Abstract
Background:
Nosocomial urinary tract infections (UTIs) are common in catheterized patients. Fungal UTI has become an important nosocomial problem over the past decade. The microbiology of candiduria is rapidly evolving and new trends are being reported.
Aims:
To study the microbiological trends and antifungal resistance profile of Candida in urine of catheterized chronic liver disease (CLD) patients at a super specialty hepatobiliary tertiary-care center.
Materials and Methods:
urine samples were collected by sterile technique, processed by semi-quantitative method as per the standard protocols. Direct microscopic examination of urine sample was also done to look for the presence of pus cells, red blood cells, casts, crystals or any bacterial or fungal element.
Result:
A total of 337 yeast isolates were obtained from catheterized patients, non-albicans Candida spp. emerged as the predominant pathogen and was responsible for 67.06% of nosocomial fungal UTI. Candida tropicalis accounted for 34.71% of the cases, whereas Candida albicans grew in 32.93%, Candida glabrata 16.32%, rare Candida spp. Nearly 11.5% (Candida hemolunii to be confirmed by molecular methods). Antifungal sensitivity varied non-albicans species except C. tropicalis, Candida parapsilosis were more often resistant to antifungal drugs.
Conclusion:
Nosocomial Candida UTIs in CLD patients is common, due to the cumulative pressure of contributing factors such as urinary instrumentation and prolonged use of broad-spectrum antibiotics. Non-albicans Candida were found to outnumber C. albicans in catherized CLD patients. Risk of strain persistence is also higher with non-albicans Candida. Thus, species identification and susceptibility testing is a must for appropriate management of such patients.
Keywords: Candida, candiduria, catheterized, chronic liver disease, nosocomial
Introduction
During the past 30 years, Candida in urine has evolved from a scientific curiosity described mainly in case reports to a common finding in hospitalized patients, yet it still intrigues the clinicians, as it may represent colonization of perineum, urinary catheter or bladder; at the same time it may be a harbinger of a more grave illness such as pyelonephritis/cystitis, or hematogenous seeding of the renal cortex in the course of disseminated candidiasis.
Usually, urine culture is sent for unexplained fever, elevated white blood cell (WBC) count, or less cogent reasons, such as cloudy or smelly urine and no follow-up study carried out to determine the significance of candiduria. To complicate things pyuria can be associated with both long-term and intermittent catheterization, interstitial nephritis and other tubulointerstitial disorders with inflammation, in addition to infection.
Diagnostic issues
Renal infection is seen with as few as 10,000-15,000 yeasts/mL in patients without catheters, however, no correlation with biopsy-proven renal infection could be established with colony counts of 20,000-100,000 cfu/mL.[1] Thus, as few as 10,000 cfu/mL may mean infection and 10,000-100,000 cfu/mL colonization? Ambiguity obscures pseudohyphae formation as a marker for invasion, as Candida glabrata, cannot make pseudohyphae and Candida albicans can be induced to form pseudohyphae by varying the pH and nutrients in the urine. Further, Antibody-coated yeasts cells in urine were shown to be a non-specific.[2] Cast containing yeast is specific for upper urinary tract infection (UTI), but insensitive.[3]
We undertook this study to determine the significance of candiduria in catheterized chronic liver disease (CLD) patients, identify the prevailing microbiological trends and antifungal resistance.
Materials and Methods
The study was carried out during January 2011 to May 2013 at a hepatobiliary tertiary care hospital on CLD patients admitted to the liver coma intensive care unit (ICU).
Inclusion criteria
Only pure growth isolates growing in significant count (≥104) cfu/mL, from two consecutive urine samples of catheterized CLD patients, admitted in the ICU for more than 72 h; collected using sterile technique were included. The second sample was collected after removing the old catheter and inserting a new one. These isolates were from samples where direct microscopic examination showed concomitant pyuria.
Exclusion criteria
Samples where yeast was isolated in the absence of pyuria, or as a mixed growth or when repeat sample could not be obtained, were excluded from analysis, isolates obtained from non-catheterized patients or from patients whose duration of admission was <72 h in the hospital were also excluded. If the second sample was collected without removal of the catheter it was rejected.
