Abstract
The widespread application of cell penetrating agents to clinical therapeutics and imaging agents relies on the ability to prepare them on a large scale and to readily conjugate them to their cargos. Dendritic analogues of cell penetrating peptides, with multiple guanidine groups on their peripheries offer advantages as their high symmetry allows them to be efficiently synthesized, while orthogonal functionalities at their focal points allow them to be conjugated to cargo using simple synthetic methods. Their chemical structures and properties are also highly tunable as their flexibility and the number of guanidine groups can be tuned by altering the dendritic backbone or the linkages to the guanidine groups. This review describes the development of cell-penetrating dendrimers based on several different backbones, their structure-property relationships, and comparisons of their efficacies with those of known cell penetrating peptides. The toxicities of these dendritic guanidines are also reported as well as their application towards the intracellular delivery of biologically significant cargos including proteins and nanoparticles.
Keywords: cell penetrating peptide, dendrimer, guanidine, drug delivery, cell-uptake, toxicity
1. Introduction
In recent decades there has been significant interest in the preparation of new materials for the delivery of drugs and imaging agents. This interest is motivated mainly by the problematic properties of these molecules. For example, while genomics and proteomics are revealing many promising drug candidates based on peptides and oligonucleotides, these molecules are often unable to reach their target sites within cells as they are not capable of traversing cell membranes. On the other hand, cell penetrating peptides (CPPs) are a class of molecules that exhibit the ability to cross biological barriers. They were discovered from serendipitous observations on the HIV-1 Tat trans-activating factor and on the Drosophila Antennapedia transcription factor [1,2,3,4]. Since then, the search for other CPPs has been ongoing, either by the screening of natural proteins or by rational design [5,6,7,8,9,10,11,12,13,14,15,16].
The functions common to all CPPs include their abilities to rapidly enter cells and their high water solubilities [17]. While the main classes of CPPs differ from one another in structure [17,18] (Table 1), they are generally composed of large fractions of basic amino acids and in particular often contain a high concentrations of guanidinium moieties [18,19,20,21,22,23]. Indeed, the translocation capabilities of CPPs are strongly influenced by the presence of positively charged residues, particularly arginine [13,14,15,24]. The observation of this primary structure-function relationship has thus far led to the development of 1) peptide analogues where residues are replaced by positively charged ones such as arginine [13,23,24,25] and 2) nonpeptide analogues that contain positively charged functionalities including guanidine groups [23,24,26,27,28,29,30,31,32].
Table 1.
Amino acids sequences of the main CPPs.
| Tat49-57 | RKKRRQRRR |
| Polyarginine | RRRRRRRRR |
| Decalysine | KKKKKKKKKK |
| Penetratin | RQIKIWFQNRRMKWKK |
| Transportan | GWTLNSAGYLLGKINLKALAALAKKIL |
| MPG | GALFLGFLGAAGSTMGAWSQPKKKRKV |
| Pep1 | KETWWETWWTEWSQPKKKRKV |
Several studies have been carried out in order to elucidate the role of the arginine residues in the HIV Tat49-57 (RKKRRQRRR) sequence [33]. For example, the replacement of each residue in this sequence by alanine showed that arginine and lysine residues were essential to the cell-uptake [24]. When the non-charged glutamine was replaced by an alanine (RKKRRARRR), cell-uptake was less affected. The conclusion was that positive charge was important for uptake. Subsequently, it was found that a lysine 9-mer (KKKKKKKKK) performed less well than the original Tat49-57 sequence. In marked contrast, the corresponding arginine 9-mer (RRRRRRRRR) was superior to the Tat reference [24]. These studies therefore showed an obvious link between translocation and arginine residues. Experiments have also been conducted to investigate the effects of oligomer length. Studies on the HIV Tat49-57 sequence revealed that truncations led to less effective uptake [24]. In a study on oligoarginines comprising 6–20 arginine residues, a maximum activity was found for the 15-mer [13,34]. For cost reasons concerning synthesis, the 8-mer (RRRRRRRR) was chosen by Wender and coworkers [35] but this number also seemed to be the most efficient in several other cases [36].
The interesting finding that oligomers comprising either all D or all L amino acids worked similarly suggested that the backbone of the transporter was not critical to uptake [13]. Indeed it has been shown that interdigitation of an arginine backbone with α, β, γ, or δ amino acids [25] or even replacement of the peptide backbone with an oligocarbamate [30] improved cellular uptake relative to the corresponding arginine oligomers. By investigating different backbones, it was shown that the main backbone requirement for cellular uptake of arginine-rich peptides was conformational freedom, an essential parameter to afford an optimized interaction with the cell membrane moieties [33].
Overall, research on various linear analogues of CPPs has revealed the importance of multiple guanidine groups and the possibility to vary the backbone and the spacing between guanidines without loss of cell-uptake efficiency [23,25,30,37,38]. As a next step, if CPPs are to be used to deliver biologically active cargoes into cells, another important design consideration should be their ease of synthesis. While peptides are often prepared by iterative syntheses on solid supports, the preparation of large quantities is still quite costly and may prove limiting for the widespread pharmaceutical application of these materials. The preparation of other non-peptide linear oligomers by convergent solution syntheses also involves many synthetic reactions and purification steps [23,24,25,30]. Therefore, the development of cell penetrating molecules that can be efficiently prepared on larger scales and which can be readily conjugated to their cargos would represent an important step towards extending their utility in drug delivery applications. Dendrimer synthesis, once optimized, can be efficient and cost-effective, as indicated by the increasing numbers of dendrimers available commercially on a multi-gram scale. For example, one can purchase 2 g of a 3rd generation polyamidoamine (PAMAM) dendrimer for less than US $400 and functionalize it with guanidines in a single step. In contrast, for a similar price one can only purchase less than 10 mg of the Tat49-57 peptide (depending on the supplier). In addition, as the backbones are tunable their flexibilities can be optimized [39,40]. Furthermore, dendrimers can be designed to be resistant to rapid biodegradation, unlike many linear peptides [41,42,43,44]. Because of these potential advantages, dendrimers represent promising backbones for the display of multiple guanidine residues. This review is focused on the use of dendritic guanidines as analogues of cell-penetrating peptides. It is an attempt to summarize the state-of-the-art in this field. The following sections will successively discuss the unique properties of dendrimers, their syntheses and functionalization with guanidine groups, the cell penetrating capabilities of the resulting dendritic guanidines, their structure-property relationships and their conjugation to cargo.
2. Dendrimers as Cell-Penetrating Agents
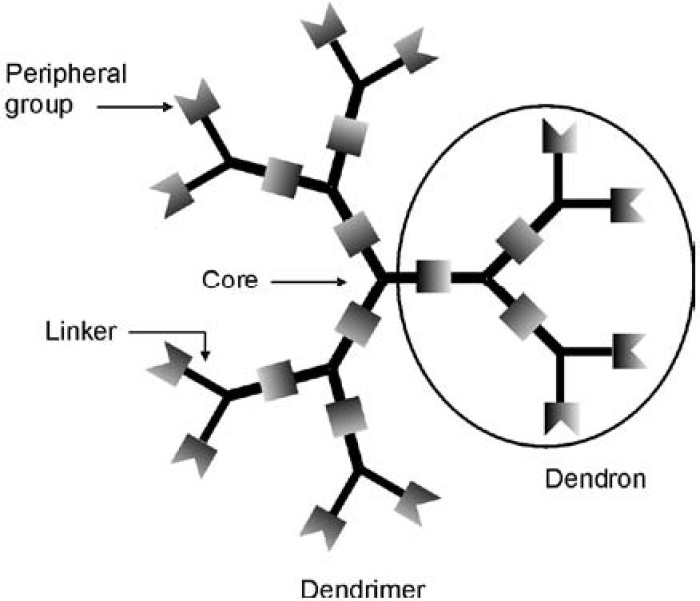
While peptides are typically linear macromolecules, highly branched structures are also found widely in nature, where the branching and display of multiple terminal functionalities enable the enhancement of functions. At the interface of polymer and synthetic organic chemistry, “dendrimers” have emerged over the past couple of decades as a new class of highly branched molecules. Named based on the Greek word dendron, meaning “tree”, dendrimers are macromolecules that have structures resembling those of trees (Figure 1). As dendrimers are synthesized from branched monomer units in a stepwise manner, monodisperse products are often obtained. In addition, it is possible to achieve precise control over their molecular shape, dimensions, density, polarity, flexibility and solubility by choosing different building/branching units and surface functional groups [45,46,47,48,49]. Generally, dendrimers are globe- or ellipsoid-shaped, and they consist of three distinct regions: (1) a central core, (2) repeated branches, and (3) surface functional groups [50]. The central core should be a molecule with at least two reactive functional groups. The repeated branches are organized in a series of radially concentric layers called ‘’generations’’ [51]. When iterative growth is conducted from focal point or core molecules containing one reactive group, wedge-shaped structures, commonly referred to as “dendrons” result.
Figure 1.
Schematic of a dendrimer structure.
Dendritic structures have been recognized to be ideal building blocks for biomedical applications because of their monodispersities, high loading capacities, large-scale production, and bioconjugation capabilities [39,52,53,54]. Most pertinent to their development as analogues of CPPs, the properties of dendrimers are often dominated by their peripheral groups, particularly at high generations owing to the exponential increase in the number of peripheral units with each generation. Thus dendrimers may serve as useful backbones for the conjugation of multiple guanidine groups. Tuning the dendrimer or dendron generation allows the number of guanidine groups to be controlled, while the distance between gaunidines and the flexibilities of the structures can be determined by the selection of the branching monomers and linkers.
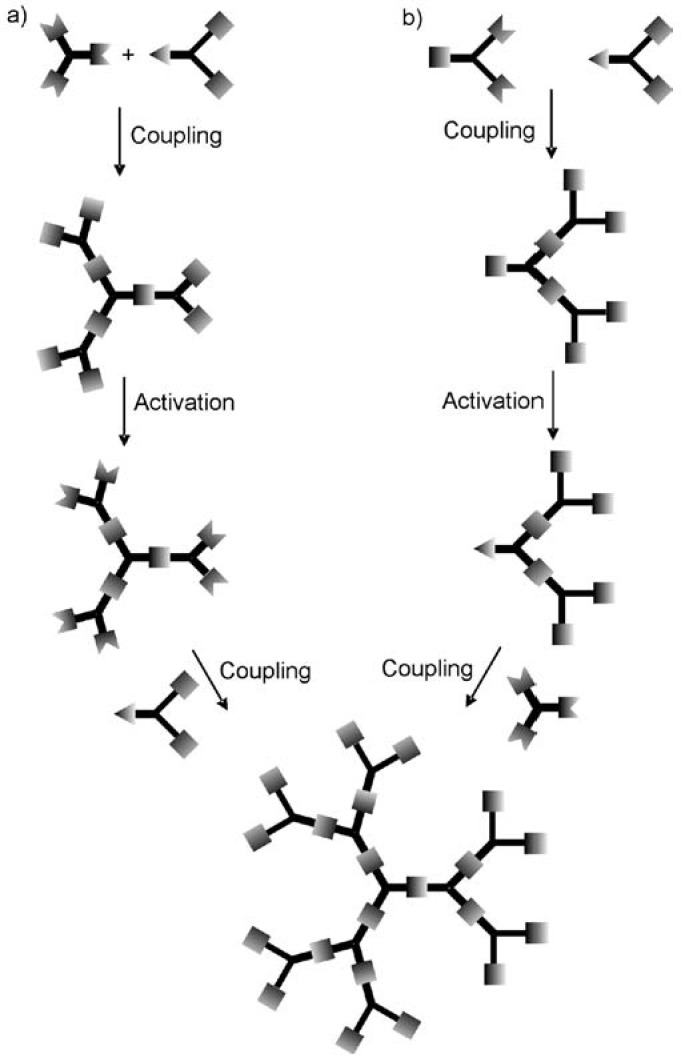
Generally, there are two approaches used to synthesize dendrimers: the divergent method and the convergent one [40,55]. In the divergent approach, the dendrimer is grown outwards from the core by the repetition of coupling and activation steps as shown in Figure 2a. Repetition of these coupling and activation steps provides an exponential increase in the number of peripheral groups, and reactions at each step. In the convergent approach (Figure 2b), growth initiates from what will become the dendrimer periphery and progresses towards the core. First the peripheral groups are coupled to each branch of the monomer, while keeping the focal point of the monomer in an unreacted form. This focal point can then be activated and subsequently coupled to another monomer unit. This reaction sequence of coupling and activation continues until the desired generation is reached, and then the resulting dendritic fragments, referred to as “dendrons” are finally coupled to a core molecule. The convergent approach often affords dendrimers with perfect structural homogeneity due to the small number of coupling reactions performed at each step and the possibility to remove flawed structures by purification methods such as chromatography [51,55,56]. However, the divergent approach is the preferred one for the large scale industrial preparation of dendrimers because the quantity of dendrimer sample increases with each generation and the removal of excess reagents by techniques such as precipitation, distillation, or ultrafiltration is facilitated by their differences in mass. In some cases, a divergent synthesis can yield a monodisperse product [57,58,59]. In other cases minor structural flaws resulting from incomplete couplings or side reactions can exist, [47,60] but the polydispersities are still as low or lower than can be achieved in the best controlled polymerizations. Although the focus of this review is not to describe the syntheses of the dendrimer backbones in detail, these approaches have allowed for the preparation of several families of dendrimers such as the PAMAM [61], polypropyleneimine (PPI) [62], polyester [63,64,65,66], amino acid [67,68,69,70,71], carbohydrate [72,73] and various hydrophobic dendrimers [74,75]. At this stage, the syntheses of dendrimers is a sufficiently well developed field such that many dendrimer backbones can be prepared on a multigram or larger scale, and several dendrimer backbones including the PAMAM, PPI, polyesters, and polylysines are even commercially available. The ready availability of these backbones provides an additional advantage in their development as analogues of CPPs.
Figure 2.
Comparison of the (a) divergent and (b) convergent approaches to dendrimer synthesis.
