Abstract
Background
The only definitive treatment for end-stage organ failure is orthotopic transplantation. Lung extracellular matrix (ECM) holds great potential as a scaffold for lung tissue engineering since it retains the complex architecture, biomechanics and topological specificity of the lung. Decellularization of human lungs rejected from transplantation could provide “ideal” biological scaffolds for lung tissue engineering, but the availability of such lungs remains limited. The present study was designed to determine whether porcine lung could serve as a suitable substitute of human lung to study tissue-engineering therapies.
Methods
Human and porcine lungs were procured, sliced into sheets, and decellularized using three different methods. Compositional, ultrastructural, and biomechanical changes to the ECM were characterized. The suitability of LECM for cellular re-population was evaluated by assessing the viability, growth, and metabolic activity of human lung fibroblasts (hMRC-5s), human small airway epithelial cells (hSAECs), and human adipose-derived mesenchymal stem cells (hMSCs) over a period of seven days.
Results
Decellularization using CHAPS, 3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate, showed the best maintenance of both human and porcine LECM, with similar retention of ECM proteins, except for elastin. Human and porcine LECM supported the cultivation of pulmonary cells in a similar way, except that the human LECM was stiffer and resulted in higher metabolic activity of the cells than porcine LECM.
Conclusions
Porcine lungs can be decellularized using CHAPS to produce lung ECM scaffolds with properties resembling those of human lungs, for pulmonary tissue engineering. We propose that porcine lung ECM can be an excellent screening platform for the envisioned human tissue engineering applications of decellularized lungs.
Keywords: lung, extracellular matrix, ECM, biomaterials, bioengineering, pulmonary tissue engineering, in vitro studies, stem cells
INTRODUCTION
Lung transplantation is currently the only definitive treatment for nearly 25 million patients with end-stage lung disease [1]. The supply of donor lungs is limited, and long-term outcomes of transplantation remain hampered by immunosuppressive regimens [1]. To address these challenges, tissue-engineering approaches are now being developed that use scaffolds and cells to create functional lung substitutes.
Due to the complex hierarchical structure of the lung, successful strategies will require a highly specialized matrix that can support the engraftment, growth, and function of a diverse population of cells. Only limited success in engineering lung tissue has been achieved until recently, when two parallel landmark studies introduced a new paradigm by using native extracellular matrix (ECM) [2-4] that has been shown to provide the cells with topologically specific signals and attachment sites inherent to native tissues [5-10]. The plausibility of bioengineering lungs was shown by generating a three-dimensional scaffold via decellularization of rat lungs, reseeding pulmonary cells onto the endothelial and epithelial surfaces of the scaffold, and achieving functional gas exchange of the resulting graft for a period of several hours, both in vitro and in vivo [11].
If scaled up to human lungs, tissue engineering could potentially expand the pool of donor organs, particularly if lungs marginally unsuitable for transplant could be improved upon by processing and seeding with the recipient patient's autologous stem cells (provided the patient suffers no inherent relevant genetic defect, e.g., cystic fibrosis). Additionally, there would be improved immunocompatibility due to the presence of autologous cells and gradual remodeling of the lung parenchyma. We propose that the “conditioning” of donor lungs by a combination of perfusion treatments and cell seeding could improve the quality of marginal lungs without structural defect to a level acceptable for transplantation.
Because healthy donor lungs are always used for transplantation, the availability of human lungs for tissue engineering studies is limited to those rejected for transplantation, where rejection is based on standard functional criteria such as inferior PaO2/FiO2, low compliance, or infection. For this reason, we considered porcine lungs, which are readily available, as a xenogeneic alternative to human lungs for research purposes. Porcine tissues have found use for developing human tissue engineering strategies in a variety of applications [e.g., 12]. The goal of the present study was to determine if porcine lung could be used as a tissue-engineering platform representative of human lungs.
