Abstract
Background
The policy in a number of countries is to provide people with a terminal illness the choice of dying at home. This policy is supported by surveys indicating that the general public and patients with a terminal illness would prefer to receive end of life care at home.
Objectives
To determine if providing home-based end of life care reduces the likelihood of dying in hospital and what effect this has on patients’ symptoms, quality of life, health service costs and care givers compared with inpatient hospital or hospice care.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library) to October 2009, Ovid MED-LINE(R) 1950 to March 2011, EMBASE 1980 to October 2009, CINAHL 1982 to October 2009 and EconLit to October 2009. We checked the reference lists of articles identified for potentially relevant articles.
Selection criteria
Randomised controlled trials, interrupted time series or controlled before and after studies evaluating the effectiveness of home-based end of life care with inpatient hospital or hospice care for people aged 18 years and older.
Data collection and analysis
Two authors independently extracted data and assessed study quality. We combined the published data for dichotomous outcomes using fixed-effect Mantel-Haenszel meta-analysis. When combining outcome data was not possible we presented the data in narrative summary tables.
Main results
We included four trials in this review. Those receiving home-based end of life care were statistically significantly more likely to die at home compared with those receiving usual care (RR 1.33, 95% CI 1.14 to 1.55, P = 0.0002; Chi 2 = 1.72, df = 2, P = 0.42, I2 = 0% (three trials; N=652)). We detected no statistically significant differences for functional status (measured by the Barthel Index), psychological well-being or cognitive status, between patients receiving home-based end of life care compared with those receiving standard care (which included inpatient care). Admission to hospital while receiving home-based end of life care varied between trials and this was reflected by high levels of statistically significant heterogeneity in this analysis. There was some evidence of increased patient satisfaction with home-based end of life care, and little evidence of the impact this form of care has on care givers.
Authors’ conclusions
The evidence included in this review supports the use of end of life home-care programmes for increasing the number of patients who will die at home, although the numbers of patients being admitted to hospital while receiving end of life care should be monitored. Future research should also systematically assess the impact of end of life home care on care givers.
Medical Subject Headings (MeSH): *Attitude to Death, *Home Care Services, Hospice Care [*psychology], Patient Preference [*psychology], Randomized Controlled Trials as Topic
MeSH check words: Adult, Humans
BACKGROUND
From surveys of the preferences of the general public and patients with a terminal illness there is a growing consensus that, given adequate support, most people would prefer to receive end of life care at home (Department of Health 2008; Higginson 2000). The preferences of patients who do not have care givers are less clear. In some countries, namely the US, Australia and Canada, the number of people dying at home has increased (Decker 2006), whereas in others, for example the UK, Italy and Japan, it has declined. Paradoxically the UK has more palliative care services than any other country in the European Union (Centeno 2007) and is seen as being a leader for service development in palliative care (Agelopoulos 2009), which includes the provision of home-care teams. Despite this, only a minority die at home; in 2008 it was estimated to be 18% of deaths compared with 58% of deaths in NHS hospitals (Department of Health 2008). Explanations for the larger proportion of people dying in hospital include poorly co-ordinated services with variable standards of provision making it difficult for people to be transferred between settings (National Audit Office 2008). The National Audit Office 2008 emphasises how improved collaboration between health and social care, and acute and community services, could improve the quality of care, reduce emergency admissions and allow more people to die in the place of their choosing. A recent study examining these trends highlights the impact a growing ageing population will have on the number of people dying in hospital unless major changes are made to the way services are provided (Gomes 2008).
The rationale for end of life care at home is complex as it reflects the policy objective of providing patients and their families with a choice of where and when they want care. While a policy supporting choice is broadly endorsed (Agelopoulos 2009; Department of Health 2008), it brings with it conceptual and methodological difficulties for those evaluating the effectiveness of these types of services, and further challenges to those responsible for implementing these interventions. One difficulty underpinning the concept of choice in this context is that while more people want to die at home they also recognise the practical and emotional difficulties of exercising this choice. For example, patients with a terminal illness express concern about being a ‘burden’ to family and friends, worry about their families seeing them in distress or having to get involved with intimate aspects of care (Gott 2004). Therefore, while their preferred place of care may be home, the reality is that preferences can reasonably change over time.
OBJECTIVES
To determine if providing home-based end of life care reduces the likelihood of dying in hospital and what effect this has on patients’ symptoms, quality of life, health service costs and care givers compared with inpatient hospital or hospice care. The following questions are addressed:
Are patients who receive end of life care at home more likely to die at home than those who are allocated to inpatient hospital or hospice care?
Do patients who receive end of life care at home have better symptom control than those who are allocated to inpatient hospital or hospice care?
Does patient and care giver satisfaction differ between end of life care at home and inpatient hospital care?
Do the costs to health services alter as a result of providing end of life care at home?
Do patients receiving end of life care at home have an increased risk of unplanned or precipitous admission to hospital?
METHODS
Criteria for considering studies for this review
Types of studies
We included the following types of studies.
Randomised controlled trials (RCT)
Interrupted time series (ITS)
Controlled before and after (CBA) studies
We excluded CBA studies with fewer than two intervention sites and two control sties. We also excluded interrupted time series without a clearly defined point in time when the intervention occurred and at least three data points before and three after the intervention.
Types of participants
The review includes evaluations of end of life care at home for patients, aged 18 years and over, who are at the end of life and require terminal care.
Types of interventions
Studies comparing end of life care at home with inpatient hospital or hospice care are included. The end of life care at home (which may be referred to as terminal care at home, hospital at home or hospice at home) studies may include patients referred directly from the community who therefore have no physical contact with the hospital, or those referred from the emergency room or hospital inpatient services. We used the following definition to determine if studies should be included in the review: end of life care at home is a service that provides active treatment for continuous periods of time by healthcare professionals in the patient’s home for patients who would otherwise require hospital or hospice inpatient end of life care.
Types of outcome measures
Place of death
Patients’ preferred place of death
Control of symptoms (pain, breathlessness, nausea and vomiting, constipation, terminal agitation)
Delay in care (medical, nursing or domiciliary care) from point of referral to intervention (end of life home care/hospice at home or inpatient care)
Family or care giver stress
Family or care giver unable to continue caring
Patient anxiety
Family/care giver anxiety
Unplanned/precipitous admission or discharge
Search methods for identification of studies
We searched the following databases: the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library) to October 2009, Ovid MEDLINE(R) 1950 to March 2011, EM-BASE 1980 to October 2009, CINAHL 1982 to October 2009 and EconLit to October 2009. Full details of the search terms used are in Appendix 1. We checked the reference lists of articles identified electronically for evaluations of end of life home care and obtained potentially relevant articles. We sought unpublished studies by contacting providers and researchers who were known to be involved in this field. We developed a list of contacts using the existing literature and following discussion with researchers in the area.
Data collection and analysis
One author (SS) read all the abstracts in the records retrieved by the electronic searches to identify publications that appeared to be eligible for this review. Three authors (SS, BW and SSt) independently read these publications and selected studies for the review according to the pre-specified inclusion criteria. We resolved disagreements by discussion. We assessed the quality of eligible trials using the criteria described by the Cochrane Effective Practice and Organisation of Care (EPOC) Group (see ‘METHODS USED IN REVIEWS’, ‘ASSESSMENT OF METHODOLOGICAL QUALITY’ under ‘GROUP DETAILS’ in The Cochrane Library). Two authors (SS and BWor SS and SSt) completed data extraction independently using a checklist developed by EPOC, modified and amended for the purposes of this review (see ‘METHODS USED IN REVIEWS’ under ‘GROUP DETAILS’). We combined the published data for dichotomous outcomes using fixedeffect Mantel-Haenszel meta-analysis (Deeks 1998). The pooled effect is expressed as a risk ratio for end of life home care compared with usual hospital care; values > 1 indicate outcomes favouring end of life care at home, and < 1 for other outcomes. We quantified heterogeneity using Cochran’s Q (Cochran 1954) and the I2 statistic, the latter quantifying the percentage of the total variation across studies that is due to heterogeneity rather than chance (Higgins 2003); smaller percentages suggest less observed heterogeneity. Statistical significance throughout was taken at the two-sided 5% level (2P < 0.05) and data are presented as the estimated effect with 95% confidence intervals. When combining outcome data was not possible because of differences in the reporting of outcomes, we presented the data in narrative summary tables. The study by Jordhøy 2000 was a cluster-randomised trial; this was taken into account in the published analysis for some of the outcomes by testing the significance of differences between treatment groups using bootstrap estimation to fit regression models, allowing for clustering (Jordhøy 2000). However, for the outcomes place of death and admission to hospital no confidence intervals were reported, therefore we adjusted the data entered into the meta-analysis using an estimate of the intra-correlation coefficient (ICC) of 0.02; we obtained this from the Aberdeen database of ICCs (http://www.abdn.ac.uk/hsru/research/research-tools/study-design). We contacted the authors for an estimate of the ICC but have not received these data.
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
From 4264 abstracts we identified four published trials, three trials where the participant was randomised and one cluster-randomised trial, for inclusion in this review (Brumley 2007; Grande 2000; Hughes 1992; Jordhøy 2000). Two of the RCTs were conducted in the US (Brumley 2007; Hughes 1992), one in Norway (Jordhøy 2000) and one in the UK (Grande 2000).
The mean age of participants ranged from 63 years to 74 years old, with numbers of men versus women being roughly equal. Between 17% and 36% of participants lived alone (Brumley 2007; Grande 2000; Jordhøy 2000). The diagnosis of trial participants varied. In one trial, conducted in the US, 21% of participants had a diagnosis of late-stage chronic obstructive pulmonary disease, 33% of heart failure and 47% of cancer, with an estimated life-expectancy of 12 months or less (Brumley 2007). The most common diagnosis in the second trial conducted in the US was cancer, with 73% in the intervention group and 80% in the control group having this diagnosis (Hughes 1992). In Grande 2000, conducted in the UK, 86% of participants had a diagnosis of cancer and the survival from referral was a median of 11 days. The Jordhøy 2000 trial conducted in Norway recruited participants with incurable malignant diseases, excluding those with haematological malignant disease other than lymphoma.
