Abstract
Objective:
Chronic periodontitis (CP) is associated with increased levels of blood reactive oxygen species (ROS). So, treatment of CP may lead to decrease in blood ROS. However, not much literature is available comparing the effect of surgical and non-surgical periodontal treatment on blood ROS levels. Reactive oxygen metabolites (ROMs) are a useful measure of blood ROS. The aim of this study was to investigate the effect of periodontal treatment on plasma ROM levels in CP patients.
Materials and Methods:
Thirty CP patients and 15 controls were monitored. Plasma samples were collected at baseline and the clinical parameters were recorded. The CP patients were randomly divided into two groups: Scaling and root planing (Group II) and periodontal flap surgery (Group III). Both groups were re-evaluated 1 and 2 months after therapy. Clinical parameters were reviewed, plasma samples collected, and ROM levels were determined using a spectrophotometric technique.
Results:
At baseline, the ROM levels for Group II and Group III were 519.8 ± 62.4 and 513.4 ± 74.7 CARR U, respectively, which were higher than Group I value (282.9 ± 23.9, P < 0.001). Periodontal treatment in CP patients resulted in improvement of clinical parameters and a highly significant reduction in plasma ROM level (P < 0.001) after 2 months. Also, there was a more significant reduction in plasma ROM levels in Group III as compared to Group II (P < 0.05).
Conclusions:
In CP patients, surgical periodontal treatment was more effective in lowering the plasma ROM levels than when non-surgical periodontal treatment was performed alone and, therefore, may be more beneficial in reducing systemic oxidative stress.
Keywords: Clinical trial, periodontal disease, plasma, reactive oxygen species
INTRODUCTION
Periodontitis is regarded as “an inflammatory lesion, mediated by complex host–parasite interactions, that leads to the loss of connective tissue attachment to the root surface cementum and adjacent alveolar bone.”[1] It is one of the most common inflammatory diseases affecting adults worldwide.[2] An emerging body of evidence is associating oxidative stress to the pathogenesis of periodontal tissue destruction. Oxidative stress occurs when the pro-oxidant and antioxidant balance shifts in favor of the former, leading to potential damage.[3] It is now understood that while the primary etiological agent for periodontitis is predominantly gram-negative anaerobic or facultative bacteria within the subgingival biofilm which initiate the destruction, the majority of the periodontal tissue damage is caused by an inappropriate host response to these microorganisms and their products.[4] Bacteria and their products stimulate accumulation of polymorphonuclear leukocytes at the gingival site and subsequently the release of proteolytic enzymes and reactive oxygen species (ROS).[4] ROS include molecules like hydrogen peroxide, hypochlorous acid, singlet oxygen, and ozone, and their excessive production by polymorphonuclear leukocytes is one of the pathologic features in the periodontal lesion.[5] Numerous studies have shown that an excess of ROS and depletion of antioxidant levels in the gingival crevicular fluid are responsible for the chronic local activation of periodontal inflammation and tissue destruction.[6,7] ROS can cause periodontal tissue destruction by oxidizing DNA, proteins, lipids, and important enzymes such as antiproteases, stimulating proinflammatory cytokines’ release through depletion of intracellular thiol compounds, and activating nuclear factor-kappa B (NF-κB).[8]
With the progression of periodontitis, ROS produced in the periodontal lesion may diffuse into the blood stream and cause systemic oxidative stress. Numerous studies have suggested a positive association between periodontitis and blood ROS levels.[9,10] It is also known that oxidative stress makes a significant contribution to a variety of human diseases such as diabetes, arthritis, adult respiratory distress syndrome, heart disease, stroke, liver disease, acquired immunodeficiency syndrome (AIDS), Alzheimer's disease, Parkinson's disease, alcoholism, and many more.[11] So, it is hypothesized that periodontitis-induced increased levels of circulating ROS could be detrimental to systemic health, in which case, periodontal treatment should be effective in maintaining systemic health by decreasing ROS.
