Abstract
The resulting pain is the main symptom of acute pancreatitis and it should be alleviated as soon as possible. NSAIDs are the first line therapy for pain and they are generally administered to acute pancreatitis patients upon admission to the hospital. In addition, these drugs have also been used to prevent post-endoscopic cholangiopancreatography (ERCP) acute pancreatitis. On the other hand, there are several reports indicating that NSAIDs may be the actual cause of acute pancreatitis. We carried out a literature search on PubMed/MEDLINE; all full text papers published in from January 1966 to November 2009 on the use of NSAIDs in acute pancreatitis were collected; the literature search was also supplemented by a review of the bibliographies of the papers evaluated. Thus, in this article, we will systematically review the current literature in order to better illustrate the role of NSAIDs in acute pancreatitis, in particular: i) NSAIDs as a cause of acute pancreatitis; ii) their use to prevent post-retrograde ERCP pancreatitis and iii) their efficacy for pain relief in the acute illness of the pancreas.
Keywords: acute pancreatitis, cytokines, inflammation, arachidonic acid, prostaglandins, leukotrienes, phospholipase A2
1. Introduction
Pain is the main feature of acute pancreatitis (AP) and the majority of patients are admitted to the hospital for this reason. Management of AP is limited to supportive care and the treatment of complications when they develop. These patients require regular hospital admission, fluid administration, bowel rest and pain management.
There are no extensive studies on the pharmacological control of pain in acute pancreatitis patients [1,2,3,4], which is quite surprising given the importance of this symptom during the course of the disease. There is also a lack of evidence regarding the degree of efficacy of the various pharmacological substances used to treat the different forms of acute pancreatitis. On the other hand, there are several reports concerning the possibility that non-steroidal anti-inflammatory drugs (NSAIDs) may actually induce acute pancreatitis [5,6,7,8,9,10,11,12,13,14,15,16,17]. NSAIDs have also been used to prevent retrograde endoscopic cholangiopancreatography (ERCP)-induced acute pancreatitis [18]. Thus, in this article, we will systematically review the current literature in order to better illustrate the role of NSAIDs in acute pancreatitis, in particular i) NSAIDs as a cause of acute pancreatitis; ii) their use in preventing post-ERCP pancreatitis and iii) their efficacy for pain relief in the acute illness of the pancreas.
2. Literature Search
A search was made using the U.S. National Library of Medicine National Institutes of Health PubMed/MEDLINE database in order to select the data existing in the literature on NSAIDs and acute pancreatitis covering the period from January 1966 to November 2009. The Medical Subject Headings (MESH) terms used were “Anti-inflammatory Agents, Non-Steroidal” (explanatory variable) and “Pancreatitis” or “Pancreatitis, Acute Necrotizing” (outcome variables). We identified additional studies through a physical search of bibliographies from primary studies, review articles and key journals, and through contacts with experts in the field. For studies with multiple re-analyses, only the most recent article with the largest population was chosen. The investigators independently screened all articles for those that met broad inclusion criteria. A total of 92 citations were found [1,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95]. Of these 92 papers, 79 were excluded [5,6,7,8,9,10,11,12,13,14,15,16,17,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84] because they contained data regarding diseases other than acute pancreatitis and used drugs other than NSAIDs, or were case reports or letters, comments and review articles not containing data which met the aims of the study or were duplicate publications. Thus, 13 papers were considered for the present study; they were four studies [92,93,94,95] regarding the incidence of acute pancreatitis in NSAID consumers, five randomized studies [18,87,88,89,90] regarding the prophylactic use of NSAIDs in preventing post-ERCP pancreatitis together two meta-analytic studies on this topic [85,86] and two studies regarding the efficacy of NSAIDs in the pain control in acute pancreatitis patients [1,91].
3. NSAIDs as Inducers of Acute Pancreatitis
There are numerous case reports on the association of the use of indomethacin, piroxicam, ketoprofen, naproxen, rofecoxib and celecoxib with acute pancreatitis. Even if these reports are of importance in pointing out the possible risks of this class of drugs, we should stress the fact that NSAIDs are widely used in the general population of developed countries. A study was carried out in Italy between March and September 2002 evaluating the use of generic drugs in a free-living population demonstrated that 20% of the NSAID users were over 65 years of age and 18% were chronic users (daily or frequent use for more than six months). NSAID use was significantly higher in women, both for overall and for chronic use. The older age groups showed an increasing risk of chronic NSAID use because the presence of headache (25%), osteoarticular pain (19%), unspecified pain (15%) and osteoarthrosis (9%). More than 50% of all the NSAIDs were prescribed by physicians whereas about 44% were taken as self-treatment or following the advice of a pharmacist, relative, friend, etc. Thus, two studies exploring the frequency of acute pancreatitis during the course of NSAID assumption found that this phenomenon is negligible: Ibanez et al. [95] studied a total of 48,678 hospitalized patients using the medical records and 554 (1.1%) were diagnosed as experiencing an adverse drug reaction. After excluding upper gastrointestinal bleeding (226 cases) and certain bone marrow blood dyscrasias (42 cases), 286 patients with drug-induced events leading to hospital admission were identified in two years. Six cases of drug-induced pancreatitis were found: three related to diclofenac, one to oral contraceptives, one related to clomifene, and the remaining one to chlortalidone. In addition, biliary lithiasis was identified in two of the patients with diclofenac-related pancreatitis and in the clomifene-related case, and although the stones were not located in the common bile duct, this finding could also explain the biliary etiology of pancreatitis. In conclusion, only one case were considered by the authors related to NSAIDs use. These data were confirmed by a population-based study carried out in 1993 [93]. In this latter study the authors found that a causal relationship of pancreatitis attributed to piroxicam was found in only one case out of 100,000 users of diclofenac, naproxen or piroxicam. On the contrary, in a case-control study carried out in Sweden in 2002 [94], among 2,453 acute pancreatitis patients and 2,245 controls, the authors found that only one case and three controls had taken indomethacin, but 25 cases and 36 controls had taken diclofenac and, in a multivariate analysis, the adjusted odds ratio (OR) was 2.1 (95% CI: 1.2–3.4) for the use of NSAIDs between cases and controls. Furthermore, in a population-based case-control study including 3,083 cases of acute pancreatitis and 30,830 population controls [92], it was found that 0.7% of the cases and 0.4% of controls were current users (within 90 days before admission into the study) of celecoxib and 0.6% of cases and 0.4% of controls were former users (within 91 to 365 days before admission into the study) of this drug; 0.6% of the cases and 0.4% of controls were current users of rofecoxib and 0.8% of cases and 0.4% of controls were former users of rofecoxib; finally, 18.2% of the cases and 7.4% of controls were current users of NSAIDs and 11.9% of cases and 9.8% of controls were former users of NSAIDs. Thus, the adjusted OR for other non-steroidal anti-inflammatory drugs was 2.7 (95% CI: 2.4–3.0) with a substantial variation in risk between the individual drugs; the highest risk was for diclofenac (OR 5.0, 95% CI: 4.2–5.9) and the lowest for naproxen (OR 1.1, 95% CI: 0.7–1.7). In conclusion, there is a risk for acute pancreatitis patients taking NSAIDs and, in clinical practice, it seems that naproxen should be the preferred analgesic for limiting the risk of development of acute pancreatitis.
