Abstract
Background
Cryptococcal meningitis is the most common cause of adult meningitis in Africa, yet neurocognitive outcomes are unknown. We investigated the incidence and predictors of neurologic impairment among cryptococcal survivors.
Methods
HIV-infected, antiretroviral-naive Ugandans with cryptococcal meningitis underwent standardized neuropsychological testing at 1, 3, 6, and 12 months. A quantitative neurocognitive performance z-score (QNPZ) was calculated based on population z-scores from HIV-negative Ugandans (n=100). Comparison was made with an HIV-infected, non-meningitis cohort (n=110).
Results
Among 78 cryptococcal meningitis survivors with median CD4 count of 13 cells/μL (interquartile range: 6-44), decreased global cognitive function occurred through 12 months compared with the HIV-infected, non-cryptococcosis cohort (QNPZ-6 at 12 months, P=0.036). Tests of performance in eight cognitive domains was impaired 1 month after cryptococcal diagnosis; however, cryptococcal meningitis survivors improved their global neurocognitive function over 12 months with residual impairment (mean z-scores<-1), only in domains of motor speed, gross motor and executive function at 12 months. There was no evidence that neurocognitive outcome was associated with initial demographics, HIV parameters, or meningitis severity. Paradoxically, persons with sterile CSF cultures after 14 days of induction amphotericin therapy had worse neurocognitive outcomes than those still culture-positive at 14 days (P=0.002).
Conclusions
Cryptococcal meningitis survivors have significant short-term neurocognitive impairment with marked improvement over the first 12 months. Few characteristics related to severity of cryptococcosis, including Cryptococcus burden, were associated with neurocognitive outcome.
Keywords: Cryptococcal Meningitis, AIDS, Patient Outcome Assessment, Neurologic Manifestations, Neurobehavioral Manifestations, Intellectual disability, Cerebrospinal Fluid
Introduction
HIV-related neurologic complications are a significant cause of global morbidity and mortality. Cryptococcal meningitis is the most common central nervous system (CNS) infection in persons with AIDS worldwide and the most common cause of meningitis in Sub-Saharan Africa (Durski et al. 2013; Jarvis et al. 2010; Park et al. 2009). Despite antiretroviral therapy (ART) availability, 6-month survival is <50% in Africa (Kambugu et al. 2008; Park et al. 2009; Butler et al. 2012).
Acute neurologic complications of cryptococcosis commonly include increased intracranial pressure and associated neuropathies (Kambugu et al. 2008; Bicanic et al. 2009a). Less common manifestations include focal cryptococcomas, cerebrovascular accidents (Batista Leite et al. 2004), optic neuropathy with blindness (Johnston et al. 1992; Claus and Portegies 1998), and hearing loss (Kwartler et al. 1991). After ART initiation, further neurologic morbidity can occur from immune reconstitution inflammatory syndrome (IRIS), a paradoxical immune-mediated recurrent aseptic meningitis (Haddow et al. 2010; Boulware et al. 2010).
Long-term neurocognitive outcomes after cryptococcosis have not been well described. One study of 15 HIV/cryptococcal survivors reported neurocognitive deficits in verbal fluency and psychomotor domains (Levine et al. 2008). Whether the deficits represented acute or long-term sequelae is unclear. Case reports of reversible dementia in immunocompetent persons with cryptococcal meningitis suggest deficits could be transient (Ala et al. 2004; Hoffmann et al. 2009). To better characterize the incidence, severity of, and risk factors for neurocognitive disability, we assessed detailed neurocognitive function over 12 months following AIDS-related cryptococcal meningitis.
Methods
Participants
This prospective cohort study evaluated neurological outcomes on ART after meningitis as a nested sub-study of the Cryptococcal Optimal ART Timing (COAT) trial (clinicaltrials.gov: NCT01075152). Persons presenting with suspected meningitis to Mulago Hospital in Kampala, Uganda, from November 2010 until September 2013 had cryptococcal meningitis diagnosed by CSF quantitative culture and/or CSF cryptococcal antigen (CRAG) (Immy, Norman, Oklahoma). Cryptococcal patients were eligible for enrollment if age >18 years, HIV-infected, ART-naïve, and receiving amphotericin-based therapy. Exclusion criteria were: >2 current or previous CNS infections or other neurologic diagnoses, prior cryptococcosis, receiving chemotherapy or immunosuppressants, pregnancy or breastfeeding, inability to follow up, or significant co-morbidities. From November 2010 to April 2012 patients were enrolled in the COAT trial and thereafter patients were enrolled in an observational study. Written informed consent for study participation was obtained. Ethics approval occurred from: Makerere University, University of Minnesota, and Uganda National Council of Science and Technology.
Antifungal and Antiretroviral treatment
Cryptococcal therapy consisted of amphotericin B (0.7-1.0 mg/kg/day) combined with fluconazole 800mg/day as induction therapy for 14 days. Thereafter, enhanced consolidation therapy began with fluconazole 800mg/day for 3 weeks until CSF culture was sterile, followed by fluconazole 400mg/day for 8 weeks, then fluconazole 200mg/day secondary prophylaxis. Therapeutic lumbar punctures were performed routinely using manometers on day 1, 7, and 14 of induction therapy and additionally as needed for intracranial pressure control. Subjects enrolled in the COAT trial were randomized to initiate ART with efavirenz, zidovudine (AZT), and lamivudine (3TC) either at 7-11 days or five weeks after cryptococcal diagnosis. Timing of ART initiation was masked to investigators performing neurocognitive examinations. Subjects enrolled in the observational study, starting April 2012, initiated ART five weeks after cryptococcal diagnosis.
Clinical Evaluation
Cryptococcal meningitis survivors had a “baseline” neurocognitive evaluation conducted approximately 1-month after diagnosis. Follow-up neurocognitive evaluations were performed at 3, 6, and 12 months. Earlier neurocognitive testing was not performed due to frequent delirium associated with meningitis. Two trained research assistants performed neurocognitive assessments in either English or Luganda.
A battery of neuropsychological tests evaluated eight neuropsychological domains. These tests have been previously validated in sub-Saharan African populations (Sacktor et al. 2005; Wong et al. 2007; Nakasujja et al. 2010). The test battery included: World Health Organization-University of California-Los Angeles Auditory Verbal Learning Test (WHO-UCLA AVLT) (Maj et al. 1993), Digit Span Forward and Backward, Semantic Verbal Fluency (Spreen and Strauss 1998), Timed Gait (Robertson et al. 2006), Grooved Pegboard (Klove 1963), Finger Tapping (Reitan 1969), Weschler Adult Intelligence Scale Symbol Digit (Wechsler 1981), and Color Trails 1 and 2 (D'Elia et al. 1996). Table 1 provides further test details.
Table 1.
