Abstract
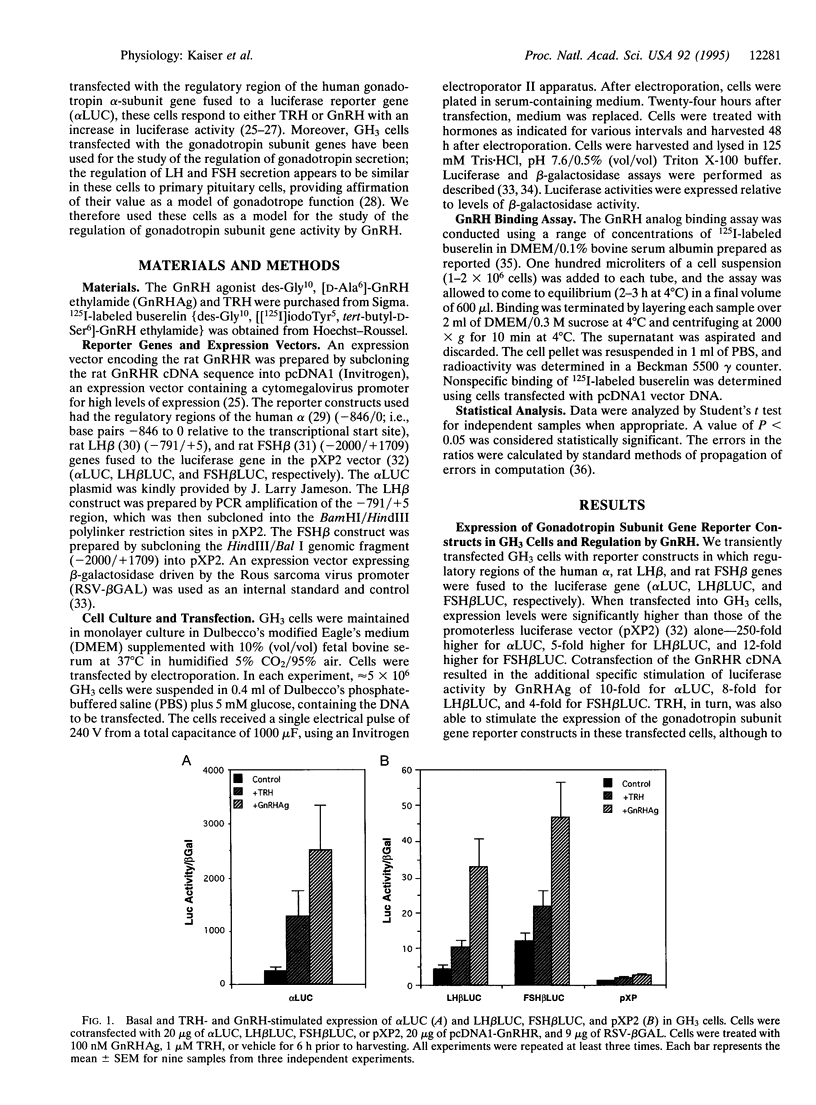
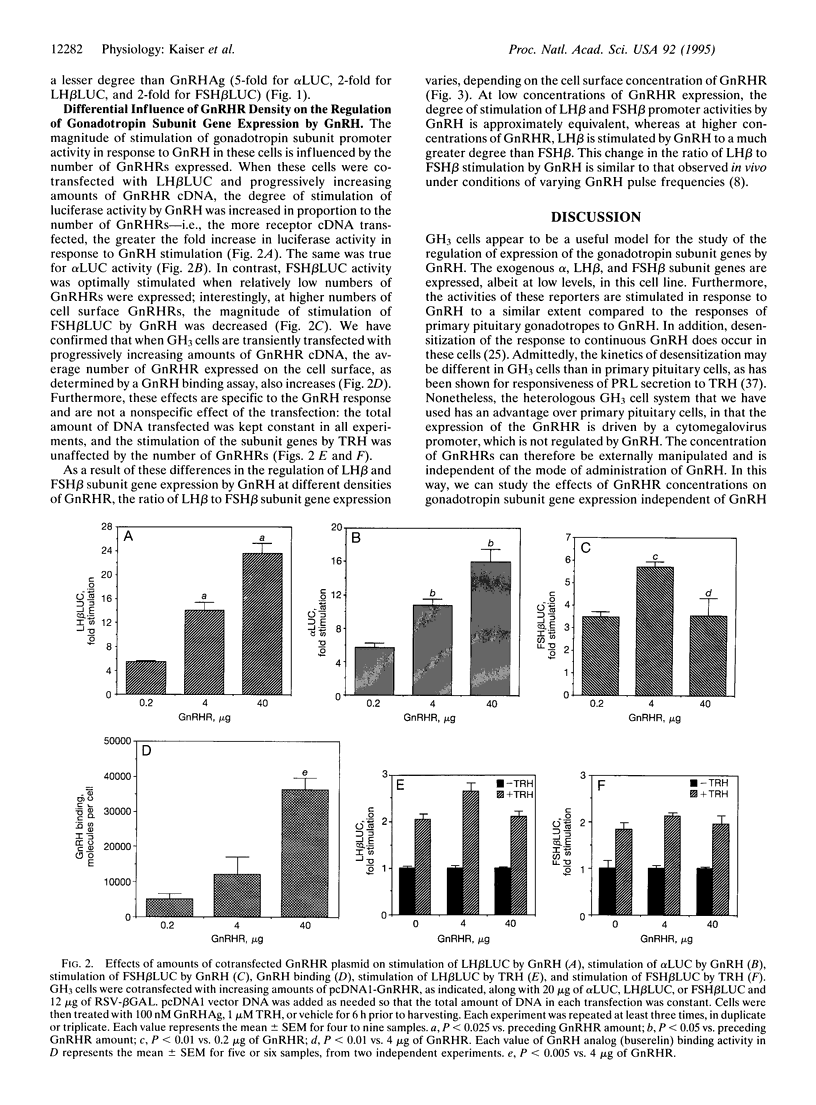
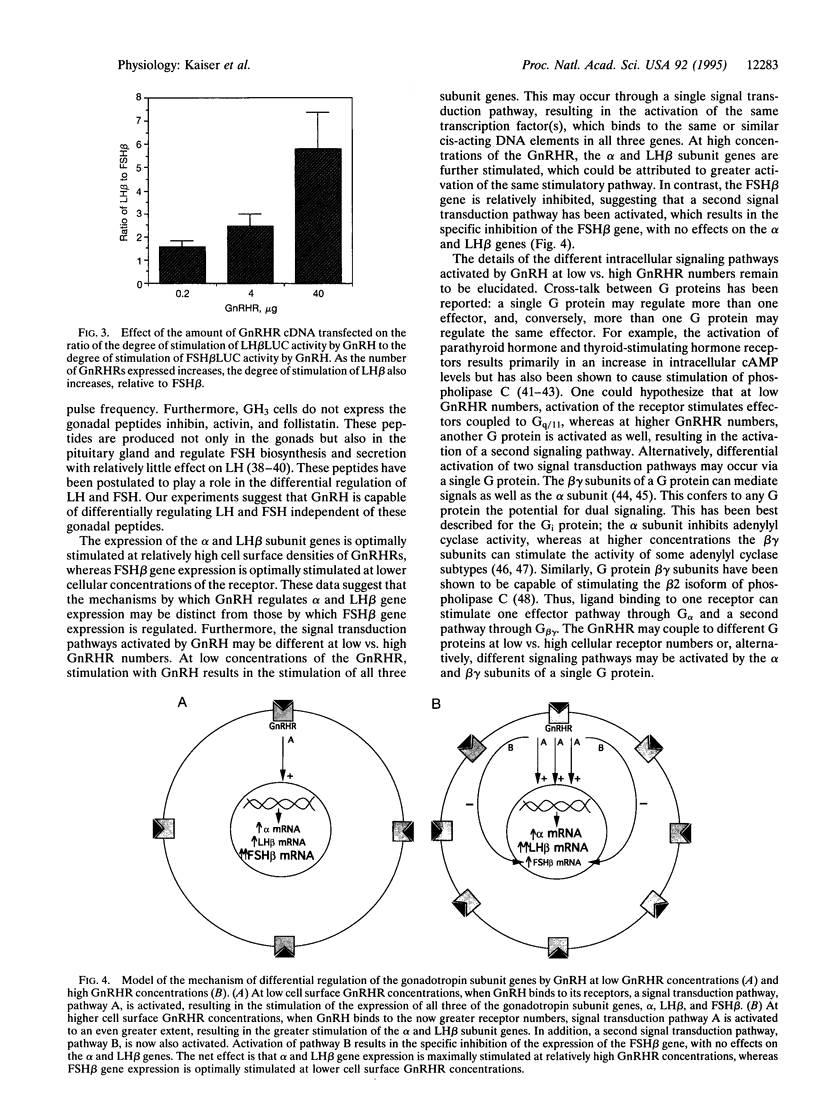
The hypothalamic hormone gonadotropin-releasing hormone (GnRH) is released in a pulsatile fashion, with its frequency varying throughout the reproductive cycle. Varying pulse frequencies and amplitudes differentially regulate the biosynthesis and secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) by pituitary gonadotropes. The mechanism by which this occurs remains a major question in reproductive physiology. Previous studies have been limited by lack of available cell lines that express the LH and FSH subunit genes and respond to GnRH. We have overcome this limitation by transfecting the rat pituitary GH3 cell line with rat GnRH receptor (GnRHR) cDNA driven by a heterologous promoter. These cells, when cotransfected with regulatory regions of the common alpha, LH beta, or FSH beta subunit gene fused to a luciferase reporter gene, respond to GnRH with an increase in luciferase activity. Using this model, we demonstrate that different cell surface densities of the GnRHR result in the differential regulation of LH and FSH subunit gene expression by GnRH. This suggests that the differential regulation of gonadotropin subunit gene expression by GnRH observed in vivo in rats may, in turn, be mediated by varying gonadotrope cell surface GnRHR concentrations. This provides a physiologic mechanism by which a single ligand can act through a single receptor to regulate differentially the production of two hormones in the same cell.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allgeier A., Offermanns S., Van Sande J., Spicher K., Schultz G., Dumont J. E. The human thyrotropin receptor activates G-proteins Gs and Gq/11. J Biol Chem. 1994 May 13;269(19):13733–13735. [PubMed] [Google Scholar]
- Aragay A. M., Katz A., Simon M. I. The G alpha q and G alpha 11 proteins couple the thyrotropin-releasing hormone receptor to phospholipase C in GH3 rat pituitary cells. J Biol Chem. 1992 Dec 15;267(35):24983–24988. [PubMed] [Google Scholar]
- Belchetz P. E., Plant T. M., Nakai Y., Keogh E. J., Knobil E. Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science. 1978 Nov 10;202(4368):631–633. doi: 10.1126/science.100883. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L. Receptor-to-effector signaling through G proteins: roles for beta gamma dimers as well as alpha subunits. Cell. 1992 Dec 24;71(7):1069–1072. doi: 10.1016/s0092-8674(05)80056-x. [DOI] [PubMed] [Google Scholar]
