Abstract
RNA-RNA interactions play critical roles in many cellular processes but studying them is difficult and laborious. Here, we describe an experimental procedure, termed crosslinking ligation and sequencing of hybrids (CLASH), which allows high-throughput identification of sites of RNA-RNA interaction. During CLASH, a tagged bait protein is UV crosslinked in vivo to stabilise RNA interactions and purified under denaturing conditions. RNAs associated with the bait protein are partially truncated, and the ends of RNA-duplexes are ligated together. Following linker addition, cDNA library preparation and high-throughput sequencing, the ligated duplexes give rise to chimeric cDNAs, which unambiguously identify RNA-RNA interaction sites independent of bioinformatic predictions. This protocol is optimized for studying miRNA targets bound by Argonaute proteins, but should be easily adapted for other RNA-binding proteins and classes of RNA. The protocol requires around 5 days to complete, excluding the time required for high-throughput sequencing and bioinformatic analyses.
Keywords: CLASH, CLIP, RNA-RNA interactions, Argonaute, AGO1, microRNA, miRNA target identification, RNA crosslinking, UV crosslinking
INTRODUCTION
The crucial role of RNA interactions in many cellular processes, including translation and splicing, has long been well established. However, interest in the identification of targets for RNA-interactions has been increased by a slew of recent discoveries, including the regulatory potential of miRNAs and lncRNAs and the direct participation of RNA in transcription regulation (for recent reviews see refs 1-3). Moreover, advances in sequencing technology have highlighted the startling complexity of the transcriptome and revealed ever-increasing numbers of different RNA species. Deep sequencing has revealed that most of the eukaryotic genome is transcribed4 but the biological functions of the majority of transcripts remain unclear.
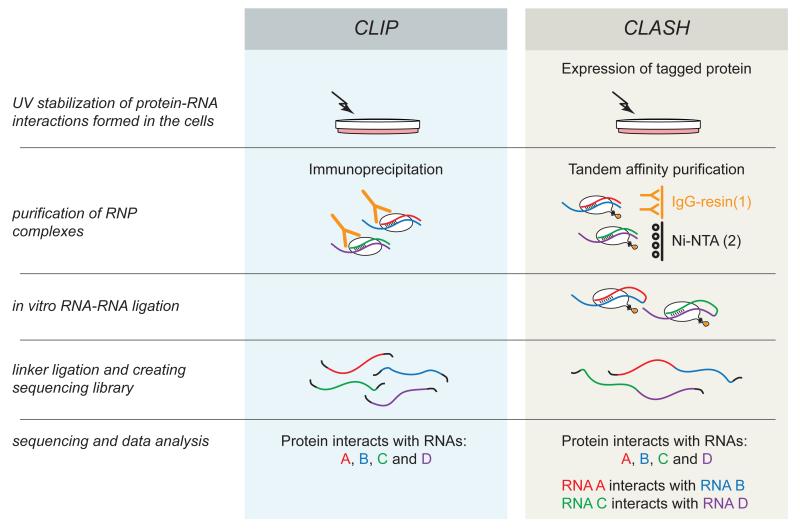
Many RNA species function via interactions with proteins and/or other RNA molecules. A key advance in high-throughput analyses of ribonucleoprotein (RNP) complexes was development of the CLIP technique (crosslinking and immunoprecipitation) by the Darnell lab5. CLIP is based on the stabilization of protein-RNA complexes in their cellular environment by UV crosslinking, immunoprecipitation of RNPs, and the isolation and sequencing of bound RNAs. Many modifications of CLIP have subsequently been reported, including HITS-CLIP (high-throughput sequencing of RNA isolated by crosslinking immunoprecipitation)6 PAR-CLIP (photoactivatable-ribonucleoside-enhanced crosslinking and immunoprecipitation)7 and iCLIP (individual-nucleotide resolution UV crosslinking and immunoprecipitation)8. Originally applied to the neuronal splicing factor, Nova, CLIP-based techniques have allowed the characterization of the RNA targets for many proteins. While all these techniques directly reveal only protein-RNA interactions, the data can greatly aid bioinformatic predictions of RNA-RNA interactions. The first experimentally supported high-throughput prediction of miRNA targets was achieved by HITS-CLIP analyses of the Argonaute (AGO) family of miRNA-binding proteins9.
The CLASH technique described here is the first reported high-throughput method for the direct identification of RNA-RNA interactions. It was derived from a further modified version of CLIP called CRAC (UV cross-linking and analysis of cDNAs)10. In CRAC, the immunoprecipitation step is replaced by purification of tandem tagged proteins on an affinity resin. This eliminates the necessity for high-affinity antibodies and allows for very stringent purification of RNPs under denaturing conditions. This ensures a low recovery of background RNA and allows the analysis of individual proteins within very stable complexes (e.g. pre-ribosomes) that would resist the semi-denaturing conditions used in CLIP approaches. In the course of CRAC experiments performed on the proteins binding to snoRNA-rRNA duplexes, we observed that a small proportion of the RNA-RNA hybrids were ligated together and gave rise to chimeric cDNAs in the sequencing data (Fig. 1). CLASH was initially developed as a bioinformatic pipeline for the analysis of these chimeras and allowed the direct identification of interacting RNA molecules and their structures11. We subsequently enhanced the efficiency of chimera recovery in CLASH analyses by modifying the experimental protocol, with a crucial additional ligation step allowing enrichment for RNA-RNA chimeras. CLASH allows the identification of RNA-RNA interactions without bioinformatic predictions and we recently applied it to characterize the human miRNA interactome12.
Figure 1.
Comparison of the principles of the CLIP and CLASH protocols.
miRNAs are short non-coding RNAs involved in the post-transcriptional regulation of gene expression. Bound by AGO proteins, they direct effector RNP complexes to the target RNAs by the means of complementarity. Each miRNA can bind many targets and each target can be regulated by multiple miRNAs, thus creating a huge network of interactions and mutual dependencies between RNA molecules. The patterns of miRNA–target mRNA basepairing have been extensively studied, but many known interactions match poorly with the established canonical rules (for review see ref 13). Defining more comprehensive miRNA targeting rules requires an experimental method that is independent of pre-existing knowledge and assumptions. Using CLASH for this purpose, we identified more than 18,000 miRNA–mRNA interactions, revealing a broad picture of the miRNA interactome in HEK 293 cells12. This protocol is specifically adjusted for studying miRNA targets bound by AGO proteins. It should, however, be readily adjusted for other RNA-binding proteins and types of RNA, as discussed further below.
Overview of the procedure
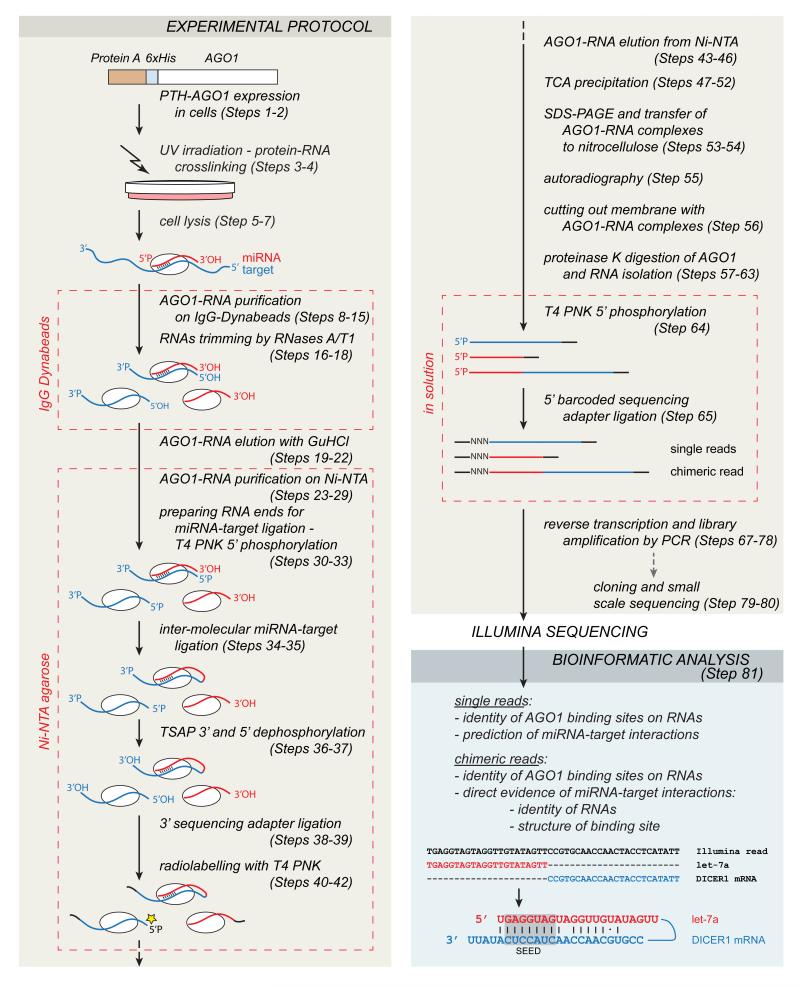
Living cells expressing PTH (Protein A-TEV cleavage site – 6xHis)-tagged AGO are UV irradiated (254 nm) to covalently couple existing protein-RNA complexes. Cells are lysed, PTH-tagged AGO is stringently purified under denaturing conditions, protein-bound RNA-RNA duplexes are trimmed by RNases and interacting RNA strands are ligated together to form chimeric guide–target molecules (Fig. 2). All AGO interacting RNAs are subsequently isolated and used to produce a cDNA library ready for Illumina sequencing.