Neuropsychological test battery and neurocognitive domains evaluated.
| Test | Test Description | Cognitive Domains |
|---|---|---|
| WHO-UCLA AVLT-Total* | Subjects are asked to recall a list of words. The test is similar to the Rey Auditory Verbal Learning test, however words have been selected to be recognizable to a variety of cultures | Verbal learning |
| WHO-UCLA AVLT-Delayed Recall* | Similar to WHO-UCLA AVLT, but subjects are asked to recall the same list of words in a delayed recall phase | Verbal memory |
| Digit Span Forward and Backward | Subjects are given a series of digits of increasing length and are asked to repeat them in forward or backward order | Attention, Working memory |
| Semantic Verbal Fluency | Subjects are given 60 seconds to produce as many words as possible within a specific category such as ‘animals’ | Language fluency (Verbal) |
| WAIS-III Symbol Digit | Subjects are asked to match symbols to numbers as quickly as possible over 120 seconds using a visual reference.; also referred to as a Digit Symbol Substitution Test. | Speed of information processing, Concentration |
| Color Trails 1 | Subjects connect encircled numbers scattered on a page in sequence during a set amount of time. This test is similar to the Trail Making Test but has been formulated to minimize cultural bias by not using any letters or written instructions | Speed of information processing, Attention |
| Color Trails 2 | Similar to The Color Trails 1 but each number is printed in two different colors, and subjects are asked to maintain the numerical sequence while alternating colors | Executive function |
| Timed Gait | The time for subjects to walk out and back 10 meters is recorded | Gross motor |
| Grooved Pegboard | Subjects are timed while placing pegs which each have a key along one side in holes in various orientations in a pegboard with either their dominant or non-dominant hand | Fine motor |
| Finger tapping | Subjects tap as rapidly as possible using the index finger on a specially adapted tapper for five 10-second trials | Motor speed |
WHO-ULCA AVLT = World Health Organization-University of California-Los Angeles Auditory Verbal Learning test; WAIS = Wechsler Adult Intelligence Scale
Raw scores for each test were standardized to create age and education-adjusted z-scores using HIV-uninfected Ugandans as reference; z-scores above zero denote above-average neurocognitive function, and scores below zero denote below-average neurocognitive function (Robertson et al. 2007; Sacktor et al. 2005). A quantitative neurocognitive performance z-score (QNPZ-8), assessing global neurocognitive function, was calculated as the mean of eight individual z-scores, including Symbol Digit, WHO-UCLA AVLT immediate and delayed recall, Verbal Fluency, Color trails1 and 2, Finger Tapping and Grooved Pegboard (mean of each of the hands). Comparison data for HIV-infected Ugandans were available for the above tests with the exception of verbal fluency and finger tapping (Robertson et al. 2007); consequently, a QNPZ-6 score omitting these tests was also calculated. We defined “impaired” neurocognitive function as a z-score<-1 (i.e. one standard deviation (SD) below the HIV-negative population average) and “severe impairment” as a z-score<-2.
Subjects who started but did not complete a test due to visual difficulties, fatigue, or physical limitations were permitted to skip tests. Uncompleted tests were assigned values equal to the mean z-score for the cryptococcal cohort minus 2 standard deviations. Missed study visits were rare during follow-up at 3 months (n=4), 6 months (n=2), and 12 months (n=5).
The International HIV Dementia Scale (IHDS) and a measure for depression, the Center for Epidemiologic Studies Depression (CES-D) scale, were also administered to study participants (Sacktor et al. 2005; Myer et al. 2008; Kaharuza et al. 2006; Radloff 1977). All clinical laboratory evaluations were performed onsite at a College of American Pathologists (CAP)-certified laboratory.
Comparison Cohorts, HIV-infected and HIV-negative Controls
We used for comparison neurocognitive evaluations from two previously described Ugandan cohorts of HIV-negative (n=100) individuals (Sacktor et al. 2005) and HIV-infected (n=110) individuals without known or past CNS opportunistic infections (Robertson et al. 2007; Sacktor et al. 2005). A global score across six domains (QNPZ-6) was calculated for comparison with the HIV-infected cohort as finger tapping and verbal fluency were not assessed in the prior HIV-infected comparison cohort. The HIV-infected cohort included persons without known active or past CNS infection and with WHO Stage II (n=21), III (n=69), or IV (n=20) HIV, of whom 49 were receiving ART (Robertson et al. 2007).
Data Analysis
Demographic and clinical characteristics were compared using Student's t-tests or Chi-square statistics. Mean z-scores were calculated for each neurocognitive test and for the total QNPZ-8 scores at each time point. Mean QNPZ-6 scores and mean z-scores for each neurocognitive test were compared between the two HIV-infected cohorts via Student's t-tests. To assess the intra-person change over time in the cryptococcal cohort, mean z-scores were compared using paired t-tests.
Longitudinal linear mixed effects regression assessed the QNPZ-8 score versus clinical parameters at meningitis diagnosis including Glasgow coma scale, Karnofsky score, CD4 count, plasma HIV RNA viral load, CSF white blood cell (WBC) count, CSF opening pressure, and quantitative CSF fungal culture. The mixed effects models included a random intercept for each subject, to account for intra-subject correlation, and were adjusted for the month of follow-up. Analysis for the effect of culture sterility at the end of amphotericin therapy on QNPZ-8 scores was performed with and without adjustment for baseline CSF fungal burden, CSF WBCs, CD4 count, and HIV viral load. Linear fixed effects regression assessed cross-sectional relationships between neurocognitive performance and HIV parameters during follow-up. Statistical significance was considered for p-values <0.05. Analyses were conducted in SAS version 9.3 (SAS Institute, Cary, NC).
Results
From November 2010 to September 2013, 78 participants with cryptococcal meningitis completed up to four follow-up neurocognitive exams over 12 months. Nine individuals died during follow-up, occurring after 1 month (n=5), after 3 months (n=3), and after 6 months (n=1) of follow-up. Baseline clinical and demographic characteristics of the cryptococcal survivors and reference cohorts are presented in Table 2. Participants had advanced AIDS and severe cryptococcal disease as evidenced by low CD4 cell counts (median 13, Interquartile range: 6 to 44 cells/ L), high HIV viral loads, and high CSF quantitative cryptococcal cultures. At meningitis presentation, mean (±SD) CSF opening pressure was 301 (±140) mmH2O with mean quantitative cryptococcal culture of 4.3 (±1.6) log10 CFU/mL of CSF. Among 39 participants with CSF opening pressures >250 mmH2O at diagnosis (57% of 68 with opening pressures reported), 18 (46%) had at least 2 additional therapeutic lumbar punctures (LP) and 17 (43%) had one repeat LP in the first seven days following meningitis diagnosis. Compared to the HIV-negative and HIV-infected non-meningitis subjects, the cryptococcal survivors were of similar age and level of education, but were more often male.
Table 2.
