Abstract
Objective
People with mental disorders are estimated to die 25 years younger than the general population, and heart disease (HD) is a major contributor to their mortality. We assessed whether Veterans Affairs (VA) health system patients with mental disorders were more likely to die from HD than patients without these disorders, and whether modifiable factors may explain differential mortality risks.
Methods
Subjects included VA patients who completed the 1999 Large Health Survey of Veteran Enrollees (LHSV) and were either diagnosed with schizophrenia, bipolar disorder, other psychotic disorders, major depressive disorder or other depression diagnosis or diagnosed with none of these disorders. LHSV data on patient sociodemographic, clinical and behavioral factors (e.g., physical activity, smoking) were linked to mortality data from the National Death Index of the Centers for Disease Control and Prevention. Hierarchical multivariable Cox proportional hazards models were used to assess 8-year HD-related mortality risk by diagnosis, adding patient sociodemographic, clinical and behavioral factors.
Results
Of 147,193 respondents, 11,809 (8%) died from HD. After controlling for sociodemographic and clinical factors, we found that those with schizophrenia [hazard ratio (HR)=1.25; 95% confidence interval (95% CI): 1.15–1.36; P<.001] or other psychotic disorders (HR=1.41; 95% CI: 1.27–1.55; P<.001) were more likely to die from HD than those without mental disorders. Controlling for behavioral factors diminished, but did not eliminate, the impact of psychosis on mortality. Smoking (HR=1.32; 95% CI: 1.26–1.39; P<.001) and inadequate physical activity (HR=1.66; 95% CI: 1.59–1.74; P<.001) were also associated with HD-related mortality.
Conclusions
Patients with psychosis were more likely to die from HD. For reduction of HD-related mortality, early interventions that promote smoking cessation and physical activity among veterans with psychotic disorders are warranted.
Keywords: Heart-disease-related mortality, Mental disorders, Modifiable risk factors, Mortality
1. Background
Mental disorders, including schizophrenia, bipolar disorder and major depressive disorder, affect up to 20% of the US population and are leading causes of disability, social and occupational dysfunctions, and premature mortality [1–3]. Heart disease (HD) is one of the leading causes of death among persons with mental disorders [4,5], and it has been associated with 25–30 years of premature mortality among patients diagnosed with schizophrenia [4,6].
There are many reasons for the increased risk of HD and subsequent premature mortality in persons with mental disorders. Notably, a substantial percentage of persons with schizophrenia have significantly higher rates of obesity [7], diabetes and hypertension compared to the general population [8]. The prevalence of negative health behaviors, including smoking [9] and limited physical activity [10], is also higher among patients diagnosed with schizophrenia and other mental disorders than among the general population [8,11]. Medications that are increasingly being used to manage psychosis, notably second-generation antipsychotics, are associated with weight gain, contributing to an increased risk of HD [12,13]. Furthermore, community mental health programs, which are often the primary source of care for persons with chronic mental illnesses, often lack adequate access to and coordination with general medical services [14,15].
Prior studies have mainly focused on HD-related mortality in schizophrenia and have not compared mortality among patients with other mental disorders, including bipolar disorder or depression. Moreover, studies have not assessed the specific mechanisms of increased HD-related mortality in persons with mental disorders. Existing studies on HD mortality risk in patients with mental disorders have also been limited in several important ways. First, they were either based on selective clinical trial samples for a single disorder (e.g., schizophrenia) [8] or based on institutionalized populations [6], which do not fully represent patient populations in usual care. Second, comparison groups have been primarily based on the US general population rather than on more comparable patient samples (e.g., nonpsychiatric patients from the same treatment settings). Most studies have lacked specific information on cause-specific mortality, assessing all-cause mortality risks as opposed to disease-specific mortality risks, leading to less precise assessments of explanatory risk factors. Finally, most studies have lacked sufficient clinical or behavioral measures to assess the specific mechanisms of HD-related mortality risk in patients with mental disorders [5,16,17].
Understanding which mental disorder diagnoses are most vulnerable to HD-related mortality and which specific clinical or health behaviors contribute to increased HD-related mortality can assist providers in prioritizing intervention efforts. The Veterans Affairs (VA), with its integrated health care system and extensive clinical and mortality data, provides an ideal setting for the study of the specific effects of behavioral and clinical factors on HD-related mortality in this group. Based on a comprehensive national survey of VA patients receiving care, we (a) compared HD-related mortality rates among VA patients with schizophrenia, bipolar disorder, other psychotic disorder or depression to HD-related mortality rates among VA patients without these diagnoses; (b) determined whether patients with mental disorders were more likely to die from HD than those without these disorders; and (c) identified clinical or behavioral factors that explained the impact of mental disorders on HD mortality.
2. Methods
VA patients who had completed the 1999 Large Health Survey of Veteran Enrollees (LHSV) and who had been diagnosed with schizophrenia, bipolar disorder, other psychotic disorder or major depression or had none of these diagnoses in 1998–1999 were included. Administered in 1999, the LHSV is the largest survey ever conducted of veterans using VA health services and is one of the largest comprehensive assessments of health status, sociodemographics, behaviors and utilization within a national patient population [18]. The reliability and validity of the LHSV questions have been demonstrated elsewhere [19–21].
2.1. Study population
Data from the LHSV were linked to the VA’s National Psychosis Registry (NPR), the National Registry of Depression (NARDEP) and a random sample cohort of veterans without mental disorders, all of which were developed and maintained by the National VA Serious Mental Illness Treatment Research and Evaluation Center [22]. NPR includes comprehensive administrative data on diagnosis, utilization and cost for all VA patients with an International Classification of Diseases, Ninth Revision (ICD-9) diagnosis of schizophrenia, bipolar disorder or other psychotic disorder. NARDEP [23] includes these data for all VA patients with an ICD-9 diagnosis of depression. The random sample of VA patients included those who made at least one inpatient or outpatient visit within the VA during fiscal year (FY) 1998 or 1999. In the VA, providers record diagnoses using standardized forms for each encounter. Professional coders verify diagnoses and assign corresponding ICD-9 codes. This study received approval from local institutional review boards, and informed consent from patients was not required for this secondary data analysis.