Recently, reactive oxygen metabolites (ROMs) were recognized as a useful measure of ROS.[12,13,14] This analysis measures the whole oxidant capacity of blood against N, N-diethyl-para-phenylenediamine in acidic buffer. The main components of ROM are hydroperoxides, which are intermediate oxidative products of lipids, peptides, and amino acids. Despite the fair oxidant power, hydroperoxides in the plasma are relatively stable compared to their parent free radicals; therefore, their levels can be detected. This test is based on the reaction of plasma samples with transition metal ions to form alkoxy and peroxy radicals. Recently, Tamaki et al.[15] and D‘Aiuto et al.[16] in their study have shown that in patients with chronic periodontitis (CP), non-surgical periodontal therapy was effective in reducing plasma ROMs. However, the effect of surgical periodontal therapy on plasma ROMs has not yet been explored. Systematic review has shown that surgical debridement is a more effective method for the treatment of moderate to severe CP in terms of attachment level gain and reduction in gingival inflammation.[17] Hence, it would be interesting to compare the effects of surgical to non-surgical periodontal treatment with respect to plasma ROM levels.
Since there is limited literature on the effect of treatment on the blood ROM levels, this study was conducted with an objective to compare the ROM levels between patients with CP and controls, and to investigate the effects of non-surgical and surgical periodontal treatment on the plasma ROM levels in patients with CP.
MATERIALS AND METHODS
Sample size calculation
A sample size of 15 per group was required for the detection of a significant difference in clinical parameters (80% power; two-sided 5% significance level).
Subject recruitment
Forty-five subjects from the Out-patient Department of Bapuji Dental College and Hospital, Davangere were enrolled for this randomized, longitudinal, and interventional study, and monitored over a period of 2 months. Subjects included 15 periodontally healthy controls (Group I: 7 men and 8 women aged 26-49 years; mean age: 32.8 ± 6.38 years) and 30 CP patients. The CP patients were further randomly and equally divided into two groups – Group II (nine men and six women aged 25-47 years; mean age: 35.6 ± 5.79 years) and Group III (eight men and seven women aged 25-48 years; mean age: 35.8 ± 7.08 years). The control group was composed of individuals who presented with good oral hygiene, no evidence of interproximal attachment loss, probing depth ≤3 mm at all sites on all teeth, and no gingival swelling, redness, bleeding on probing (BOP), or tooth mobility.[18] The patients with periodontal problems had been diagnosed with moderate to severe chronic generalized periodontitis based on the clinical and radiographic criteria proposed by the 1999 International World Workshop for Classification of Periodontal Diseases and Conditions.[19] It was ensured that they had teeth with 30% periodontal bone loss and ≥4-mm-deep periodontal pockets. The gingiva showed the characteristics signs of chronic inflammation and had BOP.
All subjects were between 25 and 50 years of age and systemically healthy. The exclusion criteria were <15 teeth, pregnancy and lactation, previous or current smoking, and the use of antioxidant supplements (e.g., vitamin C, vitamin E, or coenzyme Q10), anti-inflammatory medications like nonsteroidal anti-inflammatory drugs (NSAIDs), or antibiotics within the previous 3 months. All subjects lived in the same geographic area (Davangere city, India) and were of a middle-class socioeconomic status and had similar traditional nutrition habits. The research protocol was reviewed and approved by the Institutional Review Board (IRB), Bapuji Dental College and Hospital, and a written informed consent was obtained from all the selected patients prior to commencement of the study.
Periodontal treatment
At baseline, the clinical parameters were recorded for all subjects and the plasma samples were collected for assessment of ROM levels. No treatment was done after sample collection in Group I patients. In Group II patients, sample collection was followed by non-surgical periodontal treatment. This included oral hygiene instructions and scaling and root planing (SRP). The treatment consisted of an average of four 45-min sessions over 3 weeks. The SRP was performed with manual and ultrasonic instrumentation. Clinical data and plasma samples were re-evaluated at 1 and 2 months after therapy to assess the changes. In Group III patients, after the sample collection at baseline, the subjects were appointed for surgical pocket therapy. At first, oral hygiene instructions were given and SRP was done similar to that performed for Group II patients. The patients were re-evaluated at 4 weeks and periodontal flap surgery was performed in areas of persisting pocket depth. At follow-up visits of 1 and 2 months postoperatively, the clinical data and plasma samples were re-evaluated to assess the changes.