Characteristics of participants with Cryptococcal meningitis and controls with and without HIV
| Variable | HIV+ Cryptococcal Meningitis N=78 | HIV+ controls without meningitis N=110 | HIV− negative controls N=100 |
|---|---|---|---|
| Age | 35 (±8) | 36 (±9) | 31 (±7) |
| Males (N, %) | 48 (62%) | 31 (28%) | 47 (47%) |
| Years of Education | 8 (±4) | 9 (±5) | 10 (±4) |
| WHO Clinical Stage 4 (N, %) | 78 (100%) | 20 (18%) | -- |
| Clinical Factors at Baseline | |||
| Glasgow Coma Scale < 15 (N, %) | 19 (24%) | 0 (0%) | 0 (0%) |
| Karnofsky Score | 48 (±11) | 73 (±12) | 100 |
| Seizures (N, %) | 12 (15%) | -- | 0 (0%) |
| CD4+ T cells/μL | 40 (±69) | 176 (±191) | |
| HIV Viral Load, log10 copies/mL | 5.4 (±0.5) | ||
| CSF Profile at Cryptococcal Meningitis Diagnosis | |||
| Opening pressure, mmH2O | 301 (±140) | ||
| White blood cell count, cells/μL | 61 (±95) | ||
| C. neoformans quantitative culture, log10 CFU/mLa | 4.3 (±1.6) | ||
| Follow-up at 1 month | |||
| CES-D > 16 (N, %)b | 55 (72%) | ||
| Karnofsky Score | 61 (±10) | ||
| Int'l HIV Dementia Scale ≤10 (N, %)c | 71 (93%) | ||
Data presented are mean (± SD), unless otherwise noted.
Restricted to CSF specimens collected within 2 days of starting amphotericin, n=64
Center for Epidemiology Studies Depression Scale (CES-D) scores >16 suggest depression symptomatology, n=76
International HIV Dementia Scale ≤10 are suggestive of HIV dementia, n=77.
Neurocognitive function
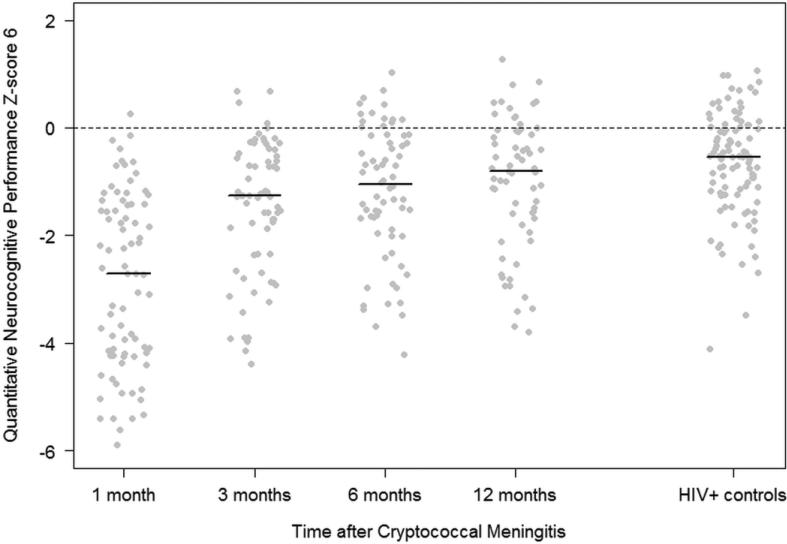
Overall, one month after cryptococcal meningitis, 89% (69/78) of survivors had an impaired global neurocognitive function score, QNPZ-8<-1, and 55% (43/78) had severe impairment with QNPZ-8 <-2. Average z-scores across study participants demonstrated neurocognitive impairment (z-score< -1) in each cognitive domain tested, except for one test of attention and working memory (Digit Span Forward mean (±SD) z-score = −0.7, (±1.0)) and verbal fluency (mean z-score = −1.0, (±0.6)). Severe deficits (mean z-score<-2) were seen in tests of verbal learning, verbal memory, speed of information processing, executive function, motor speed, and fine and gross motor function (Table 3). Compared with an HIV-infected cohort, cryptococcal survivors had significantly decreased QNPZ-6 scores (P<0.001, Figure 1) with significant specific deficits in tests of all domains (all P<0.001, except for Digit Span Forward test P=0.008). Scores for all domains were also significantly lower than the HIV-negative reference cohort (all P<0.001). One month after cryptococcal meningitis diagnosis, 93% of the participants had IHDS score ≤10 suggestive of HIV dementia, while 73% had a CES-D score >16 suggestive of depression symptomatology.
Table 3.
Neurocognitive domains tested among cryptococcal meningitis survivors over 1 year
| Neurocognitive Domain | Test | 1 month (N = 78) | 3 months (N = 69) | 6 months (N = 68) | 12 months (N = 64) | HIV+ controls (N = 110) | 1 month vs. HIV+ controls | 3 months vs. HIV+ controls | 6 months vs. HIV+ controls | 12 months vs. HIV+ controls |
|---|---|---|---|---|---|---|---|---|---|---|
| Global Function | QNPZ-8 | −2.6 (±1.4) | −1.4 (±1.1)a | −1.2 (±1.1)b | −1.0 (±1.1) | |||||
| Global Function | QNPZ-6 | −2.8 (±1.6) | −1.5 (±1.3)a | −1.2 (±1.2)b | −1.0 (±1.2) | −0.7 (±1.0) | <0.001 | <0.001 | 0.003 | 0.036 |
| Verbal learning | AVLT- total | −2.6 (±1.6) | −1.5 (±1.4)a | −1.3 (±1.8) | −1.0 (±1.3) | −1.0 (±1.4) | <0.001 | 0.006 | 0.116 | 0.567 |
| Verbal memory | AVLT- recall | −2.5 (±2.1) | −1.3 (±1.6)a | −1.1 (±1.7) | −0.9 (±1.4) | −0.9 (±1.3) | <0.001 | 0.114 | 0.537 | 0.879 |
| Attention, working memory | Digit span forward | −0.7 (±1.0) | −0.5 (±1.1) | −0.4 (±1.0) | −0.3 (±1.2) | −0.2 (±1.0) | 0.008 | 0.11 | 0.187 | 0.433 |
| Digit span backward | −1.7 (±1.3) | −1.3 (±1.1)b | −1.0 (±1.2) | −1.0 (±1.1) | −0.8 (±1.3) | <0.001 | 0.018 | 0.271 | 0.179 | |
| Language | Verbal fluency | −1.0 (±0.6) | −0.7 (±0.6)a | −0.6 (±0.8) | −0.4 (±0.8)b | |||||
| Speed of information processing | Symbol digit | −2.2 (±1.1) | −1.5 (±0.9)a | −1.3 (±1.1)b | −1.1 (±1.2) | −0.5 (±1.3) | <0.001 | <0.001 | <0.001 | 0.003 |
| Color trails 1 | −3.9 (±3.1) | −1.3 (±2.0)a | −0.9 (±1.8)b | −0.9 (±1.8) | −0.6 (±1.9) | <0.001 | 0.002 | 0.071 | 0.079 | |
| Executive function | Color trails 2 | −3.8 (±2.2) | −2.6 (±2.1)a | −2.3 (±2.2) | −2.2 (±2.7) | −1.2 (±1.7) | <0.001 | <0.001 | <0.001 | 0.003 |
| Motor speed | Finger-tapping | −2.7 (±1.4) | −1.7 (±1.2)a | −1.6 (±1.2) | −1.5 (±1.2) | |||||
| Fine motor | Grooved pegboard - dominant hand | −2.2 (±2.6) | −0.4 (±1.7)a | −0.1 (±1.5) | −0.2 (±1.7) | 0.6 (±1.1) | <0.001 | <0.001 | <0.001 | <0.001 |
| Grooved pegboard - non-dominant hand | −1.9 (±2.2) | −0.8 (±2.0)a | −0.3 (±1.7)b | 0.1 (±1.6)b | −0.7 (±1.9) | <0.001 | 0.775 | 0.153 | 0.004 | |
| Gross motor | Timed gait | −13.7 (±5.4) | −9.5 (±5.4)a | −8.5 (±4.1) | −7.9 (±3.5) | −0.3 (±2.3) | <0.001 | <0.001 | <0.001 | <0.001 |
P<0.001 change from prior visit.