Study inclusion criteria were as follows. First, patients had to have received VA care in FY 1998 or 1999, either with a diagnosis of schizophrenia, bipolar disorder, other psychotic disorder, major depressive disorder or other depression diagnosis, or as part of the random sample of VA patients with no diagnosis of these conditions. Second, patients had to have completed the LHSV, to have received VA care in FY 2000 and to be alive at the start of calendar year 2000. In this study, patients with mental disorders were identified based on ICD-9 diagnoses of schizophrenia (including schizoaffective disorder: ICD-9 codes 295.0–295.4, 295.6, 295.7, 295.8 and 295.9), bipolar disorder (ICD-9 codes 296.0–296.1 and 296.4–296.8), other psychotic disorders (ICD-9 codes 297–297.9 and 298–298.9), major depressive disorder (ICD-9 codes 296.2 and 296.3, including codes for severe episodes with psychotic features) or other depressive disorder. Based on previously established definitions from NARDEP, other depressive disorders included the following ICD-9 code diagnoses: 311 (depressive disorder not otherwise specified), 300.4 (dysthymia), 309.0 (adjustment disorder with depressed mood), 293.83 (mood disorder due to medical condition), 296.90 (mood disorder), 309.1 (prolonged depressive reaction), 296.99 (other specified affective disorders) and 301.12 (chronic depressive personality disorder), of which the majority were diagnosed with depressive disorder not otherwise specified or dysthymia. [24]. Since patients may be diagnosed with multiple (and sometimes mutually exclusive) conditions, we used the most frequently occurring serious mental illness diagnosis recorded in all encounters in FY 1998–1999 to classify patients using the following hierarchy: schizophrenia, bipolar disorder, other psychotic disorder, major depressive disorder or other depression. In cases where encounter data revealed an equal number of each diagnosis, we categorized the patient as having the more debilitating condition using the above hierarchy. The World Health Organization’s Global Burden of Disease Study [1] identifies schizophrenia as the most debilitating mental disorder.
Given our focus on identifying the risk factors of HD-related mortality in VA patients diagnosed with different mental disorders, we wanted to include all patients diagnosed with schizophrenia, bipolar disorder, other psychosis, major depressive disorder or other depression in FY 1998 or 1999 who had also completed the LHSV. Our random sample of 100,000 patients receiving care in FY 1998 or 1999 was selected regardless of psychiatric diagnosis status, as many patients were also included in the population of patients with mental disorders from NPR or NARDEP. This allowed for an enriched sample of VA patients with mental disorders and a comparable sample of patients randomly selected from the same time period. Hence, our total sample size was 147,193, including patients diagnosed with schizophrenia (N=22,817), bipolar disorder (N=15,203), other psychosis (N=7336), major depressive disorder (N=34,952) or other depressive disorder diagnoses (N=50,813). Our total random sample of patients who also completed the survey without any of these mental disorder diagnoses was 16,072.
2.2. Data
LHSV data on patient, clinical and behavioral factors were linked to mortality data from FY 2000–2006 from the Centers for Disease Control and Prevention (CDC)’s National Death Index (NDI) Plus database. Additional data, including age (as of the LHSV survey date), gender, service-connected disability and medical comorbidities, were available from VA electronic medical record data from FY 1998 to FY 1999. Data on other psychiatric diagnoses, including substance use disorders (alcohol and illicit drug use) and anxiety disorders were also ascertained. Having a VA-service-connected disability acquired primarily during or because of military service qualifies a veteran for unlimited outpatient care.
Using the LHSV and administrative data, we operationalized available patient sociodemographic factors that are thought to confound the association between mental disorder diagnosis and HD mortality [8,16,17]. From the LHSV, we ascertained self-reported patient race/ethnicity (categorized as black, white or other), education (categorized as high school or less, some college or college graduate) and marital status (categorized as married or unmarried). Additional patient factors from the LHSV included support (having someone to take the patient to the doctor—yes or no; living alone or not), financial hardship (defined as not having sufficient money to buy food — yes or no) and reliance on VA care (VA use only, compared to any non-VA services use in 1999).
Clinical factors, including risk factors of HD-related mortality, were ascertained from VA electronic data. We focused, in particular, on current (1998–1999) diagnoses of conditions considered risk factors or risk equivalents of HD, including diabetes, cardiovascular disease, cerebrovascular disease, hypertension or hyperlipidemia, based on ICD-9 codes that have been previously established in this cohort [25]. Other medical comorbidities were defined using the Charlson Comorbidity Index for administrative data [26], modified to exclude diabetes, myocardial infarction, chronic heart failure, cerebrovascular disease or dementia. The Charlson Comorbidity Index classifies categories of comorbidity by severity (e.g., cancers, HIV): 0 (no condition), 1 (condition present in time period) or 1.14 (more severe condition present in time period). Scores range from 0 to 18, with higher scores indicating a greater burden of medical comorbidity. Charlson scores were categorized because of nonnormal distribution. Data on co-occurring psychiatric comorbidities not considered mutually exclusive with psychosis or depression [dementia, posttraumatic stress disorder (PTSD)] and any hospitalization occurring in 1998–1999 were also ascertained from VA data and treated as potential confounders to mortality risk.