- Carroll R. S., Corrigan A. Z., Gharib S. D., Vale W., Chin W. W. Inhibin, activin, and follistatin: regulation of follicle-stimulating hormone messenger ribonucleic acid levels. Mol Endocrinol. 1989 Dec;3(12):1969–1976. doi: 10.1210/mend-3-12-1969. [DOI] [PubMed] [Google Scholar]
- Clapham D. E., Neer E. J. New roles for G-protein beta gamma-dimers in transmembrane signalling. Nature. 1993 Sep 30;365(6445):403–406. doi: 10.1038/365403a0. [DOI] [PubMed] [Google Scholar]
- Crowley W. F., Jr, Filicori M., Spratt D. I., Santoro N. F. The physiology of gonadotropin-releasing hormone (GnRH) secretion in men and women. Recent Prog Horm Res. 1985;41:473–531. doi: 10.1016/b978-0-12-571141-8.50015-9. [DOI] [PubMed] [Google Scholar]
- Dalkin A. C., Haisenleder D. J., Ortolano G. A., Ellis T. R., Marshall J. C. The frequency of gonadotropin-releasing-hormone stimulation differentially regulates gonadotropin subunit messenger ribonucleic acid expression. Endocrinology. 1989 Aug;125(2):917–924. doi: 10.1210/endo-125-2-917. [DOI] [PubMed] [Google Scholar]
- Delbeke D., Kojima I., Dannies P. S. Comparison of patterns of prolactin release in GH4C1 cells and primary pituitary cultures. Mol Cell Endocrinol. 1985 Nov;43(1):15–22. doi: 10.1016/0303-7207(85)90037-1. [DOI] [PubMed] [Google Scholar]
- Edlund T., Walker M. D., Barr P. J., Rutter W. J. Cell-specific expression of the rat insulin gene: evidence for role of two distinct 5' flanking elements. Science. 1985 Nov 22;230(4728):912–916. doi: 10.1126/science.3904002. [DOI] [PubMed] [Google Scholar]
- Federman A. D., Conklin B. R., Schrader K. A., Reed R. R., Bourne H. R. Hormonal stimulation of adenylyl cyclase through Gi-protein beta gamma subunits. Nature. 1992 Mar 12;356(6365):159–161. doi: 10.1038/356159a0. [DOI] [PubMed] [Google Scholar]
- Gershengorn M. C. Mechanism of signal transduction by TRH. Ann N Y Acad Sci. 1989;553:191–196. doi: 10.1111/j.1749-6632.1989.tb46641.x. [DOI] [PubMed] [Google Scholar]
- Gharib S. D., Roy A., Wierman M. E., Chin W. W. Isolation and characterization of the gene encoding the beta-subunit of rat follicle-stimulating hormone. DNA. 1989 Jun;8(5):339–349. doi: 10.1089/dna.1.1989.8.339. [DOI] [PubMed] [Google Scholar]
