Abstract
Rapid advances in DNA synthesis techniques have made it possible to engineer viruses, biochemical pathways and assemble bacterial genomes. Here, we report the synthesis of a functional 272,871 bp designer eukaryotic chromosome, synIII, which is based on the 316,617 bp native Saccharomyces cerevisiae chromosome III. Changes to synIII include TAG/TAA stop-codon replacements, deletion of subtelomeric regions, introns, tRNAs, transposons and silent mating loci as well as insertion of loxPsym sites to enable genome scrambling. SynIII is functional in S. cerevisiae. Scrambling of the chromosome in a heterozygous diploid reveals a large increase in “a mater” derivatives resulting from loss of the MATα allele on synIII. The total synthesis of synIII represents the first complete design and synthesis of a eukaryotic chromosome, establishing S. cerevisiae as the basis for designer eukaryotic genome biology.
Saccharomyces cerevisiae has a genome size of ~12 megabases (MBs) distributed among 16 chromosomes. The entire genome encodes ~6000 genes of which ~5000 are individually nonessential (1). Which of these non-essential genes are simultaneously dispensable? While a number of studies have successfully mapped pairwise “synthetic lethal” interactions between gene knockouts, those methods do not scale well to 3 or more gene combinations because the number of combinations rises exponentially. Our approach to address this question is to produce a synthetic yeast genome with all nonessential genes flanked by loxPsym sites to enable inducible evolution and genome reduction (a process referred to as SCRaMbLEing) in vivo (2, 3). The availability of a fully synthetic S. cerevisiae genome will allow direct testing of evolutionary questions such as “what is the maximum number of nonessential genes that can be deleted without a catastrophic loss of fitness?” and “what is the catalog of viable 3-gene, 4-gene, … n-gene deletions that survive under a given growth condition?” that are not otherwise easily approachable in a systematic unbiased fashion. Engineering and synthesis of viral and bacterial genomes have been reported in the literature (4–11). An international group of scientists, has embarked on constructing a designer eukaryotic genome, Sc2.0 (www.syntheticyeast.org), and here we report the total synthesis of the first complete designer yeast chromosome.
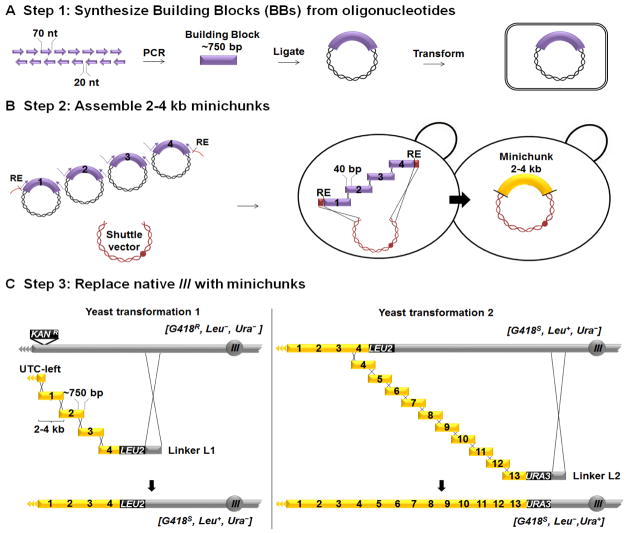
Yeast chromosome III, the third smallest in S. cerevisiae (316,617 bp), containing the MAT locus determining mating type, was the first chromosome sequenced (12). We designed synIII according to fitness, genome stability and genetic flexibility principles developed for the Sc2.0 genome (2). The native sequence was edited in silico using a series of deletion, insertion, and base substitution changes to produce the desired “designer” sequence (Figures 1, S1, S2 and Supplementary Text). The hierarchical wet-lab workflow used to construct synIII (Fig. 2) consisted of three major steps: 1) The 750 bp “building blocks” (BBs) were produced starting from overlapping 60- to 79-mer oligonucleotides and assembled using standard PCR methods (13, 14) by undergraduate students in the Build-A-Genome class at Johns Hopkins University (Fig. 2A) (15). The arbitrary naming scheme for the different sized DNA molecules used in the Sc2.0 project is explained in Fig. S3. 2) The 133 synIIIL (+ centromere) BBs and 234 synIIIR BBs were assembled into 44 and 83 overlapping DNA “minichunks” of ~2–4 kb respectively (Table S1, Figures 2B and S4) (16, 17). 3) All adjacent minichunks for synIII were designed to overlap one another by one BB to facilitate further assembly in vivo by homologous recombination in yeast (18, 19). Using an average of 12 minichunks and alternating selectable markers in each experiment, the native sequence of S. cerevisiae III was systematically replaced by its synIII counterpart in eleven successive rounds of transformation (Fig. 2C; Table S2) (20, 21).