The intervention in three trials was multidisciplinary care, which included specialist palliative care nurses, family physicians, palliative care consultants, physiotherapists, occupational therapists, nutritionists and social care workers. In one trial the focus of the intervention was on nursing care, which was only available for the last two weeks of life. In three trials, nursing care was available for 24 hours if required; in the trial conducted in Norway the smallest urban district did not have access to 24-hour care. The intervention evaluated by Jordhøy 2000 was hospital-based at the Palliative Medicine Unit which provided community outreach. The intervention had four components: (1) all inpatient and outpatient hospital services were provided at the Palliative Medicine Unit unless care elsewhere was required for medical reasons; (2) the Palliative Medicine Unit served as a link to the community services and the palliative care physician and community nurse were defined as the main care givers; (3) predefined guidelines were used to keep optimal interaction between services; and (4) community professionals were offered an educational programme which included bedside training and 6 to 12 hours of lectures every six months. The lectures addressed the most frequent symptoms and difficulties in palliative care. Follow-up consultations were with the community staff.
Patients received end of life care at home for a maximum of 14 days in the trial by Grande 2000 and for an average of 68 days in the trial by Hughes 1992. Duration of care was not reported in the other two trials (Brumley 2007; Jordhøy 2000); although survival time was reported it is not possible to link survival time to duration of the intervention as patients moved between care settings.
Two trials described an educational component. In one this was for the patients and their families and included identifying goals of care and the expected course of the disease and outcomes, as well as the likelihood of success of various treatments (Brumley 2007). In the other trial an educational programme was provided for community staff (Jordhøy 2000). In two of the trials the service was co-ordinated by a nurse (Grande 2000; Jordhøy 2000); one was physician-led (Hughes 1992), and in one a core team of physician, specialist nurse and social worker managed care across settings and provided assessment, evaluation, planning, care delivery, follow up, monitoring and continuous reassessment of care (Brumley 2007).
The care that the control group received varied across trials and thus reflected differences in health systems and the way standard care is delivered. In two trials this was described as including home care (though not specialised end of life care), acute inpatient care, primary care services and hospice care (Brumley 2007; Grande 2000). In one trial the control group received inpatient care at a Veterans Administration (VA) hospital (Hughes 1992), and in another trial conventional care was shared among the hospital departments and the community, with no well-defined routine (Jordhøy 2000).
Risk of bias in included studies
The method of randomisation was clearly described in two trials (Brumley 2007; Grande 2000) as was concealment of allocation. Blinding was not possible in any of the trials but all four trials addressed incomplete outcome data and collected baseline data. In one trial the intervention group had access to input available to the control group (e.g. care was supplemented by GP and other community care when less than 24-hour hospital at home input was provided) (Grande 2000). There was no evidence of selective reporting of outcome data in three of the trials (Grande 2000; Hughes 1992; Jordhøy 2000).
Effects of interventions
See: Summary of findings for the main comparison Patient outcomes for home-based end of life care
Place of death
We were able to combine data from three trials to assess the effectiveness of end of life home care on dying at home. We found that those receiving end of life home care were statistically significantly more likely to die at home compared with those receiving usual care (risk ratio (RR) 1.33, 95% confidence interval (CI) 1.14 to 1.55, P = 0.0002; Chi2 = 1.72, df = 2, P = 0.42, I2 = 0%; N= 652); usual care included hospice care, inpatient care and routinely available primary health care. In one of the trials included in this analysis 61% (n = 113/186) of patients allocated to end of life home care actually received this form of care (Grande 2000). One trial reported that patients who died at home were the youngest (median age: intervention 66 years, control 65 years), men (56% versus 65%) and living with spouses (80% versus 69%); whereas patients who died in nursing homes were older (median age: intervention 74 years, control 78 years), women (63% and 67%) and not living with spouses (69% and 64%) (Jordhøy 2000). One trial reported data on numbers dying in hospital and in a nursing home; there was no statistically significant difference between groups for either location (RR 1.12, 95% CI 0.82 to 1.52. P = 0.49); (RR 0.47, 95% CI 0.17 to 1.32, P = 0.15).
Patient outcomes
No statistically significant difference was detected for functional status (measured by the Barthel Index), psychological well-being or cognitive status, between patients receiving end of life home care and those receiving inpatient care (Hughes 1992). Grande 2000 obtained patient outcome data from GPs, district nurses and informal care givers as previous attempts to obtain data directly from patients proved unsuccessful. Outcomes focused on the need for additional support with care and symptoms (pain, nausea/vomiting, constipation, diarrhoea, breathlessness, anxiety and depression). Reported differences between the two groups varied by assessor. For example, a statistically significant difference was detected in care givers’ reports of pain control between the two groups (difference of −0.48 points on a four-point scale, 95% CI −0.93 to −0.03); no statistically significant difference was detected between the two groups for GPs or district nurse assessments. A statistically significant difference was detected between the two groups for GPs’ assessment of depression (difference on a four-point scale −0.6, 95% CI −0.90 to −0.20) and anxiety (difference −0.40, 95% CI −0.80 to −0.02); no statistically significant difference was detected between the two groups for assessments made by informal care givers or district nurses (Grande 2000).
Patient satisfaction
Patients receiving end of life home care reported greater satisfaction than those in the hospital group (P = 0.02) at one-month follow up (Hughes 1992). This difference disappeared at six months follow up, which may reflect a reduced sample size due to the death of a number of these patients. Brumley 2007 reports similar findings, with greater satisfaction reported by those receiving end of life home care at 30 days (OR 3.37, 95% CI 1.42 to 8.10) and no evidence of a statistically significant difference at 60 days (Brumley 2007).
Admitted to hospital
Initially we combined data from all four trials for this outcome and found no statistically significant difference between groups for admission to hospital (RR 0.93, 95% CI 0.82 to 1.05). However, due to the high level of heterogeneity (Chi2 = 25.63, df = 3, P < 0.0001, I2 = 88%) we have not retained this pooled analysis.
Hospital length of stay
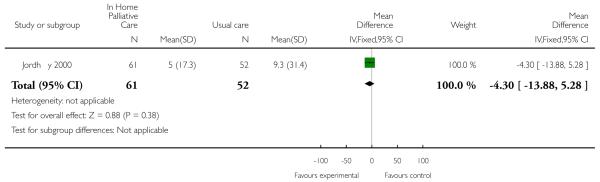
Hughes 1992 examined differences in hospital length of stay in six ways according to the type of bed the patient used, for example a private hospital bed, a Veterans Administration (VA) general bed, or an emergency room bed. Two of the tests revealed a reduction in length of stay for patients receiving end of life home care. Overall use of VA hospital beds was lower for patients allocated to end of life home care compared with those allocated to hospital care, though the difference was not statistically significant. These patients spent on average 10 days (SD 13.3) in any VA hospital bed, compared with 15.9 days (SD 15.86) for the control group (mean difference −5.9 days, 95% CI 0.78 to 11). A comparison was also made of the days spent in a general VA bed. Patients allocated to end of life home care spent on average 5.63 days (SD 10) in a general VA bed, compared with 12.06 days (SD 15.2) for the control group (mean difference −6.43, 95% CI 2.55 to 10.3) (Hughes 1992). Jordhøy 2000 reported a reduction in the number of inpatient days for patients receiving end of life home care that was not statistically significant (difference −4.3, 95% CI −9.19 to 0.59).
Use of other health services
Hughes 1992 examined differences in the use of 15 services such as emergency room visits, rehabilitation and use of private hospitals. The results from all but one of these comparisons were not statistically significant and are not reported. The one statistically significant finding was in the use of outpatient services, with those receiving end of life home care making fewer visits (difference 1.86, 95% CI −3.2 to −0.53, P = 0.01) (Hughes 1992).
Staff views on the provision of services
Grande 2000 reported the views of GPs, district nurses and informal care givers in terms of the provision of services. A statistically significant difference was detected for the perception by district nurses that there should have been additional help for the care givers looking after the patients (difference 0.45, 95% CI 0.12 to 0.77), and that there should have been additional help with night nursing (difference −0.60 on a three-point scale, 95% CI −0.86 to −0.34); this was an on-treatment analysis.
Cost
One trial analysed cost based on the use of health services reported by the patients and confirmed by the providers (Hughes 1992). No statistically significant difference was detected between those receiving the intervention and the control group in overall net healthcare costs. This trial provided no detail on the measurement and valuation of benefits, or on the volume of resources used. Average costs obtained from provider units were used to compare the costs of end of life home care with hospital care. A second trial (Brumley 2007) reported that the average cost per day incurred by those receiving end of life home care was significantly lower than those receiving standard care (mean difference −117.50, t = −2.417, P = 0.02).
Care giver outcomes
Care givers of patients receiving end of life home care reported higher satisfaction compared with care givers in the control group at one-month follow up (Hughes 1992). This difference disappeared at six months, which may reflect a reduced sample size. At six months follow up, care givers of patients in the end of life home care group who had survived more than 30 days reported a decrease in psychological well-being compared with care givers looking after patients in the control group. Grande 2004 found no statistically significant difference between groups for care giver bereavement response six months following death.
DISCUSSION
Despite the widespread support for models of care that better serve the needs of patients at the end of their life, there is only moderate evidence supporting the effectiveness of end of life home care. This is not surprising given the difficulties in conducting research in this area.
Summary of main results
Those receiving end of life home care were statistically significantly more likely to die at home compared with those receiving usual care; there was substantial variability in the data for admission to hospital during end of life home care. The point in a patient’s illness that end of life home care was provided varied between trials, as did the duration of care. For example in one trial median survival from recruitment was 11 days (Grande 2000) and in another it was 196 days (Brumley 2007). There is some evidence indicating higher levels of patient satisfaction for those allocated to end of life home care at one-month follow up. Two of the four trials reported data on care giver outcomes with one of these trials reporting that care givers of patients with a terminal illness receiving end of life home care experienced greater satisfaction than those receiving hospital care (Hughes 1992). However, they experienced lower morale if the patient survived for more than 30 days.
One trial (Hughes 1992), conducted in the US, examined cost in some detail and did not report a statistically significant difference in overall net health costs between end of life home care and hospital care. A second trial (Brumley 2007), also conducted in the US, reported that the average cost per day incurred by those receiving end of life home care was significantly lower than those receiving standard care (mean difference −117.50, t = −2.417, P = 0.02).