Sample collection and processing: Urine samples were collected using sterile technique - urine catheter was clamped (non-traumatic clamp/gate clip) few centimeters distal to the sampling port. Once sufficient urine collected in the tube, sampling port was wiped with an alcohol swab. After drying, the tube stabilized below the sampling port, needle inserted at an angle of 45°; and urine aspirated, transferred to the sterile container and sent to the lab within 1 h of drawing. Direct microscopic examination of urine sample was carried out to look for WBC, red blood cell, casts, crystals or any bacterial or fungal element. Sample processing was carried out immediately by semi-quantitative method as per the standard protocols
A repeat sample was requested after a catheter change where yeast was isolated as a pure growth in a significant (>104) colony count, to rule out contamination or colonization of the catheter. Multiple samples were accepted to confirm persistence or clearance of candiduria
Identification and susceptibility testing: Species identification and antifungal sensitivity was carried out in VITEK 2 compact system by YST ID (Bio Merieux, Mumbai, India) and VITEK YS 01 card respectively, as per the manufacturers’ instructions
Strain persistence: defined as repeated isolation of same species (14 days) without any episode of clearance, despite a catheter change
Candidemia: 8-10 mL of blood was aseptically drawn from the patients 3-5 days after detecting candiduria and cultured in BacT Alert (Bio Merieux) as per the manufacturers’ instructions. Isolation of the same species from the blood culture was recorded.
Results
During the period from January 2011 to May 2013 we received 9284 urine samples. 3713 (39.93%) catheterized patients grew pure Candida isolates in a significant count. A second sample, collected after removing the old catheter and inserting a new one, grew yeast in 337 (9.98%) samples. No significant fungal growth was seen in 3376 (90.9%) on repeat sampling.
Non-albicans Candida spp. emerged as the predominant pathogen 67.06% (226).
Candida tropicalis 34.7% (117), C. albicans 32.93% (111). C. glabrata 16.3% (55), rare Candida spp. 11.5% (39) (Candida hemolunii to be confirmed by molecular methods) and Candida parapsilosis 2.3% (8) were responsible for nosocomial fungal UTIs [Table 1].
Table 1.
Distribution of Candida spp. in the study group (n=337)

Antifungal susceptibility
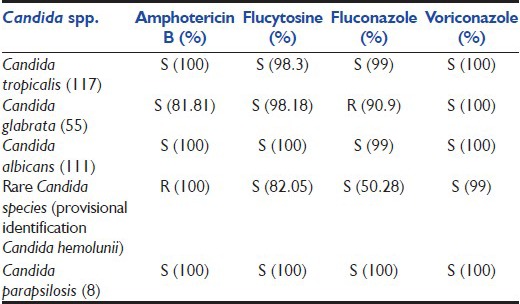
C. tropicalis, C. parapsilosis, C. albicans were fully susceptible to all antifungals tested, namely Amphotericin B, Flucytosine, Fluconazole, Voriconazole, others displayed variable amount of resistance. Resistance and higher MICs (though in sensitive range) were demonstrated by C. glabrata and rare Candida spp. [Table 2].
Table 2.
Sensitivity pattern of Candida spp. (n=330)
Strain persistence
In total 16 patients persistently grew Candida in cultures. Same species was isolated in 15 patients on repeat sampling (more than 14 days later). Of these persistent strains, only three were C. albicans, four C. tropicalis, three C. parapsilosis, Three C. glabrata, two C. hemolunii, one patient growing C. tropicalis, grew C. hemolunii later. Thus, strain persistence as determined in the follow-up samples was very high (15/16, 93.75%). The chances that the primary strain causing candiduria would be persistent were significantly higher with non-albicans Candida spp. when compared with C. albicans. Candiduria evolved to candidemia in 3.5% (11) patients.
Discussion
CLD patients are known to have various functional immune abnormalities, ignoring a possible Candida infection in such patients can have fatal consequences. Obtaining a repeat urine sample post catheter change is a simple but a highly discriminatory exercise; clearly demonstrated in our study as 90.9% of Candida cleared post catheter change.
