Abstract
Objectives
Mental fatigability refers to the failure to sustain participation in tasks requiring mental effort. Older adults with vascular risk are at particular risk for experiencing mental fatigability. The present study (1) tested a new way of measuring objective mental fatigability by examining its association with perceived mental fatigability; and (2) identified psychological, physiological, and situational factors that would be associated with mental fatigability.
Methods
A cross-sectional study was conducted with 49 community-dwelling participants aged 75+ years with vascular risk. A 20-minute fatigability-manipulation task was used to induce mental fatigability and develop objective and perceived mental fatigability measures. Objective fatigability was calculated by the change of reaction time over the course of the task. Perceived fatigability was calculated by the change of fatigue self-reported before and after the task. A set of potential psychological, physiological, and situational predictors were measured.
Results
There was a significant increase in reaction time and self-reported fatigue to the fatigability manipulation task, indicating occurrence of objective and perceived mental fatigability. Reaction time and self-reported fatigue were moderately, but significantly correlated. Higher levels of executive control and having a history of more frequently engaging in mental activities were associated with lower objective mental fatigability. None of the examined factors were associated with perceived mental fatigability.
Conclusion
Objective and perceived mental fatigability were sensitive to our fatigability-manipulation task. While these two measures were correlated, they were not associated with the same factors. These findings need to be validated in a large study with a more heterogeneous sample and a greater variety of fatigability-manipulation tasks.
Keywords: Mental fatigability, executive control, history of mental activities, reaction time
Introduction
Fatigue is one of the most common complaints in community-dwelling older adults [1]. Its effects include a diminished capacity to maintain activities of daily living, and reduced participation in leisure activities that protect cognitive, physical, and psychosocial well-being [2-4]. Fatigue is a multidimensional concept that can be experienced as general tiredness (i.e., trait, chronic subjective fatigue) or as the expectation and experience of becoming tired in response to activities, which then leads to difficulty in maintaining these activities at a desired level of performance (i.e., acute state, fatigability) [5-8]. Perhaps surprisingly, older adults may not necessarily report more fatigue (i.e., chronic subjective fatigue) than their younger counterparts; however, they do demonstrate a higher likelihood of becoming tired or tiring faster during an activity (i.e., fatigability) [9]. The factors underlying why older adults experience greater functional effects of fatigue are still unclear, but is important to note that chronic subjective fatigue and fatigability are not necessarily correlated. An older adult may complain that he is “tired all the time,” but still lead an active life and have near-normal functional capacity while another person who has the same complaint of chronic fatigue may live in a physically and mentally restricted manner and be functionally impaired. These differences may be explained by the variability in an individual's fatigability [10]. Therefore, recent work has begun to focus on fatigability in order to answer the question, “When is fatigue a problem?” A comprehensive understanding of fatigability may help us understand when and how fatigue symptoms translate to poor function in old age.
Mental fatigability, the failure to remain engaged in tasks or activities requiring sustained mental efforts, is problematic but rarely recognized by the medical community [11-13]. Neurologically, mental fatigability reflects dysfunctional cerebral activity in the basal ganglia, involving contributions from the frontal regions (including the prefrontal cortex (PFC) and the anterior cingulate cortex), thalamus, and the amygdala [14]. Behaviorally, mental fatigability may not only affect mental activities (e.g., motivation, action control) [15], but also the level of physical activities engaged in by an individual [16, 17].
The most common approach to measuring mental fatigability is via self-report, but numerous issues (e.g., construct contamination, see discussion by Leavitt [18]) may affect the utility of such measures. There is a need for complementary objective measurement that would provide an estimation of fatigability that is free of the issues present in self-report measures. Physical fatigability is measured by performance during a physical task that requires sustained energy (i.e., assessing decreased muscle movements over time) [7, 13]. Previous studies of mental fatigability have attempted to directly apply this approach to mental fatigability by measuring declined accuracy during a cognitive task. Unfortunately, results of these attempts have revealed inconsistent associations between self-report mental fatigability and performance during cognitive tasks [18]. Education may greatly compensate for mental fatigability meaning one's accuracy in the cognitive tasks may not necessarily decline over time even though they have become fatigued. Other dimensions of cognitive performance may be more sensitive to the effects of mental fatigability. Two studies in patients with multiple sclerosis identified a significant and consistent relationship between self-report fatigability and speed of processing assessed by cognitive tests requiring sustained mental efforts [19, 20]. Another recent study found accuracy rate in cognitive tasks was only significantly correlated to self-reported mental fatigability in the group whose reaction time (RT) increased across the executive-attention demanding mental tasks [21]. Increased RT may be a more reliable measure for an objective (i.e., performance-based) mental fatigability and incorporating sustained attention tasks may be important when designing a mental task that can induce mental fatigability.
Mental fatigability is likely influenced by multiple factors. Cognitive factors such as executive function have been shown to influence mental fatigability [22]. Physiological factors such as sleep quality may also affect the generation of mental fatigability [23]. A recent conceptual framework proposed that both idiopathic and disease-related fatigue is influenced by psychological (i.e., both affective and cognitive), physiological (i.e., functional and health related), and situational (i.e., individual's environment related) factors [24, 25]. It is unclear whether this conceptual framework can be applied in the context of mental fatigability.
Specific groups of older adults are at particular risk for experiencing mental fatigability. Vascular risk (e.g., Type 2 diabetes, hypertension, dyslipidemia, and smoking) is the most common health condition in older adults, affecting at least half of the elderly population in the U.S. [26]. Those with vascular risk factors provide an ideal model to study fatigability in old age since fatigue and fatigability are prevalent in this group [12, 27, 28]. Additionally, recent work by our group found that older adults with vascular risk factors did not participate in adequate physical and cognitive leisure activities [29], and fatigability was suspected to be a main barrier to the engagement in such activities [7]. Identifying more reliable measures of mental fatigability and understanding the factors associated with this phenomenon is needed to better understand how to identify, prevent, or treat mental fatigability in older adults, especially those with vascular risk.