P<0.05 change from prior visit.
Outcomes for each test are expressed as mean (±SD) z-scores where a score of 0 equals the population average and −1 indicates one standard deviation below average. The quantitative neurocognitive performance z-score-8 (QNPZ-8) includes the mean of 8 individual z-scores: WHO-UCLA AVLT immediate and delayed recall, verbal fluency, color trails 1 and 2, finger tapping and grooved pegboard (mean of each of the hands). The QNPZ-6 omitted verbal fluency and finger tapping. At 1, 3, 6, and 12 months, respectively, 54%, 22%, 28%, and 22% of individuals had at least one test that was started but unable to be completed. The most frequently uncompleted test was color trails 2.
Figure 1. Neurocognitive function of cryptococcal meningitis survivors over 12 months compared with HIV-infected and HIV-negative population controls.
Figure 1 displays the neurocognitive function of cryptococcal meningitis survivors compared with an HIV-infected Ugandan cohort (n=110). The z-scores were normalized to an HIV-negative adult Ugandan population (n=100), adjusted for age and education (population average z-score = 0). The summary quantitative neurocognitive performance z-score (QNPZ-6) was collected after cryptococcal diagnosis at 1 month (n=78), 3 months (n=69), 6 months (n=68), and 12 months (n=64). The QNPZ-6 was composed of the average z-score of: grooved pegboard (mean of both hands), symbol digit, WHO-UCLA auditory verbal learning test (both immediate and delayed recall), and color trails 1 and 2. Each individual score is depicted by a dot and the black line depicts the median score for each group. Nine individuals (12%) died during follow-up between 1 and 12 months.
After 3 months of follow-up, a significant improvement in neurocognitive function had occurred with the mean QNPZ-8 score increasing from -2.6 at 1 month to -1.4 at 3 months (P<0.001). Still, 59% (41/69) of cryptococcal survivors had impaired neurocognitive function with QNPZ-8 scores<-1, and 25% (17/69) had severe impairment (QNPZ-8<-2). Although there were persistent deficits at 3 months, neurocognitive function significantly improved in all cognitive domains tested, except tests of attention (compared with z-scores at 1 month, all P<0.01 except Digit Span Forward P=0.21 and Digit Span Backward P=0.04).
By 6 months, cryptococcal survivors demonstrated additional improvement in nearly all neurocognitive domains. At 6 months, the majority of individuals (53%) were within 1 standard deviation of the HIV-negative Ugandan population norms and the QNPZ-8 score improved from -1.4 to -1.2 between 3 and 6 months (P=0.007). Global severe impairment (QNPZ-8 < -2) was present in 22% of individuals. Impairments persisted in tests of verbal learning, verbal memory and motor speed with mixed results for verbal learning, verbal memory, speed of information processing, and motor speed, and with severe impairment in executive and gross motor function. Compared to the HIV-infected non-meningitis cohort, at 6 months there were residual deficits in tests of speed of information processing (symbol digit P<0.001), executive function (P<0.001) and gross motor function (P<0.001). Fine motor function had improved to HIV-negative population norms.
Overall, compared with the HIV-infected cohort, global neurocognitive impairment, as assessed by the QNPZ-6, was significantly worse among cryptococcal survivors at each evaluation: 1 month (P<0.001), 3 months (P<0.001), and 6 months (P=0.003). By 12 months after cryptococcal meningitis, among 64 subjects tested, only 41% (26/64) had global neurocognitive impairment (QNPZ-8<-1) and 20% (13/64) had severe impairment (QNPZ-8<-2). The mean QNPZ-6 was -1.0, and remained significantly lower than among the HIV-infected cohort (QNPZ-6 mean = -0.7, P=0.04). However, compared with assessments earlier after cryptococcal meningitis diagnosis, substantial improvement was observed over the first year of HIV and antifungal therapies. At 12 months, of the 8 domains tested, impairment (mean z-score < -1) persisted in executive function, motor speed, and gross motor function with severe impairment (mean z-score <-2) in executive and gross motor function.