Overall completeness and applicability of evidence
All trials were conducted in a developed country, with two in the US, one in the UK and one in Norway between 1992 and 2007. A total of 694 participants were recruited by three trials, and in one trial, three clusters were randomised (N = 434 participants). Around a quarter of participants lived alone. Patient survival times varied, indicating that they were recruited at different stages of their illness. In Grande 2000, participants had a median survival of 11 days from referral, participants recruited to the cluster trial in Norway had an estimated life-expectancy of between two to nine months (Jordhøy 2000), and in the trial conducted in the US of 12 months or less (Brumley 2007). Admissions to hospital also varied, which may be explained by the different healthcare systems, the configuration of existing community-based services and support provided to care givers. Despite these differences, the evidence does support the implementation of end of life home-care programmes, with access to 24-hour care, to support more people dying at home.
Quality of the evidence
The quality of evidence included in this review reflects the difficulties in conducting research in this area. An inevitably high mortality resulted in a loss of power, trials were unblinded and patients crossed over between intervention and control groups. In addition, measuring symptoms and quality of life is difficult, and may have to be done by a proxy (e.g. a nurse, doctor or care giver). However each of these groups can form different impressions, which are then reflected in their assessments of the patient (Grande 2000). There is a risk that some of the results may have occurred by chance as several of the studies conducted a large number of statistical tests. Finally, and most importantly, there are ethical concerns with randomising patients at the end of their life rather than letting them exercise their choice of where they want to be cared for.
Potential biases in the review process
Only one review author reviewed the abstracts and applied the inclusion criteria to produce a long list of potential eligible studies. Two review authors independently applied eligibility criteria and assessed these studies for inclusion, extracted data and assessed the scientific quality. We only identified one abstract of an ongoing trial (Stern 2006) and did not identify subsequent publication of these trial results. We did not identify any unpublished randomised data to include in this review, therefore there is a risk that we have excluded studies that could contribute to this review.
Agreements and disagreements with other studies or reviews
Previous systematic reviews include one published by Smeenk 1998, which compared home-care programmes for patients with incurable cancer to routinely available home care. Studies in which the control group received hospital care were excluded from this review. In addition to noting the poor descriptions of the intervention and control groups’ care, Smeenk 1998 reported that the evidence supporting home-care programmes is inconclusive. Zimmermann 2008 published a systematic review of specialised palliative care across a range of settings. They also concluded that methodological limitations contribute to a weak evidence base.
AUTHORS’ CONCLUSIONS
Implications for practice
The evidence included in this review supports the use of end of life home-care programmes for increasing the number of patients who will die at home, although the numbers of patients being admitted to hospital and the time spent at home while receiving end of life care should be monitored. The organisation of end of life home care will depend on the configuration of existing services as caring for more patients at home will place additional demands on primary care. For example, the trial in Norway concluded that a service with restrictive night services and staff with no specific training in palliative care limited the number of patients who could be admitted. The authors suggest that a more advanced and extensive end of life home-care service may be necessary to substantially increase the proportion of days in home care (Jordhøy 2000). The model of end of life care evaluated by Grande 2000 restricted end of life care to two weeks; this could have led to difficulties in withdrawing a service if a patient had not died within the two-week time frame. The need for access to 24-hour care was highlighted by all of the trials included in this review.
Implications for research
Given that the average age at death is predicted to increase and that those dying are likely to have increasingly complex co-morbidities (Gomes 2008), attention should be given to testing different models of end of life home care. A patient preference design comparing different models of end of life home care could be considered but may limit patient numbers (Grande 2000) and further reduce the generalisability of the results. Prospective audit with robust methods of data collection to document patients’ transfer between care settings also has a place. Key research outcomes should include facilitating patient choice, place of death, the control of patients’ symptoms, transfer to other care settings, impact on healthcare resources and care giver burden. The burden on care givers can be substantial as they provide assistance with a complex range of care needs (Kleinman 2009). This burden can contribute to psychological and physical morbidity.
There are many examples of innovative models of care, with several using a whole-systems approach. Examples are care pathways (Chan 2010; www.mcpcil.org.uk/liverpool_care_pathway) and the Marie Curie Delivering Choice programme. This latter programme includes community service models that provide 24-hour care and aim to strengthen co-ordination between services Agelopoulos 2009). Commissioners of health care require some evidence on how best to organise these services and the major gap in the evidence is around cost-effectiveness. The lack of precision around estimates of admission, or transfer, to hospital could have a major bearing on cost. This needs to be addressed, given the high costs of care at the end of life in developed countries.
PLAIN LANGUAGE SUMMARY.
Home-based end of life care
A number of countries have invested in health services to provide care at home to patients with a terminal illness who wish to die at home. This investment is backed by surveys of the preferences of the general public and patients with a terminal illness, which indicate that most people would prefer to receive end of life care at home. We systematically reviewed the literature to see if the provision of end of life home care reduces the likelihood of dying in hospital and what effect this has on patients’ symptoms, quality of life, health service costs and care givers compared with inpatient hospital or hospice care. We included four trials in our review and report that the provision of end of life home care does increase the probability of dying at home. However, it is not clear if this also results in more people being transferred to hospital during this phase of their illness. There are few data on the impact these services have on family members and lay care givers.
ACKNOWLEDGEMENTS
Professor Steve Iliffe assisted with data extraction for one of the trials (Hughes 1992). We would like to acknowledge the peer review contribution from Mike Bennett, Luciana Ballini, Camilla Zimmermann, Álvaro Sanz, Andy Oxman and Craig Ramsay.
SUMMARY OF FINDINGS FOR THE MAIN COMPARISON
| Patient outcomes for home-based end of life care | ||||||
| Patient or population: patients with a terminal illness | ||||||
| Settings: Norway, UK, USA | ||||||
| Intervention: home-based end of life care | ||||||
| Comparison: a combination of services which could include routine (not specialised) home care, acute inpatient care, primary care services and hospice care | ||||||
| Outcomes |
Illustrative comparative risks* (95% CI)
|
Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
|
| ||||||
| Control | Patient outcomes | |||||
|
| ||||||
| Dying at home Follow up: 6 to 24 months |
Study population
|
RR 1.33 (1.14 to 1.55) | 652 (3 studies) | ⊕⊕⊕⊕ high | In one trial eligible patients were assigned treatment according to the district (cluster) in which they lived | |
| 444 per 1000 | 591 per 1000 (506 to 688) | |||||
|
| ||||||
|
Medium-risk population
| ||||||
| 510 per 1000 | 678 per 1000 (581 to 790) | |||||
|
| ||||||
| Admission to hospital Follow up: 6 to 24 months | See comment | See comment | Estimates ranged from a relative increase in risk of admission to hospital of 2.61 to a relative reduction in risk of 0.62 | 823 (4 studies) | ⊕⊕⊕○ moderate | Data were not pooled due to the high degree of heterogeneity for this outcome |
|
| ||||||
| Patient satisfaction Follow up: 1 to 6 months | See comment | See comment | Not calculated | 199 (2 studies)1 | ⊕⊕○○ low | Increased satisfaction reported at 1 month, not at 6 months |
| Carer burden Follow up 6 months | See comment | See comment | Not calculated | 155 (2 studies) | ⊕⊕○○ low | One study demonstrated a reduction in psychological well-being for care givers of patients who had survived more than 30 days |
The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: confidence interval; RR: risk ratio
GRADE Working Group grades of evidence:
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
Description of the scale used to measure satisfaction was not reported in one of the trials.