The objectives of the current study were two-fold. First, we examined the relationship between a novel, objective (i.e., performance-based) measure of mental fatigability that entailed consecutive assessment of RT to a task requiring sustained mental effort and perceived (i.e., self-reported) mental fatigability. We hypothesized that the correlation between the RT measure of mental fatigability and perceived mental fatigability would be greater than the correlation between the accuracy measure of the fatigability-manipulation task and perceived fatigability. Second, we aimed to identify psychological (i.e., subjective chronic fatigue, executive control, and depressive symptoms), physiological (i.e., vascular risk, sleepiness, anti-inflammatory and beta-blocker medications), and situational (i.e., history of mental activities) predictors of objective and perceived mental fatigability.
Methods
Design
An exploratory cross-sectional study was conducted by recruiting participants enrolled in a regional cohort study designed to identify blood-based predictors for incident dementia [30]. Community-dwelling older adults aged 75 years or older were invited to participate in a series of neuropsychological, functional, and neuropsychiatric tests conducted by a group of clinicians. This group of clinicians also reviewed each individual's medication list. Individuals with mild cognitive impairment, dementia due to Alzheimer's disease, or who were cognitively healthy were eligible for participating in the cohort study. Only individuals who were cognitively healthy per cohort study protocol were referred to the present study within one month of their annual cohort study visit. Additional inclusion criteria for the present study were: English speaking, capacity to provide informed consent, presence of at least one of the following vascular risk (hypertension, high cholesterol, diabetes, as confirmed by relevant medications; and self-report smoking), and adequate auditory and visual acuity for testing. Exclusion criteria included self-reported history of stroke, sleep disorder, or major depression. The study was approved by the University of Rochester institutional review board.
A total of 71 individuals from the cohort study were referred to the present study during our eight months recruitment period: 7 declined to participate, 13 were found to be ineligible for the present study, and 2 cancelled the study appointment for unknown reasons. Written consent was obtained from the 49 eligible participants and these individuals then completed the study protocol.
Measurements
Mental fatigability
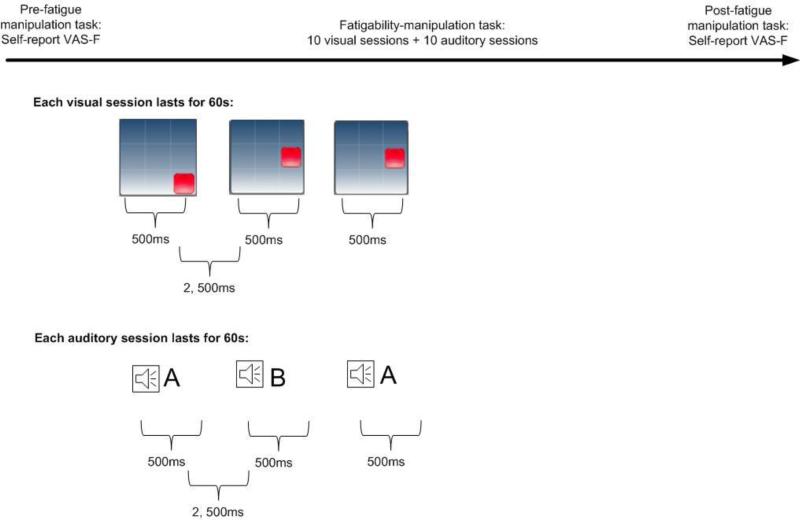
Fatigability-manipulation task (see Figure 1)
Figure 1. Study Procedure.
The protocol included 10 visual sessions and 10 auditory sessions. The computer program used a locked periodic design in which the visual or auditory stimuli were presented to the participants during epochs of 60 seconds per session. Each stimulus lasted for 500 milliseconds (ms), and the inter-stimulus interval lasted 2,500 ms. Performance-based fatigability was computed based on the reaction time to the stimulus over sessions. Perceived fatigability was computed based on VAS-F before and after fatigue manipulation task.
In the present study, we employed a component of a popular cognitive training program called the N-back task [31] as the fatigability-manipulation task. Performing the N-back task requires capacity of processing speed, working memory, and sustained attention [32]. We utilized a 20 minute 1-back paradigm, given the following considerations: First, a previous study found that, when examining RT, mental load was similar between 1-, 2-, and 3-back task in older adults. More importantly, compared to 2- or 3-back task, 1-back task reflected the greatest efforts in locating resources from the PFC to manage cognitive challenges, which lead to greatest probability of breakdown (i.e., generating of fatigability) of brain function [32]. Conversely, the capacity to perform 2- and 3-back, from the very beginning, may have already been beyond the capacity to activate compensatory function from the PFC in older adults, which may lead to frustrations and confound the interpretation of fatigability [32]. Second, our previous work showed that a laboratory cognitive task lasting less than 20 minutes was able to induce significantly cardiovascular response (i.e., heart rate variability) in older adults [33]. Meanwhile, performance decrements have consistently been seeing in 20 to 30 minutes fatigability manipulation tasks [34]. Thus, we utilized the 20-minute protocol to ensure that we would be able to induce mental fatigability and to secure the safety of participants who had vascular risk.
The fatigability-manipulation task included 20 sessions of 1-back computerized tasks consisting of 10 sessions of visual stimuli and 10 sessions of auditory stimuli. During a task with visual stimuli, participants were presented with a sequence of red squares, appearing in one of the eight different loci on the screen. Participants were required to press the “Visual” button on the touch screen whenever the currently presented stimulus was at the same location as the one 1 position back in the sequence. During a task with auditory stimuli, participants were presented with a sequence of letters spoken in a female voice. There were eight candidate letters, selected on the basis of their distinctiveness. Participants were required to press the “Audio” button if the current letter matched the letter back in the sequence. Positions of square locations and sequence of letters spoken in the tasks were determined randomly. The computer program used a locked periodic design in which the visual or auditory stimuli were presented to the participants during epochs of 60 seconds per session. Each stimulus lasted for 500 milliseconds, and the inter-stimulus interval lasted 2,500 milliseconds. No responses were required for non-targets. Two types of data were recorded: response accuracy and the time taken to respond to the stimulus (RT). Standardized instruction and 2-3 minute practices were provided to help participants fully understand the tasks before the 20-minute task period. The task was operated as an app on an iPad (Brian Williams, Tnxbai© 2008). Due to technical problems, we failed to record one participant's data during the task.
