Abstract
Background:
Local drug delivery agents can effectively deliver the antimicrobial drugs in bactericidal concentration, and have shown improved clinical outcomes when used as an adjunct to mechanical therapy. The aim of this study was to evaluate the efficacy of a xanthan-based chlorhexidine gel versus herbal extracts’ gel as an adjunct to periodontal therapy in the treatment of chronic periodontitis.
Materials and Methods:
A total of 150 sites, age group of 30-50 years, periodontal pockets measuring 5-8 mm and diagnosed with chronic periodontitis were selected for the study. The selected sites were randomized in five groups: Scaling and root planing (SRP) alone (Group A), SRP + Chlosite gel (Group B), SRP + Herbal gel (Group C), Chlosite gel alone (Group D) and Herbal gel alone (Group E). Clinical parameters such as Plaque Index, Gingival Index, probing pocket depth and clinical attachment level were recorded at baseline and 1- and 3-month intervals.
Results:
After 3 months, there were statistically significant reductions in all the clinical parameters for Groups B and C compared with Group A. There was no significant reduction in all clinical parameters between Group D and E where no mechanical therapy was performed.
Conclusion:
The results indicate that the local application of herbal gel can be comparably used as chlorhexidine gel in the treatment of chronic periodontitis as an adjunct to mechanical periodontal therapy.
Keywords: Antimicrobial agents, chlorhexidine gel, herbal gel, local drug delivery, periodontitis
INTRODUCTION
Periodontitis is a chronic inflammatory disease, and the primary etiologic factor is a subset of specific bacteria from the oral flora. The microbial flora induce an immune response within the connective tissue of the gingiva that result in prolonged release of inflammatory mediators that disrupt tissue homeostasis and lead to attachment loss.[1] The successful management of destructive periodontal diseases may require the administration of antimicrobial agents as an adjunct to conventional periodontal therapy.[2] Local drug delivery systems are designed to deliver the antimicrobial agents in periodontal pockets to treat periodontal infections and to compensate for the limitation of the mechanical and surgical therapy, further preventing early microbial recolonization.[3,4,5] Herbal products are safe in comparison with the synthetics for human use. The adjunctive use of herbal products has been successfully used in effective treatment of periodontal diseases.[6] Various herbs that have a significant role in dentistry are Azarachtica indica (Neem), Mimusops elengi (Bakul), Acacia arabica (Babul), Anacylus pyrethrum D.C. (Akarkara), etc.[7]
The present study was undertaken to evaluate the efficacy of a xanthan-based chlorhexidine gel (Chlosite) versus a herbal extracts’ gel (formulation of three medicinal plants - bark of Mimusops elengi (Bakul), bark of Acacia arabica (Babul) and extract of Punica granatum (Pomegranate) - as an adjunct to periodontal therapy in subjects with chronic periodontitis.
MATERIALS AND METHODS
A randomized, controlled, split mouth clinical study was conducted, and the inclusion criteria for patient selection were the age group of 30-50 years. Patients diagnosed with chronic periodontitis with at least three nonadjacent interproximal sites with 4-8 mm of probing pocket depth (PPD) with no contraindication to periodontal therapy were included in the study. Patients were required to not have received any periodontal and antibiotic therapy in the last 6 months and were to be co-operative and committed to maintain oral hygiene. Patients who were smokers, tobacco and alcohol abusers, with any systemic illness (hypertension, diabetes, etc.), pregnant and lactating females, using antimicrobial mouthwash in the past 2 months and those requiring periodontal surgery were excluded from the study.
A total of 150 sites from 30 patients were selected for the study, in which 10 patients had undergone orthodontic treatment and suffered from periodontal problems. In a total of 15 patients, three sites in each patient were identified for the study in which mechanical therapy was performed. In another 15 patients, two sites in each patient were identified for the study in which no mechanical therapy was performed. The duration of the study was 3 months. A duly signed informed consent was obtained from the patients. On the screening day, patient evaluation was followed by impressions for the fabrication of acrylic stents required for the measurement of pocket depths and level of attachment in the control and test sites during the study period.[8]
After being assessed for eligibility and periodontal status, study subjects (selected sites) were assigned randomly to the five groups selected for the study. The five treatment groups were Control Group (A): Scaling and root planing alone (SRP), Test Group (B): SRP with Chlosite gel application, Test Group (C): SRP with Herbal gel application, Test Group (D): Chlosite gel application with no SRP and Test Group (E): Herbal gel application with no SRP.