- Gharib S. D., Wierman M. E., Shupnik M. A., Chin W. W. Molecular biology of the pituitary gonadotropins. Endocr Rev. 1990 Feb;11(1):177–199. doi: 10.1210/edrv-11-1-177. [DOI] [PubMed] [Google Scholar]
- Haisenleder D. J., Dalkin A. C., Ortolano G. A., Marshall J. C., Shupnik M. A. A pulsatile gonadotropin-releasing hormone stimulus is required to increase transcription of the gonadotropin subunit genes: evidence for differential regulation of transcription by pulse frequency in vivo. Endocrinology. 1991 Jan;128(1):509–517. doi: 10.1210/endo-128-1-509. [DOI] [PubMed] [Google Scholar]
- Haisenleder D. J., Ortolano G. A., Dalkin A. C., Ellis T. R., Paul S. J., Marshall J. C. Differential regulation of gonadotropin subunit gene expression by gonadotropin-releasing hormone pulse amplitude in female rats. Endocrinology. 1990 Dec;127(6):2869–2875. doi: 10.1210/endo-127-6-2869. [DOI] [PubMed] [Google Scholar]
- Hinkle P. M., Tashjian A. H., Jr Receptors for thyrotropin-releasing hormone in prolactin producing rat pituitary cells in culture. J Biol Chem. 1973 Sep 10;248(17):6180–6186. [PubMed] [Google Scholar]
- Hoffman A. R., Crowley W. F., Jr Induction of puberty in men by long-term pulsatile administration of low-dose gonadotropin-releasing hormone. N Engl J Med. 1982 Nov 11;307(20):1237–1241. doi: 10.1056/NEJM198211113072003. [DOI] [PubMed] [Google Scholar]
- Hsieh K. P., Martin T. F. Thyrotropin-releasing hormone and gonadotropin-releasing hormone receptors activate phospholipase C by coupling to the guanosine triphosphate-binding proteins Gq and G11. Mol Endocrinol. 1992 Oct;6(10):1673–1681. doi: 10.1210/mend.6.10.1333052. [DOI] [PubMed] [Google Scholar]
- Huckle W. R., Conn P. M. Molecular mechanism of gonadotropin releasing hormone action. II. The effector system. Endocr Rev. 1988 Nov;9(4):387–395. doi: 10.1210/edrv-9-4-387. [DOI] [PubMed] [Google Scholar]
- Jameson L., Chin W. W., Hollenberg A. N., Chang A. S., Habener J. F. The gene encoding the beta-subunit of rat luteinizing hormone. Analysis of gene structure and evolution of nucleotide sequence. J Biol Chem. 1984 Dec 25;259(24):15474–15480. [PubMed] [Google Scholar]