4. The Prophylactic Use of NSAIDs for Preventing Post-ERCP Acute Pancreatitis
In various prospective studies, the frequency of post-ERCP pancreatitis ranges from 1 to 14%. After exposure to trigger events, injury to the gland occurs in an extremely rapid time interval. In experimental models of acute pancreatitis, it has been suggested that digestive enzyme activation might occur within the acinar cells and it has been shown that, in the early stages of acute pancreatitis induced by secretagogues or by diet, there is a co-localization of digestive enzymes and lysosomal hydrolases within large cytoplasm vacuoles; this co-localization mechanism might result in activation of the digestive enzyme. The trigger events which may determine the final effect of acute pancreatitis during ERCP is still unknown; mechanical, chemical, enzymatic, and microbiological factors may be involved as well as factors related to the patient and the physician [97]. Finally, the hypothesis of the activation of chemokines by endoscopic maneuvers as a cause of acute pancreatitis cannot be ruled out [97]. Recent studies [98,99,100,101] have indicated the usefulness of ERCP as a model for studying the early inflammatory response in acute pancreatitis. In their study, Kiviniemi et al. [99] found that, in uncomplicated cases, acute phase response determined by serum C-reactive protein levels was rare and did not parallel the serum amylase or lipase levels. However, Blanchard et al. [102] hypothesized that cytokines may be produced primarily by pancreatic parenchymal cells. Reasoning that the ductal epithelium is the cell type most likely to be exposed to noxious stimuli in common causes of pancreatitis, such as ERCP and passage of a gallstone, they examined the response of well-differentiated pancreatic ductal adenocarcinoma cell lines to stimuli known to stimulate cytokine production in other cells. CAPAN-1 and CAPAN-2 cells were incubated with endotoxins or TNF-alpha and the supernatant was assayed for production of IL-1, IL-6 and IL-8 by ELISA. The cells were assayed for activation of the transcription factor NF-kappa B by electrophoretic mobility shift assay. These authors found no detectable production of IL-1 by either cell line. CAPAN-1 cells had a concentration-dependent production of IL-6 and IL-8 in response to both endotoxins and TNF-alpha. CAPAN-2 cells had a concentration-dependent production of IL-6 and IL-8 in response to TNF-alpha. They had low level expression of IL-8 which was unaffected by any concentration of lipopolysaccharide (LPS) and no detectable production of IL-6 in response to LPS. On the basis of these findings, the authors concluded that pancreatic duct cells may play an active part in the pathogenesis of acute pancreatitis through the production of cytokines. More recently, we also found [103] that ERCP maneuvers significantly increase the serum levels of C-reactive protein, amyloid A and IL-6 in patients who did not develop acute pancreatitis, thus confirming the data of Blanchard et al. [102]. However, the administration of IL-10 in reducing the incidence of pancreatitis after therapeutic ERCP in humans is still under debate [104,105]. NSAIDs are potent inhibitors of prostaglandins, phospholipase A2 and neutrophil-endothelial interaction; all of these are believed to play an important role in the pathogenesis of acute pancreatitis [106,107,108]. NSAIDs are also inexpensive, easily administered and have a favorable risk profile when given as a one-time dose, making them an attractive option in the pharmacological prevention of post-procedural pancreatitis [86]. Five studies have been published in the last decade on the efficacy of NSAIDs in preventing pancreatitis induced by ERCP [18,87,88,89,90]. Three studies utilized diclofenac; in two studies, diclofenac was administered in the form of a suppository at a dosage of 100 mg immediately after ERCP [18,87] and, in the third study, diclofenac was given by mouth at a dosage of 50 mg 30–90 min before the ERCP and 4–6 h after the procedure. Another two studies utilized indomethacin [88,90], given as suppositories at a dosage of 100 mg before ERCP examination. All the studies except one [87] demonstrated that NSAIDs prevented post-procedural acute pancreatitis in a significant manner. Two subsequent meta-analyses [85,86] on this topic concluded that the widespread prophylactic administration of NSAIDs may significantly reduce the risk of acute pancreatitis after ERCP, resulting in major clinical and economic benefits. However, we should point out that even if the studies considered were randomized controlled trials, they had different modalities of ERCP procedures (e.g., number of cannulations, number of pancreatic duct injections, whether a sphincterotomy was performed) as well as in pharmacological manipulation (e.g., choice of drug, route of delivery, timing of administration); all these differences render the studies heterogeneous and suggest the need to plan more rigorous studies on this topic.
5. NSAIDs for Treating Pain in Acute Pancreatitis
The pathological activation of sensory neurons and inflammatory sequelae constitute what is known as neurogenic inflammation which appears to be important in many organ systems, including the pancreas. The destruction of the pancreatic parenchyma during acute pancreatitis quickly induces an inflammatory reaction at the site of injury. The initial cellular response involves the infiltration of polymorphonuclear leukocytes into the perivascular regions of the pancreas. Within a few hours, macrophages and lymphocytes accumulate and phagocyte-derived oxygen radicals participate in a primary injury to the pancreatic capillary endothelial cells. The increased microvascular permeability facilitates margination and extravascular migration of additional neutrophils and monocytes, amplifying the inflammatory process [109] and leads to many metabolic consequences including pain, fever, hypotension, acidosis and acute respiratory distress syndrome. Pain in acute pancreatitis is also due to the release of tachykinin substance P and calcitonin-gene-related peptide. Factors which stimulate primary sensory neurons include hydrogen ions, heat, leukotrienes, arachidonic acid metabolites, bradykinins and proteases, such as trypsin [110].
The mechanisms of action of NSAIDs come from their ability to inhibit cyclooxygenase-dependent prostanoid formation [111]. Other NSAID mechanisms which have recently been proposed, but have not been completely proven, include intracellular inhibition of phosphodiesterase, inhibition of bradykinin levels, and the uncoupling of G-protein-membrane protein interactions [112,113]. Other studies have suggested that the major analgesic mechanism for NSAIDs is through a central mechanism while the major anti-inflammatory mechanism occurs at a peripheral site of action [114].