3. Syntheses of Guanidine Functionalized Dendrimers
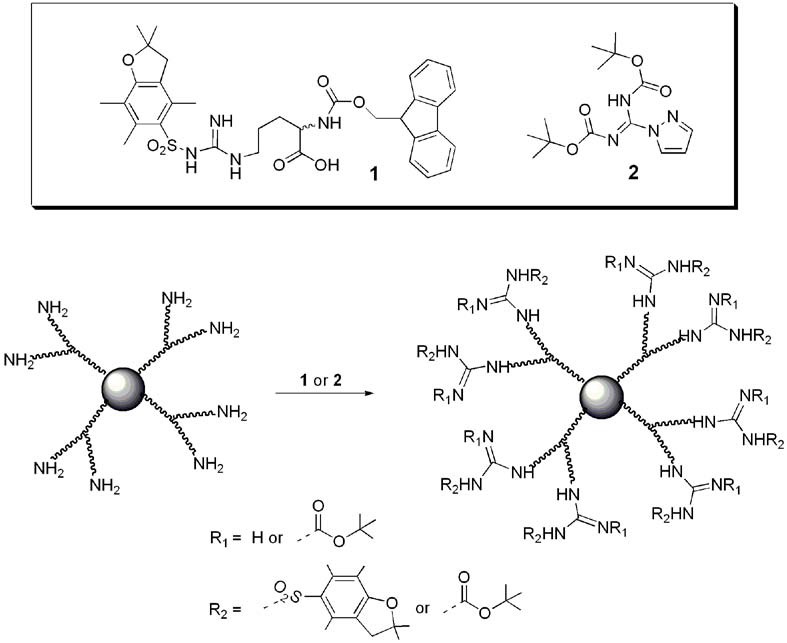
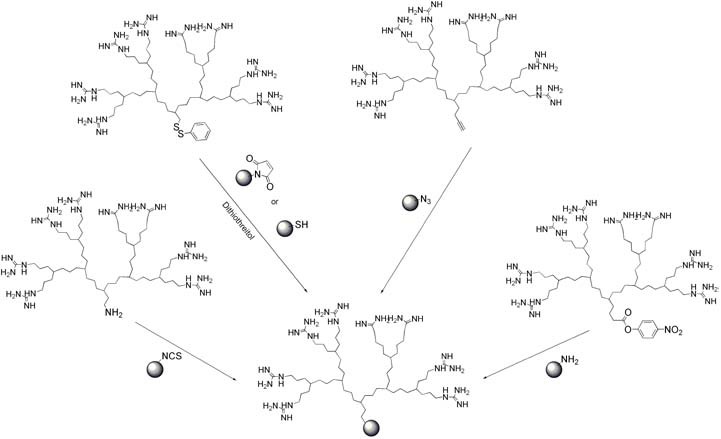
With many diverse dendrimer backbones readily available commercially or through chemical synthesis, the guanidine end group is generally introduced after the dendrimer synthesis by a final functionalization step. This usually involves guanidinylation of terminal amino groups on the dendrimer using either fluorenylmethyloxycarbonyl (Fmoc) protected arginine (1) [76] or 1H-pyrazole-1-carboxamidine (2) [77] (Figure 3). The guanidine moiety of these molecules is generally introduced in a protected form and the protecting group is removed from the dendrimer in the final step.
Figure 3.
Introduction of the guanidine functionality to a dendrimer’s peripheral amine groups using Fmoc protected arginine (1) or 1H-pyrazole-1-carboxamidine (2).
Fmoc-protected arginine has been used to functionalize polyamide based dendrimers [78,79,80,81,82,83,84] (generally PAMAM) [79,80,81,82,83,84]) and PPI dendrimers [85]. The pyrazole carboxamidine represents an efficient alternative to increase the reactivity of the coupling and was used to functionalize polyamide [29,86], PPI [87,88,89,90,91], melamine [92] and polyether based dendrimers [93]. In the case of polyester based dendrimers [94], pyrazole carboxamidine 2 was used to create a guanidine functionalized precursor with a carboxylic acid moiety 4 that was then coupled to the polyester dendrimer by using carbodiimide chemistry (Figure 4).
Figure 4.
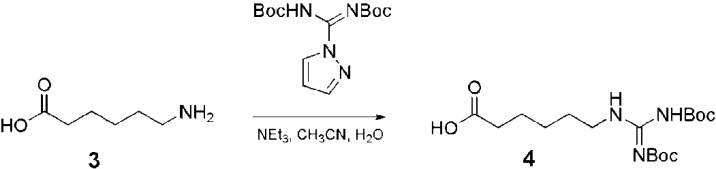
Synthesis of a guanidine functionalized precursor having a carboxylic acid moiety for coupling to a polyester dendrimer periphery [94].
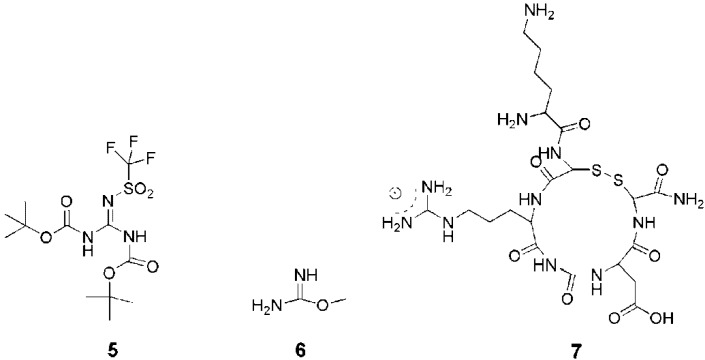
Occasionally, other precursors such as N,N’-diBoc-N’’-triflylguanidine (5) [26,95,96,97], O-methyl-isourea hydrochloride (6) [98,99] or an arginine-containing tri/tetrapeptide 7 [100,101,102] (RGD or CRGD to target αvβ3 Integrins) have also been used to introduce guanidine moieties (Figure 5).
Figure 5.
Precursors for the introduction of guanidine moieties.
4. Structures and Cellular Internalization of Guanidine Functionalized Dendrimers
The translocation of a branched-chain arginine transporter was first investigated with success by Futaki et al. [27]. It was shown that peptide clusters formed by approximately eight arginine residues played a crucial role in translocation. Moreover, flexibility in the positions of the positive charges in these peptides confirmed that a continuous arrangement of the arginine residues was not required. As described above, dendrimers are promising alternative branched backbones for the multivalent display of guanidine functionalities due to their relatively economical syntheses and abilities to display varying numbers of guanidine moieties in a controlled manner.
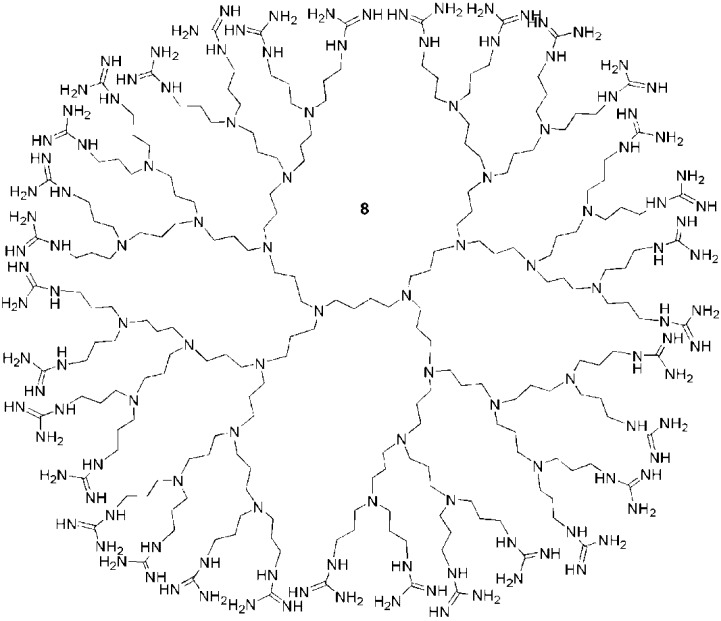
Initially, guanidine functionalized PPI dendrimers such as 8 (Figure 6) were investigated as analogues of CPPs by Paleos and coworkers. The first studies on these macromolecules were focused on liposome internalization of 4th and 5th generation dendrimers [89,90]. With a 4th generation dendrimer, liposome uptake was enhanced by the number of guanidine groups. For example, for 0, 6, or 12 guanidines on the dendrimer surface, the liposome uptake was 25%, 60% and 80% respectively. Moreover, for equal concentrations of guanidine moieties, the higher generation dendrimers proved to be more effective in interacting with liposomes. This behavior was attributed to the multivalent effect of the dendrimer [103,104]. Nevertheless, this interaction enhancement with increasing dendrimer generation was not correlated with liposome penetration efficiency. An investigation of 3rd and 4th generation guanidine functionalized PPI dendrimers bearing either 16 or 32 guanidine groups respectively showed that the lower generation dendrimer was more effective in penetrating the liposomes [87]. It was concluded from this work with liposomes that the constraints imposed by the size and the dense surface functionalization inhibited effective liposome internalization of the higher generations despite the multivalent effect leading to a stronger interaction. The effects of decorating the surface with other functionalities were also tested. For example, guanidine functionalized PPI dendrimers were partially actetylated [87] or partially hyroxylated [88]. On A549 lung carcinoma cells, partially acetylated derivatives of the 3rd and 4th generation dendrimers showed enhanced translocation abilities compared to the non-acetylated derivatives, an effect attributed to their increased hydrophobicities. In this case, the number of guanidines, eight for the 3rd generation and 14 for the 4th generation, was crucial. Cell uptake was localized to the nucleus for the 3rd generation dendrimer and to the cytosol for the 4th generation dendrimer [87]. The introduction of hydroxyl groups was accomplished by the ring opening of propylene oxide and led to a decrease in internalization of a 4th generation dendrimer in HEK 293 and COS-7 cells [88]. However, in this latter case, the partially hydroxylated dendrimers were less toxic.
Figure 6.
A guanidine functionalized 4th generation PPI dendrimer [89,90].
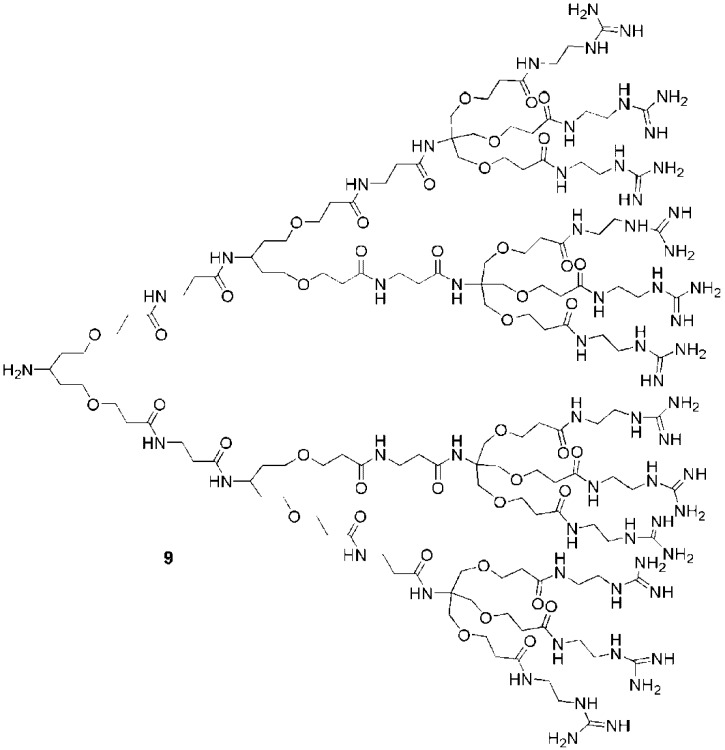
The second backbone investigated for the development of guanidine functionalized dendrimers was a polyamide. The first studies on these dendrimers were performed by Goodman and coworkers who synthesized dendrons with focal point amine groups for coupling to cargos [26]. These dendrons such as 9 (Figure 7) were internalized efficiently into HeLa S3 cells as well as human cervical carcinoma cells. Dendrimers with different numbers of guanidines (1, 3, 6, 9, or 12) on their surfaces were tested. The dendrimers bearing 6, 9 or 12 guanidines exhibited better uptake than dendrimers with 1 or 3 guanidines, suggesting that 6 guanidine groups were sufficient to achieve good translocation. A comparative assay with Tat49-57 conjugates revealed that dendrons with 9 guanidine groups exhibited the same cell penetrating capabilities as Tat49-57. Nevertheless, the dendron was not able to cross the nuclear membrane as efficiently as the Tat49-57 conjugate. Although the presence of a fully complexed Tat49-57-chromophore in the nucleus had not been demonstrated in this work, it has since then been elegantly confirmed by using Cre recombinase as cargo that the Tat conjugate reaches the nucleus without compromising the survival and competency of the cells [105,106].
Figure 7.
A polyamide dendrimer with 12 peripheral guanidines [26].
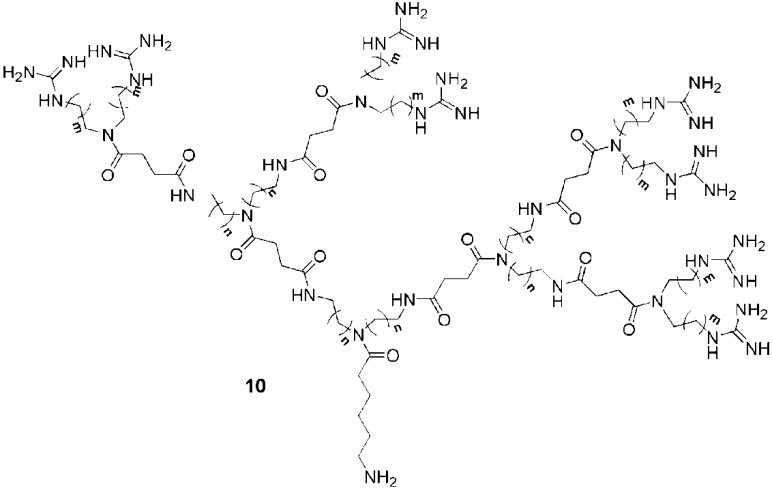
In other work, the prospect of new guanidine-based transporters prompted Wender and coworkers to study tunable guanidine functionalized polyamide dendrimers [29]. These authors prepared a series of 9 dendrons, all bearing 9 guanidine groups on their peripheries (Figure 8). Tunable spacers (n or m, see Figure 8) incorporated throughout the dendron backbones permitted a study of the effect of spacer length on cell uptake efficiency in the human T cell line Jurkat. The results revealed that the longest hydrocarbon spacers led to faster rates of uptake into cells. For dendrons such as 10 (n = 5, m = 2 or 5), the efficiencies exceeded those of the linear oligoarginine transporter (9-mer). The increased uptake of the dendrons with the longer spacers may be attributed to the increased flexibilities of the backbones as was observed for the linear CPPs; however, the incorporation of longer spacers also introduced increased hydrophobicity which may have enhanced the interaction of the molecules with membranes and thus their uptake. In order to separate the effects of flexibility and hydrophobicity, it would be necessary to compare molecules having hydrophilic versus hydrophobic spacers.