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled trial | |
|---|---|---|
| Participants | Age: | |
| Mean age 74 year SD 12.0 | ||
| Sex: | ||
| 51% men (n = 151) | ||
| 49% women (n = 146) | ||
| Ethnicity | ||
| 37% belonged to an ethnic minority group | ||
| 18% were Asian/Pacific Islanders | ||
| 13% Hawaiian | ||
| 4% Latino | ||
| 2% other | ||
| Place of residence | ||
| 66% lived in their own home or apartment 8% lived in the home of a family member | ||
| 74% resided with a family member, primarily a spouse or a child | ||
| 26% lived alone | ||
| Condition: | ||
| Late-stage chronic obstructive pulmonary disease (COPD) (21%); congestive heart failure (CHF) (33%) or cancer with a life-expectancy of 12 months or less (47%); participants visited the emergency department or hospital at least once within the previous year; and scored 70% or less on the Palliative Performance Scale. Life expectancy was assessed by the primary care physician who responded to the question ‘Would you be surprised if this patient died in the next year?’ | ||
| Number recruited: 718 referred to the study, 408/718 excluded, 196 did not meet eligibility criteria, 67 were eligible for and admitted to hospice care, 59 refused, 38 died before enrolment, 26 were part of another research project, and 22 moved out of the area or could not be contacted. 310 terminally ill participants were randomly allocated: T = 155, C = 155. In the intervention group 8/155 died before receiving palliative care, while in the control group 5/155 withdrew from the study. This left 297 available for analysis | ||
|
| ||
| Interventions | Multi-disciplinary team which included a physiotherapist, occupational therapist, speech therapist, dietician, social worker, bereavement co-ordinator, counsellor, chaplain, pharmacist, palliative care physician and a specialist nurse trained in symptom control and biopsychosocial interventions. The specialist nurse provided education, discussed goals of care and the expected course of the disease and expected outcomes as well as the likelihood of success of various treatment and interventions. 24-hour care was available if required | |
| The service was co-ordinated by a core team of physician, specialist nurse and social worker who managed care across settings and provided assessment, evaluation, planning, care delivery, follow up, monitoring and continuous reassessment of care. The service was not time-limited and was provided until death or transfer to a hospice | ||
| Control care: followed Medicare guidelines, services included home health services, acute care services, primary care services and hospice care | ||
|
| ||
| Outcomes | Reid-Gundlach Satisfaction with Services instrument was used to measure overall satisfaction with services, perception of service providers and likelihood of positive recommendations of services to others. Palliative Performance Scale was used to measure severity of illness | |
| Data were also collected retrospectively from health maintenance organisation (HMO) service utilisation databases at each site, from time patient enrolled in study until time of death or end of study period. Medical service use data: costs for all standard medical care and costs associated with the palliative care programme. Service data: number of emergency department visits, physician office visits, hospital days, skilled nursing facility days, home health and palliative visits, palliative physician home visits and days in hospice. Service costs calculated using actual costs for contracted medical services (Colorado) and proxy cost estimates for all services provided within the HMO | ||
|
| ||
| Notes | Healthcare system: US healthcare system, not for profit HMOs. Two-group model, closed panel, non-profit HMOs providing integrated healthcare services in Hawaii and Colorado. The Colorado site has more than 500 physicians representing all medical specialities and sub specialities in 16 separate ambulatory medical offices spread across a greater metropolitan area. The HMO contracts with outside providers for emergency department, hospital, home health and hospice care to serve its 477,000 person membership, which spans the 6-county Denver metropolitan area. The Hawaii site is located in Oahu and serves approximately 224,000 members, with 12 medical offices in Oahu, 3 in Maui and 3 on the Big Island. A medical group of 317 physicians provide care. In contrast to Colorado, the HMO provides all outpatient and most inpatient care, and it also has an internal home health agency | |
|
| ||
| Risk of bias | ||
|
| ||
| Bias | Authors’ judgement | Support for judgement |
|
| ||
| Random sequence generation (selection bias) | Low risk | Group assignment was determined by blocked randomisation using a computer-generated random number chart, stratified according to study site |
|
| ||
| Allocation concealment (selection bias) | Low risk | Once eligibility was determined, the intake clerk contacted the evaluators, who randomly assigned patients to the palliative care intervention or usual care |
|
| ||
| Blinding (performance bias and detection bias) | High risk | |
| All outcomes | ||
|
| ||
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8/155 died in the intervention group before the intervention was delivered; 5/155 withdrew from the control group |
| During the course of the study (maximum follow-up time at 120 days) 75% (n = 225) participants died | ||
|
| ||
| Baseline measures | Low risk | Palliative Performance Scale, demographic data |
|
| ||
| Protection against contamination | Low risk | Both groups had access to hospice care, the control group did not have access to the intervention (an interdisciplinary home-based healthcare programme) |
| Methods | RCT | |
|---|---|---|
| Participants | Requiring terminal care: treatment = 186 (87% with a diagnosis of cancer); control = 43 (86% with a diagnosis of cancer) | |
| Living alone: treatment 21%, control 17% | ||
| Mean age: treatment 72 (SD 11); control 73 (SD 14) | ||
| Male 50%, female 54% | ||
| Survival from referral for both groups a median of 11 days | ||
|
| ||
| Interventions | Referred from primary or secondary care | |
| 6 qualified nurses, 2 nursing aides, a co-ordinator (RGN level), agency staff providing 24-hour care if required for a maximum of 2 weeks, most had Marie Curie experience. Intervention patients could also access standard care | ||
| Control group received standard care: hospital care or hospice care, with input from the GP and district nurses, Marie Curie nursing, Macmillan nursing, social services and private nursing | ||
|
| ||
| Outcomes | Symptoms and support, GP visits, place of death and admission to hospital | |
|
| ||
| Notes | UK study | |
|
| ||
| Risk of bias | ||
|
| ||
| Bias | Authors’ judgement | Support for judgement |
|
| ||
| Random sequence generation (selection bias) | Low risk | 4:1 randomisation ratio (HAH:control) to ensure sufficient admissions to hospital at home. Random numbers from a random number table were used |
|
| ||
| Allocation concealment (selection bias) | Low risk | Allocation for each referral was assigned from a random number table by the researcher and concealed in sequentially numbered, opaque sealed envelopes |
|
| ||
| Blinding (performance bias and detection bias) | High risk | |
| All outcomes | ||
|
| ||
| Incomplete outcome data (attrition bias) | Low risk | Response rates: 144/198 (73%) for carers, 225/228 (99%) district nurses, 194/228 (85%) primary care physicians |
| All outcomes | ||
|
| ||
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
|
| ||
| Baseline measures | Low risk | Demographic data |
|
| ||
| Protection against contamination | High risk | Intervention was contaminated by other input available to the control group (e.g. supplemented by GP and other community care when less than 24-hour hospital at home input was provided) |
| Methods | RCT | |
|---|---|---|
| Participants | Patients who had an estimated life expectancy of < 6 months were recruited. Patients | |
| requiring terminal care (73% in the intervention group had a diagnosis of cancer and 80% in the control group). | ||
| Number of patients in 3 years: | ||
| Treatment = 83 | ||
| Control = 85 | ||
| Average age: | ||
| Treatment: = 65.7 years | ||
| Control = 63.3 years | ||
|
| ||
| Interventions | Hospital at home | |
| Type of service: physician-led | ||
| Skill mix and size of team: nurses; 1 physiotherapist; 1 dietitian; 1 social worker; health technicians | ||
| Control group: inpatient hospital care | ||
|
| ||
| Outcomes | Mortality | |
| Functional status | ||
| Psychological well-being | ||
| Cognitive status | ||
| Patient satisfaction | ||
| Readmission | ||
| Cost | ||
| Inpatient hospital days | ||
| Use of other health services | ||
| Carer satisfaction | ||
| Carer morale | ||
| Follow up: | ||
| 1 month | ||
| 6 months | ||
|
| ||
| Notes | US study | |
|
| ||
| Risk of bias | ||
|
| ||
| Bias | Authors’ judgement | Support for judgement |
|
| ||
| Random sequence generation (selection bias) | Unclear risk | Not described |
|
| ||
| Allocation concealment (selection bias) | Unclear risk | Not described |
|
| ||
| Blinding (performance bias and detection bias) | High risk | |
| All outcomes | ||
|
| ||
| Incomplete outcome data (attrition bias) | Low risk | All stated outcomes reported |
| All outcomes | ||
|
| ||
| Baseline measures | Low risk | |
| Methods | Cluster-randomised controlled trial (3 pairs of clusters stratified into pairs according to the number of inhabitants older than 60 years, and if area was urban or rural) Originally 8 clusters, 2 urban districts with the smallest number of inhabitants > 60 years were merged with larger ones | |
|
| ||
| Participants | Patients with incurable malignant disease, life-expectancy of 2 to 9 months (estimated at referral) and age older than 18 years. Patients with haematological malignant disorders other than lymphomas were excluded from the trial | |
| Median age | ||
| T = 70 years (range 38 to 90) | ||
| C = 69 years (range 37 to 93) | ||
| Sex (number male): | ||
| T = 132/235 (56%) | ||
| C-98/199 (49%) | ||
| Living alone: | ||
| T = 70/235 (30%) | ||
| C = 71/199 (36%) | ||
| Relatives in the same neighbourhood | ||
| T= 214/235 (91%) | ||
| C= 179/199 (90%) | ||
| Receiving home help at the time of recruitment | ||
| T= 26/235 (11%) | ||
| C = 45/199 (23%) | ||
| Number recruited from March 1995 to November 1997 | ||
| 434/707 referred patients were included | ||
| T = 235 | ||
| C = 199 | ||
| Numbers of patients per cluster | ||
| Cluster 1 | ||
| T = 134 | ||
| C = 116 | ||
| Cluster 2 | ||
| T = 77 | ||
| C= 65 | ||
| Cluster 3 | ||
| T= 24 | ||
| C= 18 | ||
|
| ||
| Interventions | A hospital-based intervention co-ordinated by the Palliative Medicine Unit with community outreach. The intervention had been operational for 2 years and 8 months. The Palliative Medicine Unit provided supervision and advice and joined visits at home. The community nursing office determined the type and amount of home care and nursing home care offered | |
| Multidisciplinary, involving palliative care team, community team, patients and families Specialist palliative care nurses provided care in the home with a family physician and palliative care consultants (n = 3) Physiotherapy, nutrition and social care available. Access to a priest. 24-hour care was limited with the smallest urban district not having access to 24-hour care | ||
| Educational programme for community staff including bedside teaching and 6 to 12 hours of lectures every 6 months | ||
| Access to informal help | ||
| T = 187/235 (80%) | ||
| C= 140/199 (70%) | ||
| Control group: conventional care is shared among the hospital departments and the community | ||
|
| ||
| Outcomes | Time at home, place of death, admissions to hospital, health-related quality of life, admission to nursing home, survival Follow up of maximum 2 years | |
|
| ||
| Notes | Healthcare system: the Norwegian Public Health Service which provides hospital and community care. The intervention was linked to the Trondheim University Hospital The Norwegian Public Health Service provides hospital and community care. Eight community healthcare districts participated: 6 districts of Trondheim city (population 141,000) and 2 neighbouring rural communities (Malvik: population 10,000 and Melhus: population 13,000) | |
| Community services in all the districts are similar: include family physicians, home-care nursing and nursing homes. One family physician manpower-year serves around 1500 inhabitants. A mean of 30 manpower-years of home-care nurses’ or nurse-assistants’ time are available per 1000 inhabitants older than 67 years. All except the smallest urban district provides 24-hour home-care service. However, night service is limited to short visits or telephone consultations. Number of nursing home beds (short and long-term) is restricted to 20 beds per 100 inhabitants older than 80 years. In each district, home-care and nursing home services are co-ordinated at a common community nursing office, which decides the type and amount of service that a referred patient will be offered. Hospital services for all 8 districts are provided by Trondheim University Hospital. Palliative Medicine Unit has 12 inpatient beds, an outpatient clinic and a consultant team that works in and out of the hospital, including 2 palliative care nurses, a social worker, a priest, a nutritionist and a part-time physiotherapist. During the study, 3 fulltime physicians were employed. The team only worked daytime hours | ||
|
| ||
| Risk of bias | ||
|
| ||
| Bias | Authors’ judgement | Support for judgement |
|
| ||
| Random sequence generation (selection bias) | High risk | Eligible patients were assigned treatment according to the district (cluster) in which they lived |
|
| ||
| Allocation concealment (selection bias) | High risk | Cluster-randomised controlled trial of 8 local community healthcare districts stratified into pairs according to the number of inhabitants older than 60 years and whether the areas were rural or urban. Two small urban districts were merged with larger ones, making a total of 3 clusters |
|
| ||
| Blinding not possible, reliable measures of outcome used | Blinding (performance bias and detection bias) | |
| All outcomes | ||
|
| ||
| Selective reporting (reporting bias) | Low risk | |
|
| ||
| Baseline measures | Low risk | |
|
| ||
| Protection against contamination | Low risk | Intervention was not available to control groups |
C = control
HAH = hospital at home
HMO = health maintenance organisation
RGN = registered general nurse
T = treatment
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Brumley 2003 | A non-randomised study (it is described as a ‘non-equivalent comparison group’) and compares a palliative care programme with home care |
| Enguidanos 2005 | Non-equivalent study design |
| Hughes 1990 | Intervention does not provide end of life home care |
| Hughes 2000 | Intervention is not an alternative to inpatient hospital or hospice care |
| McCusker 1987 | Non-randomised study using routinely collected data |
| McWhinney 1994 | No outcome data reported; authors describe the challenges of conducting a trial in this area |
| Stern 2006 | Abstract only, no outcome data reported. Full article not identified |
DATA AND ANALYSES
Comparison 1. Patient outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Functional status | Other data | No numeric data | ||
| 2 Psychological well-being | Other data | No numeric data | ||
| 3 Cognitive status | Other data | No numeric data | ||
| 4 Patient satisfaction | Other data | No numeric data | ||
| 5 Pain | Other data | No numeric data | ||
| 6 Survival time from referral to death | Other data | No numeric data | ||
| 7 Mortality | Other data | No numeric data | ||
| 8 Dying at home | 3 | 652 | Risk Ratio (M-H, Fixed, 95% CI) | 1.33 [1.14, 1.55] |
| 9 Time spent at home in the last 2 weeks of life | Other data | No numeric data | ||
| 10 Dying in a nursing home | Other data | No numeric data | ||
| 11 Admitted to hospital | 4 | Risk Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 12 Dying in hospital | 1 | 113 | Risk Ratio (M-H, Fixed, 95% CI) | 1.12 [0.82, 1.52] |
| 13 Dying in a nursing home | 1 | 113 | Risk Ratio (M-H, Fixed, 95% CI) | 0.47 [0.17, 1.32] |
| 14 GPs’ ratings of patients anxiety | Other data | No numeric data | ||
| 15 GPs’ ratings of patients depression | Other data | No numeric data | ||
| 16 Severity of illness | Other data | No numeric data | ||
| 17 Number of inpatient days | 1 | 113 | Mean Difference (IV, Fixed, 95% CI) | −4.30 [−13.88, 5.28] |
Comparison 2. Resource use and cost.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Health service use | Other data | No numeric data | ||
| 2 Cost | Other data | No numeric data | ||
| 3 Inpatient days | Other data | No numeric data | ||
Comparison 3. Staff views.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 District nurse views | Other data | No numeric data | ||
Comparison 4. Carer outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Carer satisfaction | Other data | No numeric data | ||
| 2 Carer morale | Other data | No numeric data | ||
Analysis 1.1. Comparison 1 Patient outcomes, Outcome 1 Functional status.