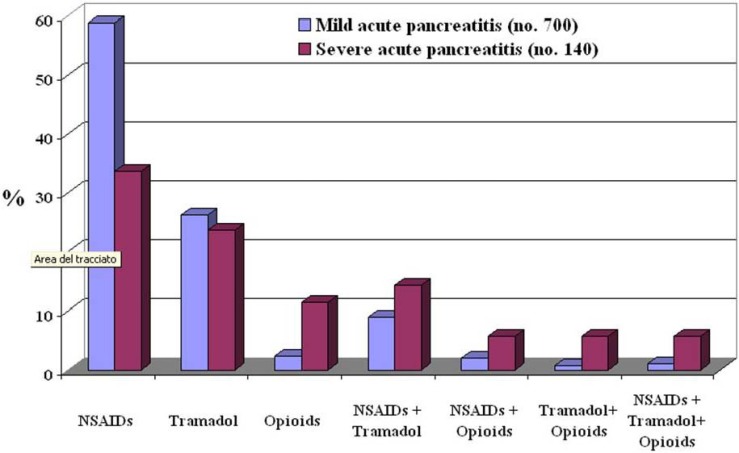
Only two studies exist in the literature on pain control with NSAIDs in patients with acute pancreatitis. The first paper was published in 1985 [1] and the other one in 2008 [91]. In the first controlled double-blind study, indomethacin, in the form of suppositories at a dosage of 50 mg twice daily for seven consecutive days, was compared to identical-looking placebo suppositories. There were 14 patients in the active treatment group and 16 in the placebo group. As expected, the number of days with pain and the number of opiate injections were significantly less in patients treated with indomethacin; most importantly, bleeding from the gastrointestinal tract was not seen in the indomethacin-treated group. In the second study, metamizole was compared to morphine; 8 patients with acute pancreatitis were randomized to receive 10 mg of morphine subcutaneously every 4 h and 8 patients received 2 g every 8 h of metamizole intravenously. Pain scores were recorded every 4 h during the first 48 h after admission using a visual analogical scale. Pethidine was also additionally administered as a rescue therapy. Seventy-five percent of the patients achieved pain relief in the metamizole group versus 37.5% in the morphine group within 24 h of hospitalization, but this difference was not statistically significant; the mean time for achieving pain relief was shorter in the metamizole group even if in a non-significant manner. At the end of the study (48 h after admission), 75% of patients achieved pain relief in the metamizole group versus 50% in the morphine group. Three patients in each group needed pethidine; two out of three achieved pain control in the metamizole group vs. none of the three in the morphine group. From the data of this study, intravenous metamizole shows a non-significant association with quicker pain relief than morphine given subcutaneously in acute pancreatitis but, most importantly, this drug seems to have the same efficacy as opiates in controlling acute pancreatitis pain. Currently, there is only one study which has evaluated how pain is routinely treated in patients with acute pancreatitis [115]. This study demonstrates that analgesics were graded according to the severity of the pain. In fact, patients with mild acute pancreatitis received mainly NSAIDs and tramadol whereas patients with severe pancreatitis received a high percentage of opioids or an association of analgesics comprising NSAIDs, tramadol and opioids. Data on analgesic administration were available in 840 of the 1,173 patients (71.6%) (Figure 1); NSAIDs were administered in 459 patients (54.6%), tramadol in 216 (25.7%), opioids in 32 (3.8%) and a combination of the various drugs in the remaining 133 (13.8%) patients. There was a significant difference in the type of analgesics administered between patients with mild and those with severe disease (P < 0.001); NSAIDs and tramadol were used more frequently in patients with mild disease whereas opioids associated with NSAIDs and or tramadol were used more frequently in patients with severe disease. The duration of analgesic treatment was significantly longer in patients with severe acute pancreatitis in comparison to those suffering from the mild form; NSAIDs: 3.0 ± 2.3 days in patients with mild acute pancreatitis and 6.7 ± 10.1 days in patients with severe acute pancreatitis (P < 0.001); tramadol: 3.2 ± 2.3 days in patients with mild acute pancreatitis and 8.0 ± 11.7 days in patients with severe acute pancreatitis (P < 0.001).
Figure 1.
Type of analgesics administered to 700 patients with mild acute pancreatitis and to 140 with severe acute pancreatitis.
6. Conclusions
The answers to the questions posed as to whether NSAIDs may cause acute pancreatitis, whether their prophylactic use is able to prevent post-ERCP pancreatitis, and whether they are capable of controlling pain in acute pancreatitis are the following: 1) there is a risk for acute pancreatitis associated with the use of NSAIDs and, in clinical practice, it seems that naproxen should be the preferred analgesic in limiting the risk of development of acute pancreatitis; 2) both diclofenac and indomethacin may significantly reduce the risk of acute pancreatitis after ERCP resulting in major clinical and economic benefits and, finally, 3) NSAIDs are able to control the pain in acute pancreatitis patients. However, further clinical studies on the best NSAID to be used in clinical practice are needed. An example comes from the use of diclofenac; this is a drug largely used to treat pain in acute pancreatitis. It is useful in preventing post-ERCP pancreatitis, but it is considered the major NSAID responsible for inducing acute pancreatitis in the general population.
References and Notes
- 1.Ebbehoj N., Friis J., Svendsen L.B., Bulow S., Madsen P. Indomethacin treatment of acute pancreatitis. A controlled double-blind trial. Scand. J. Gastroenterol. 1985;20:798–800. doi: 10.3109/00365528509088825. [DOI] [PubMed] [Google Scholar]
- 2.Jakobs R., Adamek M.U., von Bubnoff A.C., Riemann J.F. Buprenorphine or procaine for pain relief in acute pancreatitis. A prospective randomized study. Scand. J. Gastroenterol. 2000;35:1319–1323. doi: 10.1080/003655200453692. [DOI] [PubMed] [Google Scholar]
- 3.Stevens M., Esler R., Asher G. Transdermal fentanyl for the management of acute pancreatitis pain. Appl. Nurs. Res. 2002;15:102–110. doi: 10.1053/apnr.2002.29532. [DOI] [PubMed] [Google Scholar]
- 4.Kahl S., Zimmermann S., Pross M., Schulz H.U., Schmidt U., Malfertheiner P. Procaine hydrochloride fails to relieve pain in patients with acute pancreatitis. Digestion. 2004;69:5–9. doi: 10.1159/000076541. [DOI] [PubMed] [Google Scholar]
