIDDM. Endocrinol Jpn. 1987;34(5):745–753. doi: 10.1507/endocrj1954.34.745. [DOI] [PubMed] [Google Scholar]
- Khetarpaul N, Chauhan BM. Effect of natural fermentation on phytate and polyphenol content and in vitro digestibility of starch and protein of pearl millet (Pennisetum typhoideum) J Sci Food Agric. 1991;55:189–195. [Google Scholar]
- Khodr B, Khalil Z. Modulation of inflammation by reactive oxygen species: implications for aging and tissue repair. Free Radical Biol Med. 2001;30:1–8. doi: 10.1016/s0891-5849(00)00378-6. [DOI] [PubMed] [Google Scholar]
- King L. Impaired wound healing in patients with diabetes. Nurs Stand. 2001;15(38):39–45. doi: 10.7748/ns2001.06.15.38.39.c3039. [DOI] [PubMed] [Google Scholar]
- Knudsen KEB, Munck L. Dietary fibre contents and compositions of sorghum and sorghum-based foods. J Cereal Sci. 1985;3:153–164. [Google Scholar]
- Kurup PG, Krishnamurthy S. Glycemic response and lipemic index of rice, ragi and tapioca as compared to wheat diet in human. Indian J Exp Biol. 1993;31:291–293. [PubMed] [Google Scholar]
- Lakshmi Kumari P, Sumathy S. Effect of consumption of finger millet on hyperglycemia in non-insulin dependent diabetes mellitus (NIDDM) subjects. Plant Foods Hum Nutr. 2002;57:205–213. doi: 10.1023/a:1021805028738. [DOI] [PubMed] [Google Scholar]
- Lebovitz HE. Effect of the postprandial state on non traditional risk factors. Am J Cardiol. 2001;88:204–205. [Google Scholar]
- Lee SH, Chung IM, Cha YS, Park Y. Millet consumption decreased serum concentration of triglyceride and C-reactive protein but not oxidative status in hyperlipidemic rats. Nutr Res. 2010;30:290–296. doi: 10.1016/j.nutres.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Lopez HW, Levrat MA, Guy C, Messanger A, Demigne C, Remesy C. Effects of soluble corn bran arabinoxylans on cecal digestion, lipid metabolism, and mineral balance (Ca, Mg) in rats. J Nutr Biochem. 1999;10:500–509. doi: 10.1016/s0955-2863(99)00036-4. [DOI] [PubMed] [Google Scholar]
- Maillard MN, Soum MH, Boivia P, Berset C. Antioxidant activity of barley and malt: relationship with phenolic content. LWT Food Sci Technol. 1996;3:238–244. [Google Scholar]
- Malleshi NG (2003) Decorticated finger millet (Eleusine coracana). US Patent No: 2003/0185951
- Malleshi NG, Chakravarthy MS, Kumar S (1995) A process for the preparation of milled malted flour. Indian Patent No:184879
- Malleshi NG, Hadimani NA, Rangaswami C, Klopfenstein CF. Physical and nutritional qualities of extruded weaning foods containing sorghum, pearl millet, finger millet blended with mung beans and non-fat dried milk. Plant Foods Hum Nutr. 1996;49(3):181–189. doi: 10.1007/BF01093214. [DOI] [PubMed] [Google Scholar]
- Mangala SL, Malleshi NG, Mahadevamma S, Tharanathan RN. Resistant starch from different processed rice and ragi. Eur Food Res Technol. 1999;209:32–37. [Google Scholar]
- Marlett JA. Analysis of dietary fiber in human foods. In: Kritchevsky D, Bonfield C, Anderson JW, editors. Dietary fibre: Chemistry, physiology and health effects. New York: Plenum; 1990. pp. 31–48. [Google Scholar]
- Martinez-Flores HE, Chang YK, Bustos FM, Sinencio FS (1999) Extrusion-cooking of cassava starch with different fiber sources: effect of fibers on expansion and physicochemical properties. Adv Extrusions 271–278
- Matuschek E, Towo E, Svanberg U. Oxidation of polyphenols in phytate reduced high tannin cereals: effect on different phenolic groups and on in vitro accessible iron. J Agric Food Chem. 2001;49:5630–5638. doi: 10.1021/jf0108157. [DOI] [PubMed] [Google Scholar]
- McDonough CM, Rooney LW, Earp CA. Structural characteristics of Eleusine coracana (finger millet) using scanning and fluorescence microscopy. Food Microstr. 1986;5:247–256. [Google Scholar]