Figure 8.
Guanidine functionalized polyamide dendrimers incorporating difference spacers (n,m = 1,2 or 5) [29].
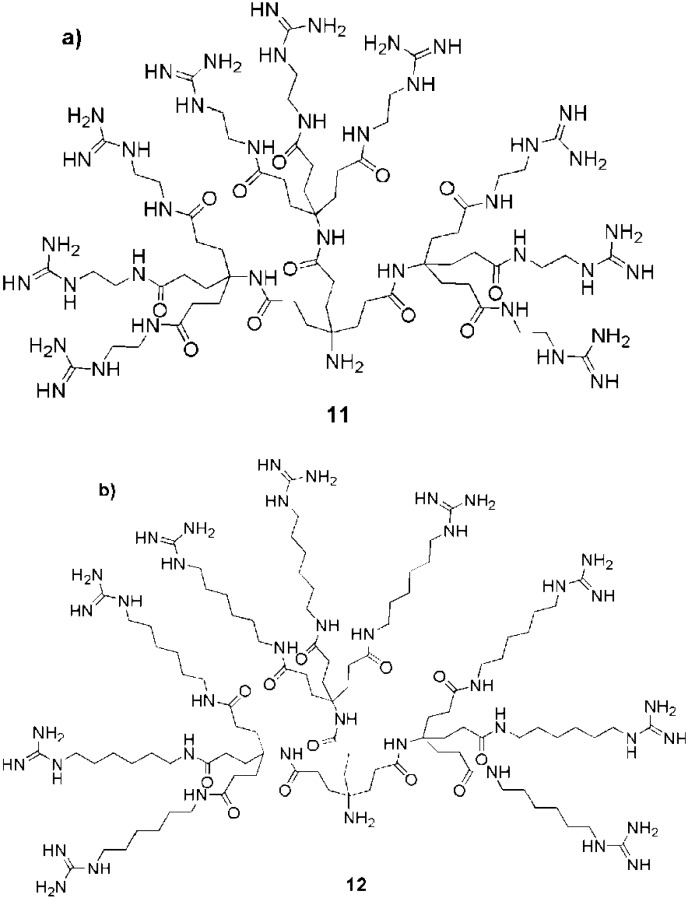
The effects of spacers were further investigated by Harth et al. They prepared two different dendrons, each bearing 9 peripheral guanidines but with two different spacers: C2 length (11) or C6 length (12) (Figure 9) [95]. Fluorescent conjugates of these dendrons were tested with NIH-3T3 and HMEC cells and the conjugate with the longer spacer was internalized more rapidly.
Figure 9.
Guanidine functionalized dendrimers having either (a) C2 or (b) C6 spacers [95].
Moreover, while the dendron with the longer spacer was localized in the cell nuclei after internalization, the other conjugate was taken up into cytosolic compartments. Dendron 12, containing the C6 spacer was also used to create protein conjugates [96,97] but the nuclear localization observed with the dendron alone was lost in these cases.
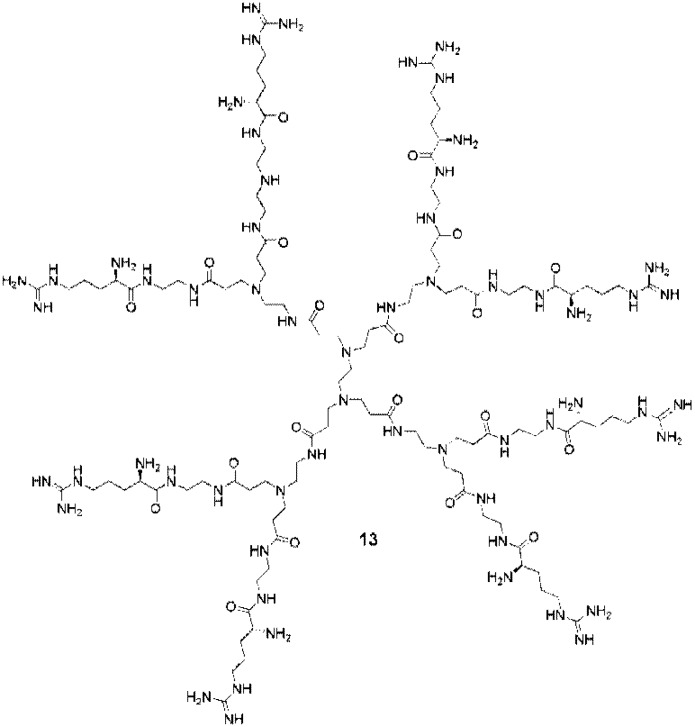
Another polyamide-based dendrimer investigated for cell penetration was the well-known PAMAM dendrimer. The first attempt to functionalize PAMAM dendrimers with guanidine groups was performed by Park and coworkers [80]. These authors compared the transfection efficiencies of an arginine functionalized PAMAM dendrimer 13 (Figure 10) with a lysine functionalized PAMAM dendrimer.
Figure 10.
A PAMAM dendrimer functionalized with guanidine groups [85].
The results obtained using 293, HepG2, Neuro 2A and vascular smooth muscle cells strongly evidenced the efficiency of the guanidinylated dendrimer and its potential as a good nonviral gene carrier. This first study, carried out using a 4th generation dendrimer with 58 peripheral guanidines opened doors to vulnerable-cell transfection [79]. Further studies on the 4th generation dendrimer were also done by the same group to evaluate its ability to cross monolayers of CaCo-2 cells [81]. The results suggested a paracellular transport mechanism (apical-to-basolateral or basolateral-to-apical) revealing the potential for these macromolecules to be used in oral absorption applications.
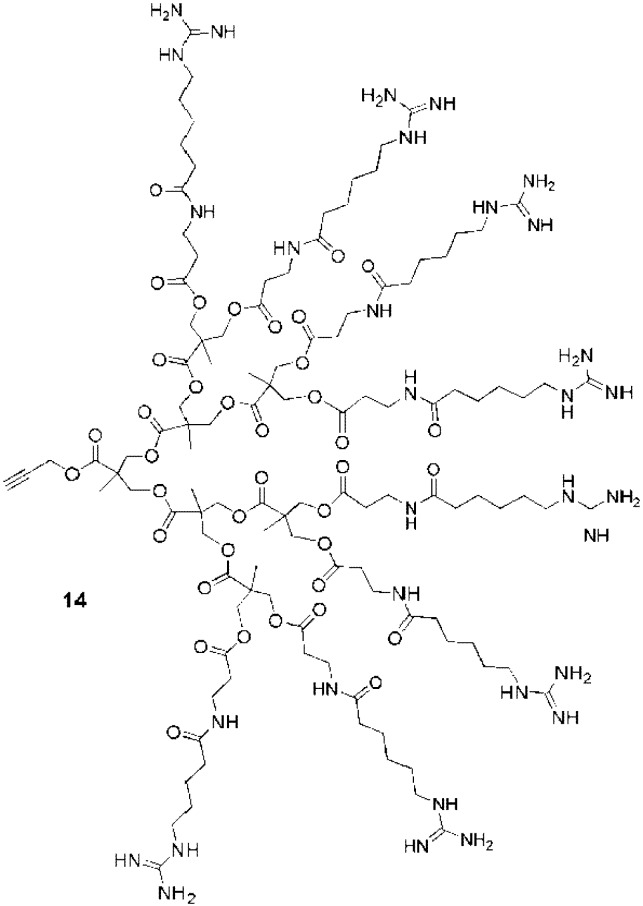
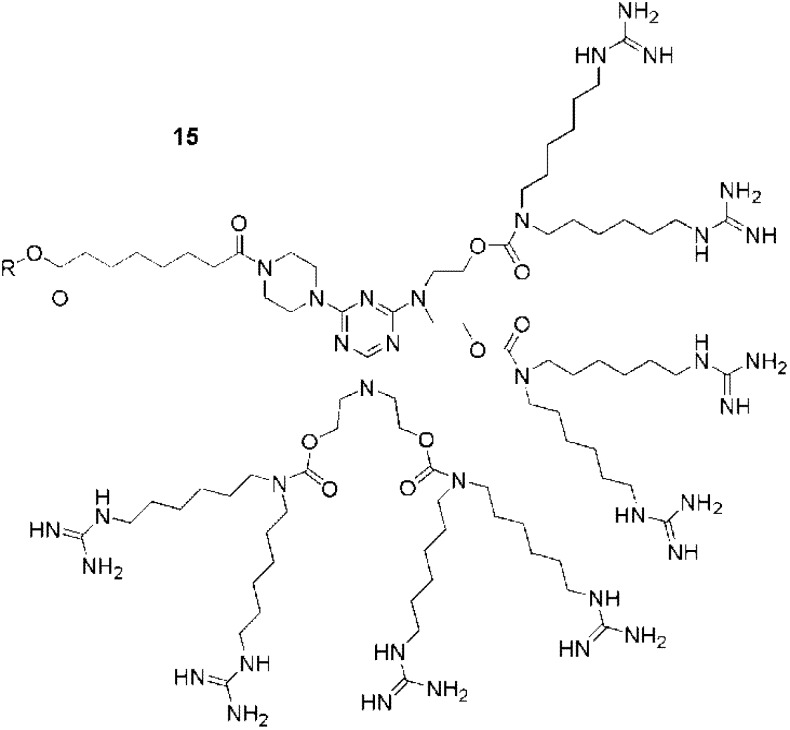
Two other guanidine functionalized dendrimers were also developed for cell penetration. A 3rd generation polyester dendron (14, Figure 11) bearing 8 peripheral guanidines exhibited cell-penetrating efficiencies similar to those of Tat49-57 in GL261 mouse glioma cells [94]. Interestingly, this dendron presents a clickable propargyl group at its focal point for conjugation to cargo. The second example was a polycarbamate-based dendron (15, Figure 12), also bearing 8 guanidines. Coupled with different cargos, this CPP analogue gave efficient transfection results in vivo (in mice) [98,99]. From the studies described above, it is evident that guanidinylated dendrimers are able to act as CPP analogues, with efficiencies similar to those of the peptide Tat49-57. A wide variety of dendrimer backbones have been functionalized with guanidine moieties, and the effects of the linkers, dendrimer backbones, and the numbers of guanidines have also been investigated. As for CPPs, these studies have suggested the importance of the flexibilities and conformational freedom of the guanidine moieties in the molecules. With dendrimers, this freedom was easily achieved by tuning the length of the spacers between the guanidine groups and the dendrimer peripheries. It should also be considered that the lengths of these spacers may also affect the overall hydrophobicities of the structures, which may in turn affect their uptake. Furthermore, it was also shown that the structures of the dendrimers impacted the cellular localization (cytosolic vs. nuclear), thus suggesting that dendrimers as CPPs analogues are also useful to cross different types of biological membranes.
Figure 11.
Guanidine functionalized polyester dendron with a ‘clickable’ focal point [94].
Figure 12.
Table 2.
Guanidinylated dendrimers used for cell-uptake studies.
| Backbone | Guanidines | Cargo | Cell-line | Reference |
|---|---|---|---|---|
| Poly(propylene imine) | 0,6,12 | Betamethasone derivatives | Liposomes | [89] |
| Poly(propylene imine) | 4,8 | - | Liposomes | [90] |
| Poly(propylene imine) | 8,14 | FITC | Human lung carcinoma (A549) | [87] |
| Poly(propylene imine) | 16,32 | - | Liposomes | |
| Poly(propylene imine) | 0-32 | DNA | HEK293, COSY7 | [88] |
| Poly(propylene imine) | 8 | DNA | HeLa, 293 cells | [85] |
| Polyamide | 3,6,9,12 | GFP, FITC | HeLa S3 and Human cervical carcinoma | [26] |
| Polyamide | 9 | FITC | NIH-3T3 fibroblasts and HMEC | [95] |
| Polyamide | 9 | IgG-Antibodies | Hep-2 (RSV-GFP) | [97] |
| Polyamide | 9 | Polymer nanoparticles | NIH-3T3 fibroblasts | [96] |
| Polyamide (dendrigrafts) | 12 | FITC | Human lung carcinoma (A549) | [86] |
| Polyamide | 8 | FITC | Human lymphocyte (T cell line Jurkat) | [29] |
| PAMAM | 58 | DNA | 293, HepG2, Neuro2A, rat vascular smooth muscle cells | [80] |
| PAMAM | 58 | DNA,RNA | Primary cortical cells (Neurons, glial cells) | [79] |
| PAMAM | 16,32,64 | DNA,RNA | 293, HUVECs | [81] |
| PAMAM | 64 | DNA | HepG2, HeLa, SMCs, HUVECs | [82] |
| PAMAM | 31,59,60,116 | DNA | HeLa, 293, A549 | [83] |
| Polycarbonate | 8 | Oligonucleotides | mouse | [99] |
| Polycarbonate | 8 | Oligonucleotides | mouse | [98] |
| Polyester | 8 | Fe3O4 nanoparticles | GL261 mouse glioma | [94] |
5. Cellular Internalization Mechanism
CPPs can enter cells through multiple mechanisms. Theoretically, both endocytosis and diffusion to the cytosol can occur. Wender highlighted that for CPPs a universal uptake mechanism is not very probable [23]. However, he defined two common aspects of any mechanistic possibilities derived from the guanidine group: an association occurs first and is followed by the cell uptake itself.
The first association step involves ionic and hydrogen bonding interactions between the negatively charged carboxylates, phosphates and sulfates belonging to membrane constituents and the guanidine groups, which are protonated and thus positively charged at physiological pH. It was shown that the bidentate hydrogen bond network formed with guanidinium groups is particularly strong and important. For example, monomethylation or dimethylation of the guanidine groups strongly affected the cell uptake with decreases of 80% and 95% respectively [107]. Moreover, the spatial orientation of the guanidiniums was another key point to facilitate the interaction with the membrane. Space and freedom were required because negatively charged functionalities on the membrane diffuse and repel each other [23,24]. Also of importance were the organizational [108] and multivalent effects [103,104] that act synergistically to enhance the binding.