Figure 2.
Overview of the experimental steps in the CLASH technique and the outcome of the basic bioinformatic analysis. (Adapted from ref. 12 with permission.)
Bioinformatic analyses of CLASH data provides two types of information; precise AGO binding sites on RNAs (similar to information obtained from CRAC/CLIP methods) and RNA-RNA hybrids that are formed within the AGO RNA-binding pocket (specific to CLASH technique).
Advantages of the method
CLASH is currently the only technique that allows for direct mapping of RNA-RNA interactions on a large scale. It has various significant features:
It utilizes UV irradiation that specifically stabilizes direct protein-RNA interactions14. As it is applied to living cells, recovered complexes should represent a snap-shot of physiological interactions. Only brief irradiation is required (~ 1 min), offering the future possibility of time resolved studies and kinetic analyses.
Protein-RNA complexes are purified stringently under denaturing conditions, which results in low background; more details on the background recovery in the method are provided in ref. 12.
Several samples can be prepared in parallel as the procedure is streamlined by the use of the small spin columns for enzymatic reactions instead of tubes.
The CLASH method is flexible and should be easily adapted to the analysis of other RNA-RNA interactions.
Limitations and future improvements
Stringent purification of protein-RNA complexes is a crucial step of the protocol. CLASH, therefore, strictly depends on the presence of a tagged protein in the cells. To date CLASH has been applied only in established cell cultures, but this limitation may be overcome by using viral vectors for primary cell or organ cultures or knock-in of tagged constructs for in vivo studies on animals. To allow more physiological studies we, and our collaborators, are preparing a genetically modified mouse in which endogenous AGO2 is replaced with a tagged protein (FLAG-6xHis).
Another limitation of the current protocol arises from low efficiency of RNA-RNA ligation. The number of chimeric reads in the sequencing data is quite variable and usually lower than 2% of all sequencing reads. A good cDNA library obtained using the current protocol is expected to yield about 15,000 unique miRNA-mRNA interaction sites. This is a substantial number but does not saturate miRNA interactions in cells. A reliable comparison of alterations in miRNA targets under changing physiological conditions would require more complete coverage. Obtaining even higher numbers of RNA-RNA hybrids from each sample should therefore be a priority in the next optimization stages.
Applications and future uses of the method
AGO-CLASH is the method of choice for looking for new phenomena in miRNA biology: observation of new patterns of target binding, identifying novel targets or RNA regulators. Additional modifications to increase the efficiency of RNA-RNA ligation (as discussed below) would facilitate a range of applications; for example, studying the dynamic of miRNA interactions in changing physiological conditions, comparison of targets of various miRNA family members or the influences of diverse miRNA modifications on targeting efficiency. As CLASH uses a simple concept of creating chimeric RNAs from two interacting RNA molecules it should be easily applied to studies of various biological processes that involve ternary complexes: RNA-binding protein and 2 interacting RNA molecules. Depending on the initial phosphorylation state or modifications of the ends of interacting RNA molecules different orders of the enzymatic reactions should be considered (more details in “Preparing RNA ends for ligation” section, below). In some cases linker-mediated ligation may give better results than a direct guide:target RNA ligation strategy.
In the case of miRNAs, the interacting RNA molecules form a basepaired duplex that is buried within the AGO protein. However, this structural arrangement is not essential, since interactions between snoRNAs, their rRNA targets can be identified by CLASH using proteins that bind adjacent to the RNA duplex11. CLASH is particularly suited to analyses in which one protein binds to a wide variety of RNA-RNA duplexes. Possible future applications include the identification of targets for the many long non-protein coding RNAs (lncRNAs). Interactions between miRNAs and lncRNAs have been identified in this way12 demonstrating its feasibility. Pre-mRNA packaging factors or hnRNP proteins might be suitable as bait proteins for these CLASH analyses. The large datasets generated are also likely to be of value in training future biophysical computational models of miRNA-mRNA interactions.
Experimental design
Initial preparation steps – tagging of AGO1
In ref. 12, we used the AGO1 protein with a tripartite tag (PTH) added at the N-terminus. This consists of (1) two immunoglobulin-binding Z domains from Staphylococcus aureus Protein A; (2) TEV protease cleavage site; (3) 6xHis-tag (Fig. 2). However, in the protocol we do not include a TEV cleavage step for the following reasons. Firstly, we observed that 6xHis-AGO1 generated by the TEV cleavage is retained by the remaining Protein A-IgG-Dynabeads complex. As endogenous AGO1 does not bind IgG-Dynabeads, this suggests that this retention is caused by a non-specific binding of AGO1 to the cleaved protein A region of the tag, which is retained on the column.
Secondly, performing RNA-RNA ligation directly after TEV cleavage, without AGO1 denaturation and additional stringent purification on Ni-NTA, significantly reduced ligation efficiency and increased background of the method. Instead we elute AGO1-RNA complexes from IgG-Dynabeads using denaturing conditions.
We believe that the only part of the PTH tag crucial for the success of the CLASH method is the 6xHis-tag, and the first purification step (purification on IgG-Dynabeads, steps 8-15) can be performed using any good efficiency tag in place of Protein A. A FLAG-PreScission cleavage site-6xHis tag was successfully tested in the lab for proteins other than AGO1. Good antibodies should also be suitable for the first purification step, but this has not yet been confirmed.
Initial preparation steps – cell culture
The first stage of the CLASH technique, not described in detail in this protocol, is the preparation of cells expressing the tagged protein. In our experiments we used Flp-In T-REx 293 cell line stably transfected with pcDNA5/FTR/TO vector expressing PTH-tagged human AGO1 upon induction with doxycycline (described in detail in ref. 12). However transient transfection of 293 cells and possibly other cell lines is also a possible option.
Purification of AGO complexes (Steps 8-15 and 23-29)
Stringent purification of the AGO-RNA complexes before the RNA-RNA ligation step is very important for the success of the CLASH procedure. Protein-RNA crosslinking is not an efficient process and we expect that almost all of the recovered interactions originate from complexes where one RNA strand is cross-linked to AGO and the other RNA molecule is maintained by RNA-RNA base pairing. Therefore, one of the main concerns is to what extent the RNA-RNA interactions are stable throughout the procedure and how much background arises from random RNA-RNA interactions formed after cell lysis. For that purpose we prepared a control experiment with mixed human and yeast lysates, looking for the inter-species chimeric sequences (described in more detail in ref. 12). For this protocol the background appeared to be less than 2%, however for the alternative protocol with less stringent purification (no guanidine hydrochloride wash before RNA-RNA ligation) it was close to 10%.
To minimize the background we used highly denaturing 6 M guanidine hydrochloride in this protocol, possibly sacrificing the numbers of identified RNA-RNA interactions and creating bias towards more stable ones. However, a higher recovery of RNA-RNA interactions at the cost of reduced specificity might be achieved by careful optimization of the Ni-NTA wash conditions, if this was desired for other applications.
RNase digestion (Steps 16-18)
A crucial step in the CLASH procedure is fragmentation of protein-bound RNAs with RNases. In our study we used a mixture of RNase A and T1, however, other RNases are probably also suitable, and have been used successfully in related CLIP and PAR-CLIP techniques15. Overdigestion with RNase can generate very short RNA fragments, which are less readily mapped unambiguously to the genome or transcriptome database following cloning and sequencing, and can lead to RNA cleavage within protein binding sites and significantly biased results15. Conversely, long chimeric cDNAs may not be fully sequenced, potentially leading to one fragment failing to be mapped. Moreover, the non-basepaired loop region between two ligated RNA molecules is usually shorter than 10 nucleotides12, suggesting that fragments with short single stranded overhangs might be preferentially ligated. Suboptimal RNase treatment can also influence other steps in the procedure; e.g. long RNAs can bind directly to the nitrocellulose. Commercial RNase preparations are very concentrated and it can be difficult to obtain a reproducible dilution directly from the original stock. It is therefore convenient to prepare a larger volume of pre-diluted stock (we use 1:20 dilution in water), prior to optimisation of final RNase concentration and incubation time, and do not change it between experiments.
Oligonucleotide design
5′ linkers for cDNA cloning were ordered from Integrated DNA Technologies (IDT). These carried a 5′ blocking group (inverted dideoxythymidine; invddT) followed by four DNA residues, an RNA sequence complementary to primers present on Illumina sequencing plates, three random ribonucleotides to allow PCR duplicates to be identified, and a five to seven ribonucleotide “barcode” to allow multiple samples to be sequenced together.
Preparing RNA ends for ligation (Steps 30-33)
In the AGO-CLASH protocol we take advantage of the short length of miRNAs that tend not to be trimmed by RNases and therefore retain their natural 3′ OH ends. These do not have to be enzymatically modified for RNA-RNA ligation (the expected phosphorylation state of RNA molecules along the CLASH protocol is depicted in Fig. 2).