Functional status.
| Study | Heading 1 | Heading 2 |
|---|---|---|
| Hughes 1992 | At 6 months: treatment mean: 72 (n = 18) control mean: 69.31 (n = 16) |
High attrition in both groups due to death. The Barthel Self-Care Index with modified scoring system was used. No p value given, insufficient data to calculate CI |
Analysis 1.2. Comparison 1 Patient outcomes, Outcome 2 Psychological well-being.
Psychological well-being
| Study | Heading 1 | Heading 2 |
|---|---|---|
| Hughes 1992 | At 6 months: treatment mean: 1.54 (n = 17) control mean: 1.57 (n = 14) |
High attrition in both groups due to death. Philadelphia Geriatric Morale Scale used (shortened version). No p value given, insufficient data to calculate CI |
Analysis 1.3. Comparison 1 Patient outcomes, Outcome 3 Cognitive status.
Cognitive status
| Study | Heading 1 | Heading 2 |
|---|---|---|
| Hughes 1992 | At 6 months: treatment mean: 8.33 (n=18) control mean: 8.86 (n=14) |
High attrition in both groups due to death. Short Portable Mental Status Questionnaire used (10 items). No p value given, insufficient data to calculate CI |
Analysis 1.4. Comparison 1 Patient outcomes, Outcome 4 Patient satisfaction.
Patient satisfaction
| Study | Heading 1 | Heading 2 |
|---|---|---|
| Brumley 2007 | Satisfaction measured by the Reid-Gundlack Satisfaction with Service instrument Rates of satisfaction increased in the intervention group at 30 days OR = 3.37; 95% CI = 1.42-8.10; P = .006 (n=216) At 60 days OR 1.79 95% CI 0.65 to 4.96 (n=168) |
|
| Hughes 1992 | At one month: p = .02 At 6 months: treatment mean: 2.72 (n = 17) control mean: 2.45 (n = 14) |
Insufficient data to calculate CI. No p value given, insufficient data to calculate CI. 17 item questionnaire derived from the National Hospice Study |
Analysis 1.5. Comparison 1 Patient outcomes, Outcome 5 Pain.
Pain
| Study | Heading 1 | Heading 2 |
|---|---|---|
| Grande 2000 | Pain assessed by the caregiver Treatment: 2.52 (0.93) Control: 3.0 (1.10) Z = 1.971, p < 0.05 |
A 4 point scale with a lower score indicating less of a problem. This is not significant if patients allocated to HAH but not receiving it are excluded |
Analysis 1.6. Comparison 1 Patient outcomes, Outcome 6 Survival time from referral to death.
Survival time from referral to death
| Study | |
| Brumley 2007 | Intervention arm : 196 + 164 days |
| Comparator arm : 242+ 200 days | |
| t test p=0.03 | |
| Kaplan-Meier survival analysis did not show significant differences in survival time between the 2 groups | |
| Grande 2000 | Treatment group (allocated and admitted to hospital at home): median 16 days |
| Allocated and not admitted to hospital at home: median 8 days | |
| Z = 3.005, p < 0.003 | |
| Jordhøy 2000 | Median survival |
| T=99 days (95% CI 79 to 119 days) | |
| C=127 days (95% CI 88 to 166 days) | |
Analysis 1.7. Comparison 1 Patient outcomes, Outcome 7 Mortality.
Mortality
| Study | Heading 1 | Heading 2 |
|---|---|---|
| Hughes 1992 | At 6 months: | |
| treatment: 68/86 (79.1%) | ||
| control: 66/85 (77.6%) |
Analysis 1.8. Comparison 1 Patient outcomes, Outcome 8 Dying at home.
Review: Hospital at home: home-based end of life care
Comparison: 1 Patient outcomes
Outcome: 8 Dying at home
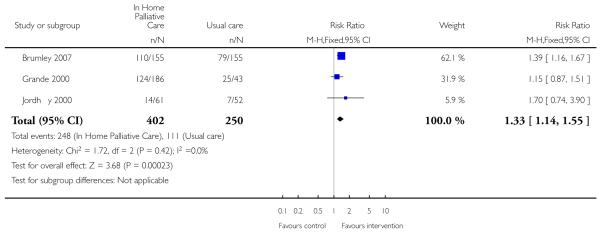
|
Analysis 1.9. Comparison 1 Patient outcomes, Outcome 9 Time spent at home in the last 2 weeks of life.
Time spent at home in the last 2 weeks of life
| Study | Heading 1 | Heading 2 |
|---|---|---|
| Grande 2000 | Treatment: 152/186 82% | |
| Control: 34/44 77% | ||
| X 2 = 0.557, df = 1, p = 0.455 |
Analysis 1.10. Comparison 1 Patient outcomes, Outcome 10 Dying in a nursing home.
Dying in a nursing home
| Study | |
| Jordhøy 2000 | Intervention group: 19/235 (9%) |
| Control group: 36/199 (21%) | |
| P < 0.05, adjusted for prognostic factors and baseline imbalances | |
| Factors predictive of death in nursing homes, individually and according to final logistic regression model, were: female (OR 2.09, p=0.01), age (OR=1.08, p<0.01), living with spouse (OR 0.53, p=0.02) and home care at entry to study (OR 2.52, p<0.01) | |
| After allowance for these factors, difference in nursing home deaths between these 2 groups was still significant (p= 0.01) | |
Analysis 1.11. Comparison 1 Patient outcomes, Outcome 11 Admitted to hospital.
Review: Hospital at home: home-based end of life care
Comparison: 1 Patient outcomes
Outcome: 11 Admitted to hospital
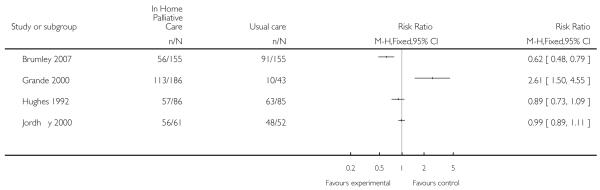
|
Analysis 1.12. Comparison 1 Patient outcomes, Outcome 12 Dying in hospital.
Review: Hospital at home: home-based end of life care
Comparison: 1 Patient outcomes
Outcome: 12 Dying in hospital
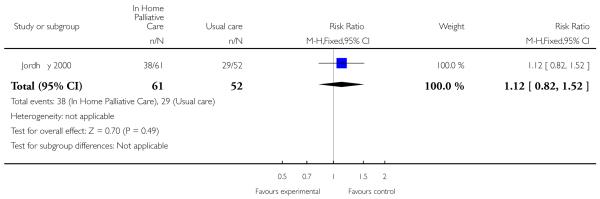
|
Analysis 1.13. Comparison 1 Patient outcomes, Outcome 13 Dying in a nursing home.
Review: Hospital at home: home-based end of life care
Comparison: 1 Patient outcomes
Outcome: 13 Dying in a nursing home
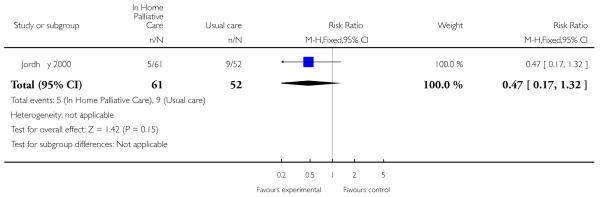
|
Analysis 1.14. Comparison 1 Patient outcomes, Outcome 14 GPs' ratings of patients anxiety.