After the first association step, cell-uptake occurs. Theoretically, either endocytosis or diffusion processes permit molecules to enter cells. It is generally accepted that diffusion only occurs for small molecules (molecular weight < 3,000 g・mol-1) and involves passive or active transport through the membrane, depending on the molecule [109]. It was shown that endocytosis is certainly involved for CPPs, because at 4 ºC, a temperature known to inhibit endocytosis, a significant decrease is generally observed [110,111]. It is important to note that some uptake is observed under conditions that inhibit endocytosis, suggesting that simple diffusion probably also occurs [13,112,113]. Numerous models for understanding how this direct diffusion of CPPs could occur have been suggested as an inverted micelle model [3], a carpet model [114] or an adaptive translocation model [107]. More details concerning the endocytosis/direct diffusion of CPPs can be found in recent reviews by Wender et al. [23] and Nakase et al. [115].
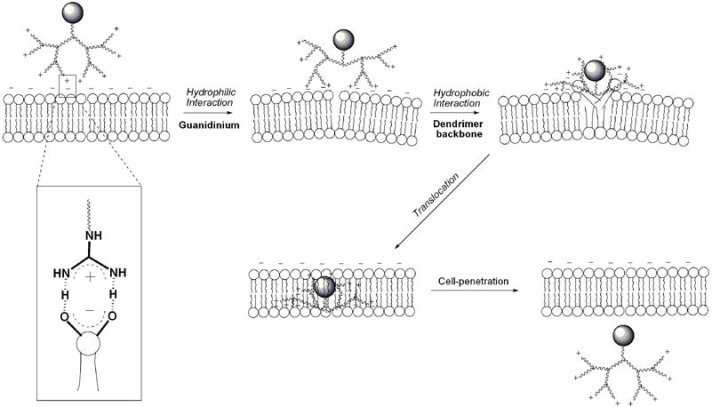
For dendrimers, all of the elements required for strong interactions between the guanidiniums and the negatively charged membranes were found to be important, including the use of flexible spacers for conformational freedom, and the incorporation of multiple guanidines for organizational and multivalent effects [116]. For guanidine functionalized dendrimers, Paleos and coworkers proposed a mechanism based on liposome interaction studies that is in principle, applicable to cells [28] (Figure 13). This mechanism is based on the possibility for dendrimers to exhibit adaptive solubility behaviour [117,118], the ability to become hydrophobic or hydrophilic depending on the environment. After charge neutralization, a change in the dendrimer’s conformation could occur, leading to the exposure of the dendrimer’s hydrophobic interior to the interior of the cell membrane. By this mechanism, the dendrimer may be translocated easily by simple diffusion. This mechanism is certainly concomitant to endocytosis, which may be more important if large or highly hydrophilic cargo is attached to the dendrimer.
Figure 13.
Postulated mechanism of direct cell penetration for a guanidine functionalized dendrimer/dendron.
6. Toxicity of Guanidine Functionalized Dendrimers
One drawback of the use of polycationic vectors is their potential cell toxicity [119,120]. It is important to highlight that toxicity is a relative concept, strongly dependent on the concentration used. Moreover the toxicity as well as the cell-uptake efficiency are cell-line dependent.
There are several recent examples of the use of CPPs as therapeutic vectors, in which they were found to be well tolerated in vivo [7,121,122,123]. Nevertheless, CPPs have also revealed potentially toxic behaviour [124,125,126]. For example, the full-length Tat protein exerted a toxic action on primary rat neuronal cultures, inducing neuronal cell death that was correlated with the time of exposure [127]. The neurotoxic effects of this Tat protein had also been previously revealed [128] as well as for the basic region Tat49–57 alone [129]. This basic domain was also able to induce endothelial cell apoptosis [130]. Although at 100 μM Tat48–60 did not induce any significant toxicity during a period of 24 h on HeLa cells [4], at concentrations as high as 1 mM, Tat49–57 demonstrated a toxic effect in all the cell lines tested [131]. The precise mechanisms leading to this cytotoxicity still remain unclear but the membrane-disrupting potential of CPPs appears to be correlated with the hydrophobic moment of the peptides [125]. Moreover, the toxicity of CPPs depends heavily on the peptide concentration, on the cargo molecule attached and on the coupling strategy used [126].
First, it is important to emphasize that there is nothing inherently concerning about dendrimers or dendrons. Dendrimers are increasingly being used in a wide variety of biomedical applications [132,133] where a number of dendrimer backbones have been found to be well tolerated. For example, polyester dendrimers have been investigated as carriers of the anticancer drug doxorubicin, and even at doses higher than that of the free drug, the delivery system was less toxic [134]. In addition, PAMAM dendrimers functionalized with the anticancer drug methotrexate and folic acid as a targeting group have been found to be well tolerated in vivo [135]. Another significant example is the dendrimer-based microbiocide VivaGelTM, which is in clinical trials for the prevention of HIV or HSV-2 (genital herpes) transmission [136]. On the other hand, polycationic polymers and dendrimers such as PAMAM and poly(ethyleneimine) (PEI) have also been demonstrated to be toxic under some circumstances. They can induce the formation of nanoscale holes in model lipid membranes and cause dye leakage in cell culture experiments at concentrations in the range of 200 nM [137,138,139,140,141,142]. As a reference, this concentration is lower than the 1 mM concentration cited above for the CPP. Nevertheless, a variety of studies have demonstrated that cationic drug delivery systems (including CPPs) can buffer endolysosomal acidity. This has been termed the “proton sponge” effect [143], and it has been proposed that due to lysosomal swelling and rupture [144,145,146] this is important for allowing an efficient delivery.
In general, the toxicities of dendrimers have been found to be dependent on the charge state of the dendrimer backbone and the peripheral groups [142]. Therefore, it is of interest to evaluate the potential toxicities of dendrimers with peripheral guanidines. Some studies have been carried out towards this goal. For example, 4th generation PPI based dendrimers were toxic with or without guanidinium groups, both exhibiting relative cell viabilities of approximately 50% at concentrations of 30 μg・mL-1 (after 24 h) [88]. The guanidine functionalized dendrimers were only slightly more toxic. Similar results were also obtained for melamine based dendrimers [92]. The latter were also hemolytic with a relative hemolysis of 50% (after 24 h) below 10 μg・mL-1. A 5th generation PPI dendrimer bearing 64 guanidine moieties was significantly more toxic (10 μM for 50% cell viability after 6 days) than the PPI with primary amine groups (40 μM for 50% cell viability after 6 days) [91]. Guanidine functionalized PPI dendrimers showed marked dose dependent cytotoxic effects as well as generation effects meaning that the number of guanidine groups was correlated to the toxicity [86,87]. A second generation dendrimer was consequently less toxic with HeLa and 293 cells, and even with only 8 guanidines, was efficiently internalized by the cells without showing toxicity [85]. Another interesting finding was that the toxicity was correlated to the efficiency of the internalization of polyamide based dendrimers when the effects of spacers with different degrees of flexibility were studied [29]. Nevertheless, other polyamide-based guanidinylated dendrimers were not toxic during the internalization [26,95]. Thus far there is only one example demonstrating the use of a dendritic guanidines in vivo and no toxicity was detected. This work involved carbamate based dendrons, which were successfully used for transfection in mice [98,99].
In some cases, it has been possible to reduce the toxicities of otherwise toxic cell penetrating dendrimers. Studies have focused on the use of lower generation dendrimers [85,86] when these lower generations have exhibited sufficient cell penetration. Another option has been to decorate the surface with two functionalities such as guanidines for cell uptake and hydroxyls for biocompatibility [88]. However a decrease in cell uptake was observed in this case. A third option was recently introduced using the PAMAM backbone, with or without the guanidines. The peripheral primary amines were first functionalized to provide a hydroxylated PAMAM dendrimer and then this dendrimer was functionalized with arginine and used for cell internalization with increased biocompatibility [81,82].
Overall, it is difficult to quantitatively compare the toxicities of CPPs with guanidinylated dendrimers as they have not been measured side by side on the same cell lines using the same assays. Such studies will be pertinent in future work in this field. However, based on the successful use of dendrimers in other biomedical applications and the preliminary results described above, it appears that it should be possible to develop relatively non-toxic cell penetrating dendrimers. The key to this success will be the choice of a dendrimer backbone that is preferably uncharged and non-toxic such as the polyester or polycarbamate, as well as careful tuning of the hydrophobic/hydrophilic balance and minimizing the number of guanidines to that required for activity.
7. Conjugation of Guanidine Functionalized Dendrimers to Cargo
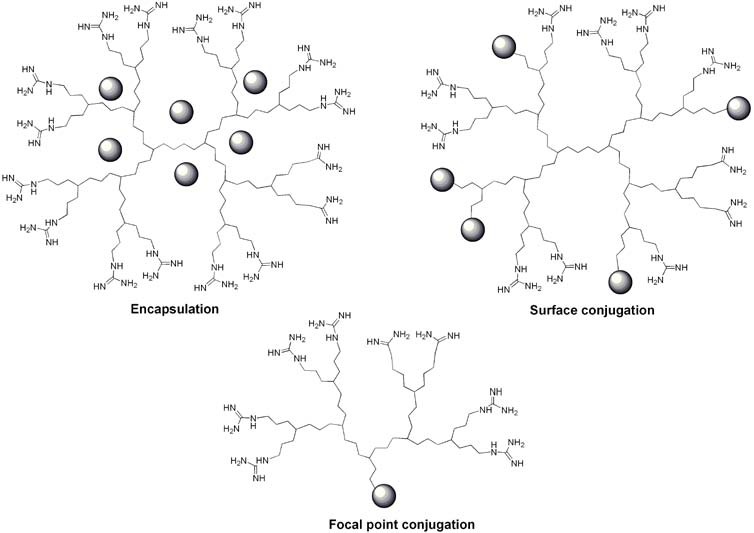
Interest in CPPs rapidly increased when it became evident that they were able to act as vectors for the delivery of other molecules such as proteins [147,148,149,150], peptides [149,151,152,153], nucleic acids [154,155,156,157,158,159,160], imaging agents [161,162,163], nanocarriers [164,165,166,167,168], liposomes [164,169,170,171], and several small molecules [33,172,173,174]. Many molecules with anticancer, anti-inflammatory or antimicrobial activities have been successfully associated with dendrimers, either covalently or noncovalently [175]. First of all, dendrimers are particularly well-suited to encapsulate/complex many small molecules [39,176] (Figure 14a).
Figure 14.
Guanidine functionalized dendrimers and their association with cargo: (a) encapsulation (b) surface conjugation (c) focal point conjugation.
These cargos can physically interact with dendrimers through either the encapsulation into void spaces, the association with surface groups, or a mixture of both. Driving forces for these interactions are hydrogen bonding, van der Waals interactions, and electrostatic attractions between opposite charges on the dendrimers and the cargos [177]. While the complexation of molecules within guanidinylated dendrimers has been investigated much less extensively, studies carried out thus far have yielded highly promising results. For example, guanidinylated PAMAM dendrimers have been used to encapsulate macromolecules such as DNA and RNA [79,80,81,82,83,85,88] for transfection applications. The conclusion of these studies was that guanidinylated PAMAM dendrimers were highly potent for transfection, even with vulnerable cells. For mechanistic studies, Paleos and coworkers have encapsulated betamethasone derivatives as dye molecules into guanidine functionalized dendrimers, but these molecules are also commonly used as anti-inflammatory agents [89].
Alternatively, different approaches have been developed to covalently conjugate the cargo. The first possibility is to conjugate cargo molecules to the dendrimer periphery (Figure 14b). This approach is often chosen [133] and has for example been used to synthesize polyester dendrimers as carriers of the anticancer drug doxorubicin [134] or to produce the dendrimer-based microbiocide VivaGelTM, which is in clinical trials for the prevention of HIV or HSV-2 (genital herpes) transmission [136]. With guanidinylated dendrimers this approach has also begun to be explored. For example, the partial reaction of nucleophilic surface functionalities on the dendrimer with activated fluorescein dye molecules (FITC), enabled the evaluation of the cellular internalization of the conjugate [84,86,87]. The second possibility was to couple dendrons to cargo molecules at their focal points (Figure 14c, Figure 15).
Figure 15.
Conjugation to cargo via the dendron focal point.
For example, dye molecules have been coupled with focal point amine groups of several different dendrons in order to study their cell uptake [26,29,95]. In addition, by using the focal point approach, Goodman and coworkers developed a coupling using a disulfide linker that enabled the conjugation of green fluorescent protein (GFP) to a dendritic guanidine [26]. Interestingly, the replacement of the low molecular weight fluorescein dye molecule with GFP did not change the translocation activity of their dendron. A disulfide linker was also used to couple dendritic guanidines to antibodies [97] and to polymer nanoparticles [96]. These conjugates were efficiently internalized by mammalian cells. For MRI applications, a polyester-based dendritic guanidine with a focal point alkyne was conjugated to azide functionalized Fe3O4 nanoparticles via a Cu(I) catalyzed “click” reaction [94]. This was demonstrated to enhance the cell uptake of the nanoparticles relative to control unfunctionalized nanoparticles, thus increasing the ability to detect the cells by MRI. For transfection applications, oligocarbamate-based dendrons were coupled to oligonucleotides [98,99]. In order to accomplish this, the dendrons were first functionalized to bear activated esters at their focal points, with which amines were subsequently reacted. This ‘dendrimeric octa-guanidine’ was highly effective to restore the dystrophin expression in both skeletal and cardiac muscles in the dystrophic mdx mice, as was the case with a CPP [178]. Moreover, Morcos et al. highlighted that this dendrimeric octaguanidine was highly potent for tissue penetration [98].
All of these results indicate that guanidinylated dendrimers can be successfully conjugated to a wide variety of cargos ranging from small molecules to biomacromolecules and nanoparticles. While much of this work is recent, it appears thus far that the dendrimers significantly enhance the cell uptake of their cargo, despite sizes approaching the tens of nanometres range. In addition, the controlled and step-wise nature of dendrimer chemistry enables the preparation of well-defined conjugates, particularly using the focal point approach. Future work in this area will likely involve the optimization of conjugation methods, the selection of the best transporters, and further biological studies of the conjugates.