- 5.Mennecier D., Ceppa F., Sinayoko L., Corberand D., Harnois F., Thiolet C., Farret O. Acute pancreatitis after treatment by celecoxib. Gastroenterol. Clin. Biol. 2007;31:668–669. doi: 10.1016/s0399-8320(07)91915-6. [DOI] [PubMed] [Google Scholar]
- 6.Heluwaert F., Pofelski J., Germain E., Roblin X. Piroxicam and acute pancreatitis. Gastroenterol. Clin. Biol. 2006;30:635–636. doi: 10.1016/s0399-8320(06)73248-1. [DOI] [PubMed] [Google Scholar]
- 7.Mete D., Milon A., Belon G., Gatina J.H. Acute pancreatitis and ketoprofen. Gastroenterol. Clin. Biol. 2001;25:721–722. [PubMed] [Google Scholar]
- 8.Maroy B. Benign acute pancreatitis probably due to taking ketoprofen. Therapie. 1998;53:602–603. [PubMed] [Google Scholar]
- 9.Flamenbaum M., Abergel A., Marcato N., Zenut M., Kemeny J.L., Cassan P. Regressive fulminant hepatitis, acute pancreatitis and renal insufficiency after taking ketoprofen. Gastroenterol. Clin. Biol. 1998;22:975–976. [PubMed] [Google Scholar]
- 10.Aygencel G., Akbuga B., Keles A. Acute pancreatitis following naproxen intake. Eur. J. Emerg. Med. 2006;13:372. doi: 10.1097/01.mej.0000224428.51623.b2. [DOI] [PubMed] [Google Scholar]
- 11.Mahjoub W., Jarboui S., Ben Moussa M., Abdesselem M.M., Zaouche A. Indomethacin-induced pancreatitis: A second case report. JOP. 2006;7:321–323. [PubMed] [Google Scholar]
- 12.Memis D., Akalin E., Yucel T. Indomethacin-induced pancreatitis: A case report. JOP. 2005;6:344–347. [PubMed] [Google Scholar]
- 13.Nind G., Selby W. Acute pancreatitis: A rare complication of celecoxib. Intern. Med. J. 2002;32:624–625. doi: 10.1046/j.1445-5994.2002.00298.x. [DOI] [PubMed] [Google Scholar]
- 14.Amaravadi R.K., Jacobson B.C., Solomon D.H., Fischer M.A. Acute pancreatitis associated with rofecoxib. Am. J. Gastroenterol. 2002;97:1077–1078. doi: 10.1111/j.1572-0241.2002.05646.x. [DOI] [PubMed] [Google Scholar]
- 15.Baciewicz A.M., Sokos D.R., King T.J. Acute pancreatitis associated with celecoxib. Ann. Intern. Med. 2000;132:680. doi: 10.7326/0003-4819-132-8-200004180-00027. [DOI] [PubMed] [Google Scholar]
- 16.Carrillo-Jimenez R., Nurnberger M. Celecoxib-induced acute pancreatitis and hepatitis: A case report. Arch. Intern. Med. 2000;160:553–554. doi: 10.1001/archinte.160.4.553. [DOI] [PubMed] [Google Scholar]
- 17.Castiella A., Lopez P., Bujanda L., Arenas J.I. Possible association of acute pancreatitis with naproxen. J. Clin. Gastroenterol. 1995;21:258. doi: 10.1097/00004836-199510000-00022. [DOI] [PubMed] [Google Scholar]
- 18.Murray B., Carter R., Imrie C., Evans S., O'Suilleabhain C. Diclofenac reduces the incidence of acute pancreatitis after endoscopic retrograde cholangiopancreatography. Gastroenterology. 2003;124:1786–1791. doi: 10.1016/s0016-5085(03)00384-6. [DOI] [PubMed] [Google Scholar]
- 19.Kim K.H., Kim T.N., Jang B.I. A case of acute pancreatitis caused by 5-aminosalicylic acid suppositories in a patient with ulcerative colitis. Korean J. Gastroenterol. 2007;50:379–383. [PubMed] [Google Scholar]
- 20.Montano Loza A., Garcia Correa J., Gonzalez Ojeda A., Fuentes Orozco C., Davalos Cobian C., Rodriguez Lomeli X. Prevention of hyperamilasemia and pancreatitis after endoscopic retrograde cholangiopancreatography with rectal administration of indomethacin. Rev. Gastroenterol. Mex. 2006;71:262–267. [PubMed] [Google Scholar]
- 21.Vlasov A.I., Berezin V.A., Gerasimenko A.V., Mosina L.M., Saushev I.V. Dimephosphon in complex therapy of acute edematous pancreatitis. Vestn. Khir. Im. I. I. Grek. 2003;162:81–84. [PubMed] [Google Scholar]
- 22.Bulychev V.F., Vakhrushev Ia.M. Therapy of patients with chronic pancreatitis of alcoholic etiology by dalagrin and laser therapy of the blood. Klin. Med. (Mosk) 2000;78:43–45. [PubMed] [Google Scholar]
- 23.Schworer H., Ramadori G. Acute pancreatitis--adverse effect of 5-aminosalicylic acid (mesalazine) in various galenic dosage forms. Dtsch. Med. Wochenschr. 2000;125:1328–1330. doi: 10.1055/s-2000-8072. [DOI] [PubMed] [Google Scholar]
- 24.Glintborg B. Pancreatitis in a patient with Crohn disease treated with mesalazine and azathioprine. Ugeskr. Laeger. 2000;162:4553–4554. [PubMed] [Google Scholar]
- 25.Miyasaka Y., Ono K., Nagayama K., Murakami T., Noguchi O., Uchihara M., Izumi N., Miyake S., Kubota K., Enomoto N., Tanaka Y., Marumo F., Sato C. A case of pancreatic pleural effusion and ascites treated successfully with conservative measures including octreotide and nafamostat mesilate. Nippon Shokakibyo Gakkai Zasshi. 1996;93:937–941. [PubMed] [Google Scholar]
- 26.Skomarovskii A.T., Zemskov V.S., Skomarovskii A.A. Use of dalargin in the treatment of acute cholecystopancreatitis complicated by obstructive jaundice. Klin. Khir. 1996;8:13–14. [PubMed] [Google Scholar]
- 27.Zheng M.H., Xia H.H., Chen Y.P. Rectal administration of NSAIDs in the prevention of post-ERCP pancreatitis: A complementary meta-analysis. Gut. 2008;57:1632–1633. [PubMed] [Google Scholar]
- 28.Lankisch P.G. Indomethacin may reduce the incidence and severity of acute pancreatitis after ERCP. Am. J. Gastroenterol. 2008;103:244. doi: 10.1111/j.1572-0241.2007.01562_5.x. [DOI] [PubMed] [Google Scholar]
- 29.Bai Y., Duowu Z., Zhaoshen L. Is indomethacin a new hope for post-ERCP pancreatitis? Am. J. Gastroenterol. 2007;102:2103. doi: 10.1111/j.1572-0241.2007.01324_1.x. [DOI] [PubMed] [Google Scholar]
- 30.Wagh M.S., Sherman S. Indomethacin for post-ERCP pancreatitis prophylaxis: Another attempt at the Holy Grail. Am. J. Gastroenterol. 2007;102:984–986. doi: 10.1111/j.1572-0241.2007.01163.x. [DOI] [PubMed] [Google Scholar]