Performance-based/objective fatigability was calculated as increased RT over the fatigability-manipulation task. We averaged the time to make correct response to the stimuli within one session; a total of 20 RTs were developed. This method has been validated in previous studies [18, 21]. We also computed the accuracy rate (the ratio of the total correct responses to the total required responses) per session for comparison. Self-report/perceived fatigability was calculated as the change in self-reported acute fatigue before and after engaging in the fatigability-manipulation task [5, 7]. Participants were presented with an18-item visual analogue scale to evaluate fatigue severity (VAS-F). This instrument measures varying aspects of fatigue (e.g., “concentrating is a tremendous chore”, etc.) and participants indicated their response by marking on a 10-cm analogue rating line [35]. The length of the line between 0 and the place the participant indicated the level of fatigue was recorded for each item. A mean score was calculated for the 18 items; higher scores indicated a higher level of fatigue. The scale has been validated in adults with and without chronic illnesses across a wide range of ages [36]. In the present study, the internal consistency of the VAS-F before and after fatigability-manipulation task was 0.88 and 0.94, respectively. In addition, we also used two items that clearly described only mental fatigue “Concentrating is a tremendous chore” and “Carrying on a conversation is a tremendous chore” as a supplemental analysis for self-report/perceived fatigability. Of note, the items were chosen simply based on their expression, which has not been confirmed by any statistical analysis (e.g., factor analysis). We present this supplemental analysis as an Appendix.
Potential predictors of fatigability
Psychological predictors
Subjective chronic fatigue, a trait measure, was assessed by a mean score of the 20-item Multidimensional Fatigue Inventory [37]. This 20-item measure captured five domains of chronic fatigue: mental fatigue, physical fatigue, general fatigue, reduced motivation, and reduced activities. Internal consistency was 0.89 in this study. Executive control was measured by three neuropsychological tests: Trail making test A and B [38], Stroop word and color test [39], and Digit span forward and backward [40]. These are commonly used cognitive tests for working memory, attention, and executive function in elderly groups [41]. Seven performance scores were calculated: Trail making test A, Trail making test B, Stroop word reading, Stroop color naming, Stroop interference, Digit span forward, and Digit span backward. Each of the seven scores was then standardized. A composite score was developed by averaging the standardized scores. Depressive symptoms were measured by the 15-item Geriatric Depression Scale (GDS) [42]. Participants responded “yes” or “no” to questions related to their depressive symptoms during the past week. Total depressive symptoms were summed.
Physiological predictors
Vascular risk was calculated as the total number of vascular risk factors (e.g., hypertension, high cholesterol, smoking, and diabetes) reported. Sleepiness was measured by the 8-item Epworth scale [43]. Participants responded to questions related to their sleepiness (in contrast to just feeling tired) under different situations using a scale ranging from “would never doze” to “high chance of dozing”. A mean score was computed with higher scores indicating more sleepiness. Internal consistency of the scale in this study was 0.68. Anti-inflammatory and beta-blocker medication use were extracted from participants’ medication list.
Situational predictors
History of mental activities was measured by the frequency of engaging in six mentally stimulating activities over the past year in participants’ leisure time: reading, doing word games, playing cards, attending lectures, writing, and using a computer. Participants responded using a six-point rating scale from “Never” to “Daily” [44].
Additionally, participants’ age, sex, and years of education were collected by self-report.
Procedure
All tests were conducted in the CogT laboratory (PI: F.L.). During the laboratory visit, the participant was first asked to sit quietly and relax for 5 to 10 minutes to adapt to the environment. The participant then completed measures of history of mental activities and subjective chronic fatigue, as well as the neuropsychological tests. The participant then engaged in the mental fatigue manipulation task. Additional self-report measures were completed following the manipulation. The average length of laboratory visit was 90 minutes.
Data analysis
IBM SPSS 19.0 was used for data analysis. Descriptive analysis was first performed to describe sample characteristics. Because of its non-normal distribution, RT was log-transformed.
We thereafter performed the following analyses to examine whether objective and perceived fatigability were inducible over the fatigability-manipulation task. To examine objective fatigability, a generalized estimating equation (GEE) with an unstructured working correlation matrix was used. RT was the dependent variable, and age, sex, education, and the session of fatigability-manipulation task as predictors: YRT= β0 + β1age + β2sex + β3education + β4session + ε. Any significant main effects of session would indicate a significant change of RT over the fatigue manipulation task; an increase indicated an occurrence of objective fatigability. Next, to examine perceived fatigability, repeated measure ANOVA was used. Self-reported fatigue before and after the fatigue manipulation task was the dependent variable with age, sex, and education as predictors. A significant main effect of session from the repeated measure indicated a significant change in self-report fatigue; an increase indicated an occurrence of perceived fatigability.
Next, we examined the correlation between objective and perceived fatigability controlling for age, sex, and education. Because self-report fatigue was only assessed twice, we first used GEE model to develop a “predictive value of mean of response” for self-report fatigue by adjusting time of the fatigability-manipulation task. We then examined the correlation between the adjusted self-report fatigue and RT over the fatigability-manipulation task using GEE: YRT= β0 + β1age + β2sex + β3education + β4adjusted self-report fatigue + ε.
As a comparison with RT, we also performed the analyses above using accuracy rate as the dependent variable. Given the skewness of accuracy data, we dichotomized the data into 100% accurate vs. less than 100% accurate.