GPs’ ratings of patients anxiety
| Study | Heading 1 | Heading 2 |
|---|---|---|
| Grande 2000 | Treatment: (n = 127) 2.10 (0.95) Control: (n = 30) 2.5 (0.97) Z= 2.101 |
Intention to treat A 4 point scale with lower scores indicating less of a problem. No difference was detected for the ratings reported by district nurses and informal carers |
Analysis 1.15. Comparison 1 Patient outcomes, Outcome 15 GPs' ratings of patients depression.
GPs’ ratings of patients depression
| Study | Heading 1 | Heading 2 |
|---|---|---|
| Grande 2000 | Treatment: (n = 125) 1.62 (0.76) Control: (n = 27) 2.19 (1.08) Z = 2.603, p < 0.009 |
Intention to treat A 4 point scale with lower scores indicating less ofa problem. No difference was detected for the ratings reported by district nurses and informal carers |
Analysis 1.16. Comparison 1 Patient outcomes, Outcome 16 Severity of illness.
Severity of illness
| Study | Heading 1 | Heading 2 |
|---|---|---|
| Brumley 2007 | Severity of illness measured by the Palliative Performance Scale | Data not reported |
Analysis 1.17. Comparison 1 Patient outcomes, Outcome 17 Number of inpatient days.
Review: Hospital at home: home-based end of life care
Comparison: 1 Patient outcomes
Outcome: 17 Number of inpatient days
|
Analysis 2.1. Comparison 2 Resource use and cost, Outcome 1 Health service use.
Health service use
| Study | Heading 1 | Heading 2 |
|---|---|---|
| Brumley 2007 | Controlling for survival, age, severity of illness and primary disease | Service costs were calculated using actual costs for contracted medical services in Colorado and proxy cost estimates for all services provided within the HMO as services within the HMO are not billed separately. |
| Adjusted mean cost | ||
| T=$12,670 sd $12,523 | ||
| C=$20,222 sd $30,026 | Costs were based on figures from 2002 | |
| Average cost per day incurred by those on intervention arm ($95.30) was significantly lower than that of comparator group ($212.80) (t = -2.417; P = .02). | Hospitalisation and emergency department cost estimates were calculated using aggregated data from more than 500,000 HMO patient records and include ancillary services such as laboratory and radiology. Costs of physician office visits included nurse and clerk expenses. | |
| Home health and palliative care visits were calculated using average time spent on each visit and multiplying that by the cost for each discipline’s reimbursement rate. Proxy costs generated for hospital days and emergency department visits were significantly lower than the actual costs received from contracted providers. | ||
| Total cost variable was constructed by aggregating costs for physician visits, emergency department visits, hospital days, skilled nursing facility days and home health or palliative days accumulated from the point of study enrollment until the end of the study period or death | ||
| Grande 2000 | GP workload in penultimate week of life: evening home visits | |
| Treatment mean 0.17 (0.46) | ||
| Control mean 0.61 (1.42) | ||
| Z = 2.295 | ||
| P < 0.022 | ||
| GP workload: night visits in penultimate week of life | ||
| Treatment mean 0.04 (0.20) | ||
| Control mean 0.26 (0.55) | ||
| Z = 3.61 | ||
| P < 0.0003 | ||
| GP workload in last week of life: | ||
| Evening home visits: | ||
| Treatment mean 0.17 (0.46) | ||
| Control mean: 0.61 (1.42) | ||
| Number in each group: | ||
| Treatment 150-1 | ||
| Control:37-8 | ||
| Night time visits | ||
| Treatment mean: 0.04 (0.2) | ||
| Control mean: 0.26 (0.55) | ||
| Number in each group: | ||
| Treatment 150-1 | ||
| Control:37-8 | ||
| Primary and secondary care services in last 2 weeks of life: failed to detect a difference | ||
| Hughes 1992 | At 6 months: | 95% CI not calculated as equal variances can not be assumed |
| VA services | ||
| outpatient visits mean (SD) at 6 months treatment: 0.73 (1.9) control: 2.59 (6.1) difference: 1. | Comparisons were made with 13 other types of service, these are not reported | |
| 86 | ||
| p = 0.01 |
Analysis 2.2. Comparison 2 Resource use and cost, Outcome 2 Cost.
Cost
| Study | |
|---|---|
| Hughes 1992 | 1986 prices (average costs) |
| Home care: | |
| treatment: $1,001 | |
| control = $343 | |
| p = <0.001 | |
| Insufficient data to calculate CI | |
| VA hospital: | |
| treatment: $1,795 | |
| control: $3,434 | |
| p < 0.02 | |
| Insufficient data to calculate CI | |
| VA general bed: | |
| treatment: $1,310 | |
| control: $2,807 | |
| p < 0.02 | |
| Insufficient data to calculate CI | |
| Cost of all institutional care: | |
| treatment: $2341.79 | |
| control: $3757.37 | |
| p = 0.05 | |
| Insufficient data to calculate CI | |
| Net health care costs per capita: | |
| treatment mean: $4,248.68 | |
| control mean: $3,479.36 | |
| Insufficient data to calculate CI |
Analysis 2.3. Comparison 2 Resource use and cost, Outcome 3 Inpatient days.
Inpatient days
| Study | |
|---|---|
| Hughes 1992 | At 6 months mean (SD): |
| General bed days: | |
| treatment = 5.63 (10) control = 12.06 (15.2) mean difference 6.43 days p = 0.002 | |
| 95%CI 2.55 to 10.3 | |
| All VA hospital days: | |
| treatment: 9.94 (13.3) control = 15.86 (20.1) mean difference 5.92 | |
| p = 0.03 | |
| 95% CI 0.78 - 11 | |
| Jordhoy 2000 | Mean (SD) number of inpatient days |
| T=5.0 (17.3) N=235 | |
| C=9.3 (31.4) N=199 |
Analysis 3.1. Comparison 3 Staff views, Outcome 1 District nurse views.
District nurse views
| Study | Heading 1 | Heading 2 |
|---|---|---|
| Grande 2000 | District nurse thought there should be additional help for the carer | 3 point scale with lower scores indicating less ofa problem No difference was detected for the ratings reported by GPs and informal carers |
| Treatment: (n = 141) 1.81 (0.87) | ||
| Control: (n = 31) 1.36 (0.60) | A 3 point scale with lower scores indicating less of a problem | |
| Z= 2.838 | ||
| P < 0.005 | No difference was detected for the ratings reported by GPs and informal carers | |
| District nurse thought there should be more help with night nursing | ||
| Treatment: (n = 143) 1.43 (0.64) | ||
| Control: (n = 33) 2.03 (0.84) | ||
| Z = 4.012 | ||
| P < 0.0001 |
Analysis 4.1. Comparison 4 Carer outcomes, Outcome 1 Carer satisfaction.
Carer satisfaction
| Study | Heading 1 | Heading 2 |
|---|---|---|
| Hughes 1992 | At 1 month: | |
| p = 0.005 | ||
| Carers in the treatment group reported a greater level of satisfaction | ||
| At 6 months: NS |
Analysis 4.2. Comparison 4 Carer outcomes, Outcome 2 Carer morale.
Carer morale
| Study | Heading 1 | Heading 2 |
|---|---|---|
| Hughes 1992 | At 1 month: NS | Confidence intervals not calculated as no numbers reported |
| At 6 months: | ||
| for patients surviving | ||
| > 30 days: p = 0.03 |
Appendix 1. Search strategy
MEDLINE search terms
exp Home Care Services/
exp Hospitalization/
Terminal Care/
Palliative Care/
Hospice Care/
or/2-5
1 and 6
(hospital adj2 home).tw.
(home-based adj2 hospital-based).tw.
home hospitali?ation.tw.
(hospice adj2 home).tw.
(((terminal or palliative or hospice* or respite) adj2 (care or support)) and home).tw.
((death or dying) adj2 (place or home)).tw.
((end adj2 life) and home).tw.
or/8-14
7 or 15
EMBASE search terms
EMBASE RCT filter (Cochrane Handbook) random$; factorial$; crossover$; cross over$; cross-over$; placebo$; doubl$ adj blind$; singl$ adj blind$; assign$; allocat$; volunteer$; and index terms, known as EMTREE terms: crossover-procedure; double-blind procedure; randomized controlled trial; single-blind procedure.