- 31.Antonopoulos S., Mikros S., Kokkoris S., Protopsaltis J., Filioti K., Karamanolis D., Giannoulis G. A case of acute pancreatitis possibly associated with combined salicylate and simvastatin treatment. JOP. 2005;6:264–268. [PubMed] [Google Scholar]
- 32.Boix E., Lopez P., Perez-Mateo M., Pico A. Lanreotide autogel is a therapeutic option for patients who develop acute pancreatitis after somatostatin analog treatment. J. Endocrinol. Invest. 2004;27:613–614. doi: 10.1007/BF03347488. [DOI] [PubMed] [Google Scholar]
- 33.Machala W., Wachowicz N., Komorowska A., Gaszynski W. The use of drotrecogin alfa (activated) in severe sepsis during acute pancreatitis - two case studies. Med. Sci. Monit. 2004;10:CS31–CS36. [PubMed] [Google Scholar]
- 34.Oiofinlade O. Diclofenac reduces the incidence of acute pancreatitis after endoscopic retrograde cholangiopancreatography. Gastroenterology. 2004;126:632. doi: 10.1053/j.gastro.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 35.O'Connor A.S., Navab F., Germain M.J., Freeman J.K., Mulhern J.G., O'Shea M.H., Lipkowitz G.S., Madden R.L., Braden G.L. Pancreatitis and duodenitis from sarcoidosis, successful therapy with mycophenolate mofetil. Dig. Dis. Sci. 2003;48:2191–2194. doi: 10.1023/b:ddas.0000004525.62906.29. [DOI] [PubMed] [Google Scholar]
- 36.Toubanakis C., Batziou E., Sipsas N., Galanopoulos G., Tzivras M., Archimandritis A. Acute pancreatitis after long-term therapy with mesalazine, and hyperamylasaemia associated with azathioprine in a patient with ulcerative colitis. Eur. J. Gastroenterol. Hepatol. 2003;15:933–934. doi: 10.1097/00042737-200308000-00019. [DOI] [PubMed] [Google Scholar]
- 37.Freeman M.L. Prevention of post-ERCP pancreatitis: Pharmacologic solution or patient selection and pancreatic stents? Gastroenterology. 2003;124:1977–1980. doi: 10.1016/S0016-5085(03)00553-5. [DOI] [PubMed] [Google Scholar]
- 38.Famularo G., Bizzarri C., Nicotra G.C. Acute pancreatitis caused by ketorolac tromethamine. J. Clin. Gastroenterol. 2002;34:283–284. doi: 10.1097/00004836-200203000-00021. [DOI] [PubMed] [Google Scholar]
- 39.Yang C.C., Deng J.F., Lin T.J. Pancytopenia, hyperglycemia, shock, coma, rhabdomyolysis, and pancreatitis associated with acetaminophen poisoning. Vet. Hum. Toxicol. 2001;43:344–347. [PubMed] [Google Scholar]
- 40.Adachi E., Okazaki K., Matsushima Y., Seno H., Uchida K., Nakase H., Kawanami C., Nakamura T., Chiba T. Acute pancreatitis secondary to 5-aminosalicylic acid therapy in a patient with ulcerative colitis. Int. J. Pancreatol. 1999;25:217–221. doi: 10.1007/BF02925970. [DOI] [PubMed] [Google Scholar]
- 41.Fernandez J., Sala M., Panes J., Feu F., Navarro S., Teres J. Acute pancreatitis after long-term 5-aminosalicylic acid therapy. Am. J. Gastroenterol. 1997;92:2302–2303. [PubMed] [Google Scholar]
- 42.Debongnie J.C., Dekoninck X. Sulfasalazine, 5-ASA and acute pancreatitis in Crohn's disease. J. Clin. Gastroenterol. 1994;19:348–349. doi: 10.1097/00004836-199412000-00024. [DOI] [PubMed] [Google Scholar]
- 43.Choi C.W., Kang D.H., Kim G.H., Eum J.S., Lee S.M., Song G.A., Kim D.U., Kim I.D., Cho M. Nafamostat mesylate in the prevention of post-ERCP pancreatitis and risk factors for post-ERCP pancreatitis. Gastrointest. Endosc. 2009;69:e11–e18. doi: 10.1016/j.gie.2008.10.046. [DOI] [PubMed] [Google Scholar]
- 44.Chen C., Xu S., Wang W.X., Ding Y.M., Yu K.H., Wang B., Chen X.Y. Rosiglitazone attenuates the severity of sodium taurocholate-induced acute pancreatitis and pancreatitis-associated lung injury. Arch. Med. Res. 2009;40:79–88. doi: 10.1016/j.arcmed.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 45.Xiping Z., Jie Z., Qin X., Guanghua F., Yang C., Tongfa J., Qi X. Influence of baicalin and octreotide on NF-kappaB and p-selectin expression in liver and kidney of rats with severe acute pancreatitis. Inflammation. 2009;32:1–11. doi: 10.1007/s10753-008-9096-9. [DOI] [PubMed] [Google Scholar]
- 46.Sha H., Ma Q., Jha R.K., Xu F., Wang L., Wang Z., Zhao Y., Fan F. Resveratrol ameliorates hepatic injury via the mitochondrial pathway in rats with severe acute pancreatitis. Eur. J. Pharmacol. 2008;601:136–142. doi: 10.1016/j.ejphar.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 47.Xiping Z., Hua T., Hanqing C., Li C., Zhiwei W., Keyi W., Wei Y., Yun L., Qingyu L., Qing H., Fei W. The protecting effects and mechanisms of Baicalin and Octreotide on heart injury in rats with SAP. Mediators Inflamm. 2007;2007:19469. doi: 10.1155/2007/19469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barkay O., Niv E., Santo E., Bruck R., Hallak A., Konikoff F.M. Low-dose heparin for the prevention of post-ERCP pancreatitis: A randomized placebo-controlled trial. Surg. Endosc. 2008;22:1971–1976. doi: 10.1007/s00464-007-9738-2. [DOI] [PubMed] [Google Scholar]
- 49.Wang L., Ma Q., Chen X., Sha H., Ma Z. Effects of resveratrol on calcium regulation in rats with severe acute pancreatitis. Eur. J. Pharmacol. 2008;580:271–276. doi: 10.1016/j.ejphar.2007.10.068. [DOI] [PubMed] [Google Scholar]
- 50.Cheng B.Q., Liu C.T., Li W.J., Fan W., Zhong N., Zhang Y., Jia X.Q., Zhang S.Z. Ethyl pyruvate improves survival and ameliorates distant organ injury in rats with severe acute pancreatitis. Pancreas. 2007;35:256–261. doi: 10.1097/MPA.0b013e318064678a. [DOI] [PubMed] [Google Scholar]
- 51.Szabolcs A., Tiszlavicz L., Kaszaki J., Posa A., Berko A., Varga I.S., Boros I., Szuts V., Lonovics J., Takacs T. Zerumbone exerts a beneficial effect on inflammatory parameters of cholecystokinin octapeptide-induced experimental pancreatitis but fails to improve histology. Pancreas. 2007;35:249–255. doi: 10.1097/mpa.0b013e318070d791. [DOI] [PubMed] [Google Scholar]