The following clinical parameters were recorded: Plaque Index (PI),[9] Gingival Index (GI),[10] PPD and clinical attachment level (CAL) using the UNC-15 probe. All the clinical parameters were recorded at regular intervals, i.e. at baseline and at 1 and 3 months. Periodic recall was performed every 10 days for reapplication of the respective gels into the pockets and the parameters were recorded after three applications, i.e. at 1 month.
The materials used in the study were the Chlosite gel (Chlorhexidine gel) and the herbal gel, which was prepared using extracts of medicinal plants selected for the study. Chlosite is a local drug delivery system (GHIMAS, Italy). It contains chlorhexidine in xanthan gel. The gel is a unique combination of two formulations: Chlorhexidine digluconate 0.5% and chlorhexidine dihydrochloride 1%.[11,12] The medicinal plants selected for the herbal gel formulation used in the present study are the bark of Mimusops elengi (Bakul), bark of Acacia arabica (Babul) and seeds of Punica granatum (pomegranate).
The Carbopol-based herbal gel was formulated using the bark of Mimusops elengi, Acacia arabica and Punica granatum, which were dried in an oven at 40°C for about 24 h and the specimens were ground. The ground material was washed with water four times (500 mL each) after every 8 h and was filtered and concentrated in a rotary vacuum evaporator at 100 mmHg at a temperature of 50°C. The concentrated extract was freeze-dried in freeze dryer and then dried completely in a lyophilizer. The dry extract was weighed and dissolved in the required amount of distilled water to prepare concentrated 50% hydroalcoholic solution and was subjected to chromatographic analysis. This procedure was performed at the National Botanical Research Institute, Lucknow.
The chromatographic profile of the extracts of the medicinal plants using high profile thin layer chromatography (HPTLC) was performed using precoated thin layer chromatography (TLC) plates (Aluchrosep silica gel 60 UV 254) of 0.2-mm thickness that were activated by heating them for 30 min at 100°C. The extracts of the genuine and the market samples were used for comparative the HPTLC analysis. A known quantity of tests (10 mg/mL) and standard (1 mg/mL) was applied on the TLC plate. Rectangular glass chambers or twin trough chambers were used for TLC development. The developed plates were observed under UV light at 254 nm and 366 nm (CAMAG Reprostar 3) and the data were transferred and analyzed by the computer.[13]
The microbiological evaluation of the herbal extracts for their antimicrobial activity and the minimum inhibitory concentration (MIC) of each extract was evaluated. Nutrient broth was prepared and the plaque sample was collected from the periodontal pocket with paper points that was suspended in a screw-capped vial containing nutrient broth. The collected microbes were cultured by inoculating them into fresh nutrient broth medium and incubated at 37°C for 30 h in the lab. This resulted in bacterial growth (turbidity) showing sufficient microbial growth. The nutrient broth containing microorganisms was then inoculated in different concentrations of herbal extracts (1-10%) contained in separate test tubes. It was further incubated at 37°C for 24-48 h. The solution showing maximum antimicrobial activity was confirmed by no growth of microbes and was subsequently selected as the optimum concentration for the formulation of the herbal gel.[14] The following concentrations of the herbal crude extract were added in the Carbopol 974-0.75% w/v. The aqueous extract of Mimusops elengi – 8% w/v, aqueous extract of Acacia arabica - 7% w/v and aqueous extract of Punica granatum (pomegranate) - 8% w/v were also added. Distilled water was further added to make the quantity of the gel to 100 mL. The dispersion so obtained was then mechanically stirred at room temperature for 1 h. 1 M NaOH was added to neutralize the sample to pH 7.4 and the gel was then allowed to equilibrate for at least 16 h at room temperature. It was kept in the refrigerator at 25°C so as to maintain the activity and the stability of the gel.
