Abstract
Intravenous immunoglobulin [IVIg] is useful for treating several clinical conditions and is largely considered safe, without major adverse events. Here we report a case of acute ST elevation myocardial infarction associated with high dose IVIg administration in a previously healthy 69-year-old male patient of Guillain Barre syndrome. The case is being reported to emphasize the need for treating physicians to be aware of thrombotic complications associated with IVIg. The thrombotic complications associated with IVIg are reviewed in brief, and the measures to reduce them are discussed.
Keywords: Adverse events, intravenous immunoglobulin, myocardial infarction, thrombosis
Introduction
Intravenous immunoglobulin [IVIg] is used for treatment of a variety of autoimmune, rheumatologic, and immunodeficiency [both primary and secondary] disorders and can be life-saving in several of these conditions. Although it has good safety profile,[1] it can be rarely associated with serious adverse events. Thrombotic complications, both arterial and venous, are uncommon adverse events associated with IVIg use.[2] Here, we report a case of acute ST elevation anterior wall myocardial infarction [MI] associated with high dose [2 g/kg] IVIg administration in a previously healthy 69-year-old male patient of Guillain Barre syndrome [GBS]. The case is being reported to draw the attention of treating physicians to this uncommon, but serious adverse event associated with IVIg administration. We have reviewed the literature on thrombotic complications associated with IVIg in brief and measures to reduce them are discussed.
Case Report
A previously healthy, 69-year-old male manual laborer without addictions was admitted with complaints of progressive weakness of all the 4 limbs for 3 days. The weakness initially started in the lower limbs and then ascended rapidly to involve the upper limbs and neck muscles, forcing him to confine himself to bed. There was no history of preceding diarrheal illness, upper respiratory tract infection, or exposure to organophosphate insecticides.
On admission to our hospital [day-4 of illness], he was found to have symmetric areflexic quadriparesis with weakness of neck flexors [power in lower limbs: Grade 1/5, upper limbs: Grade 2/5, neck flexors: Grade 2/5] but extraocular and facial muscles, sensations, bowel and bladder were not affected. A provisional diagnosis of GBS was made. The patient was started on intravenous immunoglobulin [Norglobin™ (5 g/100 ml), containing maltose as excipient, marketed by Claris Lifesciences, Ahmedabad (India), dose: 0.4 g/kg/day × 5 days], along with venous thrombosis prophylaxis [enoxaparin] from day-4. He later had progressive paralysis with involvement of lower cranial nerves and respiratory muscles, needing endotracheal intubation, mechanical ventilation and nasogastric tube feeding in the intensive care unit from day-5. The patient had autonomic dysfunction with episodes of hypertension and tachycardia on days- 4 and 5, which resolved by day-6.
On day-8, when the last dose of IVIg was just about to be completed, patient had sudden unexplained episode of hypotension [systolic blood pressure: 70 mm Hg] with profuse sweating and restlessness. An electrocardiogram [Figures 1-3] showed ST segment elevation in leads V1 to V4, suggesting acute anterior wall ST elevation MI. Patient's blood pressure rose with inotropes [norepinephrine and dopamine], and he received thrombolytic therapy [streptokinase-15,00,000 units] within 1 hour. Inotropes were tapered gradually and stopped after 8 hours. Echocardiography revealed hypokinesia of the interventricular septum and anterior wall of left ventricle with an ejection fraction of 40%. Troponin T was positive. Patient additionally received antiplatelet drugs [aspirin, clopidogrel], atorvastatin and enoxaparin. Metoprolol and enalapril were added from day-9, after achievement of hemodynamic stability.
Figure 1.
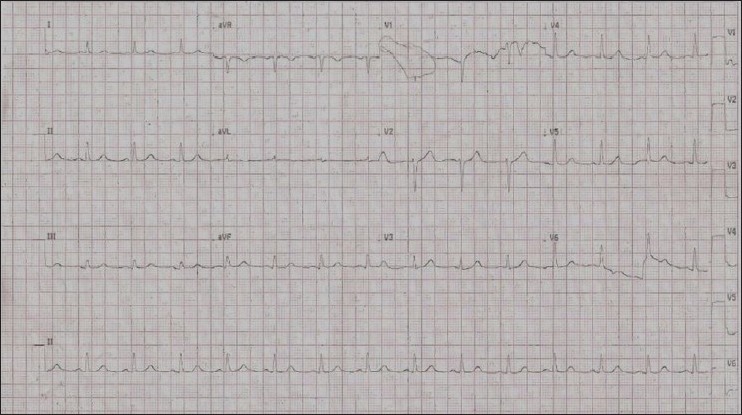
ECG on admission [day 4 of illness], showing normal pattern
Figure 3.