While decellularization by perfusion has been described for murine and rat lungs [11, 13, 14], very little is known about perfusion of lungs from larger mammals. To facilitate our comparison of human and porcine tissue, we assessed the decellularization of human and porcine lungs using three different methods: (i) sodium dodecyl sulfate (SDS), (ii) 3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), and (iii) a 3-Step method consisting of Tween-20®, sodium deoxycholate, and peracetic acid. Structural, compositional, and biomechanical changes to human and porcine lung ECM were assessed to determine the outcomes of decellularization and to select the optimal method. Decellularized human and porcine lung ECM was then seeded with human lung fibroblasts (hMRC-5s), human small airway epithelial cells (hSAECs), and human adipose-derived mesenchymal stem cells (hMSCs) to evaluate their capacity to support the growth of seeded human cells (Figure 1). The collected data support the suitability of porcine lungs as a source of ECM for pulmonary tissue engineering applications.
Figure 1. Overall Approach.
Decellularized slices from human and porcine lungs were compared with respect to their histomorphology, biochemical composition, mechanical properties and ability to support the growth and metabolism of cultured cells. Three different methods of decellularization and three different types of human cells (lung fibroblasts, small airway epithelial cells, mesenchymal cells) were evaluated.
MATERIAL AND METHODS
Organ Harvest
Three human lungs and three porcine lungs were harvested and similar regions of the lower left lobes were used for decellularization and characterization. Human lungs rejected for transplantation were procured from the New York Organ Donor Network (NYODN) under a protocol approved by the Institutional Review Board at Columbia University. The porcine lungs were harvested from Yorkshire pigs (40—50kg) after the animals were used in another research study not affecting lungs and euthanized, under a protocol approved by the Columbia University Institutional Animal Care and Use Committee.
Decellularization
Upon harvest, lungs were cleared of blood and immediately frozen at −80°C until use. The lungs were partially thawed and sectioned to 2mm-thick sheets that were washed with 2X phosphate buffered saline (PBS) for 15min and placed in a series of decellularization solutions on an orbital shaker, using one of the following three protocols:
SDS: Four 2hr washes with 1.8mM sodium dodecyl sulfate (SDS), each followed by dH2O (5 min) and 2X PBS (15 min)
CHAPS: Four 2hr washes with 8mM 3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), each followed by dH2O (5 min) and 2X PBS (15 min)
3-Step method: 2hr wash with 3% Tween-20®, 2hr wash with 4% sodium deoxycholate, 1hr wash with 0.1% peracetic acid.
All slices were then subjected to alternating 1X PBS and dH2O washes (two of each). 7mm-diameter discs of decellularized tissue were then punched using a biopsy punch under sterile conditions and used in experiments. Pen/Strep (5% each) was added to all solutions to eliminate native and pathological bacteria from the lung ECM.
For all assays, three random samples were collected from 3 different lungs (n=9 for each human and porcine) before and after decellularization.
DNA
DNA content of decellularized tissues was quantified using Quant-iT™ PicoGreen® dsDNA Assay kit (Invitrogen) according to the manufacturer's instructions. Tissue samples were weighed and digested in 125μg/mL papain (Sigma) overnight at 60°C. Fluorescence was measured at 520nm with excitation at 480nm. DNA was quantified using a standard curve prepared using λ-phage DNA, and normalized to the tissue wet weight.
Histological Analysis
Tissue samples were fixed in 3.7% formalin, embedded in paraffin, and sectioned to 5μm thickness. General evaluation was performed using hematoxylin and eosin, and distributions of matrix proteins were evaluated using Masson's Trichrome (collagen), Alcian blue (proteoglycans), and Van Gieson's (elastic fibers) stains. All samples were stained at the same time and imaged under the same exposure and imaging conditions.
Collagen
Tissue samples were initially weighed and digested in 0.1mg/mL pepsin overnight at 4°C (15mg of tissue per 1mL of digest solution). Collagen was then quantified using the Sircol™ Collagen Assay kit (Biocolor) according to the manufacturer's instructions.
Sulfated GAG
Sulfated glycosaminoglycans (sGAG) were quantified using the Dimethylene Blue (DMB) Dye Assay. Tissue samples were weighed and digested in 1□□ μg/mL papain (Sigma) overnight at 60°C (45 mg tissue per mL of digest solution), mixed with DMB dye (1:8 ratio), and absorbance was immediately measured at 595nm with reference wav and normalized to the tissue wet weight.