CINAHL search terms
exp Home Health Care/ or Home Nursing/
Hospitalization/
Terminal Care/
Palliative Care/
Hospice Care/
Hospice and Palliative Nursing/
or/2-6
1 and 7
TI (hospital* N2 home) or AB (hospital* N2 home)
TI (home-based N2 hospital-based) or AB (home-based N2 hospital-based)
TI (home hospitalisation) or TI (home hospitalization) or AB (home hospitalisation) or AB (home hospitalization)
TI (hospice N2 home) or AB (hospice N2 home)
TI (terminal care N5 home) or TI (palliative care N5 home) or TI (respite care N5 home) or AB (terminal care N5 home) or AB (palliative care N5 home) or AB (respite care N5 home)
TI (“place of death” or “dying at home” or “death at home” or “die at home” or “home death”) or AB (“place of death” or “dying at home” or “death at home” or “die at home” or “home death”)
TI (“end of life” N5 home) or AB (“end of life” N5 home)
or/9-15
-
8 or 16
Used SIGN filter (updated to take account of new subject headings)
TI (clinic* trial*) or AB (clinic* trial*)
TI (singl* blind* or doubl* blind* or treb* blind* or tripl* blind*) or TI (singl* mask* or doubl* mask* or treb* mask* or tripl* mask*) or AB (singl* blind* or doubl* blind* or treb* blind* or tripl* blind*) or AB (singl* mask* or doubl* mask* or treb* mask* or tripl* mask*)
TI Placebo* or AB Placebo*
TI (allocated N2 random*) or AB (allocated N2 random*)
(MH “Clinical Trials+”) or (MH “Random Assignment”) or (MH “Double-Blind Studies”) or (MH “Placebos”)
Or/18-22
17 and 23
CENTRAL search terms
exp Home Health Care/ or Home Nursing/
Hospitalization/
Terminal Care/
Palliative Care/
Hospice Care/
Hospice and Palliative Nursing/
or/2-6
1 and 7
(hospital* NEAR2/ home):ti or (hospital* NEAR2/ home):ab
(home-based NEAR2/ hospital-based):ti or (home-based NEAR2/ hospital-based):ab
(home hospitalisation):ti or (home hospitalization):ti or (home hospitalisation):ab or (home hospitalization):ab(hospice NEAR2/ home):ti or (hospice NEAR2/ home):ab
(terminal care NEAR5/ home):ti or (palliative care NEAR5/ home):ti or (respite care NEAR5/ home):ti or (terminal care NEAR5/ home):ab or (palliative care NEAR5/ home):ab or (respite care NEAR5/ home):ab
(“place of death” or “dying at home” or “death at home” or “die at home” or “home death”):ti or (“place of death” or “dying at home” or “death at home” or “die at home” or “home death”):ab
(“end of life” NEAR5/ home):ti or (“end of life” NEAR5/ home):ab
or/9-15
8 or 16
Econlit search terms
ti:(hospital* N2 home) or ab:(hospital* N2 home)
ti:(home-based N2 hospital-based) or ab:(home-based N2 hospital-based)
ti:(home hospitalisation) or ti:(home hospitalization) or ab:(home hospitalisation) or ab:(home hospitalization)ti:(hospice N2 home) or ab:(hospice N2 home)
ti:(terminal care N5 home) or ti:(palliative care N5 home) or ti:(respite care N5 home) or ab:(terminal care N5 home) or ab:(palliative care N5 home) or ab:(respite care N5 home)
ti:(“place of death” or “dying at home” or “death at home” or “die at home” or “home death”) or ab:(“place of death” or “dying at home” or “death at home” or “die at home” or “home death”)
ti:(“end of life” N5 home) or ab:(“end of life” N5 home)
or/9-15
9. 8 or 16
FEEDBACK
Feedback on Review, 5 December 2012
Summary
I would like to draw attention to some fundamental errors in this review.
The review states that “Studies comparing end of life care at home with inpatient hospital or hospice care are included”. Surely, this means that in an included controlled trial, one arm is allocated to home care, and one arm to in-hospital or in-hospice care, at the point of admission or for early discharge during an admission. As the authors state “We used the following definition to determine if studies should be included in the review: end of life care at home is a service that provides active treatment for continuous periods of time by healthcare professionals in the patient’s home for patients who would otherwise require hospital or hospice inpatient end of life care.” However, in none of the included studies is this the case. All studies are comparing different intensities of home care services, sometimes specialist inpatient units are also part of the intervention, with both intervention and control groups able to use hospital or hospice services.
This is what the articles say:
1. Grande GE:
Intervention (BMJ article):
Hospital at home provides practical home nursing care for up to 24 hours a day for up to two weeks. The service was used mainly for terminal care during the last two weeks of life. The hospital at home team consisted of six qualified nurses, two nursing auxiliaries, and a nurse coordinator.
Agency nurses were also used as required.
Both patients allocated to hospital at home and control patients could receive the standard care services provided in the district. The intervention group, however, could also receive hospital at home. Thus the trial compared hospital at home and standard care versus standard care only.
Standard care comprised care in hospital or hospice or care at home with input from general practice, district nursing, Marie Curie nursing, Macmillan nursing, evening district nursing, social services, a flexible care nursing service, or private care. Or in their Palliative Medicine article:
Both CHAH and control patients could receive the standard care provided locally. This included care in hospital or hospice, or care at home with input from GP, district nursing, Marie Curie nursing, Macmillan nursing, evening district nursing, Social Services, private care and a Flexible Care nursing service. The latter was a home nursing service, similar to Marie Curie nursing, but funded by the community NHS Trust and available to all diagnostic groups. Thus the trial compared CHAH and standard care with standard care only.
2. Hughes
“The Edward Hines, Jr. VA Hospital has had a Hospital-Based Home Care (HBHC) program since 1971….(the primary aim of the study was about cost but) we also sought to compare the attributes of the Hines model of care with traditional community home care services to which control group patients could be referred.”
3. Jordhoy
Conventional care is shared among the hospital departments and the community, according to diagnosis and medical needs. No well-defined routines exist.
Palliative-care intervention:
The Palliative Medicine Unit has 12 inpatient beds, an outpatient clinic, and a consultant team that works in and out of the hospital…. We compared the palliative-care intervention with conventional care (control).
4. Brumley
This was a randomized, controlled trial conducted at two separate managed care sites to test the replicability and the effectiveness of an In-home Palliative Care (IHPC) program…. Each patient enrolled in the intervention arm received customary and usual standard care within individual health benefit limits in addition to the IHPC program…. Usual care consisted of standard care to meet the needs of the patients and followed Medicare guidelines for home healthcare criteria.
There would seem to me to be a major lack of understanding of what Hospital at Home means.
Could you please inform me of how the Cochrane Collaboration will address these major flaws?
Submitter agrees with default conflict of interest statement:
I work in a public hospital and in a public hospital in the home unit. I am also President of the Hospital in the Home Society of Australasia, which is a not for profit organisation.
Gideon Caplan Occupation Director, Post Acute Care Services
Reply
Response
As we mention in the discussion of our systematic review, conducting research in the area of end of life care is complex. One of the difficulties is that the care needs and preferences for place of death 1 of people approaching the end of their life can change rapidly; as a result they may require care from different groups of healthcare professionals and in different settings. In the trials included in our systematic review this resulted in a cross over between intervention and control groups (mentioned in the discussion of this systematic review). Finally, and most importantly, there are ethical concerns with not allowing people approaching the end of their life to choose where they want to be cared for. An added challenge for a systematic review in this area is that the evidence cuts across different health systems, again something we mention in the discussion: ‘the care that the control group received varied across trials and thus reflected differences in health systems and the way standard care is delivered.’
1 Munday D, Petrova M, Dale J. Exploring preferences for place of death with terminally ill patients: qualitative study of experiences of general practitioners and community nurses in England. BMJ 2009; 338: b2391 doi:10.1136/bmj.b2391
Our response to the points you make for each of the included studies is below.
Feedback
1. Grande GE:
Intervention (BMJ article): Hospital at home provides practical home nursing care for up to 24 hours a day for up to two weeks. The service was used mainly for terminal care during the last two weeks of life. The hospital at home team consisted of six qualified nurses, two nursing auxiliaries, and a nurse coordinator. Agency nurses were also used as required.
Both patients allocated to hospital at home and control patients could receive the standard care services provided in the district. The intervention group, however, could also receive hospital at home. Thus the trial compared hospital at home and standard care versus standard care only. Standard care comprised care in hospital or hospice or care at home with input from general practice, district nursing, Marie Curie nursing, Macmillan nursing, evening district nursing, social services, a flexible care nursing service, or private care.
Or in their Palliative Medicine article:
Both CHAH and control patients could receive the standard care provided locally. This included care in hospital or hospice, or care at home with input from GP, district nursing, Marie Curie nursing, Macmillan nursing, evening district nursing, Social Services, private care and a Flexible Care nursing service. The latter was a home nursing service, similar to Marie Curie nursing, but funded by the community NHS Trust and available to all diagnostic groups. Thus the trial compared CHAH and standard care with standard care only.
Response
People receiving specialist end of life home care could also be admitted to inpatient care, hospice care and access primary care services (SS, SS, BW).
Feedback
2. Hughes
“The Edward Hines, Jr. VA Hospital has had a Hospital-Based Home Care (HBHC) program since 1971….(the primary aim of the study was about cost but) we also sought to compare the attributes of the Hines model of care with traditional community home care services to which control group patients could be referred.”
Response
The control group also received inpatient care (SS, SS, BW).
Feedback
3. Jordhoy
Conventional care is shared among the hospital departments and the community, according to diagnosis and medical needs. No well-defined routines exist.
Palliative-care intervention: The Palliative Medicine Unit has 12 inpatient beds, an outpatient clinic, and a consultant team that works in and out of the hospital…. We compared the palliative-care intervention with conventional care (control).
Response
We gave additional detail in the included studies table: A hospital-based intervention co-ordinated by the Palliative Medicine Unit with community outreach. The intervention had been working for 2 years and 8 months. The Palliative Medicine Unit provided supervision and advice and joined visits at home. The community nursing office determined the type and amount of home care and home nursing offered. The care was multidisciplinary, involving a palliative care team, community team, patients and families. Specialist palliative care nurses provided care in the home with a family physician and palliative care consultants (n = 3). Physiotherapy, nutrition and social care were available as was access to a priest. 24-hour care was limited; the smallest urban district had no access to 24-hour care.
In addition we asked the authors for additional data and to clarify that their trial was eligible for the review (SS, SS, BW).
Feedback
4. Brumley
This was a randomized, controlled trial conducted at two separate managed care sites to test the replicability and the effectiveness of an In-home Palliative Care (IHPC) program…. Each patient enrolled in the intervention arm received customary and usual standard care within individual health benefit limits in addition to the IHPC program…. Usual care consisted of standard care to meet the needs of the patients and followed Medicare guidelines for home healthcare criteria.
Response
The difference between the intervention and the control group was that the control group did not receive specialised 24 hour ‘in home palliative care’ while those allocated to the intervention had access to it until death or transfer to a hospice (see included studies table) (SS, SS, BW).
Feedback
Could you please inform me of how the Cochrane Collaboration will address these major flaws?
Response
The feedback was submitted to the EPOC feedback editor, who then passed it on to the authors and the EPOC managing editor. The authors drafted a response, which was approved by the feedback editor and an additional EPOC editor.
Contributors
Sasha Shepperd
Bee Wee
Sharon Straus
WHAT’S NEW
Last assessed as up-to-date: 31 March 2011.
| Date | Event | Description |
|---|---|---|
| 18 January 2012 | Feedback has been incorporated | Feedback submitted; feedback and responses included in “Feedback section” |
HISTORY
Review first published: Issue 7, 2011
Footnotes
DECLARATIONS OF INTEREST: Bee Wee is a full time salaried employee of the National Health Service in United Kingdom. Her responsibilities include the provision of specialist palliative care services (in the hospice, community and hospital), service development, education and research in palliative care. Neither she, nor her organisation, stands to gain or lose from the conclusions of this review, but like other services within the National Health Service, the conclusions of this review may inform future service development and/or commissioning.