Fig. 1. SynIII design.

Representative synIII design segments for loxPsym site insertion (A & B) and stop codon TAG to TAA editing (C) are shown. Green diamonds represent loxPsym sites embedded in the 3′ UTR of non-essential genes and at several other landmarks. Fuchsia circles indicate synthetic stop codons (TAG recoded to TAA). Complete maps of designed synIII chromosome with common and systematic ORF names, respectively, are shown in Figures S1 & S2.
Fig. 2. SynIII construction.
(A) Building block (BB) synthesis. 750 bp BBs (purple) were synthesized from oligonucleotides at Johns Hopkins University by students in the Build-AGenome course. (B) Assembly of minichunks. 2–4 kb minichunks (yellow) were assembled by homologous recombination in S. cerevisiae (Table S1). Adjacent minichunks were designed to encode overlap of one BB to facilitate downstream assembly steps. Minichunks were flanked by a rare cutting restriction enzyme (RE) site, XmaI or NotI. (C) Direct replacement of native yeast chromosome III with pools of synthetic minichunks. Eleven iterative one-step assemblies and replacements of native genomic segments of yeast chromosome III were carried out using pools of overlapping synthetic DNA minichunks (Table S2), encoding alternating genetic markers (LEU2 or URA3), which enabled complete replacement of native III with synIII in yeast.
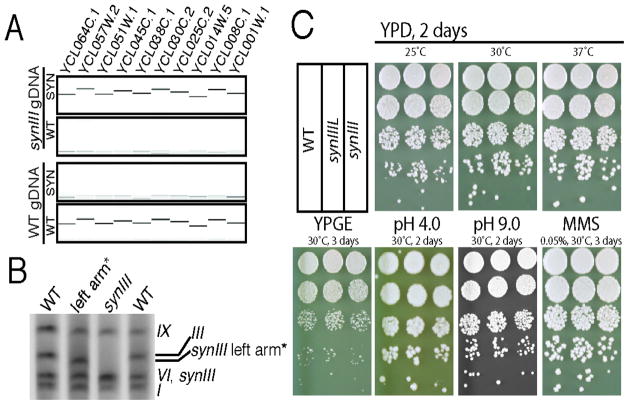
PCRTag analysis (2) revealed the presence of synIII synthetic PCRTags and absence of native PCRTags (Fig. 3A; see Supplementary Text, Figures S5, S6 & S7 for complete set of PCRTag analyses). The smaller size of synIII and intermediates in its full synthesis as compared to the native yeast chromosome was demonstrated by pulsed-field gel electrophoresis (Figures 3B and S8) (22). Analysis of the intermediate strains revealed that the starting strain had some unexpected rearrangements in at least two chromosomes and that an additional rearrangement occurred during the assembly process; these did not affect synIII (Fig. S8). These abnormalities were eliminated through back-crossing the synIIIL intermediate strain to strain BY4742 (Table S3), yielding a MATα strain with an electrophoretic karyotype perfectly matching BY4742 but for the expected altered length III (compare lane 97 to 97* in Fig. S8). Southern blot analyses using arm-specific radiolabeled probes further verified and validated the structure of the left and right arm telomere ends of synIII, which had been specified by the UTC sequence (Fig. S9). Restriction fragment sizes on Southern blots are compatible with the deletion of HML, HMR, and much of each subtelomere (Fig. S9). This was further confirmed by complete genome sequencing of the synIII strain.