Virtually any Candida species may be associated with candiduria. Earlier studies report C. albicans as the predominant isolate (50-70%).[4] However, now there is a paradigm shift toward non-albicans Candida. Non-albicans species C. glabrata adapts well to urine properties such as substrate availability, osmolality and pH; fluconazole prophylaxis promotes their selection.[5] C. parapsilosis, a common cause of candidemia in both adults and neonates, is uncommonly isolated from urine of adults. It is found more often in urine from neonates associated with systemic infection.[4,6] C. hemolunii, inherently resistant to many antifungals, is being increasingly reported; a case of catheter related candidemia has also been reported.[7]
As there is geographical, institutional and species specific variation in antifungal susceptibility patterns, determination of species and antifungal susceptibility is important. In our study most isolates of C. albicans, C. parapsilosis and C. tropicalis were sensitive to fluconazole, flucytosine, amphotericin B, voriconazole, though some strains demonstrated an increase MICs to these drugs. Primary resistance in C. albicans is uncommon, though resistance has been described in human immunodeficiency virus patients on long term antifungal prophylaxis, closely linked to advanced acquired immunodeficiency syndrome and the cumulative dose of azoles.[8,9]
Isolates of C. glabrata and rare Candida spp. (C. hemolunii) were resistant to many antifungals. This has been reported earlier in patients on prolonged treatment with fluconazole and has been attributed to, mechanisms such as permeability barrier, alteration in protein profile or genetic material and haploid state of C. glabrata.[10] Increase in MIC of fluconazole has been reported in C. tropicalis after 2 weeks and for amphotericin B after 7 weeks, some studies have demonstrated a change in MIC of fluconazole in 24-48 h only.[11,12] Absolute (100%) resistance of C. hemolunii for fluconazole, amphotericin B is highly alarming as these are the most commonly used antibiotics, especially as empirical therapy. C. haemulonii is considered to be an emerging yeast pathogen for which the optimal strategy of patient management has yet to be elucidated. Several cases of C. haemulonii candidemia have been reported; most were resistant to both fluconazole and amphotericin B and failed to eradicate C. haemulonii candidemia.[7,13,14] However, classical methods of identification and the usual commercial systems are not able to identify these rare clinical isolates. Identification of these species should be based on molecular methods, such as sequence analysis of internal transcribed spacer regions and matrix-assisted laser desorption ionization-time of flight mass spectrometry.[15]
Strain persistence was very high (15/16, 93.5%) especially with non-albicans Candida. Studies have shown persistence of same strain in cases of recurrent or persistent candiduria, though they may differ in phenotypic characteristics.[16]
Conclusions
Candida in urine is highly influenced by the manner of collection; repeat sampling should be done in catheterized patients to rule out contamination/colonization of bladder.
Catheterized CLD patients admitted in the ICU have associated risk factors and may develop nosocomial UTI due to Candida spp. In the presence of associated risk factors, there is a definite risk of invasive candidiasis following candiduria; hence, aggressive approach is warranted by the clinicians. A change in trend with shift toward non-albicans Candida spp. as the predominant pathogen causing nosocomial UTI is ominous, as they are more often resistant, difficult to treat and chances that these strains would remain persistent are higher. Therefore, species identification and susceptibility testing is a must for better clinical outcomes and management.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Navarro EE, Almario JS, Schaufele RL, Bacher J, Walsh TJ. Quantitative urine cultures do not reliably detect renal candidiasis in rabbits. J Clin Microbiol. 1997;35:3292–7. doi: 10.1128/jcm.35.12.3292-3297.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harding SA, Merz WG. Evaluation of antibody coating of yeasts in urine as an indicator of the site of urinary tract infection. J Clin Microbiol. 1975;2:222–5. doi: 10.1128/jcm.2.3.222-225.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Argyle C, Schumann GB, Genack L, Gregory M. Identification of fungal casts in a patient with renal candidiasis. Hum Pathol. 1984;15:480–1. doi: 10.1016/s0046-8177(84)80084-2. [DOI] [PubMed] [Google Scholar]