- 52.Zhang X.P., Zhang L., Yang P., Zhang R.P., Cheng Q.H. Protective effects of baicalin and octreotide on multiple organ injury in severe acute pancreatitis. Dig. Dis. Sci. 2008;53:581–591. doi: 10.1007/s10620-007-9868-3. [DOI] [PubMed] [Google Scholar]
- 53.Seo S.W., Jung W.S., Piao T.G., Hong S.H., Yun K.J., Park R.K., Shin M.K., Song H.J., Park S.J. Selective cyclooxygenase-2 inhibitor ameliorates cholecystokinin-octapeptide-induced acute pancreatitis in rats. World J. Gastroenterol. 2007;13:2298–2304. doi: 10.3748/wjg.v13.i16.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xue D., Zhang W., Zhang Y., Wang H., Zheng B., Shi X. Adjusting effects of baicalin for nuclear factor-kappaB and tumor necrosis factor-alpha on rats with caerulein-induced acute pancreatitis. Mediat. Inflamm. 2006;5:26295. doi: 10.1155/MI/2006/26295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao H., Lu H.G., Shi Y.B., Zhao L.M., Bai C., Wang X. Role of enteral nutrition supplemented with ebselen and EHEC in pancreatitis-associated multiple organ dysfunction in rats. Inflamm. Res. 2006;55:423–429. doi: 10.1007/s00011-006-6008-z. [DOI] [PubMed] [Google Scholar]
- 56.Kalyoncu N.I., Alhan E., Ercin C., Kural B.V. Effects of dual inhibitor of cyclooxygenase and 5-lipoxygenase on acute necrotizing pancreatitis in rats. Hepatogastroenterology. 2006;53:597–602. [PubMed] [Google Scholar]
- 57.Shapiro H., Singer P., Halpern Z., Bruck R. Polyphenols in the treatment of inflammatory bowel disease and acute pancreatitis. Gut. 2007;56:426–435. doi: 10.1136/gut.2006.094599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Almeida J.L., Jukemura J., Coelho A.M., Patzina R.A., Machado M.C., da Cunha J.E. Inhibition of cyclooxygenase-2 in experimental severe acute pancreatitis. Clinics (Sao Paulo) 2006;61:301–306. doi: 10.1590/s1807-59322006000400005. [DOI] [PubMed] [Google Scholar]
- 59.Alsfasser G., Warshaw A.L., Thayer S.P., Antoniu B., Laposata M., Lewandrowski K.B., Fernandez-del Castillo C. Decreased inflammation and improved survival with recombinant human activated protein C treatment in experimental acute pancreatitis. Arch. Surg. 2006;141:670–676. doi: 10.1001/archsurg.141.7.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lefter L.P., Dajbog E., Scripcariu V., Dragomir C. Safety and efficacy of conservative management in acute severe pancreatitis. Chirurgia (Bucur.) 2006;101:135–139. [PubMed] [Google Scholar]
- 61.Cosen-Binker L.I., Binker M.G., Cosen R., Negri G., Tiscornia O. Influence of nitric oxide-donating nonsteroidal anti-inflammatory drugs on the evolution of acute pancreatitis. Shock. 2006;25:190–203. doi: 10.1097/01.shk.0000192122.91166.a8. [DOI] [PubMed] [Google Scholar]
- 62.Letoha T., Kusz E., Papai G., Szabolcs A., Kaszaki J., Varga I., Takacs T., Penke B., Duda E. In vitro and in vivo nuclear factor-kappaB inhibitory effects of the cell-penetrating penetratin peptide. Mol. Pharmacol. 2006;69:2027–2036. doi: 10.1124/mol.105.019653. [DOI] [PubMed] [Google Scholar]
- 63.Ma Z.H., Ma Q.Y., Wang L.C., Sha H.C., Wu S.L., Zhang M. Effect of resveratrol on peritoneal macrophages in rats with severe acute pancreatitis. Inflamm. Res. 2005;54:522–527. doi: 10.1007/s00011-005-1388-z. [DOI] [PubMed] [Google Scholar]
- 64.Lau H.Y., Bhatia M. The effect of CP96, 345 on the expression of tachykinins and neurokinin receptors in acute pancreatitis. J. Pathol. 2006;208:364–371. doi: 10.1002/path.1899. [DOI] [PubMed] [Google Scholar]
- 65.Reding T., Bimmler D., Perren A., Sun L.K., Fortunato F., Storni F., Graf R. A selective COX-2 inhibitor suppresses chronic pancreatitis in an animal model (WBN/Kob rats): Significant reduction of macrophage infiltration and fibrosis. Gut. 2006;55:1165–1173. doi: 10.1136/gut.2005.077925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao Y.F., Zhai W.L., Zhang S.J., Chen X.P. Protection effect of triptolide to liver injury in rats with severe acute pancreatitis. Hepatobiliary Pancreat. Dis. Int. 2005;4:604–608. [PubMed] [Google Scholar]
- 67.Alhan E., Kalyoncu N.I., Ercin C., Kural B.V. Effects of the celecoxib on the acute necrotizing pancreatitis in rats. Inflammation. 2004;28:303–309. doi: 10.1007/s10753-004-6055-x. [DOI] [PubMed] [Google Scholar]
- 68.Huang J., Moochhala S.M., Moore P.K., Bhatia M. Flurbiprofen and HCT1026 protect mice against acute pancreatitis-associated lung injury. Shock. 2005;24:182–187. doi: 10.1097/01.shk.0000172093.16033.12. [DOI] [PubMed] [Google Scholar]
- 69.Ma Z.H., Ma Q.Y. Resveratrol: A medical drug for acute pancreatitis. World J. Gastroenterol. 2005;11:3171–3174. doi: 10.3748/wjg.v11.i21.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Madanick R.D., O'Loughlin C.J., Barkin J.S. Diclofenac reduces the incidence of acute pancreatitis after endoscopic retrograde cholangiopancreatography. Dig. Dis. Sci. 2005;50:879–881. doi: 10.1007/s10620-005-2658-x. [DOI] [PubMed] [Google Scholar]
- 71.O'Brien G., Shields C.J., Winter D.C., Dillon J.P., Kirwan W.O., Redmond H.P. Cyclooxygenase-2 plays a central role in the genesis of pancreatitis and associated lung injury. Hepatobiliary Pancreat. Dis. Int. 2005;4:126–128. [PubMed] [Google Scholar]
- 72.Meng Y., Zhang M., Xu J., Liu X.M., Ma Q.Y. Effect of resveratrol on microcirculation disorder and lung injury following severe acute pancreatitis in rats. World J. Gastroenterol. 2005;11:433–435. doi: 10.3748/wjg.v11.i3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang R., Uchiyama T., Alber S.M., Han X., Watkins S.K., Delude R.L., Fink M.P. Ethyl pyruvate ameliorates distant organ injury in a murine model of acute necrotizing pancreatitis. Crit. Care Med. 2004;32:1453–1459. doi: 10.1097/01.ccm.0000130835.65462.06. [DOI] [PubMed] [Google Scholar]