Elastin
Elastin was quantified using the Fastin™ Elastin Assay kit (Biocolor) according to the manufacturer's instructions. Tissue samples were weighed, and water-soluble α–elastin was extracted via three hot 0.25M oxalic acid extractions, which were combined for each sample (35mg of tissue per 1mL of solution).
Scanning Electron Microscopy
Tissue samples were frozen, lyophilized, coated with a thin layer of gold under vacuum, and mounted onto a cylindrical stage for SEM imaging with a JEOL JSM 5600LV scanning electron microscope at 350X.
Immunohistochemistry
Samples for immunohistochemistry were fixed in formalin for 30min, embedded in paraffin, cut to 8μm, and mounted on slides. Sections were deparaffinized, subjected to boiling citrate buffer for 16 minutes for antigen retrieval, and blocked with 10% Normal Goat Serum in PBS for 2hrs at room temperature. Primary antibody staining was performed for 2hrs at 4°C using the following primary antibodies: Collagen IV (Rb pAb to Coll IV (ab6586) diluted 1:200), Laminin (Rb pAb to Laminin (ab11575) diluted 1:100), Elastin (Rb pAb to Elastin (ab21610) diluted 1:200), and Fibronectin (Rb pAb to Fibronectin (ab23750) diluted 1:200). The secondary antibody (Goat pAb to Rb IgG (ab98464) diluted 1:200) was incubated for 1hr at room temperature. Completed sections were mounted in Vectashield Mounting Medium with DAPI, cover-slipped, and imaged using an Olympus IX81 microscope at 10X.
Mechanical Testing
Mechanical testing was conducted with an Instron testing machine model 5848 with a 10N load cell. Using a custom-made mold, samples were cut into 3cm by 1cm pieces from randomly selected transverse sections of the lower left lobe, as detailed in Figure 7. This single orientation and the lower left lobe were selected and consistently maintained to minimize the effects of lung anisotropy on mechanical data. The two ends of the strips were secured with sandpaper to prevent slippage, mounted on the Instron, and a pre-load of 0.003N was set. Samples were kept hydrated with 1X PBS at room temperature. Uniaxial stretch of 20% was applied at a rate of 1% strain/sec and frequencies of 0.25, 0.50, or 0.75 Hz (all samples were tested at the same grip-to-grip distance for consistency).
Figure 7. Preparation of Lung Samples for Mechanical Characterization.
Slices were obtained from the lower left lobes of human and porcine lungs and decellularized by three different methods. Samples were randomly obtained from the transverse sections of the lung and tested in uniaxial tensile strain.
Cell Culture
Human lung fibroblasts (hMRC-5s) were obtained from ATCC (www.atcc.org) and cultured in DMEM with 10% FBS and 1% pen/strep under standard culture conditions. Human small airway epithelial cells (hSAECs) were kindly provided by Dr Gao and Dr Minna (Dallas, TX) and were cultured in Small Airway Growth Media from ATCC. Human adipose-derived mesenchymal stem cells (hMSCs) were kindly provided by Dr. Gimble (Baton Rouge, LA) and cultured in DMEM/F12 (1:1) with 10% fetal bovine serum (FBS) and 1% pen/strep.
Growth Study
Cells were passaged by trypsinization after 7 days, by which time 70–80% confluency had been reached, seeded onto decellularized lung punches at an initial density of 2.5×104 cells/mL lung matrix, and cultured for 7 days. Cell growth was assessed by quantifying DNA content of re-cellularized lung scaffolds using a Quant-iT™ PicoGreen® dsDNA assay kit.
Metabolic Activity
Metabolic activity of the cells during the growth study was measured using Alamar Blue® reagent according to the manufacturer's instructions (Life Technologies). The reagent was added at various time points to the cells in culture and incubated for 9-12hrs before samples were collected and absorbance was measured at 570nm with reference wavelength of 600nm.
Live/Dead Imaging
Cells were stained with a Live/Dead® kit (Invitrogen). Calcein-AM was used to indicate live cells (green), and ethidium homodimer-1 was used to indicate dead cells (red). Samples were imaged with an Olympus Fluoview FV1000 confocal microscope at 20X.
Statistics
A two-way ANOVA was performed with a Bonferroni ad hoc test. P values less than 0.05 were considered significant.