Trimming of longer RNA molecules by either RNase A or T1 leaves ends (3′P and 5′OH) that are incompatible with ligation. The order of enzymatic repair reactions should therefore be reversed (5′ and 3′ TSAP dephosphorylation first, then 5′ T4 PNK phosphorylation). Short RNAs with unusual end modifications may require the inclusion of additional enzymatic steps. Note that, while this protocol is predicted to yield only chimeras with the miRNA sequence positioned 5′ to the mRNA-derived sequence (Fig. 2), the opposite orientation was also recovered at lower frequency.
CLASH controls
In CLASH experiments, three kinds of controls are useful: (1) A negative control sample prepared from cell line without tagged AGO protein. This is an important control that indicates the amount of RNA background. This control sample should give little if any signal by autoradiography (Fig. 3a) and not give rise to much PCR product (Fig. 3b). However this is not a very good negative control for the analysis of sequencing data. (2) A useful negative control for the bioinformatic analysis, is a sample prepared from a cell line that carries a different tagged protein; preferentially one that is involved in a different biological process. This can give information on the common, unspecific RNA contaminants. (3) A control sample prepared with mixed lysates of different species (see “Purification of AGO1 complexes” section, above and ref. 12 for more details). This is especially useful sample when modifying the experimental protocol described here, as it gives information on the recovery of unspecific RNA-RNA complexes that do not originate from physiological interactions.
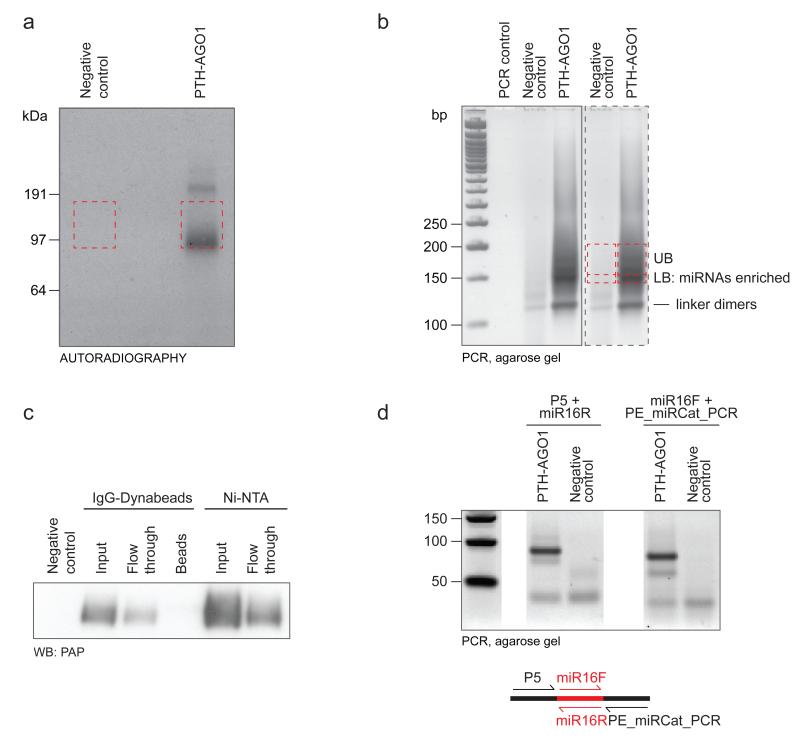
Figure 3.
Examples of gel analyses at different steps in the CLASH protocol. a: SDS-PAGE purification of the AGO1 protein, visualized by autoradiography of the radioactively labelled, crosslinked RNA (Step 55). Red dashed lines indicate the regions excised for elution of the RNA-protein complexes. b: MetaPhor agarose separation of the PCR amplified DNA library ready for sequencing (Step 76). Gel in the dashed box is a fragment of the original scan (solid box) repeated for clarity of the picture. Red dashed lines indicate the regions excised for preferential recovery of miRNAs (LB) and chimeric cDNAs/targets (UB). c: Recovery of the tagged AGO1 on IgG-Dynabeads and Ni-NTA resin (Step 25); western blot with peroxidase-anti-peroxidase (WB: PAP) soluble complex antibody recognizing Protein A tag; beads: residual AGO1 after elution; eluate fraction from IgGDynabeads is coinciding with Ni-NTA input d: MetaPhor agarose separation of the control RT-PCR products generated with miR-16 specific primers.
Bioinformatic analysis (Step 81)
Basic bioinformatic analysis of CLASH data involves: (1) pre-processing of Illumina sequence reads i.e. quality control and linker trimming; (2) mapping reads to the transcriptomic or genomic database; (3) identification of the chimeric reads defined as two high quality hits to distinct regions of the database (4) determination of the RNA-RNA interaction sites and basepairing by the RNA secondary structure prediction algorithms.
The bioinformatic pipeline for analysis of the AGO1 CLASH data was described in ref. 12. More detailed descriptions of the bioinformatics, further discussion of variants in the analyses, and their influence on its outcome are described in ref. 16.
MATERIALS
REAGENTS
CRITICAL If you are planning to use a different supplier than suggested, check that the enzymes are not His-tagged (information not usually included in the enzyme information sheet). Be aware that commercial enzymes are frequently contaminated with RNA derived from the organism used for recombinant protein production. While it is possible to remove contaminating RNAs by Gu-HCl washes on the Ni-NTA beads, enzymatic reactions performed in solution can be significantly affected.
CRITICAL All the reagents used in Steps 17 -71 should be RNase-free. DEPC treatment not required.
PTH-AGO1-Flp-InT-REx 293 cells created from Flp-In T-REx 293 cell line (Life Technologies, R780-07) stably transfected with pcDNA5/FTR/TO vector (Life Technologies, V6520-20) expressing PTH-tagged human AGO1 (described in detail in ref. 12).
DMEM with 4.5 mg l−1 glucose (Life Technologies # 41966)
Foetal Bovine Serum (FBS; Sigma-Aldrich, F2442)
Dulbecco’s PBS (DPBS; Life Technologies, #14190)
Doxycycline (Sigma-Aldrich, D9891) CAUTION Harmful if swallowed.
Peroxidase-anti-peroxidase soluble complex antibody (PAP; Sigma-Aldrich, P1291)
Dynabeads M-270 Epoxy (Life Technologies, #14301)
IgG antibody from rabbit serum (Sigma-Aldrich, I5006)
rATP, 100 mM (Promega, cat. no. E6011). Prepare aliquots and store them at −20 °C for at least 1 year CR ITICAL Avoid repeated thawing and freezing.
ATP, 10 mM (New England BioLabs, supplied with T4 RNA ligase 1). Store it at −20 °C, or for long-term storage (more than 1 month), store it at −70 °C.
Bovine serum albumin (BSA; Sigma-Aldrich, A3294), dissolved in water at 10mg ml−1
Guanidine hydrochloride (Gu-HCl; Sigma-Aldrich, G4505) CAUTION Harmful if swallowed.
Ni-NTA Superflow beads (QIAGEN, #30410)
RNasin (Promega, N2111)
PEG 8000 (Sigma-Aldrich, P1458)
-
miRCat-33 Conversion Oligos Pack (Illumina compatible 3′ adapter and RT primer, Integrated DNA Technologies, #51-01-13-10) C = 10 μM in water. After dissolving, aliquot and store in −80°C for at least 1 year.
CRITICAL Always use fresh aliquot.
Illumina-compatible L5 adapters (r stands for ribonucleotide, rN indicates a random ribonucleotide): 5′-invddT-ACACrGrArCrGrCrUrCrUrUrCrCr
GrArUrCrU-rNrNrN-bar code-3′, list of bar codes used: -L5Aa: 5′-rUrArA
rGrC-3′OH; L5Ab: 5′-rArUrUrArGrC-3′OH; L5Ac: 5′-rGrCrGrCrArGrC-3′OH; L5Cc: 5′-rArCrTrCrArGrC-3′OH; L5Cd: 5′-rGrArCrTrTrArGrC-3′OH
(Integrated DNA Technologies, custom order); stock concentration = 100 μM in water. Prepare aliquots after dissolving. They can be stored at −80 °C for at least 1 year.
CRITICAL Always use fresh aliquots of the linker.
P5 PCR primer: AATGATACGGCGACCACCGAGATCTACACTCTTTCCCTACACGACGCTCTTCCGA TCT (Integrated DNA Technologies, custom order), C = 10 μM in water. After dissolving, aliquot and store at −20°C for at least 3 years.
PE_miRCat_PCR primer: CAAGCAGAAGACGGCATACGAGATCGGTCTCGGCATTCCTGGCCTTGGCACCC GAGAATTCC (Integrated DNA Technologies, custom order), C= 10 μM in water. After dissolving, aliquot and store at −20°C for at least 3 years.
miR-16 specific PCR primers: miR-16F: GCAGCACGTAAATATTGGCG; miR-16R: GCCAATATTTACGTGCTGCTA
32P-γ-ATP (Perkin Elmer, 6000 Ci/mmol, NEG502Z) CAUTION Radioactive material, especially dangerous when ingested or inhaled. Use protective clothing, Plexiglas shielding and monitor for surface contamination.