Zones of inhibition were measured for Chlosite and herbal and carbopol gel. Chlorhexidine gel (Chlosite) and herbal gel was accurately measured (30 μL) and taken as the sample. Plain gel (30 μL) was taken as the control, which contained neither chlorhexidine nor the herbal formulation, to ensure that the gel base did not have any antimicrobial activity of its own. One hundred microliters of the microbial suspension was added in liquid agar nutrient medium, which was then poured into Petri plates and allowed to cool till solidification. Wells were made in the solidified nutrient agar medium containing microbes. The formulations of herbal gel, Chlosite gel and plain gel were added in the marked wells. Petri plates were incubated for 24-48 h at 37°C. Zones of inhibition were observed and measured for Chlosite gel and herbal and plain gel. For both the Chlosite and the herbal gel, the diameters of the inhibition zone were 4.09 ± 0.73 mm and 3.89 ± 1.27 mm, respectively, after 30 h, showing the comparable antimicrobial activity against the microbes [Figure 1]. A clear zone was observed for the plain gel containing neither chlorhexidine nor herbal extracts, showing that the gel base has no pharmacological activity of its own.[15,16]
Figure 1.
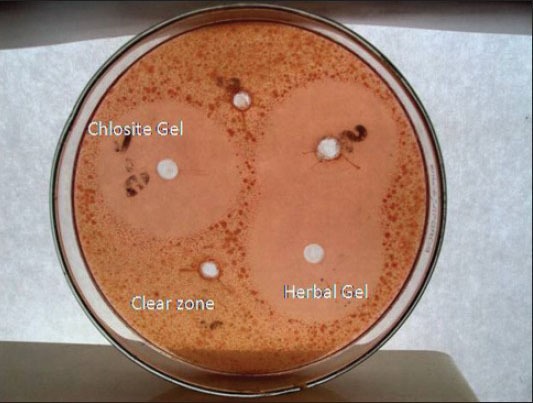
Zones of inhibition of Chlosite, herbal and plain gels (clear zone)
SRP was performed on selected sites using ultrasonic and hand instruments. After thorough SRP, the pocket was irrigated with normal saline and then dried with paper points. Chlorhexidine (Chlosite) and herbal gel were applied directly from the syringe into the pocket. It should be ensured that a slight amount of gel is extruded to confirm the proper placement of gel in the pocket. Coe Pak was placed to ensure that the gel remains in the site for the required period.[17] Patients were instructed to carry out normal oral hygiene procedures and not to use any mouth rinses, floss or oral irrigation devices and were asked to report immediately if pain, swelling or any other problem occurred.
RESULTS
Data analysis
The baseline and 1- and 3-month values were compared for changes that occurred over time, i.e. changes in PI, GI, PPD reduction and CAL. The Student t-test was used to evaluate and establish differences between baseline and 1- and 3-month values.
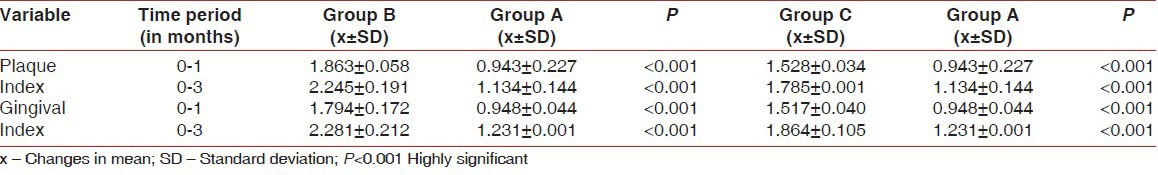
The mean changes in PI and GI between Group B and C with Group A are given in Table 1. All the patients showed statistically significant improvements in both the clinical parameters with the baseline levels.
Table 1.