- Kaiser U. B., Jakubowiak A., Steinberger A., Chin W. W. Regulation of rat pituitary gonadotropin-releasing hormone receptor mRNA levels in vivo and in vitro. Endocrinology. 1993 Aug;133(2):931–934. doi: 10.1210/endo.133.2.8393779. [DOI] [PubMed] [Google Scholar]
- Kaiser U. B., Katzenellenbogen R. A., Conn P. M., Chin W. W. Evidence that signalling pathways by which thyrotropin-releasing hormone and gonadotropin-releasing hormone act are both common and distinct. Mol Endocrinol. 1994 Aug;8(8):1038–1048. doi: 10.1210/mend.8.8.7527898. [DOI] [PubMed] [Google Scholar]
- Kaiser U. B., Zhao D., Cardona G. R., Chin W. W. Isolation and characterization of cDNAs encoding the rat pituitary gonadotropin-releasing hormone receptor. Biochem Biophys Res Commun. 1992 Dec 30;189(3):1645–1652. doi: 10.1016/0006-291x(92)90266-n. [DOI] [PubMed] [Google Scholar]
- Kakar S. S., Musgrove L. C., Devor D. C., Sellers J. C., Neill J. D. Cloning, sequencing, and expression of human gonadotropin releasing hormone (GnRH) receptor. Biochem Biophys Res Commun. 1992 Nov 30;189(1):289–295. doi: 10.1016/0006-291x(92)91556-6. [DOI] [PubMed] [Google Scholar]
- Katt J. A., Duncan J. A., Herbon L., Barkan A., Marshall J. C. The frequency of gonadotropin-releasing hormone stimulation determines the number of pituitary gonadotropin-releasing hormone receptors. Endocrinology. 1985 May;116(5):2113–2115. doi: 10.1210/endo-116-5-2113. [DOI] [PubMed] [Google Scholar]
- Katz A., Wu D., Simon M. I. Subunits beta gamma of heterotrimeric G protein activate beta 2 isoform of phospholipase C. Nature. 1992 Dec 17;360(6405):686–689. doi: 10.1038/360686a0. [DOI] [PubMed] [Google Scholar]
- Kay T. W., Jameson J. L. Identification of a gonadotropin-releasing hormone-responsive region in the glycoprotein hormone alpha-subunit promoter. Mol Endocrinol. 1992 Nov;6(11):1767–1773. doi: 10.1210/mend.6.11.1282668. [DOI] [PubMed] [Google Scholar]
- Kirk S. E., Dalkin A. C., Yasin M., Haisenleder D. J., Marshall J. C. Gonadotropin-releasing hormone pulse frequency regulates expression of pituitary follistatin messenger ribonucleic acid: a mechanism for differential gonadotrope function. Endocrinology. 1994 Sep;135(3):876–880. doi: 10.1210/endo.135.3.8070381. [DOI] [PubMed] [Google Scholar]
- Knobil E. The neuroendocrine control of the menstrual cycle. Recent Prog Horm Res. 1980;36:53–88. doi: 10.1016/b978-0-12-571136-4.50008-5. [DOI] [PubMed] [Google Scholar]