- 4.Storfer SP, Medoff G, Fraser VJ, Powderly WG, Dunagan WC. Candiduria: retrospective review in hospitalized patients. Infect Dis Clin Pract. 1994;3:23–9. [Google Scholar]
- 5.Snydman DR. Shifting patterns in the epidemiology of nosocomial Candida infections. Chest. 2003;123(5 Suppl):500S–3. doi: 10.1378/chest.123.5_suppl.500s. [DOI] [PubMed] [Google Scholar]
- 6.Pemán J, Cantón E, Linares-Sicilia MJ, Roselló EM, Borrell N, Ruiz-Pérez-de-Pipaon MT, et al. Epidemiology and antifungal susceptibility of bloodstream fungal isolates in pediatric patients: A Spanish multicenter prospective survey. J Clin Microbiol. 2011;49:4158–63. doi: 10.1128/JCM.05474-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim S, Ko KS, Moon SY, Lee MS, Lee MY, Son JS. Catheter-related candidemia caused by Candida haemulonii in a patient in long-term hospital care. J Korean Med Sci. 2011;26:297–300. doi: 10.3346/jkms.2011.26.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Millon L, Manteaux A, Reboux G, Drobacheff C, Monod M, Barale T, et al. Fluconazole-resistant recurrent oral candidiasis in human immunodeficiency virus-positive patients: Persistence of Candida albicans strains with the same genotype. J Clin Microbiol. 1994;32:1115–8. doi: 10.1128/jcm.32.4.1115-1118.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Troillet N, Durussel C, Bille J, Glauser MP, Chave JP. Correlation between in vitro susceptibility of Candida albicans and fluconazole-resistant oropharyngeal candidiasis in HIV-infected patients. Eur J Clin Microbiol Infect Dis. 1993;12:911–5. doi: 10.1007/BF01992164. [DOI] [PubMed] [Google Scholar]
- 10.Fidel PL, Jr, Vazquez JA, Sobel JD. Candida glabrata: Review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin Microbiol Rev. 1999;12:80–96. doi: 10.1128/cmr.12.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santhanam J, Fairuz A, Ooi KF, Husni M. Development of resistance in Candida tropicalis: an in vitro study [poster] Malaysian Journal Of Community Health. 2002;8:50–3. [Google Scholar]
- 12.Blumberg HM, Hendershot EF, Lott TJ. Persistence of the same Candida albicans strain despite fluconazole therapy. Documentation by pulsed-field gel electrophoresis. Diagn Microbiol Infect Dis. 1992;15:545–7. doi: 10.1016/0732-8893(92)90106-4. [DOI] [PubMed] [Google Scholar]
- 13.Khan ZU, Al-Sweih NA, Ahmad S, Al-Kazemi N, Khan S, Joseph L, et al. Outbreak of fungemia among neonates caused by Candida haemulonii resistant to amphotericin B, itraconazole, and fluconazole. J Clin Microbiol. 2007;45:2025–7. doi: 10.1128/JCM.00222-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruan SY, Kuo YW, Huang CT, Hsiue HC, Hsueh PR. Infections due to Candida haemulonii: Species identification, antifungal susceptibility and outcomes. Int J Antimicrob Agents. 2010;35:85–8. doi: 10.1016/j.ijantimicag.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Cendejas-Bueno E, Kolecka A, Alastruey-Izquierdo A, Theelen B, Groenewald M, Kostrzewa M, et al. Reclassification of the Candida haemulonii complex as Candida haemulonii (C. haemulonii group I), C. duobushaemulonii sp. nov. (C. haemulonii group II), and C. haemulonii var. vulnera var. nov.: Three multiresistant human pathogenic yeasts. J Clin Microbiol. 2012;50:3641–51. doi: 10.1128/JCM.02248-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain M, Dogra V, Mishra B, Thakur A, Loomba PS, Bhargava A. Candiduria in catheterized intensive care unit patients: Emerging microbiological trends. Indian J Pathol Microbiol. 2011;54:552–5. doi: 10.4103/0377-4929.85091. [DOI] [PubMed] [Google Scholar]