Comparison of plaque index and gingival index from baseline to 3 months between Groups B and C (test groups) and Group A (control group)
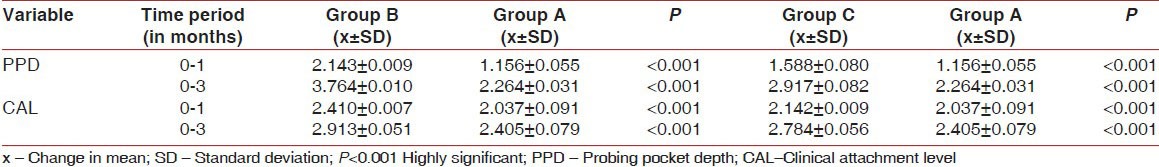
The mean changes in PPD and CAL gain between Group B and C with Group A are given in Table 2.
Table 2.
Comparison of probing pocket depth and clinical attachment level gain from baseline to 3 months between Groups B and C (test groups) and Group A (control group)
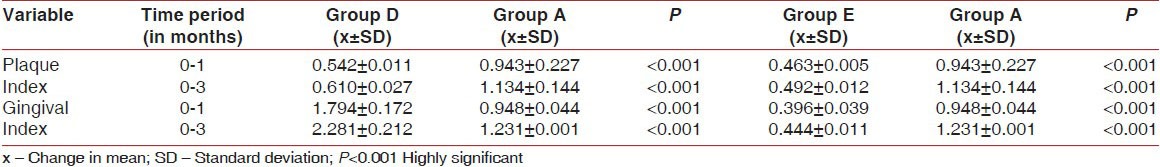
The mean changes in PI and GI between Group D and E with Group A are given in Table 3.
Table 3.
Comparison of plaque index and gingival index from baseline to 3 months between Groups D and E (test groups) with Group A (control group)
The mean changes in PPD and CAL gain between Group D and E with Group A are given in Table 4.
Table 4.
Comparison of probing pocket depth and clinical attachment level gain from baseline to 3 months between Groups D and E (test groups) with Group A (control group)
DISCUSSION
Local administrations of antimicrobial agents are commonly preferred as they provide a higher dosage of the drug in the periodontal pocket therefore decreasing its unfavorable systemic side-effects.[18] Chlosite (Ghimas Company, Italy), a xanthan-based 1.5% chlorhexidine gel, when locally delivered in the pocket allows higher concentration of the drug in gingival crevicular fluid as compared with that of mouthwash. Xanthan is a naturally occurring, biocompatible, saccharidic polymer and its cross-linkage controls the release of drug.[19] When in contact with water, it forms a three-dimensional pseudoplastic reticulum, which is capable of holding and maintaining various substances in suspension.[20] Swelling-controlled erosional process allows for sustained release of the drug. It also undergoes a progressive process of imbibition and is absorbed from the pocket within 10-30 days of injection. It contains a combination of two formulations: Chlorhexidine digluconate - 0.5% (fast releasing) and Chlorhexidine dihydrochloride - 1% (slow releasing).[21] Chlorhexidine digluconate is liberated on the first day and achieves a concentration greater than 100 ug/mL. This concentration is maintained for an average of 6-9 days, and is much more than the MIC for chlorhexidine (0.10 ug/mL). Microorganisms of high susceptibility to chlorhexidine include P. gingivalis, P. intermedia, F. nucleatum, C. rectus, T. forsythia, H. aphrophilus, etc.[22] The limited life span of antimicrobials due to resistance because of indiscriminate use necessitates the continuous search for alternatives. Nature has been a source of medicinal plants for thousands of years, and a number of modern drugs have been isolated from natural sources.[23,24] Therefore, the effectiveness of herbs, i.e. Mimusops elengi and Acacia arabica, against periodontal diseases was assessed. The extract of the bark of Mimusops elengi is used for treating periodontal diseases. Lupeol, saponin and tannin are the pharmacologically active agents isolated from the bark of the plant. Lupeol present in the bark has an anti-inflammatory activity and tannins have antibacterial and antioxidant properties.[25,26]