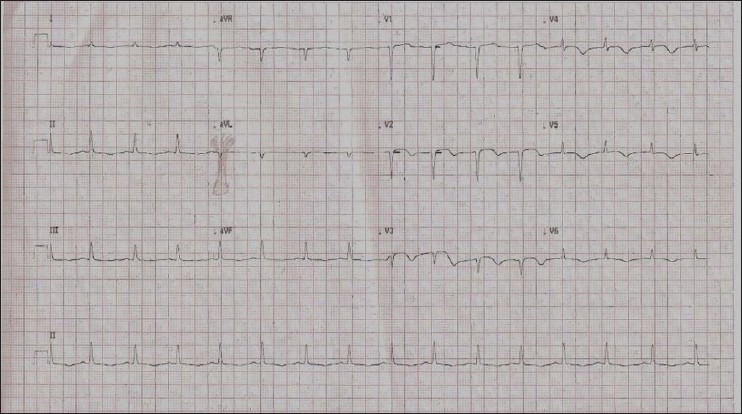
ECG 30 minutes after thrombolysis with streptokinase [15,00,000 units]
Figure 2.
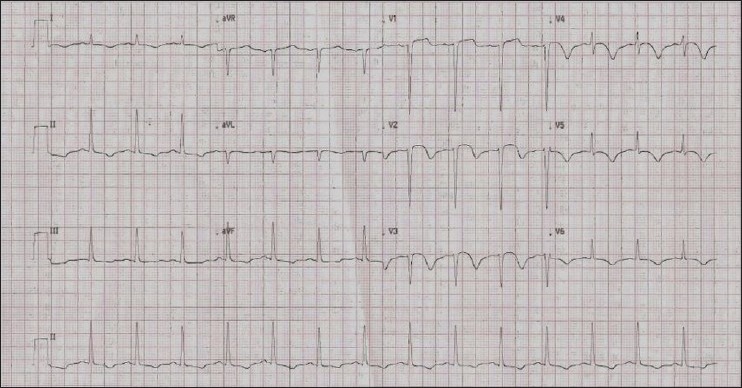
ECG on day 8, when the patient developed sudden hypotension, showing Q waves with ST elevation in V1-V3, T inversions in all other leads suggesting anterior wall ST elevation MI
Laboratory evaluation on admission [day-4] had revealed normal plasma glucose levels, HbA1c [5.8%], serum electrolytes, lipid profile, renal and liver functions. He had microcytic hypochromic anemia [Hb: 9.4 g/dl]. Nerve conduction studies done on day-11 revealed diffuse sensory-motor demyelinating polyneuropathy. Urine porphobilinogen test was negative. ELISA for HIV [human immunodeficiency virus], HBsAg [hepatitis B surface antigen] and HCV [hepatitis C virus] was negative. He was gradually weaned off from ventilator on day-16 and extubated on day-17. He was discharged on day-20 with partial recovery of muscle power [grade 2/5 in all 4 limbs] on oral hematinics.
Discussion
IVIg comprises of gamma globulins derived from purified pooled plasma of thousands of donors, consisting mainly of polyspecific IgG. Its immunomodulatory and anti-inflammatory actions have enabled its effective use in several autoimmune and inflammatory clinical conditions. Adverse reactions are reported in 24-36% of patients after high dose [2 g/kg] IVIg,[2] most being mild and transient such as headaches, flushing, fever, chills, fatigue, nausea, diarrhea, blood pressure changes and tachycardia. More serious adverse effects like acute renal failure, thrombotic events, aseptic meningitis, hematologic complications and anaphylactic reactions are much rarer.
Thrombotic complications reported in association with high dose IVIg include both venous [deep venous thrombosis, pulmonary embolism, hepatic veno-occlusive disease] and arterial events [acute myocardial infarction, ischemic stroke, thrombosis of arteriovenous fistulae created for hemodialysis, spinal cord ischemia], the latter being 4 times more common than the former.[3] Thrombotic events are reported in most clinical conditions where IVIg has been used, with an estimated incidence of 0.15-1.2% per treatment course.[3] Arterial thromboses are more commonly associated with atherosclerotic risk factors such as advanced age, smoking, obesity, dyslipidemia, diabetes mellitus, hypertension, renal dysfunction, and prior coronary or cerebrovascular disease and occur early (77% within 24 hours) after IVIg administration. Venous thromboses occur later (54% more than 24 hours after IVIg administration) and are associated with risk factors such as obesity and immobility.[3] The risk of thrombosis increases significantly when multiple cardiovascular risk factors co-exist.[4]
Autonomic dysfunction [AD] in GBS patients per se has been reported anecdotally to cause MI by catecholamine surge and coronary spasm.[5] In our patient, MI followed few days after the initial AD subsided. Our patient, unlike other reported cases of MI with multiple atherosclerotic risk factors,[6,7] did not have smoking, obesity, dyslipidemia, diabetes mellitus, hypertension, renal dysfunction and prior coronary or cerebrovascular disease as risk factors. Advanced age was the only risk factor he had. MI occurred during the fifth day of IVIg infusion, in concert with reported timing of occurrence.[3,6,7] The infarction was confirmed by electrocardiography, echocardiography and elevated troponin T.