Hybond-C Extra membrane (GE Healthcare, RPN303E)
Kodak BioMax MS Autoradiography Film (#8222648)
MetaPhor agarose (Lonza, #50180)
SYBRSafe (Life Technologies, S33102)
cOmplete Protease inhibitors, EDTA-free (Roche Applied Science, #11873580001)
RNace-IT (Agilent, #400720) diluted 1:20 with water, store at 4°C for at least 2 years
SeeBlue Plus2 Pre-Stained Standard (Life Technologies, LC5925)
NuPAGE LDS Sample Buffer 4X (Life Technologies, N0007)
NuPAGE 4-12% polyacrylamide Bis-Tris gels (Life Technologies, NP0335)
NuPAGE SDS MOPS running buffer (Life Technologies, NP0001)
NuPage Transfer Buffer (Life Technologies, NP00061)
GlycoBlue (Life Technologies, AM9515)
MinElute PCR purification kit (QIAGEN, #28004)
MinElute Gel extraction kit (QIAGEN, #28604)
GeneRuler 50 bp DNA ladder (Thermo Scientific, SM0371)
Qubit dsDNA HS Assay Kit (Life Technologies, Q32854)
6 x DNA Loading dye (Thermo Scientific, R0611)
T4 PNK, T4 Polynucleotide Kinase (New England BioLabs, M0201L)
T4 RNA ligase 1 (New England BioLabs, M0204L)
T4 RNA ligase reaction buffer, 10× (supplied with T4 RNA ligase 1)
T4 RNA ligase 2 truncated, K227Q (New England BioLabs, M0351L)
TSAP, Thermosensitive Alkaline Phosphatase (Promega, M9910)
Proteinase K (Roche Applied Science, #03115836001)
SuperScript III Reverse Transcriptase (Life Technologies, #18080-044)
5 x First strand buffer (supplied with SuperScript III Reverse Transcriptase)
0.1M DTT (supplied with SuperScript III Reverse Transcriptase)
RNase H (New England BioLabs, M0297L)
TaKaRa LA Taq (Clontech, RR002M)
10X LA PCR Buffer ll (Mg2+ plus) supplied with TaKaRa LA Taq
dNTPs 2.5mM (supplied with TaKaRa LA Taq). Aliquot and store in −80°C. CRITICAL Always use fresh aliquot.
TOPO TA Cloning® Kit for Sequencing, with One Shot® TOP10 Chemically Competent E. coli (Life Technologies, K4575-40)
-
Phenol (Sigma-Aldrich, P4557, used without Equilibration Buffer)
CAUTION Phenol is toxic on inhalation, on contact with skin or if swallowed, causes severe skin burns and eye damage. Use a hood, protective clothing, eyes protection and gloves.
Tris (Life Technologies, 15504-020)
HCl (Thermo Fisher Scientific, 10000180)
NaCl (Thermo Fisher Scientific, 10326390)
NP-40 (Roche Applied Science, #11754599001)
EDTA, Ethylenediaminetetraacetic acid (Thermo Fisher Scientific, 10213570)
Glycerol (Thermo Fisher Scientific, 10336040)
BME, β-mercaptoethanol (Sigma-Aldrich, M3148) CAUTION Toxic if swallowed or if inhaled, fatal in contact with skin, causes serious eye damage. Use a hood, protective clothing, eyes protection and gloves. Avoid release to the environment.
MgCl2 (Sigma-Aldrich, M8266)
TCA, 100% trichloroacetic acid (Sigma-Aldrich, 91228) CAUTION Causes severe skin burns and eye damage. Wear protective clothing, eyes protection and gloves.
Acetone (Thermo Fisher Scientific, 10162180)
Imidazole (Sigma-Aldrich, I2399)
SDS, Sodium dodecyl sulphate (Sigma-Aldrich, L4390)
Methanol (Thermo Fisher Scientific, 11976961)
Ethanol (Hayman Limited, AR100-X)
Boric acid (Thermo Fisher Scientific, 10263370)
Sodium acetate (Thermo Fisher Scientific, 10122350)
Chlorophorm (Thermo Fisher Scientific, 10293850)
Isoamyl alcohol (Sigma-Aldrich, I9392)
EQUIPMENT
Humidified 37°C, 5% CO2 incubator
Cell culture hood
150mm cell culture dishes (Thermo Scientific; #157150)
Crosslinker - Stratalinker 1800 (Stratagene) with UV bulbs, λ = 254nm (USHIO, #3000016)
Cell scrapers
Filter units for buffers sterilization (Thermo Scientific, Rapid-Flow Disposable Filter Units, CN membrane)
Magnetic rack for 15ml conical tubes (Life Technologies, Dynal MPC-15)
Magnetic rack for microcentrifuge tubes (Life Technologies, CS15000)
Rotating wheel for microcentrifuge tubes
Spin Columns with snap-caps (Thermo Scientific, #69725)
Cooling plate for microcentrifuge tubes
Thermoblock with shaking (Eppendorf Thermomixer comfort)
Refrigerated centrifuge for conical tubes (Sorvall Legend RT)
Refrigerated benchtop centrifuge (Eppendorf, #5417R)
SDS-PAGE tank XCell SureLock® Mini-Cell (Life Technologies, EI0001)
Mini Trans-Blot® Electrophoretic Transfer Cell (Bio-Rad, #170-3930)
Power supply unit
Phosphorescent rulers for autoradiography (Sigma-Aldrich, R8133)
Thermocycler (Bio-Rad, DNA Engine)
Agarose gel electrophoresis cell
Gel scanner (FLA-5100)
Qbit Fluorometer (Life Technologies, Q32857)
Film developer
Vortex
Filter tips (Starlab)
1.5 ml microcentrifuge tubes
15 ml conical tubes
Disposable pipettes
Pipette aid (e.g. Pipetboy)
Radioactivity monitor
Transparency film (e.g. 3M CG6000)
Scalpels
LB agar plates with Ampicillin for bacterial selection
REAGENT SETUP
PCI, Phenol-chloroform-isoamyl alcohol solution reagents mixed in volume proportions 25:24:1. Store at 4°C. Discard if the colour of the mixture changes.
10 x TBE buffer 890 mM Tris, 890 mM Boric acid, 20 mM EDTA. Store at room temperature.
CRITICAL All buffers listed below should be filter sterilized before use and, unless stated otherwise, stored at 4°C for at least a year.
Lysis buffer Mix 50mM Tris-HCl (pH 7.8), 300 mM NaCl, 1% NP-40 (vol/vol., use 50% stock), 5 mM EDTA (pH 8.0), 10% glycerol (vol/vol. use 50% stock) in the deionized water. Add 5mM BME and protease inhibitors just before use.
LS-IgG buffer Mix 50 mM Tris-HCl (pH 7.8), 300 mM NaCl, 0.5 % NP-40 (vol/vol.), 2.5 % glycerol (vol/vol.), 5 mM MgCl2 in the deionized water. Add 5mM BME just before use.
HS-IgG buffer Mix 50 mM Tris-HCl (pH 7.8), 800 mM NaCl, 0.5 % NP-40 (vol/vol.), 2.5 % glycerol (vol/vol.), 10 mM MgCl2 in the deionized water. Add 5mM BME just before use.
PNK-WB buffer Mix 50 mM Tris-HCl (pH 7.8), 50 mM NaCl, 0.5 % NP-40 (vol/vol.), 10 mM MgCl2 in the deionized water. Add 5mM BME just before use.
5 x PNK buffer Mix 250 mM Tris-HCl (pH 7.5), 250 mM NaCl, 2.5 % NP-40 (vol/vol.), 50 mM MgCl2, 50 mM BME. After filtering aliquot buffer into small portions and keep frozen at −20°C.
Ni-WBI buffer Mix 50 mM Tris-HCl (pH 7.8), 300 mM NaCl, 0.1 % NP-40 (vol/vol.), 10 mM Imidazole (pH 8.0), 6 M Gu-HCl in deionized water. Add 5mM BME just before use. Protect from light.
Ni-WBII buffer Mix 50 mM Tris-HCl (pH 7.8), 300 mM NaCl, 0.1 % NP-40 (vol/vol.), 10 mM Imidazole (pH 8.0) in the deionized water. Add 5mM BME just before use. Protect from light.
Ni-EB buffer Mix 50 mM Tris-HCl (pH 7.8), 50 mM NaCl, 0.1 % NP-40 (vol/vol.), 150 mM Imidazole (pH 8.0) in the deionized water. Add 5 mM BME just before use. Protect from light.
Proteinase K buffer Mix 50 mM Tris-HCl (pH 7.8), 50 mM NaCl, 0.1 % NP-40 (vol/vol.), 10 mM Imidazole (pH 8.0), 1% SDS (wt/vol., use 10% stock), 5 mM EDTA (pH 8.0) and 5 mM BME in the deionized water. Protect from light.
IgG coated Dynabeads Prepared according to protocol published in ref. 17.
PROCEDURE
Cell culture TIMING 48 hours
1 Seed PTH-AGO1-Flp-In T-REx 293 or control Flp-In T-REx 293 cells onto 150mm dishes at about 20% confluency and grow overnight in DMEM with 10% FBS.