Finally, to examine predictors of objective and perceived fatigability, GEE and repeated ANOVA were used separately for RT and self-reported fatigue. In separate models, each potential psychological, physiological, and situational factor was entered as a predictor along with age, sex, education, and session of fatigability-manipulation task. For GEE model, the model was: YRT= β0 + β1age + β2sex + β3education + β4session + β5factor + β6session × factor + ε. A significant main effect of the factor indicated a significant difference in the RT by the factor. A significant interaction term involving task sessions would indicate different rates of change in RT over session as a function of the factor. Of note, only a significant interaction term indicated the particular factor's effect on fatigability. A similar procedure using repeated ANOVA was used to identify significant factors associated with perceived fatigability. We only conducted univariate instead of multivariate analyses, because a sample size of ≥ 68 or larger would have been needed for simultaneously testing at least two predictors when targeting a small effect size between predictors and outcome (0.15), power at 0.80, and alpha at 0.05. Alpha was set at 0.05.
Results
Sample Characteristics
Table 1 shows the demographic and health characteristics of the sample. The average age of the sample was 82.73. Fourteen participants had only one vascular risk factor (28.6%), 31 had two (63.3%), and 4 had three (8.2%). No one was an active smoker. Chronic subjective fatigue was comparable to an age-matched healthy sample [45]. Executive control was comparable to age- and education-adjusted normative data from the literature [46, 47]. We excluded individuals with major depression, leaving the sample with fewer depressive symptoms relative to an age-matched community sample (e.g., [48]).
Table 1.
Demographic and Health Characteristics (N = 49)
| Age, Mean (SD) | 82.73 (3.03) |
| Years of education, Mean (SD) | 15.69 (2.23) |
| Male, n (%) | 22 (44.9%) |
| Smoking, n (%) | 0 (0) |
| Diabetes, n (%) | 8 (16.3%) |
| Hypertension, n (%) | 43 (87.8%) |
| High cholesterol, n (%) | 37 (75.5%) |
| Anti-inflammatory medication, n (%) | 28 (57.1%) |
| Beta-blocker, n (%) | 26 (53.1%) |
| History of mental activities, Mean (SD) | 3.61 (0.78) |
| Subjective chronic fatigue, Mean (SD) | 10.47 (3.32) |
| Executive control (Z composite score), Mean (SD) | −0.02 (0.56) |
| • Trail making test A (reversed) | 264.10 (10.87) |
| • Trail making test B (reversed) | 215.35 (35.98) |
| • Stroop word reading | 43.21 (10.21) |
| • Stroop color naming | 37.12 (10.37) |
| • Stroop interference | 45.44 (6.19) |
| • Digit span forward | 6.57 (1.56) |
| • Digit span backward | 4.61 (1.00) |
| Sleepiness, Mean (SD) | 0.82 (0.41) |
| Depressive symptoms, Mean (SD) | 0.86 (1.24) |
Descriptive and Correlational Data of Objective and Perceived Mental Fatigability
After controlling for age, sex, and education, RT increased significantly over time (B= 0.006, SE = 0.002, p = .002), indicating an occurrence of objective mental fatigability. In contrast, response accuracy showed no significant change across the task (B = -0.048, SE = 0.037, p = .19). The type of stimuli (auditory vs. visual) of the fatigability-manipulation task did not affect RT (B = 0.031, SE = 0.026, p = .24).
After controlling for age, sex, and education, self-report fatigue increased significantly after the fatigability-manipulation task (F(1, 45) = 5.33, p = .026), indicating an occurrence of perceived mental fatigability.
We then examined the correlation between objective and perceived mental fatigability using GEE adjusting for age, sex, and education. Self-report fatigue was significantly and positively related to RT (B = 0.291, SE = 0.095, p = .002) over the course of fatigability manipulation task, but not to accuracy rate (B = −2.205, SE = 3.093, p = .48).
Predictors for Objective and Perceived Mental Fatigability
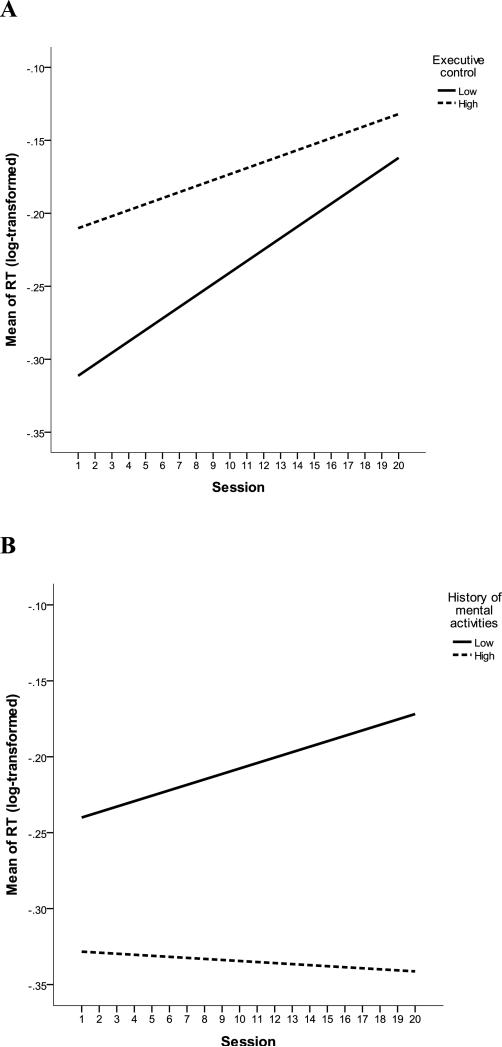
Table 2 shows the relationship between the psychological, physiological, and situational factors and objective and perceived fatigability. In terms of RT, taking anti-inflammatory medications (B = −0.201, SE = 0.098, p = .041) and reporting a lower frequency of engaging in mental activities (B = −0.251, SE = 0.106, p = .018) were significantly associated with longer level of RT. Lower executive control (B = −0.004, SE = 0.002, p = .046) and a history of less frequently engaging in mental activities (B = −0.003, SE = 0.002, p = .024) were significantly associated with faster increase in RT over sessions (also see Figure 2). Executive control and history of mental activities were not correlated after controlling for age, sex, and education (r = 0.03, p = .85).
Table 2.