- McKeown NM. Whole grain intake is favorably associated with metabolic risk factors for type 2 diabetes and cardiovascular disease in the Framingham Offspring Study. Am J Clin Nutr. 2002;76:390–398. doi: 10.1093/ajcn/76.2.390. [DOI] [PubMed] [Google Scholar]
- Miyake T, Shibamoto T. Antioxidative activities of natural compounds found in plants. J Agric Food Chem. 1997;45:1819–1822. [Google Scholar]
- Monnier VM. Nonenzymatic glycosylation, the Maillard reaction and the aging process. J Gerontol. 1990;45:105–111. doi: 10.1093/geronj/45.4.b105. [DOI] [PubMed] [Google Scholar]
- Mori H, Kawabata K, Yoshimi N. Chemopreventive effects of ferulic acid and rice germ on large bowel carcinogenesis. Anticancer Res. 1999;19:3775–3779. [PubMed] [Google Scholar]
- Morris JN, Marr JW, Clayton DG. Diet and heart: a postscript. Br Med J. 1977;2:1307–1314. doi: 10.1136/bmj.2.6098.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namikii M. Antioxidants/antimutagens in food. Crit Rev Food Sci Nutr. 1990;29:273–300. doi: 10.1080/10408399009527528. [DOI] [PubMed] [Google Scholar]
- Navita G, Sumathi P. Effect of primary processing on dietary fiber profile of selected millets. J Food Sci Technol. 1992;29(5):314–315. [Google Scholar]
- Ohta T, Yamasaki S, Egashira Y, Sanada H. Antioxidant activity of corn bran hemicellulose fragments. J Agric Food Chem. 1994;42:653–656. [Google Scholar]
- Ohta T, Semboku N, Kuchii A, Egashira Y, Sanada H. Antioxidant activity of corn bran cell-wall fragments in the LDL oxidation system. J Agric Food Chem. 1997;45:1644–1648. [Google Scholar]
- Onyango C, Noetzold H, Bley T, Henle T. Proximate composition and digestibility of fermented and extruded uji from maize-finger millet blend. LWT Food Sci Technol. 2004;37:827–832. [Google Scholar]
- Onyango C, Noetzold H, Ziems A, Hofmann T, Bley T, Henle T. Digestibility and antinutrient properties of acidified and extruded maize–finger millet blend in the production of uji. LWT Food Sci Technol. 2005;38:697–707. [Google Scholar]
- Patel JC, Dhirawani MK, Dharne RD. Ragi in the management of diabetes mellitus. Indian J Med Sci. 1968;22:28–29. [PubMed] [Google Scholar]
- Periago MJ, Englyst HN, Hudson GJ. The influence of thermal processing on the non-starch polysaccharide (NSP) content and in vitro digestibility of starch in peas (Pisum sativum L) LWT Food Sci Technol. 1996;29(1):33–40. [Google Scholar]
- Periago MJ, Ros G, Casas JL. Non-starch polysaccharides and in vitro starch digestibility of raw and cooked chick peas. J Food Sci. 1997;62(1):93–96. [Google Scholar]
- Plaami SP. Content of dietary fiber in foods and its physiological effects. Food Rev Int. 1997;13(1):29–76. [Google Scholar]
- Prachure AA, Kulkarni PR. Effect of food processing treatments on generation of resistant starch. Int J Food Sci Nutr. 1997;48:257–260. doi: 10.3109/09637489709028570. [DOI] [PubMed] [Google Scholar]
- Premavalli KS, Roopa S, Bawa AS. Effect of variety and processing on the carbohydrate profile of finger millet. Trends Carbohydr Chem. 2004;9:109–113. [Google Scholar]
- Rajasekaran NS, Nithya M, Rose C, Chandra TS. The effect of finger millet feeding on the early responses during the process of wound healing in diabetic rats. Biochim Biophys Acta. 2004;1689:190–201. doi: 10.1016/j.bbadis.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Ramachandra G, Virupaksha TK, Shadaksharaswamy M. Relationship between tannin levels and in vitro protein digestibility in finger millet (Eleusine coracana Gaertn) J Agric Food Chem. 1977;25:1101–1104. doi: 10.1021/jf60213a046. [DOI] [PubMed] [Google Scholar]
- Ramananthan MK, Gopalan C. Effect of different cereals on blood sugar levels. Indian J Med Sci. 1957;45:255–262. [PubMed] [Google Scholar]
- Ramulu P, Udayasekhara Rao P. Effect of processing on dietary fiber content of cereals and pulses. Plant Foods Hum Nutr. 1997;50(3):249–257. doi: 10.1007/BF02436061. [DOI] [PubMed] [Google Scholar]