2 The next day induce cells for PTH-AGO1 production with Doxycycline (final concentration 0.5 μg ml−1) for 36 hours. Use 4 plates per CLASH sample.
UV-Crosslinking TIMING about 10 min per CLASH sample
3 At this stage cells should be ~ 90% confluent. Briefly wash growing cells once with room temperature DPBS.
4 Place the culture plates without lids on ice tray and into Stratalinker. Irradiate at λ=254 nm with power settings 400mJ cm−2.
CRITICAL STEP Proceed with one plate at a time to reduce time between DPBS wash and cell lysis, thus limiting cells exposure to changed conditions.
Cell lysis TIMING 1 hr
5 Directly after crosslinking lyse the cells on the dish by adding 2.5 ml of ice-cold Lysis buffer and incubate for 5 min on ice.
6 When all the dishes are ready scrape the cells and collect the pooled lysates to 15 ml conical tubes.
7 Incubate the samples for further 10 min on ice, then centrifuge at 6,400g for 30 min at 4°C. Prepare the western blot sample (“IgG-Dynabeads input”) by boiling 15 μl lysate with 5 μl NuPAGE LDS Sample Buffer for 5 min, store it at −20°C until further use at Step 25.
PAUSE POINT Cell lysates can be either used directly or aliquoted, frozen in liquid nitrogen and stored at −80°C for up to 6 months.
IgG-Dynabeads loading and washing TIMING 90 min
8 Wash the IgG coated Dynabeads 3 times with 5 ml of ice-cold DPBS.
CRITICAL STEP Washing the beads directly before loading is essential for the removal of IgG released from the beads during storage. Free IgG competes with IgG-coated beads decreasing AGO biding efficiency.
9 Add 20 mg IgG-Dynabeads to each sample of freshly prepared or thawed lysates from Step 7. Incubate the samples with rotation for 40 min at 4°C.
CRITICAL STEP Frozen lysates should be thawed slowly on ice.
10 Place the conical tubes in the magnetic rack for about 30 seconds until the beads settle and supernatant becomes clear. Then carefully remove the supernatant. Prepare the western blot sample (“IgG-Dynabeads flow through”) by boiling 15 μl supernatant with 5 μl NuPAGE LDS Sample Buffer for 5 min, store it at −20°C until further use at Step 25. Discard remaining supernatant.
11 Wash the beads briefly by resuspending them in 10 ml of ice cold LS-IgG buffer, allow the beads to pellet in the magnetic rack and discard supernatant.
12 Wash the beads twice with ice-cold HS-IgG buffer, this time incubating them for 5 min in the buffer with rotation at 4°C. Pellet the beads and discard supernatant.
13 Wash the beads once briefly with 10 ml of ice cold LS-IgG buffer. Pellet the beads and discard supernatant.
14 Wash the beads once briefly with 10 ml ice-cold PNK-WB buffer. Pellet the beads and discard supernatant.
15 Gently resuspend the beads with a pipette in 1ml ice-cold PNK-WB buffer and transfer them to the 1.5 ml microcentrifuge tubes. Pellet the beads in the magnetic rack and discard the supernatant.
RNase digestion on Dynabeads TIMING 15 min
16 Prepare the RNase digestion buffer by adding 1 μl of diluted RNace-IT (0.5 u) to 500 μl pre-warmed PNK-WB buffer per each sample.
CRITICAL STEP This step might require optimization, more details in “Experimental design; RNase digestion” section
17 Add prepared RNase digestion buffer to the IgG-Dynabeads from Step 15 and incubate for 7 min at 20°C.
CRITICAL STEP During the digestion, tap the samples gently every 30 seconds to prevent Dynabeads from settling.
18 Place the samples briefly on ice to slow down the reaction, then settle the beads on the magnetic rack. Remove the RNase digestion buffer completely and discard it.
Elution from IgG-Dynabeads TIMING 40 min
19 Add 500 μl Ni-WBI buffer to the beads and incubate for 10 min at room temperature with rotation.
20 Pellet the beads and collect the supernatant.
21 Repeat the elution twice more, once with 500 μl and once with 1 ml of Ni-WBI buffer. Pool all the eluate fractions together. Prepare the western blot sample (“Ni-NTA input”) as follows: 10 μl eluate + 90 μl water + 100 μl 10% TCA, incubate for 40 min on ice, then proceed as in Steps 48-52. Store the sample at −20°C until further use at Step 25.
22 Wash the Dynabeads after elution three times with 1 ml PNK-WB and resuspend beads in 1ml PNK-WB. Prepare the western blot sample (“IgG-Dynabeads beads”) by mixing 15 μl IgG-Dynabeads suspension + 135 μl water + 50 μl NuPAGE LDS Sample Buffer, boil for 5 min and store it at −20°C until further use at Step 25. Use 20 μl per gel lane.
Ni-NTA agarose loading and washing TIMING 3 hours + 6 hours western blot (optional)
23 Wash Ni-NTA beads twice with Ni-WBI buffer and prepare 50% beads suspension in Ni-WBI buffer for all the samples together. Add 80 μl of prepared Ni-NTA suspension to each eluate from Step 21 and incubate for 2 hours at 4°C with rotation.
24 Spin the beads at 1,000g for 10 seconds, 4°C and gently aspirate the supernatant. Prepare the western blot sample (“Ni-NTA flow through”) using 10 μl supernatant and proceed as in Step 21.
25 Perform western blot (optional) to estimate AGO recovery during purification steps, using samples from Steps 7, 10, 21, 22 and 24. SDS-PAGE and transfer procedures are described in Steps 53-54. For the tagged AGO detection use peroxidase-anti-peroxidase soluble complex antibody (1:10000 dilution).
26 Wash the beads twice with 1ml ice-cold Ni-WBI for 10 min at 4°C with rotation.
27 Transfer the beads to spin columns. Keep the columns in 1.5 ml microcentifuge tubes.
28 Wash the beads twice with 0.75 ml ice-cold Ni-WBII, discard the supernatant. The same wash collection tubes can be used multiple times throughout the procedure.
CRITICAL STEP All washes are carried out under gravity flow. Close lid to get rid of the remaining buffer.
CRITICAL STEP It is very important to carefully rinse the column with Ni-WBII. Ni-WBI used in Step 26 contains Gu-HCl and any remains can influence the subsequent enzymatic reactions.
29 Wash the beads 3 times with 0.75 ml ice-cold PNK-WB buffer, discard the supernatant. Dry the beads by closing the lid of the column and subsequently close the bottom of the column with the supplied snap cap.
RNA phosphorylation with T4 PNK TIMING 3 hours
30 To each column add 80 μl of PNK reaction mixture, prepared in bulk for all the samples together, volumes per sample as follows. Incubate for 2.5 hours at 20°C.
| Reagent | Volume (μl) per reaction | Final concentration |
|---|---|---|
| Water | 57 μl | |
| 5 x PNK buffer | 16 μl | |
| 100 mM rATP | 0.8 μl | 1 mM |
| RNAsin | 2 μl | 1 U μl−1 |
| T4 PNK | 4 μl | 0.5 U μl−1 |
| Mix total volume | 80 μl | |
31 Remove the snap cap and wash the beads twice with 0.5 ml ice-cold buffer Ni-WBI, discard the washes.
CRITICAL STEP To prevent quick dripping from the column, open the lid first, then open the snap cap and quickly place the column in the microcentrifuge collection tube.
CRITICAL STEP To limit the column contact area with Ni-WBI, add the buffer directly to the bottom of the column. Ni-WBI has to be rinsed off well as it contains Gu-HCl that can affect the subsequent enzymatic reactions.
32 Wash the beads twice with 0.75 ml ice-cold buffer Ni-WBII, discard the washes.
33 Wash the beads 3 times with 0.75 ml ice-cold buffer PNK-WB, discard the washes.
Inter-molecular RNA-RNA ligation TIMING overnight
34 Close the bottom of the column with snap cap, add 160 μl of ligation mixture prepared in bulk for all the samples together, volumes per sample as follows. Incubate the samples overnight at 16 °C with gentle rotation.
| Reagent | Volume (μl) per reaction | Final concentration |
|---|---|---|
| Water | 118.4 μl | |
| 5 x PNK buffer | 32 μl | |
| 100 mM rATP | 1.6 μl | 1 mM |
| RNAsin | 4 μl | 1 U μl−1 |
| T4 RNA ligase 1 | 4 μl | 0.25 U μl−1 |
| Mix total volume | 160 μl | |
35 Wash the beads as in Steps 31 – 33
Dephosphorylation with TSAP TIMING 1 hour
36 Close the bottom of the column with snap cap, add 80 μl of reaction mixture prepared in bulk for all the samples together, volumes per sample as follows. Incubate the samples for 45 min at 20 °C.