Clinical measurements
Clinical parameters assessed were gingival index (GI),[20] plaque index (PI),[21] sulcus bleeding index (SBI),[22] probing depth (PD), and clinical attachment level (CAL). PD and CAL were measured using an UNC-15 probe at six sites (mesio-buccal, mid-buccal, disto-buccal, mesio-lingual, mid-lingual, and disto-lingual) per tooth. CAL was measured as the distance (in millimeters) from the cemento-enamel junction to the bottom of the periodontal pocket. All clinical measurements were performed by a single investigator (first author).
Measurement of plasma ROM levels
While maintaining aseptic precautions, 2 ml of venous blood was collected from the antecubital vein at chair side and was immediately kept on ice and centrifuged at 3000× g for 5 min. The plasma aliquots thus obtained were stored at −80°C until analysis. The measurements of plasma ROM level were performed using a spectrophotometer, according to previously reported methods.[15,23] Twenty microliters of the plasma sample was mixed with 1 ml of the prepared acetate buffer and 20 μl of the chromogenic substrate and incubated for 5 min at 37°C. Absorbance was recorded at 505 nm using a spectrophotometer. The measurements were expressed in Carratelli unit (CARR U). It has been established that 1 CARR U corresponds to 0.08 mg/dl hydrogen peroxide.[23,14]
Statistical analyses
The clinical and plasma data were tabulated and subjected to statistical analysis using SPSS 16.0J software. Data were expressed as mean ± standard deviation (X ± SD). Analysis of variance (ANOVA) test was done for multiple group comparisons, followed by post-hoc Tukey's test for group-wise comparison. Changes in clinical and plasma parameters for each group were tested by paired t-test. Unpaired t-test was done to compare the postoperative changes in Group II and Group III. The statistical significance of correlations among variables was determined using the Spearman rank correlation coefficient. All analyses were performed using a software program. A P < 0.05 was considered significant, <0.001 was considered statistically highly significant, and >0.05 was considered statistically non-significant.
RESULTS
Cross-sectional analysis at baseline
At baseline, there were significant differences in mean GI, PI, SBI, PD, and CAL between the controls and the patients with periodontitis (P < 0.01) [Table 1]. The ROM levels at baseline for Group II and Group III were 519.8 ± 62.4 and 513.4 ± 74.7 CARR U, respectively, which were higher than Group I value (282.9 ± 23.9, P < 0.001). In patients with periodontitis, the plasma ROM level was positively correlated with SBI, PD, and CAL [Tables 2 and 3].
Table 1.
Comparison of clinical and plasma parameters at baseline between Groups I, II, and III (cross-sectional analysis at baseline)
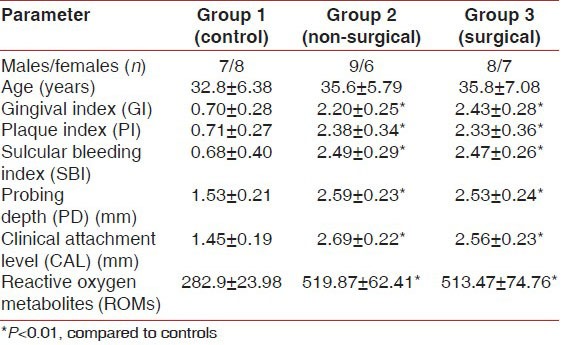
Table 2.