8. Conclusions and Perspectives
Dendrimers and dendrons bearing multiple peripheral guanidine moieties are promising analogues of CPPs. Although this is a new field with much of the progress occurring over the last several yeras, the syntheses of dendrimers is a sufficiently well developed field that a diverse range of dendrimer backbones can be readily prepared on relatively large scales and several reagents have been demonstrated to efficiently introduce guanidines to their peripheries. The many different available dendrimer backbones and linkers have allowed structure-activity relationships to be explored. This research has revealed that guanidine functionalized dendrimers can penetrate cells as efficiently as the HIV Tat49-57 peptide and that the incorporation of longer linkers into the dendrimer structure is important for this activity. It may also play a role in determining the subcellar localization of the transporter, an aspect that requires further study. In addition, the ability to tune the dendrimer’s generation has allowed the effect of varying the number of peripheral guanidine groups to be easily explored and it has generally been found that the optimal number is between 6 and 8. While higher numbers of guanidines lead to stronger interactions with membranes, the larger sizes of these molecules can make them more challenging to internalize and can lead to increased toxicity.
The covalent and non-covalent conjugation of guanidine functionalized dendrons to biologically relevant cargo is an area that is just beginning to be developed. Thus far, some exciting results have emerged demonstrating that dendritic guanidines can facilitate the cellular internalization of even large cargo such as DNA, proteins, and nanoparticles. Nevertheless, cell viability upon internalization needs to be confirmed for these promising systems by the use of functional assays that require cell survival, such as the delivery of Cre recombinase [105,106]. Moreover, most of the research concerning guanidinylated dendrons has been carried out in vitro, so future efforts will need to focus on translating these results to animal models of disease. As part of this work, the in vivo toxicities and biodistribution behaviour of these molecules must be further explored before such systems can be applied in the clinic. It is known that cationic carriers, including CPPs can sometimes exhibit toxicity and research thus far has revealed that dendritic guanidines may not be an exception to this. Moreover, in order to be internalized by the target cells, the system must effectively reach the target site and evade undesired uptake by the reticuloendothelial system. The approaches for achieving this will be highly dependent on the target but may involve active targeting strategies and/or masking the cationic nature of protonated guanidines prior to their arrival at the target. Other promising applications involving the in vivo biodistribution and targeting of dendrimers, such as those outlined above, suggest that this work can be successful. Thus, in the future it is likely that the challenge in this field will be the conjugation of dendritic guanidines to a wider array of cargo aimed at specific therapies or imaging applications. In addition, the in vitro and in vivo efficacies and biocompatibilities of these systems must be explored. Nevertheless, significant progress has been made in this field during a relatively short time period and the results thus far demonstrate that dendritic guanidines are promising molecules for intracellular delivery and are worthy of further attention.
Acknowledgements
We thank the Natural Sciences and Engineering Research Council of Canada, the Ontario Centers of Excellence, and the Canada Research Chairs Program for Funding.
References and Notes
- 1.Frankel A.D., Pabo C.O. Cellular Uptake of the Tat Protein From Human Immunodeficiency Virus. Cell. 1988;55:1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- 2.Joliot A.H., Triller A., Volovitch M., Pernelle C., Prochiantz A. Alpha-2,8-Polysialic Acid is the Neuronal Surface Receptor of Antennapedia Homeobox Peptide. New Biol. 1991;3:1121–1134. [PubMed] [Google Scholar]
- 3.Derossi D., Joliot A.H., Chassaing G., Prochiantz A. The Third Helix of the Antennapedia Homeodornain Translocates through Biological Membrane. J. Biol. Chem. 1994;269:10444–10450. [PubMed] [Google Scholar]
- 4.Vives E., Brodin P., Lebleu B. A Truncated HIV-1 Tat Protein Basic Domain Rapidly Translocates through the Plasma Membrane and Accumulates in the Cell Nucleus. J. Biol. Chem. 1997;272:16010–16017. doi: 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
- 5.Morris M.C., Vidal P., Chaloin L., Heitz F., Divita G. A New Peptide Vector for Efficient Delivery of Oligonucleotides into Mammalian Cells. Nucleic Acids Res. 1997;25:2730–2736. doi: 10.1093/nar/25.14.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pooga M., Hallbrink M., Zorko M., Langel U. Cell Penetration by Transportan. FASEB J. 1998;12:67–77. doi: 10.1096/fasebj.12.1.67. [DOI] [PubMed] [Google Scholar]
- 7.Morris M.C., Deshayes S., Heitz F., Divita G. Cell-penetrating Peptides: From Molecular Mechanisms to Therapeutics. Biol. Cell. 2008;100:201–217. doi: 10.1042/BC20070116. [DOI] [PubMed] [Google Scholar]
- 8.Oehlke J., Scheller A., Wiesner B., Krause E., Beyermann M., Klauschenz E., Melzig M., Bienert M. Cellular Uptake of an Alpha-helical Amphipathic Model Peptide with the Potential to Deliver Polar Compounds into the Cell Interior Non-endocytically. Biochim. Biophys. Acta. 1998;1414:127–139. doi: 10.1016/s0005-2736(98)00161-8. [DOI] [PubMed] [Google Scholar]
- 9.Abes S., Moulton H.M., Clair P., Prevot P., Youngblood D.S., Wu R.P., Iversen P.L., Lebleu B. Vectorization of Morpholino Oligomers by the (R-Ahx-R)4 Peptide Allows Efficient Splicing Correction in the Absence of Endosomolytic Agents. J. Control. Release. 2006;116:304–313. doi: 10.1016/j.jconrel.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Ivanova G.D., Arzumanov A., Abes R., Yin H., Wood M.J., Lebleu B., Gait M.J. Improved Cell-penetrating Peptide-PNA Conjugates for Splicing Redirection in HeLa Cells and Exon Skipping in Mdx Mouse Muscle. Nucleic Acids Res. 2008;36:6418–6428. doi: 10.1093/nar/gkn671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mae M., El Andaloussi S., Lundin P., Oskolkov N., Johansson H.J., Guterstam P., Langel U. A Stearylated CPP for Delivery of Splice Correcting Oligonucleotides Using a Non-covalent Co-incubation Strategy. J. Control. Release. 2009;134:221–227. doi: 10.1016/j.jconrel.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 12.De Coupade C., Fittipaldi A., Chagnas V., Michel M., Carlier S., Tasciott E., Darmon A., Ravel D., Kearsey J., Giacca M., Cailler F. Novel Human-derived Cell-penetrating Peptides for Specific Subcellular Delivery of Therapeutic Biomolecules. Biochem. J. 2005;390:407–418. doi: 10.1042/BJ20050401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell D.J., Kim D.T., Steinman L., Fathman C.G., Rothbard J.B. Polyarginine Enters Cells More Efficiently Than Other Polycationic Homopolymers. J. Pept. Res. 2000;56:318–325. doi: 10.1034/j.1399-3011.2000.00723.x. [DOI] [PubMed] [Google Scholar]
- 14.Futaki S., Suzuki T., Ohashi W., Yagami T., Tanaka S., Ueda K., Sugiura Y. Arginine-rich Peptides. J. Biol. Chem. 2001;276:5836–5840. doi: 10.1074/jbc.M007540200. [DOI] [PubMed] [Google Scholar]
- 15.Futaki S., Nakase I., Tadokoro A., Takeuchi T., Jones A.T. Arginine-rich Peptides and Their Internalization Mechanisms. Bio. Soc. Trans. 2007;35:784–787. doi: 10.1042/BST0350784. [DOI] [PubMed] [Google Scholar]
- 16.Ward B., Seal B.L., Brophy C.M., Panitch A. Design of a Bioactive Cell-penetrating Peptide: When a Transduction Domain Does More Than Transduce. J. Pept. Sci. 2009;15:668–674. doi: 10.1002/psc.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mueller J., Kretzschmar I., Volkmer R., Boisguerin P. Comparison of Cellular Uptake Using 22 CPPs in 4 Different Cell Lines. Bioconjug. Chem. 2008;19:2363–2374. doi: 10.1021/bc800194e. [DOI] [PubMed] [Google Scholar]
- 18.Said Hassane F., Saleh A.F., Abes R., Gait M.J., Lebleu B. Cell Penetrating Peptides: Overview and Applications to the Delivery of Oligonucleotides. Cell. Mol. Life Sci. 2009;67:715–726. doi: 10.1007/s00018-009-0186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heitz F., Morris M.C., Divita G. Twenty Years of Cell-penetrating Peptides: From Molecular Mechanisms to Therapeutics. Br. J. Pharmacol. 2009;157:195–206. doi: 10.1111/j.1476-5381.2009.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langel U. Cell-penetrating Peptides: Processes and Applications. CRC Press; London, UK: 2002. [Google Scholar]
- 21.Lindsay M.A. Peptide-Mediated Cell Delivery: Application in Protein Target Validation. Curr. Opin. Pharmacol. 2002;2:587–594. doi: 10.1016/S1471-4892(02)00199-6. [DOI] [PubMed] [Google Scholar]
- 22.Bennett R.P., Dalby B. Protein Delivery Using VP22. Nat. Biotechnol. 2002;20:20–20. doi: 10.1038/nbt0102-20. [DOI] [PubMed] [Google Scholar]
- 23.Wender P.A., Galliher W.C., Goun E.A., Jones L.R., Pillow T.H. The Design of Guanidinium-rich Transporters and Their Internalization Mechanisms. Adv. Drug Delivery Rev. 2008;60:452–472. doi: 10.1016/j.addr.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wender P.A., Mitchell D.J., Pattabiraman K., Pelkey E.T., Steinman L., Rothbard J.B. The Design, Synthesis, and Evaluation of Molecules that Enable or Enhance Cellular Uptake: Peptoid Molecular Transporters. Proc. Natl. Acad. Sci. USA. 2000;97:13003–13008. doi: 10.1073/pnas.97.24.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rothbard J.B., Kreider E., VanDeusen C.L., Wright L., Wylie B.L., Wender P.A. Arginine-Rich Molecular Transporters for Drug Delivery: Role of Backbone Spacing in Cellular Uptake. J. Med. Chem. 2002;45:3612–3618. doi: 10.1021/jm0105676. [DOI] [PubMed] [Google Scholar]
- 26.Chung H., Harms G., Seong C.M., Choi B.H., Min C., Taulane J.P., Goodman M. Dendritic Oligoguanidines as Intracellular Translocators. Biopolymers. 2004;76:83–96. doi: 10.1002/bip.10597. [DOI] [PubMed] [Google Scholar]
- 27.Futaki S., Nakase I., Suzuki T., Youjun Y., Sugiura Y. Translocation of Branched-Chain Arginine Peptides through Cell Membranes: Flexibility in the Spatial Disposition of Positive Charges in Membrane-Permeable Peptides. Biochemistry. 2002;41:7925–7930. doi: 10.1021/bi0256173. [DOI] [PubMed] [Google Scholar]
- 28.Pantos A., Tsogas I., Paleos C.M. Guanidinium Group: A Versatile Moiety Inducing Transport and Multicompartmentalization in Complementary Membranes. Biochim. Biophys. Acta. 2008;1778:811–823. doi: 10.1016/j.bbamem.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Wender P.A., Kreider E., Pelkey E.T., Rothbard J., VanDeusen C.L. Dendrimeric Molecular Transporters: Synthesis and Evaluation of Tunable Polyguanidino Dendrimers That Facilitate Cellular Uptake. Org. Lett. 2005;7:4815–4818. doi: 10.1021/ol051496y. [DOI] [PubMed] [Google Scholar]
- 30.Wender P.A., Rothbard J.B., Jessop T.C., Kreider E.L., Wylie B.L. Oligocarbamate Molecular Transporters: Design, Synthesis, and Biological Evaluation of a New Class of Transporters for Drug Delivery. J. Am. Chem. Soc. 2002;124:13382–13383. doi: 10.1021/ja0275109. [DOI] [PubMed] [Google Scholar]
- 31.Yu J., Huang J., Jiang H., Quan J., Cho M., Cho C. Guanidinylated Poly(allyl amine) as a Gene Carrier. J. Appl. Polym. Sci. 2009;112:926–933. [Google Scholar]
- 32.Ziegler A. Thermodynamic Studies and Binding Mechanisms of Cell-penetrating Peptides with Lipids and Glycosaminoglycans. Adv. Drug Delivery Rev. 2008;60:580–597. doi: 10.1016/j.addr.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Goun E.A., Pillow T.H., Jones L.R., Rothbard J.B., Wender P.A. Molecular Transporters: Synthesis of Oligoguanidinium Transporters and Their Application to Drug Delivery and Real-T ime Imaging. Chem. Bio. Chem. 2006;7:1497–1515. doi: 10.1002/cbic.200600171. [DOI] [PubMed] [Google Scholar]