| Reagent | Volume (μl) per reaction | Final concentration |
|---|---|---|
| Water | 54 μl | |
| 5 x PNK buffer | 16 μl | |
| RNAsin | 2 μl | 1 U μl−1 |
| TSAP | 8 μl | 0.1 U μl−1 |
| Mix total volume | 80 μl | |
37 Wash the beads as in Steps 31 – 33
3′ end adapter ligation on beads TIMING 6.5 hours
38 Close the bottom of the column with snap cap, add 80 μl of ligation mixture prepared in bulk for all the samples together, volumes per sample as follows. Incubate samples for 6 hours at 16 °C.
| Reagent | Volume (μl) per reaction | Final concentration |
|---|---|---|
| Water | 18 μl | |
| 5 x PNK buffer | 16 μl | |
| 25 % PEG 8000 | 32 μl | 10 % |
| 10 μM miRCat-33 3′-linker | 8 μl | 1 μM |
| RNAsin | 2 μl | 1 U μl−1 |
| T4 RNA ligase 2, truncated, K227Q | 4 μl | 10 U μl−1 |
| Mix total volume | 80 μl | |
39 Wash the beads as in Steps 31 – 33
Radioactive labelling of RNA TIMING 1 hour 15 min
40 Close the bottom of the column with snap cap, add 80 μl reaction mixture prepared in bulk for all the samples together, volumes per sample as follows. Incubate samples for 30 min at 37°C.
| Reagent | Volume (μl) per reaction | Final concentration |
|---|---|---|
| Water | 55 μl | |
| 5 x PNK buffer | 16 μl | |
| 32P-γ-ATP | 3 μl | |
| RNAsin | 2 μl | 1 U μl−1 |
| T4 PNK | 4 μl | 0.5 U μl−1 |
| Mix total volume | 80 μl | |
CAUTION Always be careful working with radioactive material. Consult local rules for detailed guidelines.
41 Wash the beads extensively with Ni-WBI, each wash volume is 0.5 ml.
CRITICAL STEP Wash the beads multiple times at this stage to remove free 32P-γ-ATP, reduce the risk of exposure to radioactivity and contamination. Monitor the radioactivity in the flow through and repeat washes until this falls to ~10 cpm.
42 Wash the beads as in Steps 31 – 33.
Elution of PTH-AGO-RNA complexes from Ni-NTA beads TIMING 30 min
43 Add 200 μl Ni-EB buffer to the beads, close the snap cap and incubate in thermoblock for 5 min at room temperature with gentle mixing.
44 Collect the supernatant.
45 Repeat the elution twice more, once with 200 μl and once with 600 μl Ni-EB buffer.
46 Pool all the eluate fractions together (total volume ~ 1ml).
TCA precipitation TIMING 2 hours
47 Add 2 μg BSA and 200 μl 100% TCA and incubate for 40 min on ice.
48 Centrifuge the samples in table top centrifuge; 20,000g, 30 min, 4°C.
49 Discard supernatant.
CAUTION Note that at this stage supernatant can still be radioactive as it contains unincorporated 32P-γ-ATP.
50 Wash the pellets containing protein-RNA complexes twice with 1 ml ice cold acetone. Vortex the samples well during each wash and spin pellets with max speed (20,000g ) for 10 min at 4°C. Discard supernatants.
51 Dry the pellets at room temperature (~ 5 minutes; or at 37°C, but do not over dry them).
52 Resuspend the pellets in 15 μl water, add 5 μl 4 x NuPAGE LDS Sample Buffer and heat the samples for 10 min at 65°C.
CRITICAL STEP Protein pellets after TCA precipitation are easier to resuspend in water than in pre-diluted 1 x NuPAGE LDS Sample Buffer.
CRITICAL STEP The NuPAGE buffer system is quite resistant to small changes in the pH of the samples, but observe carefully the colour of the samples in the NuPAGE LDS Sample Buffer. If it changes add 1 μl of 1M Tris pH 7.8 or more, until the colour comes back to normal
PAUSE POINT Prepared samples can be frozen at −20 °C for a few days.
TROUBLESHOOTING
SDS-PAGE and transfer of protein-RNA complexes TIMING 4.5 hours
53 Thaw the samples, separate proteins and pre-stained protein standard on the 4-12% polyacrylamide Bis-Tris NuPAGE gel using NuPAGE SDS MOPS running buffer, run at a constant voltage U = 150 V until the dye reaches the bottom of the gel (about 30 min).
CRITICAL STEP It is important to use NuPAGE SDS MOPS buffer as, unlike home-made buffers, it does not change its pH significantly during the run. Increase in buffer pH can cause RNA degradation.
CRITICAL STEP To reduce the risk of sample cross-contamination separate neighbouring samples with empty lanes.
TROUBLESHOOTING
54 Transfer gel separated protein-RNA complexes to nitrocellulose membrane in the cooled wet-transfer tank using NuPage Transfer Buffer supplemented with 10% methanol for 2 hours at constant voltage U = 100 V.
55 Air dry the nitrocellulose membrane for 30 seconds, wrap it in the cling film, attach the phosphorescent ruler and expose it on the autoradiography film for an hour (or longer as needed).
CRITICAL STEP Attaching phosphorescent ruler to the cling film before exposition is critical as it is the only way to precisely align the nitrocellulose membrane with the exposed autoradiography film.
PAUSE POINT Nitrocellulose membrane can be frozen at −20 °C for at least a week.
TROUBLESHOOTING
RNA purification TIMING 5 hours to overnight + 4 hours
56 Align carefully the nitrocellulose membrane with the developed autoradiography film using signal from phosphorescent ruler. Cut out the radioactive bands using disposable scalpels following the instructions included in Fig. 3a.
57 Place the cut out membrane pieces in the separate 1.5 ml microcentrifuge tubes. Add 400 μl Proteinase K buffer, 100 μg Proteinase K per tube and incubate the samples in the thermoblock set up for gentle mixing for 2 hours at 55°C.
CRITICAL STEP After that time, check the membrane for remaining radioactivity, which should be low in comparison to the solution.
TROUBLESHOOTING
58 Discard the membrane and perform PCI extraction of RNA from solution. Add 50 μl 3M sodium acetate pH 5.5 and 500 μl PCI and vortex the samples vigorously for 30 sec.
CAUTION Phenol is toxic on inhalation, on contact with skin or if swallowed, causes severe skin burns and eye damage. Use a hood, protective clothing, eyes protection and gloves.
59 Centrifuge samples at maximum speed (20,000g) in table top centrifuge for 5 min at room temperature. Immediately collect the upper aqueous phase (about 350 μl). Check lower organic phase for remaining radioactivity.
CRITICAL STEP Be very careful not to aspirate the lower organic fraction.
TROUBLESHOOTING
60 Add 1 μl (15 μg) GlycoBlue and 1 ml ethanol. Incubate the samples at −80°C for 30 min.
CRITICAL STEP Extended incubation time at −80°C causes adverse co-precipitation of sodium acetate. Alternatively, precipitate RNA overnight at −20 °C.
61 Centrifuge samples at maximum speed (20,000g) for 30 min at 4°C. Discard supernatant.
TROUBLESHOOTING
62 Wash the RNA containing pellets twice with 0.75 ml ice-cold 70 % ethanol (vol/vol. in water), vortex the samples well. After each wash, centrifuge samples for 10 min with the speed/temperature settings as in Step 61. Discard supernatant.
63 Air dry the pellets (~ 5 minutes, alternatively at 37°C, do not over dry the pellets).
TROUBLESHOOTING
PNK phosphorylation and 5′ adapter ligation TIMING 7 hours to overnight + 30 min
64 Resuspend each pellet in 15 μl phosphorylation mixture prepared in bulk for all the samples together, volumes per sample as follows. Incubate the samples for 30 min at 37°C.
| Reagent | Volume (μl) per reaction | Final concentration |
|---|---|---|
| T4 RNA ligase reaction buffer, 10× | 1.5 μl | |
| 10 mM ATP | 1.5 μl | 1 mM |
| T4 PNK | 1 μl | 0.67 U μl−1 |
| Water | 11 μl | |
| Mix total volume | 15 μl | |
65 Add 5 μl of 5′ adapter ligation mixture, prepared for each sample as follows. Incubate samples for 6 hours (or overnight) at 16°C.
| Reagent | Volume (μl) per reaction | Final concentration (vol = 20 μl) |
|---|---|---|
| Water | 2 μl | |
| T4 RNA ligase reaction buffer, 10× | 0.5 μl | |
| 10 mM ATP | 0.5 μl | 1 mM |
| Barcoded 5′ adapter | 1 μl | 5 μM |
| RNA ligase 1 | 1 μl | 0.5 U μl−1 |
| Mix total volume | 5 μl | |
RNA purification and concentration TIMING variable: 2.5 hours to overnight + 2 hours
66 For easier handling increase each sample volume to 400 μl with water. Proceed with PCI extraction and ethanol precipitation as in Steps 58 – 63.
PAUSE POINT Dried RNA pellets can be stored indefinitely at −20 °C.