Fig. 3. Characterization and testing of synIII strain.
(A) PCRTag analysis (one PCRTag/10 kb) of the left arm of SynIII and wild-type yeast (BY4742) DNA is shown. Analysis of the complete set of PCRTags is shown in Figures S4, S5 & S6. (B) Karyotypic analysis of synIII and synIIIL strains by pulsed-field gel electrophoresis revealed the size reduction of synIII and synIIIL compared to native III. Yeast chromosome numbers are indicated on the right side. SynIII (272,871 bp) and native chromosome VI (270,148 bp) co-migrate in the gel. Karyotypic analysis of synIII and all intermediate strains is shown in Fig. S8. (C) SynIII and synIIIL phenotyping on various types of media. Ten-fold serial dilutions of saturated cultures of wild type (BY4742), synIIIL and synIII strains were plated on the indicated media and temperatures. YPD, yeast extract peptone dextrose; YPGE, yeast extract peptone glycerol ethanol; MMS, methyl methanosulfate. Complete set of synIII and synIIIL phenotyping under various conditions is shown in Fig. S11.
DNA sequencing of the synIII strain genome revealed sequence differences at ten sites in synIII compared to our designed sequence (Table S4). Nine of the changes are base substitutions or one bp indels. Three of the nine mutations correspond to pre-existing but apparently innocuous mutations in the minichunks and BBs. Of the remainder, two correspond to the wild-type base at this position, and thus may simply reflect inheritance of wild-type sequence. Since PCRTag analysis (Table S5) was the method used to validate transformants during the 11 intermediate construction steps, the recombination events involved are “patchy” transformants with tiny patches of native DNA instead of synthetic sequence that would have been missed during the PCRTag analysis. The remaining four mutations, which must have originated during the integration process, all occur in regions of overlap in the synIII minichunks, suggesting that the homologous recombination process may be somewhat error prone relative to baseline error rates (23). The tenth change is the absence of an expected loxPsym site.
To check for negative effects of modifications on fitness of synIII-containing strains from the wild type (BY4742), we examined colony size, growth curves, and morphology under various conditions. A growth curve analysis established that synIII and the isogenic native strain had no detectable fitness difference (Fig. S10). The strains were also indistinguishable from each other on colony size tests (Fig. 3C), indicating that defects in fitness attributable to the synIIIL intermediate or synIII are very modest, with only one condition out of 21 (high sorbitol) showing a subtle fitness defect for synIII (Fig. S11). Cell morphology of all intermediate strains was similar to that of wild type (Fig. S12) except that during replacement round R3 (giving rise to strain 219 kb-synIII), a very low frequency (~1% of cells) of morphologically abnormal buds were observed (Fig. S12). We performed transcript profiling to identify possible changes in gene expression across synIII or genome-wide due to synonymous substitutions, introduction of loxPsym sites, and other changes. While ten loci are differentially expressed at genome-wide significance (p <7.4×10−6 for 5% family-wise error rate based on 6756 loci with at least one mapped read, and also corresponding to 1% false-discovery rate), eight of these correspond to loci intentionally deleted from synIII. The remaining two loci are HSP30 on synIII, ~16-fold down, and PCL1 on native chromosome XIV, ~16-fold up (Fig. S13).