Correlation between plasma ROM levels and clinical parameters in Group II at baseline
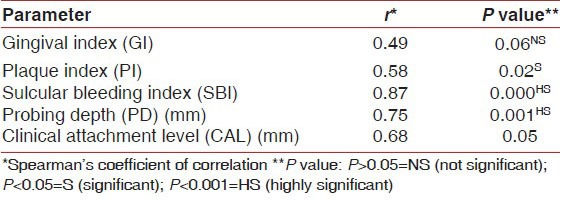
Table 3.
Correlation between plasma ROM levels and clinical parameters in Group III at baseline
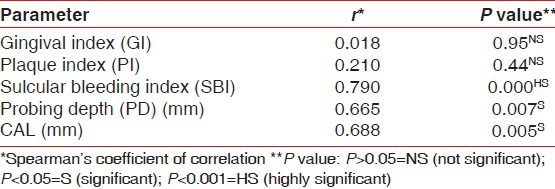
Longitudinal changes in clinical and plasma parameters in patients with periodontitis
Mean GI, PI, SBI, PD, and CAL values improved at 1 month (P < 0.001) and 2 months (P < 0.001) after periodontal treatment in both Group II and Group III [Tables 4 and 5]. All CP patients showed a significant reduction in plasma ROM level when re-evaluated 1 month (P < 0.001) and 2 months (P < 0.001) after periodontal treatment.
Table 4.
Changes in clinical parameters after periodontal treatment in Group II (longitudinal changes)
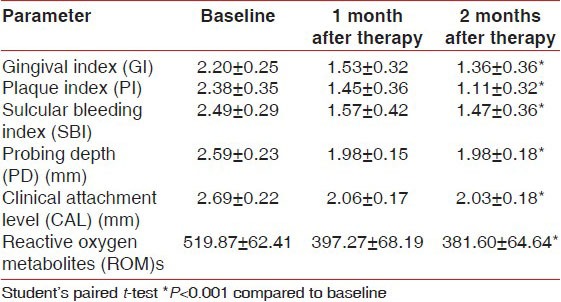
Table 5.
Changes in clinical parameters after periodontal treatment in Group III (longitudinal changes)
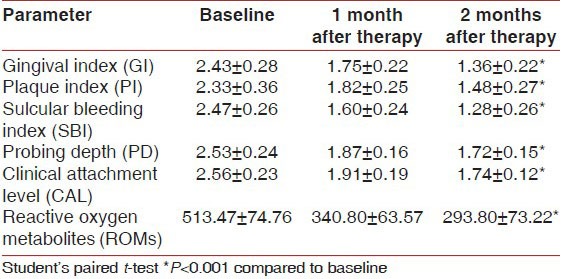
Comparison of plasma parameters between Group II and Group III
The plasma ROM level showed statistically more significant decrease in Group III as compared to Group II at the end of 2 months (P < 0.05) [Graph 1].
Graph 1.
Mean difference in plasma ROM levels at different time intervals between Group II and Group III

DISCUSSION
To the best of our knowledge, this is the first study comparing the effects of non-surgical and surgical periodontal therapy on the plasma ROM levels in patients with CP. The plasma ROM levels measured at baseline were significantly higher in patients with CP (Group II and Group III) as compared to controls. As plasma ROM is considered to be a reliable indicator of ROS in blood,[12,13,14] its increase in an otherwise healthy CP patient indicates that periodontal disease can increase the ROS levels in blood. Thus, our study establishes that there exists a positive association between periodontitis and blood ROS levels and reiterates that ROS produced in the periodontal lesion may spread to the blood stream. Previous studies performed by Akalin et al.,[9] Baltacioglu et al.,[10] Tamaki et al.,[15] and Wei et al.[24] have shown similar results.
In patients with periodontitis (Group II and Group III), the plasma ROM level was positively correlated with PD, CAL, and SBI at baseline. Increased PD and CAL are pathognomonic for periodontitis, excellent indicators of past destruction of the periodontal attachment apparatus, and can also be used to monitor the progression of periodontitis.[25] Bleeding is one of the earliest signs of gingival inflammation and indicates active nature of the disease.[26] Therefore, we found that in periodontitis patients, the plasma level of ROM increased with an increase in periodontal breakdown, as reflected by PD and CAL,[27] and the present disease activity, as mirrored by SBI. Tamaki et al.[15] also reported positive correlation of plasma ROM levels with PD, CAL, and the percentage of sites with BOP, but not with plaque level.