Reverse transcription TIMING 2.5 hours
67 Resuspend each pellet in 13 μl of RT mix I prepared in bulk for all the samples together, volumes per sample as follows.. Incubate samples for 3 min at 80°C, then immediately place samples on ice for 5 min.
| Reagent | Volume (μl) per reaction | Final concentration (vol = 20 μl) |
|---|---|---|
| Water | 8 μl | |
| 2.5 mM dNTPs | 4 μl | 0.5 mM |
| 10 μM miRCat-33 primer | 1 μl | 0.5 μM |
| Mix total volume | 13 μl | |
68 To each sample add 6 μl RT mix II prepared in bulk for all the samples together, volumes per sample as follows. Incubate for 3 min at 50°C.
| Reagent | Volume (μl) per reaction | Final concentration (vol = 20 μl) |
|---|---|---|
| 5 x First strand buffer | 4 μl | |
| 0.1 M DTT | 1 μl | 5 mM |
| RNAsin | 1 μl | 2 U μl−1 |
| Mix total volume | 6 μl | |
69 Add 1 μl Superscript III Reverse Transcriptase and incubate samples for 60 min at 50°C.
70 To inactivate reverse transcriptase incubate samples for 15 min at 65°C.
71 To degrade template RNA add 2 μl RNase H and incubate samples for 30 min at 37°C.
PAUSE POINT Prepared cDNA can be stored indefinitely at −20°C.
PCR amplification of the library TIMING 1 hour
72 To PCR amplify the library prepare 200 μl PCR mixture for each sample as follows, and divide into 4 PCR tubes for the amplification.
| Reagent | Volume (μl) per reaction | Final concentration |
|---|---|---|
| Water | 140 μl | |
| 10 x LA buffer | 20 μl | |
| 2.5 mM dNTP | 20 μl | 0.25 mM |
| 10 μM primer P5 | 4 μl | 0.2 μM |
| 10 μM primer PE_miRCat_PCR |
4 μl | 0.2 μM |
| Template cDNA from Step 71 | 10 μl | |
| TaKaRa LA Taq | 2 μl | 0.05 U μl−1 |
| Mix total volume | 200 μl | |
For amplification use following program:
| Step | Temperature | Time | Number of cycles |
|---|---|---|---|
| Initial denaturation | 95°C | 2 min | 1 |
| Denaturation | 98°C | 20 sec | |
| Annealing | 52°C | 20 sec | 19-24 |
| Extension | 68°C | 20 sec | |
| Final extension | 72°C | 5 min | 1 |
CRITICAL STEP Limit the number of PCR cycles to reduce the proportion of PCR duplicates in the sequencing result.
CRITICAL STEP To confirm the quality of the library, a small scale PCR amplification can initially be performed with 1 μl template. However, before high-throughput sequencing, increase the sequencing depth by PCR amplification of the entire cDNA library. It is also informative to set up additional PCR reaction with miRNA specific primers (see “Anticipated results” section).
PAUSE POINT Samples can be frozen and stored for up to a year at −20°C. However, freezing is not recommended if library quality will be checked by cloning and small scale sequencing. In this case, follow the procedure without break until Step 77 or 79.
Size selection of the DNA fragments in the library TIMING 4 hours
73 Prepare the 3% MetaPhor agarose gel in TBE buffer with SYBR Safe according to manufacturer’s instructions. Gel can be prepared in advance (i.e. a day before) and stored at 4°C wrapped in cling film.
74 Concentrate the DNA library from Step 72 using MinElute PCR Purification Kit according to the manufacturer’s instructions. Elute the sample with 20 μl buffer EB provided with the kit. Add 4 μl 6 x DNA loading dye to each sample.
75 Place the gel tank on ice, load the samples and 50bp GeneRuler DNA ladder and run the gel at constant voltage (80 V) for about 2 hours until Bromophenol Blue reaches the edge of the gel.
CRITICAL STEP Unless your samples carry barcoded linkers, separate neighbouring samples with empty lanes to reduce the risk of cross-contamination.
TROUBLESHOOTING
76 Scan the gel on the gel scanner and print the scan in its original size. Place the print underneath the transparency film and gel on its top. Carefully overlay the gel with the scan and cut out gel slices with DNA fragments of desired length following the instructions included in Fig. 3b and in the “ANTICIPATED RESULTS” section.
CRITICAL STEP: This is an important step in the library preparation as it allows enrichment of chimeric reads vs. miRNA reads.
TROUBLESHOOTING
77 Isolate DNA fragments from the gel using MinElute Gel Extraction Kit, following manufacturer’s instructions. Elute the sample with 20 μl buffer EB from the kit.
CRITICAL STEP To increase cloning efficiency dissolve agarose slice in QG buffer at room temperature, instead of 50°C suggested by the kit manufacturer.
PAUSE POINT It is possible to freeze the sample at −20°C at this point and come back to the cloning procedure the next day. However, this can affect cloning efficiency.
78 Measure the concentration of the cDNA library using Qbit Fluorometer and Qubit dsDNA HS Assay Kit following manufacturer’s instructions. The library is ready for high-throughput sequencing.
Cloning of the cDNA library and small scale sequencing (Optional)
79 To check the quality of the cDNA library by small scale sequencing, set up cloning reaction using TOPO-TA Cloning Kit according to manufacturer’s instructions. Use 2 μl of the library from Step 78 per 6 μl cloning mixture. Transform the Top10 competent bacteria that are supplied with kit and grow them on the LB agar plates with Ampicillin.
CRITICAL STEP Handle the Top10 bacteria supplied with the TOPO-TA Cloning Kit with utmost care, they are extremely sensitive.
TROUBLESHOOTING
80 Send colonies or purified plasmids for Sanger sequencing.
Bioinformatic analysis TIMING Basic analysis lasts ~5 hrs
81 For the description of the analysis see “Experimental design, Bioinformatic analysis” section and ref 12. A recently developed software package for CLASH data analysis is available and described in detail in ref. 16.
TROUBLESHOOTING
Troubleshooting
Troubleshooting advice can be found in Table 1.
Table 1.
Troubleshooting table
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 52 | Slight change in the colour of the NuPAGE LDS Sample Buffer | Low pH of the sample caused by the remaining TCA | Add 1 μl of 1 M Tris buffer pH 7.8 |
| 53 | Uneven migration of the samples in the gel | Low pH of the sample caused by the remaining TCA | Carefully observe the colour of the samples from Step 52. If the problem persists add a further wash with ice-cold acetone. |
| 55 | Additional bands on the autoradiography film - radioactive signal in the negative control at the level of tagged AGO | Cross-contamination of the samples | Repeat the experiment taking care not to mix the samples |
| Additional bands on the autoradiography film – at different levels than tagged AGO | Background proteins bound to the beads | Extend the wash in Step 26. | |
| Radioactive smear in the whole lanes of the gel, visible at autoradiography film | Unspecific RNA bound to AGO-RNA complexes | Extend the wash in Step 26; add more RNace-IT | |
| Low signal on the autoradiography film | Low expression of tagged-AGO | Optimize conditions of tagged-AGO expression; expose the gel longer | |
| Low binding efficiency to IgG-Dynabeads | Check binding efficiency by western blot (see comments to Step 25 in the “Anticipated results” section); coat the Dynabeads once more and wash them well directly before the experiment. | ||
| Inefficient cross-linking | Optimize cross-linking conditions | ||
| RNA degradation | Ensure that you work in RNase-free conditions | ||
| 57 | High radioactive signal on the nitrocellulose membrane. | Inefficient Proteinase K treatment or other unrecognized reason | May try adding more Proteinase K, and incubating samples for an additional hour. However, from our experience, RNAs retention on the membrane is a result of preceding steps and cannot be reversed at this stage. |
| 59 | Significant radioactivity in the organic phase | Inefficient Proteinase K treatment; remains of protein bind to RNA prevent it from entering aqueous phase | Take fresh Proteinase K aliquot; add more Proteinase K and/or incubate samples longer. See also troubleshooting comments to Step 57. |
| 61 | Pellet loose or floating in supernatant | traces of organic phase aspirated during PCI extraction | Take more care collecting upper aqueous phase. |
| 63 | RNA pellets difficult to resuspend | Pellets over dried | Take more care drying pellets |
| 75 | Samples diffuse from wells while loading | Samples contain leftover ethanol from Step 74 | Incubate remaining samples for a few min at 50°C, add more loading dye |
| 76 | No PCR products despite strong signal on the autoradiography film | Problems with enzymatic reactions | Replace enzymes, check buffers |
| Problems with ligation of sequencing adapters | After resuspending in water, divide the adapters into small aliquots; use fresh aliquot for each experiment; (see comments to Step 76 in the “Anticipated results” section) | ||
| Unique PCR product of approximate size of 120 bp | Only PCR amplified linker dimers visible on the gel; problems with enzymatic reactions | See other troubleshooting comments to Step 76 | |
| PCR products smaller then 140bp migrate in one relatively sharp band | Excessive RNase digestion in Step 17 | Optimize RNase digestion conditions. See “Experimental design: RNase digestion” section | |
| 79 | No bacterial colonies on the plates | Unsuccessful TOPO-TA cloning | Check if your polymerase leaves 3′ A-overhangs in the PCR products; if not add A-overhangs postamplification according to the protocol supplied with the TOPO-TA cloning kit |
| Top10 bacteria do not survive transformation | During transformation be very gentle; do not pipette bacteria and do not shake them after heat shock before spreading on the plate | ||
| 81 | Sequencing returns multiple similar sequences that cannot be mapped to the database | RNA contamination coming from commercial enzymes (frequently bacterial rRNA) | Change enzyme supplier |
| Problem with 3′ end adapter ligation | miRCat-33 primer used for reverse transcription is complementary to bacterial rRNA. Therefore, a problem with 3′ end adapter ligation results in enrichment of cDNA/PCR product in bacterial rRNA. | ||
| No reads mapping to miRNA in the sequencing result | Significant experimental problems | Repeat the experiment |
TIMING
Steps 1-2: Cell culture: 48 hours
Steps 3-4: UV-Crosslinking: about 10 min per CLASH sample
Steps 5-7: Cell lysis: 1 hour
Steps 8-15: IgG-Dynabeads loading and washing: 90 min
Steps 16-18: RNase digestion on Dynabeads: 15 min
Steps 19-22: Elution from IgG-Dynabeads: 40 min
Steps 23-29: Ni-NTA agarose loading and washing: 3 hours + 6 hours western blot (optional)
Steps 30-33: RNA phosphorylation with T4 PNK: 3 hours
Steps 34-35: Inter-molecular RNA-RNA ligation: overnight
Steps 36-37: Dephosphorylation with TSAP: 1 hour
Steps 38-39: 3′ end adapter ligation on beads: 6.5 hours
Steps 40-42: Radioactive labelling of RNA: 1 hour 15 min
Steps 43-46: Elution of PTH-Ago1-RNA complexes from Ni-NTA beads: 30 min
Steps 47-52: TCA precipitation: 2 hours
Steps 53-55: SDS-PAGE and transfer of protein-RNA complexes: 4.5 hours
Steps 56-63: RNA purification: variable, 5 hours to overnight and 4 hours
Steps 64-65: PNK phosphorylation and 5′ adapter ligation: variable, 7 hours to overnight + 30 min
Step 66: RNA purification and concentration: variable, 2.5 hours to overnight + 2 hours
Steps 67-71: Reverse transcription: 2.5 hours
Step 72: PCR amplification of the library: 1 hour
Steps 73-78: Size selection of the DNA fragments in the library: 4 hours
Steps 79-80 (optional): Cloning of the cDNA library and small scale sequencing: variable
Step 81: Bioinformatic analysis: basic analysis lasts ~5 hours
ANTICIPATED RESULTS
The CLASH procedure is long and contains relatively few steps at which progress can be controlled. It is therefore useful to collect samples from all the purification fractions for clues that may help future troubleshooting.