The inclusion of hundreds of designed changes in the synthetic chromosome, including the removal of 11 tRNA genes, said to be important sites of cohesin loading, might result in subtle or overt destabilizing effects on the synthetic chromosome; alternatively, removal of repetitive DNA sequences might increase stability by reducing the likelihood of “ectopic” recombination events involving two different repeat copies. Because of the 98 loxPsym sites added to synIII (and all the other changes), it was important to evaluate the genome integrity and the loss rate of the chromosome in the absence of Cre expression. PCRTag analysis revealed that synIII is stable over 125 mitotic generations in 30 independent lineages (Fig. 4A). To evaluate the loss rate of synIII, we used the “a-like faker” assay in which MATα cells carrying synIII were monitored for acquiring the ability to mate as MATa cells, a consequence of losing chromosome III (24). Despite the extensive chromosome engineering, the frequency of MATα/synIII loss was not significantly different from that of the wild-type control (Fig. 4B).
Fig. 4. Genomic stability of the synIII strain.
(A) PCRTag analysis of synIII strain after ~125 generations. We assayed for the loss of 58 different segments lacking essential genes in the absence of SCRaMbLEing; no losses were observed after over 200,000 segment-generations analyzed; reported frequency is a maximum estimate of segment loss frequency per generation. (B) Evaluation of the loss rate of synIII chromosome using a-like faker assay. No significant change in the loss frequency was observed, although the absolute loss rate value is modestly higher in synIII. (C) SCRaMbLE leads to a gain of mating type a behavior in synIII heterozygous diploids. Frequency of a-mater and α-mater colonies post-SCRaMbLE (induction with estradiol) in synIII/III and III/III strains. Complete SCRaMbLE analysis is shown in Fig. S18.
It is not known whether cohesin accumulation at a tRNA gene region directly depends on the presence of the tRNA gene, nor its effect on chromosome stability is clear. We compared the map of cohesin binding sites on native chromosome and synIII using ChIPseq analysis (Fig. S14). The overall cohesin binding pattern is quite similar between the two chromosomes. However, at three tRNA genes that show a prominent peak in the native chromosome, that peak is reduced or in one case, the glutamine tRNA gene tQ(UUG)C, completely absent from synIII (Fig. S14). Thus, we conclude that tRNA genes and their documented interactions with both cohesin and condensin (25, 26) are dispensable for high levels of chromosome stability. We also compared the replication dynamics of synIII and native III (Supplementary Text, Table S9 and Fig. S15) and saw few dramatic changes in dynamics in spite of several ARS sequences having been deleted.
SCRaMbLEing in haploid strains containing chromosome synIII, leads to lethality via essential gene loss (Fig. S16). We looked for more subtle effects of SCRaMbLE in a heterozygous MATa/α (mating incompetent) diploid strain with a synthetic MATα chromosome and a native MATa chromosome (Fig S17). We introduced the Cre-EBD plasmid into such strains as well as to WT MATa/α diploids, and very briefly induced with estradiol. In spite of the minimal level of SCRaMbLEing induced, we observed a massive increase in the frequency of “a mater” derivatives in the nativeIII/synIII heterozygous strains (Figures 4C & S18). Such a-mater derivatives can arise from the loss of the MATα locus, because such “MAT-less” strains express a-specific genes. PCRTag mapping of several such derivatives showed that these variants had indeed lost different sections of synIII, all of which included the MAT locus (Fig. S18).
The total synthesis of the synIII chromosome represents a major step towards the design and complete synthesis of a novel eukaryotic genome structure – using the model Saccharomyces cerevisiae as the basis for a synthetic designer genome “Sc2.0”. The many changes made to synIII, including intron deletion, tRNA gene removal, and loxPsym sites and PCRTags introduction, do not appear to significantly decrease the fitness or alter the transcriptome or the replication timing of the synIII strain, supporting the very pliable nature of the yeast genome and potentially allowing for much more aggressively redesigned future genome versions. Sc2.0 represents just one of myriad possible arbitrary genome designs, and we anticipate that synthetic chromosome design will become a new means of posing specific evolutionary and mechanistic questions about genome structure and function. Rapid advances in synthetic biology coupled with ever decreasing costs of DNA synthesis suggest that it will soon become feasible to engineer new eukaryotic genomes, including plant and animal genomes, with synthetic chromosomes encoding desired functions and phenotypic properties based on specific design principles.