Baseline Predictors of Future Neurocognitive Function
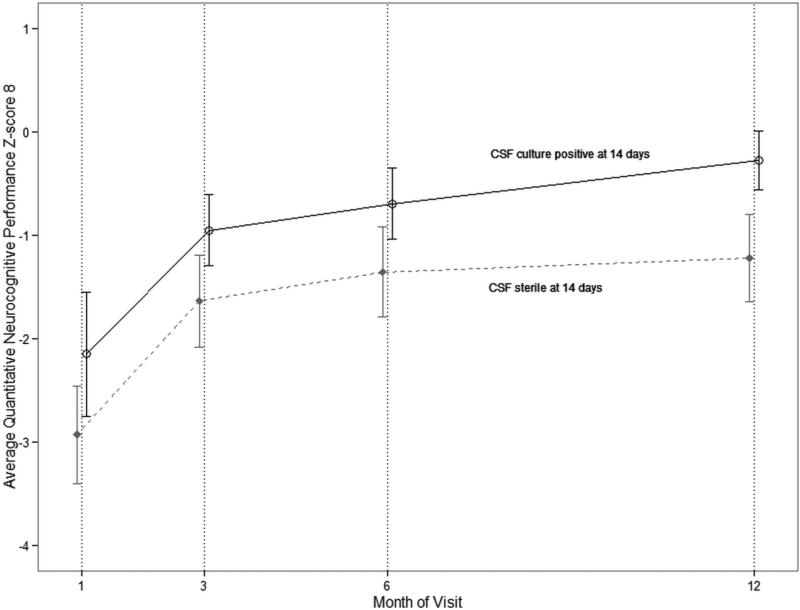
Initial clinical and laboratory characteristics at cryptococcal meningitis presentation were not associated with neurocognitive function over the course of follow-up as assessed by QNPZ-8 scores including altered mental status (Glasgow Coma Scale<15, P=0.21), Karnofsky score (P=0.15), CSF opening pressure (P=0.96), CSF WBC count (P=0.53), quantitative fungal CSF culture (P=0.47), pre-ART CD4 count (P=0.09), or pre-ART HIV RNA viral load (P=0.85). Also unassociated was day 14 CSF opening pressure (P=0.28). HIV RNA viral load at 3 and 6 months follow-up were unrelated to QNPZ-8 scores at the corresponding time points (3 months, P=0.59; 6 months, P=0.77). At 3 months, there was no association between CD4 count and QNPZ-8 (P=0.74) and marginal association at 6 months (P=0.09); however, at 12 months higher CD4 counts were significantly associated with higher QNPZ-8 scores (0.03 points greater QNPZ-8 per 10-cell/μL CD4 increase, 95% confidence interval (CI): 0.003, 0.05; P=0.02). Paradoxically, individuals with sterile CSF cultures at day 14 had significantly worse QNPZ-8 scores over 12 months than persons culture-positive after 14 days of amphotericin (adjusted P=0.003) (Figure 2).
Figure 2. Association of global neurocognitive outcome with CSF culture status at day 14 of amphotericin induction therapy.
The figure displays the mean QNPZ-8 score over 12 months of follow-up stratified by CSF culture status after 14 days of amphotericin 0.7-1.0mg/kg/day with fluconazole 800mg/day induction therapy. Error bars represent 95% confidence intervals. Individuals with a sterile CSF culture at day 14 (n=25) had worse QNPZ-8 scores at any given visit over 12 months than individuals with a non-sterile CSF (n=21) at day 14 (P=0.002). Those with sterile cultures had worse outcomes (adjusted QNPZ-8 mean difference = -0.9, 95% CI: -1.4 to -0.3; P=0.002) when adjusted for baseline CSF fungal burden, CSF WBC count, pre-ART CD4 and pre-ART HIV viral load in a linear regression model incorporating a random intercept for intra-person repeated measures. These results did not change when adjusted for ART timing (difference = -0.90; 95% CI: -1.4 to -0.3; P=0.002). The baseline fungal burden for those with a non-sterile day 14 CSF culture was a mean (±SD) of 4.8 (±1.2) log10 CFU/mL of CSF and for those with a sterile day 14 CSF culture was 3.8 (±1.9) log10 CFU/mL. Twenty-three individuals did not have a CSF culture results available after 14 days of amphotericin, and these individuals had QNPZ-8 scores similar to those with a negative culture. Among those with missing culture status at day 14, the QNPZ-8 mean (±SD) were at 1 month = -2.6 (± 1.5), 3 months = -1.6 (±1.2), 6 months = -1.3 (±1.1), and 12 months = -1.6 (±1.2).
The occurrence of suspected immune reconstitution inflammatory syndrome (IRIS) was uncommon. Only 7.7% (6/78) of participants were diagnosed with probable IRIS during 6 months of follow-up after cryptococcal diagnosis. IRIS was not associated with lower QNPZ-8 scores at 6 months (P=0.77). Additionally, there was no difference in neurocognitive performance by timing of early vs. deferred ART initiation (at 1 month early ART (n= 26), QNPZ-8 = -2.57 (±1.41); deferred ART (n=52), QNPZ-8 = -2.61 (±1.39); P =0.92).
Throughout follow-up, global QNPZ-8 scores were significantly associated with Karnofsky score at the same visit with each 10-point increase in Karnofsky score associated with a 0.5 point increase in QNPZ-8 score (95% CI: 0.4, 0.6; P<0.001). Depression symptomatology was associated with worse neurocognitive performance; individuals with CES-D score >16 had a 0.7 lower QNPZ-8 score at any given visit (95% CI: -1.0, -0.5; P<0.001).
Activities of Daily Living
Functionally at 1 month, 85% (44/52) reported being unable to work, 19% (10/53) reported being unable to track their own medications. By 6 months, 24% (16/66) reported inability to work, 5% (3/66) reported decreased efficiency and difficulty maintaining focus at work, and 5% (3/66) reported working with “a great deal of difficulty.” Two individuals (3% of 65 assessed) reported being unable to track their medications at the 6-month exam. By 12 months only 7 participants (of 62 assessed) indicated an inability to work. These individuals unable to work had lower QNPZ-8 scores (-2.1 (±1.5)) and were more likely to have depressive symptomatology (CES-D score >16 in 67%) at the 12-month exam than individuals who were able to work (mean QNPZ-8 score = -0.8±0.1 and 9% with depressive symptomatology; P=0.06 and P=0.003, respectively). At 12 months, one individual indicated being unable to independently track medications.
Discussion
HIV-infected Ugandans with cryptococcal meningitis had significantly worse neurocognitive performance in all cognitive domains and in gross motor function in the short-term compared with HIV-infected and HIV-negative Ugandans without CNS infections. However, cryptococcal meningitis survivors demonstrated significant improvement over the first 12 months. With ongoing treatment, over half of cryptococcal survivors recovered to within one standard deviation of the reference HIV-negative population by 6 months, and the vast majority reported being able to work by 6 months. Cryptococcal meningitis survivors at 12 months, however, had severe impairment in both executive and gross motor function as well as persistent impairment in verbal learning and motor speed, which was statistically worse than HIV-infected Ugandans without meningitis. All individuals received the same ART regimen, but at one-month of follow-up there was no difference in neurocognitive function between those who received “early” ART beginning at one-week after cryptococcal diagnosis, and those who started ART at the one-month visit.
There was also no association with baseline fungal burden or initial severity of meningitis illness with long-term neurocognitive outcomes, except those with sterile cultures at 14 days had statistically worse global neurocognitive function than those with persistently positive C. neoformans cultures. This suggests that immune responses or amphotericin toxicity may possibly play a larger role in neurologic damage than the Cryptococcus organism. Amphotericin causes cellular activation via toll-like receptor (TLR) 2 and CD14 (Razonable et al. 2005; Sau et al. 2003). TLR2 is present on microglia cells, and amphotericin activation of microglial cells can trigger interleukin (IL)-1beta, IL-6, and tumor necrosis factor-alpha (Motoyoshi et al. 2008). We speculate that those who achieved greater uptake of amphotericin into the CNS may have sterilized their CSF faster (Bicanic et al. 2007; Bicanic et al. 2009b); however, they also may have had more microglial cell activation and neuronal damage, manifesting as persistently worse neurocognitive performance.