The bark constituents of Acacia arabica are used to treat stomatitis and gingival bleeding. Its bark contains tannin (24-42%), which has analgesic, anti-inflammatory, astringent and immunoregulatory properties. There are cyanogenic glycosides in addition to several enzymes such as oxidases, peroxidases and pectinases that have shown to exhibit antimicrobial properties. The antibacterial activity of Acacia arabica was assessed against strains of Aggregatibacter actinomycetemcomitans, Capnocytophaga, Porphyromonas gingivalis, Prevotella intermedia and Treponema denticola.[15,26] Pomegranate is currently finding important applications in the field of dental health. Clinical studies have shown that pomegranate-containing mouthwashes may fight dental plaque and tartar formation by inhibiting the activities of microorganisms that cause plaque by suppressing their ability to adhere to the surface of the tooth. Pomegranate's active components, including polyphenolic flavanoids (e.g., punicalagins and ellagic acid), are believed to prevent gingivitis through a number of mechanisms including reduction of oxidative stress in the oral cavity, antioxidant activity, anti-inflammatory effects and anti-bacterial effects; pomegranate rinsing also lowered the activity of alfa glucuronidase, an enzyme that breaks down sucrose while it increased the activities of ceruloplasmin, an antioxidant enzyme.[27] Formulations are designed for the application to flow into the pocket for the purpose they are incorporated with rheological properties that assist retention at the site of application.[28,29]
It is also reported in studies that lupeol is one of the major pharmacologically active ingredients in Mimusops elengi. It has anti-inflammatory and antimicrobial properties.[30,31] Therefore, to assess the presence of lupeol in the collected samples of Mimusops elengi, HPTLC was performed by using lupeol as a standardized chemical marker. The fingerprint profiles of lupeol and Mimusops elengi were observed at 254 nm and 366 nm, and the spots were detected at the same level [Figure 2], signifying the presence of lupeol in the Mimusops elengi sample selected for the study. Flavanoids and tannins are the active components of Punica granatum (pomegranate), which have anti-microbial and antioxidant properties. Therefore, to assess the presence of tannins in the collected samples of Punica granatum (pomegranate), HPTLC was performed using tannins as the standardized chemical marker shown in Figure 3.
Figure 2.
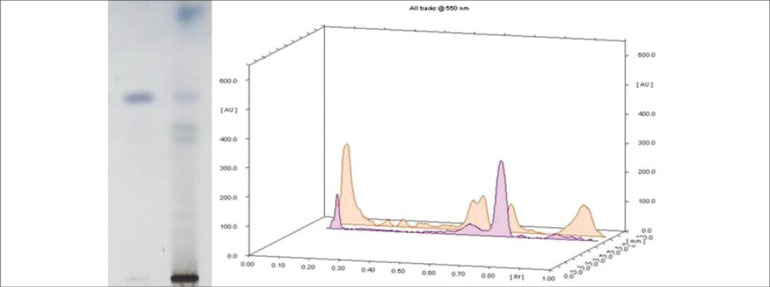
Chromatographic analysis of Mimusops elengi and lupeol as standard chemical marker
Figure 3.
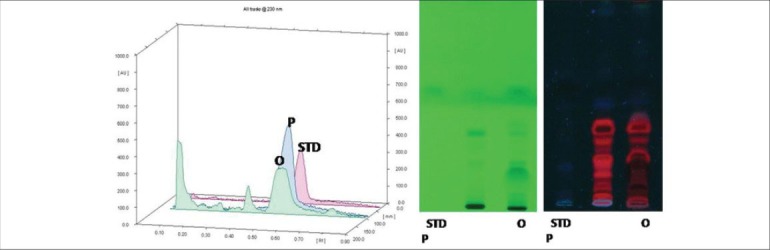
Chromatographic analysis of Acacia arabica (P) and Punica granatum (O) with tannin as standard chemical marker (STD)
CONCLUSION
From the results of the study, the conclusion drawn was that test sites where Chlosite gel and herbal gel were employed displayed a statistically significant reduction in all the clinical parameters (PI, GI, PPD and CAL) after treatment as compared with control sites that showed minimal changes in clinical parameters with SRP alone.