Predictors of Objective and Perceived Fatigability (N = 48)a
| Objective fatigabilityb (Response time) | Perceived fatigabilityb (Self-report fatigue) | |||||
|---|---|---|---|---|---|---|
| Psychological factor | Session | Factor | Factor × session | Session | Factor | Factor × session |
| Subjective chronic fatigue | 0.005 (0.005), p = .26 | −0.050 (0.062), p = .42 | 0 (0.002), p = .81 | 5.26, p = .027 | 30.38, p < .001 | 0.08, p = .78 |
| Executive control | 0.006 (0.001), p < .001 | 0.121 (0.091), p = .18 | −0.004 (0.002), p = .046 | 3.26, p = .08 | 3.12, p = .08 | 0.64, p = .43 |
| Depressive symptom | 0.004 (0.002), p = .11 | 0.008 (0.105), p = .94 | −0.001 (0.003), p = .80 | 5.01, p = .030 | 5.47, p = .024 | 0.61, p = .44 |
| Physiological factor | ||||||
| Vascular risk = 1 (vascular risk > 1 as reference) | 0.007 (0.002), p < .001 | 0.021 (0.101), p = .83 | −0.002 (0.003), p = .56 | 5.31, p = .026 | 1.70, p = .20 | 1.06 p = .31 |
| Sleepiness | 0.003 (0.003), p = .27 | −0.379 (0.196), p = .06 | 0.003 (0.003), p = .40 | 3.59, p = .07 | 9.27, p = .004 | 0.43, p = .52 |
| Taking anti-inflammation (not taking anti-inflammation as reference) | 0.008 (0.003), p = .008 | 0.201 (0.098), p = .041 | −0.003 (0.003), p = .28 | 4.98, p = .031 | 1.69, p = .20 | 0.20, p = .66 |
| Taking beta-blocker (not taking beta-blocker as reference) | 0 (0.004), p = .93 | 0.198 (0.104), p = .06 | 0.004 (0.004), p = .35 | 5.04, p = .030 | 1.37, p = .39 | 0.40, p = .53 |
| Situational factor | ||||||
| History of mental activities | 0.014 (0.005), p = .009 | −0.251 (0.106), p = .018 | −0.003 (0.002), p = .024 | 3.91, p = .05 | 0.02, p = .90 | 1.95, p = .17 |
A participant failed to attend the fatigability-manipulation task
Controlled for age, education, and sex.
Figure 2.
Executive Control (2A) and History of Mental Activities (2B) Influenced Objective Mental Fatigability. Note, RT was adjusted for age, sex, and education. Executive control and History of mental activities were divided based on their median scores for display purpose. “Session” refers to the timeline of the fatigue manipulation task; each session lasted for 1 minute, and there were a total of 20 sessions.
In terms of perceived mental fatigability, lower subjective chronic fatigue (F(1, 44) = 30.38, p < .001), sleepiness (F(1, 44) = 9.27, p = .004), and depressive symptoms (F(1, 44) = 5.47, p = .024) were significantly related to overall lower self-reported fatigue. None of the predictors were related to the change of self-report fatigue over sessions.
Vascular risk burden and beta-blocker medications were not related to levels or changes of RT or self-reported fatigue over sessions.
Discussion
In the present study, we were able to capture objective and perceived mental fatigability using a fatigability-manipulation task in a group of adults aged 75 years and older with vascular risk. This is a population at high risk for cognitive decline [49] and for whom fatigue is a common and problematic symptom [11-13]. Consistent with the literature [7], there was a significant increase in RT, but no change in accuracy rate over the fatigability-manipulation task. Importantly, RT over the course of fatigability manipulation task, but not accuracy rate, was significantly correlated, though only moderately, with perceived fatigability. These results suggest that RT in task requiring sustained mental effort may be a better index of objective mental fatigability than accuracy. However, the objective and perceived measures of mental fatigability were predicted by different factors. Higher levels of executive control and a history of more frequently engaging in mental activities protected older adults from objective mental fatigability, but no factor predicted the perceived fatigability. Understanding the discrepancy in these associated factors would facilitate the understanding of the underlying processes in objective and perceived fatigability.
The moderate but significant correlation between objective and perceived mental fatigability warrants further discussion. In general, previous studies failed to find such an association when utilizing performance-based measures based on accuracy [18]. Our study is among the first to demonstrate a significant association through the utilization of an objective mental fatigability based on RT. However, it must be acknowledged that the association was moderate. Previous imaging studies suggest that increased RT in the N-back task reflects a decreased capacity to utilize the compensatory function of PFC, especially within the dorsolateral and medial regions [32]. Perceived fatigability is generated from basal ganglia and travels to the reward circuit of the PFC located within the orbitofrontal and medial regions [14]. Hence, there is definite overlap and correlation between objective and perceived mental fatigability in terms of the neural regions regulating these responses, but the strength of such correlation may not be substantial. Furthermore, the length of the fatigability-manipulation task was only 20 minutes, while other fatigability manipulation protocols can be as long as 3 hours [50]. Participants in the present study may have only just approached the threshold for the efficient use of PFC while the feeling of fatigue may have not yet been generated. Such discrepancy may affect the strength of correlation between objective and perceived mental fatigability as well; however, such interpretation requires relevant brain imaging investigation.
This is the first pilot study using a conceptual framework of related psychological, physiological, and situational factors [24, 25] to explore predictors of mental fatigability. Interestingly, despite the significant correlation between perceived and objective mental fatigability, we found different associated predictors for the two dimensions of mental fatigability. A history of engaging in mental activities and higher levels of executive control were associated with lower objective mental fatigability. History of mental activities in the present study was derived from several common mental leisure activities in the past, and frequently engaging in these activities was found to protect cognitive function against acute stress related cardiovascular response in our previous study [33], suggests its possible role in protecting brain function against fatigability. Executive control here mostly reflected a combination of executive function, working memory, and attention. As discussed, mental fatigability may be related to functional abnormality of the communication between the basal ganglia and PFC, a brain region supporting multiple domains of executive control [14]. Next, although subjective chronic fatigue, sleepiness, and depressive symptoms were significantly associated with the level of self-report fatigue before and after the fatigability-manipulation task, we failed to identify any significant factors related to perceived fatigability. All available predictors are essentially chronic factors and future research may consider acute psychological factors (e.g., acute perceived stress) as potential predictors of perceived fatigability [24]. Finally, the different predictor profiles for objective and perceived mental fatigability provide a potential framework to simultaneously improve the two types of mental fatigability by either tailoring future interventions to individual's health characteristics or primarily targeting and promoting those health characteristics found to be protective against fatigability.