- 34.Wright L.R., Rothbard J.B., Wender P.A. Guanidinium Rich Peptide Transporters and Drug Delivery. Curr. Prot. Pept. Sci. 2003;4:105–124. doi: 10.2174/1389203033487252. [DOI] [PubMed] [Google Scholar]
- 35.Wender P.A., Jessop T.C., Pattabiraman K., Pelkey E.T., VanDeusen C.L. An Efficient, Scalable Synthesis of the Molecular Transporter Octaarginine Via a Segment Doubling Strategy. Org. Lett. 2001;3:3229–3232. doi: 10.1021/ol0161108. [DOI] [PubMed] [Google Scholar]
- 36.Futaki S., Ohashi W., Suzuki T., Niwa M., Tanaka S., Ueda K., Harashima H., Sugiura Y. Stearylated Arginine-Rich Peptides: A New Class of Transfection Systems. Bioconjug. Chem. 2001;12:1005–1011. doi: 10.1021/bc015508l. [DOI] [PubMed] [Google Scholar]
- 37.Potocky T.B., Menon A.K., Gellman S.H. Effects of Conformational Stability and Geometry of Guanidinium Display on Cell Entry by Beta-Peptides. J. Am. Chem. Soc. 2005;127:3686–3687. doi: 10.1021/ja042566j. [DOI] [PubMed] [Google Scholar]
- 38.Rueping M., Mahajan Y., Sauer M., Seebach D. Cellular Uptake Studies with Beta-Peptides. Chem. Bio. Chem. 2002;3:257–259. doi: 10.1002/1439-7633(20020301)3:2/3<257::AID-CBIC257>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 39.Medina S.H., El-Sayed M.E.H. Dendrimers as Carriers for Delivery of Chemotherapeutic Agents. Chem. Rev. 2009;109:3141–3157. doi: 10.1021/cr900174j. [DOI] [PubMed] [Google Scholar]
- 40.Tomalia D.A. Birth of a New Macromolecular Architecture: Dendrimers as Quantized Building Blocks for Nanoscale Synthetic Polymer Chemistry. Prog. Polym. Sci. 2005;30:294–324. [Google Scholar]
- 41.Cheng Y., Xu T. The Effect of Dendrimers on the Pharmacodynamic and Pharmacokinetic Behaviors of Non-Covalently or Covalently Attached Drugs. Eur. J. Med. Chem. 2008;43:2291–2297. doi: 10.1016/j.ejmech.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 42.Dutta R.C. Drug Carriers in Pharmaceutical Design: Promises and Progress. Curr. Pharm. Des. 2007;13:761–769. doi: 10.2174/138161207780249119. [DOI] [PubMed] [Google Scholar]
- 43.Wang W. Instability, Stabilization, and Formulation of Liquid Protein Pharmaceuticals. Int. J. Pharm. 1999;185:129–188. doi: 10.1016/S0378-5173(99)00152-0. [DOI] [PubMed] [Google Scholar]
- 44.Pini A., Falciani C., Bracci L. Branched Peptides as therapeutics. Curr. Protein Pept. Sci. 2008;9:468–477. doi: 10.2174/138920308785915227. [DOI] [PubMed] [Google Scholar]
- 45.Buhleier E., Wehner W., Vogtle F. "Cascade"- and "Nonskid-Chain-like" Syntheses of Molecular Cavity Topologies. Synthesis. 1978;55:155–158. [Google Scholar]
- 46.Tomalia D.A., Baker H., Dewald J., Hall M.G.K., Martin S., Roek J., Ryder J., Smith P. A New Class of Polymers : Starbust-Dendritic Macromolecules. Polym. J. 1985;17:117–132. [Google Scholar]
- 47.Tomalia D.A., Naylor A.M., Goddard W.A. Starburst Dendrimers: Molecular-Level Control of Size, Shape, Surface Chemistry, Topology, and Flexibility from Atoms to Macroscopic Matter. Angew. Chem. Int. Ed. 1990;29:138–175. doi: 10.1002/anie.199001381. [DOI] [Google Scholar]
- 48.Frechet J.M.J., Tomalia D. Dendrimers and Other Dentritic Polymers. John Wiley and Sons; Chichester, New York, NY, USA: 2001. [Google Scholar]
- 49.Newkome G.R., Moorefield C.N., Vogtle F. Dendrimers and Dendrons: Concepts, Syntheses, Applications. Wiley-VCH; Weinheim, Germany: 2001. [Google Scholar]
- 50.Aulenta F., Hayes W., Rannard S. Dendrimers: A New Class of Nanoscopic Containers and Delivery Devices. Eur. Polym. J. 2003;39:1741–1771. [Google Scholar]
- 51.Caminade A., Laurent R., Majoral J. Characterization of Dendrimers. Adv. Drug Delivery Rev. 2005;57:2130–2146. doi: 10.1016/j.addr.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 52.Gillies E.R., Frechet J.M.J. Dendrimers and Dendritic Polymers in Drug Delivery. Drug Discov. Today. 2005;10:35–43. doi: 10.1016/S1359-6446(04)03276-3. [DOI] [PubMed] [Google Scholar]
- 53.Boas U., Heegaard P.M.H. Dendrimers in Drug Research. Chem. Soc. Rev. 2004;33:43. doi: 10.1039/b309043b. [DOI] [PubMed] [Google Scholar]
- 54.Lee C.C., MacKay J.A., Frechet J.M.J., Szoka F.C. Designing Dendrimers for Biological Applications. Nat. Biotechnol. 2005;23:1517–1526. doi: 10.1038/nbt1171. [DOI] [PubMed] [Google Scholar]
- 55.Hawker C.J., Frechet J.M.J. Preparation of Polymers with Controlled Molecular Architecture. A New Convergent Approach to Dendritic Macromolecules. J. Am. Chem. Soc. 1990;112:7638–7647. doi: 10.1021/ja00177a027. [DOI] [Google Scholar]
- 56.Ashton P.R., Boyd S.E., Brown C.L., Jayaraman N., Nepogodiev S.A., Stoddart J.F. A Convergent Synthesis of Carbohydrate-Containing Dendrimers. Chem. Eur. J. 1996;2:1115–1128. [Google Scholar]
- 57.Ma X., Tang J., Shen Y., Fan M., Tang H., Radosz M. Facile Synthesis of Polyester Dendrimers from Sequential Click Coupling of Asymmetrical Monomers. J. Am. Chem. Soc. 2009;131:14795–14803. doi: 10.1021/ja9037406. [DOI] [PubMed] [Google Scholar]
- 58.Sahota H., Lloyd P.M., Yeates S.G., Derrick P.J., Taylor P.C., Haddleton D.M. Characterization of Aromatic Polyester Dendrimers by Matrix-Assisted Laser Desorption Ionization Mass Spectrometry. J. Chem. Soc., Chem. Commun. 1994:2445–2446. [Google Scholar]
- 59.Ihre H., De Jesus O.L.P., Frechet J.M.J. Fast and Convenient Divergent Synthesis of Aliphatic Ester Dendrimers by Anhydride Coupling. J. Am. Chem. Soc. 2001;123:5908–5917. doi: 10.1021/ja010524e. [DOI] [PubMed] [Google Scholar]
- 60.Baytekin B., Werner N., Luppertz F., Engeser M., Bruggemannb J., Bitter S., Henkel R., Felder T., Schalley C.A. How Useful is Mass Spectrometry for the Characterization of Dendrimers? “Fake Defects” in the ESI and MALDI Mass Spectra of Dendritic Compounds. Int. J. Mass Spectrom. 2006;249-250:138–148. doi: 10.1016/j.ijms.2006.01.016. [DOI] [Google Scholar]
- 61.Esfand R., Tomalia D.A. Poly(amidoamine) (PAMAM) Dendrimers: From Biomimicry to Drug Delivery and Biomedical Applications. Drug Discov. Today. 2001;6:427–436. doi: 10.1016/s1359-6446(01)01757-3. [DOI] [PubMed] [Google Scholar]
- 62.de Bradander-van den Berg E.M.M., Meijer E.W. Poly(propylenimine) Dendrimers: Large-Scale Synthesis via Heterogeneously Catalyzed Hydrogenation. Angew. Chem. Int. Ed. 1993;32:1308–1311. doi: 10.1002/anie.199313081. [DOI] [Google Scholar]
- 63.Morgan M.T., Nakanishi Y., Kroll D.J., Griset A.P., Carnahan M.A., Wathier M., Oberlies N.H., Manikumar G., Wani M.C., Grinstaff M.W. Dendrimer-Encapsulated Camptothecins: Increased Solubility, Cellular Uptake, and Cellular Retention Affords Enhanced Anticancer Activity In vitro. Cancer Res. 2006;66:11913–11921. doi: 10.1158/0008-5472.CAN-06-2066. [DOI] [PubMed] [Google Scholar]
- 64.Grinstaff M.W. Biodendrimers: New Polymeric Biomaterials for Tissue Engineering. Chem. Eur. J. 2002;8:2838–2846. [PubMed] [Google Scholar]
- 65.Goodwin A.P., Lam S.S., Frechet J.M.J. Rapid, Efficient Synthesis of Heterobifunctional Biodegradable Dendrimers. J. Am. Chem. Soc. 2007;129:6994–6995. doi: 10.1021/ja071530z. [DOI] [PubMed] [Google Scholar]
- 66.Padilla De Jesus O.L., Ihre H.R., Gagne L., Frechet J.M.J., Szoka F.C. Polyester Dendritic Systems for Drug Delivery Applications: In Vitro and In Vivo Evaluation. Bioconjug. Chem. 2002;13:453–461. doi: 10.1021/bc010103m. [DOI] [PubMed] [Google Scholar]
- 67.Newkome G.R., Lin X., Weis C.D. Polytryptophane Terminated Dendritic Macromolecules. Tetrahedron: Asymmetry. 1991;2:957–960. doi: 10.1016/S0957-4166(00)86135-2. [DOI] [Google Scholar]
- 68.Kono K., Fukui T., Takagishi T., Sakurai S., Kojima C. Preparation of Poly(ethylene glycol)-Modified Poly(amidoamine) Dendrimers with a Shell of Hydrophobic Amino Acid Residues and Their Function as a Nanocontainer. Polymer. 2008;49:2832–2838. [Google Scholar]
- 69.Ranganathan D., Kurur S. Synthesis of Totally Chiral, Multiple Armed, Poly Glu and Poly Asp Scaffoldings on Bifunctional Adamantane Core. Tetrahedron Lett. 1997;38:1265–1268. doi: 10.1016/S0040-4039(97)00054-3. [DOI] [Google Scholar]
- 70.Kim Y., Zeng F., Zimmerman S.C. Peptide Dendrimers from Natural Amino Acids. Chem. Eur. J. 1999;5:2133–2138. [Google Scholar]
- 71.Kress J., Rosner A., Hirsch A. Depsipeptide Dendrimers. Chem. Eur. J. 2000;6:247–257. doi: 10.1002/(SICI)1521-3765(20000117)6:2<247::AID-CHEM247>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 72.Roy R. A Decade of Glycodendrimer Chemistry. Trend Glycosci. Glycotech. 2003;15:291–310. [Google Scholar]
- 73.Röckendorf N., Lindhorst T.K. Glycodendrimers. Top. Curr. Chem. 2001;217:202–238. [Google Scholar]
- 74.Patri A.K., Majoros I.J., Baker J.R. Dendritic Polymer Macromolecular Carriers for Drug Delivery. Curr. Opin. Chem. Biol. 2002;6:466–471. doi: 10.1016/s1367-5931(02)00347-2. [DOI] [PubMed] [Google Scholar]
- 75.Frechet J.M.J. Dendrimers and Supramolecular Chemistry. Proc. Natl. Acad. Sci. USA. 2002;99:4782–4787. doi: 10.1073/pnas.082013899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carpino L.A., Han G.Y. 9-Fluorenylmethoxycarbonyl Amino-protecting Group. J. Org. Chem. 1972;37:3404–3409. [Google Scholar]
- 77.Bernatowicz M.S., Wu Y., Matsueda G.R. 1H-Pyrazole-1-carboxamidine Hydrochloride an Attractive Reagent for Guanylation of Amines and its Application to Peptide Synthesis. J. Org. Chem. 1992;57:2497–2500. [Google Scholar]
- 78.Kasai S., Nagasawa H., Shimamura M., Uto Y., Hori H. Design and Synthesis of Antiangiogenic/Heparin-Binding Arginine Dendrimer Mimicking the Surface of Endostatin. Bio. Med. Chem. Lett. 2002;12:951–954. doi: 10.1016/s0960-894x(02)00066-5. [DOI] [PubMed] [Google Scholar]
- 79.Kim J.-B., Choi J.S., Nam K., Lee M., Park J.-S., Lee J.-K. Enhanced Transfection of Primary Cortical Cultures using Arginine-grafted PAMAM Dendrimer, PAMAM-Arg. J. Control. Release. 2006;114:110–117. doi: 10.1016/j.jconrel.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 80.Choi J.S., Nam K., Park J., Kim J., Lee J., Park J. Enhanced Transfection Efficiency of PAMAM Dendrimer by Surface Modification with L-Arginine. J. Control. Release. 2004;99:445–456. doi: 10.1016/j.jconrel.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 81.Nam H.Y., Hahn H.J., Nam K., Choi W., Jeong Y., Kim D., Park J.S. Evaluation of Generations 2, 3 and 4 Arginine Modified PAMAM Dendrimers for Gene Delivery. Int. J. Pharm. 2008;363:199–205. doi: 10.1016/j.ijpharm.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 82.Nam H.Y., Nam K., Hahn H.J., Kim B.H., Lim H.J., Kim H.J., Choi J.S., Park J.-S. Biodegradable PAMAM ester for enhanced transfection efficiency with low cytotoxicity. Biomaterials. 2009;30:665–673. doi: 10.1016/j.biomaterials.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 83.Kim T.-I., Bai C.Z., Nam K., Park J.-S. Comparison Between Arginine Conjugated PAMAM Dendrimers with Structural Diversity for Gene Delivery systems. J. Control. Release. 2009;136:132–139. doi: 10.1016/j.jconrel.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 84.Pisal D.S., Yellepeddi V.K., Kumar A., Kaushik R.S., Hildreth M.B., Guan X., Palakurthi S. Permeability of Surface-Modified Polyamidoamine (PAMAM) Dendrimers Across Caco-2 Cell Monolayers. Int. J. Pharm. 2008;350:113–121. doi: 10.1016/j.ijpharm.2007.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim T., Baek J., Bai C.Z., Park J.S. Arginine-conjugated Polypropylenimine Dendrimer as a Non-toxic and Efficient Gene Delivery Carrier. Biomaterials. 2007;28:2061–2067. doi: 10.1016/j.biomaterials.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 86.Tsogas I., Theodossis T., Sideratou Z., Paleos C.M., Collet H., Rossi J.C., Romestand B., Commeyras A. Interaction and Transport of Poly(L-lysine) Dendrigrafts through Liposomal and Cellular Membranes: The Role of Generation and Surface Functionalization. Biomacromolecules. 2007;8:3263–3270. doi: 10.1021/bm700668w. [DOI] [PubMed] [Google Scholar]
- 87.Tsogas I., Sideratou Z., Tsiourvas D., Theodossiou T.A., Paleos C.M. Interactive Transport of Guanidinylated Poly(propylene imine)-Based Dendrimers through Liposomal and Cellular Membranes. Chem. Bio. Chem. 2007;8:1865–1876. doi: 10.1002/cbic.200700289. [DOI] [PubMed] [Google Scholar]