Our study builds upon a cross-sectional study of 15 U.S. HIV/cryptococcal-infected patients compared with persons living with AIDS without cryptococcosis, which identified significantly worse global cognitive function (Levine et al. 2008). Specific deficits were described in verbal fluency and fine motor domains, but there were no deficits in abstraction/executive function, as we identified in the current study. Factors possibly contributing to disparate results include study design, sample size, length of time from CNS infection, and patient population. The current study included a larger sample size, well-defined standardized management regimens, and evaluation of changes in neurocognitive performance over time. An additional strength of the current study is that cryptococcal survivors were compared with both HIV-infected and HIV-negative cohorts from the same region in Uganda, with similar demographics and cultural and socioeconomic backgrounds.
Prior literature suggests that, among individuals with HIV-associated neurocognitive disorder (HAND), deficits in neuropsychological tests were indicative of a >5-fold increased likelihood of dependence in performing instrumental activities of daily living, such as managing money and medications (Heaton et al. 2004). Poor executive function was also found to independently predict poor instrumental activities of daily living performance (Heaton et al. 2004). These patterns, and our experience, suggest that most cryptococcal meningitis survivors will require family/caregiver support, and this should be recognized in both linkage to care and in pre-ART counseling, as this impairment could increase the risk for HIV and meningitis treatment failure. The participants with cryptococcosis in the current study were counseled regarding treatment adherence and counseling extensively addressed finding and training appropriate caregivers prior to hospital discharge.
Factors other than cryptococcosis may influence neurocognitive function. Depression is also associated with mild cognitive impairment, and depression symptoms are common in HIV/AIDS populations. Our study found 73% of cryptococcal survivors had depression symptomatology (CES-D score>16) at 1 month. This incidence is slightly higher than another study reporting CES-D>16 in 54% of HIV-infected Ugandans without CNS infections initiating ART (Nakasujja et al. 2010). Depressive symptoms are known to improve with ART (Nakasujja et al. 2010), and our study found that the frequency of depressive symptoms declined to less than one-third at 3 and 6 months. Consequently, improvements in depression may account for some of the improvements observed in neurocognitive function.
Additionally, HAND, which is associated with lower CD4 nadir and is also known to improve with ART, may potentially confound some differences identified in this study (Ellis et al. 2011; Sacktor et al. 2006). The HIV-infected non-meningitis control cohort had decreased frequency of WHO Clinical Stage IV HIV with 18% having CD4<200 compared with 100% of the cryptococcal-infected cohort (by definition). This may be associated with higher rates of HAND in the cryptococcal cohort. Whether the mechanism(s) leading to neurocognitive deficits after cryptococcal meningitis are related to that which causes HAND, attributable to cryptococcal infection, and/or treatment toxicity is unknown.
We acknowledge that “practice effects” may partially contribute to the improvement observed over 1 year of follow-up (Levine et al. 2004). However, the magnitude of neurocognitive improvement (mean ΔZ-score= +1.6 by 12 months) coupled with the overall clinical improvement, such as the ability to return to work, suggest that most individuals experienced real clinical improvement that was thus reflected in their neurocognitive testing.
Notably, among cryptococcal survivors, indicators of meningitis severity and risk of acute mortality, including altered mental status, increased intracranial pressure, and CSF fungal burden at diagnosis (Bicanic et al. 2009b; Graybill et al. 2000), did not predict neurocognitive performance. Previous studies in HIV-negative individuals have also reported associations between Cryptococcus-related brain MRI changes and CSF fungal burden (i.e. CSF cryptococcal antigen titer) with neurocognitive deficits (Lu et al. 2011; Chen et al. 2012), though our data suggest that HIV-infected cryptococcal survivors with a high fungal burden on presentation do not necessarily have a worse prognosis for neurocognitive recovery. HIV viral load and CD4 count were also not predictive of the neurocognitive function through 6 months; however, all participants had very low CD4 counts initially. ART timing was not a significant confounder to these analyses, as the timing of ART therapy did not affect neurocognitive outcomes, and there was not a significant association with viral load. Higher CD4 counts at 12 months were, however, associated with improved neurocognitive function; further studies are necessary to delineate possible associations with HAND, which is also associated with CD4 T cell counts and improvement on ART (Sacktor et al. 2006; Robertson et al. 2004; Wong et al. 2007).
Although predictors of long term outcome were limited, the overall prognosis of neurologic recovery is good, despite initial critical illness. This neurocognitive outcome information is useful for patients and physicians to be aware that substantial improvement does occur over time on ART, and long-term survival can be quite good (Butler et al. 2012).
In conclusion, short-term neurologic deficits followed cryptococcal meningitis. However, individuals improved significantly over time with residual neurocognitive deficits in verbal learning, executive function, motor speed, and gross motor function at 12 months. Initial clinical severity or fungal burden did not predict worse neurocognitive outcome; however, 2-week CSF culture sterility was paradoxically associated with worse neurocognitive outcome over 12 months. The CSF immune response and/or amphotericin toxicity should be further explored in future studies. Importantly, this study highlights the potential for return to a functional and independent lifestyle, with appropriate therapy, in most cryptococcal meningitis survivors despite severe cryptococcal infection and AIDS.
Acknowledgements
We thank medical care of the participants by Drs. Abdu Musubire, Henry Nabeta, and Josh Rhein as well as institutional support from Drs. Paul Bohjanen, Andrew Kambugu, and Yukari Manabe. We thank Ms. Darlisha Williams MPH for project management, Dr. Grant Botker for piloting data collection materials, the research assistants: Alice Namudde and Charles Ssebunya for the neurocognitive data collection. We thank Dr. Ned Sacktor for his pioneering work on neurocognitive function in Africa to enable comparison.
Source of Funding: This research was made possible through support from the Fogarty International Center and National Institute of Neurologic Diseases and Stroke (R21NS065713) and National Institute of Allergy and Infectious Diseases (U01AI089244, K23AI073192).
Footnotes
Conflicts of Interest: None.