Health behaviors related to HD were ascertained based on self-reported data from the LHSV, including physical activity and smoking, along with other health behaviors associated with premature morbidity and mortality, including alcohol and illicit drug use. Smoking was defined as whether the respondent reported current smoking. Adequate physical activity was ascertained based on the question “How often do you engage in regular activities (e.g., brisk walking, jogging, bicycling, etc.) long enough to work up a sweat?” The response options were none, less than once per week, one to two times per week, three to four times per week and five or more times per week. Based on guidelines for adequate physical activity in a given week [27], responses of three or more times per week were considered adequate physical activity to maintain health and to reduce the risk of HD. Alcohol use was assessed based on a question on hazardous (binge) drinking adapted from the Alcohol Use Disorders Identification Test [28]. We also included illicit drug use based on ICD-9 diagnoses from VA administrative data, as no self-reported survey questions on illicit drug use were available.
2.3. Mortality and cause of death
The NDI Plus, maintained by the National Center for Health Statistics, is a national computerized index of death record information submitted by state vital statistics offices. This federally maintained database contains the dates and causes of mortality for deaths occurring in 1979 or later. Death records are added to the NDI file annually, approximately 12 months after the end of the calendar year. NDI data were acquired and linked to all patients in the NPR, NARDEP and random sample populations between 1998 and 2006. HD-related deaths were defined using CDC cause-of-death criteria for “diseases of the heart,” including myocardial infarction, congestive heart failure, hypertensive HD and ischemic HD [International Classification of Diseases, Tenth Revision (ICD-10) codes I00–I09, I13 and I20–I51].
2.4. Analyses
The cumulative incidence of HD-related mortality was calculated across mental disorder diagnoses (schizophrenia, bipolar disorder, other psychosis or depression) and compared to those without these conditions. Multivariable Cox proportional hazards models were used to assess the risk of HD-related mortality by schizophrenia, bipolar disorder, other psychosis and depression status using the hazard ratio (HR; or risk ratio) estimate, comparing to those without these mental disorder diagnoses. Three models were run, with the first model controlling for mental disorder diagnosis and sociodemographic data only, the second model controlling for added clinical factors (e.g., co-occurring diagnoses) and the third model controlling for added behavioral variables, notably those directly related to HD risk, including smoking and inadequate physical activity. Three survival curves (displaying the time to mortality from HD for each mental health diagnosis) were run, with the first model controlling for sociodemographic factors, the second model controlling for sociodemographic and clinical factors and the third model controlling for sociodemographic, clinical and behavioral factors. The censor date was the date of death from other causes; for those still alive by the end of the observation period, the censor date was 12/31/06.
3. Results
Out of 147,193 eligible patients, 40,241 (27.3%) had died by the end of 2006. Of the 40,241 deaths, the most common cause of death was HD (N=11,809, or 8.0% of the total sample). Overall, 15.5% of the patients were diagnosed with schizophrenia, 10.3% of the patients were diagnosed with bipolar disorder and 5.0% of the patients were diagnosed with other psychotic disorder (Table 1). About one fourth of the overall sample was diagnosed with diabetes, and over half of the sample was diagnosed with hypertension. The mean Charlson score for the overall sample was 0.8 (S.D.=1.3, range 0–18), indicating a moderate comorbidity burden. Over a third of the sample was currently smoking, and about half of the sample reported inadequate physical activity.
Table 1.
Demographics of patients with and without mental disorders
| Total sample [147,193 (100.0%)] |
Schizophrenia [22,817 (15.5%)] |
Bipolar disorder [15,203 (10.3%)] |
Other psychosis [7336 (5.0%)] |
Major depressive disorder [34,952 (23.8%)] |
Other depression [50,813 (34.5%)] |
No psychiatric disorder [16,072 (10.9%)] |
χ2 | P | |
|---|---|---|---|---|---|---|---|---|---|
| Sociodemographic factors | |||||||||
| Age (years) | |||||||||
| <50 | 23.32 | 33.33 | 33.18 | 17.72 | 24.42 | 19.22 | 12.85 | 7742.5 | <.0001 |
| 50–<65 | 37.09 | 38.62 | 41.01 | 28.99 | 41.98 | 35.92 | 27.96 | ||