SOURCES OF SUPPORT
Internal sources
-
Department of Public Health, University of Oxford, UK.Part-funded SS
External sources
-
NIHR Cochrane Programme Grant, UK.Part-funded SS
-
Canada Research Chair in Knowledge Translation, Canada.Funded SSt
DIFFERENCES BETWEEN PROTOCOL AND REVIEW: End of life home care was originally part of a broader review evaluating the effectiveness of hospital at home services. As more data have become available this review has been split into three: admission avoidance hospital at home, early discharge hospital at home and home-based end of life home care.
NOTES: This review is an update; the original review was first published in Issue 1, 1998 of The Cochrane Library (Shepperd 2005). The original review has been separated into three distinct reviews: Hospital at home admission avoidance, Hospital at home early discharge, and Hospital at home: home-based end of life care. The titles have been changed for consistency. Hospital at home admission avoidance (Shepperd 2008) and Hospital at home early discharge (Shepperd 2009) are published in The Cochrane Library.
References to studies included in this review
- Brumley 2007 [Published data only] .Brumley R, Enguidanot S, Jamison P, Seitz R, Morgenstern N, Saito S, et al. Increased satisfaction with care and lower costs: results of a randomized trial of in-home palliative care. Journal of the American Geriatrics Society. 2007;S5:993–1000. doi: 10.1111/j.1532-5415.2007.01234.x. [DOI] [PubMed] [Google Scholar]
- Grande 2000 [Published data only] .Grande G, Farquhar MC, Barclay SIG. Caregiver bereavement outcome: relationship with hospital at home, satisfaction with care and home death. Journal of Palliative Care. 2004;20(2):69–77. [PubMed] [Google Scholar]; Grande GE, Todd CJ, Barclay SI, Farquhar MC. A randomised controlled trial of a hospital at home service for the terminally ill. Palliative Medicine. 2000;14:375–85. doi: 10.1191/026921600701536200. [DOI] [PubMed] [Google Scholar]; Grande GE, Todd CJ, Barclay SI, Farquhar MC. Does hospital at home for palliative care facilitate death at home? Randomised controlled trial. BMJ. 1999;319:1472–5. doi: 10.1136/bmj.319.7223.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes 1992 [Published data only] .Hughes SL, Cummings J, Weaver F, Manheim L, Braun B, Conrad K. A randomized trial of the cost effectiveness of VA hospital-based home care for the terminally ill. Health Services Research. 1992;26:801–17. [PMC free article] [PubMed] [Google Scholar]
- Jordhøy 2000 [Published data only] .Jordhøy MS, Fayers P, Saltnes T, Ahlner-Elmqvist M, Jannert M, Kaasa S. A palliative care intervention and death at home: a cluster randomized trial. Lancet. 2000;356:888–93. doi: 10.1016/s0140-6736(00)02678-7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Brumley 2003 [Published data only] .Brumley RD, Enguidanos S, Cherin DA. Effectiveness of a home-based palliative care program for end-of-life. Journal of Palliative Medicine. 2003;6(5):715–24. doi: 10.1089/109662103322515220. [DOI] [PubMed] [Google Scholar]
- Enguidanos 2005 [Published data only] .Enguidanos SM, Cherin D, Brumley R. Home-based palliative care study: site of death, and costs of medical care for patients with congestive heart failure, chronic obstructive pulmonary disease, and cancer. Journal of Social Work in End-of-life & Palliative Care. 2005;1(3):37–56. doi: 10.1300/J457v01n03_04. [DOI] [PubMed] [Google Scholar]
- Hughes 1990 [Published data only] .Hughes SL, Cummings J, Weaver F, Manheim LM, Conrad KJ, Nash K. A randomized trial of Veterans Administration home care for severely disabled veterans. Medical Care. 1990;28(2):135–45. doi: 10.1097/00005650-199002000-00004. [DOI] [PubMed] [Google Scholar]
- Hughes 2000 [Published data only] .Hughes SL, Weaver FM, Giobbie-Hurder A, Manheim L, Henderson W, Kubal JD, et al. Department of Veterans Affairs Cooperative Study Group on Home-Based Primary Care. Effectiveness of team-managed home-based primary care: a randomized multicenter trial. JAMA. 2000;284(22):2877–85. doi: 10.1001/jama.284.22.2877. [DOI] [PubMed] [Google Scholar]
- McCusker 1987 [Published data only] .McCusker J, Stoddard AM. Effects of an expanding home care program for the terminally ill. Medical Care. 1987;25:373–85. doi: 10.1097/00005650-198705000-00002. [DOI] [PubMed] [Google Scholar]
- McWhinney 1994 [Published data only] .McWhinney IR, Bass MJ, Donner A. Evaluation of a palliative care service: problems and pitfalls. BMJ. 1994;309:1340–2. doi: 10.1136/bmj.309.6965.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern 2006 [Published data only] .Stern A, Goldsmith C, Thabane L, Abernathy T, Cohen R, Jadad A, et al. The efficacy of home telehealth in palliative care for oncology patients and their caregivers. Journal of Palliative Care. 2006;22:208–9. [Google Scholar]
Additional references
- Agelopoulos 2009 .Agelopoulos N, Tate T. The Marie Curie Delivering Choice Programme. European Journal of Palliative Care. 2009;16:290–4. [Google Scholar]
- Centeno 2007 .Centeno C, Clark D, Lynch T, Rocafort J, Praill D, de Lima L, et al. Facts and indicators on palliative care development in 52 countries of the WHO European region: results of an EAPC task force. Palliative Medicine. 2007;21:463–71. doi: 10.1177/0269216307081942. [DOI] [PubMed] [Google Scholar]
- Chan 2010 .Chan R, Webster J. End-of-life care pathways for improving outcomes in caring for the dying. Cochrane Database of Systematic Reviews. 2010;(1) doi: 10.1002/14651858.CD008006.pub2. DOI: 10.1002/14651858.CD008006.pub2. [DOI] [PubMed] [Google Scholar]
- Cochran 1954 .Cochrane WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–29. [Google Scholar]
- Decker 2006 .Decker SL, Higginson IJ. A tale of two cities: factors affecting place of cancer death in London and New York. European Journal of Public Health. 2006;17(3):285–90. doi: 10.1093/eurpub/ckl243. [DOI] [PubMed] [Google Scholar]
- Deeks 1998 .Deeks J, Bradburn M, Bilker W, Localio R, Berlin J. Much ado about nothing: meta-analysis for rare events. 6th Cochrane Colloquium, Baltimore. 1998 [Google Scholar]
- Department of Health 2008 .Department of Health . End of life care strategy - promoting high quality care for all adults at the end of life. Department of Health; 2008. [Google Scholar]
- Gomes 2008 .Gomes B, Higginson IJ. Where people die (1974 to 2030): past trends, future projections and implications for care. Palliative Medicine. 2008;22:33–41. doi: 10.1177/0269216307084606. [DOI] [PubMed] [Google Scholar]
- Gott 2004 .Gott M, Seymour J, Bellamy G, Clark D, Ahmedzai S. Older people’s views about home as a place of care at the end of life. Palliative Medicine. 2004;18:460–7. doi: 10.1191/0269216304pm889oa. [DOI] [PubMed] [Google Scholar]
- Grande 2004 .Grande G, Farquhar MC, Barclay SIG. Caregiver bereavement outcome: relationship with hospital at home, satisfaction with care and home death. Journal of Palliative Care. 2004;20(2):69–77. [PubMed] [Google Scholar]
- Higgins 2003 .Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analysis. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higginson 2000 .Higginson IJ, Sen-Gupta GJA. Place of care in advanced cancer: a qualitative systematic literature review of patient preferences. Journal of Palliative Medicine. 2000;3(3):287–300. doi: 10.1089/jpm.2000.3.287. [DOI] [PubMed] [Google Scholar]
- Kleinman 2009 .Kleinman A. Caregiving: the odyssey of becoming more human. Lancet. 2009;373:292–3. doi: 10.1016/s0140-6736(09)60087-8. [DOI] [PubMed] [Google Scholar]
- National Audit Office 2008 .National Audit Office . End of Life Care. The Stationary Office; 2008. http://www.nao.org.uk/publications/0708/end_of_life_care.aspx. [Google Scholar]
- Shepperd 2005 .Shepperd S, Iliffe S. Hospital at home versus in-patient hospital care. Cochrane Database of Systematic Reviews. 2005;(3) doi: 10.1002/14651858.CD000356.pub2. DOI: 10.1002/14651858.CD000356.pub2. [DOI] [PubMed] [Google Scholar]
- Shepperd 2008 .Shepperd S, Doll H, Angus R, Clarke M, Iliffe S, Kalra L, Ricauda NA, Wilson A. Hospital at home admission avoidance. Cochrane Database of Systematic Reviews. 2008;(4) doi: 10.1002/14651858.CD007491. DOI: 10.1002/14651858.CD007491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepperd 2009 .Shepperd S, Doll H, Broad J, Gladman J, Iliffe S, Langhorne P, Richards S, Martin F, Harris R. Hospital at home early discharge. Cochrane Database of Systematic Reviews. 2009;(1) doi: 10.1002/14651858.CD000356.pub3. DOI: 10.1002/14651858.CD000356.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeenk 1998 .Smeenk FWJM, va Haastregt JCM, de Witte LP, Crebolder HFJM. Effectiveness of home care programmes for patients with incurable cancer on their quality of life and time spent in hospital: a systematic review. BMJ. 1998;316:1939–44. doi: 10.1136/bmj.316.7149.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann 2008 .Zimmermann C, Riechelmann R, Kryzanowska M, Rodin G, Tannock I. Effectiveness of specialised palliative care - a systematic review. JAMA. 2008;299(14):1698–709. doi: 10.1001/jama.299.14.1698. [DOI] [PubMed] [Google Scholar]
- * . Indicates the major publication for the study