- 74.Slogoff M.I., Ethridge R.T., Rajaraman S., Evers B.M. COX-2 inhibition results in alterations in nuclear factor (NF)-kappaB activation but not cytokine production in acute pancreatitis. J. Gastrointest. Surg. 2004;8:511–519. doi: 10.1016/j.gassur.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 75.Warzecha Z., Dembinski A., Ceranowicz P., Konturek S., Tomaszewska R., Stachura J., Nakamura T., Konturek P.C. Inhibition of cyclooxygenase-2 reduces the protective effect of hepatocyte growth factor in experimental pancreatitis. Eur. J. Pharmacol. 2004;486:107–119. doi: 10.1016/j.ejphar.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 76.Foitzik T., Hotz H.G., Hotz B., Wittig F., Buhr H.J. Selective inhibition of cyclooxygenase-2 (COX-2) reduces prostaglandin E2 production and attenuates systemic disease sequelae in experimental pancreatitis. Hepatogastroenterology. 2003;50:1159–1162. [PubMed] [Google Scholar]
- 77.Mentes A., Batur Y., Bayol U. Salycylate--induced pancreatic injury in the cat: A preliminary study. Rom. J. Gastroenterol. 2002;11:309–312. [PubMed] [Google Scholar]
- 78.Gukovsky I., Reyes C.N., Vaquero E.C., Gukovskaya A.S., Pandol S.J. Curcumin ameliorates ethanol and nonethanol experimental pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;284:G85–G95. doi: 10.1152/ajpgi.00138.2002. [DOI] [PubMed] [Google Scholar]
- 79.Hirata M., Hayashi I., Yoshimura K., Ishii K., Soma K., Ohwada T., Kakita A., Majima M. Blockade of bradykinin B(2) receptor suppresses acute pancreatitis induced by obstruction of the pancreaticobiliary duct in rats. Br. J. Pharmacol. 2002;135:29–36. doi: 10.1038/sj.bjp.0704462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Su S.B., Motoo Y., Xie M.J., Taga H., Sawabu N. Antifibrotic effect of the herbal medicine Saiko-keishi-to (TJ-10) on chronic pancreatitis in the WBN/Kob rat. Pancreas. 2001;22:8–17. doi: 10.1097/00006676-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 81.Su S.B., Motoo Y., Xie M.J., Sakai J., Taga H., Sawabu N. Expression of pancreatitis-associated protein (PAP) in rat spontaneous chronic pancreatitis: Effect of herbal medicine Saiko-keishi-to (TJ-10) Pancreas. 1999;19:239–247. doi: 10.1097/00006676-199910000-00004. [DOI] [PubMed] [Google Scholar]
- 82.Andersen V., Sonne J., Larsen S. Antipyrine, oxazepam, and indocyanine green clearance in patients with chronic pancreatitis and healthy subjects. Scand. J. Gastroenterol. 1999;34:813–817. doi: 10.1080/003655299750025750. [DOI] [PubMed] [Google Scholar]
- 83.Griesbacher T., Lembeck F. Effects of the bradykinin antagonist, HOE 140, in experimental acute pancreatitis. Br. J. Pharmacol. 1992;107:356–360. doi: 10.1111/j.1476-5381.1992.tb12751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Henry D., Lim L.L., Garcia Rodriguez L.A., Perez Gutthann S., Carson J.L., Griffin M., Savage R., Logan R., Moride Y., Hawkey C., Hill S., Fries J.T. Variability in risk of gastrointestinal complications with individual non-steroidal anti-inflammatory drugs: Results of a collaborative meta-analysis. Br. Med. J. 1996;312:1563–1566. doi: 10.1136/bmj.312.7046.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dai H.F., Wang X.W., Zhao K. Role of nonsteroidal anti-inflammatory drugs in the prevention of post-ERCP pancreatitis: A meta-analysis. Hepatobiliary Pancreat. Dis. Int. 2009;8:11–16. [PubMed] [Google Scholar]
- 86.Elmunzer B.J., Waljee A.K., Elta G.H., Taylor J.R., Fehmi S.M., Higgins P.D. A meta-analysis of rectal NSAIDs in the prevention of post-ERCP pancreatitis. Gut. 2008;57:1262–1267. doi: 10.1136/gut.2007.140756. [DOI] [PubMed] [Google Scholar]
- 87.Cheon Y.K., Cho K.B., Watkins J.L., McHenry L., Fogel E.L., Sherman S., Schmidt S., Lazzell-Pannell L., Lehman G.A. Efficacy of diclofenac in the prevention of post-ERCP pancreatitis in predominantly high-risk patients: A randomized double-blind prospective trial. Gastrointest. Endosc. 2007;66:1126–1132. doi: 10.1016/j.gie.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 88.Montano Loza A., Rodriguez Lomeli X., Garcia Correa J.E., Davalos Cobian C., Cervantes Guevara G., Medrano Munoz F., Fuentes Orozco C., Gonzalez Ojeda A. Effect of the administration of rectal indomethacin on amylase serum levels after endoscopic retrograde cholangiopancreatography, and its impact on the development of secondary pancreatitis episodes. Rev. Esp. Enferm. Dig. 2007;99:330–335. doi: 10.4321/s1130-01082007000600005. [DOI] [PubMed] [Google Scholar]
- 89.Khoshbaten M., Khorram H., Madad L., Ehsani Ardakani M.J., Farzin H., Zali M.R. Role of diclofenac in reducing post-endoscopic retrograde cholangiopancreatography pancreatitis. J. Gastroenterol. Hepatol. 2008;23:e11–e16. doi: 10.1111/j.1440-1746.2007.05096.x. [DOI] [PubMed] [Google Scholar]
- 90.Sotoudehmanesh R., Khatibian M., Kolahdoozan S., Ainechi S., Malboosbaf R., Nouraie M. Indomethacin may reduce the incidence and severity of acute pancreatitis after ERCP. Am. J. Gastroenterol. 2007;102:978–983. doi: 10.1111/j.1572-0241.2007.01165.x. [DOI] [PubMed] [Google Scholar]
- 91.Peiro A.M., Martinez J., Martinez E., de Madaria E., Llorens P., Horga J.F., Perez-Mateo M. Efficacy and tolerance of metamizole versus morphine for acute pancreatitis pain. Pancreatology. 2008;8:25–29. doi: 10.1159/000114852. [DOI] [PubMed] [Google Scholar]
- 92.Sorensen H.T., Jacobsen J., Norgaard M., Pedersen L., Johnsen S.P., Baron J.A. Newer cyclo-oxygenase-2 selective inhibitors, other non-steroidal anti-inflammatory drugs and the risk of acute pancreatitis. Aliment. Pharmacol. Ther. 2006;24:111–116. doi: 10.1111/j.1365-2036.2006.02959.x. [DOI] [PubMed] [Google Scholar]