| ≥65 | 39.60 | 28.04 | 25.81 | 53.29 | 33.60 | 44.86 | 59.18 | ||
| Male | 93.67 | 95.23 | 90.31 | 95.94 | 91.68 | 94.11 | 96.52 | 913.0 | <.0001 |
| Race/ethnicity | |||||||||
| Black | 12.42 | 22.05 | 8.79 | 18.20 | 10.02 | 9.88 | 12.80 | 3403.4 | <.0001 |
| White | 72.21 | 59.58 | 76.47 | 65.31 | 74.32 | 75.59 | 73.93 | ||
| Other | 15.37 | 18.37 | 14.74 | 16.49 | 15.66 | 14.53 | 13.27 | ||
| Education | |||||||||
| Less than high school | 22.84 | 21.25 | 12.84 | 31.17 | 20.03 | 25.66 | 28.00 | 2394.6 | <.0001 |
| High school graduate or general equivalency diploma | 32.87 | 38.23 | 30.82 | 32.68 | 31.33 | 32.42 | 32.12 | ||
| College or more | 44.40 | 40.52 | 56.34 | 36.15 | 48.64 | 41.91 | 39.88 | ||
| Someone to take patient to the doctor | 82.75 | 80.11 | 79.52 | 83.83 | 81.31 | 83.86 | 88.61 | 698.2 | <.0001 |
| Financial hardship | 22.72 | 26.27 | 26.90 | 23.05 | 25.26 | 21.42 | 12.17 | 1496.2 | <.0001 |
| Living alone | 28.49 | 32.18 | 31.88 | 26.17 | 28.99 | 27.71 | 22.50 | 514.0 | <.0001 |
| Service connection | 48.04 | 59.39 | 48.79 | 47.01 | 49.68 | 45.14 | 37.27 | 2139.4 | <.0001 |
| Any non-VA care use | 46.14 | 40.03 | 45.32 | 41.77 | 46.08 | 47.89 | 52.14 | 688.2 | <.0001 |
| Clinical/severity factors | |||||||||
| Diabetes diagnosis | 23.24 | 21.65 | 19.55 | 25.34 | 23.17 | 24.60 | 23.87 | 223.0 | <.001 |
| Cardiovascular disease | 16.81 | 10.97 | 12.36 | 26.88 | 17.14 | 19.84 | 14.46 | 1704.5 | <.001 |
| Hypertension diagnosis | 55.38 | 46.81 | 48.37 | 59.05 | 55.52 | 60.08 | 57.32 | 1499.2 | <.001 |
| Hyperlipidemia diagnosis | 35.17 | 29.46 | 32.86 | 28.80 | 37.93 | 37.72 | 34.28 | 758.9 | <.001 |
| Cerebrovascular disease | 2.81 | 1.99 | 1.90 | 7.80 | 2.66 | 3.21 | 1.60 | 888.9 | <.001 |
| Any hospitalization (FY 1998–1999) | 30.96 | 35.68 | 38.89 | 47.75 | 31.45 | 28.55 | 15.67 | 3554.0 | <.001 |
| Other psychiatric disorders | |||||||||
| Dementia diagnosis | 4.41 | 4.70 | 3.70 | 22.33 | 3.30 | 3.74 | 1.07 | 6189.4 | <.001 |
| PTSD diagnosis | 22.59 | 21.99 | 30.17 | 23.60 | 33.23 | 19.35 | 2.86 | 6654.7 | <.001 |
| Behavioral factors | |||||||||
| Current smoker | 36.80 | 49.21 | 44.30 | 33.43 | 36.53 | 33.94 | 23.28 | 3317.9 | <.001 |
| Hazardous drinking | 24.15 | 22.16 | 21.44 | 20.22 | 23.59 | 25.18 | 29.17 | 412.0 | <.001 |
| Physical activity less than three times per week | 47.39 | 45.63 | 43.95 | 57.96 | 48.87 | 49.41 | 38.73 | 1012.4 | <.001 |
| Illicit drug use disorder | 10.52 | 15.70 | 18.05 | 15.19 | 11.13 | 7.65 | 1.68 | 3529.3 | <.001 |
| Pr>F | |||||||||
| Charlson score (mean±S.D.) | 0.8±1.3 | 0.7±1.2 | 0.7±1.3 | 1.0±1.5 | 0.9±1.4 | 0.9±1.4 | 0.7±1.2 | <.001 | |
| Charlson score range | 0–18 | 0–15 | 0–18 | 0–12 | 0–16 | 0–17 | 0–16 | ||
| Age in years (mean±S.D.) | 59.7±13.5 | 56.0±12.6 | 55.5±12.7 | 64.1±14.4 | 58.3±12.9 | 61.3±13.4 | 65.0±13.0 | <.001 |
Compared to those without a mental disorder diagnosis, patients diagnosed with schizophrenia or bipolar disorder were younger, and those diagnosed with bipolar disorder were more likely to be female and college educated. For clinical factors, those with other psychotic disorders were the most likely to be diagnosed with dementia, whereas those with depression were the most likely to be diagnosed with hyperlipidemia. For health behaviors, patients with schizophrenia were the most likely to be current smokers, and those with bipolar disorder were the least likely to report adequate physical activity (Table 1).
Compared to those without a mental disorder diagnosis, patients with other psychotic disorders were the most likely to die from HD. Those diagnosed with schizophrenia who died from HD were more likely to die at a younger age (68.6 years) than those without a mental disorder diagnosis (76.5 years; P<.001) (see Table 2).
Table 2.
Mortality from HD, by mental disorder diagnosis
| Died of HD [n (%)] |
Age of death [mean (S.D.)] |
|
|---|---|---|
| Schizophrenia (N=22,817) | 1579 (6.92) | 68.6 (12.0) |
| Bipolar disorder(N=15,203) | 921 (6.06) | 69.3 (12.2) |
| Other psychosis diagnosis (N=7336) | 877 (11.95)** | 76.5 (9.8) |
| Major depressive disorder only (N=34,952) | 2559 (7.32) | 71.8 (11.1) |
| Other depression (N=50,813) | 4422 (8.70) | 74.1 (10.5) |
| No mental disorder diagnosis (N=16,072) | 1380 (8.59) | 76.5 (9.3) |
| Total sample (N=147,193) | 11,738 (7.97) | 72.9 (11.1) |
HD-related deaths were deaths defined using CDC cause-of-death criteria for “diseases of the heart,” including myocardial infarction, congestive heart failure, pulmonary HD, hypertensive HD and ischemic HD (ICD-10 codes I00–I09, I11, I13 and I20–I51).
P<.001 compared to patients without a mental disorder diagnosis.