References
- Ala TA, Doss RC, Sullivan CJ. Reversible dementia: a case of cryptococcal meningitis masquerading as Alzheimer's disease. J Alzheimers Dis. 2004;6(5):503–508. doi: 10.3233/jad-2004-6507. [DOI] [PubMed] [Google Scholar]
- Batista Leite AG, Vidal JE, Filho FB, Nogueira RS, Penalva de Oliveira AC. Cerebral infarction related to cryptococcal meningitis in an HIV-infected patient: case report and literature review. Brazilian J Infect Dis. 2004;9(2):175–179. doi: 10.1590/s1413-86702004000200008. [DOI] [PubMed] [Google Scholar]
- Bicanic T, Brouwer AE, Meintjes G, Rebe K, Limmathurotsakul D, Chierakul W, Teparrakkul P, Loyse A, White NJ, Wood R, Jaffar S, Harrison T. Relationship of cerebrospinal fluid pressure, fungal burden and outcome in patients with cryptococcal meningitis undergoing serial lumbar punctures. AIDS. 2009a;23(6):701–706. doi: 10.1097/QAD.0b013e32832605fe. doi:10.1097/QAD.0b013e32832605fe00002030-200903270-00008 [pii. [DOI] [PubMed] [Google Scholar]
- Bicanic T, Meintjes G, Wood R, Hayes M, Rebe K, Bekker LG, Harrison T. Fungal burden, early fungicidal activity, and outcome in cryptococcal meningitis in antiretroviral-naive or antiretroviral-experienced patients treated with amphotericin B or fluconazole. Clin Infect Dis. 2007;45(1):76–80. doi: 10.1086/518607. doi:10.1086/518607. [DOI] [PubMed] [Google Scholar]
- Bicanic T, Muzoora C, Brouwer AE, Meintjes G, Longley N, Taseera K, Rebe K, Loyse A, Jarvis J, Bekker LG, Wood R, Limmathurotsakul D, Chierakul W, Stepniewska K, White NJ, Jaffar S, Harrison TS. Independent association between rate of clearance of infection and clinical outcome of HIV associated cryptococcal meningitis: analysis of a combined cohort of 262 patients. Clin Infect Dis. 2009b;49(5):702–709. doi: 10.1086/604716. doi:doi:10.1086/604716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware DR, Meya DB, Bergemann TL, Wiesner DL, Rhein J, Musubire A, Lee SJ, Kambugu A, Janoff EN, Bohjanen PR. Clinical features and serum biomarkers in HIV immune reconstitution inflammatory syndrome after cryptococcal meningitis: a prospective cohort study. PLoS Med. 2010;7(12):e1000384. doi: 10.1371/journal.pmed.1000384. doi:10.1371/journal.pmed.1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler EK, Boulware DR, Bohjanen PR, Meya DB. Long term 5-year survival of persons with cryptococcal meningitis or asymptomatic subclinical antigenemia in Uganda. PLoS One. 2012;7(12):e51291. doi: 10.1371/journal.pone.0051291. doi:10.1371/journal.pone.0051291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Chang CC, Chang WN, Tsai NW, Lui CC, Lin WC, Chen NC, Chen C, Huang CW, Lu CH. Neuro-psychological Sequelae in HIV-negative Cryptococcal Meningitis after Complete Anti-fungal Treatment. Acta Neurol Taiwan. 2012;21(1):8–17. [PubMed] [Google Scholar]
- Claus JJ, Portegies P. Reversible blindness in AIDS-related cryptococcal meningitis. Clin Neurol Neurosurg. 1998;100:51–52. doi: 10.1016/s0303-8467(97)00119-4. [DOI] [PubMed] [Google Scholar]
- D'Elia LF, Satz P, Uchiyama CL, White T. Color Trails Test: Professional Manual. Psychological Assessment Resources; Odessa, FL: 1996. [Google Scholar]
- Durski KN, Kuntz KM, Yasukawa K, Virnig BA, Meya DB, Boulware DR. Cost-effective diagnostic checklists for meningitis in resource-limited settings. J Acquir Immune Defic Syndr. 2013;63(3):e101–108. doi: 10.1097/QAI.0b013e31828e1e56. doi:10.1097/QAI.0b013e31828e1e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ, Badiee J, Vaida F, Letendre S, Heaton RK, Clifford D, Collier AC, Gelman B, McArthur J, Morgello S, McCutchan JA, Grant I. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS. 2011;25(14):1747–1751. doi: 10.1097/QAD.0b013e32834a40cd. doi:10.1097/QAD.0b013e32834a40cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybill JR, Sobel J, Saag M, van Der Horst C, Powderly W, Cloud G, Riser L, Hamill R, Dismukes W. Diagnosis and management of increased intracranial pressure in patients with AIDS and cryptococcal meningitis. The NIAID Mycoses Study Group and AIDS Cooperative Treatment Groups. Clin Infect Dis. 2000;30(1):47–54. doi: 10.1086/313603. doi:CID990496 [pii] [DOI] [PubMed] [Google Scholar]
- Haddow LJ, Colebunders R, Meintjes G, Lawn SD, Elliott JH, Manabe YC, Bohjanen PR, Sungkanuparph S, Easterbrook PJ, French MA, Boulware DR. Cryptococcal immune reconstitution inflammatory syndrome in HIV-1-infected individuals: proposed clinical case definitions. Lancet Infect Dis. 2010;10(11):791–802. doi: 10.1016/S1473-3099(10)70170-5. doi:10.1016/S1473-3099(10)70170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, McCutchan JA, Reicks C, Grant I. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004;10(3):317–331. doi: 10.1017/S1355617704102130. doi:10.1017/s1355617704102130. [DOI] [PubMed] [Google Scholar]
- Hoffmann M, Muniz J, Carroll E, De Villasante J. Cryptococcal meningitis misdiagnosed as Alzheimer's disease: complete neurological and cognitive recovery with treatment. J Alzheimers Dis. 2009;16(3):517–520. doi: 10.3233/JAD-2009-0985. doi:10.3233/jad-2009-0985. [DOI] [PubMed] [Google Scholar]
- Jarvis JN, Meintjes G, Williams A, Brown Y, Crede T, Harrison TS. Adult meningitis in a setting of high HIV and TB prevalence: findings from 4961 suspected cases. BMC infectious diseases. 2010;10:67. doi: 10.1186/1471-2334-10-67. doi:10.1186/1471-2334-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston SR, Corbett EL, Foster O, Ash S, Cohen J. Raised intracranial pressure and visual complications in AIDS patients with cryptococcal meningitis. J Infect. 1992;24(2):185–189. doi: 10.1016/0163-4453(92)92954-h. [DOI] [PubMed] [Google Scholar]
- Kaharuza MF, Bunnell R, Moss S, Purcell WD, Bikaako-Kajura W, Wamai N, Downing R, Solberg P, Coutinho A, Mermin J. Depression and CD4 cell count among persons with HIV infection in Uganda. AIDS Behav. 2006;10(S105-111) doi: 10.1007/s10461-006-9142-2. [DOI] [PubMed] [Google Scholar]