- Kuphal D., Janovick J. A., Kaiser U. B., Chin W. W., Conn P. M. Stable transfection of GH3 cells with rat gonadotropin-releasing hormone receptor complementary deoxyribonucleic acid results in expression of a receptor coupled to cyclic adenosine 3',5'-monophosphate-dependent prolactin release via a G-protein. Endocrinology. 1994 Jul;135(1):315–320. doi: 10.1210/endo.135.1.8013367. [DOI] [PubMed] [Google Scholar]
- Loumaye E., Catt K. J. Homologous regulation of gonadotropin-releasing hormone receptors in cultured pituitary cells. Science. 1982 Feb 19;215(4535):983–985. doi: 10.1126/science.6296998. [DOI] [PubMed] [Google Scholar]
- McArdle C. A., Gorospe W. C., Huckle W. R., Conn P. M. Homologous down-regulation of gonadotropin-releasing hormone receptors and desensitization of gonadotropes: lack of dependence on protein kinase C. Mol Endocrinol. 1987 Jun;1(6):420–429. doi: 10.1210/mend-1-6-420. [DOI] [PubMed] [Google Scholar]
- Muyan M., Ryzmkiewicz D. M., Boime I. Secretion of lutropin and follitropin from transfected GH3 cells: evidence for separate secretory pathways. Mol Endocrinol. 1994 Dec;8(12):1789–1797. doi: 10.1210/mend.8.12.7535895. [DOI] [PubMed] [Google Scholar]
- Nordeen S. K. Luciferase reporter gene vectors for analysis of promoters and enhancers. Biotechniques. 1988 May;6(5):454–458. [PubMed] [Google Scholar]
- Papavasiliou S. S., Zmeili S., Khoury S., Landefeld T. D., Chin W. W., Marshall J. C. Gonadotropin-releasing hormone differentially regulates expression of the genes for luteinizing hormone alpha and beta subunits in male rats. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4026–4029. doi: 10.1073/pnas.83.11.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst W. C., Snyder L. A., Schuster D. I., Brosius J., Sealfon S. C. Sequence alignment of the G-protein coupled receptor superfamily. DNA Cell Biol. 1992 Jan-Feb;11(1):1–20. doi: 10.1089/dna.1992.11.1. [DOI] [PubMed] [Google Scholar]
- Reinhart J., Mertz L. M., Catt K. J. Molecular cloning and expression of cDNA encoding the murine gonadotropin-releasing hormone receptor. J Biol Chem. 1992 Oct 25;267(30):21281–21284. [PubMed] [Google Scholar]