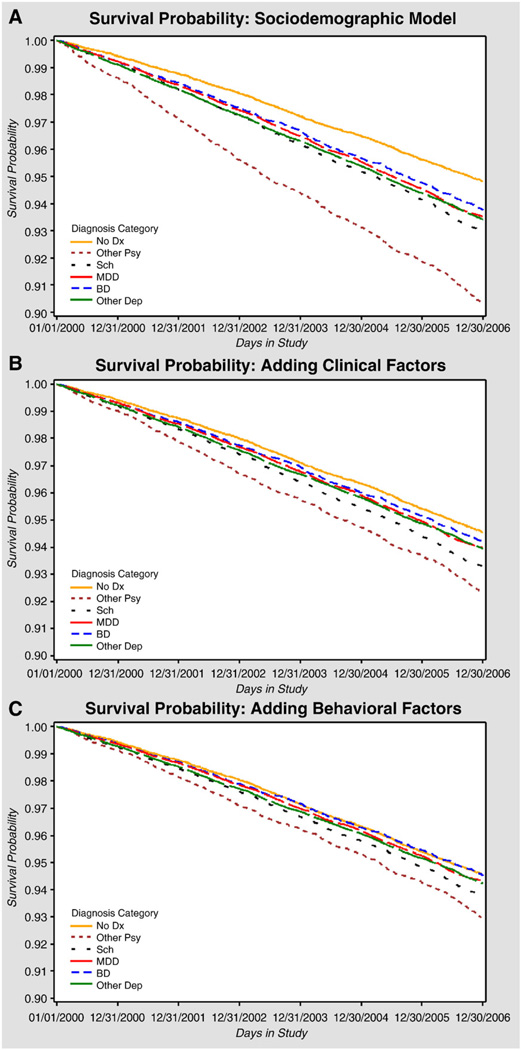
Results from the four HD mortality risk models are presented in Table 3 and Fig. 1A–C. After controlling for sociodemographic factors, we found that those diagnosed with other psychotic disorders were the most likely to die from HD by the end of 2006 [HR=1.89; 95% confidence interval (95% CI): 1.72–2.08; P<.001] compared to those without a mental disorder diagnosis. Patients with schizophrenia (HR=1.37; 95% CI: 1.26–1.49; P<.05), bipolar disorder (HR=1.24; 95% CI: 1.13–1.36; P<.001), major depressive disorder (HR=1.26; 95% CI: 1.17–1.35; P<.001) or other depression (HR=1.28; 95% CI: 1.19–1.37; P<.05) were also more likely to die from HD than those without a mental disorder diagnosis. However, after adding clinical factors, we found that the magnitude of the HRs was diminished, suggesting that clinical factors explained some of the differential in mortality within these patient groups. Diagnoses of diabetes (HR=1.52; 95% CI: 1.44–1.57; P<.001) and dementia (HR=1.31; 95% CI: 1.25– 1.46; P<.001) were strongly associated with HD mortality, yet did not fully explain the association between other psychotic disorder diagnosis and mortality. PTSD diagnosis was associated with a lower mortality in the model that adjusted for sociodemographic and clinical factors (HR=0.83; 95% CI: 0.78–0.88; P<.001).
Table 3.
Multivariable results: Cox proportional HRs for mortality from HD (HD without pulmonary HD)
| Sociodemographic model (123,287 observations) |
Adding clinical factors (123,287 observations) |
Adding behavioral factors (117,892 observations) |
|
|---|---|---|---|
| Schizophrenia | 1.37 (1.26–1.49)** | 1.25 (1.15–1.36)** | 1.17 (1.07–1.28)* |
| Bipolar disorder | 1.24 (1.13–1.36)** | 1.09 (0.99–1.20) | 1.04 (0.94–1.14) |
| Other psychosis | 1.89 (1.72–2.08)** | 1.41 (1.27–1.55)** | 1.30 (1.18–1.45)** |
| Major depressive disorder | 1.26 (1.17–1.35)** | 1.09 (1.01–1.17)* | 1.04 (0.97–1.13) |
| Other depression diagnosis | 1.28 (1.19–1.37)** | 1.10 (1.03–1.18)* | 1.06 (0.99–1.14) |
| Demographic factors | |||
| Male (vs. female) | 2.01 (1.74–2.33)** | 1.90 (1.65–2.20)** | 1.91 (1.65–2.21)** |
| Age 50–64 (vs. <50) years | 2.38 (2.17–2.62)** | 2.03 (1.85–2.23)** | 2.01 (1.82–2.22)** |
| Age ≥65 (vs. <50) years | 8.26 (7.54–9.04)** | 5.64 (5.14–6.19)** | 6.07 (5.50–6.70)** |
| Black (vs. White) | 0.83 (0.77–0.90)** | 0.75 (0.69–0.80)** | 0.74 (0.69–0.80)** |
| Other (vs. White) | 0.74 (0.69–0.80)** | 0.73 (0.68–0.78)** | 0.74 (0.68–0.79)** |
| Less than high school (vs. college) | 1.36 (1.29–1.43)** | 1.25 (1.19–1.32)** | 1.16 (1.10–1.23)** |
| High school graduate (vs. college) | 1.13 (1.08–1.19)** | 1.11 (1.05–1.17)** | 1.06 (1.01–1.12)* |
| No one to take patient to the doctor | 0.98 (0.92–1.04) | 0.99 (0.94–1.06) | 0.98 (0.92–1.05) |
| Financial hardship | 1.03 (0.97–1.09) | 0.99 (0.94–1.05) | 0.94 (0.89–1.00) |
| Any non-VA use | 1.05 (1.01–1.10)* | 1.22 (1.17–1.27)** | 1.22 (1.17–1.28)** |
| Living alone | 1.15 (1.10–1.21)** | 1.18 (1.12–1.23)** | 1.19 (1.14–1.25)** |
| Service connection | 0.91 (0.87–0.95)** | 0.95 (0.91–0.99)* | 0.94 (0.90–0.98)* |
| Clinical/severity factors | |||
| Charlson score of 1 (vs. 0) | – | 1.33 (1.26–1.40)** | 1.24 (1.17–1.30)** |
| Charlson score ≥2 (vs. 0) | – | 1.39 (1.33–1.47)** | 1.31 (1.25–1.38)** |
| Diabetes diagnosis | – | 1.51 (1.44–1.57)** | 1.52 (1.45–1.59)** |
| Cardiovascular disease diagnosis | – | 1.68 (1.60–1.77)** | 1.63 (1.55–1.72)** |
| Hypertension diagnosis | – | 1.38 (1.32–1.46)** | 1.40 (1.33–1.47)** |
| Hyperlipidemia diagnosis | – | 0.86 (0.82–0.89)** | 0.88 (0.84–0.92)** |
| Cerebrovascular diagnosis | – | 0.87 (0.80–0.96)* | 0.83 (0.75–0.91)* |
| Hospitalization (1998–1999) | – | 1.45 (1.38–1.52)** | 1.39 (1.33–1.46)** |
| Other psychiatric disorders | |||
| Dementia diagnosis | – | 1.35 (1.25–1.46)** | 1.31 (1.21–1.42)** |
| PTSD diagnosis | – | 0.83 (0.78–0.88)** | 0.81 (0.76–0.86)** |
| Behavioral factors | |||
| Current smoker | – | – | 1.32 (1.26–1.39)** |
| Hazardous drinking | – | – | 0.95 (0.90–1.00) |
| Illicit drug use disorder | – | – | 1.07 (0.98–1.16) |
| Physical activity less than once a week | – | – | 1.66 (1.59–1.74)** |
Results are expressed as HR (95% CI).