- Rao BSN, Prabhavati J. Tannin content of foods commonly consumed in India and its influence on ionizable iron. J Sci Food Agric. 1982;33:89. doi: 10.1002/jsfa.2740330116. [DOI] [PubMed] [Google Scholar]
- Rao PU, Deosthale YG. In vitro availability of iron and zinc in white and colored ragi (Eleusine coracana): Role of tannin and phytate. Plants Foods Hum Nutr. 1988;38:35–41. doi: 10.1007/BF01092308. [DOI] [PubMed] [Google Scholar]
- Rao MVSSTS, Muralikrishna G. Non-starch polysaccharides and bound phenolic acids from native and malted finger millet (ragi, Eleusine coracana, Indaf-15) Food Chem. 2001;72:187–192. [Google Scholar]
- Rao MVSSTS, Muralikrishna G. Evaluation of the antioxidant properties of free and bound phenolic acids from native and malted finger millet (ragi, Eleusine coracana Indaf-15) J Agric Food Chem. 2002;50:889–892. doi: 10.1021/jf011210d. [DOI] [PubMed] [Google Scholar]
- Rao MVSSTS, Muralikrishna G. Structural analysis of arabionoxylans isolated from native and malted finger millet (Eleusine coracana, ragi) Carbohydr Res. 2004;339:2457–2463. doi: 10.1016/j.carres.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Rao RSP, Muralikrishna G. Water soluble feruloyl arabinoxylans from rice and ragi: changes upon malting and their consequence on antioxidant activity. Phytochem. 2006;67:91–99. doi: 10.1016/j.phytochem.2005.09.036. [DOI] [PubMed] [Google Scholar]
- Rao RSP, Muralikrishna G. Structural characteristics of water-soluble ferulyl arabinoxylans from rice (Oryza sativa) and ragi (finger millet, Eleusine corcana): variations upon malting. Food Chem. 2007;104:1160–1170. [Google Scholar]
- Rao RSP, Manohar RS, Muralikrishna G. Functional properties of water-soluble non-starch polysaccharides from rice and ragi: effect on dough characteristics and baking quality. LWT Food Sci Technol. 2007;40:1678–1686. [Google Scholar]
- Rasmussen O, Winther C, Hermansen K. Glycemic responses to different types of bread in IDDM patients: studies at constant insulinaemia. Eur J Clin Nutr. 1991;45(2):97–103. [PubMed] [Google Scholar]
- Rohn S, Rawel HM, Kroll J. Inhibitory effects of plant phenols on the activity of selected enzymes. J Agric Food Chem. 2002;50:3566–3571. doi: 10.1021/jf011714b. [DOI] [PubMed] [Google Scholar]
- Roopa S, Premavalli KS. Effect of processing on starch fractions in different varieties of finger millet. Food Chem. 2008;106:875–882. [Google Scholar]
- Sagum R, Arcot J. Effect of domestic processing methods on the starch. Non-starch polysaccharides and in vitro starch and protein digestibility of 3 varieties of rice with varying levels of amylose. Food Chem. 2000;70:107–111. [Google Scholar]
- Saldivar S (2003) Cereals: dietary importance. In: Caballero B, Trugo L, Finglas P (ed) Encyclopedia of Food Sciences and Nutrition, Reino Unido: Academic Press, Agosto, London, pp 1027–1033
- Saito N, Sakai H, Sekihara H, Yajima Y. Effect of an α-glucosidase inhibitor (voglibose), in combination with sulphonilureas, on glycemic control in type II diabetes patients. J Int Med Res. 1998;26:219–232. doi: 10.1177/030006059802600501. [DOI] [PubMed] [Google Scholar]
- Scalbert A. Antimicrobial properties of tannins. Phytochem. 1991;30:3875–3883. [Google Scholar]
- Seetharam A, Ravikumar RL. Blast resistance in finger millet—its inheritance and biochemical nature. In: Riley KW, Gupta SC, Seetharamn A, Mushonga JN, editors. Advances in small millets. New York: International Science Publisher; 1994. pp. 449–465. [Google Scholar]
- Shahidi F, Janitha PK, Wanasundara PD. Phenolic antioxidants. Crit Rev Food Sci Nutr. 1992;32:67–103. doi: 10.1080/10408399209527581. [DOI] [PubMed] [Google Scholar]
- Shamai K, Bianco-Peled H, Shimoni E. Polymorphism of resistant starch type III. Carbohydr Polym. 2003;54:363–369. [Google Scholar]
- Shankara P (1991) Investigations on pre-harvest and post harvest aspects of finger millet. Ph. D. thesis, University of Mysore, India