Supplementary Material
Acknowledgments
This work was supported by grants from National Science Foundation (MCB 0718846) to J.D.B, J.S.B, and S.C., and from Microsoft to J.S.B. S.M. and S.C were supported by a grant from National Institutes of Health (GM077291 to S.C.), H.M. by a fellowship from Fondation pour la 6 Recherche Médicale and a Pasteur-Roux fellowship, S.R. by an Exploratory Research Grant from MSCRF, L.A.M. by a fellowship from the National Sciences and Engineering Research Council of Canada, S.M.R. by a fellowship from the U.S Department of Energy, and J.S.D. by a fellowship from JHU Applied Physics Laboratory. We thank Dan Gibson for helpful suggestions regarding the isothermal assembly reaction, Ed Louis and Dan Gottschling for advice on synthetic telomere design, and Leonid Teytelman and Jasper Rine for advice on silent cassette DNA.
Footnotes
We dedicate this publication to the memory of Har Gobind Khorana, who synthesized the first yeast tRNA gene.
Author Contributions
N.A., H.M., J.S.B., J.D.B. and S.C. designed experiments. J.D.B. and S.M.R. designed synIII. N.A., H.M., L.A.M., S.R., G.S., S.M.R., J.S.D., Z.K., Y.C., Z.G., V.L., S.M., K.K., N.A., G.F. and S.C. performed experiments. N.A., H.M., G.S., R.K., J.D.B. and S.C. analyzed data. N.A., H.M., J.D.B. and S.C. wrote the manuscript. JHU Build-A-Genome course students (author names denoted with‡) synthesized most of the building blocks (BBs) for synIII; H.M., G.S., S.M.R., J.S.D., L.Z.S., E.M.C., Y.C., K.Z., J.S.H., M.H., J.T. and J.D.B. taught the Build-AGenome course. S.C. led the effort on the construction and assembly of synIII.
The synIII sequences have been deposited at GenBank with accession numbers KJ463385 (the “as designed” reference sequence version 3.3_41) and KC880027 (the actual physical sequence in strain HMSY011, sequence version 3.3_42).
The authors declare no competing financial interests.
“This manuscript has been accepted for publication in Science. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencemag.org/. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.”
References
- 1.Goffeau A, et al. Life with 6000 genes. Science. 1996;274:546, 563–7. doi: 10.1126/science.274.5287.546. [DOI] [PubMed] [Google Scholar]
- 2.Dymond JS, et al. Synthetic chromosome arms function in yeast and generate phenotypic diversity by design. Nature. 2011;477:471–476. doi: 10.1038/nature10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dymond J, Boeke J. The Saccharomyces cerevisiae SCRaMbLE system and genome minimization. Bioeng Bugs. 2012;3:168–171. doi: 10.4161/bbug.19543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cello J, Paul AV, Wimmer E. Chemical synthesis of poliovirus cDNA: generation of infectious virus in the absence of natural template. Science. 2002;297:1016–1018. doi: 10.1126/science.1072266. [DOI] [PubMed] [Google Scholar]
- 5.Chan LY, Kosuri S, Endy D. Refactoring bacteriophage T7. Mol Syst Biol. 2005;1:2005.0018. doi: 10.1038/msb4100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Posfai G, et al. Emergent properties of reduced-genome Escherichia coli. Science. 2006;312:1044–1046. doi: 10.1126/science.1126439. [DOI] [PubMed] [Google Scholar]
- 7.Gibson DG, et al. Complete chemical synthesis, assembly, and cloning of a Mycoplasma genitalium genome. Science. 2008;319:1215–1220. doi: 10.1126/science.1151721. [DOI] [PubMed] [Google Scholar]
- 8.Lartigue C, et al. Genome transplantation in bacteria: changing one species to another. Science. 2007;317:632–638. doi: 10.1126/science.1144622. [DOI] [PubMed] [Google Scholar]