Charlson score was categorized because of nonnormal distribution.
P<.05.
P<.001.
Fig. 1.
(A–C) Survival curves for the Cox proportional hazards models assessing HD mortality risk by mental disorder diagnosis. (A) represents the analysis controlling for sociodemographic factors (gender, age, race, education, marital status, support, financial hardship, non-VA use, living alone and service connection). (B) adds clinical factors (Charlson score, cardiovascular disease, diabetes, hypertension, hyperlipidemia, cerebrovascular disease, any hospitalization and other psychiatric diagnoses, including dementia, PTSD and substance use diagnosis). (C) adds health behaviors (smoking, alcohol use and physical activity).
After additionally controlling for health behaviors, we found that those with schizophrenia (HR=1.17; 95% CI: 1.07–1.28; P<.05) or other psychotic disorders (HR=1.30; 95% CI: 1.18–1.45; P<.001) were still more likely to die from HD than those without a mental disorder (see Fig. 1C). Behavioral factors significantly associated with an increased risk of HD mortality included smoking (HR=1.32; 95% CI: 1.26–1.39; P<.001) and inadequate physical activity (HR=1.66; 95% CI: 1.59–1.74; P<.001).
4. Discussion
A substantial number of VA patients had died from HD by the end of 2006. Patients with mental disorders also experienced a substantial burden of HD risk factors, including diabetes and hypertension, and many were current smokers and reported inadequate physical activity.
Patients with psychosis (schizophrenia or other psychotic disorder diagnoses) were the most likely to die from HD. Yet we were surprised to find that the adjusted relative risks of HD mortality were not significantly different for those with bipolar disorder or depression compared to patients without these diagnoses. Prior research has suggested that persons with bipolar disorder have a similar HD risk factor profile as those with schizophrenia [29]. We also found that while dementia was strongly associated with HD mortality risk, having a PTSD diagnosis may have been protective. This finding may reflect treatment engagement rather than psychopathology, as those diagnosed with this condition may be more likely to receive VA services [30].
Clinical and behavioral factors largely contributed to, but did not fully explain, HD-related mortality among patients with other psychotic disorders. Notably, the magnitude of this risk substantially diminished for those with schizophrenia (from 37% to 17%) after controlling for clinical and behavioral factors, including medical comorbidity, smoking and physical inactivity. Smoking and physical inactivity were the behavioral factors most strongly associated with HD-related mortality. Smoking, one of the strongest predictors of mortality in general, is common among individuals with schizophrenia and has been thought to help mitigate psychotic symptoms. Physical inactivity is also more prevalent in persons with psychotic disorders than in the general population, given that they are more likely institutionalized, with little opportunities for adequate exercise. Still, the fact that psychosis was independently associated with a greater HD-related mortality suggests that these patients are especially vulnerable to poor outcomes independent of health behaviors.
To our knowledge, this is the largest study to comprehensively examine how modifiable risk factors explain HD-related mortality within a large national survey of patients with and without mental disorders. Several studies have reported an increased risk of HD-related mortality among those with schizophrenia, but relatively few studies have compared mortality risk across other mental disorder diagnoses [17,31,32]. The importance of including patients with other mental disorder diagnoses beyond schizophrenia is reflected in our findings that patients diagnosed with other psychotic disorders were most at risk for HD mortality in our study and may represent an especially vulnerable patient group.
In addition, previous studies did not include comprehensive patient-level clinical or behavioral data that could explain the differential in HD mortality between mental disorder diagnoses and ultimately inform the development and implementation of treatment interventions for this group. In a meta-analysis, Saha et al. [31] reported a standard HD mortality estimate of 2.5 for patients with schizophrenia, yet there was no quantification of modifiable clinical or behavioral factors that explained this increased risk. Osborn et al. [32] found that UK patients with schizophrenia were about three times more likely to die from HD; however, while the analysis controlled for smoking and antipsychotic use, it did not control for co-occurring medical conditions (e.g., diabetes, obesity), which might have explained these associations. Understanding the specific modifiable risk factors associated with HD-related mortality in persons with mental disorders can inform interventions to reduce mortality risk in this group [33–35].
Despite the use of comprehensive patient-level survey and electronic data from a large national sample of patients with and without mental disorders, there are limitations to this study that warrant consideration. First, this study was not based on an inception cohort, and patients with mental illness may have had variable exposures to their illness that could change the association between disease and outcome over time. No follow-up LHSV was available to assess changes in risk factors over time. Second, the VA did not have complete laboratory and vital signs data for all patients at the time of the survey to confirm clinical diagnoses, notably hypertension and hyperlipidemia. Third, we were unable to assess the effect of medication use, notably atypical antipsychotics, on HD mortality risk. Nonetheless, we controlled for potential sequelae of atypical antipsychotics, including hyperlipidemia and diabetes diagnoses, and including these medications as confounders may have led to overadjustment.