Accumulated evidence suggests that activities offering novel cognitive stimuli or requiring constant cognitive effort, as seen in mentally fatiguing tasks, have the potential to prevent and slow cognitive decline [51]. However, experiencing these benefits requires sustained engagement with the activities. Mental fatigability may threaten an individual's ability and motivation to sustain their engagement, thus lessening the likelihood of reaping the benefits of these activities. As previously emphasized, older adults with vascular risk are at high risk for cognitive decline and fatigue [49]. Findings from the present study may have important implications for designing appropriate cognitive training paradigms to prevent cognitive decline for older adults with vascular risk.
A limitation of this study is the small sample size, which may have reduced statistical power, especially for examining the factors associated with perceived fatigability. Given the small sample and the exploratory nature of the study, we examined individual predictors with mental fatigability instead of multiple predictors in a single analysis. For example, although we found executive control and history of mental activities both predicted objective fatigability but were not statistically correlated, they are still theoretically related. Thus, future studies need to test how these predictors interact to predict mental fatigability. Second, we focused on a sample of adults 75 years of age and older with vascular disease risk. We recognize that by targeting this group the threshold for inducing mental fatigability may have been lowered, especially under the circumstance of lack of appropriate control. However, previous work has established this group as being high risk for experiencing increases in mental fatigability and subsequent declines in cognitive functioning [49]. Therefore, we deemed it appropriate to use this sample as the first test of the new approach to assessing mental fatigability objectively as this phenomenon is known to be common in this group. Although we found that neither vascular risk nor beta-blocker use was a significant risk factor for mental fatigability, this protocol needs to be tested in a more heterogeneous older adult sample to determine the validity of applying it to understanding idiopathic mental fatigability in old age. Third, our fatigability-manipulation protocol was based on a synthesis of relevant findings from the literature [32] as well as our own previous work [33]. We sought to design a protocol that achieved a balance of challenge versus frustration while also being safe for participants to complete. However, even though our study found a moderate association between perceived and objective fatigability, we still cannot ascertain if the increased RT, as seen in objective fatigability, was due to boredom, since a previous study suggested monotonous task may lead to underload mental efforts and boredom [52]. Generally, two types of fatigability-manipulation protocols are utilized by investigators in this field – one focusing on working memory (e.g., N-back task), and one on inhibition (e.g., Stroop Word Color) [50]. Future studies may consider applying both protocols to enhance the ecological validity of the mental fatigability task, since the mental fatigability encountered in older adults’ daily lives usually result from multiple stimuli. Also, appropriate physiological arousal measures (e.g., heart rate variability) may be incorporated to differentiate between mental fatigability and boredom [52]. Finally, we were not able to compare other approaches that utilize RT in measuring objective mental fatigability. For example, a recent study assessed intraindividual variability of RT in younger adults; and intraindividual variability of RT had a higher correlation with chronic subjective fatigue than mean RT [50]. However, the protocol was much lengthier (at least 90 minutes) and this would likely be a barrier to its use with older adults. Regardless, comparisons of different approaches of eliciting mental fatigability deserve future investigation.
Despite the small sample size and exploratory design, we observed a number of significant associations between key measures of interest that support a new approach to conceptualizing two dimensions of mental fatigability. This work also provides preliminary evidence for important individual characteristics including executive function and regular engagement in mentally stimulating activities, as predictors of objective mental fatigability. Together, these findings provide an important foundation for a future validation study that will clarify whether these features of mental fatigability can be incorporated into individualized cognitive training interventions aimed at maintaining cognitive well-being in older adults.
Highlights.
Objective and perceived mental fatigability are correlated.
Objective and perceived mental fatigability are not predicted by the same factors.
Understanding mental fatigability may implicate the prevention of cognitive decline.
Acknowledgement
The authors would like to thank the Drs Harriet Kitzman and Duje Tadin's comments on the original manuscripts. The authors would like to thank the following people in assistance of data collection: Eileen Johnson, Judith Brasch, and Marian Moskow. The authors also would like to thank IQ booster (the app) developer Brian Williams for sharing the code with the project and technical support.
Data collection of the present project was supported by Sigma Theta Tau International Small Grants to F. Lin. The manuscript preparation was supported by the University of Rochester CTSA award number KL2 TR000095 from the National Center for Advancing Translational Sciences of the National Institutes of Health to F. Lin. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Appendix
Appendix.
Analyses using two mental fatigue related items from VAS-F as the measure for self-report/perceived fatigability.
| 1. Correlation between objective and perceived mental fatigability: | |||
|---|---|---|---|
| We examined the correlation between objective and perceived mental fatigability using GEE adjusting for age, sex, and education. Self-report fatigue was significantly and positively related to RT (B = 0.333, SE = 0.109, p = .002) over the course of fatigability manipulation task. | |||
| 2. Predictors of perceived mental fatigability (control for age, sex, and education): | |||
| Psychological factor | Session | Factor | Factor × session |
| Subjective chronic fatigue | 0.05, p = .83 | 3.53, p = .067 | 0.01, p = .91 |
| Executive control | 0.46, p = .50 | 0.56, p = .46 | 4.75, p = .035 |
| Depressive symptom | 0.04, p = .85 | 2.27, p = .14 | 0.41, p = .53 |
| Physiological factor | |||
| Vascular risk = 1 (vascular risk > 1 as reference) | 0.004, p = .95 | 0.09, p = .91 | 0.75, p = .48 |
| Sleepiness | 0.18, p = .67 | 4.03, p = .051 | 0.32, p = .57 |
| Taking anti-inflammation (not taking anti-inflammation as reference) | 0.08, p = .79 | 0.32, p = .58 | 0.17, p = .69 |
| Taking beta-blocker (not taking beta-blocker as reference) | 0.02, p = .89 | 1.14, p = .29 | 0.31, p = .58 |
| Situational factor | |||
| History of mental activities | 0.01, p = .94 | 0.09, p = .76 | 1.29, p = .26 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interest Statement
The authors have no competing interests to report.