In our study, the CP patients were randomly assigned to either non-surgical periodontal therapy (Group II) or surgical periodontal therapy (Group III) and the therapeutic effects were assessed 1 and 2 months after treatment. In both groups, plasma ROM levels decreased significantly following periodontal therapy. Since periodontal therapy is known to decrease periodontal inflammation, it can be safely assumed that resolution of periodontal inflammation by periodontal treatment decreases plasma ROS levels and, thereby, reduces oxidative stress.
In Group II patients, non-surgical periodontal treatment resulted in reduction of mean plasma ROM level at 1 and 2 months after therapy. Similar results were shown after 1 month by D’Aiuto et al.[16] and after 1 and 2 months by Tamaki et al.[15] But Tamaki et al. observed that 2 months post non-surgical periodontal therapy, the plasma ROM levels were similar to those found in control subjects. In our study, although there was a significant decrease in ROM levels after 2 months, the levels did not match those of the controls. Thus, it is plausible that non-surgical periodontal treatment alone was not adequate in completely reducing the periodontal inflammation and, thereby, the oxidative stress. This may be attributed to the persistence of pocket depth following SRP alone and the inability of the patients to maintain these areas, leading to incomplete resolution of periodontal inflammation and disease activity.
In Group III patients, non-surgical periodontal treatment was followed by surgical pocket therapy in areas of persisting pocket depth. The primary purpose of raising a periodontal flap is for the operator to get better accessibility and visibility, which further allows for a more thorough root surface debridement. Our results show that surgical periodontal therapy was able to reduce the clinical parameters more effectively than when only non-surgical periodontal treatment was done, indicating that in moderate to advanced CP patients, surgical periodontal treatment leads to better treatment end point. Interestingly, the plasma ROM levels after 2 months of surgical periodontal therapy matched those found in control subjects. Also, Group III showed statistically significant decrease in plasma ROM levels when compared to Group II both after 1 and 2 months. Therefore, it is reasonable to contemplate that surgical periodontal treatment was more efficient in decreasing the periodontal inflammation, and by doing so, it reduces the blood ROS levels more successfully. It is also expected that surgical periodontal therapy, by decreasing the periodontal oxidative stress, will be more effective in preventing ROS-mediated future tissue destruction.
Therefore, this study provides ample evidence that periodontitis, a common potential source of low-grade inflammation, can lead to a systemic oxidative stress state. The excessive circulating ROS thus produced can oxidize DNA, lipids, and proteins, thereby contributing to tissue damage. Animal studies have already revealed that periodontal inflammation induces oxidative tissue damage in the aorta and liver with increasing serum ROS.[28,29] These results may well be extrapolated to humans, and so, it can be said that periodontitis-induced high oxidative stress can possibly lead to a similar tissue damage. That being said, it is conceivable that the reduction of circulating ROS by periodontal treatment offers clinical benefits in terms of reducing the risk for future systemic disease.
During the experimental period, changes in plasma ROM level associated with generic factors such as hygiene, diet, or exercise were assumed to be comparable between the treatment and control groups, which was a limitation of the study.