- 9.Wang HH, et al. Programming cells by multiplex genome engineering and accelerated evolution. Nature. 2009;460:894–898. doi: 10.1038/nature08187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibson DG, et al. Creation of a bacterial cell controlled by a chemically synthesized genome. Science. 2010;329:52–56. doi: 10.1126/science.1190719. [DOI] [PubMed] [Google Scholar]
- 11.Isaacs FJ, et al. Precise manipulation of chromosomes in vivo enables genome-wide codon replacement. Science. 2011;333:348–353. doi: 10.1126/science.1205822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oliver SG, et al. The complete DNA sequence of yeast chromosome III. Nature. 1992;357:38–46. doi: 10.1038/357038a0. [DOI] [PubMed] [Google Scholar]
- 13.Richardson SM, Wheelan SJ, Yarrington RM, Boeke JD. GeneDesign: rapid, automated design of multikilobase synthetic genes. Genome Res. 2006;16:550–556. doi: 10.1101/gr.4431306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stemmer WP, Crameri A, Ha KD, Brennan TM, Heyneker HL. Single-step assembly of a gene and entire plasmid from large numbers of oligodeoxyribonucleotides. Gene. 1995;164:49–53. doi: 10.1016/0378-1119(95)00511-4. [DOI] [PubMed] [Google Scholar]
- 15.Dymond JS, et al. Teaching synthetic biology, bioinformatics and engineering to undergraduates: the interdisciplinary Build-a-Genome course. Genetics. 2009;181:13–21. doi: 10.1534/genetics.108.096784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Annaluru N, et al. Assembling DNA fragments by USER fusion. Methods Mol Biol. 2012;852:77–95. doi: 10.1007/978-1-61779-564-0_7. [DOI] [PubMed] [Google Scholar]
- 17.Gibson DG, et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods. 2009;6:343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- 18.Ma H, Kunes S, Schatz PJ, Botstein D. Plasmid construction by homologous recombination in yeast. Gene. 1987;58:201–216. doi: 10.1016/0378-1119(87)90376-3. [DOI] [PubMed] [Google Scholar]
- 19.Larionov V, et al. Specific cloning of human DNA as yeast artificial chromosomes by transformation-associated recombination. Proc Natl Acad Sci U S A. 1996;93:491–496. doi: 10.1073/pnas.93.1.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibson DG, et al. One-step assembly in yeast of 25 overlapping DNA fragments to form a complete synthetic Mycoplasma genitalium genome. Proc Natl Acad Sci U S A. 2008;105:20404–20409. doi: 10.1073/pnas.0811011106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muller H, et al. Assembling large DNA segments in yeast. Methods Mol Biol. 2012;852:133–150. doi: 10.1007/978-1-61779-564-0_11. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz DC, Cantor CR. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984;37:67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- 23.Lynch M, et al. A genome-wide view of the spectrum of spontaneous mutations in yeast. Proc Natl Acad Sci U S A. 2008;105:9272–9277. doi: 10.1073/pnas.0803466105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuen KW, et al. Systematic genome instability screens in yeast and their potential relevance to cancer. Proc Natl Acad Sci U S A. 2007;104:3925–3930. doi: 10.1073/pnas.0610642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D’Ambrosio C, et al. Identification of cis-acting sites for condensin loading onto budding yeast chromosomes. Genes Dev. 2008;22:2215–2227. doi: 10.1101/gad.1675708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lengronne A, et al. Cohesin relocation from sites of chromosomal loading to places of convergent transcription. Nature. 2004;430:573–578. doi: 10.1038/nature02742. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.