- 93.Jick H., Derby L.E., Garcia Rodriguez L.A., Jick S.S., Dean A.D. Nonsteroidal antiinflammatory drugs and certain rare, serious adverse events: A cohort study. Pharmacotherapy. 1993;13:212–217. [PubMed] [Google Scholar]
- 94.Blomgren K.B., Sundström A., Steineck G., Genell S., Sjöstedt S., Wiholm B.E. A Swedish case-control network for studies of drug-induced morbidity: Acute pancreatitis. Eur. J. Clin. Pharmacol. 2002;58:275–283. doi: 10.1007/s00228-002-0471-4. [DOI] [PubMed] [Google Scholar]
- 95.Ibáñez L., Laporte J.R., Carné X. Adverse drug reactions leading to hospital admission. Drug Saf. 1991;6:450–459. doi: 10.2165/00002018-199106060-00005. [DOI] [PubMed] [Google Scholar]
- 96.Motola D., Vaccheri A., Silvani M.C., Poluzzi E., Bottoni A., De Ponti F., Montanaro N. Pattern of NSAID use in the Italian general population: A questionnaire-based survey. Eur. J. Clin. Pharmacol. 2004;60:731–738. doi: 10.1007/s00228-004-0826-0. [DOI] [PubMed] [Google Scholar]
- 97.Pezzilli R., Romboli E., Campana D., Corinaldesi R. Mechanisms involved in the onset of post-ERCP pancreatitis. J.O.P. 2002;3:162–168. [PubMed] [Google Scholar]
- 98.Kunz D., Bank U., Ittenson A., Schulz H.U., Sokolowski A. Alteration of immunological functions in acute pancreatitis. Eur. J. Clin. Chem. Clin. Biochem. 1993;31:A35–A36. [Google Scholar]
- 99.Kiviniemi H., Juvonen T., Makela J. Acute phase response in patients with uncomplicated endoscopic retrograde cholangiopancreatography. H.P.B. Surg. 1994;8:129–131. doi: 10.1155/1994/69467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Oezcueruemez-Porsch M., Kunz D., Hardt P.D., Fadgyas T., Kress O., Schulz H.U., Schnell-Kretschmer H., Temme H., Westphal S., Luley C., Kloer H.U. Diagnostic relevance of interleukin pattern, acute-phase proteins, and procalcitoninin early phase of post-ERCP pancreatitis. Dig. Dis. Sci. 1998;43:1763–1769. doi: 10.1023/a:1018887704337. [DOI] [PubMed] [Google Scholar]
- 101.Kaw M., Singh S. Serum lipase, C-reactive protein, and interleukin-6 levels in ERCP-induced pancreatitis. Gastrointest. Endosc. 2001;54:435–440. doi: 10.1067/mge.2001.117763. [DOI] [PubMed] [Google Scholar]
- 102.Blanchard J.A., Barve S., Joshi-Barve S., Talwalker R., Gates L.K. Cytokine production by CAPAN-1 and CAPAN-2 cell lines. Dig. Dis. Sci. 2000;45:927–932. doi: 10.1023/a:1005573024448. [DOI] [PubMed] [Google Scholar]
- 103.Pezzilli R., Gabbrielli A., Morselli-Labate A.M., D'Alessio P., Barakat B., Costamagna G., Dibenedetti F., Massa M., Merlini G., Melzi d'Eril G.M. Does gabexate mesilate affect serum concentrations of acute phase proteins after endoscopic retrograde cholangiopancreatography examination? Hepatogastroenterology. 2003;50:851–855. [PubMed] [Google Scholar]
- 104.Deviere J., Le Moine O., Van Laethem J.L., Eisendrath P., Ghilain A., Severs N., Cohard M. Interleukin 10 reduces the incidence of pancreatitis after therapeutic endoscopic retrograde cholangiopancreatography. Gastroenterology. 2001;120:498–505. doi: 10.1053/gast.2001.21172. [DOI] [PubMed] [Google Scholar]
- 105.Sherman S., Cheng C.L., Costamagna G., Binmoeller K.F., Puespoek A., Aithal G.P., Kozarek R.A., Chen Y.K., Van Steenbergen W., Tenner S., Freeman M., Monroe P., Geffner M., Deviere J. Interleukin-10 ERCP Study Group, authors. Efficacy of recombinant human interleukin-10 in prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis in subjects with increased risk. Pancreas. 2009;38:267–274. doi: 10.1097/MPA.0b013e31819777d5. [DOI] [PubMed] [Google Scholar]
- 106.Gross V., Leser H.G., Heinisch A., Schölmerich J. Inflammatory mediators and cytokines. New aspects of pathophysiology and assessment of severity of acute pancreatitis? Hepatogastroenterology. 1993;40:522–530. [PubMed] [Google Scholar]
- 107.Makela A., Kuusi T., Schroeder T. Inhibition of serum phospholipase A2 in acute pancreatitis by pharamacologic agents in vitro. Scand. J. Clin. Lab. Invest. 1997;57:401–408. doi: 10.3109/00365519709084587. [DOI] [PubMed] [Google Scholar]
- 108.Davies N.M., Anderson K.E. Clinical phamacokinetics of diclofenac. Therapeutic insights and pitfalls. Clin. Pharamakokinet. 1997;33:184–213. doi: 10.2165/00003088-199733030-00003. [DOI] [PubMed] [Google Scholar]
- 109.Pezzilli R. Pharmacotherapy for acute pancreatitis. Expert Opin. Pharmacother. 2009;10:2999–3014. doi: 10.1517/14656560903382630. [DOI] [PubMed] [Google Scholar]
- 110.Liddle R.A., Nathan J.D. Neurogenic inflammation and pancreatitis. Pancreatology. 2004;4:551–559. doi: 10.1159/000082180. [DOI] [PubMed] [Google Scholar]
- 111.Ferreira S.H., Vane J.R. New aspects of the mode of action of nonsteroidal anti-inflammatory drugs. Annu. Rev. Pharmacol. 1974;53:57–73. [Google Scholar]
- 112.Swift J.Q., Garry M.G., Roszkowski M.T., Hargreaves K.M. Effect of flurbiprofen on tissue levels of immunoreactive bradykinin and acute post-operative pain. J. Oral. Maxillofac. Surg. 1993;51:112–116. doi: 10.1016/s0278-2391(10)80002-3. [DOI] [PubMed] [Google Scholar]
- 113.Cashman J.N. The mechanisms of action of NSAIDs in analgesia. Drugs. 1996;5:13–23. doi: 10.2165/00003495-199600525-00004. [DOI] [PubMed] [Google Scholar]
- 114.Malmberg A.B., Yaksh T.L. Hyperalgesia mediated by spinal glutamate or substance P receptor blocked by spinal cyclooxygenase inhibition. Science. 1992;257:1276–1279. doi: 10.1126/science.1381521. [DOI] [PubMed] [Google Scholar]
- 115.Pezzilli R., Uomo G., Gabbrielli A., Zerbi A., Frulloni L., De Rai P., Castoldi L., Cavallini G., Di Carlo V. ProInf-AISP Study Group, authors. A prospective multicentre survey on the treatment of acute pancreatitis in Italy. Dig. Liver Dis. 2007;39:838–846. doi: 10.1016/j.dld.2007.05.014. [DOI] [PubMed] [Google Scholar]