Step 7: Before starting the CLASH procedure it is worth determining the expression level of the tagged AGO protein by western Blot. The aim should be to express the tagged protein at close to endogenous levels. Overexpression risks generating spurious binding sites. Note that the protein A tag should be cleaved in order to compare the amount of tagged and endogenous proteins, since it will bind to antibodies used for western blotting.
Step 25: By comparing the amount of tagged AGO in the cell lysate with AGO remaining in the flow through from IgG-Dynabead loading, it is possible to estimate the Dynabeads IgG-coating efficiency and per cent protein bound by the IgG-Dynabeads relative to the cell lysate input. Independent of the amount of the IgG beads used per sample, we routinely observe about 30 % protein loss at this stage (Fig. 3c). At the stage of Ni-NTA loading we also observe up to 25% loss in the amount of AGO (Fig. 3c).
Step 50: Following TCA precipitation, higher radioactivity of the samples than negative control prepared from cell line without tagged AGO protein is expected..
Step 56: The strength of signal obtained by autoradiography can vary significantly from experiment to experiment. However, with successful procedure a clear signal should be visible within one hour. There should be a single main band in each sample lane corresponding to crosslinked AGO-RNA complexes at the level of the orange band in the pre-stained SeeBlue Plus 2 standard. An additional band is frequently visible above this, corresponding to AGO-RNA complexes. The negative control prepared from cell line without tagged AGO protein should not give clear radioactive bands, but some signal in the whole lane may be visible after an overnight exposure, due to background RNA.
Step 76: A typical agarose gel picture is presented in Fig. 3b. A fast migrating band of about 120bp is frequently visible, both in the samples and negative controls. This corresponds to the ligated adapter dimers and its intensity varies significantly between experiments. 20bp above that, a broad band, relatively sharper at the bottom and smeared at the top, is expected. The lower part of the band mostly corresponds to cloned miRNAs, whereas the upper, smeared part is enriched for targets and chimeric sequences. This upper band should appear only in the crosslinked samples. Separately isolating the DNA fragments of different sizes allows enrichment for the target and chimeric sequences in the sequence data. We generally use 3 fold more of the upper band (UB: targets + chimeras) than the lower band (LB: miRNAs). In case of doubts, preparing additional control PCR reactions with primers that amplify a specific miRNA can confirm that the crosslinking and linker ligations were successful.. For studies on HEK 293 cells we used primers specific for the highly expressed miR-16; a typical gel picture is shown in Fig. 3d.
Step 78: Because the amount of cDNA in the library depends on multiple factors it is difficult to give any expected concentration. To have enough material for the small scale and high-throughput sequencing without creating unnecessary PCR duplicates, adjust the number of PCR cycles to obtain about 20-80 ng of DNA.
Step 80: A small scale sequencing analysis should provide the following information: (1) types of RNAs present in the sequencing data; expect many miRNAs for AGO-CLASH. (2) The average length of cDNA inserts, which reflects effectiveness of RNase treatment. (3) The presence of bacterial rRNA contamination.
ACKNOWLEDGEMENTS
We thank Rebecca Holmes, Grzegorz Kudla and Alex Tuck for helpful comments on the manuscript. This work was supported by Wellcome Trust funding [077248]. Work in the Wellcome Trust Centre for Cell Biology is supported by Wellcome Trust core funding [092076].
Footnotes
COMPETING FINANTIAL INTERESTS The authors declare that they have no competing financial interests.
REFERENCES
- 1.Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annual review of biochemistry. 2010;79:351–79. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- 2.Hu W, Alvarez-Dominguez JR, Lodish HF. Regulation of mammalian cell differentiation by long non-coding RNAs. EMBO reports. 2012;13:971–83. doi: 10.1038/embor.2012.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guil S, Esteller M. Cis-acting noncoding RNAs: friends and foes. Nature structural & molecular biology. 2012;19:1068–75. doi: 10.1038/nsmb.2428. [DOI] [PubMed] [Google Scholar]
- 4.Djebali S, et al. Landscape of transcription in human cells. Nature. 2012;489:101–8. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ule J, et al. CLIP identifies Nova-regulated RNA networks in the brain. Science (New York, N.Y.) 2003;302:1212–5. doi: 10.1126/science.1090095. [DOI] [PubMed] [Google Scholar]
- 6.Licatalosi DD, et al. HITS-CLIP yields genome-wide insights into brain alternative RNA processing. Nature. 2008;456:464–9. doi: 10.1038/nature07488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hafner M, et al. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell. 2010;141:129–41. doi: 10.1016/j.cell.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.König J, et al. iCLIP reveals the function of hnRNP particles in splicing at individual nucleotide resolution. Nature structural & molecular biology. 2010;17:909–15. doi: 10.1038/nsmb.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chi SW, Zang JB, Mele A, Darnell RB. Argonaute HITS-CLIP decodes microRNA-mRNA interaction maps. Nature. 2009;460:479–86. doi: 10.1038/nature08170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Granneman S, Kudla G, Petfalski E, Tollervey D. Identification of protein binding sites on U3 snoRNA and pre-rRNA by UV cross-linking and high-throughput analysis of cDNAs. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:9613–9618. doi: 10.1073/pnas.0901997106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kudla G, Granneman S, Hahn D, Beggs JD, Tollervey D. Cross-linking, ligation, and sequencing of hybrids reveals RNA-RNA interactions in yeast. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:10010–10015. doi: 10.1073/pnas.1017386108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helwak A, Kudla G, Dudnakova T, Tollervey D. Mapping the Human miRNA Interactome by CLASH Reveals Frequent Noncanonical Binding. Cell. 2013;153:654–65. doi: 10.1016/j.cell.2013.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pasquinelli AE. MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nature reviews. Genetics. 2012;13:271–82. doi: 10.1038/nrg3162. [DOI] [PubMed] [Google Scholar]
- 14.Greenberg JR. Ultraviolet light-induced crosslinking of mRNA to proteins. Nucleic acids research. 1979;6:715–32. doi: 10.1093/nar/6.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kishore S, et al. A quantitative analysis of CLIP methods for identifying binding sites of RNA-binding proteins. Nature methods. 2011;8:559–64. doi: 10.1038/nmeth.1608. [DOI] [PubMed] [Google Scholar]
- 16.Travis AJ, Moody J, Helwak A, Tollervey D, Kudla G. hyb: a bioinformatics pipeline for the analysis of CLASH (crosslinking, ligation and sequencing of hybrids) data. Methods. 2013 doi: 10.1016/j.ymeth.2013.10.015. doi:10.1016/j.ymeth.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oeffinger M, et al. Comprehensive analysis of diverse ribonucleoprotein complexes. Nature Methods. 2007;4:951–956. doi: 10.1038/nmeth1101. [DOI] [PubMed] [Google Scholar]