RESULTS
Decellularized human and porcine lung tissue slices appeared translucent, with visible conduits throughout the matrix (Figure 2A). Decellularization consistently removed >95% of the nuclear material (Figure 2B). H&E staining showed no discernible nuclei (Figure 2C). Histological analysis revealed retention of small anthracotic aggregations (black regions) in some human lung sections; these regions were generally avoided during imaging.
Figure 2. Characterization of Lung Decellularization using the SDS, CHAPS, and 3 Step Methods.
(A) Macroscopic images of human and porcine lung tissue slices before and after decellularization. Scale bar: 5 mm. (B) DNA quantification before and after decellularization. Data represent mean ± SE (n=9 for each group). * indicates p < 0.05. (C) H&E stain of three decellularization methods at 40X. Scale bar: 50 μm.
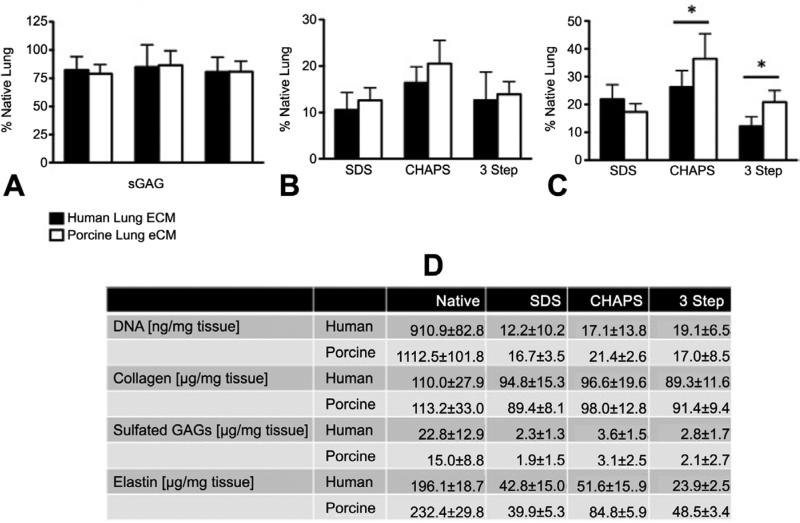
A number of assays were used to compare the relative retention of major ECM components following decellularization. Both human and porcine LECM retained 80% of total collagen regardless of the decellularization method used (Figure 3A,D). However, all three methods substantially decreased the amount of sGAG (Figure 3B,D), with no apparent differences between the human and porcine LECM. There was a significant difference between human and porcine LECM in the amount of elastin retained after decellularization using CHAPS and the 3-Step method (Figure 3C). These quantitative results were consistent with histological staining using Masson's Trichrome (collagen, blue), Alcian Blue (sGAG, blue), and Van Gieson's (elastic fibers, black) (Figure 4). Collagen IV was found in the basement membrane of both native and decellularized human and porcine tissue with no apparent differences between decellularization methods (Figure 5A). In contrast, laminin and fibronectin were not well retained in either porcine or human LECM, with fibronectin most depleted by the SDS method (Figure 5B-C). Finally, there was higher retention of elastin for CHAPS compared to the SDS and 3 Step methods (Figure 5D).
Figure 3. Characterization of collagen, sulfated glycosaminoglycans, and elastin using SDS, CHAPS, and 3 Step Method.
(A) Collagen content; (B) Sulfated glycosaminoglycan content; (C) Elastin content. Data represent mean ± SE (n=9 for each group). * indicates p < 0.05. (D) Biochemical compositions of the human and porcine lung tissues, in their native state and following decellularization by three different methods.
Figure 4. Histological evaluation of human and porcine lung tissues following decellularization by one of the three methods.
Masson's Trichrome (collagen, blue), Alcian Blue (sGAG, blue), and Van Gieson's (elastic fibers, black) staining of decellularized human and porcine lung tissue at 20X objective. Scale bar represents 100 μm.
Figure 5. Distributions of Extracellular Matrix Proteins.
Representative immunohistochemical stains are shown for: (A) Collagen IV, (B) Laminin, (C) Fibronectin, and (D) Elastin. All images were acquired with a 10X objective. Scale bar: 100μm.