- Kambugu A, Meya DB, Rhein J, O'Brien M, Janoff EN, Ronald AR, Kamya MR, Mayanja-Kizza H, Sande MA, Bohjanen PR, Boulware DR. Outcomes of cryptococcal meningitis in Uganda before and after the availability of highly active antiretroviral therapy. Clin Infect Dis. 2008;46(11):1694–1701. doi: 10.1086/587667. doi:10.1086/587667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klove H. Clinical neuropsychology. Med Clin North Am. 1963;47:1647–1658. [PubMed] [Google Scholar]
- Kwartler JA, Linthicum FH, Jahn AF, Hawke M. Sudden hearing loss due to AIDS-related cryptococcal meningitis--a temporal bone study. Otolaryngol Head Neck Surg. 1991;104(2):265–269. doi: 10.1177/019459989110400219. [DOI] [PubMed] [Google Scholar]
- Levine AJ, Hinkin CH, Ando K, Santangelo Gianni, Martinez M, Valdes-Sueiras M, Saxton EH, Mathisen G, Commins DL, Moe A, Singe CFJ. An exploratory study of long-term neurocognitive outcomes following recovery from opportunistic brain infections in HIV+ adults. J Clin Exp Neuropsychol. 2008;30(7):836–843. doi: 10.1080/13803390701819036. doi:10.1080/13803390701819036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AJ, Miller EN, Becker JT, Selnes OA, Cohen BA. Normative data for determining significance of test-retest differences on eight common neuropsychological instruments. The Clinical neuropsychologist. 2004;18(3):373–384. doi: 10.1080/1385404049052420. doi:10.1080/1385404049052420. [DOI] [PubMed] [Google Scholar]
- Lu CH, Chen HL, Chang WN, Tsai NW, Wang HC, Yang TM, Lin YJ, Lin CP, Chen CC, Cheng BC, Lin WC. Assessing the chronic neuropsychologic sequelae of human immunodeficiency virus-negative cryptococcal meningitis by using diffusion tensor imaging. AJNR American journal of neuroradiology. 2011;32(7):1333–1339. doi: 10.3174/ajnr.A2489. doi:10.3174/ajnr.A2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maj M, D'Elia L, Satz P, Janssen R, Zaudig M, Uchiyama C, Starace F, Galderisi S, Chervinsky A. Evaluation of two new neuropsychological tests designed to minimize cultural bias in the assessment of HIV-1 seropositive persons: a WHO study. Arch Clin Neuropsychol. 1993;8(2):123–135. [PubMed] [Google Scholar]
- Motoyoshi A, Nakajima H, Takano K, Moriyama M, Kannan Y, Nakamura Y. Effects of Amphotericin B on the expression of neurotoxic and neurotrophic factors in cultured microglia. Neurochemistry international. 2008;52(6):1290–1296. doi: 10.1016/j.neuint.2008.01.012. doi:10.1016/j.neuint.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Myer L, Smit J, Liezel LR, Parker S, Sein DJ, Seedat S. Common mental disorders among HIV- infected individuals in South Africa: prevalence, predictors, and validation of brief psychiatric rating scales. AIDS. 2008;22(2):146–158. doi: 10.1089/apc.2007.0102. [DOI] [PubMed] [Google Scholar]
- Nakasujja N, Skolasky RL, Musisi S, Allebeck P, Robertson K, Ronald A, Katabira E, Clifford DB, Sacktor N. Depression symptoms and cognitive function among individuals with advanced HIV infection initiating HAART in Uganda. BMC Psychiatry. 2010;10(1):44. doi: 10.1186/1471-244X-10-44. doi:10.1186/1471-244x-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23(4):525–530. doi: 10.1097/QAD.0b013e328322ffac. doi:10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self report depression scale for research in the general population. App Psychol Meas. 1977;3(1):385–401. [Google Scholar]
- Razonable RR, Henault M, Lee LN, Laethem C, Johnston PA, Watson HL, Paya CV. Secretion of proinflammatory cytokines and chemokines during amphotericin B exposure is mediated by coactivation of toll-like receptors 1 and 2. Antimicrob Agents Chemother. 2005;49(4):1617–1621. doi: 10.1128/AAC.49.4.1617-1621.2005. doi:10.1128/AAC.49.4.1617-1621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM. Manual for Administration of Neuropsychological Test Batteries for Adults and Children. Indiana University Press; Bloomington: 1969. [Google Scholar]
- Robertson KR, Nakasujja N, Wong M, Musisi S, Katabira E, Parsons TD, Ronald A, Sacktor N. Pattern of neuropsychological performance among HIV positive patients in Uganda. BMC neurology. 2007;7:8. doi: 10.1186/1471-2377-7-8. doi:10.1186/1471-2377-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson KR, Parsons TD, Sidtis JJ, Hanlon Inman T, Robertson WT, Hall CD, Price RW. Timed Gait test: normative data for the assessment of the AIDS dementia complex. J Clin Exp Neuropsychol. 2006;28(7):1053–1064. doi: 10.1080/13803390500205684. doi:10.1080/13803390500205684. [DOI] [PubMed] [Google Scholar]
- Robertson KR, Robertson WT, Ford S, Watson D, Fiscus S, Harp AG, Hall CD. Highly active antiretroviral therapy improves neurocognitive functioning. Journal of acquired immune deficiency syndromes. 2004;36(1):562–566. doi: 10.1097/00126334-200405010-00003. [DOI] [PubMed] [Google Scholar]
- Sacktor N, Nakasujja N, Skolasky R, Robertson K, Wong M, Musisi S, Ronald A, Katabira E. Antiretroviral therapy improves cognitive impairment in HIV+ individuals in sub-Saharan Africa. Neurology. 2006;67(2):311–314. doi: 10.1212/01.wnl.0000225183.74521.72. doi:10.1212/01.wnl.0000225183.74521.72. [DOI] [PubMed] [Google Scholar]
- Sacktor NC, Wong M, Nakasujja N, Skolasky RL, Selnes OA, Musisi S, Robertson K, McArthur JC, Ronald A, Katabira E. The International HIV Dementia Scale: a new rapid screening test for HIV dementia. Aids. 2005;19(13):1367–1374. [PubMed] [Google Scholar]
- Sau K, Mambula SS, Latz E, Henneke P, Golenbock DT, Levitz SM. The antifungal drug amphotericin B promotes inflammatory cytokine release by a Toll-like receptor- and CD14-dependent mechanism. The Journal of biological chemistry. 2003;278(39):37561–37568. doi: 10.1074/jbc.M306137200. doi:10.1074/jbc.M306137200. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 2nd ed. Oxford University Press; New York: 1998. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Revised. The Psychological Corporation; San Antonio: 1981. [Google Scholar]
- Wong MH, Robertson K, Nakasujja N, Skolasky R, Musisi S, Katabira E, McArthur JC, Ronald A, Sacktor N. Frequency of and risk factors for HIV dementia in an HIV clinic in sub-Saharan Africa. Neurology. 2007;68(5):350–355. doi: 10.1212/01.wnl.0000252811.48891.6d. doi:10.1212/01.wnl.0000252811.48891.6d. [DOI] [PubMed] [Google Scholar]