CONCLUSIONS
To conclude, our study shows that CP patients showed higher levels of plasma ROM than periodontally healthy controls, and there exists a positive correlation between plasma ROM and clinical parameters at baseline like PD, CAL, and SBI. Also, surgical periodontal therapy was more effective in reducing the periodontal inflammation than non-surgical periodontal treatment alone and, thus, may offer additional benefits in the reduction of systemic oxidative stress. Further longitudinal studies of larger stratified populations will be required to validate the impact of periodontal disease and treatment on systemic oxidative stress.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Page RC, Kornman KS. The pathogenesis of human periodontitis: An introduction. Periodontol 2000. 1997;14:9–11. doi: 10.1111/j.1600-0757.1997.tb00189.x. [DOI] [PubMed] [Google Scholar]
- 2.Locker D, Slade GD, Murray H. Epidemiology of periodontal disease among older adults: A review. Periodontol 2000. 1998;16:16–33. doi: 10.1111/j.1600-0757.1998.tb00113.x. [DOI] [PubMed] [Google Scholar]
- 3.Sies H. New York: Academic Press; 1991. Oxidative stress: Oxidants and antioxidants. [DOI] [PubMed] [Google Scholar]
- 4.Lamster IB, Novak MJ. Host mediators in gingival crevicular fluid: Implications for the pathogenesis of periodontal disease. Crit Rev Oral Biol Med. 1992;3:31–60. doi: 10.1177/10454411920030010501. [DOI] [PubMed] [Google Scholar]
- 5.Chapple IL, Matthews JB. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol 2000. 2007;43:160–232. doi: 10.1111/j.1600-0757.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 6.Ingarro LJ. Signal transduction mechanisms involving nitric oxide. Biochem Pharmacol. 1991;41:485–90. doi: 10.1016/0006-2952(91)90618-f. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert P, Tramini P, Sieso V, Piva MT. Alkaline phosphatase isozyme activity in serum from patients with chronic periodontitis. J Periodont Res. 2003;38:362–5. doi: 10.1034/j.1600-0765.2003.00388.x. [DOI] [PubMed] [Google Scholar]
- 8.Wei PF, Ho KY, Ho YP, Wu YM, Yang YH, Tsai CC. The investigation of glutathione peroxidase, lactoferrin, myeloperoxidase and interleukin-1beta in gingival crevicular fluid: Implications for oxidative stress in human periodontal diseases. J Periodontal Res. 2004;39:287–93. doi: 10.1111/j.1600-0765.2004.00744.x. [DOI] [PubMed] [Google Scholar]
- 9.Akalin FA, Baltacioglu E, Alver A, Karabulut E. Lipid peroxidation levels and total oxidant status in serum, saliva and gingival crevicular fluid in patients with chronic periodontitis. J Clin Periodontol. 2007;34:558–65. doi: 10.1111/j.1600-051X.2007.01091.x. [DOI] [PubMed] [Google Scholar]
- 10.Baltacioglu E, Akalýn FA, Alver A, Deger O, Karabulut E. Protein carbonyl levels in serum and gingival crevicular fluid in patients with chronic periodontitis. Arch Oral Biol. 2008;53:716–22. doi: 10.1016/j.archoralbio.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 11.McCord JM. The evolution of free radicals and oxidative stress. Am J Med. 2000;108:652–9. doi: 10.1016/s0002-9343(00)00412-5. [DOI] [PubMed] [Google Scholar]
- 12.Iamele L, Fiocchi R, Vernocchi A. Evaluation of an automated spectrophotometric assay for reactive oxygen metabolites in serum. Clin Chem Lab Med. 2002;40:673–6. doi: 10.1515/CCLM.2002.115. [DOI] [PubMed] [Google Scholar]
- 13.Christou K, Markoulis N, Moulas AN, Pastaka C, Gourgoulianis KI. Reactive oxygen metabolites (ROMs) as an index of oxidative stress in obstructive sleep apnea patients. Sleep Breath. 2003;7:105–10. doi: 10.1007/s11325-003-0105-9. [DOI] [PubMed] [Google Scholar]