- 88.Tziveleka L., Psarra A.G., Tsiourvas D., Paleos C.M. Synthesis and Characterization of Guanidinylated Poly(propylene imine) Dendrimers as Gene Transfection Agents. J. Control. Release. 2007;117:137–146. doi: 10.1016/j.jconrel.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 89.Pantos A., Tsiourvas D., Nounesis G., Paleos C.M. Interaction of Functional Dendrimers with Multilamellar Liposomes: Design of a Model System for Studying Drug Delivery. Langmuir. 2005;21:7483–7490. doi: 10.1021/la0510331. [DOI] [PubMed] [Google Scholar]
- 90.Sideratou Z., Foundis J., Tsiourvas D., Nezis I.P., Papadimas G., Paleos C.M. A Novel Dendrimeric “Glue” for Adhesion of Phosphatidyl Choline-Based Liposomes. Langmuir. 2002;18:5036–5039. doi: 10.1021/la020150i. [DOI] [PubMed] [Google Scholar]
- 91.Cordes H., Boas U., Olsen P., Heegaard P.M.H. Guanidino- and Urea-Modified Dendrimers as Potent Solubilizers of Misfolded Prion Protein Aggregates under Non-cytotoxic Conditions. Dependence on Dendrimer Generation and Surface Charge. Biomacromolecules. 2007;8:3578–3583. doi: 10.1021/bm7006168. [DOI] [PubMed] [Google Scholar]
- 92.Chen H., Neerman M.F., Parrish A.R., Simanek E.E. Cytotoxicity, Hemolysis, and Acute in Vivo Toxicity of Dendrimers Based on Melamine, Candidate Vehicles for Drug Delivery. J. Am. Chem. Soc. 2004;126:10044–10048. doi: 10.1021/ja048548j. [DOI] [PubMed] [Google Scholar]
- 93.Okuro K., Kinbara K., Tsumoto K., Ishii N., Aida T. Molecular Glues Carrying Multiple Guanidinium Ion Pendants via an Oligoether Spacer: Stabilization of Microtubules against Depolymerization. J. Am. Chem. Soc. 2009;131:1626–1627. doi: 10.1021/ja800491v. [DOI] [PubMed] [Google Scholar]
- 94.Martin A.L., Bernas L.M., Rutt B.K., Foster P.J., Gillies E.R. Enhanced Cell Uptake of Superparamagnetic Iron Oxide Nanoparticles Functionalized with Dendritic Guanidines. Bioconjugate Chem. 2008;19:2375–2384. doi: 10.1021/bc800209u. [DOI] [PubMed] [Google Scholar]
- 95.Huang K., Voss B., Kumar D., Hamm H.E., Harth E. Dendritic Molecular Transporters Provide Control of Delivery to Intracellular Compartments. Bioconjug. Chem. 2007;18:403–409. doi: 10.1021/bc060287a. [DOI] [PubMed] [Google Scholar]
- 96.Hamilton S.K., Harth E. Molecular Dendritic Transporter Nanoparticle Vectors Provide Efficient Intracellular Delivery of Peptides. ACS Nano. 2009;3:402–410. doi: 10.1021/nn800679z. [DOI] [PubMed] [Google Scholar]
- 97.Hamilton S.K., Ikizler M.R., Wallen C., Wright P.F., Harth E. Effective Delivery of IgG-antibodies Into Infected Cells via Dendritic Molecular Transporter Conjugate IgGMTw. Mol. BioSyst. 2008;4:1209–1211. doi: 10.1039/b816645e. [DOI] [PubMed] [Google Scholar]
- 98.Wu B., Li Y., Morcos P.A., Doran T.J., Lu P., Lu Q.L. Octa-guanidine Morpholino Restores Dystrophin Expression in Cardiac and Skeletal Muscles and Ameliorates Pathology in Dystrophic mdx Mice. Mol. Therapy. 2009;17:864–871. doi: 10.1038/mt.2009.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li Y., Morcos P.A. Design and Synthesis of Dendritic Molecular Transporter that Achieves Efficient in Vivo Delivery of Morpholino Antisense Oligo. Bioconjug. Chem. 2008;19:1464–1470. doi: 10.1021/bc8001437. [DOI] [PubMed] [Google Scholar]
- 100.Almutairi A., Rossin R., Shokeen M., Hagooly A., Ananth A., Capoccia B., Guillaudeu S., Abendschein D., Anderson C.J., Welch M.J., Frechet J.M.J. Biodegradable Dendritic Positron-emitting Nanoprobes for the Noninvasive Imaging of Angiogenesis. Proc. Natl. Acad. Sci. USA. 2009;106:685–690. doi: 10.1073/pnas.0811757106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lesniak W.G., Kariapper M.S.T., Nair B.M., Tan W., Hutson A., Balogh L.P., Khan M.K. Synthesis and Characterization of PAMAM Dendrimer-Based Multifunctional Nanodevices for Targeting rvâ3 Integrins. Bioconjug. Chem. 2007;18:1148–1154. doi: 10.1021/bc070008z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Boswell C.A., Eck P.K., Regino C.A.S., Bernardo M., Wong K.J., Milenic D.E., Choyke P.L., Brechbiel M.W. Synthesis, Characterization, and Biological Evaluation of Integrinαvβ3-Targeted PAMAM Dendrimers. Mol. Pharm. 2008;5:527–539. doi: 10.1021/mp800022a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mammen M., Choi S., Whitesides G.M. Polyvalent Interactions in Biological Systems: Implications for Design and Use of Multivalent Ligands and Inhibitors. Angew. Chem. Int. Ed. 1998;37:2754–2794. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 104.Kitov P.I., Bundle D.R. On the Nature of the Multivalency Effect: A Thermodynamic Model. J. Am. Chem. Soc. 2003;125:16271–16284. doi: 10.1021/ja038223n. [DOI] [PubMed] [Google Scholar]
- 105.Xu Y., Liu S., Yu G., Chen J., Chen J., Xu X., Wu Y., Zhang A., Dowdy S.F., Cheng G. Excision of Selectable Genes from Transgenic Goat Cells by a Protein Transducible TAT-Cre Recombinase. Gene. 2008;419:70–74. doi: 10.1016/j.gene.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 106.Gitton Y., Tibaldi L., Dupont E., Levi G., Joliot A. Efficient CPP-mediated Cre Protein Delivery to Developing and Adult CNS Tissues. BMC Biotechnol. 2009;9:1–11. doi: 10.1186/1472-6750-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rothbard J.B., Jessop T.C., Lewis R.S., Murray B.A., Wender P.A. Role of Membrane Potential and Hydrogen Bonding in the Mechanism of Translocation of Guanidinium-rich Peptides into Cells. J. Am. Chem. Soc. 2004;126:9506–9507. doi: 10.1021/ja0482536. [DOI] [PubMed] [Google Scholar]
- 108.Onda M., Yoshihara K., Koyano H., Ariga K., Kunitake T. Molecular Recognition of Nucleotides by the Guanidinium Unit at the Surface of Aqueous Micelles and Bilayers. A Comparison of Microscopic and Macroscopic Interfaces. J. Am. Chem. Soc. 1996;118:8524–8530. doi: 10.1021/ja960991+. [DOI] [Google Scholar]
- 109.Seddon A.M., Casey D., Law R.V., Gee A., Templer R.H., Ces O. Drug Interactions with Lipid Membranes. Chem. Soc. Rev. 2009;38:2509–2519. doi: 10.1039/b813853m. [DOI] [PubMed] [Google Scholar]
- 110.Drin G., Cottin S., Blanc E., Rees A.R., Temsamani J. Studies on the Internalization Mechanism of Cationic Cell-penetrating Peptides. J. Biol. Chem. 2003;278:31192–31201. doi: 10.1074/jbc.M303938200. [DOI] [PubMed] [Google Scholar]
- 111.Richard J.P., Melikov K., Vives E., Ramos C., Verbeure B., Gait M.J., Chernomordik L.V., Lebleu B. Cell-penetrating Peptides. A Reevaluation of the Mechanism of Cellular Uptake. J. Biol. Chem. 2003;278:585–590. doi: 10.1074/jbc.M209548200. [DOI] [PubMed] [Google Scholar]
- 112.Maiolo J.R., Ferrer M., Ottinger E.A. Effects of Cargo Molecules on the Cellular Uptake of Arginine-rich Cell-penetrating Peptides. Biochim. Biophys. Acta. 2005;1712:161–172. doi: 10.1016/j.bbamem.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 113.Suzuki T., Futaki S., Niwa M., Tanaka S., Ueda K., Sugiura Y. Possible Existence of Common Internalization Mechanisms among Arginine-rich Peptides. J. Biol. Chem. 2002;277:2437–2443. doi: 10.1074/jbc.M110017200. [DOI] [PubMed] [Google Scholar]
- 114.Pouny Y., Rapaport D., Mor A., Nicolas P., Shai Y. Interaction of Antimicrobial Dermaseptin and its Fluorescently Labeled Analogues with Phospholipids Membranes. Biochemistry. 1992;31:12416–12423. doi: 10.1021/bi00164a017. [DOI] [PubMed] [Google Scholar]
- 115.Nakase I., Takeuchi T., Tanaka G., Futaki S. Methodological and Cellular Aspects That Govern the Internalization Mechanisms of Arginine-rich Cell-penetrating Peptides. Adv. Drug Deliv. Rev. 2008;60:598–607. doi: 10.1016/j.addr.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 116.Theodossiou T.A., Pantos A., Tsogas I., Paleos C.M. Guanidinylated Dendritic Molecular Transporters: Prospective Drug Delivery Systems and Application in Cell Transfection. Chem. Med. Chem. 2008;3:1635–1643. doi: 10.1002/cmdc.200800190. [DOI] [PubMed] [Google Scholar]
- 117.Rothbard J.B., Jessop T.C., Wender P.A. Adaptive Translocation: The Role of Hydrogen Bonding and Membrane Potential in the Uptake of Guanidinium-rich Transporters into Cells. Adv. Drug Delivery Rev. 2005;57:495–504. doi: 10.1016/j.addr.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 118.Tsogas I., Tsiourvas D., Nounesis G., Paleos C.M. Modeling Cell Membrane Transport: Interaction of Guanidinylated Poly(propylene imine) Dendrimers with a Liposomal Membrane Consisting of Phosphate-based Lipids. Langmuir. 2006;22:11322–11328. doi: 10.1021/la0620861. [DOI] [PubMed] [Google Scholar]
- 119.Douglas K.L., Piccirillo C.A., Tabrizian M. Cell Line-dependent Internalization Pathways and Intracellular Trafficking Determine Transfection Efficiency of Nanoparticle Vectors. Eur. J. Pharm. Biopharm. 2008;68:676–687. doi: 10.1016/j.ejpb.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 120.Godbey W.T., Wu K.K., Mikos A.G. Poly(ethylenimine)-mediated Gene Delivery Affects Endothelial Cell Function and Viability. Biomaterials. 2001;22:471–480. doi: 10.1016/s0142-9612(00)00203-9. [DOI] [PubMed] [Google Scholar]
- 121.Olson E.S., Aguilera T.A., Jiang T., Ellies L.G., Nguyen Q.T., Wong E.H., Gross L.A., Tsien R.Y. In vivo Characterization of Activatable Cell Penetrating Peptides for Targeting Protease Activity in Cancer. Integr. Biol. (Camb). 2009;5:382–393. doi: 10.1039/b904890a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vives E. Present and Future of Cell-Penetrating Peptide Mediated Delivery Systems: "Is the Trojan Horse too Wild to go only to Troy?". J. Control. Release. 2005;109:77–85. doi: 10.1016/j.jconrel.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 123.Dietz G.P., Bähr M. Synthesis of Cell-Penetrating Peptides and their Application in Neurobiology. Methods Mol. Biol. 2007;399:181–198. doi: 10.1007/978-1-59745-504-6_13. [DOI] [PubMed] [Google Scholar]
- 124.Scheller A., Oehlke J., Wiesner B., Dathe M., Krause E., Beyermann M., Melzig M., Bienert M. Structural Requirements for Cellular Uptake of Alpha-helical Amphipathic Peptides. J. Pept. Sci. 1999;5:185–194. doi: 10.1002/(SICI)1099-1387(199904)5:4<185::AID-PSC184>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 125.Hallbrink M., Floren A., Elmquist A., Pooga M., Bartfai T., Langel U. Cargo Delivery Kinetics of Cell-Penetrating Peptides. Biochim. Biophys. Acta. 2001;1515:101–109. doi: 10.1016/s0005-2736(01)00398-4. [DOI] [PubMed] [Google Scholar]
- 126.El-Andaloussi S., Järver P., Johansson H.J., Langel U. Cargo-Dependent Cytotoxicity and Delivery Efficacy of Cell-Penetrating Peptides: A Comparative Study. Biochem. J. 2007;407:285–292. doi: 10.1042/BJ20070507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bonavia R., Bajetto A., Barbero S., Albini A., Noonan D.M., Schettini G. HIV-1 Tat causes Apoptotic Death and Calcium Homeostasis Alterations in Rat Neurons. Biochem. Biophys. Res. Commun. 2001;288:301–308. doi: 10.1006/bbrc.2001.5743. [DOI] [PubMed] [Google Scholar]
- 128.Nath A., Psooy K., Martin C., Knudsen B., Magnuson D.S., Haughey N., Geiger J.D. Identification of a human immunodeficiency virus type 1 Tat epitope that is neuroexcitatory and neurotoxic. J. Virol. 1996;70:1475–1480. doi: 10.1128/jvi.70.3.1475-1480.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Benjouad A., Mabrouk K., Moulard M., Gluckman J.C., Rochat H., Van Rietschoten J., Sabatier J.M. Cytotoxic Effect on Lymphocytes of Tat from Human Immunodeficiency Virus (HIV-1) Fed. Eur. Biochem. Soc. Lett. 1993;319:119–124. doi: 10.1016/0014-5793(93)80049-z. [DOI] [PubMed] [Google Scholar]
- 130.Jia H., Lohr M., Jezequel S., Davis D., Shaikh S., Selwood D., Zachary I. Cysteine-Rich and Basic Domain HIV-1 Tat Peptides Inhibit Angiogenesis and Induce Endothelial Cell Apoptosis. Biochem. Biophys. Res. Commun. 2001;283:469–479. doi: 10.1006/bbrc.2001.4790. [DOI] [PubMed] [Google Scholar]
- 131.Trehin R., Merkle H.P. Chances and Pitfalls of Cell Penetrating Peptides for Cellular Drug Delivery. Eur. J. Pharm. Biopharm. 2004;58:209–223. doi: 10.1016/j.ejpb.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 132.Tomalia D.A., Reyna L.A., Svenson S. Dendrimers as Multi-Purpose Nanodevices for Oncology Drug Delivery and Diagnostic Imaging. Biochem. Soc. Trans. 2007;35:61–67. doi: 10.1042/BST0350061. [DOI] [PubMed] [Google Scholar]