Proposed mechanisms for thrombosis include some of the following- platelet activation, increased blood viscosity, contamination of IVIg with activated coagulation factors, induced arterial vasospasm, production of vasoconstrictive cytokines and vasculitis.[8] IVIg products from different manufacturers might differ considerably, and factors such as osmolality, pH and excipient used [sodium or sugar], along with volume load and infusion rates determine the adverse effects.[9] It is thought that salt-based high viscosity products might be responsible for initiation of thrombosis and MI.[9]
It is recommended to assess carefully, the overall risk of MI, stroke and venous thromboembolism, along with risk benefit ratio before IVIg use.[4,7] Maintenance of hydration, a slower rate of infusion [100 mg/kg/hour], combined with antiplatelet therapy [aspirin-300 mg/day] and anticoagulation [enoxaparin-1 mg/kg/day] have been suggested during IVIg infusion to reduce IVIg-related thrombosis.[9,10]
To conclude, IVIg administration in high doses can be rarely associated with thrombotic complications such as acute MI. Adequate hydration and slower rate of infusion, along with careful patient selection based on the risk-benefit assessment may reduce this complication.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Tufan F, Kamali S, Erer B, Gul A, Inanc M, Ocal L, et al. Safety of high-dose intravenous immunoglobulin in systemic autoimmune diseases. Clin Rheumatol. 2007;26:1913–5. doi: 10.1007/s10067-007-0694-y. [DOI] [PubMed] [Google Scholar]
- 2.Katz U, Achiron A, Sherer Y, Shoenfeld Y. Safety of intravenous immunoglobulin (IVIG) therapy. Autoimmun Rev. 2007;6:257–9. doi: 10.1016/j.autrev.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Paran D, Herishanu Y, Elkayam O, Shopin L, Ben-Ami R. Venous and arterial thrombosis following administration of intravenous immunoglobulins. Blood Coagul Fibrinolysis. 2005;16:313–8. doi: 10.1097/01.mbc.0000172694.85233.a8. [DOI] [PubMed] [Google Scholar]
- 4.Caress JB, Hobson-Webb L, Passmore LV, Finkbiner AP, Cartwright MS. Case-control study of thromboembolic events associated with IV immunoglobulin. J Neurol. 2009;256:339–42. doi: 10.1007/s00415-009-0969-0. [DOI] [PubMed] [Google Scholar]
- 5.Mukerji S, Aloka F, Farooq MU, Kassab MY, Abela GS. Cardiovascular complications of the Guillain-Barré syndrome. Am J Cardiol. 2009;104:1452–5. doi: 10.1016/j.amjcard.2009.06.069. [DOI] [PubMed] [Google Scholar]
- 6.Elkayam O, Paran D, Milo R, Davidovitz Y, Almoznino-Sarafian D, Zeltser D, et al. Acute myocardial infarction associated with high dose intravenous immunoglobulin infusion for autoimmune disorders: A study of four cases. Ann Rheum Dis. 2000;59:77–80. doi: 10.1136/ard.59.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marie I, Maurey G, Hervé F, Hellot MF, Levesque H. Intravenous immunoglobulin-associated arterial and venous thrombosis; report of a series and review of the literature. Br J Dermatol. 2006;155:714–21. doi: 10.1111/j.1365-2133.2006.07390.x. [DOI] [PubMed] [Google Scholar]
- 8.Hefer D, Jaloudi M. Thromboembolic events as an emerging adverse effect during high-dose intravenous immunoglobulin therapy in elderly patients: A case report and discussion of the relevant literature. Ann Hematol. 2004;83:661–5. doi: 10.1007/s00277-004-0895-2. [DOI] [PubMed] [Google Scholar]
- 9.Vo AA, Cam V, Toyoda M, Puliyanda DP, Lukovsky M, Bunnapradist S, et al. Safety and adverse events profiles of intravenous gammaglobulin products used for immunomodulation: A single-center experience. Clin J Am Soc Nephrol. 2006;1:844–52. doi: 10.2215/CJN.01701105. [DOI] [PubMed] [Google Scholar]
- 10.Huang L, Kanellis J, Mulley W. Slow and steady. Reducing thrombotic events in renal transplant recipients treated with IVIg for antibody-mediated rejection. Nephrology (Carlton) 2011;16:239–42. doi: 10.1111/j.1440-1797.2010.01399.x. [DOI] [PubMed] [Google Scholar]