- 14.Komatsu F, Kagawa Y, Sakuma M, Kawabata T, Kaneko Y, Otgontuya D, et al. Investigation of oxidative stress and dietary habits in Mongolian people, compared to Japanese people. Nutr Metab (Lond) 2006;3:21. doi: 10.1186/1743-7075-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamaki N, Tomofuji T, Ekuni D, Yamanaka R, Yamamoto T, Morita M. Short-term effects of non-surgical periodontal treatment on plasma level of reactive oxygen metabolites in patients with chronic periodontitis. J Periodontol. 2009;80:901–6. doi: 10.1902/jop.2009.080640. [DOI] [PubMed] [Google Scholar]
- 16.D’Aiuto F, Nibali L, Parkar M, Patel K, Suvan J, Donos N. Oxidative stress, systemic inflammation, and severe periodontitis. Jour Dent Res. 2010;89:1241–6. doi: 10.1177/0022034510375830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heitz-Mayfield LJ, Trombelli L, Heitz F, Needleman I, Moles D. A systematic review of the effect of surgical debridement vs non-surgical debridement for the treatment of chronic periodontitis. J Clin Periodontol. 2002;29(Suppl 3):92–102. doi: 10.1034/j.1600-051x.29.s3.5.x. [DOI] [PubMed] [Google Scholar]
- 18.Alpagot T, Wolff LF, Smith QT, Tran SD. Risk indicators for periodontal disease in a racially diverse urban population. J Clin Periodontol. 1996;23:982–8. doi: 10.1111/j.1600-051x.1996.tb00524.x. [DOI] [PubMed] [Google Scholar]
- 19.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 20.Loe H, Silness J. Periodontal disease in pregnancy I. Prevalence and Severity. Acta Odont Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 21.Silness J, Loe H. Periodontal disease in pregnancy II. Correlation between oral hygiene and periodontal conditions. Acta Odont Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 22.Muhlemann HR, Son S. Gingival sulcus bleeding: A leading symptom in initial gingivitis. Helv Odontol Acta. 1971;15:107–13. [PubMed] [Google Scholar]
- 23.Tamaki N, Tomofuji T, Maruyama T, Ekuni D, Yamanaka R, Takeuchi N, et al. Relationship between periodontal condition and plasma reactive oxygen metabolites in patients in the maintenance phase of periodontal treatment. J Periodontol. 2008;79:2136–42. doi: 10.1902/jop.2008.080082. [DOI] [PubMed] [Google Scholar]
- 24.Wei D, Zhang XL, Wang YZ, Yang CX, Chen G. Lipid peroxidation levels, total oxidant status and superoxide dismutase in serum, saliva and gingival crevicular fluid in chronic periodontitis patients before and after periodontal therapy. Aust Dent J. 2010;55:70–8. doi: 10.1111/j.1834-7819.2009.01123.x. [DOI] [PubMed] [Google Scholar]
- 25.Ryan ME. Clinical attachment level change as an outcome measure for therapies that slow the progression of periodontal disease. J Int Acad Periodontol. 2005;7:162–71. [PubMed] [Google Scholar]
- 26.Greenstein G. The role of bleeding upon probing in the diagnosis of periodontal disease. A literature review. J Periodontol. 1984;55:684–8. doi: 10.1902/jop.1984.55.12.684. [DOI] [PubMed] [Google Scholar]
- 27.Klock KS, Gjerdet NR, Haugejorden O. Periodontal attachment loss assessed by linear and area measurements in vitro. J Clin Periodontol. 1993;20:443–7. doi: 10.1111/j.1600-051x.1993.tb00386.x. [DOI] [PubMed] [Google Scholar]
- 28.Ekuni D, Tomofuji T, Sanbe T, Irie K, Azuma T, Maruyama T, et al. Periodontitis-induced lipid peroxidation in rat descending aorta is involved in the initiation of atherosclerosis. J Periodontal Res. 2009;44:434–42. doi: 10.1111/j.1600-0765.2008.01122.x. [DOI] [PubMed] [Google Scholar]
- 29.Tomofuji T, Ekuni D, Yamanaka R, Kusano H, Azuma T, Sanbe T, et al. Chronic administration of lipopolysaccharide and proteases induces periodontal inflammation and hepatic steatosis in rats. J Periodontol. 2007;78:1999–2000. doi: 10.1902/jop.2007.070056. [DOI] [PubMed] [Google Scholar]