- Sharavathy MK, Urooj A, Puttaraj S. Nutritionally important starch fractions in cereal based Indian food preparations. Food Chem. 2001;75:241–247. [Google Scholar]
- Shobana S, Malleshi NG. Preparation and functional properties of decorticated finger millet (Eleusine coracana) J Food Eng. 2007;79:529–538. [Google Scholar]
- Shobana S, Usha Kumari SR, Malleshi NG, Ali SZ. Glycemic response of rice, wheat and finger millet based diabetic food formulations in normoglycemic subjects. Int J Food Sci Nutr. 2007;58(5):363–372. doi: 10.1080/09637480701252229. [DOI] [PubMed] [Google Scholar]
- Shobana S, Sreerama YN, Malleshi NG. Composition and enzyme inhibitory properties of finger millet (Eleusine coracana L.) seed coat phenolics: mode of inhibition of α-glucosidase and pancreatic amylase. Food Chem. 2009;115:1268–1273. [Google Scholar]
- Shobana S, Harsha MR, Platel K, Srinivasan K, Malleshi NG. Amelioration of hyperglycaemia and its associated complications by finger millet (Eleusine coracana L.) seed coat matter in streptozotocin-induced diabetic rats. Br J Nutr. 2010;104(12):1787–1795. doi: 10.1017/S0007114510002977. [DOI] [PubMed] [Google Scholar]
- Siwela M, Taylor JRN, de Milliano WAJ, Duodu KG. Occurrence and location of tannins in finger millet grain and antioxidant activity of different grain types. Cereal Chem. 2007;84:169–174. [Google Scholar]
- Siwela M, Taylor JRN, de Milliano WAJ, Duodu KG. Influence of phenolics in finger millet on grain and malt fungal load, and malt quality. Food Chem. 2010;121:443–449. [Google Scholar]
- Sripriya G, Chandrasekharan K, Murty VS, Chandra TS. ESR spectroscopic studies on free radical quenching action of finger millet (Eleusine coracana) Food Chem. 1996;57(4):537–540. [Google Scholar]
- Sripriya G, Antony U, Chandra TS. Changes in carbohydrate, free amino acids, organic acids, phytate and HCl extractability of minerals during germination and fermentation of finger millet (Eleusine coracana) Food Chem. 1997;58(4):345–350. [Google Scholar]
- Tharanathan RN, Mahadevamma S. Grain legumes—a boon to human nutrition. Trends Food Sci Technol. 2003;14:507–518. [Google Scholar]
- Thebaudin JY, Lefebvre AC, Harrington M, Bourgeois CM. Dietary fibre: nutritional and technological interest. Trends Food Sci Technol. 1997;8:41–48. [Google Scholar]
- Thomas M, Leelamma S, Kurup PA. Neutral detergent fiber from various foods and its hypocholesterolemic action in rats. J Food Sci Technol. 1990;27(5):290–293. [Google Scholar]
- Thompson LU. Potential health benefits and problems associated with antinutrients in foods. Food Res Int. 1993;26:131–149. [Google Scholar]
- Toeller M. α-Glucosidase inhibitors in diabetes: efficacy in NIDDM subjects. Eur J Clin Invest. 1994;24:31–35. doi: 10.1111/j.1365-2362.1994.tb02253.x. [DOI] [PubMed] [Google Scholar]
- Tovey FI. Aetiology of duodenal ulcer: an investigation into the buffering action and the effect on pepsin of bran and unrefined carbohydrate foods. Postgrad Med J. 1974;50:683. doi: 10.1136/pgmj.50.589.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovey FI. Diet and duodenal ulcer. J Gastroenterol Hepatol. 1994;9:177–185. doi: 10.1111/j.1440-1746.1994.tb01240.x. [DOI] [PubMed] [Google Scholar]
- Unlu E, Faller JF. Formation of resistant starch by a twin screw extruder. Cereal Chem. 1998;75(3):346–350. [Google Scholar]
- Viswanath V, Urooj A, Malleshi NG. Evaluation of antioxidant and antimicrobial properties of finger millet polyphenols (Eleusine coracana) Food Chem. 2009;114:340–346. [Google Scholar]
- Wadikar DD, Vasudish CR, Premavalli KS, Bawa AS. Effect of variety and processing on antinutrients in finger millet. J Food Sci Technol. 2006;43(4):370–373. [Google Scholar]
- Willett WC. Diet and health: what should we eat? Science. 1994;264:532–537. doi: 10.1126/science.8160011. [DOI] [PubMed] [Google Scholar]