The ultrastructural morphologies of human and porcine LECM before and after decellularization were similar, as evidenced by electron microscopy (Figure 6). Native lung slices showed smooth surfaces that were disrupted following decellularization, resulting in a more fibrillar structure and a rougher topographical profile. Decellularization using SDS showed the most fibrillar ultrastructure of all the methods (Figure 6). Overall, there were no major differences in ultrastructural morphology between human and porcine LECM.
Figure 6. Ultrastructure of Decellularized Lung Tissues.
Representative scanning electron micrographs are show for all experimental groups. Scale bar: 50μm.
Mechanical testing of decellularized human and porcine LECM was performed as shown in Figure 7, for transverse sections of the lower left lobe, and significant differences were observed (Figure 8A-B). Both the tangential modulus and peak stress experienced at a 20% strain were higher for human than porcine LECM. CHAPS decellularization resulted in the highest tangential modulus and peak stress for human and porcine LECM. The most compliant LECM was observed for the 3-Step and SDS methods in the human and porcine LECM, respectively. The peak stress and tangential modulus for all decellularization methods correlated with changes in elastin content, as shown in Figure 8C-D.
Figure 8. Mechanical Properties of Lung Tissue.
Representative uniaxial stress-strain curves for (A) human and (B) porcine lung ECM, in their native state and following decellularization. Linear correlation was detected between the elastin content and the maximum stress (C) and tangential modulus (D) at 20% strain for decellularized human and porcine lung ECM. Data represent Mean ± SE (n ≥ 9).
Since CHAPS-decellularized tissue showed the best retention of LECM structure, human lung fibroblasts (hMRC-5s), human small airway epithelial cells (hSAECs), and human adipose-derived mesenchymal stem cells (hMSCs) were cultured on CHAPS-decellularized human and porcine LECM. All cell types proliferated at comparable rates over a 7-day period (Figure 9), were fully viable over 7 days of culture (Figure 10A-B) and had comparable metabolic rates (Figure 10C-D). However, hSAECs were more metabolically active in human than porcine LECM over 7 days of culture (Figure 10C-D).
Figure 9. Growth Curves of Three Human Cell Types on CHAPS-Decellularized Lung Scaffolds.
Growth curves for 7 days culture of three different types of cells on CHAPS-decellularized ■ human and porcine lung ECM (A) Δ human lung fibroblasts (hMRC-5s), (B) human small airway epithelial cells (hSAECs), and (C) human adipose-derived mesenchymal stem cells (hMSCs). Data represent Mean ± SE (n ≥ 9). * p < 0.05.
Figure 10. Viability and Metabolic Activity of Three Human Cell Types on CHAPS-Decellularized Lung Scaffolds.
(A-B) Cell viability (live cells stained green for calcein-AM, dead cells stained red for ethidium homodimer-1) for human lung fibroblasts (hMRC-5s), human small airway epithelial cells (hSAECs), and human adipose-derived mesenchymal stem cells (hMSCs) after 1 and 7 days of culture on decellularized lung matrix. Scale bar: 40 μm. (C-D) Metabolic cell activity measured after 1 and 7 days of culture. Data represent Mean ± SE (n = 9).
COMMENT
The present study assessed the feasibility of using porcine lung as a source of ECM for lung tissue engineering, towards engineering a whole lung or lung lobe for transplantation. Tissue slices were decellularized using three different methods: (1) SDS, (2) CHAPS, and (3) 3 Step method consisting of Tween-20®, sodium deoxycholate, and peracetic acid. Structural changes to the lung ECM were assessed histologically and using quantitative biochemical, and biomechanical methods. In addition, the growth and metabolism of human lung fibroblasts (hMRC-5s), human small airway epithelial cells (hSAECs), and human adipose-derived mesenchymal stem cells (hMSCs) in human and porcine LECM were compared. Within the decellularization protocols and parameters investigated, CHAPS resulted in the best retention of LECM integrity and composition compared to SDS and 3 Step methods. Except for mechanical properties and the metabolism of hSAECs, a few differences were found between the human and porcine LECM.