- Schneider H., Feyen J. H., Seuwen K. A C-terminally truncated human parathyroid hormone receptor is functional and activates multiple G proteins. FEBS Lett. 1994 Sep 5;351(2):281–285. doi: 10.1016/0014-5793(94)00878-7. [DOI] [PubMed] [Google Scholar]
- Smith M. A., Vale W. W. Desensitization to gonadotropin-releasing hormone observed in superfused pituitary cells on Cytodex beads. Endocrinology. 1981 Mar;108(3):752–759. doi: 10.1210/endo-108-3-752. [DOI] [PubMed] [Google Scholar]
- Stanislaus D., Janovick J. A., Jennes L., Kaiser U. B., Chin W. W., Conn P. M. Functional and morphological characterization of four cell lines derived from GH3 cells stably transfected with gonadotropin-releasing hormone receptor complementary deoxyribonucleic acid. Endocrinology. 1994 Nov;135(5):2220–2227. doi: 10.1210/endo.135.5.7956945. [DOI] [PubMed] [Google Scholar]
- Stojilković S. S., Chang J. P., Ngo D., Catt K. J. Evidence for a role of protein kinase C in luteinizing hormone synthesis and secretion. Impaired responses to gonadotropin-releasing hormone in protein kinase C-depleted pituitary cells. J Biol Chem. 1988 Nov 25;263(33):17307–17311. [PubMed] [Google Scholar]
- Tang W. J., Gilman A. G. Type-specific regulation of adenylyl cyclase by G protein beta gamma subunits. Science. 1991 Dec 6;254(5037):1500–1503. doi: 10.1126/science.1962211. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Yasumura Y., Levine L., Sato G. H., Parker M. L. Establishment of clonal strains of rat pituitary tumor cells that secrete growth hormone. Endocrinology. 1968 Feb;82(2):342–352. doi: 10.1210/endo-82-2-342. [DOI] [PubMed] [Google Scholar]
- Tsutsumi M., Zhou W., Millar R. P., Mellon P. L., Roberts J. L., Flanagan C. A., Dong K., Gillo B., Sealfon S. C. Cloning and functional expression of a mouse gonadotropin-releasing hormone receptor. Mol Endocrinol. 1992 Jul;6(7):1163–1169. doi: 10.1210/mend.6.7.1324422. [DOI] [PubMed] [Google Scholar]
- Wildt L., Häusler A., Marshall G., Hutchison J. S., Plant T. M., Belchetz P. E., Knobil E. Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology. 1981 Aug;109(2):376–385. doi: 10.1210/endo-109-2-376. [DOI] [PubMed] [Google Scholar]
- Ying S. Y. Inhibins, activins, and follistatins: gonadal proteins modulating the secretion of follicle-stimulating hormone. Endocr Rev. 1988 May;9(2):267–293. doi: 10.1210/edrv-9-2-267. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]