References
- 1.Avlund K. Fatigue in older adults: an early indicator of the aging process? Aging Clin Exp Res. 2010;22:100–15. doi: 10.1007/BF03324782. [DOI] [PubMed] [Google Scholar]
- 2.Wijeratne C, Hickie I, Brodaty H. The characteristics of fatigue in an older primary care sample. J Psychosom Res. 2007;62:153–8. doi: 10.1016/j.jpsychores.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Yu DS, Lee DT, Man NW. Fatigue among older people: a review of the research literature. Int J Nurs Stud. 2009;47:216–28. doi: 10.1016/j.ijnurstu.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Lin F, Chen D, Vance DE, Ball JK, Mapstone M. Longitudinal associations of subjective fatigue, cognitive function, and everyday performance in old age. International Psychogeriatrics. 2013;25:275–85. doi: 10.1017/S1041610212001718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeLuca J. Fatigue as a window to the brain. The MIT Press; 2005. [Google Scholar]
- 6.Alexander NB, Taffet GE, Horne FM, Eldadah BA, Ferrucci L, Nayfield S, et al. Bedside-to-Bench conference: research agenda for idiopathic fatigue and aging. J Am Geriatr Soc. 2011;58:967–75. doi: 10.1111/j.1532-5415.2010.02811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eldadah BA. Fatigue and fatigability in older adults. PM R. 2011;2:406–13. doi: 10.1016/j.pmrj.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 8.Chaudhuri A, Behan PO. Fatigue in neurological disorders. Lancet. 2004;363:978–88. doi: 10.1016/S0140-6736(04)15794-2. [DOI] [PubMed] [Google Scholar]
- 9.Hickie IB, Hooker AW, Hadzi-Pavlovic D, Bennett BK, Wilson AJ, Lloyd AR. Fatigue in selected primary care settings: sociodemographic and psychiatric correlates. Med J Aust. 1996;164:585–8. doi: 10.5694/j.1326-5377.1996.tb122199.x. [DOI] [PubMed] [Google Scholar]
- 10.Bailey A, Channon S, Beaumont JG. The relationship between subjective fatigue and cognitive fatigue in advanced multiple sclerosis. Mult Scler. 2007;13:73–80. doi: 10.1177/1352458506071162. [DOI] [PubMed] [Google Scholar]
- 11.Schepens SL, Kratz AL, Murphy SL. Fatigability in osteoarthritis: effects of an activity bout on subsequent symptoms and activity. J Gerontol A Biol Sci Med Sci. 2012;67:1114–20. doi: 10.1093/gerona/gls076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manty M, Ekmann A, Thinggaard M, Christensen K, Avlund K. Fatigability in basic indoor mobility in nonagenarians. J Am Geriatr Soc. 2012;60:1279–85. doi: 10.1111/j.1532-5415.2012.04034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schnelle JF, Buchowski MS, Ikizler TA, Durkin DW, Beuscher L, Simmons SF. Evaluation of two fatigability severity measures in elderly adults. J Am Geriatr Soc. 2012;60:1527–33. doi: 10.1111/j.1532-5415.2012.04062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeLuca J, Genova HM, Capili EJ, Wylie GR. Functional neuroimaging of fatigue. Phys Med Rehabil Clin N Am. 2009;20:325–37. doi: 10.1016/j.pmr.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Boksem MA, Meijman TF, Lorist MM. Mental fatigue, motivation and action monitoring. Biol Psychol. 2006;72:123–32. doi: 10.1016/j.biopsycho.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Kratz AL, Schepens SL, Murphy SL. Effects of cognitive task demands on subsequent symptoms and activity in adults with symptomatic osteoarthritis. Am J Occup Ther. 2013;67:683–91. doi: 10.5014/ajot.2013.008540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcora SM, Staiano W, Manning V. Mental fatigue impairs physical performance in humans. J Appl Physiol (1985) 2009;106:857–64. doi: 10.1152/japplphysiol.91324.2008. [DOI] [PubMed] [Google Scholar]
- 18.Leavitt VM, DeLuca J. Central fatigue: issues related to cognition, mood and behavior, and psychiatric diagnoses. PM R. 2011;2:332–7. doi: 10.1016/j.pmrj.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 19.Andreasen AK, Spliid PE, Andersen H, Jakobsen J. Fatigue and processing speed are related in multiple sclerosis. Eur J Neurol. 2011;17:212–8. doi: 10.1111/j.1468-1331.2009.02776.x. [DOI] [PubMed] [Google Scholar]
- 20.Holtzer R, Foley F. The relationship between subjective reports of fatigue and executive control in multiple sclerosis. J Neurol Sci. 2009;281:46–50. doi: 10.1016/j.jns.2009.02.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holtzer R, Shuman M, Mahoney JR, Lipton R, Verghese J. Cognitive fatigue defined in the context of attention networks. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2011;18:108–28. doi: 10.1080/13825585.2010.517826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Persson J, Larsson A, Reuter-Lorenz PA. Imaging fatigue of interference control reveals the neural basis of executive resource depletion. J Cogn Neurosci. 2013;25:338–51. doi: 10.1162/jocn_a_00321. [DOI] [PubMed] [Google Scholar]
- 23.Lehmann P, Eling P, Kastrup A, Grothues O, Hildebrandt H. Self-reported sleep problems, but not fatigue, lead to decline in sustained attention in MS patients. Mult Scler. 2013;19:490–7. doi: 10.1177/1352458512457719. [DOI] [PubMed] [Google Scholar]
- 24.Cho J, Martin P, Margrett J, MacDonald M, Johnson MA, Poon LW, et al. Multidimensional predictors of fatigue among octogenarians and centenarians. Gerontology. 2012;58:249–57. doi: 10.1159/000332214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen LH, Li CY, Shieh SM, Yin WH, Chiou AF. Predictors of fatigue in patients with heart failure. J Clin Nurs. 2010;19:1588–96. doi: 10.1111/j.1365-2702.2010.03218.x. [DOI] [PubMed] [Google Scholar]