Results indicate that the local application of herbal gel can be comparably used as chlorhexidine gel (Chlosite) in the treatment of chronic periodontitis as an adjunct to mechanical periodontal therapy and can produce significant clinical benefits when compared with SRP alone.
ACKNOWLEDGEMENT
The authors would like to thank the National Botanical Research Institute, Lucknow, India, for the chromatographic analysis used in the study. They would also like to thank the Central Institute of Medicinal and Aromatic Sciences, Lucknow, for their assistance in making the study run as smoothly as possible. The authors report no conflicts of interest related to this study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Jorgensen MG, Slots J. The ins and outs of periodontal antimicrobial therapy. J Calif Dent Assoc. 2002;30:297–305. [PubMed] [Google Scholar]
- 2.Jeffcoat AK, Bray KS, Ciancio SG, Dentino AR, Fine DH, Gordon JM, et al. Adjunctive use of a subgingival controlled release chlorhexidine chip reduces probing depth and improves attachment level compared with scaling and root planing alone. J Periodontal. 1998;69:989–97. doi: 10.1902/jop.1998.69.9.989. [DOI] [PubMed] [Google Scholar]
- 3.Galgut P. Periodontal diseases- New ways of managing these conditions. Dentist. 2007;6:12–4. [Google Scholar]
- 4.Shah N, Bhavsar N, Jathal B. Local delivery of antimicrobial agents in periodontal therapy. JISP Journal of Indian Society of Periodontology. 1999;2:26–9. [Google Scholar]
- 5.Rams TE, Slots J. Local drug delivery of antimicrobial agents in periodontal pockets. Periodontol2000. 1996;10:139–59. doi: 10.1111/j.1600-0757.1996.tb00072.x. [DOI] [PubMed] [Google Scholar]
- 6.Joy PP, Thomas J, Mathew S, Skaria B.P. Medicinal Plants. In: Bose TK, Kabir J, Das P, Joy PP, editors. Tropical Horticulture. Vol. 2. Calcutta: Naya Prokash; 2001. pp. 450–4. [Google Scholar]
- 7.Akhtar N, Ali M, Alam MS. Herbal drugs used in dental care. Pharma Rev. 2005;10:43–6. [Google Scholar]
- 8.Lekovic V, Camargo PH, Weinlaender M, Nedic M, Aleksic Z, Kenney EB. Comparison between enamel matrix protein used alone or in combination with porous bone mineral in treatment of intrabony periodontal defects in humans. J Periodontol. 2000;71:1110–6. doi: 10.1902/jop.2000.71.7.1110. [DOI] [PubMed] [Google Scholar]
- 9.Silness P, Loe H. Periodontal disease in pregnancy. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 10.Loe H, Silness J. Periodontal disease in pregnancy I Prevalence and Severity. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 11.Maurstad G, Stokke BT. Metastable and stable states of xanthan polyelectrolyte complexes studies by atomic force microscopy. Biopolymers. 2004;74:199–213. doi: 10.1002/bip.20073. [DOI] [PubMed] [Google Scholar]
- 12.Italy: Manufactured by GHIMAS; 2000. Literature on Chlosite. Xanthan gel with chlorhexidine for periodontal and peri-implant infectious diseases. [Google Scholar]
- 13.Akadémiai Kiadó AZ. Quantitative HPTLC analysis of the eugenol content of leaf powder and capsule formulation of Ocimum sanctum. J Plant Chromatogr. 2007;20:135–8. [Google Scholar]
- 14.Andrews JM. Determination of minimum inhibitory concentration. Journal of Antimicrobial Chemotherapy. 2001;48(Suppl):5–16. doi: 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- 15.Durre S, Muhammad AR. In vitro antibacterial activity of extracts of mimusops elengi against gram positive and gram negative bacteria. African Journal of microbiology research. 2009;3:458–62. [Google Scholar]