In previous studies, whole rat lungs were decellularized via perfusion decellularization and then re-populated with neonatal rat lung epithelial cells and vascular endothelial cells [3,4,11]. Pulmonary epithelial cells attached and grew within the matrix much better than on tissue culture plates, suggesting that the acellular matrix provided important cues for directing cellular organization and growth.
Other studies have investigated the effects of different decellularization methods on the composition and re-cellularization of mouse lungs [13,14], as the first step toward engineering ex vivo functional lung tissue. However, perfusion decellularization described for mouse and rat lungs may prove more challenging for human lungs due to significant differences in size, diffusion distances, and the anatomy of the airway and vascular networks [3,4,11,13,14]. To maintain uniform decellularization of human-sized lung, it is important to understand the effectiveness of each of the reagents used and how they affect lung matrix structure. In addition, access to an abundant source of human-sized lungs is needed for in vitro testing and the development of an optimal perfusion system that would fully decellularize human lungs. To assess these parameters, we analyzed the ultrastructure, biomolecular composition, biomechanical properties (in transverse sections of the lower left lobe, to minimize the effects of lung anisotropy), and cellular attachment and growth of three relevant cell types for decellularized human and porcine lungs.
The results of the present study are consistent with those of Peterson et al., in which CHAPS (a zwitterionic detergent) was the best reagent for decellularizing lung tissue based on the greatest retention of most of the ECM proteins studied [3,15]. The effects of SDS and CHAPS on lung matrix protein retention are also consistent between the two studies.
Decellularization reduces the total amount of collagen and sulfated glycosaminoglycans to approximately 80% and 20% of native tissue, respectively (Figure 3A-B). In our study, elastin content was also significantly higher in the porcine CHAPS group than in the human CHAPS group (Figure 3C), a result that may be related to the increased compliance observed in the mechanical testing of CHAPS-decellularized porcine LECM. Although it is difficult to conclusively correlate the retention of ECM components with the mechanical behavior observed, there appears to be a relationship between the amount of elastin and the stiffness of LECM at low strain (Figure 8C-D). Similar trends can be found for collagen and sGAG content (Figure 3D). Alternatively, the relationship between ECM content and the mechanical properties may be due to the decellularization method itself and not strictly to the retention of ECM components. The specific contributions of structural proteins and tissue anisotropy to the mechanical properties of LECM remain to be investigated in greater detail.
The end-goal of decellularizing human lung tissue is to obtain a native-like scaffold for re-population with lung cells or their precursors. Since the ultrastructure of the matrix and the specific extracellular proteins and molecules it contains may provide lung-specific cues for stem cells, it is important to identify the decellularization protocol that best preserves this environment. With the exception of mechanical properties under low strains (0-20%), there appear to be parallels between human and porcine lungs based on the degradation or loss of ECM proteins during decellularization (Figures 4, 5).
Strains of up to 20% were considered based on the radial volumetric expansion of lungs at inspiratory capacity. Differences between the stress and tangential modulus at 20% strain in human and porcine lungs (Figures 8A-B) could be a result of storage at −80°C prior to processing and testing. Other possibilities include differences in the health and age of the human and porcine lungs tested. While porcine lungs came from healthy pigs, human lungs were those rejected for transplantation that underwent changes in their LECM related to the reason for rejection. Additionally, older tissues may have more cross-linking and different overall distributions of fibers. The exact reason for this mechanical difference between human and porcine LECM remains unknown.
Three cell types were cultured on slices of human and porcine LECM to determine if the decellularization method affected cellular attachment and growth. CHAPS-decellularized human and porcine LECM showed similar growth rates for human lung fibroblasts (hMRC-5s), small airway epithelial cells (hSAECs), and human adipose-derived mesenchymal stem cells (hMSCs), suggesting that all three cell types attach and proliferate at similar rates (Figure 9). These findings obtained for slices of human and porcine lungs decellularized on a shaker are consistent with previous studies of perfusion decellularization of mouse lungs [14]. In contrast to previous studies, our study showed substantial growth of hMSCs on LECM [13]. Interestingly, hSAECs displayed higher mitochondrial activity on human LECM when compared to those on porcine LECM (Figure 10C-D). While epithelial cells may be more sensitive to species-specific matrix, the exact reasons remain unknown.