- 26.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaltsas G, Vgontzas A, Chrousos G. Fatigue, Endocrinopathies, and Metabolic Disorders. PM&R. 2011;2:393–8. doi: 10.1016/j.pmrj.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 28.Melamed S, Shirom A, Toker S, Berliner S, Shapira I. Burnout and risk of cardiovascular disease: evidence, possible causal paths, and promising research directions. Psychol Bull. 2006;132:327–53. doi: 10.1037/0033-2909.132.3.327. [DOI] [PubMed] [Google Scholar]
- 29.Lin F, Friedman E, Quinn J, Chen DG, Mapstone M. Effect of leisure activities on inflammation and cognitive function in an aging sample. Arch Gerontol Geriatr. 2012;54:e398–404. doi: 10.1016/j.archger.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mapstone M, Cheema AK, Fiandaca MS, Zhong X, Mhyre TR, Macarthur LH, et al. Plasma phospholipids identify antecedent memory impairment in older adults. Nat Med. 2014 doi: 10.1038/nm.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaeggi SM, Buschkuehl M, Jonides J, Shah P. Short- and long-term benefits of cognitive training. Proc Natl Acad Sci U S A. 2011;108:10081–6. doi: 10.1073/pnas.1103228108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mattay VS, Fera F, Tessitore A, Hariri AR, Berman KF, Das S, et al. Neurophysiological correlates of age-related changes in working memory capacity. Neurosci Lett. 2006;392:32–7. doi: 10.1016/j.neulet.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 33.Lin F, Heffner K, Mapstone M, Chen DG, Porsteisson A. Frequency of Mentally Stimulating Activities Modifies the Relationship Between Cardiovascular Reactivity and Executive Function in Old Age. Am J Geriatr Psychiatry. 2013 doi: 10.1016/j.jagp.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mackworth NH. The breakdown of vigilance durning prolonged visual search. Quarterly Journal of Experimental Psychology. 1948;1:6–21. [Google Scholar]
- 35.Lee KA, Hicks G, Nino-Murcia G. Validity and reliability of a scale to assess fatigue. Psychiatry Res. 1991;36:291–8. doi: 10.1016/0165-1781(91)90027-m. [DOI] [PubMed] [Google Scholar]
- 36.Dittner AJ, Wessely SC, Brown RG. The assessment of fatigue: A practical guide for clinicians and researchers. Journal of Psychosomatic Research. 2004;56:157–70. doi: 10.1016/S0022-3999(03)00371-4. [DOI] [PubMed] [Google Scholar]
- 37.Smets EMA, Garssen B, Bonke B, De Haes JCJM. The multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. Journal of Psychosomatic Research. 1995;39:315–25. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 38.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and motor skills. 1958;8:271–6. [Google Scholar]
- 39.Golden CJ, Freshwater SM. Stroop color and word test. 1978 [Google Scholar]
- 40.Guarch J, Marcos T, Salamero M, Blesa R. Neuropsychological markers of dementia in patients with memory complaints. Int J Geriatr Psychiatry. 2004;19:352–8. doi: 10.1002/gps.1074. [DOI] [PubMed] [Google Scholar]
- 41.Bossers WJ, van der Woude LH, Boersma F, Scherder EJ, van Heuvelen MJ. Recommended measures for the assessment of cognitive and physical performance in older patients with dementia: a systematic review. Dement Geriatr Cogn Dis Extra. 2012;2:589–609. doi: 10.1159/000345038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clinical gerontologist. 1986;5:165–73. [Google Scholar]
- 43.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 44.Lachman ME, Agrigoroaei S, Murphy C, Tun PA. Frequent cognitive activity compensates for education differences in episodic memory. Am J Geriatr Psychiatry. 18:4–10. doi: 10.1097/JGP.0b013e3181ab8b62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hagglund L, Boman K, Lundman B, Brulin C. Depression among elderly people with and without heart failure, managed in a primary healthcare setting. Scand J Caring Sci. 2008;22:376–82. doi: 10.1111/j.1471-6712.2007.00540.x. [DOI] [PubMed] [Google Scholar]
- 46.Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19:203–14. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 47.Van der Elst W, Van Boxtel MP, Van Breukelen GJ, Jolles J. The Stroop color-word test: influence of age, sex, and education; and normative data for a large sample across the adult age range. Assessment. 2006;13:62–79. doi: 10.1177/1073191105283427. [DOI] [PubMed] [Google Scholar]
- 48.Milaneschi Y, Cesari M, Simonsick EM, Vogelzangs N, Kanaya AM, Yaffe K, et al. Lipid peroxidation and depressed mood in community-dwelling older men and women. PLoS One. 2013;8:e65406. doi: 10.1371/journal.pone.0065406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Panza F, Frisardi V, Capurso C, Imbimbo BP, Vendemiale G, Santamato A, et al. Metabolic syndrome and cognitive impairment: current epidemiology and possible underlying mechanisms. J Alzheimers Dis. 2010;21:691–724. doi: 10.3233/JAD-2010-091669. [DOI] [PubMed] [Google Scholar]
- 50.Wang C, Ding M, Kluger BM. Change in intraindividual variability over time as a key metric for defining performance-based cognitive fatigability. Brain Cogn. 2014;85C:251–8. doi: 10.1016/j.bandc.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Valenzuela M, Sachdev P. Can cognitive exercise prevent the onset of dementia? Systematic review of randomized clinical trials with longitudinal follow-up. Am J Geriatr Psychiatry. 2009;17:179–87. doi: 10.1097/JGP.0b013e3181953b57. [DOI] [PubMed] [Google Scholar]
- 52.Pattyn N, Neyt X, Henderickx D, Soetens E. Psychophysiological investigation of vigilance decrement: boredom or cognitive fatigue? Physiol Behav. 2008;93:369–78. doi: 10.1016/j.physbeh.2007.09.016. [DOI] [PubMed] [Google Scholar]