- 16.Mohmmadreza A, Bahram I, Ghassem A, Gita E, Alireza AB, Mahdi A. The effect of locally delivered xanthan based chlosite gel with scaling and root planning in the treatment of chronic periodontitis: Microbial findings. J Dental Research. 2008;5:30–3. [Google Scholar]
- 17.Abrishami M, Iramloo B, Ansari G, Eslami G, Akbarzadeh BaghebanA, Anaraki M. The effect of Locally Delivered Xanthan-Based Chlosite gel with scaling and Root Planing in the treatment of chronic Periodontitis: Microbial Findings. Dent Res J. 2008;5:47–52. [Google Scholar]
- 18.Stabholz A, Sela WN, Friedman M, Golomb G, Soskolne A. Clinical and microbiological effects of sustained release chlorhexidine in periodontal pockets. J Clin Periodontal. 1986;13:783–8. doi: 10.1111/j.1600-051x.1986.tb00882.x. [DOI] [PubMed] [Google Scholar]
- 19.Maurstad G, Stokke BT. Metastable and stable states of xanthan polyelectrolyte complexes studies by atomic force microscopy. Biopolymers. 2004;74:199–213. doi: 10.1002/bip.20073. [DOI] [PubMed] [Google Scholar]
- 20.Zeng WM. Oral controlled release formulation for highly water soluble drugs: Drug-sodium alginate xanthan gum-zinc acetate matrix. Drug Dev Ind Pharm. 2004;30:491–5. doi: 10.1081/ddc-120037479. [DOI] [PubMed] [Google Scholar]
- 21.Andreopoulos AG, Tarantili PA. Xanthan gum as a carrier for controlled release of drugs. J Biomater Appl. 2001;16:34–46. doi: 10.1106/XBFG-FYFX-9TW9-M83U. [DOI] [PubMed] [Google Scholar]
- 22.Pratten J, Barnett P, Wilson M. Composition and susceptibility to chlorhexidine of multispecies biofilms of oral bacteria. Appl Environ Microbiol. 1998;64:3515–9. doi: 10.1128/aem.64.9.3515-3519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abbas AM, Abdul MM. An evaluation of antimicrobial activities of Mimusops elengi Linn. Res J Agric Biol Sci. 2008;6:871–4. [Google Scholar]
- 24.Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–82. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rangama B, Panagoda GJ. Antibiotic Activity of Tephrosia purpurea (Fabaceae) and Mimusops elengi (Sapotaceae) against some clinical bacterial isolates. Peradeniya Univ Res Sess. 2007;12:132–54. [Google Scholar]
- 26.Gazi MI. The finding of antiplaque features in Acacia arabica type of chewing gum. J Clin Periodontol. 1991;18:75–7. doi: 10.1111/j.1600-051x.1991.tb01123.x. [DOI] [PubMed] [Google Scholar]
- 27.Menezes SM, Cordeiro LN, Viana GS. Punica granatum (pomegranate) extract is active against dental plaque. J Herb Pharmacother. 2006;6:79–92. [PubMed] [Google Scholar]
- 28.Jones DS, Woolfson AD, Brown AF, O’Neil MJ. Mucoadhesive syringeable drug delivery systems for controlled application of metronidazole to the periodontal pocket in vitro release kinetic syringeability, mechanical and mucoadhesive properties. J Control Rel. 1997;49:71–9. [Google Scholar]
- 29.Sahani RV. Development and Characterization of some herbodental formulations for periodontitis. Int Online Pharma J. 2002;2:342–34. [Google Scholar]
- 30.New Delhi, India: Council of Scientific and Industrial Research (CSIR); 1956. Glossary of Indian Medicinal Plants. National Institute of Science and Communication Information Resources; p. 179. [Google Scholar]
- 31.2nd English ed. New Delhi: Controller of Publications; 2003. Ayurvedic pharmacopoeia committee. The Ayurvedic Formulary of India, Part I 29-31. [Google Scholar]