In summary, our study suggests that porcine lung is a promising substitute for human lung for in vitro studies of decellularization and human cell-ECM interactions for lung tissue engineering. Porcine lungs can serve as the platform to optimize decellularization protocols for eventual translation to human lungs. Since all donor human lungs that meet transplant criteria are used for transplantation, an alternative source of lungs is needed for research. Our data show that porcine lung ECM serves as a suitable and readily available substitute for human lung tissue for pulmonary tissue engineering research and applications.
ACKNOWLEDGEMENTS
We are grateful to Dr Boning Gao and Dr John Minna (University of Texas Southwestern Medical Center, Dallas, TX) for providing human small airway epithelial cells, Dr Jeff Gimble (Louisiana State University, Baton Rouge, LA) for providing human adipose-derived mesenchymal stem cells, and Dr Dr. Kara Spiller and Dr. Sarah Huang for their expert advice. Funding support for this work was provided by the Irving Institute for Translational Research (CaMPR grant UL1RR024156).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting Presentation: 48th Annual Meeting of the Society of Thoracic Surgeons. Fort Lauderdale, Florida, January 29th, 2012.
REFERENCES
- 1.Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR). OPTN / SRTR 2010 Annual Data Report. H.R.a.S.A. Department of Health and Human Services, Healthcare Systems Bureau, Division of Transplantation; 2011. pp. 108–110. [Google Scholar]
- 2.Petersen TH, et al. Bioreactor for the LongTerm Culture of Lung Tissue. Cell transplantation. 2010 doi: 10.3727/096368910X544933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen TH, et al. Tissue-engineered lungs for in vivo implantation. Science (New York, NY) 2010;329(5991):538–541. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song JJ, et al. Enhanced in vivo function of bioartificial lungs in rats. The Annals of thoracic surgery. 2011;92(3):998–1005. doi: 10.1016/j.athoracsur.2011.05.018. discussion 1005-6. [DOI] [PubMed] [Google Scholar]
- 5.Badylak SF, Taylor D, Uygun K. Whole-Organ Tissue Engineering: Decellularization and Recellularization of Three-Dimensional Matrix Scaffolds. Annual review of biomedical engineering. 2010 doi: 10.1146/annurev-bioeng-071910-124743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGowan SE. Extracellular matrix and the regulation of lung development and repair. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1992;6(11):2895–2904. [PubMed] [Google Scholar]
- 7.Petersen T, Calle E. Strategies for lung regeneration. Materials Today. 2011 [Google Scholar]
- 8.Song JJ, Ott HC. Organ engineering based on decellularized matrix scaffolds. Trends in molecular medicine. 2011;17(8):424–432. doi: 10.1016/j.molmed.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Sellaro TL, et al. Maintenance of human hepatocyte function in vitro by liver- derived extracellular matrix gels. Tissue Eng Part A. 2010;16(3):1075–82. doi: 10.1089/ten.tea.2008.0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sellaro TL, et al. Maintenance of hepatic sinusoidal endothelial cell phenotype in vitro using organ-specific extracellular matrix scaffolds. Tissue Eng. 2007;13(9):2301–10. doi: 10.1089/ten.2006.0437. [DOI] [PubMed] [Google Scholar]
- 11.Ott HC, et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16(8):927–33. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 12.Chu CR, Szczodry M, Bruno S. Animal models for cartilage regeneration and repair. Tissue Eng Part B Rev. 2010;16(1):105–15. doi: 10.1089/ten.teb.2009.0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daly AB, et al. Initial binding and recellularization of decellularized mouse lung scaffolds with bone marrow-derived mesenchymal stromal cells. Tissue Eng Part A. 2012;18(1-2):1–16. doi: 10.1089/ten.tea.2011.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallis JM, et al. Comparative assessment of detergent-based protocols for mouse lung de-cellularization and re-cellularization. Tissue Eng Part C Methods. 2012;18(6):420–32. doi: 10.1089/ten.tec.2011.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petersen TH, et al. Matrix Composition and Mechanics of Decellularized Lung Scaffolds. Cells, tissues, organs. 2012;195(3):222–31. doi: 10.1159/000324896. [DOI] [PMC free article] [PubMed] [Google Scholar]