Furthermore, the lack of treatment data was a limitation, and the roles of continuity of care and treatment engagement in mortality risk were not assessed. Treatment engagement may have led to the “protective” effect of being diagnosed with a specific condition (e.g., PTSD). In VA electronic data, diagnosis is often synonymous with treatment. The possible effect of treatment may have also explained the finding that depression and bipolar disorder had relatively less influence on HD mortality than psychotic disorders. Hence, the roles of treatment continuity and mortality across different mental disorder diagnoses should be explored further. We were also unable to control for the treatment effects of diabetes, hypertension or other HD risk factors (e.g., antihypertensives, lipid-lowering drugs, etc.); thus, residual confounding may have occurred. Moreover, we were unable to control for current psychiatric symptoms. Finally, the focus on VA patients may limit the generalizability of our findings.
Nonetheless, our findings have important implications for reducing disparities in HD-related mortality among persons with psychotic disorders within and beyond the VA setting. HD is prevalent, is associated with up to a 20% shorter life expectancy and is the most common cause of death in persons with mental disorders [4]. Our study found that VA patients with psychosis were the most likely to die from HD, but also elucidates specific modifiable behavioral risk factors that contribute to this public health crisis. For the reduction of HD-related mortality, programs should consider smoking-cessation strategies or interventions to promote exercise among patients who are able to benefit from physical activity interventions. Moreover, interventions to improve physical activity that focus on reducing HD risk should be age appropriate (e.g., lower impact for older individuals) and, more importantly, should address behavior change within the context of psychotic symptoms and co-occurring conditions, notably dementia. In addition, interventions to reduce the risk of HD should be integrated with existing programs such as geriatric psychiatry or rehabilitation so that the management of medical comorbidity and HD risk factors is addressed in light of individuals’ current functional status. With an emphasis on integrated mental health services, the VA is poised to take the next step towards implementing programs that aim to reduce the risk of HD-related mortality in patients with psychosis.
Acknowledgments
This work was supported by the Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service. This work was also completed with the support of the VA National Serious Mental Illness Treatment Research and Evaluation Center and the National Institute of Mental Health. We would like to acknowledge the VA Office of Quality and Performance for providing access to LHSV data.
References
- 1.Murray CJ, Lopez AD. Evidence-based health policy — lessons from the Global Burden of Disease Study. Science. 1996;274(5288):740–743. doi: 10.1126/science.274.5288.740. [DOI] [PubMed] [Google Scholar]
- 2.Harris E, Barraclough B. Excess mortality of mental disorder. Br J Psychiatry. 1998;173:11–53. doi: 10.1192/bjp.173.1.11. [DOI] [PubMed] [Google Scholar]
- 3.Stewart WF, Ricci JA, Chee E, Hahn SR, Morganstein D. Cost of lost productive work time among US workers with depression. JAMA. 2003;289:3135–3144. doi: 10.1001/jama.289.23.3135. [DOI] [PubMed] [Google Scholar]
- 4.Hennekens C. Increasing global burden of cardiovascular disease in general populations and patients with schizophrenia. J Clin Psychiatry. 2007;68(Suppl 4):4–7. [PubMed] [Google Scholar]
- 5.Angst F, Stassen HH, Clayton PJ, Angst J. Mortality of patients with mood disorders: follow-up over 34–38 years. J Affect Disord. 2002;68:167–181. doi: 10.1016/s0165-0327(01)00377-9. [DOI] [PubMed] [Google Scholar]
- 6.Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3:A42. [PMC free article] [PubMed] [Google Scholar]
- 7.Casey DE, Haupt DW, Newcomer JW, Henderson DC, Sernyak MJ, Davidson M, et al. Antipsychotic-induced weight gain and metabolic abnormalities: implications for increased mortality in patients with schizophrenia. J Clin Psychiatry. 2004;65(Suppl 7):4–18. [quiz 9–20]. [PubMed] [Google Scholar]
- 8.Goff DC, Sullivan LM, McEvoy JP, Meyer JM, Nasrallah HA, Daumit GL, et al. A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophr Res. 2005;80:45–53. doi: 10.1016/j.schres.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Hennekens CH, Hennekens AR, Hollar D, Casey DE. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150:1115–1121. doi: 10.1016/j.ahj.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Kilbourne AM, Rofey DL, McCarthy JF, Post EP, Welsh D, Blow FC. Nutrition and exercise behavior among patients with bipolar disorder. Bipolar Disord. 2007;9:443–452. doi: 10.1111/j.1399-5618.2007.00386.x. [DOI] [PubMed] [Google Scholar]
- 11.Newcomer JW. Medical risk in patients with bipolar disorder and schizophrenia. J Clin Psychiatry. 2006;67:e16. [PubMed] [Google Scholar]
- 12.American Diabetes Association. Consensus development conference on antipsychotic drugs and obesity and diabetes. Clin Psychiatry. 2004;65:267–272. doi: 10.4088/jcp.v65n0219. [DOI] [PubMed] [Google Scholar]
- 13.Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19(Suppl 1):1–93. doi: 10.2165/00023210-200519001-00001. [DOI] [PubMed] [Google Scholar]