- 133.Nanjwade B.K., Bechra H.M., Derkar D.K., Manvi F.V., Nanjwade V.K. Dendrimers: Emerging Polymers for Drug-Delivery Systems. Eur. J. Pharm. Sci. 2009;38:185–196. doi: 10.1016/j.ejps.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 134.Lee C.C., Gillies E.R., Fox M.E., Guillaudeu S.J., Fréchet J.M., Dy E.E., Szoka F.C. A Single Dose of Doxorubicin-Functionalized Bow-tie Dendrimer Cures Mice Bearing C-26 Colon Carcinomas. Proc. Natl. Acad. Sci. USA. 2006;103:16649–16654. doi: 10.1073/pnas.0607705103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Majoros I.J., Williams C.R., Becker A., Baker J.R. Methotrexate Delivery via Folate Targeted Dendrimer-based Nanotherapeutic Platform. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009;1:502–510. doi: 10.1002/wnan.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Halford B. Dendrimers Branch Out. Chem. Eng. News. 2005;83:30–36. [Google Scholar]
- 137.Leroueil P.R., Hong S., Mecke A., Baker J.R., Orr B.G., Banaszak-Holl M.M. Nanoparticle Interaction with Biological Membranes: Does Nanotechnology Present a Janus Face? Acc. Chem. Res. 2007;40:335–342. doi: 10.1021/ar600012y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Leroueil P.R., Berry S.A., Duthie K., Han G., Rotello V.M., McNerny D.Q., Baker J.R., Orr B.G., Banaszak Holl M.M. Wide Varieties of Cationic Nanoparticles Induce Defects in Supported Lipid Bilayers. Nano Lett. 2008;8:420–424. doi: 10.1021/nl0722929. [DOI] [PubMed] [Google Scholar]
- 139.Fischer D., Li Y., Ahlemeyer B., Krieglstein J., Kissel T. In Vitro Cytotoxicity Testing of Polycations: Influence of Polymer Structure on Cell Viability and Hemolysis. Biomaterials. 2003;24:1121–1131. doi: 10.1016/S0142-9612(02)00445-3. [DOI] [PubMed] [Google Scholar]
- 140.Hong S., Bielinska A.U., Mecke A., Keszler B., Beals J.L., Shi X., Balogh L., Orr B.G., Baker J.R., Banaszak-Holl M.M. Synthetic and Natural Polycationic Polymer Nanoparticles Interact Selectively with Fluid-Phase Domains of DMPC Lipid Bilayers. Bioconjug. Chem. 2004;15:774–782. [Google Scholar]
- 141.Hong S., Leroueil P.R., Janus E.K., Peters J.L., Kober M.M., Islam M.T., Orr B.G., Baker J.R., Banaszak Holl M.M. Interaction of Polycationic Polymers with Supported Lipid Bilayers and Cells: Nanoscale Hole Formation and Enhanced Membrane Permeability. Bioconjug. Chem. 2006;17:728–734. doi: 10.1021/bc060077y. [DOI] [PubMed] [Google Scholar]
- 142.Malik N., Wiwattanapatapee R., Klopsch R., Lorenz K., Frey H., Weener J.W., Meijer E.W., Paulus W., Duncan R. Dendrimers: Relationship Between Structure and Biocompatibility in Vitro, and Preliminary Studies on the Biodistribution of 125I-Labelled PAMAM Dendrimers in Vivo. J. Control. Release. 2000;65:133–148. doi: 10.1016/S0168-3659(99)00246-1. [DOI] [PubMed] [Google Scholar]
- 143.Behr J.P. Synthetic Gene-transfer Vectors. Acc. Chem. Res. 1993;26:274–278. doi: 10.1021/ar200213g. [DOI] [PubMed] [Google Scholar]
- 144.Akinc A., Thomas M., Klibanov A.M., Langer R.J. Exploring Polyethylenimine-mediated DNA Transfection and the Proton Sponge Hypothesis. Gene Med. 2005;7:657–663. doi: 10.1002/jgm.696. [DOI] [PubMed] [Google Scholar]
- 145.Sonawane N.D., Szoka F.C., Verkman A.S. Chloride Accumulation and Swelling in Endosomes Enhances DNA Transfer by Polyamine-DNA Polyplexes. J. Biol. Chem. 2003;278:44826–44831. doi: 10.1074/jbc.M308643200. [DOI] [PubMed] [Google Scholar]
- 146.Kichler A., Leborgne C., Coeytaux E., Danos O. Polyethylenimine-mediated Gene Delivery: A Mechanistic Study. J. Gene Med. 2001;3:135–144. doi: 10.1002/jgm.173. [DOI] [PubMed] [Google Scholar]
- 147.Fawell S., Seery J., Daikh Y., Moore C., Chen L.L., Pepinsky B., Barsoum J. Tat-mediated Delivery of Heterologous Proteins into Cells. Proc. Natl. Acad. Sci. USA. 1994;91:664–668. doi: 10.1073/pnas.91.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wadia J.S., Dowdy S.F. Tat Peptide-mediated Cellular Delivery: Back to Basics. Adv. Drug Delivery Rev. 2005;57:559–577. doi: 10.1016/j.addr.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 149.Wadia J.S., Dowdy S.F. Transmembrane Delivery of Protein and Peptide Drugs by TAT-mediated Transduction in the Treatment of Cancer. Adv. Drug Delivery Rev. 2005;57:579–596. doi: 10.1016/j.addr.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 150.Fittipaldi A., Giacca M. Transcellular Protein Transduction Using the Tat Protein of HIV-1. Adv. Drug Delivery Rev. 2005;57:597–608. doi: 10.1016/j.addr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 151.Hallbrink M., Floren A., Elmquist A., Pooga M., Bartfai T., Langel U. Cargo Delivery Kinetics of Cell-penetrating Peptides. Biochim. Biophys. Acta. 2001;1515:101–109. doi: 10.1016/s0005-2736(01)00398-4. [DOI] [PubMed] [Google Scholar]
- 152.Corradin S., Ransijn A., Corradin G., Bouvier J., Delgado M.B., Fernandez-Carneado J., Mottram J.C., Vergeres G., Mauel J. Novel Peptide Inhibitors of Leishmania gp63 Based on the Cleavage Site of MARCKS (Myristoylated Alanine-rich C Kinase Substrate)-Related Protein. Biochem. J. 2002;367:761–769. doi: 10.1042/BJ20020386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Datta K., Sundberg C., Karumanchi S.A., Mukhopadhyay D. The 104-123 Amino Acid Sequence of the b-domain of von Hippel-Lindau Gene Product Is Sufficient to Inhibit Renal Tumor Growth and Invasion. Cancer Res. 2001;61:1768–1775. [PubMed] [Google Scholar]
- 154.Eguchi A., Akuta T., Okuyama H., Senda T., Yokoi H., Inokuchi H., Fujita S., Hayakawa T., Takeda K., Hasegawa M., Nakanishi M. Protein Transduction Domain of HIV-1 Tat Protein Promotes Efficient Delivery of DNA into Mammalian Cells. J. Biol. Chem. 2001;276:26204–26210. doi: 10.1074/jbc.M010625200. [DOI] [PubMed] [Google Scholar]
- 155.Snyder E.L., Dowdy S.F. Protein/peptide Transduction Domains: Potential to Deliver Large DNA Molecules into Cells. Curr. Opin. Mol. Ther. 2001;3:147–152. [PubMed] [Google Scholar]
- 156.Lebleu B., Moulton H.M., Abes R., Ivanova G.D., Abes S., Stein D.A., Iversen P.L., Arzumanov A.A., Gait M.J. Cell Penetrating Peptide Conjugates of Steric Block Oligonucleotides. Adv. Drug Delivery Rev. 2008;60:517–529. doi: 10.1016/j.addr.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Abes R., Arzumanov A., Moulton H., Abes S., Ivanova G., Gait M.J., Iversen P., Lebleu B. Arginine-rich Cell Penetrating Peptides: Design, Structure-activity, and Applications to Alter Pre-mRNA Splicing by Steric-block Oligonucleotides. J. Pept. Sci. 2008;14:455–460. doi: 10.1002/psc.979. [DOI] [PubMed] [Google Scholar]
- 158.Veldhoen S., Laufer S.D., Restle T. Recent Developments in Peptide-Based Nucleic Acid Delivery. Int. J. Mol. Sci. 2008;9:1276–1320. doi: 10.3390/ijms9071276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Crombez L., Aldrian-Herrada G., Konate K., Nguyen Q., McMaster G.K., Brasseur R., Heitz F., Divita G. A New Potent Secondary Amphipathic Cell-penetrating Peptide for siRNA Delivery into Mammalian Cells. Mol. Ther. 2009;17:95–103. doi: 10.1038/mt.2008.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Abes R., Arzumanov A.A., Moulton H.M., Abes S., Ivanova G.D., Iversen P.L., Gait M.J., Lebleu B. Cell-penetrating-peptide-based Delivery of Oligonucleotides: An Overview. Bio. Soc. Trans. 2007;35:775–779. doi: 10.1042/BST0350775. [DOI] [PubMed] [Google Scholar]
- 161.Kersemans V., Kersemans K., Cornelissen B. Cell Penetrating Peptides for In Vivo Molecular Imaging Applications. Curr. Pharm. Des. 2008;14:2415–2447. doi: 10.2174/138161208785777432. [DOI] [PubMed] [Google Scholar]
- 162.Bhorade R., Weissleder R., Nakakoshi T., Moore A., Tung C.H. Macrocyclic Chelators with Paramagnetic Cations Are Internalized into Mammalian Cells via a HIV-Tat Derived Membrane Translocation Peptide. Bioconjug. Chem. 2000;11:301–305. doi: 10.1021/bc990168d. [DOI] [PubMed] [Google Scholar]
- 163.Polyakov V., Sharma V., Dahlheimer J.L., Pica C.M., Luker G.D., Piwnica-Worms D. Novel Tat-Peptide Chelates for Direct Transduction of Technetium-99m and Rhenium into Human Cells for Imaging and Radiotherapy. Bioconjug. Chem. 2000;11:762–771. doi: 10.1021/bc000008y. [DOI] [PubMed] [Google Scholar]
- 164.Torchilin V.P. Tat Peptide-mediated Intracellular Delivery of Pharmaceutical Nanocarriers. Adv. Drug Delivery Rev. 2008;60:548–558. doi: 10.1016/j.addr.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 165.Rudolph C., Schillinger U., Ortiz A., Tabatt K., Plank C., Muller R.H., Rosenecker J. Application of Novel Solid Lipid Nanoparticle (SLN)-Gene Vector Formulations Based on a Dimeric HIV-1 TAT-Peptide in Vitro and in Vivo. Pharm. Res. 2004;21:1662–1669. doi: 10.1023/B:PHAM.0000041463.56768.ec. [DOI] [PubMed] [Google Scholar]
- 166.de la Fuente J.M., Berry C.C. Tat Peptide as an Efficient Molecule To Translocate Gold Nanoparticles into the Cell Nucleus. Bioconjug. Chem. 2005;16:1176–1180. doi: 10.1021/bc050033+. [DOI] [PubMed] [Google Scholar]
- 167.Sethuraman V.A., Bae Y.H. Tat Peptide-based Micelle System for Potential Active Targeting of Anti-cancer Agents to Acidic Solid Tumors. J. Control. Release. 2007;118:216–224. doi: 10.1016/j.jconrel.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mishra R., Su W., Pohmann R., Pfeuffer J., Sauer M.G., Ugurbil K., Engelmann J. Cell-Penetrating Peptides and Peptide Nucleic Acid-Coupled MRI Contrast Agents: Evaluation of Cellular Delivery and Target Binding. Bioconjug. Chem. 2009;20:1860–1868. doi: 10.1021/bc9000454. [DOI] [PubMed] [Google Scholar]
- 169.Tseng Y.L., Liu J.J., Hong R.L. Translocation of Liposomes into Cancer Cells by Cell-Penetrating Peptides Penetratin and Tat: A Kinetic and Efficacy Study. Mol. Pharmacol. 2002;62:864–872. doi: 10.1124/mol.62.4.864. [DOI] [PubMed] [Google Scholar]
- 170.Torchilin V.P., Levchenko T.S. TAT-Liposomes: A Novel Intracellular Drug Carrier. Curr. Protein Pept. Sci. 2003;4:133–140. doi: 10.2174/1389203033487298. [DOI] [PubMed] [Google Scholar]
- 171.Torchilin V.P., Rammohan R., Weissig V., Levchenko T.S. TAT peptide on the surface of liposomes affords their efficient intracellular delivery even at low temperature and in the presence of metabolic inhibitors. Proc. Natl. Acad. Sci. USA. 2001;98:8786–8791. doi: 10.1073/pnas.151247498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kirschberg T.A., VanDeusen C.L., Rothbard J.B., Yang M., Wender P.A. Arginine-Based Molecular Transporters: The Synthesis and Chemical Evaluation of Releasable Taxol-Transporter Conjugates. Org. Lett. 2003;5:3459–3462. doi: 10.1021/ol035234c. [DOI] [PubMed] [Google Scholar]
- 173.Rothbard J.B., Garlington S., Lin Q., Kirschberg T., Kreider E., McGrane P.L., Wender P.A., Khavari P.A. Conjugation of Arginine Oligomers to Cyclosporin A Facilitates Topical Delivery and Inhibition of Inflammation. Nat. Med. 2000;6:1253–1257. doi: 10.1038/81359. [DOI] [PubMed] [Google Scholar]
- 174.Gupta B., Levchenko T.S., Torchilin V.P. Intracellular Delivery of Large Molecules and Small Particles by Cell-penetrating Proteins and Peptides. Adv. Drug Delivery Rev. 2005;57:637–651. doi: 10.1016/j.addr.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 175.Svenson S. Dendrimers as Versatile Platform in Drug Delivery Applications. Eur. J. Pharm. Bio. 2009;71:445. doi: 10.1016/j.ejpb.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 176.Samad A., Alam I., Saxena K. Dendrimers : A Class of Polymers in the Nanotechnology for the Delivery of Active Pharmaceuticals. Curr. Pharm. Des. 2009;15:2958–2969. doi: 10.2174/138161209789058200. [DOI] [PubMed] [Google Scholar]
- 177.D’Emanuele A., Attwood D. Dendrimer-drug Interactions. Adv. Drug Delivery Rev. 2005;57:2147–2162. doi: 10.1016/j.addr.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 178.Jearawiriyapaisarn N., Moulton H.M., Buckley B., Roberts J., Sazani P., Fucharoen S. Sustained Dystrophin Expression Induced by Peptide-Conjugated Morpholino Oligomers in the Muscles of mdx Mice. Mol. Ther. 2008;16:1624–1629. doi: 10.1038/mt.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]