- 14.Kilbourne AM, Irmiter C, Capobianco J, Reynolds K, Milner K, Barry K, et al. Improving integrated general medical and mental health services in community-based practices. Adm Policy Ment Health. 2008;35:337–345. doi: 10.1007/s10488-008-0177-8. [DOI] [PubMed] [Google Scholar]
- 15.Druss BG, Marcus SC, Campbell J, Cuffel B, Harnett J, Ingoglia C, et al. Medical services for clients in community mental health centers: results from a national survey. Psychiatr Serv. 2008;59:917–920. doi: 10.1176/ps.2008.59.8.917. [DOI] [PubMed] [Google Scholar]
- 16.Mensah GA, Brown DW, Croft JB, Greenlund KJ. Major coronary risk factors and death from coronary heart disease: baseline and follow-up mortality data from the Second National Health and Nutrition Examination Survey (NHANES II) Am J Prev Med. 2005;29(5 Suppl 1):68–74. doi: 10.1016/j.amepre.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 17.Laursen TM, Munk-Olsen T, Nordentoft M, Mortensen PB. Increased mortality among patients admitted with major psychiatric disorders: a register-based study comparing mortality in unipolar depressive disorder, bipolar affective disorder, schizoaffective disorder, and schizophrenia. J Clin Psychiatry. 2007;68:899–907. doi: 10.4088/jcp.v68n0612. [DOI] [PubMed] [Google Scholar]
- 18.Veterans Health Administration Office of Quality Performance and the Center for Health Quality OaER. Health Status and Outcomes of Veterans: Physical and Mental Component Summary Scores Veterans SF-36: 1999 Large Health Survey of Veteran Enrollees Executive Report. Washington, DC: Department of Veterans Affairs; 2000. [Google Scholar]
- 19.Selim AJ, Fincke G, Ren XS, Lee A, Rogers WH, Miller DR, et al. Comorbidity assessments based on patient report: results from the Veterans Health Study. J Ambul Care Manage. 2004;27:281–295. doi: 10.1097/00004479-200407000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Skinner KM, Miller DR, Spiro A, III, Kazis LE. Measurement strategies designed and tested in the Veterans Health Study. J Ambul Care Manage. 2004;27:180–189. doi: 10.1097/00004479-200404000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Skinner KM, Miller DR, Lincoln E, Lee A, Kazis LE. Concordance between respondent self-reports and medical records for chronic conditions: experience from the Veterans Health Study. J Ambul Care Manage. 2005;28:102–110. doi: 10.1097/00004479-200504000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Blow F, McCarthy J, Valenstein M. Care in the VHA for veterans with psychosis: FY2005. Annual Report on Veterans with Psychoses. 2005 [Google Scholar]
- 23.Blow F, Owen RE, Valenstein M, Austin KL, Khanjua K, McCarthy JF. Specialty care for veterans with depression in the VHA National Depression Registry Report. Ann Arbor, MI: VA National Serious Mental Illness Treatment Research and Evaluation Center; 2006. [Google Scholar]
- 24.Kilbourne AM, Welsh D, McCarthy JF, Post EP, Blow FC. Quality of care for cardiovascular disease-related conditions in patients with and without mental disorders. J Gen Intern Med. 2008;23:1628–1633. doi: 10.1007/s11606-008-0720-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kilbourne AM, McCarthy JF, Welsh D, Blow F. Recognition of co-occurring medical conditions among patients with serious mental illness. J Nerv Ment Dis. 2006;194:598–602. doi: 10.1097/01.nmd.0000230637.21821.ec. [DOI] [PubMed] [Google Scholar]
- 26.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 27.Haskell WL, Lee I, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exer. 2007;39:1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 28.Gordon AJ, Maisto SA, McNeil M, Kraemer KL, Conigliaro RL, Kelley ME, et al. Three questions can detect hazardous drinkers. J Fam Pract. 2001;50:313–320. [PubMed] [Google Scholar]
- 29.Birkenaes AB, Opjordsmoen S, Brunborg C, Engh JA, Jonsdottir H, Ringen PA, et al. The level of cardiovascular risk factors in bipolar disorder equals that of schizophrenia: a comparative study. J Clin Psychiatry. 2007;68:917–923. doi: 10.4088/jcp.v68n0614. [DOI] [PubMed] [Google Scholar]
- 30.Valenstein M, Kim HM, Ganoczy D, McCarthy JF, Zivin K, Austin KL, et al. Higher-risk periods for suicide among VA patients receiving depression treatment: prioritizing suicide prevention efforts. J Affect Disord. 2008;112:50–58. doi: 10.1016/j.jad.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saha S, Chant D, McGrath J. Meta-analyses of the incidence and prevalence of schizophrenia: conceptual and methodological issues. Int J Methods Psychiatr Res. 2008;17:55–61. doi: 10.1002/mpr.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osborn DP, Levy G, Nazareth I, Petersen I, Islam A, King MB. Relative risk of cardiovascular and cancer mortality in people with severe mental illness from the United Kingdom’ s General Practice Research Database. Arch Gen Psychiatry. 2007;64:242–249. doi: 10.1001/archpsyc.64.2.242. [DOI] [PubMed] [Google Scholar]
- 33.Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64:1123–1131. doi: 10.1001/archpsyc.64.10.1123. [DOI] [PubMed] [Google Scholar]
- 34.Brar JS, Ganguli R, Pandina G, Turkoz I, Berry S, Mahmoud R. Effects of behavioral therapy on weight loss in overweight and obese patients with schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2005;66:205–212. doi: 10.4088/jcp.v66n0208. [DOI] [PubMed] [Google Scholar]
- 35.McKibbin CL, Patterson TL, Norman G, Patrick K, Jin H, Roesch S, et al. A lifestyle intervention for older schizophrenia patients with diabetes mellitus: a randomized controlled trial. Schizophr Res. 2006;86:36–44. doi: 10.1016/j.schres.2006.05.010. [DOI] [PubMed] [Google Scholar]